Abstract
BACKGROUND:
Although pediatric cancer mortality and survival have improved in the United States over the past 40 years, differences exist by age, race/ethnicity, cancer site, and economic status. To assess progress, this study examined recent mortality and survival data for individuals younger than 20 years.
METHODS:
Age-adjusted death rates were calculated with the National Vital Statistics System for 2002–2016. Annual percent changes (APCs) and average annual percent changes (AAPCs) were calculated with joinpoint regression. Five-year relative survival was calculated on the basis of National Program of Cancer Registries data for 2001–2015. Death rates and survival were estimated overall and by sex, 5-year age group, race/ethnicity, cancer type, and county-based economic markers.
RESULTS:
Death rates decreased during 2002–2016 (AAPC, –1.5), with steeper declines during 2002–2009 (APC, –2.6), and then plateaued (APC, –0.4). Leukemia and brain cancer were the most common causes of death from pediatric cancer, and brain cancer surpassed leukemia in 2011. Death rates decreased for leukemia and lymphoma but were unchanged for brain, bone, and soft-tissue cancers. From 2001–2007 to 2008–2015, survival improved from 82.0% to 85.1%. Survival was highest in both periods among females, those aged 15 to 19 years, non-Hispanic Whites, and those in counties in the top 25% by economic status. Survival improved for leukemias, lymphomas, and brain cancers but plateaued for bone and soft-tissue cancers.
CONCLUSIONS:
Although overall death rates have decreased and survival has increased, differences persist by sex, age, race/ethnicity, cancer type, and economic status. Improvements in pediatric cancer outcomes may depend on improving therapies, access to care, and supportive and long-term care.
Keywords: cancer, epidemiology, mortality, pediatric, survival
INTRODUCTION
Cancer is the second leading cause of death after injury among people aged 1 to 19 years in the United States.1 Five-year survival for patients with pediatric cancer improved from 63% in the 1970s to 83% in the 2000s.2 Children and adolescents who survive cancer often have chronic diseases due to their treatment that require long-term care and planning.3
Despite improvements in mortality and survival for patients with pediatric cancers over the past 40 years, progress has been limited for some cancer types diagnosed in childhood, including bone and soft-tissue cancers.2,4–7 Past studies have shown higher mortality with solid tumors versus leukemia and lymphoma and have described disparities in cancer outcomes, such as lower survival for Blacks versus Whites, which warrant further exploration using more recent data.5,8–10 Thus, this study examined surveillance data for pediatric cancer since 2001 and described recent trends.
This study used data available from the National Vital Statistics System (Centers for Disease Control and Prevention), which covers all US states and the District of Columbia, to describe death rates and data available from the National Program of Cancer Registries (NPCR; Centers for Disease Control and Prevention), which covers 93% of the US population, to describe survival.11–13 Because pediatric cancer incidence and survival differ on the basis of geographic area,14,15 use of databases that cover most of the US population can help us to describe trends and disparities in outcomes and regional variations, which can inform planning. Because NPCR survival data cover 93% of the US population, it can provide a more comprehensive picture of pediatric cancer survival than past studies using the Surveillance, Epidemiology, and End Results database, that covered ≤28% of the US population at the time of these studies.5,7
MATERIALS AND METHODS
This work was a secondary analysis of deidentified data; institutional review board review was not required. Death data from 2002–2016 for individuals younger than 20 years with cancer were based on death certificate information reported to state vital statistics offices and compiled by the National Vital Statistics System. This report includes all 50 states and the District of Columbia. Population estimates for denominators for death rates were obtained from the US Census and the National Cancer Institute16 and were aggregated to the county, state, and national level. Causes of death were categorized with the International Classification of Diseases, Tenth Revision and were grouped by site codes.16 Only malignant tumors were included. County-level variables were estimated from the 2012–2016 American Community Survey17 and were reported by quartile.
Death rates were age-adjusted to the 2000 US standard population, as previously done.2,15,18 Rates were expressed per 1 million persons. The annual percent change was calculated with joinpoint regression19,20 to quantify changes in death rates. The number of joinpoints was based on the length of the period, with up to 2 joinpoints allowed (allowing different slopes for up to 3 periods).20 Joinpoint models were selected with sequential permutation tests via the Joinpoint Regression Program.21 The average annual percent change was calculated to provide a single trend estimate during 2002–2016. Trends were considered statistically significant if they were different from zero at P < .05, and they were described as increasing or decreasing only if results were statistically significant. Death rates were estimated by sex; age group; race/ethnicity; US Census region; cause of death by cancer type; and county-level urban/rural status, education, poverty (percentage of families whose incomes are below the federal poverty level), and household income.22 Trends were estimated by sex, age group, race/ethnicity, region, and cause of death by cancer type.
Survival data were available from the NPCR survival data set, as described previously.8,23,24 Data were reported by central cancer registries to the NPCR and met publication criteria for inclusion in US Cancer Statistics.25,26 Vital status was determined on the basis of linkages with the National Death Index or on active patient follow-up conducted by the state before data submission. The analysis included data through the November 2018 data submission, which represented data from 43 NPCR central cancer registries, including all US states (except for Connecticut, Hawaii, Indiana, Iowa, Kansas, Nevada, New Mexico, and South Dakota, which did not conduct active case follow-up or linkage through the 2015 death file, did not provide the full date of death, or were not NPCR states) and the District of Columbia.
The survival analysis included patients diagnosed with malignant cancer at an age < 20 years and included first primary tumors only. Cancer was defined with codes from the International Classification of Diseases for Oncology, Third Edition.27 Patients identified only by autopsy or death certificate (0.3% of patients) were excluded.
Five-year relative survival was calculated for patients with cancer diagnosed during 2001–2015 with follow-up through December 31, 2015, the most recent date available. Cases diagnosed in 2016 did not have adequate follow-up time to be included in the survival analysis. Survival was calculated on the basis of expected life tables stratified by age, sex, race/ethnicity, socioeconomic status, geography, and year.28 Relative survival was defined as the ratio of the observed all-cause survival in a group of individuals with cancer to the expected all-cause survival of a similar group of individuals in the general population.8,24,29 Relative survival was calculated via the Ederer II method.30,31 The cohort method was used to estimate survival for pediatric patients diagnosed in 2001–2007, and the complete method was used for patients diagnosed in 2012–2015 with less than 5 years of follow-up. Five-year relative survival was calculated for sex, age, race/ ethnicity, US Census region, and county-based economic status8,32 and by cancer type according to cause-of-death codes and the International Classification of Childhood Cancer (ICCC).33 Relative survival during 2001–2007 was compared with relative survival during 2008–2015. We calculated differences between relative survival estimates by comparing 95% confidence intervals (CIs), which allowed for an informal, conservative comparison of estimates, as previously done.8,24 Survival between groups was described as different if 95% CIs did not overlap. Survival differences were described as “increased” or “improved” if 2008–2015 values were higher than those in 2001–2007 and 95% CIs did not overlap.
RESULTS
A total of 30,384 cancer deaths were reported among children and adolescents aged 0 to 19 years during 2002–2016 in the United States; this represented an overall annual rate of 25 cancer deaths per 1 million (Table 1). The most common cause of cancer death during 2002–2016 was leukemia (29%), which was followed by brain and other nervous system cancers (27%) and bone and joint cancers (9%). Death rates were highest in adolescents aged 15 to 19 years (31 per 1 million) and were higher in males (27 per 1 million) than females (22 per 1 million). Death rates among non-Hispanic White, non-Hispanic Black, and Hispanic groups had overlapping 95% CIs. Death rates were highest in the Western US Census region and were highest in metropolitan areas with populations ≥ 1 million (25 per 1 million) but had overlapping 95% CIs with death rates in nonmetropolitan areas. Death rates were higher in counties in the highest quartile of residents living in poverty than counties in the lowest quartile and were highest in counties with the least educational attainment (counties where ≥15.78% of the population aged ≥25 years had less than a high school education). Pediatric cancer death rates in the highest and lowest quartiles of county-level median household income had overlapping 95% CIs.
TABLE 1.
Age-Adjusted Death Rates and Trends for Individuals Younger Than 20 Years With Malignant Cancer in the United States, 2002–2016
| APC and Year Interval for Joinpoints |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | % of Total by Characteristic | Count | Rate (95% CI) | AAPC (95% CI) | No. of Joinpoints | Years | APC (95% CI) |
|
| |||||||
| Overall | 100.0 | 30,384 | 24.5 (24.3 to 24.8) | −1.5 (−2.1 to −0.9)a | 1 | 2002–2009 | −2.6 (−3.5 to −1.6)a |
| 2009–2016 | −0.4 (−1.4 to 0.6) | ||||||
| Sex | |||||||
| Male | 56.4 | 17,149 | 27.0 (26.6 to 27.4) | −1.5 (−2.3 to −0.7)a | 1 | 2002–2009 | −3.0 (−4.3 to −1.8)a |
| 2009–2016 | 0.0 (−1.3 to 1.4) | ||||||
| Female | 43.6 | 13,235 | 21.9 (21.5 to 22.3) | −1.5 (−1.9 to −1.0)a | 0 | ||
| Age, y | |||||||
| 0–14 | 67.6 | 20,546 | 22.5 (22.2 to 22.8) | −1.5 (−2.2 to −0.7)a | 1 | 2002–2009 | −2.6 (−3.7 to −1.4)a |
| 2009–2016 | −0.3 (−1.6 to 0.9) | ||||||
| 0–4 | 21.5 | 6547 | 21.9 (21.4 to 22.4) | −1.3 (−1.9 to −0.6)a | 0 | ||
| 5–9 | 23.1 | 7026 | 23.4 (22.8 to 23.9) | −1.6 (−2.4 to −0.9)a | 1 | 2002–2011 | −2.6 (−3.3 to −1.8)a |
| 2011–016 | −0.1 (−1.8 to 2.0) | ||||||
| 10–14 | 22.9 | 6973 | 22.3 (21.7 to 22.8) | −1.4 (−2.1 to −0.6)a | 0 | ||
| 15–19 | 32.4 | 9838 | 30.6 (30.0 to 31.2) | −1.6 (−2.1 to −1.1)a | 0 | ||
| Racial/ethnic groupb | |||||||
| White, non−Hispanic | 56.8 | 17,246 | 24.5 (24.1 to 24.9) | −1.5 (−2.1 to −0.8)a | 1 | 2002–2010 | −2.4 (−3.2 to −1.6)a |
| 2010–016 | −0.2 (−1.5 to 1.2) | ||||||
| Black, non−Hispanic | 15.4 | 4690 | 24.7 (24.0 to 25.4) | −1.4 (−2.0 to −0.7)a | 0 | ||
| Hispanic | 22.2 | 6760 | 25.2 (24.6 to 25.8) | −1.7 (−2.3 to −1.1)a | 0 | ||
| AI/AN, non−Hispanic | 0.8 | 258 | 20.1 (17.8 to 22.8) | −0.9 (−3.1 to 1.3) | 0 | ||
| API, non−Hispanic | 4.5 | 1353 | 21.8 (20.7 to 23.0) | −0.7 (−2.2 to 0.8) | 0 | ||
| US Census region | |||||||
| Northeast | 16.3 | 4950 | 23.5 (22.8 to 24.1) | −1.5 (−2.1 to −0.9)a | 0 | ||
| Midwest | 21.2 | 6447 | 23.8 (23.2 to 24.4) | −1.3 (−2.0 to −0.6)a | 0 | ||
| South | 37.1 | 11,284 | 24.6 (24.1 to 25.0) | −1.2 (−2.3 to −0.2)a | 1 | 2002–2009 | −2.9 (−4.5 to −1.3)a |
| 2009–2016 | 0.5 (−1.2 to 2.3) | ||||||
| West | 25.4 | 7703 | 26.0 (25.4 to 26.6) | −2.2 (−2.8 to −1.5)a | 0 | ||
| Cause of deathc | |||||||
| Liver | 2.5 | 756 | 0.6 (0.6 to 0.7) | −0.3 (−4.3 to 4.0) | 1 | 2002–2014 | −2.9 (−4.6 to −1.1)a |
| 2014–2016 | −17.0 (−14.5 to 60.2) | ||||||
| Bones and joints | 9.0 | 2749 | 2.2 (2.1 to 2.3) | 0.2 (−1.2 to 1.7) | 1 | 2002–2010 | −1.7 (−3.6 to 0.3) |
| 2010–2016 | 2.8 (−0.1 to 5.9) | ||||||
| Soft tissue, including heart | 7.7 | 2352 | 1.9 (1.8 to 2.0) | 0.2 (−0.9 to 1.4) | 0 | ||
| Melanoma of skin | 0.5 | 147 | 0.1 (0.1 to 0.1) | ||||
| Female genital system | 0.6 | 185 | 0.1 (0.1 to 0.2) | ||||
| Male genital system | 0.5 | 144 | 0.1 (0.1 to 0.1) | ||||
| Kidney and renal pelvis | 2.5 | 749 | 0.6 (0.6 to 0.7) | −2.7 (−4.5 to −0.9)a | 0 | ||
| Brain and other nervous system | 26.9 | 8168 | 6.7 (6.5 to 6.8) | −0.6 (−1.9 to 0.8) | 2 | 2002–2009 | −2.1 (−2.9 to −1.2)a |
| 2009–2012 | 3.8 (−3.2 to 11.4) | ||||||
| 2012–2016 | −1.1 (−3.1 to 1.0) | ||||||
| Other endocrine, including thymusd | 8.2 | 2488 | 2.0 (2.0 to 2.1) | −3.4 (−4.8 to −1.9)a | 1 | 2002–2014 | −2.0 (−2.9 to −1.1)a |
| 2014–2016 | −11.3 (−20.2 to −1.4)a | ||||||
| Lymphoma | 4.9 | 1481 | 1.2 (1.1 to 1.2) | −5.1 (−6.1 to −4.0)a | 0 | ||
| Hodgkin lymphoma | 0.8 | 253 | 0.2 (0.2 to 0.2) | ||||
| Non-Hodgkin lymphoma | 4.0 | 1228 | 1.0 (0.9 to 1.0) | −4.6 (−5.7 to −3.6)a | 0 | ||
| Leukemia | 28.5 | 8651 | 7.0 (6.8 to 7.1) | −2.8 (−3.3 to −2.3)a | 0 | ||
| Acute lymphocytic leukemia | 12.4 | 3766 | 3.0 (2.9 to 3.1) | −3.6 (−4.4 to −2.8)a | 0 | ||
| Acute myeloid leukemia | 9.4 | 2847 | 2.3 (2.2 to 2.4) | −2.0 (−3.0 to −1.1)a | 0 | ||
| % of county below poverty levele | |||||||
| 1.81–11.25 | 23.2 | 7118 | 23.3 (22.8 to 23.9) | ||||
| 11.26–15.02 | 22.9 | 7033 | 23.9 (23.4 to 24.5) | ||||
| 15.03–17.80 | 22.6 | 6943 | 24.9 (24.3 to 25.5) | ||||
| 17.81–53.95 | 30.3 | 9290 | 25.8 (25.3 to 26.3) | ||||
| % with <high school education by countye | |||||||
| 1.28–9.11 | 22.3 | 6847 | 22.7 (22.2 to 23.2) | ||||
| 9.12–12.04 | 22.9 | 7017 | 23.4 (22.9 to 24.0) | ||||
| 12.05–15.77 | 25.5 | 7826 | 25.2 (24.7 to 25.8) | ||||
| 15.78–51.48 | 28.3 | 8694 | 26.6 (26.1 to 27.2) | ||||
| Household income by county, median, $e | |||||||
| 18,970–46,950 | 25.3 | 7768 | 24.6 (24.0 to 25.1) | ||||
| 46,960–55,270 | 24.2 | 7412 | 24.2 (23.7 to 24.8) | ||||
| 55,280–64,760 | 25.9 | 7951 | 25.3 (24.8 to 25.9) | ||||
| 64,770–125,670 | 23.6 | 7253 | 24.0 (23.5 to 24.6) | ||||
| Rural/urban status (2010 Census) by county and populatione | |||||||
| Metro areas with ≥1 million | 55.7 | 17,085 | 25.2 (24.8 to 25.6) | ||||
| Metro areas with 250,000 to <1 million | 20.5 | 6302 | 23.7 (23.1 to 24.3) | ||||
| Metro areas with <250,000 | 8.5 | 2620 | 23.4 (22.5 to 24.3) | ||||
| Nonmetropolitan counties | 14.3 | 4377 | 24.2 (23.5 to 24.9) | ||||
Abbreviations: AAPC, average annual percent change; AI/AN, American Indian/Alaska Native; APC, annual percent change; API, Asian/Pacific Islander; CI, confidence interval.
The source for the data is the National Vital Statistics System (National Center for Health Statistics, Centers for Disease Control and Prevention).
Rates are per 1 million persons and are age-adjusted to the 2000 US standard population. Trends were measured with APCs and AAPCs in rates and were considered to increase or decrease if the 95% CI excluded 0 (P < .05); otherwise, trends were considered stable. Trends were calculated with joinpoint regression, which allowed different slopes for as many as 3 periods. AAPCs are presented for all groups; APCs are presented if joinpoints were identified.
Significant AAPC or APC.
Hispanic persons might be of any race; 77 cases of unknown race/ethnicity during 2002–2016 were excluded.
Causes of death were grouped by site codes. Not all causes of death by cancer type are listed here by type.
Included 146 endocrine tumors located in the brain (pituitary, craniopharyneal, or pineal; see https://wonder.cdc.gov/cancer.html).
AAPCs and APCs were not available for county-level variables.
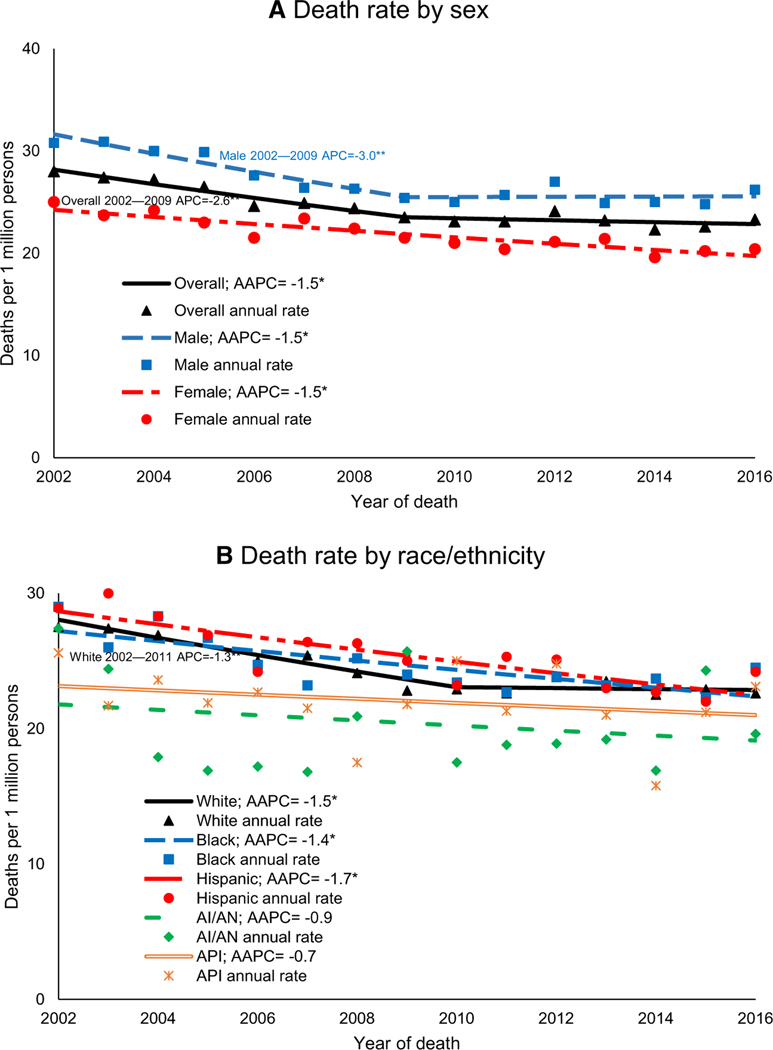
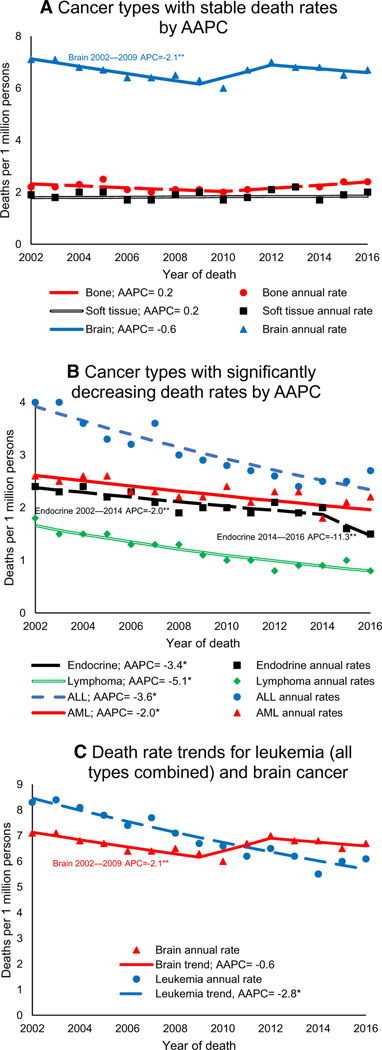
Overall pediatric cancer death rates decreased by 1.5% per year during 2002–2016 (95% CI, –2.1 to –0.9). Within this period, rates decreased during 2002–2009 and then stabilized during 2009–2016 (Table 1). During 2002–2016, pediatric cancer death rates decreased for both sexes, all age groups, non-Hispanic Whites, non-Hispanic Blacks, Hispanics, and all US Census regions (Fig. 1). For males, children aged 0 to 14 or 5 to 9 years, non-Hispanic Whites, and the South, rates first decreased and then stabilized (Table 1). During 2002–2016, deaths decreased for pediatric leukemia and lymphoma and were stable for brain, bone, and soft-tissue cancers (Fig. 2). Beginning in 2011, the death rate for brain cancer surpassed the death rate for all leukemias combined (Fig. 2).
FIGURE 1.
Trends in age-adjusted cancer death rates in persons younger than 20 years at death by (A) sex and (B) race/ethnicity (National Vital Statistics System, United States, 2002–2016). The source for the data is the National Vital Statistics System (National Center for Health Statistics, Centers for Disease Control and Prevention). Rates are per 1 million persons and are age-adjusted to the 2000 US standard population. Trends were measured with AAPCs in rates and were considered to increase or decrease if P was <.05; otherwise, trends were considered stable. Trends were calculated with joinpoint regression, which allowed different slopes for as many as 3 different periods. White, Black, AI/AN, and API persons were non-Hispanic. Hispanic persons might be of any race; 77 cases of unknown ethnicity during 2002–2016 were excluded. *Indicates a significant AAPC during 2002–2016. **Indicates a significant segment APC as listed on the corresponding segment in the figure. AAPC indicates average annual percent change; AI/ AN, American Indian/Alaska Native; APC, annual percent change; API, Asian/Pacific Islander.
FIGURE 2.
Trends in age-adjusted cancer death rates in persons younger than 20 years at death by the 7 cancer types with the highest death rates (National Vital Statistics System, United States, 2002–2016): (A) cancer types with stable death rates by AAPC, (B) cancer types with significantly decreasing death rates by AAPC, and (C) death rate trends for leukemia (all types combined) and brain cancer. The source for the data is the National Vital Statistics System (National Center for Health Statistics, Centers for Disease Control and Prevention). Rates are per 1 million persons and are age-adjusted to the 2000 US standard population. Trends were measured with AAPCs in rates and were considered to increase or decrease if P was <.05; otherwise, trends were considered stable. Trends were calculated with joinpoint regression, which allowed different slopes for as many as 3 different periods. Causes of death were grouped by site codes. Not all causes of death by cancer type are listed here by type. Endocrine included 146 endocrine tumors located in the brain (pituitary, craniopharyneal, or pineal; see https://wonder.cdc.gov/cancer.html). *Indicates a significant AAPC during 2002–2016. **Indicates a significant segment APC as listed on the corresponding segment in the figure. AAPC indicates average annual percent change; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; APC, annual percent change.
During 2001–2015, among the 185,312 patients included in the survival analysis, relative survival was 83.5% (95% CI, 83.3%−83.7%; Table 2). Relative survival for females (84.6%; 95% CI, 84.3%−84.8%) was higher than that for males (82.6%; 95% CI, 82.4%−82.9%), and relative survival for adolescents aged 15 to 19 years (84.3%; 95% CI, 84.0%−84.6%) was higher than that for children aged 0 to 14 years (81.3%; 95% CI, 82.9%−83.4%) at diagnosis. Relative survival was highest for non-Hispanic Whites and was lowest for non-Hispanic Blacks. Relative survival was highest for patients in counties with the highest economic status and in the Northeastern US Census region. Among common cancer types, relative survival was 88.2% (95% CI, 87.9%−88.6%) for patients with acute lymphocytic leukemia (ALL), 91.6% (95% CI, 91.2%−91.9%) for patients with lymphoma, and 75.3% (95% CI, 74.8%−75.8%) for patients with brain cancer. By ICCC group, patients with leukemias and lymphomas had relative survival of 83.3% (95% CI, 83.0%−83.7%) and 91.9% (95% CI, 91.5%−92.2%), respectively, whereas patients with brain, bone, and soft-tissue cancers had relative survival of 74.9% (95% CI, 74.4%−75.4%), 70.7% (95% CI, 69.6%−71.6%), and 74.1% (95% CI, 73.2%−74.9%), respectively (Supporting Table 1).
TABLE 2.
Five-Year Relative Survival of Individuals Younger Than 20 Years Diagnosed With Malignant Cancer in the United States, 2001–2015
| 2001–2015 |
2001–2007 |
2008–2015 |
||||
|---|---|---|---|---|---|---|
| Characteristic | Count | Relative Survival, % (95% CI) | Count | Relative Survival, % (95% CI) | Count | Relative Survival, % (95% CI) |
|
| ||||||
| Overall | 185,312 | 83.5 (83.3–83.7) | 89,275 | 82.0 (81.8–82.3) | 96,037 | 85.1 (84.9–85.4) |
| Sex | ||||||
| Male | 99,191 | 82.6 (82.4–82.9) | 47,970 | 81.1 (80.7–81.4) | 51,221 | 84.3 (83.9–84.6) |
| Female | 86,121 | 84.6 (84.3–84.8) | 41,305 | 83.2 (82.8–83.5) | 44,816 | 86.1 (85.7–86.4) |
| Age, y | ||||||
| 0–14 | 126,243 | 83.1 (82.9–83.4) | 60,547 | 81.6 (81.3–81.9) | 65,696 | 84.8 (84.5–85.1) |
| 0–4 | 58,728 | 83.8 (83.5–84.1) | 28,243 | 82.3 (81.9–82.8) | 30,485 | 85.3 (84.9–85.8) |
| 5–9 | 31,612 | 83.0 (82.5–83.4) | 14,820 | 81.4 (80.7–82.0) | 16,792 | 84.6 (84.0–85.2) |
| 10–14 | 35,903 | 82.2 (81.8–82.7) | 17,484 | 80.7 (80.1–81.2) | 18,419 | 84.1 (83.5–84.6) |
| 15–19 | 59,069 | 84.3 (84.0–84.6) | 28,728 | 83.0 (82.5–83.4) | 30,341 | 85.8 (85.3–86.2) |
| Racial/ethnic groupa | ||||||
| White, non–Hispanic | 112,198 | 85.2 (85.0–85.4) | 56,239 | 83.9 (83.6–84.2) | 55,959 | 86.7 (86.4–87.0) |
| Black, non–Hispanic | 22,228 | 77.8 (77.2–78.3) | 10,587 | 75.8 (74.9–76.6) | 11,641 | 79.9 (79.0–80.6) |
| Hispanic | 39,320 | 81.8 (81.3–82.2) | 17,392 | 79.5 (78.9–80.1) | 21,928 | 83.8 (83.2–84.3) |
| AI/AN, non–Hispanic | 1553 | 80.9 (78.7–82.9) | 723 | 79.8 (76.7–82.6) | 830 | 81.6 (78.4–84.4) |
| API, non–Hispanic | 7418 | 82.6 (81.7–83.5) | 3200 | 80.6 (79.2–81.9) | 4218 | 84.3 (83.1–85.5) |
| County–level economic statusb | ||||||
| Top 25% | 49,317 | 85.1 (84.8–85.4) | 23,495 | 83.6 (83.2–84.1) | 25,822 | 86.6 (86.2–87.1) |
| Middle 25%–75% | 108,946 | 83.1 (82.9–83.4) | 52,835 | 81.7 (81.3–82.0) | 56,111 | 84.7 (84.4–85.1) |
| Lower 25% | 23,050 | 81.5 (81.0–82.0) | 11,001 | 79.9 (79.1–80.6) | 12,049 | 83.2 (82.4–83.9) |
| US Census region | ||||||
| Northeast | 34,754 | 85.3 (84.9–85.7) | 17,157 | 83.9 (83.4–84.5) | 17,597 | 86.8 (86.3–87.4) |
| Midwest | 35,050 | 84.2 (83.7–84.6) | 17,344 | 83.1 (82.6–83.7) | 17,706 | 85.3 (84.8–85.9) |
| West | 44,128 | 82.8 (82.5–83.2) | 21,257 | 80.9 (80.4–81.4) | 22,871 | 84.8 (84.3–85.3) |
| South | 71,380 | 82.8 (82.5–83.1) | 33,517 | 81.2 (80.8–81.6) | 37,863 | 84.4 (84.0–84.8) |
| Cause of death | ||||||
| Liver | 2678 | 68.4 (66.6–70.2) | 1239 | 64.3 (61.5–66.9) | 1439 | 72.5 (69.9–74.9) |
| Bones and joints | 9423 | 70.7 (69.7–71.6) | 4739 | 70.1 (68.8–71.4) | 4684 | 71.3 (69.7–72.8) |
| Soft tissue, including heart | 10,829 | 75.9 (75.1–76.8) | 5298 | 75.4 (74.3–76.6) | 5531 | 76.3 (75.0–77.5) |
| Melanoma of skin | 5192 | 96.1 (95.5–96.6) | 3021 | 96.5 (95.7–97.1) | 2171 | 95.5 (94.3–96.4) |
| Female genital system | 3878 | 91.0 (90.1–91.9) | 1932 | 90.6 (89.2–91.9) | 1946 | 91.5 (90.0–92.7) |
| Male genital system | 6145 | 94.6 (93.9–95.2) | 2971 | 94.2 (93.3–95.0) | 3174 | 94.9 (94.0–95.7) |
| Brain and other nervous system | 33,631 | 75.3 (74.8–75.8) | 16,272 | 74.1 (73.4–74.7) | 17,359 | 76.7 (76.0–77.4) |
| Endocrine system | 14,385 | 87.9 (87.4–88.5) | 6362 | 86.0 (85.1–86.8) | 8023 | 89.9 (89.1–90.6) |
| Kidney and renal pelvis | 7506 | 89.3 (88.6–90.0) | 3603 | 87.4 (86.3–88.4) | 3903 | 91.3 (90.3–92.3) |
| Lymphoma | 25,365 | 91.6 (91.2–91.9) | 12,484 | 90.0 (89.5–90.6) | 12,881 | 93.2 (92.7–93.7) |
| Hodgkin lymphoma | 13,242 | 96.0 (95.6–96.3) | 6603 | 95.3 (94.8–95.8) | 6639 | 96.7 (96.2–97.2) |
| Non–Hodgkin lymphoma | 12,123 | 86.7 (86.1–87.4) | 5881 | 84.1 (83.2–85.1) | 6242 | 89.4 (88.5–90.2) |
| Leukemia | 46,102 | 83.3 (82.9–83.6) | 22,424 | 81.1 (80.6–81.6) | 23,678 | 85.7 (85.2–86.2) |
| Acute lymphocytic leukemia | 34,784 | 88.2 (87.9–88.6) | 16,782 | 86.8 (86.2–87.3) | 18,002 | 89.9 (89.3–90.3) |
| Acute myeloid leukemia | 7252 | 64.7 (63.6–65.9) | 3570 | 60.9 (59.3–62.5) | 3682 | 69.0 (67.3–70.6) |
Abbreviations: AI/AN, American Indian/Alaska Native; API, Asian/Pacific Islander; CI, confidence interval.
The source for the data is the National Program of Cancer Registries (Centers for Disease Control and Prevention). Bolding indicates nonoverlapping 95% CIs when 2001–2007 and 2008–2015 are compared.
Hispanic persons might be of any race; 2595 cases of unknown race during 2001–2015 were excluded.
Excluded 3999 cases with missing economic status.
Comparing 2001–2007 with 2008–2015, we found that the overall 5-year relative survival increased from 82.0% (95% CI, 81.8%−82.3%) to 85.1% (95% CI, 84.9%−85.4%; Table 2). Relative survival improved for both sexes, all ages, all races/ethnicities (except American Indians and Alaska Natives), all county-level economic status groupings, and all US Census regions. By cancer type, relative survival improved for leukemia, lymphoma, and brain tumors but was stable for bone and soft-tissue tumors. When we looked at survival by age at diagnosis, relative survival did not improve for adolescents aged 15 to 19 years with brain tumors (Supporting Table 2). For children aged 0 to 14 years, relative survival improved for neuroblastoma, nephroblastoma, and hepatic tumors. Acute myeloid leukemia (AML) relative survival improved from 62.3% (95% CI, 60.5%−64.0%) to 68.9% (95% CI, 67.0%−70.7%) in children aged 0 to 14 years and from 54.1% (95% CI, 51.2%−57.0%) to 66.5% (95% CI, 63.5%−69.3%) in adolescents aged 15 to 19 years.
DISCUSSION
This study used data covering all US states and the District of Columbia to describe decreasing death rates of pediatric cancer overall, and it presents more recent data than past national studies of pediatric cancer mortality.4,5,34 This study used high population coverage data to describe improvements in survival in all US Census regions and all county-level economic statuses. In light of stable or only slightly increasing pediatric cancer incidence rates over the past 2 decades,35,36 decreasing death rates are consistent with overall increasing relative survival. Although improvements in outcomes were seen in pediatric leukemia and lymphoma, this study found that pediatric bone and soft-tissue cancers have had modest or no improvements in mortality and survival.
Leukemia and brain cancer have the highest cancer incidence rates among children in the United States and account for the majority of pediatric cancer deaths. Brain cancer surpassed leukemia as the most common cancer-causing death, and this is consistent with past literature.34 ALL and AML accounted for 12.4% and 9.4% of pediatric cancer deaths, respectively, despite ALL occurring 5 times more often than AML in children aged 0 to 14 years and twice as often in adolescents aged 15 to 19 years.2
Pediatric ALL and AML death rates decreased and survival increased during 2001–2016. The decrease in pediatric leukemia death rates over the past 40 years has been attributed to advances in treatment and supportive care, which have been driven by clinical research efforts.2,5,37,38 Cooperative pediatric clinical trials have been instrumental in driving this improvement.37,38 Pediatric AML patients continue to have lower survival than pediatric ALL patients. For pediatric AML, the principal therapeutic agents have not changed significantly in recent years.39 Improvements in survival could have been driven by advances in risk stratification, the adjustment of chemotherapy dosages and timing, hematopoietic cell transplantation, salvage therapy, and better supportive care.38,39 Survival improvements have not been as robust in patients with high-risk AML (those with a higher risk of failing induction therapy) in comparison with patients with low-risk AML.39 Novel therapies such as immunotherapies might help to improve survival for patients with high-risk AML.39,40 New immunotherapies such as chimeric antigen receptor T therapy have shown effectiveness for pediatric ALL and may further improve survival for patients with pediatric ALL and reduce chemotherapy-related toxicity.41,42 Improvements in lymphoma outcomes were seen during the study period, and they may be due to the implementation of combined-modality and risk-adapted therapies.43,44
Pediatric solid tumor death rates and survival have improved overall since the 1970s, but depending on the tumor type, age of the patient, and metastatic spread, improvements have been either modest or stable during the past 2 decades.2,4,5,45 Some improvements, such as those for patients with pediatric brain tumors, could be due to advances in neuroimaging, surgical technology, radiation therapy delivery, and supportive care.46 Although this study found improvements in survival for patients with brain cancer, mortality was stable. Further evaluation of survival more than 5 years after diagnosis, which is lower for patients with pediatric brain tumors than those with leukemia and lymphoma,2 may be needed to better understand this discrepancy. Patients with neuroblastomas, who showed increased survival in this study over time, had improved outcomes in past studies in part because of advances in treatment such as the use of targeted antibody therapy.5,47 The most common pediatric bone cancers (osteosarcomas and Ewing tumors) and soft-tissue sarcomas (rhabdomyosarcomas)35 showed no survival improvement in this study. The absence of new therapeutic agents and the limited ability to optimize existing agents have contributed to the lack of progress for many of these cancers.45,48,49 Improvements in outcomes have been particularly scarce for metastatic bone and soft-tissue tumors over the past 2 or 3 decades.45 However, advances have been made to better understand the molecular and genetic characteristics of pediatric solid tumors with the goal of identifying actionable targets for therapy, and researchers are working to translate these findings into effective clinical therapies.5,49,50 Novel therapies for bone, soft-tissue, and brain tumors, such as immunotherapies, are being investigated.46,50,51
Understanding differences by age may inform interventions to improve outcomes. Consistent with past reports, this study reported that cancer death rates were higher in adolescents aged 15 to 19 years in comparison with children aged 0 to 14 years.4,5 However, death rates significantly improved in adolescents. Similarly to past analyses, the current study found improved but overall lower survival for adolescents with AML in comparison with children.52 The biological characteristics of tumors in children can differ from those seen in adolescents; for example, adolescents with AML tend to have more unfavorable cytogenetics.53 In addition, as adolescents get older, they are less often referred to and receive cancer care from Children’s Oncology Group institutions.54 As a result, adolescents increasingly are treated at institutions that do not have access to pediatric clinical trial protocols and are less likely to be enrolled in clinical trials than children.54,55 These differences may contribute to higher mortality among adolescents and less improvement in survival over time.56,57 Future progress in outcomes for adolescents may depend on advances in biologically based therapies, better understanding of clinical referral patterns, and measures to increase clinical trial enrollment.
Differences in cancer outcomes have been reported for many types of pediatric cancer and may be influenced by interrelated factors, including race/ethnicity, economic status, geographic location, access to care, and host or tumor genetic factors.8,10,58–61 In this study, although death rates had overlapping 95% CIs when we compared non-Hispanic Whites, non-Hispanic Blacks, and Hispanics, 5-year survival was highest for non-Hispanic Whites, and this might be related to these interrelated factors. For example, past studies of adolescents and young adults with Hodgkin lymphoma found lower survival among Blacks than Whites, among those with a lower socioeconomic status, and by insurance status.44,62 Other pediatric cancer types, including brain and other solid tumors such as neuroblastoma, have shown similar disparities in cancer outcomes by race/ethnicity and socioeconomic status.8,61,63
This study found higher death rates in counties with lower education levels, and this is consistent with findings from past studies examining adults with cancer.60 Higher survival from childhood cancer is associated with higher parental educational levels; however, a number of mechanisms, such as higher levels of social support and an ability to adhere to treatment regimens, may mediate this association.64,65 Moreover, the current study found higher death rates in counties with higher poverty and higher survival in counties with a higher economic status. Economically distressed communities may have fewer resources and less access to care, which could affect outcomes.58 Poverty status and health insurance coverage may also be related to differences in clinical trial enrollment.55 This study found similar death rates in nonmetropolitan areas and metropolitan areas with populations ≥ 1 million, and this is consistent with a recent study that found no significant differences in mortality based on distance from Children’s Oncology Group treatment centers55 and with another study that found no differences in pediatric cancer survival based on rural/urban status.66 Consistent mortality across rural and urban counties might be due to relatively consistent insurance coverage among pediatric patients, and this might not be as true for adolescent and young adult patients.66
A more thorough examination of the root causes of disparities in cancer mortality and survival might be invaluable for identifying potential interventions that could improve long-term outcomes. Further investigation might better elucidate how factors such as systemic inequality, health literacy, and host or tumor genetic variations affect outcomes and associated factors such as stage at presentation. Public health interventions might be able to improve childhood cancer outcomes through, for example, initiatives that increase outreach to increase clinical trial enrollment, increase access to care, and promote educational interventions addressing both patient education and physician communication.62,67,68
The findings of this report are subject to at least 4 limitations. First, the methods used to report death rates do not allow for descriptions of specific tumor subtypes. Although overall rates of leukemia and brain cancer can be described, certain notable pediatric cancer histologies, such as nephroblastoma (Wilms tumor) and neuroblastoma, were not specifically characterized. However, these subtypes were characterized by ICCC groupings in the survival analysis. Second, this study reported death rates among those younger than 20 years and thus does not reflect pediatric cancer deaths occurring in early adulthood. Because some cancer types (eg, lymphoma and bone cancer) increase in incidence during the ages of 15 to 19 years,2 death data for patients older than 19 years may be needed to more completely characterize death from these pediatric cancers. However, survival data in this report describe 5-year outcomes of patients diagnosed at an age as old as 19 years. Third, because county-level variables were used to assess economic, location, and education status, the analysis could not account for individual measures of economic status such as individual insurance status or household income. In addition to individual measures of economic status, other individual factors or potential confounders that might differentiate outcomes in patients, such as treatment, genetic factors, and comorbidities, were not available and could not be used to adjust the analysis.8 Finally, misclassification by race/ethnicity may occur, and rate numerators may underestimate Hispanics, American Indians, and Alaska Natives; this could artificially lower rates.69 Life tables for Asians and Pacific Islanders may be less reliable than those of other races.70
An estimated 360,164 people younger than 40 years in the United States had received a diagnosis of pediatric cancer as of January 1, 2015.71 Childhood cancer survivors often face long-term complications from their cancer treatment, including secondary cancers, heart disease, and infertility.2 Knowledge of mortality trends and changes in survival for pediatric cancer, especially as it relates to cancer type, can help clinicians and public health planners to address the long-term needs of pediatric cancer survivors. Further research focusing on novel therapies for those cancers with the least change in morality and survival, such as brain, bone, and soft-tissue cancers, may be essential to improving outcomes for these patients. A better understanding of the complex relationship between race/ethnicity and economic factors such as poverty, household income, and health insurance status could inform interventions to improve disparities in health outcomes.55 Continued surveillance of cancer outcomes can be used to assess population-level changes that result from new treatment strategies, such as targeted therapies, especially for cancer types with moderate to no improvements in mortality and survival over the past 2 decades. Finally, the use of updated clinical care guidelines for pediatric cancer survivors72 can affect mortality rates and survival, and surveillance data could be used to better understand outcomes for this growing survivor population.
Supplementary Material
Acknowledgments
We acknowledge state and regional cancer registry and health department personnel.
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379:2468–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Trends in childhood cancer mortality—United States, 1990–2004. MMWR Morb Mortal Wkly Rep. 2007;56:1257–1261. [PubMed] [Google Scholar]
- 5.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer. 2014;120:2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Close AG, Dreyzin A, Miller KD, Seynnaeve BKN, Rapkin LB. Adolescent and young adult oncology—past, present, and future. CA Cancer J Clin. 2019;69:485–496. [DOI] [PubMed] [Google Scholar]
- 8.Siegel DA, Li J, Ding H, Singh SD, King JB, Pollack LA. Racial and ethnic differences in survival of pediatric patients with brain and central nervous system cancer in the United States. Pediatr Blood Cancer. 2019;66:e27501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrahao R, Lichtensztajn DY, Ribeiro RC, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988–2011: a population-based observational study. Pediatr Blood Cancer. 2015;62:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Mortality—All COD, Aggregated Total U.S. (1990–2016) <Katrina/Rita Population Adjustment>, National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Released December 2018. Underlying Mortality Data Provided by NCHS. Accessed January 31, 2019. www.cdc.gov/nchs [Google Scholar]
- 12.Xu JQ, Murphy SL, Kochanek KD, Bastian B, Arias E. Deaths: final data for 2016. Natl Vital Stat Rep. 2018;67:1–76. [PubMed] [Google Scholar]
- 13.National Program of Cancer Registries. SEER*Stat Database: NPCR Survival Analytic File 2001–2015. United States Department of Health and Human Services, Centers for Disease Control and Prevention. Released June 2019, Based on the November 2018 Submission. [Google Scholar]
- 14.Ostrom QT, de Blank PM, Kruchko C, et al. Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015;16(suppl 10):x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel DA, Li J, Henley SJ, et al. Geographic variation in pediatric cancer incidence—United States, 2003–2014. MMWR Morb Mortal Wkly Rep. 2018;67:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute. U.S. mortality data, 1969–2016. Accessed July 19, 2019. https://seer.cancer.gov/mortality/
- 17.US Census Bureau. American Community Survey (ACS). Accessed January 29, 2020. https://www.census.gov/programs-surveys/acs
- 18.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47:1–16. [PubMed] [Google Scholar]
- 19.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Number of joinpoints. Accessed January 27, 2020. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/number-of-joinpoints
- 21.National Cancer Institute. Joinpoint Regression Program, version 4.6.0.0. Accessed January 31, 2019. https://surveillance.cancer.gov/joinpoint/
- 22.National Cancer Institute. County attributes. Accessed August 25, 2019. https://seer.cancer.gov/seerstat/variables/countyattribs/
- 23.Wilson RJ, Ryerson AB, Zhang K, Dong X. Relative survival analysis using the Centers for Disease Control and Prevention’s National Program of Cancer Registries surveillance system data, 2000–2007. J Registry Manag. 2014;41:72–76. [PMC free article] [PubMed] [Google Scholar]
- 24.Razzaghi H, Saraiya M, Thompson TD, Henley SJ, Viens L, Wilson R. Five-year relative survival for human papillomavirus–associated cancer sites. Cancer. 2018;124:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program. SEER*Stat Database: NPCR Survival Analytic file 2001–2005. United States Department of Health and Human Services, Centers for Disease Control and Prevention. Released June 2019, Based on the November 2018 Submission. [Google Scholar]
- 26.Centers for Disease Control and Prevention. U.S. Cancer Statistics publication criteria. Accessed January 27, 2020. https://www.cdc.gov/cancer/uscs/technical_notes/criteria/index.htm
- 27.Fritz A, Percy C, Jack A, et al. , eds. International Classification of Diseases for Oncology. 3rd ed. 1st rev. World Health Organization; 2013. [Google Scholar]
- 28.Surveillance, Epidemiology, and End Results Program. SEER*Stat Database: Expected Survival—U.S. by SES/Geography/Race (NHW, NHB, NHAIAN, NHAPI, HISP) 1992–2013, Ages 0–99, State-County (Modeled by Varied State-County-SES), National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch. Accessed June 26, 2019. https://seer.cancer.gov/
- 29.Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975–2014, Bethesda, MD: . National Cancer Institute; 2017. https://seer.cancer.gov/csr/1975_2014/. [Google Scholar]
- 30.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 31.Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260:103–117. [DOI] [PubMed] [Google Scholar]
- 32.Appalachian Regional Commission. County economic status and distressed areas in the Appalachian Region, fiscal year 2011. Accessed June 8, 2018. https://www.arc.gov/research/sourceandmethodologycountyeconomicstatusfy2007fy2016.asp
- 33.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005; 103:1457–1467. [DOI] [PubMed] [Google Scholar]
- 34.Curtin SC, Minino AM, Anderson RN. Declines in cancer death rates among children and adolescents in the United States, 1999–2014. NCHS Data Brief. 2016;257:1–8. [PubMed] [Google Scholar]
- 35.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Pediatrics. 2014;134:e945–e955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward E, Sherman RL, Henley SJ, et al. Annual report to the nation on the status of cancer, featuring cancer in men and women ages 20–49. J Natl Cancer Inst. 2019;111:1279–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Leary M, Krailo M, Anderson JR, Reaman GH. Progress in childhood cancer: 50 years of research collaboration, a report from the Children’s Oncology Group. Semin Oncol. 2008;35:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33:2949–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander TB, Wang L, Inaba H, et al. Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials. Cancer. 2017;123:3791–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonifant CL, Velasquez MP, Gottschalk S. Advances in immunotherapy for pediatric acute myeloid leukemia. Expert Opin Biol Ther. 2018;18:51–63. [DOI] [PubMed] [Google Scholar]
- 41.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finney OC, Brakke HM, Rawlings-Rhea S, et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J Clin Invest. 2019;129:2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cairo MS, Pinkerton R. Childhood, adolescent and young adult non-Hodgkin lymphoma: state of the science. Br J Haematol. 2016;173:507–530. [DOI] [PubMed] [Google Scholar]
- 44.Keegan TH, DeRouen MC, Parsons HM, et al. Impact of treatment and insurance on socioeconomic disparities in survival after adolescent and young adult Hodgkin lymphoma: a population-based study. Cancer Epidemiol Biomarkers Prev. 2016;25:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perkins SM, Shinohara ET, DeWees T, Frangoul H. Outcome for children with metastatic solid tumors over the last four decades. PLoS One. 2014;9:e100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollack IF, Agnihotri S, Broniscer A. Childhood brain tumors: current management, biological insights, and future directions. J Neurosurg Pediatr. 2019;23:261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer. 2018;65:e27227. [DOI] [PubMed] [Google Scholar]
- 49.Saraf AJ, Fenger JM, Roberts RD. Osteosarcoma: accelerating progress makes for a hopeful future. Front Oncol. 2018;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz LM, Majzner R, Davis KL, Mackall C. New developments in immunotherapy for pediatric solid tumors. Curr Opin Pediatr. 2018;30:30–39. [DOI] [PubMed] [Google Scholar]
- 51.Majzner RG, Heitzeneder S, Mackall CL. Harnessing the immunotherapy revolution for the treatment of childhood cancers. Cancer Cell. 2017;31:476–485. [DOI] [PubMed] [Google Scholar]
- 52.Rubnitz JE, Pounds S, Cao X, et al. Treatment outcome in older patients with childhood acute myeloid leukemia. Cancer. 2012;118:6253–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Creutzig U, Kutny MA, Barr R, Schlenk RF, Ribeiro RC. Acute myelogenous leukemia in adolescents and young adults. Pediatr Blood Cancer. 2018;65:e27089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musselman JR, Spector LG, Krailo MD, et al. The Children’s Oncology Group Childhood Cancer Research Network (CCRN): case catchment in the United States. Cancer. 2014;120:3007–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tai E, Hallisey E, Peipins LA, et al. Geographic access to cancer care and mortality among adolescents. J Adolesc Young Adult Oncol. 2018;7:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007;25:4616–4621. [DOI] [PubMed] [Google Scholar]
- 57.Gupta S, Pole JD, Baxter NN, et al. The effect of adopting pediatric protocols in adolescents and young adults with acute lymphoblastic leukemia in pediatric vs adult centers: an IMPACT cohort study. Cancer Med. 2019;8:2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Academies of Sciences, Engineering, and Medicine. Communities in Action: Pathways to Health Equity. National Academies Press; 2017. [PubMed] [Google Scholar]
- 59.Kehm RD, Spector LG, Poynter JN, Vock DM, Altekruse SF, Osypuk TL. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer. 2018;124:4090–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. [DOI] [PubMed] [Google Scholar]
- 61.Bhatia S.Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeRouen MC, Parsons HM, Kent EE, Pollock BH, Keegan THM. Sociodemographic disparities in survival for adolescents and young adults with cancer differ by health insurance status. Cancer Causes Control. 2017;28:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Austin MT, Nguyen H, Eberth JM, et al. Health disparities are important determinants of outcome for children with solid tumor malignancies. J Pediatr Surg. 2015;50:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Isaevska E, Popovic M, Alessi D, et al. Association between maternal education and survival after childhood cancer. Pediatr Blood Cancer. 2019;66:e27616. [DOI] [PubMed] [Google Scholar]
- 65.Syse A, Lyngstad TH, Kravdal O. Is mortality after childhood cancer dependent on social or economic resources of parents? A population-based study. Int J Cancer. 2012;130:1870–1878. [DOI] [PubMed] [Google Scholar]
- 66.Delavar A, Feng Q, Johnson KJ. Rural/urban residence and childhood and adolescent cancer survival in the United States. Cancer. 2018;125:261–268. [DOI] [PubMed] [Google Scholar]
- 67.Curry WT Jr, Barker FG II. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93:25–39. [DOI] [PubMed] [Google Scholar]
- 68.Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65:221–238. [DOI] [PubMed] [Google Scholar]
- 69.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States). Cancer Causes Control. 2006;17:771–781. [DOI] [PubMed] [Google Scholar]
- 70.National Cancer Institute. Expected survival tables. Accessed July 18, 2019. https://seer.cancer.gov/expsurvival/
- 71.National Cancer Institute. Table 28.12: childhood cancer (invasive, <20 years old at diagnosis). Accessed June 5, 2019. https://seer.cancer.gov/csr/1975_2015/browse_csr.php?sectionSEL=28&pageSEL=sect_28_table.12
- 72.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 5.0. Accessed June 6, 2019. http://www.survivorshipguidelines.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.