Summary
Invasive fungal disease (IFD) remains a challenging complication of treatment for paediatric acute leukaemia. Consensus fungal treatment guidelines recommend withholding chemotherapy to facilitate immune recovery in this setting, yet prolonged delays in leukaemia therapy increase risk of relapse. Blinatumomab, a bispecific T‐cell engager targeting cells expressing CD19, has shown promise for treatment of relapsed/refractory B‐cell acute lymphoblastic leukaemia (B‐ALL) and is associated with reduced toxicity compared to conventional chemotherapy. With close monitoring of minimal residual disease, we demonstrate that children with B‐ALL can receive repeated cycles of bridging blinatumomab whilst conventional chemotherapy is withheld during treatment and recovery from IFD.
Keywords: acute lymphoblastic leukaemia, blinatumomab, immunotherapy, invasive fungal disease, paediatric
INTRODUCTION
Over time, risk‐adapted intensification of chemotherapy has significantly improved survival for children with B‐cell acute lymphoblastic leukaemia (B‐ALL), 1 however this has led to an increase in the burden of infectious complications. Invasive fungal disease (IFD) is particularly problematic, 2 , 3 with an attributable mortality of 15%–20%. 4 Immune recovery is vital for successful treatment of IFD, and is generally achieved by delaying further chemotherapy following IFD diagnosis, yet specific guidance regarding the optimal duration of delay is lacking. 5 , 6 Any decision requires balancing the risk of leukaemia progression from delaying chemotherapy against the risk of progressive fungal infection due to further immunosuppression with continuation of chemotherapy.
Blinatumomab is a bispecific T‐cell engager which facilitates CD3 T‐cell binding and elimination of cells expressing CD19, including both normal B cells and B‐ALL blasts. Blinatumomab is less immunosuppressive with fewer infectious complications compared to standard chemotherapy in patients with relapsed/refractory B‐ALL. 7 , 8 T cell and innate immune responses are key in combating fungal infection, whilst the role of B‐cell immunity against fungi is limited. 9 Blinatumomab therefore presents an attractive option for ongoing leukaemia therapy in patients with B‐ALL and IFD to maximise immune reconstitution whilst maintaining leukaemia control. Here, we present the successful use of blinatumomab as a therapeutic bridge following diagnosis and treatment of IFD in a cohort of children with B‐ALL.
METHODS
Cases were identified from the leukaemia database at Perth Children's Hospital, the sole tertiary paediatric hospital for treatment of children with cancer in Western Australia. Inclusion criteria comprised patients with B‐ALL and IFD who received blinatumomab between 1 January 2018 to 31 December 2021. The decision to initiate blinatumomab in this setting was at the discretion of the treating oncologist. Clinical data collected included infection characteristics, time to initiation of blinatumomab, number of blinatumomab treatment cycles, lymphocyte and neutrophil counts at commencement of and during blinatumomab therapy, infection outcome, B‐ALL therapy and disease status. Minimal residual disease (MRD) was assessed by flow cytometry prior to, and following, each blinatumomab cycle. IFD was defined as possible, probable or proven based on the European Organisation for Research and Treatment of Cancer (EORTC) definitions. 10 Suspected IFD was defined as previously described (treated as IFD) for cases not fulfilling EORTC criteria. 3 This study was approved by the Child and Adolescent Health Service, Quality and Safety Committee (quality activity no. 42738) with delegated authority from the Perth Children's Hospital Human Research Ethics Committee, within which the work was undertaken. Written informed consent was obtained from each patient's parent for their clinical information to be used for publication.
RESULTS
During the study period, there were 133 children who received active treatment for B‐ALL. There were 15 patients with IFD of which eight (median age 7.5 years [range 6–15 years]; 50% female) received blinatumomab bridging therapy for nine IFD episodes (Table 1; Proven, n = 4; Possible, n = 4; Suspected n = 1). Eight IFD episodes were diagnosed in the context of treatment for primary ALL, six of which occurred during or immediately following induction chemotherapy. One episode occurred during induction chemotherapy for relapsed ALL (patient 2). Cytogenetic characteristics of the leukaemic blasts for each patient are shown in Table S1. Two IFD episodes were diagnosed concomitantly with an episode of bacterial sepsis (patients 1 and 2). Two patients were receiving micafungin prophylaxis at the time of IFD diagnosis (patients 2 and 8), three received fluconazole and three did not receive any antifungal prophylaxis.
TABLE 1.
Patient characteristics, fungal infection details, treatment and outcome
| Patient | Age (years) | Sex | Underlying diagnosis | Protocol and cycle at the time of infection MRD at the time of infection | Infection | Blinatumomab | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism and site | Treatment details | Time to initiation (weeks) | Number of cycles | MRD at the end of each blinatumomab cycle | Total time off chemo (weeks) | Infection status | Current treatment | Current ALL disease status | |||||
| 1 | 15 | M | B‐ALL (HR) | COG AALL1131 induction MRD <0.01% |
Escherichia coli (Septic shock, multi‐organ failure) Possible IFD (lung) |
IV antibiotics and liposomal amphotericin ICU, ECMO, mechanical ventilation, inotropes | 8 | 3 | <0.01% | 24 | Complete response | COG AALL1131 maintenance | Complete remission |
| COG AALL1131 interim maintenance MRD <0.01% | Possible IFD (lung) | Liposomal amphotericin followed by voriconazole | 1 | 2 | <0.01% | 13 | |||||||
| 2 | 7 | F | Relapsed B‐ALL | COG AALL1331 induction MRD <0.01% |
Escherichia coli and MSSA (Septic shock, ARDS, multi‐organ failure) Proven IFD Corprinopsis cinerea (lung) |
IV antibiotics, Liposomal amphotericin and voriconazole ICU, mechanical ventilation, inotropes Resection of mycetoma |
8 | 2 | <0.01% | 20 | Complete response | Completed treatment according to COG AALL1331 low risk arm D | Second CD19+ relapse 20 months later. Currently in third complete remission |
| 3 | 6 | M | B‐ALL (SR) | COG AALL0932 induction MRD <0.01% | Proven IFD Aspergillus fumigatus (disseminated, CNS, lung) |
Voriconazole and liposomal amphotericin Craniotomy and resection of CNS fungal lesion |
5 | 7 | <0.01% | 43 | Complete response | COG AALL0932 maintenance | Complete remission |
| 4 | 7 | F | B‐ALL (SR) | COG AALL0932 induction MRD <0.01% | Suspected IFD (CNS/ leptomeningeal) | Voriconazole | 3 | 2 | <0.01% | 12 | Complete response | COG AALL0932 maintenance | Complete remission |
| 5 | 8 | M | B‐ALL (SR) | COG AALL0932 induction MRD <0.01% | Proven IFD Aspergillus flavus (Disseminated, lung, skin) | Voriconazole and caspofungin | 3 | 3 | <0.01% | 21 | Complete response | COG AALL0932 maintenance | Complete remission |
| 6 | 5 | F | B‐ALL (VHR) | COG AALL1131 induction MRD 0.14% | Possible IFD (lung) | Liposomal amphotericin | 1 | 1 | <0.01% | 5 | Complete response | COG AALL1131 maintenance | Complete remission |
| 7 | 8 | F | B‐ALL (SR) | COG AALL1731 induction MRD <0.01% | Proven IFD Lichthaemia spp. (lung) |
Liposomal amphotericin followed by posaconazole Lobectomy |
2 | 2 | <0.01% | 12 | Complete response | COG AALL0932 maintenance | Complete remission |
| 8 | 8 | M | B‐ALL (HR) | COG AALL1731 SR‐High consolidation MRD <0.01% | Possible IFD (lung) | Liposomal amphotericin followed by voriconazole | 1 | 5 | <0.01% | 26 | Complete response | COG AALL1731 SR‐high consolidation | Complete remission |
Abbreviations: ARDS, acute respiratory distress syndrome; B‐ALL, B‐cell acute lymphoblastic leukaemia; CNS, central nervous system; COG, Children's Oncology Group; ECMO, extracorporeal membrane oxygenation; HR, high risk; ICU, intensive care unit admission; IFD, invasive fungal disease; IV, intravenous; MRD, minimal residual disease; MSSA, methicillin‐susceptible Staphylococcus aureus; SR, standard risk; VHR, very high risk.
Each cycle of blinatumomab was administered over 28 days at a target dose of 15 μg/m2/day, with intrathecal methotrexate dosed according to age on days 14 and 28. The duration of bridging blinatumomab ranged from 1 to 7 cycles (median 2 cycles). The median time from IFD diagnosis to initiation of blinatumomab was 3 weeks (range 1–8 weeks). The median time prior to recommencing conventional chemotherapy was 20 weeks (range 5–43 weeks).
Following initiation of blinatumomab, neutrophil count was maintained (>1.0 × 109/L) throughout bridging therapy in five of nine IFD episodes; four patients (patients 1 [first episode], 2, 3 and 8) had a brief self‐resolving period of neutropenia (<7 days) during blinatumomab therapy but otherwise maintained a normal neutrophil count. Lymphocyte count dropped transiently (<1.0 × 109/L) in the 2 days following the first blinatumomab cycle in seven episodes and remained normal in two. Thereafter, lymphocyte count >1.0 × 109/L was maintained throughout bridging therapy in six of nine episodes. Two patients (patients 2 and 3) had transient brief periods of lymphopenia (0.2–1.0 × 109/L) during blinatumomab and one (patient 4) had persistent lymphopenia (0.2–1.0 × 109/L) throughout blinatumomab therapy. Seven patients were in MRD remission (<0.01%) prior to commencing blinatumomab with MRD remission maintained following each cycle of blinatumomab. One patient had detectable MRD of 0.14% prior to commencing blinatumomab (patient 6) and achieved MRD remission following one cycle of blinatumomab.
The treatment administered to each patient for IFD is specified in Table 1. All patients had resolution of symptoms and signs of IFD and a complete radiological response. For patient 3, who had pulmonary aspergillosis and multiple cerebral aspergillomas, there was partial but incomplete resolution on repeat cranial imaging initially; subsequent surgical resection was undertaken prior to recommencement of chemotherapy (Figure 1).
FIGURE 1.
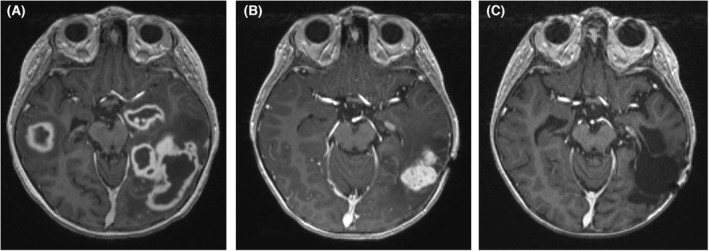
T1 weighted magnetic resonance imaging of the brain of patient 3 who had disseminated invasive aspergillosis. (A) At diagnosis – demonstrating multiple cerebral aspergillomas. (B) Repeat imaging after 8 months of antifungal therapy and seven cycles of bridging blinatumomab – showing overall improvement but a persistent left temporal lobe lesion. (C) Repeat imaging following interval resection of a left temporal aspergilloma and recommencement of conventional chemotherapy – demonstrating stable resection cavity without evidence of progressive infection.
Following blinatumomab bridging, all patients successfully recommenced the conventional chemotherapy they were receiving at the time of IFD from the timepoint at which therapy was paused. Seven patients continue to receive upfront therapy in first complete remission. Patient 2 completed all planned therapy for first relapse but suffered a second CD19+ relapse 20 months later and has completed reinduction therapy with a view to allogeneic haematopoietic stem cell transplant (HSCT). All patients remain in complete clinical, morphological and MRD remission at the end of the study period (median 24 months follow up).
DISCUSSION
In this cohort of children with B‐ALL complicated by IFD, blinatumomab bridging therapy enabled ongoing targeted treatment for leukaemia whilst delaying the significant immunosuppression associated with conventional chemotherapy. This facilitated immune recovery and allowed time for resolution of infection, contributing to successful treatment in all nine episodes.
IFD is a serious complication of ALL therapy with prevalence exceeding 10% during treatment for high‐risk primary ALL and relapsed leukaemia. 2 , 3 Successful treatment requires optimal antifungal therapy as well as host immune reconstitution. For invasive aspergillosis, consensus guidelines recommend withholding chemotherapy for “a period of several weeks of antifungal treatment and clear evidence of response” to avoid potential progression of infection in the setting of further myelosuppression. 5 However, prolonged delays in chemotherapy may increase the risk of ALL relapse. 11 Similarly, for mucormycosis, continued antifungal treatment is recommended until permanent reversal of immunosuppression. 6 Voriconazole and posaconazole, recommended agents for treatment of invasive aspergillosis and mucormycosis respectively, 5 , 6 inhibit vincristine metabolism via cytochrome P450. Concomitant use of these agents in patients with ALL may lead to severe neurotoxicity, further complicating treatment decisions. 12 The use of blinatumomab allowed for ongoing triazole antifungal therapy in seven of our patients, avoiding the need for triazole dose interruptions due to the potential for adverse drug reactions around vincristine dosing.
Blinatumomab is less immunosuppressive compared to standard chemotherapy for relapsed/refractory B‐ALL, with fewer infectious complications. 7 , 8 In clinical trials of blinatumomab in children with relapsed/refractory ALL, IFD appears rare (0%–1%). 13 This is in keeping with the understanding of the host immune response to fungal pathogens where phagocytes and T‐lymphocytes are vital, and B cells have a limited role. 9 Blinatumomab bridging therefore presents a useful option in patients with B‐ALL diagnosed with IFD during therapy, avoiding both drug interactions with triazole antifungals and the myelosuppression associated with conventional ALL therapy.
This treatment approach has been reported in a limited number of patients to date. A report from the Israeli Study Group of Childhood Leukaemia included four patients with IFD who received blinatumomab bridging. 11 The interval prior to commencing blinatumomab was more than 5 weeks (range 40–60 days). Two patients received a single blinatumomab cycle, one received two and one four. Following blinatumomab, two patients proceeded to allogeneic HSCT, one proceeded to a “chemotherapy trial” followed by maintenance and the final patient proceeded directly to maintenance therapy. Similarly, a single patient from a separate cohort in North America received one cycle of blinatumomab following IFD diagnosis prior to recommencing conventional chemotherapy. 14 There were no deaths attributed to IFD. In our cohort blinatumomab was initiated relatively promptly (within 3 weeks for most episodes) following IFD diagnosis and all patients subsequently recommenced chemotherapy following recovery from IFD according to their ALL treatment plan.
Previously blinatumomab use outside of clinical trials has been mostly limited to 1 or 2 cycles. 11 , 14 , 15 It remains uncertain as to how many cycles can safely be given in the context of bridging therapy for IFD, where ideally further chemotherapy should be withheld until at least partial resolution of infection. Our approach of monitoring MRD at the end of each blinatumomab cycle, demonstrates a model of care that allows balanced decision‐making regarding timing of reinitiation of conventional chemotherapy in conjunction with assessment of infection response. In all our patients, MRD remission was maintained following each cycle of blinatumomab, including in one patient who received seven cycles through a prolonged period without conventional chemotherapy in the setting of disseminated aspergillosis (patient 3).
The addition of blinatumomab to conventional chemotherapy is currently being assessed in primary B‐ALL (NCT03914625, NCT03643276) and incorporation of such immunotherapeutic approaches into upfront therapy may lead to a reduction in infectious complications associated with conventional chemotherapy. In the interim, as demonstrated in our cohort, blinatumomab can be utilised as bridging therapy for patients with B‐ALL in the setting of severe fungal infection, to minimise myelosuppression during treatment of IFD whilst maintaining leukaemia control.
AUTHOR CONTRIBUTIONS
Daniel K. Yeoh and Rishi S. Kotecha conceived and designed the study. Daniel K. Yeoh performed data collection and drafted the initial manuscript. Christopher C. Blyth and Rishi S. Kotecha reviewed and edited the manuscript. All authors approved the final version of the manuscript for submission.
FUNDING INFORMATION
This was an investigator‐initiated study, there are no funding sources to declare.
CONFLICT OF INTEREST
There is no conflict of interest.
PATIENT CONSENT STATEMENT
Written informed consent was obtained from each patient's parent for their clinical information to be used for publication.
Supporting information
Table S1
ACKNOWLEDGEMENT
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Yeoh DK, Blyth CC, Kotecha RS. Blinatumomab as bridging therapy in paediatric B‐cell acute lymphoblastic leukaemia complicated by invasive fungal disease. Br J Haematol. 2022;198:887–892. 10.1111/bjh.18314
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–52. [DOI] [PubMed] [Google Scholar]
- 2. Lehrnbecher T, Schöning S, Poyer F, Georg J, Becker A, Gordon K, et al. Incidence and outcome of invasive fungal diseases in children with hematological malignancies and/or allogeneic hematopoietic stem cell transplantation: results of a prospective multicenter study. Front Microbiol. 2019;10(681):681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang SS, Kotecha RS, Bernard A, Blyth CC, McMullan BJ, Cann MP, et al. Invasive fungal infections in children with acute lymphoblastic leukaemia: results from four Australian centres, 2003–2013. Pediatr Blood Cancer. 2019;66(10):e27915. [DOI] [PubMed] [Google Scholar]
- 4. Pana ZD, Roilides E, Warris A, Groll AH, Zaoutis T. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc. 2017;6(Suppl_1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cornely OA, Alastruey‐Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J‐M, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown PA, Ji L, Xu X, Devidas M, Hogan LE, Borowitz MJ, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease‐free survival in children, adolescents, and young adults with first relapse of B‐cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. 2021;325(9):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma A, Wuthrich M, Deepe G, Klein B. Adaptive immunity to fungi. Cold Spring Harb Perspect Med. 2014;5(3):a019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elitzur S, Arad‐Cohen N, Barzilai‐Birenboim S, Ben‐Harush M, Bielorai B, Elhasid R, et al. Blinatumomab as a bridge to further therapy in cases of overwhelming toxicity in pediatric B‐cell precursor acute lymphoblastic leukemia: report from the Israeli Study Group of Childhood Leukemia. Pediatr Blood Cancer. 2019;66(10):e27898. [DOI] [PubMed] [Google Scholar]
- 12. Yeoh DK, Haeusler GM, McMullan BJ, Butters C, Bryant PA, Clark JE, et al. Antifungal use in children with acute leukaemia: state of current evidence and directions for future research. J Antimicrob Chemother. 2022;77(6):1508–1524. 10.1093/jac/dkac060 [DOI] [PubMed] [Google Scholar]
- 13. Locatelli F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingebiel T, et al. Effect of blinatumomab vs chemotherapy on event‐free survival among children with high‐risk first‐relapse B‐cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. 2021;325(9):843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Contreras CF, Higham CS, Behnert A, Kim K, Stieglitz E, Tasian SK. Clinical utilization of blinatumomab and inotuzumab immunotherapy in children with relapsed or refractory B‐acute lymphoblastic leukemia. Pediatr Blood Cancer. 2021;68(1):e28718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sutton R, Pozza LD, Khaw SL, Fraser C, Revesz T, Chamberlain J, et al. Outcomes for Australian children with relapsed/refractory acute lymphoblastic leukaemia treated with blinatumomab. Pediatr Blood Cancer. 2021;68(5):e28922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


