Abstract
Background
Congenital cardiac outflow defects (COD) are the largest group of congenital heart defects, with ventricular septal defect (VSD) as the most prevalent phenotype. Increased maternal age, excessive oxidative stress and inflammation are involved in the pathophysiology of COD and enhance telomere length (TL) shortening. We investigated the association between periconception maternal TL and the risk of having a child with COD.
Methods
From a multicentre case‐control trial, 306 case mothers of a child with COD and 424 control mothers of a child without a congenital malformation were selected. Relative TL was measured by qPCR. Multivariable logistic regression was used to compute crude and adjusted odds ratios, per standard deviation decrease, between maternal T/S ratio and COD and VSD risk. Adjustments were made for maternal age. Additional adjustments were made in a second model.
Results
Shorter maternal relative TL was significantly associated with an OR of 1.29 (95% CI 1.04–1.61), p = .02, for the risk of VSD in offspring, which remained significant after an adjustment for maternal age (adjOR 1.25(95% CI 1.01–1.55), p = .04). No association between maternal TL and the risk of overall COD in offspring was observed.
Conclusion
Shorter maternal relative TL is associated with an approximately 1.3‐OR for the risk, per SD in relative TL shortening, of VSD in the offspring. These findings need further confirmation in other studies on the predictive value of maternal TL.
Keywords: biomarker, congenital heart defects, life course, maternal age, oxidative stress
1. INTRODUCTION
Congenital heart defects (CHD) are among the most common congenital malformations in newborns with approximately 1 million children born each year worldwide. Although the mortality of CHD has decreased in the past decade, 1 the quality of life of these patients is often compromised due to severe physical and psychological problems. 2
Several studies have shown a multifactorial origin of birth defects such as CHD, where interactions between genetic predispositions and environmental exposures in early pregnancy are strongly involved in the pathogenesis. 3 The most common CHD are cardiac outflow defects (COD), with ventricular septal defect (VSD) as the most prevalent phenotype, accounting for approximately 35% of CHD, with the perimembranous VSD as the most common subtype (80%). 1
The extracardiac contribution of neural crest cells together with the secondary heart field, both located in the pharynx, play an essential role in the septation process of the outflow tracts of the cardiac ventricle. 4
Maternal hyperhomocysteinemia is a risk factor for the development of neural crest‐related anomalies in offspring, such as spina bifida, cleft lip and/or palate and congenital outflow defects in offspring. 5 , 6 Hyperhomocysteinemia disturbs apoptosis and myocardialization of the cardiac outflow tract, by affecting the cardiac neural crest cells. 7 In this manner, other studies have shown the influence of one carbon metabolism on neural crest cells and thereby neural crest cells related congenital birth defects. 8 Maternal health conditions, such as maternal age, obesity, tobacco and alcohol use, poor nutrition and lifestyle, enhance oxidative stress and inflammation and are identified as risk factors for COD. 9 Telomeres are nucleoprotein structures that cap the end of chromosomes and thereby protect against unwanted recombination and degradation. In humans, TL shortens in somatic cells with advancing age due to the increased number of cellular divisions. Excessive TL shortening is an index of senescence, causes genomic instability and is associated with a higher risk of age‐related diseases. Recent findings showed that newborn TL determines the adult TL. 10 It has been suggested that TL is a long‐term biomarker of chronic oxidative stress compared with hyperhomocysteinemia as short‐term biomarker. 11 , 12
Primary prevention of COD in offspring might be possible when we have more insight into the role of TL as a biomarker in the pathogenesis to be used as future predictor of COD, and the associations with maternal health conditions. Therefore, as a first step, we aim to study the association between periconception maternal TL and the risk of having a child with a neural crest‐related COD.
2. METHODS
2.1. Study population
Data and blood samples were used from the HAVEN study (Acronym for Heart Defects, Vascular Status, Genetic Factors and Nutrition), a multicenter case‐control triad study on COD, conducted at the Department of Obstetrics and Gynaecology, Division of Obstetrics and Foetal Medicine of the Erasmus MC, University Medical Centre Rotterdam, The Netherlands. The study was designed to investigate parental health, environmental and genetic determinants in the pathogenesis and prevention of COD offspring. Data have been collected between 2003 and 2010, and the study design has previously been described in detail. 13 The HAVEN study enrolled 904 case and control mothers (Figure 1). Mothers pregnant at the study moment (n = 77), missing folate or homocysteine status (n = 94) and missing TL assessment (n = 3) were excluded from the analysis. In total, 306 case mothers of a child with COD and 424 control mothers of a child without congenital malformations were selected and included for analysis. In summary, all cases were diagnosed with COD by ultrasound and/or cardiac catheterization and/or surgery by a paediatric cardiologist. Only patients with a type of CHD that has been associated with folate, or other environmental factors before, were included, to increase the homogeneity of the case group. Control children and their parents were eligible if congenital malformations or chromosomal abnormalities were not present. The VSD group consist of perimembranous ventricular septal defects and atrioventricular septal defects. Only singletons, biological parents with no familial relationship and whom are familiar with the Dutch language (writing and reading), could participate. Fasting venous blood samples were drawn from all mothers, approximately 1 year after the periconception period of the index pregnancy, that is around 15 months. This interval was chosen to mimic the periconceptional health status of the mother and to minimize the risk of undiagnosed less severe COD in the control group. The same interval was used to obtain general information on lifestyle behaviours and demographic data by questionnaires. General questionnaire contained items on maternal age, ethnicity, education, family history, obstetrical history and periconception exposures such as folic acid supplement use and diet. After birth, data on foetal gender and pregnancy outcome were obtained from medical records. Ethnicity and educational level were classified according to the definitions of Statistics Netherlands. 14 , 15 All self‐administered questionnaires were checked by the researcher for completeness and consistency during the hospital visit. Standardized anthropometric measurements including height, weight and blood pressure were performed in the hospital to limit measurement errors. The samples, for the measurement of the biomarkers in plasma total homocysteine, in serum folate red blood cell, serum folate and in serum vitamin B12, from cases and controls were analysed over similar time frames. Appendix A, describes the measurements of the biomarkers in more detail, as previously described by Obermann‐Borst et al. 13
FIGURE 1.
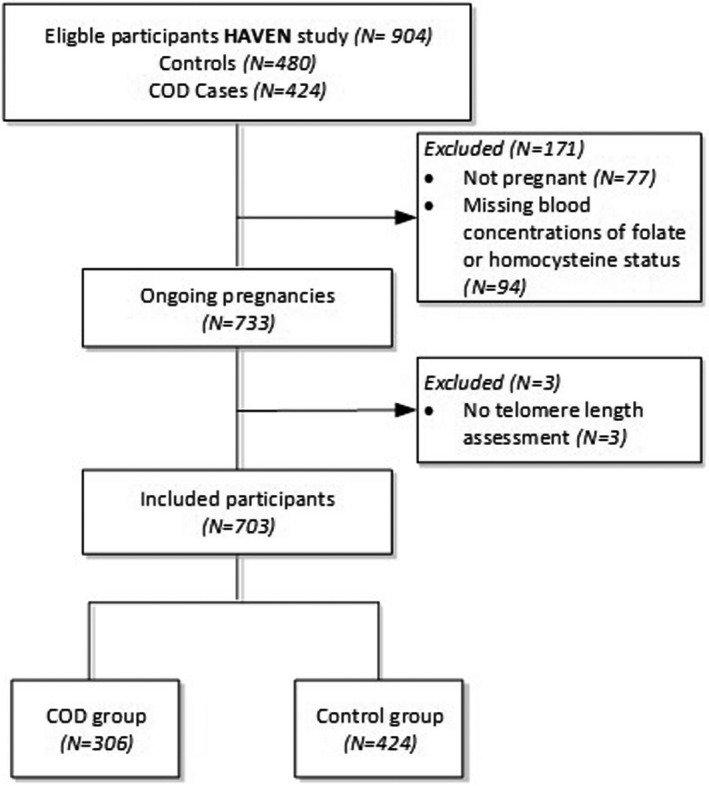
Flowchart of inclusions and exclusions of the study population in the HAVEN study (Heart Defects, Vascular Status, Genetic Factors and Nutrition). COD, congenital outflow defects
The study protocol was approved by The Central Committee on Research involving Human Subjects and the institutional review boards of all participating hospitals. All parents gave their written informed consent, as well as on behalf of their participating child.
Reporting of the study conforms to broad EQUATOR guidelines. 16
2.2. DNA isolation and Telomere length measurement
Genomic DNA from case and control mothers was randomly extracted in the same time period from EDTA blood samples (stored at −80°C until isolation) with the Reliaprep kit (Promega, Leiden, Netherlands) on a Tecan Evo robot. DNA integrity was checked for 30 randomly chosen samples on the 4200 Tapestation system (Agilent, Santa Clara, United states of America) and were found to be above >10 kbp. DNA concentrations and purities (OD260/280 and OD260/230) were measured with the Nanodrop (ThermoFisher, Waltham, United States of America). The concentrations of all samples were normalized to 50 ng/μl, and the purities were checked and found to be in the accepted range (OD260/280: 1.8–2.0 and OD260/230: 2.0–2.2).
Relative TL (TS ratio) was measured using a singleplex qPCR assay based on the method described by Cawthon 17 with minor modifications. For each sample, the telomere and 36B4 assay were run in the same well position but in different 384 wells PCR plates. Each reaction contained 5 μl reaction volume with 2 ng DNA, 1 μM of each of the telomere primers (tel1b‐forward: GGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT, tel2b‐reverse: GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCTT) or 250 nM of the 34B4 primers (36B4u‐forward: CAGCAAGTGGGAAGGTGTAATCC, 36B4d‐reverse: CCCATTCTATCATCAACGGGTACAA) and 1x Quantifast SYBR green PCR Mastermix (Qiagen, Hilden, Germany). The reactions for both assays were performed in duplicate for each sample in a QuantStudio Flex 7 real‐time PCR machine (Applied Biosystems, Waltham, United States of America). Used cycling condition were as follows: 1 cycles at 95°C for 15 min, 40 cycles at 95°C for 15 s and 60°C for 1 min. After each qPCR, a melting curve was run which showed a single peak for both assays. The cycle threshold (Ct) values and PCR efficiencies were calculated per plate using the MINER algorithm. 18 Duplicate Ct values with a Coefficient of Variance (CV) of more than 1% were repeated a second time in a different run. The average PCR efficiency per plate was 84% for the TEL assay and 79% for the 36B4 assay. This was consistent for all plates. To quantify the TEL and 36B4 assay per sample, the average Ct value (of the duplicate measurements) and the average qPCR efficiency per plate was used in the formula Q = 1/(1+PCR eff)^Ct. The TS ratio was calculated by dividing the Q of the telomere assay by the Q of the 36B4 assay. Each 384 wells PCR plate contained a set of 7 control samples. The average TS ratio of these 7 samples was used to normalize for plate batch effects. To validate the measured TS ratios, 170 randomly chosen samples were run for a second time (same DNA isolation, but different DNA dilutions). The validation experiment was performed a week later and the interclass coefficient of that experiment was 0.94. 19
2.3. Statistical analyses
Characteristics were compared between case and control mothers. Descriptive statistics of the study population were presented using means and standard deviations for normal distributed variables and median and interquartile range for skewed variables. Analysis was performed by Student's t test and Mann–Whitney U test, respectively. Frequencies (proportions) were used for categorical variables and were compared by chi‐square test.
Spearman correlations was used to evaluate correlations between maternal age, and homocysteine, and relative TL.
Multivariable logistic regression was used to compute crude and adjusted odds ratios (OR) per standard deviation (SD) decrease between the maternal T/S ratio with 95% confidence intervals (95% CI) and COD and VSD risk in offspring. The ORs were adjusted for maternal age, as known confounder for TL and COD (model 1). In a second model, we additionally adjusted for maternal body‐mass‐index, ethnicity, education and use of alcohol or smoking during pregnancy based on the characteristics of the two study populations and literature. 20 As homocysteine is an important oxidative stress marker, the analyses were also performed after adjustment for homocysteine concentrations.
This study was conducted on an available sample size of 306 case mothers and 424 control mothers. Assuming a correlation between maternal age and maternal relative TL no larger than 0.2, we can demonstrate an OR >1.24 of the standardized relative TL with 80% power based on simulation. Probability values ≤0.05 were considered statistically significant. All analyses were performed with R (R for Windows, version 3.5; R Core Team).
3. RESULTS
General characteristics, lifestyle, biomarkers as folate, homocysteine and vitamin concentrations and foetal outcomes, of case mothers and control mothers were summarized in Table 1. The interval after index pregnancy, BMI, maternal COD incidence, ethnicity, use of alcohol, smoking and folic acid supplement use were comparable between both groups. Case mothers had a significantly higher age (p = .009) with a mean age of 31.8 years in case mothers and 30.9 years in control mothers. A positive family history for COD was more common in case mothers. A significant difference was found in education level between cases and controls, where a low and high education level was more common in the case mothers. Homocysteine and folate concentrations were comparable between the groups. Birth weight of offspring was significantly lower in the group of case mothers compared with control mothers. Preterm birth was significantly more common in case mothers.
TABLE 1.
General characteristics of the study population of case mothers (COD offspring) and control mothers (offspring without congenital defects)
|
Case mothers n = 306 |
Control mothers n = 424 |
Missings | p‐value | |
|---|---|---|---|---|
| Demographics | ||||
| Maternal age, years a | 31.8 (4.7) | 30.9 (4.6) | 1 | .009 |
| Time after index pregnancy, months | 16.2 [15.2, 17.9] | 16.3 [15.3, 18.0] | 1 | .79 |
| Mode of conception, spontaneous | 289 (94.4) | 401 (94.6) | 1 | .82 |
| Body mass index, [kg/m2] | 24.0 [22.0, 28.0] | 24.0 [22.0, 27.0] | 1 | .25 |
| Education level | ||||
| Low | 86 (28.1) | 93 (22.1) | 3 | .02 |
| Intermediate | 126 (41.2) | 216 (51.3) | ||
| High | 94 (30.7) | 112 (26.6) | ||
| Geographic origin | ||||
| Western | 270 (88.2) | 356 (84.2) | 1 | .15 |
| Non‐Western | 36 (11.8) | 67 (15.8) | ||
| Mother with COD | 8 (2.6) | 4 (0.9) | 0 | .15 |
| Family history of COD | 43 (14.1) | 31 (7.3) | 0 | .009 |
| Lifestyle | ||||
| Periconception use of (n (%)) | ||||
| Alcohol | 113 (36.9) | 138 (32.5) | 4 | .51 |
| Cigarettes | 57 (18.6) | 87 (20.5) | 0 | .56 |
| Folic acid and/or (multi)vitamins | 254 (83.0) | 339 (80.1) | 0 | .38 |
| Current use of (n (%)) | ||||
| Alcohol | 157 (51.3) | 233 (55.0) | 1 | .47 |
| Cigarettes | 53 (17.3) | 78 (18.4) | 2 | .57 |
| Folic acid and/or (multi)vitamins | 83 (27.1) | 107 (25.3) | 0 | .64 |
| Biomarkers | ||||
| Vitamine B12, serum (pmol/L) | 277.5 [209.5, 361.8] | 260.0 [201.0, 357.5] | 0 | .36 |
| Plasma total homocysteine, plasma, (μmol/L) | 10.1 [8.4, 12.5] | 9.7 [8.2, 11.8] | 0 | .05 |
| Folate, serum (nmol/L) | 15.2 [12.4, 19.4] | 14.70 [12.2, 19.6] | 0 | .37 |
| Folate, red blood cell (nmol/L) | 659 [529, 802] | 634 [521, 769] | 0 | .36 |
| Birth outcomes | ||||
| Foetal gender, boys | 173 (56.5) | 234 (55.2) | 0 | .78 |
| Birthweight | 3290 [2850, 3650] | 3500 [3200, 3890] | 1 | <.001 |
| Preterm birth (<37 weeks gestation) | 48 (15.8) | 24 (5.7) | 4 | <.001 |
Abbreviation; COD, congenital outflow defects.
Presented as mean (SD). Data are presented as median [IQR] or number of individuals (proportions).
The COD phenotypes of case children (n = 306) comprised aortic valve stenosis (n = 7), atrioventricular septal defect (n = 29), perimembranous ventricular septal defect (n = 84), pulmonary valve stenosis (n = 42), coarctation of the aorta (n = 33), hypoplastic left heart syndrome (n = 12), transposition of the great arteries (n = 50), tetralogy of Fallot (n = 38) and others (n = 11). Two hundred and thirty‐five of the 306 cases were defined as isolated COD cases. Seventy‐three cases were non‐isolated and had another major structural congenital anomaly besides COD. Forty‐two of the 72 non‐isolated cases had a genetic syndrome, that is trisomy (n = 27), deletion 22q11 (n = 5), Noonan syndrome (n = 4), Beckwith‐Wiedemann syndrome (n = 1), CHARGE syndrome (n = 1), Saethre‐Chotzen syndrome (n = 1), Alagille syndrome (n = 1), Kartagener syndrome (n = 1) and Turner syndrome (n = 1). The distribution of maternal relative TL (T/S ratio) among the controls, COD cases and VSD cases were depicted in Figure 2. Maternal age revealed a significantly inverse correlation with relative TL (R = −.13; p = <.001). There was no correlation between the homocysteine concentrations in plasma and relative TL (R = .037; p = .32) (Figure A1). A positive family history for COD was not significantly associated with relative TL. A multivariable logistic model was used to determine the independent association of maternal relative TL with COD risk in offspring (Table 2A). Model 1 and model 2 showed no significant associations between shorter maternal relative TL, per decrease in standard deviation of relative TL, and the risk of COD offspring (crude OR 1.10 (95% CI 0.95–1.27), p = .22). Model 1: adjOR 1.07 (95% CI 0.92–1.24), p = .37 and Model 2: adjOR 1.07 (95% CI 0.92–1.24), p = .40. The syndromal and non‐syndromal COD group showed also a comparable non‐significant association, crude OR 1.15 (95% CI 0.84–1.58), p = .36 and crude OR 1.09 (95% CI 0.93–1.27), p = .28, respectively.
FIGURE 2.
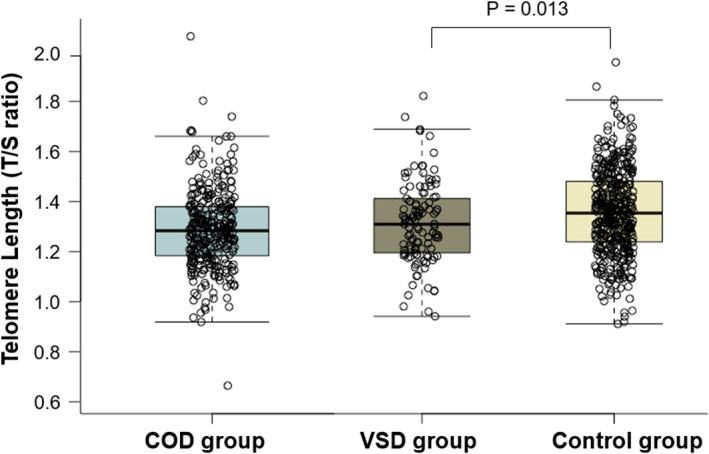
Box‐and‐whisker plot demonstrating the distribution of telomere length (T/S ratio) in control mothers (offspring without congenital defects) [N = 424] compared with total congenital outflow defect group (COD) [N = 306] and ventricular septal defect (VSD) group [N = 113]. Boxplots present median, 10th, 25th, 75th, and 90th percentile. Telomere length were compared by Mann‐Whitney U test
TABLE 2.
Maternal telomere length (T/S ratio) in association with the risk of congenital outflow defects (COD) (A) or ventricular septal defect (VSD) (B) in offspring
| A. Risk of COD | Cases/controls (n = 306/424) | Crude OR (95% CI) | p‐value | OR (95% CI) a | p‐value | OR (95% CI) b | p‐value |
|---|---|---|---|---|---|---|---|
| Total study group | 306/424 | 1.10 (0.95–1.27) | .22 | 1.07 (0.92–1.24) | .37 | 1.07 (0.92–1.24) | .40 |
| Syndromal | 42/424 | 1.15 (0.84–1.58) | .39 | 1.07 (0.79–1.48) | .65 | 1.04 (0.75–1.44) | .82 |
| Non syndromal | 261/424 | 1.09 (0.93–1.27) | .28 | 1.07 (0.92–1.25) | .39 | 1.07 (0.92–1.26) | .38 |
| B. Risk of VSD | Cases/controls (n = 113/424) | Crude OR (95% CI) | p‐value | OR (95% CI) a | p‐value | OR (95% CI) b | p‐value |
|---|---|---|---|---|---|---|---|
| Total study group | 113/424 | 1.29 (1.04–1.61) | .02 | 1.25 (1.01–1.55) | .047 | 1.24 (1.00–1.55) | .05 |
| Syndromal | 30/424 | 1.28 (0.88–1.89) | .20 | 1.19 (0.82–1.76) | .36 | 1.13 (0.77–1.69) | .54 |
| Non syndromal | 83/424 | 1.29 (1.02–1.66) | .04 | 1.26 (0.99–1.62) | .06 | 1.28 (1.00 −1.64) | .05 |
CI, confidence interval of COD or VSD risk per standard deviation (SD) decrease in maternal T/S ratio. OR for decrease in risk per SD showed as 1/OR, one SD maternal T/S ratio = 0.163. Telomere length (T/S ratio) measured at approximately 16 months after the index pregnancy.
Abbreviation: OR, odds ratio.
Risk estimates adjusted for maternal age.
Risk estimates adjusted for maternal age, maternal body‐mass‐index, ethnicity, education and use of alcohol or smoking during pregnancy.
In addition, we analysed the association between relative TL and VSD (n = 113), and demonstrated that a shorter maternal relative TL is associated with a significantly increased risk of VSD in their offspring (crude OR 1.29 (95% CI 1.04–1.61), p = .02, per standard deviation relative TL decrease). In Model 1, the association remained significant (adjOR 1.25 (95% CI 1.01–1.55), p = .04) (Table 2B). In Model 2, the association slightly attenuated (adjOR 1.24 (95% CI 1.00–1.55)). After stratification, the syndromal VSD group showed no association between shorter maternal relative TL and the risk of VSD in offspring (crude OR 1.28 (95% CI 0.88–1.89), p = .20). Whereas, the non‐syndromal VSD group showed a significant association between shorter maternal relative TL and the risk of VSD in offspring (crude OR 1.29 (95% CI 1.02–1.66), p = .04). In model 1 and model 2, this association remained, albeit not significant (adjOR 1.26 (95% CI 0.99–1.62), p = .06 and adjOR 1.28 (95% CI 1.00–1.64), respectively) (Table 2B).
Adjustment of the associations for homocysteine concentrations did not substantially affect the association between relative TL and the risk of COD and VSD offspring, Table A1.
4. DISCUSSION
This is the first study showing that shorter maternal relative TL, per standard deviation decrease, is associated with an OR of 1.29 for the risk of VSD in offspring that attenuated to an OR of 1.25 after adjustment for maternal age (adjOR 1.25 (95% CI 1.01–1.55), p = .04). Moreover, we demonstrated a significant association between shorter maternal relative TL and the non‐syndromal VSD group (crude OR 1.29 (95% CI 1.02–1.66), p = .04), where after adjustment the association remained, but lost significance.
One of the main methodological strengths of this study is the use of the standardized samples taken approximately 1 year after the periconception period of the index pregnancy, which minimizes recall bias. The fixed telomere ranking and telomere tracking in adults supports the validity of the measure of maternal TL approximately 15 months after index child as proxy for the periconception maternal TL. Moreover, we prevented misclassification of cases and controls, given that most CODs are diagnosed during the first year of life. Another strength is the detailed description of the neural crest‐related COD phenotypes and the ethnic homogeneity of case and control families. Another strength is the stratification in syndromes. As COD as part of a syndrome is more likely to be due to chromosomal abnormalities. A limitation of our study could be the measurement of TL in blood, which may not be representative for TL in the maternal oocytes or embryonic cardiac cells. However, it has been shown that leukocyte TL is highly correlated with TL of other somatic tissues from the same individual such as muscle, fat, skin and synovial tissue. This indicates that a clear intra‐individual synchronization in TL exists in adults, 21 suggesting blood cell TL to be a valid measurement. Another possible limitation of this study is that we did not measured blood cell counts. Blood cell counts could affect average TL, albeit literature is inconclusive about this association. 22 , 23 Thereby, the association between paternal factors, such as environmental risk factors and sperm quality, and the risk of congenital heart disease in the offspring could not be investigated, because of comparable paternal age in cases and controls and limited power. A limitation of our study is that there are more typical COD types such as arterial trunk and double outlet right ventricle. The representation of these group was low in our study population and we were most interested in the VSD group as this was our largest group of the HAVEN study and most common phenotype of COD. Thereby, we are aware that residual confounding can never be excluded because of the observational character of this cohort.
Our data support our hypothesis of using relative TL as biomarker for multiple exposures of oxidative stress and inflammation to assess the risk of COD offspring in the future. However, maternal age is the strongest determinant in this association. Thereby, the intricacy of TL translates in a high inter‐individual variability, when comparing same‐aged people. 24 This may be an explanation for the non‐significant association found between relative TL and the risk of COD in general. Therefore, in future studies, larger sample sizes are needed.
Previous studies in mice showed that, embryonic mice deficient in the telomerase gene show shorter TL and failure of closure of the neural tube as the main defect, suggesting that this developmental process is sensitive to telomere loss and chromosomal instability. 12 , 25 These results support our findings, considering the similar risk factors neural tube defects and COD share.
A hypothetical role of TL in neural tube defects (NTD) and COD is illustrated by the epidemiological and biological evidence of the association between hyperhomocysteinemia and increased risk of NTD and COD in offspring. 5 , 12 Hyperhomocysteinemia is a sensitive biomarker of oxidative stress and as such may reduce the synthesis or increase the damage of DNA, including the telomeres. In our study, homocysteine was comparable between the groups; however, homocysteine levels tended to be higher in cases. In our study population, no correlation between homocysteine and relative TL was found. Thereby, adjusting for homocysteine concentrations had no significant influence on the association between relative TL and the risk of COD and or VSD offspring.
Entringer et al. proposed that the effect of suboptimal intrauterine conditions on initial setting of TL and telomerase activity is mediated by the programming actions of stress‐related maternal‐placental‐foetal oxidative and metabolic pathways, in a way that accelerate cellular dysfunction, ageing and disease over the lifespan. 26 Embryogenesis in early pregnancy is sensitive to excessive oxidative stressors in the environment, resulting in structural and functional changes in cells, tissues and organ systems. Changes in TL of cells in the foetal heart may also play a role in normal cardiac development during embryogenesis and supports the association between TL and increased risk of VSD offspring. 27
The process of foetal programming of telomeres may reflect an effect shown across generations, which influences the health and well‐being of not only individuals but also their offspring. Thereby, mother–offspring correlation in TL appears to be stronger than the father‐offspring correlation, regardless of the gender of offspring. 28 These finding emphasize the importance of efforts for early prediction and prevention. It should be noted that new born TL might relate more directly to VSD, but this is not evaluated in our study. Paternal TL and paternal age also contributes to the settings of newborn TL. Thereby, it could be that a part of newborn TL, not related to maternal TL, contributes to the development or predisposition of VSD.
Other associations between TL and obstetric outcomes have also been reported. Telomerase activity is decreased or absent in placentas of foetal growth‐restricted newborns. 29 In this way, foetal growth restriction is a common occurrence during COD pregnancies.
Our findings entails a new focus in the future scientific approach of the prediction and prevention of neural crest cell‐related COD. For example, our results can form the basis for animal studies in which the pathophysiological mechanism for neural crest‐related COD can be further explored.
Moreover, it contributes to the recognition of the importance of a life course approach in women's and offspring's health. We believe there could be a role for E‐health intervention platforms, such as www.smarter.pregnancy.co.uk, 30 by improving maternal health conditions, such as an adequate intake of vegetables, fruits and folic acid supplements, stop smoking and alcohol consumption, that reduce excessive oxidative stress in women contemplating pregnancy using relative TL as a marker.
Further studies and replications of our study with larger populations and worldwide collaboration platforms are needed to confirm our findings and identify maternal relative TL as a sensitive predictive biomarker.
5. CONCLUSION
Shorter maternal relative TL, independent of the maternal chronological age effect, is associated with a higher odds, OR of 1.29, for the risk of VSD in the offspring. No association was found between maternal TL and the risk of neural crest‐related COD offspring in the total COD group. The direct biological implications of TL biology in the onset of VSD cannot be concluded from this study and should warrant further investigation in other studies on congenital malformations, and on the predictive value of maternal TL before implementation in clinical practice. For example, specify and sensitivity studies using ROC curves.
The demonstration of an underlying role for shorter maternal relative TL and the association with neural crest‐ related COD in the offspring, in particular VSD, identifies maternal TL as a possible long‐term biological marker of the long‐term oxidative stress status of women in their periconception period. Consequently, after additional research, maternal TL may be used as a biological marker in periconceptional risk assessment.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
RS initiated the study and DA reviewed the review data and wrote the first version of the article. RW, AE, JM and RS contributed to the design of the paper, cowriting of the article, revisions and gave input at all stages of the study. SW contributed to statistical analyses and interpretation of results. All authors have approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors wish to thank Pascal. P. Arp from the Department of Internal Medicine, Erasmus MC, University Medical Centre Rotterdam, Rotterdam, the Netherlands, for executing the telomere length measurements. The Bo Hjelt Foundation for Spina Bifida in memory of Madeleine Hjelt provided financial support for the conduct of the study and preparation of the manuscript. The funding source had no involvement in study design, data collection, analysis and interpretation of data, in the writing of the report nor in the decision to submit this article for publication.
APPENDIX A.
Measurements of biomarkers
Fasting venous blood samples were drawn from case and control mothers during the hospital visit. Immediately after blood sampling, an ethylenediaminetetraacetic acid tube was placed on ice and a serum separator tube was kept at room temperature. Both tubes were centrifuged at 4000 × g for 10 min at 4°C and separated within 1 h. All samples were stored at −80°C in batches within 5 months of collection. To determine total homocysteine we used liquid chromatography‐tandem mass spectrometry (LC‐MS/MS; Waters acquity UPLC premier XE, Milford, MA, USA). Serum folate and vitamin B12 were routinely determined by immunoelectrochemiluminescence immunoassay (ECLIA) on a Roche Modular E170 (Roche Diagnostics GmbH, Mannheim, Germany). Red blood cell folate was measured in the haemolysate of whole blood with ascorbic acid for stabilisation. The red blood cell folate concentration was calculated according to the following formula: (nmol/l haemolysate folate × 10/haematocrit)−(nmol/l serum folate × [1−haematocrit]/haematocrit) = nmol/l RBC folate.
The inter‐assay coefficients of variation (CV) were 5.9% at 15.3 μmol/l and 3.4% at 39.3 μmol/l for tHcy, 9.5% at 8.3 nmol/l and 3.2% at 20.2 nmol/l for folate, and 5.1% at 125 pmol/l and 2.9% at 753 pmol/l for vitamin B12.
FIGURE A1.
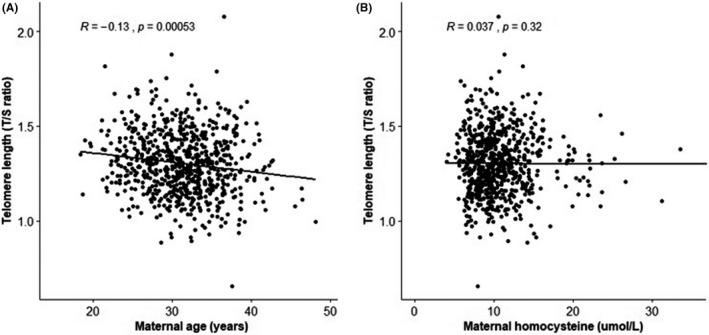
Correlations between maternal telomere length (T/S ratio) and (A) maternal age and (B) maternal plasma total homocysteine. Spearman rank correlation test is performed to test the correlations
TABLE A1.
Maternal telomere length (T/S ratio) in association with the risk of congenital outflow defects (COD) (A) or ventricular septal defect (VSD) (B) in offspring
| A. Risk of COD | Cases/controls (n = 306/424) | OR a (95% CI) | p‐value |
|---|---|---|---|
| Total study group | 306/424 | 1.07 (0.92–1.24) | .38 |
| Syndromal | 45/424 | 1.05 (0.76–1.46) | .76 |
| Non syndromal | 261/424 | 1.07 (0.92–1.25) | .39 |
| B. Risk of VSD | Cases/controls (n = 113/424) | OR a (95% CI) | p‐value |
|---|---|---|---|
| Total study group | 113/424 | 1.24 (1.01–1.55) | .049 |
| Syndromal | 30/424 | 1.18 (0.80–1.75) | .40 |
| Non syndromal | 83/424 | 1.26 (0.99–1.62) | .06 |
OR, odds ratio; CI, confidence interval of COD or VSD risk per standard deviation (SD) decrease in maternal T/S ratio. OR for decrease in risk per SD showed as 1/OR, one SD maternal T/S ratio = 0.163. Telomere length (T/S ratio) measured at approximately 16 months after the index pregnancy.
Logistic regression adjusted for maternal age and maternal homocysteine levels.
Aoulad Fares D, Wiegel RE, Eggink AJ, Willemsen SP, van Meurs JBJ, Steegers‐Theunissen RPM. Shorter periconception maternal telomere length and the risk of congenital cardiac outflow defects in the offspring. Eur J Clin Invest. 2022;52:e13784. doi: 10.1111/eci.13784
REFERENCES
- 1. Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta‐analysis of 260 studies. Int J Epidemiol. 2019;48(2):455‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawoko S, Soares JJ. Quality of life among parents of children with congenital heart disease, parents of children with other diseases and parents of healthy children. Qual Life Res. 2003;12(6):655‐666. [DOI] [PubMed] [Google Scholar]
- 3. Wlodarczyk BJ, Palacios AM, Chapa CJ, Zhu H, George TM, Finnell RH. Genetic basis of susceptibility to teratogen induced birth defects. Am J Med Genet C Semin Med Genet. 2011;157C(3):215‐226. [DOI] [PubMed] [Google Scholar]
- 4. Keyte A, Hutson MR. The neural crest in cardiac congenital anomalies. Differentiation. 2012;84(1):25‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steegers‐Theunissen RP, Boers GH, Trijbels FJ, Eskes TK. Neural‐tube defects and derangement of homocysteine metabolism. N Engl J Med. 1991;324(3):199‐200. [DOI] [PubMed] [Google Scholar]
- 6. Verkleij‐Hagoort A, Bliek J, Sayed‐Tabatabaei F, Ursem N, Steegers E, Steegers‐Theunissen R. Hyperhomocysteinemia and MTHFR polymorphisms in association with orofacial clefts and congenital heart defects: a meta‐analysis. Am J Med Genet A. 2007;143A(9):952‐960. [DOI] [PubMed] [Google Scholar]
- 7. Boot MJ, Steegers‐Theunissen RP, Poelmann RE, van Iperen L, Gittenberger‐de Groot AC. Cardiac outflow tract malformations in chick embryos exposed to homocysteine. Cardiovasc Res. 2004;64(2):365‐373. [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharya D, Khan B, Simoes‐Costa M. Neural crest metabolism: at the crossroads of development and disease. Dev Biol. 2021;475:245‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jenkins KJ, Correa A, Feinstein JA, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):2995‐3014. [DOI] [PubMed] [Google Scholar]
- 10. Bijnens EM, Zeegers MP, Derom C, et al. Telomere tracking from birth to adulthood and residential traffic exposure. BMC Med. 2017;15(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Babizhayev MA, Savel'yeva EL, Moskvina SN, Yegorov YE. Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. Am J Ther. 2011;18(6):e209‐e226. [DOI] [PubMed] [Google Scholar]
- 12. Aoulad Fares D, Schalekamp‐Timmermans S, Nawrot TS, Steegers‐Theunissen RPM. Preconception telomere length as a novel maternal biomarker to assess the risk of spina bifida in the offspring. Birth Defects Res. 2020;112(9):645‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Obermann‐Borst SA, Vujkovic M, de Vries JH, et al. A maternal dietary pattern characterised by fish and seafood in association with the risk of congenital heart defects in the offspring. BJOG. 2011;118(10):1205‐1215. [DOI] [PubMed] [Google Scholar]
- 14. Netherlands S . The Dutch standard classification of education. 2008.
- 15. Netherlands S . Definition country of origin. Voorburg/Heerlen; 1999. [Google Scholar]
- 16. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 17. Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao S, Fernald RD. Comprehensive algorithm for quantitative real‐time polymerase chain reaction. J Comput Biol. 2005;12(8):1047‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin J, Smith DL, Esteves K, Drury S. Telomere length measurement by qPCR – Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology. 2019;99:271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662‐664. [DOI] [PubMed] [Google Scholar]
- 21. Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazidi M, Penson P, Banach M. Association between telomere length and complete blood count in US adults. Arch Med Sci. 2017;13(3):601‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mollica L, Fleury I, Belisle C, Provost S, Roy DC, Busque L. No association between telomere length and blood cell counts in elderly individuals. J Gerontol A Biol Sci Med Sci. 2009;64(9):965‐967. [DOI] [PubMed] [Google Scholar]
- 24. Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12(2):509‐519. [DOI] [PubMed] [Google Scholar]
- 25. Herrera E, Samper E, Blasco MA. Telomere shortening in mTR‐/‐ embryos is associated with failure to close the neural tube. Embo J. 1999;18(5):1172‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Entringer S, de Punder K, Buss C, Wadhwa PD. The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc Lond B Biol Sci. 2018;373(1741):20170151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Booth SA, Charchar FJ. Cardiac telomere length in heart development, function, and disease. Physiol Genomics. 2017;49(7):368‐384. [DOI] [PubMed] [Google Scholar]
- 28. Broer L, Codd V, Nyholt DR, et al. Meta‐analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21(10):1163‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fragkiadaki P, Tsoukalas D, Fragkiadoulaki I, et al. Telomerase activity in pregnancy complications (Review). Mol Med Rep. 2016;14(1):16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Dijk MR, Oostingh EC, Koster MPH, Willemsen SP, Laven JSE, Steegers‐Theunissen RPM. The use of the mHealth program Smarter Pregnancy in preconception care: rationale, study design and data collection of a randomized controlled trial. BMC Pregnancy Childbirth. 2017;17(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]


