Abstract
Compassionate use is a system that provides patients with exceptional access to investigational new drugs to treat life‐threatening diseases that have no effective conventional treatments. The purpose of this study was to characterize and assess the current status of the compassionate use program’s application in Japan by evaluating expanded access clinical trials (EACTs) conducted between 2016 and 2021. In this study, a data set containing all EACTs, and pivotal clinical trials (PCTs) conducted in Japan between February 2016 and April 2021 was obtained from the Pharmaceutical and Medical Devices Agency, systemically reviewed, and analyzed. During the 5 years since EACTs began in Japan, out of 2,031 PCTs, 31 EACTs were conducted in Japan. Twenty‐four trials (77.4%) of the 31 EACTs used anticancer drugs and 5 of those trials (16.1%) were conducted in children. Furthermore, we conducted an EACT survey for drugs with a high degree of social and patient demands as recommended in the EACT notification. Among the 2,031 PCTs, we found 152 trials with high degree of social and patient demands. Of these, EACT was implemented in 17 trials (11.2%). Days from the start of the EACT to the submission of new drug applications and the approval were 9.0 (67.0–56.5) and 208.0 (172.8–308.8) days, respectively. Of the 31 EACTs conducted, 24 (77.4%) drugs have been approved as of August 2021. This first comprehensive study on EACTs clarified the current status and issues of Japan’s compassionate use system and the 5 years since the program initiated.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
In Japan, this is the first comprehensive study on expanded access clinical trials (EACTs).
WHAT QUESTION DID THIS STUDY ADDRESS?
This study clarified the current status and issues with EACTs conducted to date.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Differences and similarities between the compassionate use systems in Japan and the United States and the European Union.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
It is important to get patients to access really good medicines as early as possible by solving problems. The authors proposed a single‐patient EACT system.
After the discovery of a pharmaceutical product or drug compound, it typically takes at least 10 years to conduct nonclinical and clinical trials, obtain approval from the regulatory authorities in each region, and launch the product. 1 The marketing authorization process for new medicines is often time‐consuming and distressing for patients, underscoring the urgent need for making promising new drugs available to patients at the earliest time. Various measures, such as promoting development and shortening the review period, have been implemented to improve patient access to these new, innovative drugs. 2 , 3 , 4 , 5 , 6
Compassionate use (CU) is a system that allows unapproved investigational drugs to be used on a case‐by‐case basis to treat serious life‐threatening diseases for which existing drugs are ineffective from an ethical or humane perspective. 7 CU generally requires several unique conditions to be met. Implementation in public systems, such as laws, and the exceptional use of unapproved investigational drugs under specific conditions are among the prerequisites. Furthermore, patients with specific issues, such as those with serious or life‐threatening diseases, those unable to participate in clinical trials, those for whom no approved alternative drugs are available, and those whose self‐pay burden is not increased, are included in the prerequisites. 8 In CU, it is also critical that patients’ access to unapproved investigational drugs does not interfere with the standard clinical trial process required to approve the drugs. 9 Other terms for “CU” include “Managed Access,” “Expanded Access,” “Named Patient Supply,” “Special Access,” “Early Access,” and “Temporary Authorization for Use,” which are regulations regarding access to investigational drug use for unapproved medicines. 10
CU has been institutionalized in the United States and Europe for a relatively long time. The history of the initial discussion on CU programs led to their initiation, which was the HIV/AIDS crisis in the 1980s. 11 CU was traditionally used in the European Union, particularly in France and Italy; however, a regulatory framework was created within the EU’s Legislature in 2004. 12 This regulatory framework described in regulation number EC726/2004 is a high‐level rule in the EU’s legal system. 13 Likewise, the US Food and Drug Administration (FDA) conducts several expanded access programs (EAPs). 14 The FDA guidelines use administrative terms, such as “treatment use of investigational new drug” and “expanded access” to explain that this is the same as CU that is commonly used in the European Union. 15 In the United States, the “emergency use” and “individual patients” mechanisms are used for single patients in all phases, whereas “intermediate‐size patient population” and “treatment investigational new drug (IND)/protocol” mechanisms are used for cohort patients. In particular, these are applicable during or after the clinical trial phase. 16 The Right to Try Act of 2017 promoted the EAPs and encouraged early access to investigational drugs. 17 , 18
In Japan, the CU system was established in January 2016 as the Japanese version of the CU program (J‐CU program). 19 CU operations began with expanded access clinical trials (EACTs), also known as “clinical trials conducted from a humanitarian perspective.” Moreover, a law was enacted in Japan mandating the use of EACTs for treating life‐threatening diseases for which existing therapeutic drugs are ineffective. EACTs are typically conducted within a standard clinical trial framework after the enrollment process in pivotal clinical trials (PCTs) is complete. This practice reduces the EACT’s interference with the relevant drug development process. In addition, four conditions were set as recommendations for CU with high social and patient demands. These four conditions of high social and patient demands found in the conditions of the rule include drugs for EAPs in the United States, drugs receiving the Sakigake designation system, drugs for orphan diseases, and drugs specially requested for development by the Review Committee on Unapproved Drugs. 20 The program’s implementation, which began in 2016, was also meant to promote drugs that met these four criteria to be considered for EACTs in Japan. Moreover, all PCTs are submitted to the Pharmaceutical and Medical Devices Agency (PMDA) as clinical trial notifications. In the PCTs, the sponsor company decides whether to conduct EACTs based on the requests from patients and medical institutions and, if so, notifies the EACT body. There is no need for PMDA approval; hence, 100% of EACT requests are typically conducted. Furthermore, unlike the United States and the European Union, there is no single‐patient system or emergency CU system in Japan. Given that the trials are conducted following a process similar to that of the standard clinical trials, this has resulted in several concerns that initiating EACTs would be a lengthy process. 21
In Japan, however, CU has only been institutionalized for nearly 5 years. Given the lack of comprehensive analyses of its current status, it is unclear which steps may improve patient access to promising new drugs. Based on the abovementioned facts, this study investigated the characteristics of CU application in Japan by analyzing EACTs from 2016 to 2021. Furthermore, we propose future measures for improving implementation issues and optimizing the current CU procedure.
MATERIALS AND METHODS
Data collection
In this study, we collected all EACTs conducted to date in Japan after the “Notification on Implementation of Clinical Trials Conducted for Humanitarian Purposes (Notification No. 0122‐7, January 22, 2016).” 17 Using the Information Disclosure System, a data set containing all EACTs and PCTs conducted in Japan between February 2016 and April 2021 was obtained directly from the PMDA. This database included all EACTs and PCTs reported to the PMDA by pharmaceutical companies as clinical trial notifications and trial information, such as development codes, target indication, development phase, sponsor name, planned initiation date, and study period.
Based on EACT and PCT information obtained from the PMDA, each trial’s data were investigated using clinical trial registry databases. These data sets included information such as age, sex, trial eligibility criteria, trial exclusion criteria, end points, patient numbers, country conducting clinical trials, clinical trial venue, institution numbers, and National Clinical Trial numbers, Japic Clinical Trials Information, first submitted date, first posted date, and last posted date.
The clinical trial registry databases used included Clinical Trials.gov, Japic Clinical Trials Information, UMIN Clinical Trials Registry, and Japan Medical Association Center for Clinical Trials.
We also collected data on each trial: drug’s generic name, trade name, date of new drug application (NDA) submission to the PMDA, approval date in Japan, regulatory review fields for the PMDA, 22 therapeutic indication classifications performed according to the Japan Standard Commodity Classification numbers, 23 indications, and disease classification performed according to the International Classification of Diseases and Related Health Problems classification 24 through a publicly available database.
Clinical trial research strategy and selection criteria
First, we matched EACT and PCT data obtained from the PMDA to identify the clinical trials using EACT within PCTs. Subsequently, we used the four conditions listed in EACT notification, 19 as indicators of which EACTs should be conducted because of a high level of social and patient demands. To assess the characteristics of the CU application of EACTs in terms of these demands, we matched the clinical trials extracted for each of the four conditions with the EACT and PCT data. The clinical trial extraction method for each of the four conditions is shown below.
Drugs for which EAPs intermediate‐size IND (protocol) or treatment IND (protocol) are being conducted in the United States 25 : in Clinical Trials.gov. We extracted EAPs with an “intermediate‐size IND (protocol)” or “treatment IND (protocol)” conducted in the United States as of August 31, 2021.
Drugs designated under the Sakigake designation system: information on designated Sakigake designation drugs as of August 31, 2021, were obtained from the PMDA website.
Orphan diseases: drugs that had been designated as an orphan disease application as of August 31, 2021, were obtained from the Ministry of Health, Labour, and Welfare (MHLW) website. 26
For drugs for which development was requested by the Review Committee on Unapproved Drugs because of high medical needs, 18 information as of August 31, 2021, was obtained from the MHLW website.
The NDA and approval dates for each drug were obtained from the PMDA on the drug information website. The start date of EACT was the planned initiation date in the EACT clinical trial notification.
Data construction and outcomes evaluation
We combined the data on PCTs, EACTs, the four conditions, EACT timing, and the approval rate. Two authors (M.U. and H.M.) manually reviewed and retrieved excerpts for the identified information on each drug, PCT, and EACT. Inconsistencies were resolved through discussion. Except for cases of no applicable items, this study was prepared following the guidelines for Strengthening the Reporting of Observational Studies in Epidemiology. 27 Following that, we developed and analyzed an original database based on the collected data and we investigated the suitability of CU applications in Japan. Of the total number of drugs approved, the approval rate includes the percentage of drugs used in EACTs until August 31, 2021.
Analysis
We used descriptive statistics to characterize the drugs, EACTs, and PCTs. The median and interquartile range (IQR) was also used to present the descriptive analysis of numerical data. All analyses were conducted using the analytical tools of JMP Pro 15.
RESULTS
Number of PCTs and EACTs
Over a 5‐year period, from February 2016 to April 2021, there were 2031 PCTs for which notifications of starting clinical trials were submitted to the PMDA in Japan. Moreover, a total of 31 EACTs (26 drugs) were conducted during this time, accounting for 1.5% of all PCTs. The IQR for the number of trials per year was 5.5 (IQR: 3.5–6.5)/year. Table 1 shows the total number of PCTs and EACTs.
Table 1.
Number of EACTs and PCTs conducted between 2016 and 2021 in Japan
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Total | |
|---|---|---|---|---|---|---|---|
| Expanded access clinical trials | 6 | 8 | 4 | 5 | 6 | 2 | 31 |
| Pivotal clinical trials | 765 | 252 | 309 | 279 | 294 | 132 | 2,031 |
| %a | 0.8 | 3.2 | 1.3 | 1.8 | 2.0 | 1.5 | 1.5 |
EACTs, Expanded Access Clinical Trials; PCTs, pivotal clinical trials.
Ratio of expanded access clinical trials to pivotal trials.
Characteristics of EACTs and drugs used in EACTs
Table 2 shows the characteristics of EACTs. The most common therapeutic area for target diseases covered by EACTs was antineoplastic drugs, with 24 trials (77.4%) falling under the category of “tumors” according to the International Classification of Diseases 10 classification and 23 trials (74.2%) falling under the therapeutic classification code of “other antineoplastic drugs.” EACTs were also performed in pediatric patients; five trials (16.1%) included pediatric patients. There were 16 trials (51.6%) conducted by Japanese domestic companies, 14 (45.2%) by foreign global companies, and 1 (3.2%) as an investigator‐initiated trial for the sponsor. A detailed list of CU trials conducted during the survey period is provided in Table S1 .
Table 2.
Background of drugs and characteristics of EACTs
| Items | Number of trials | % | |
|---|---|---|---|
| Therapeutic indication classification | Oncology drugs | 23 | 74.2 |
| Peripheral nervous system | 2 | 6.5 | |
| Biological product | 2 | 6.5 | |
| Infectious diseases | 1 | 3.2 | |
| Blood derivatives | 1 | 3.2 | |
| Metabolic disease | 1 | 3.2 | |
| Alkylating agent | 1 | 3.2 | |
| Disease classification | II Neoplasms | 24 | 77.4 |
| III Diseases of the blood and certain disorders involving the immune mechanism | 1 | 3.2 | |
| IV Endocrine, nutritional, and metabolic diseases | 4 | 12.9 | |
| VI Diseases of the nervous system | 1 | 3.2 | |
| Unknown | 1 | 3.2 | |
| Age, years | ≥18 | 15 | 48.4 |
| 12–18 years | 3 | 9.7 | |
| 2–12 years | 1 | 3.2 | |
| 0–2 years | 1 | 3.2 | |
| Unknown | 11 | 35.5 | |
| Sponsor | Japanese domestic company | 16 | 51.6 |
| Global company | 14 | 45.2 | |
| Investigator‐initiated | 1 | 3.2 | |
| High degree of social and patient demands | Expanded access program in the United States | 5 | 16.1 |
| Sakigake designation | 2 | 6.5 | |
| Orphan drug designation | 16 | 51.6 | |
| Drugs for development request by MHLW | 0 | 0.0 |
EACTs, Expanded Access Clinical Trials; MHLW, Ministry of Health, Labour, and Welfare.
Status of conduct of EACTs with a high degree of social and patient demands
We reviewed the 2031 PCTs with high social and patient demands and found 152 trials with high demand. Among them, EACTs were implemented in 17 trials (11.2%). The contents of each demand were examined for items listed in “implementation of clinical trials conducted for humanitarian purposes” as drugs for which there is a high degree of social and patient demands for conducting EACTs. The number of trials conducted was investigated (Table 3 ).
Table 3.
EACTs for investigational drugs with high degree of social and patients demands
| Items | Number of pivotal clinical trials (%) | Number of conducting expanded access clinical trials (%) | Number of not conducting expanded access clinical trials (%) |
|---|---|---|---|
| High degree of social and patients demands | 152 (100.0) | 17 (11.2) | 135 (88.8) |
| Conducting expanded access program in the United States | 25 (100.0) | 5 (20.0) | 20 (80.0) |
| Sakigake designation | 16 (100.0) | 2 (12.5) | 14 (87.5) |
| Orphan drug designation | 104 (100.0) | 16 (15.4) | 88 (84.6) |
| Drugs for development request by MHLW | 31 (100.0) | 0 (0) | 31 (100.0) |
EACTs, Expanded Access Clinical Trials; MHLW, Ministry of Health, Labour, and Welfare.
Of the 25 trials in which PCTs for the same indication were being conducted in Japan among drugs for which EAPs were being conducted in the United States, 5 were EACTs (20.0%).
For “Sakigake designated drugs,” EACTs were conducted for 2 of 16 drugs (12.5%).
Among the 104 drugs designated as orphan drugs, EACTs were conducted for 16 drugs (15.4%).
Among 31 drugs for which development was requested by the Review Committee on Unapproved Drugs because of high medical needs, EACTs were conducted for 0 (0%) of the drugs.
Marketing approval rate of drugs conducting EACTs
As of August 31, 2021, of the 31 EACTs conducted, 24 (77.4%) drugs had been approved for marketing, 2 (6.5%) drugs are under reviewing after NDA by the PMDA, and 5 drugs (16.1%) are either under development or have been withdrawn (Table S1 ).
Start time and duration of EACT
We looked at the relationship between EACT initiation and NDA date for marketing approval in 24 drugs, all of which had EACTs. Notably, 13 of 24 (54.2%) EACT initiation dates occurred after the NDA, whereas 10 (41.6%) occurred within 6 months of the NDA. One EACT was initiated 305 days before the NDA. From planned EACT initiation to NDA, the median time and IQR were 9 days (IQR: −67.0 to 56.5) days. The median time between a planned EACT initiation and approval was 208.0 days (IQR: 172.8–308.8). Furthermore, EACTs were set to begin within 1 year of drug approval for 24 of 24 drugs (100%; Figure 1 ).
Figure 1.
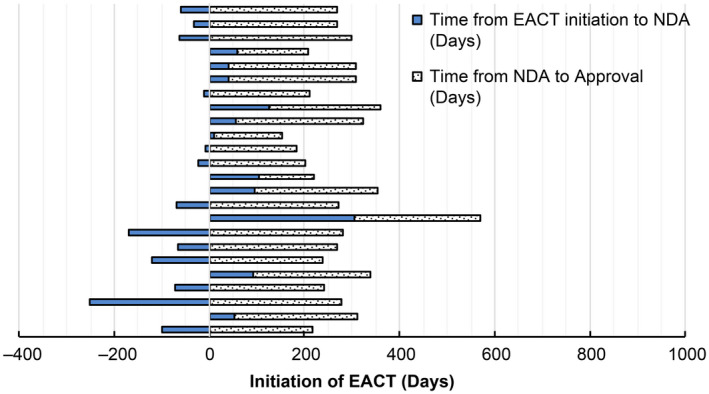
Time from initiation of expanded access clinical trials to the date of new drug application and approval. Day 0 of each drug indicates for planned initiation date of EACT. EACT, Expanded Access Clinical Trial; NDA, New Drug Application. [Colour figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
Although several reports on the result of EACT for individual drugs have been published, 28 , 29 to the best of our knowledge, this is the first study that thoroughly investigated CU and EACTs in Japan since the EACT system—the Japanese equivalent of the CU program (J‐CU program)—was established.
In 2013, at the time of introduction of the EACT system, the following three points were particularly pivotal points for discussion in Japan. 30
Guaranteeing access to unapproved drugs for patients with serious life‐threatening diseases for which no alternative therapy is available, following the US and EU systems.
Establishing an adverse reaction reporting system considering safety first.
Ensuring EACT does not interfere with the conduct of clinical trials necessary for marketing approval of the drug.
It was necessary to strike a balance among the three competing factors listed above. As such, it was decided that EACTs would not be established as a separate system and that EACTs would be conducted under the clinical trial system based on points (1) and (2) above. In addition, EACT would not be planned until patient enrollment of PCTs were completed based on point (3) above. The results of our study also confirmed that EACT is being implemented in life‐threatening diseases, such as antineoplastic drugs (Table 2 ), and that EACT is planned after the implementation of a PCT (Table S1 ).
As mentioned in the opening section, institutionalization of CU has a longer history in Europe and the United States than in Japan. Here, we would like to compare our results in this study with previous findings of the United States and the European Union. According to the findings of this study, CU in Japan began in 2016 and 31 EACTs have been conducted by August 2021 and accounting for 1.5% of the total number of PCTs in the United States. The number of expanded access programs in each year between 2010 and 2013 was between 936 and 1,199 in the United States. 16 However, there are no results in the rate of expanded access programs per pivotal trials. Therefore, it was difficult to determine whether the rate in Japan was higher or lower, however, the number of EACTs in Japan was lower than that in the United States. Regarding the characteristics of EACTs and drugs used in EACTs, the present study suggests that EACTs are being conducted for diseases for which new therapeutics are required. This is likely because of the need for early access to investigational drugs, such as those for cancer, pediatric, and rare diseases, and because unapproved drugs are being provided for the same indications as in the United States and the European Union. 31 Furthermore, in this study, we attempted to calculate the EACT implementation rates for four conditions of the high level of social and patient demands recommended in the notification of EACT in Japan. Only 20% of the drugs with expanded access in the United States are implemented in Japan, suggesting that there may be a difference between Japan and the United States.
Regarding the timing of the start of the EACTs, in our study, >50% of EACTs began after the NDA was submitted, and all trials were completed in a short period of < 1 year. According to Puthumana et al., in the United States, cohort‐type EAPs for 70% of drugs are started within 6 months before or after the NDA. 15 It is unknown whether patients are satisfied with the duration of expanded access to investigational new drugs. It may be necessary to consider an earlier start in both Japan and the United States 15 ; however, the United States has other systems of single‐patient access. There is a range of opinions from various risk–benefit perspectives to start EACTs before obtaining the data from PCTs, which help define the benefit–risk profile of the drugs with previously uncertain efficacy and safety. In addition, there may be notable advantages to starting CU programs at later stages during clinical development, such as a lower unnecessary risk to the patients and a higher likelihood of approval. Miller et al. investigated the characteristics of “expanded access” and “CU” programs registered in ClinicalTrials.gov and determined that 76% of drugs provided through these programs ultimately received FDA marketing approval. 32 In contrast, McKee investigated and focused only on single patient INDs, including individual patient INDs for emergency use, and thus excluded intermediate‐size and treatment INDs. They reported the approval rate by 5 years after the initial submission of IND was only 33%. 25 In our findings, we observed an approval rate of 24 out of 31 (77.4%) for drugs used approved for EACT in Japan (Figure 1 ), which is similar to Millar’s study.
Table 4 shows an overview of the comparison of the CU systems of Japan, the United States, and the European Union. In Japan, EACTs were only conducted as cohort‐type trials, with no CU for single patients. In contrast, in the United States, the “emergency use” and “individual patients” mechanisms apply to all phases, accounting for > 95% of trials, and “intermediate‐size patient population” and “treatment IND” mechanisms are used after the clinical trial phase, which constitutes < 5% of all EAP trials. 16 , 30 Individual EAPs in the United States, single‐patient CUs in the European Union, or nominative temporary utilization programs in France can be initiated during the early stages of clinical development. However, in the United States, treatment INDs and EU Cohort CU can only be started in the late stages of clinical trials, similar to those of the Japanese EACTs. 16 Furthermore, the Japanese system differs from other countries in that clinical trials are conducted following the same laws as standard clinical trials. Because EACTs in Japan are conducted within a standard clinical trial framework, the preparatory procedures, notification to the PMDA, and institutional review board paperwork will take time to begin, causing additional delays. Although the clinical trial system is appropriate for delivering and collecting safety information, it is a barrier to implementation for medical institutions and pharmaceutical companies. Currently, companies have no incentives to conduct EACTs in Japan. 21
Table 4.
Comparison of EACTs with CU programs conducted in the United States and Europe
| Japan | United States | Europe | |
|---|---|---|---|
| Type | Cohort |
Cohort Single patient |
Cohort Single patient (United Kingdom, France) |
| System | Performed as a clinical trial |
Separate from clinical trials (Administration within 30 days of application feasible) Emergency use system available |
Separate from clinical trials (Prior application to the regulatory authority for each country) |
| Target drugs | Investigational products for which there is high social demand and pivotal clinical trials have been conducted or are ongoing | Investigational products in the United States | Investigational products for which marketing authorization will be requested from the EMEA or those under investigation |
| Target patients | Serious or life‐threatening diseases for which there are no alternative treatments |
Serious or life‐threatening diseases for which there are no alternative treatments The expected benefit from the study drug justifies the risk |
Serious or life‐threatening diseases for which there are no alternative treatments |
| Period | From the completion of enrollment in pivotal clinical trials until approval |
Cohort: After safety confirmation and preliminary efficacy confirmation until approval Single patient: From before/after clinical trials until approval |
Cohort (Germany): Until 1 year after marketing approval |
Does not adversely affect the development of the investigational product (does not adversely affect the progress of clinical trials).
There are reasonable grounds for not being able to participate in clinical trials.
CU, compassionate use; EACTs, Expanded Access Clinical Trials; EMEA, European Medicines Evaluation Agency.
We assert that a more efficient CU system should be in place to give patients faster and more flexible access to innovative and novel investigational drugs. However, the current CU system, which is expected to be used in emergencies, such as pandemics, was rarely used in Japan during the coronavirus disease 2019 (COVID‐19) pandemic. 33 , 34 It may be necessary to operate a single‐patient IND flexibly and establish a system wherein applications can be submitted in advance to allow earlier start times. This is a complicated problem not only because of the judgment of eligibility for each patient but also because of the cost, other resources, and continuing to supply drugs, and the concern that desperate patients request access without being able to evaluate the data on benefits and risks for these unapproved compounds. However, it would be necessary to discuss whether Japan also needs this singe‐patient system. In the future, we hope that by resolving issues arising from the differences and similarities noted in the comparison between the Japanese and EU/US systems, effective drugs will be made accessible to patients at the earliest opportunity. In the United States, the Right to Try Act 17 encourages sponsors to make investigational medicines available earlier in the clinical development period, partly because it shields them from any liability associated with patient injury because of expanded access. 35 , 36 Undeniably, the CU program seeks to strike a balance between providing patients with access to unapproved drugs and treatments, protecting them from unreasonable toxicity or danger, and collecting patient safety data from patients as they take new medications. However, in Japan, we recommend establishing a single‐patient EACT system and implementing an emergency system, like the CU systems in the United States and the European Union.
This study has the following limitations:
-
•
This is a retrospective survey of published information, not a prospective study.
-
•
Information was considered based on information from past and pending EACTs. The EACTs under consideration were not included in this study. Information on the PCT was available from PMDA data, which comprised the notification of the start of PCT by pharmaceutical companies; however, it did not include completed PCTs.
In conclusion, this first retrospective survey of CU and EACTs in Japan clarified the current status and character of CU and EACTs in the 5 years since the program’s inception. The J‐CU system has only cohort‐type system. Thus, EACTs typically begin after or around the same time as NDAs, where the duration of EACTs is limited to < 1 year. We will continue to investigate whether the number of EACTs implemented in Japan is sufficient and whether the start timing is adequate. However, there is no system for early access to INDs from early stage in Japan. For one of the patient access options, we recommend establishing a single‐patient EACT system and implementing an emergency system from an early phase of clinical development similar to the CU systems in the United States and the European Union.
FUNDING
No funding was received for this work.
CONFLICT OF INTEREST
M.K. is an employee of Astellas Pharma Inc. All other authors have no competing interests for this work.
AUTHOR CONTRIBUTION
H.M., M.U., M.K., K.T., and M.Y. wrote the manuscript. H.M., M.K., K.T., and M.Y. designed the research. H.M., M.U., and M.K. performed the research. H.M. and M.U. analyzed the data. H.M. contributed new reagents/analytical tools.
ETHICAL APPROVAL
This study did not require institutional review board approval and patient informed consent because it was based on publicly available information and involved no patient records.
Supporting information
Table S1
References
- 1. Scannell, J.W. , Blanckley, A. , Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 11, 191–200 (2012). [DOI] [PubMed] [Google Scholar]
- 2. Nagai, S. Flexible and expedited regulatory review processes for innovative medicines and regenerative medical products in the US, the EU, and Japan. Int. J. Mol. Sci. 20, 3801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corrigan‐Curay, J. & Stein, P. FDA breakthrough therapy designation‐trial design and more—commentary. Clin. Pharmacol. Ther. 110, 869–870 (2021). [DOI] [PubMed] [Google Scholar]
- 4. Cherla, A. , Naci, H. , Kesselheim, A.S. , Gyawali, B. & Mossialos, E. Assessment of coverage in England of cancer drugs qualifying for US Food and Drug Administration accelerated approval. JAMA Intern. Med. 181, 490–498 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yen, E. , Davis, J.M. & Milne, C.P. Impact of regulatory incentive programs on the future of pediatric drug development. Ther. Innov. Regul. Sci. 53, 609–614 (2019). [DOI] [PubMed] [Google Scholar]
- 6. Muensterman, E.T. , Luo, Y. , Parker, J.M. , Muensterman, E.T. & Luo, J.M. Breakthrough therapy, PRIME and Sakigake: a comparison between neuroscience and oncology in obtaining preferred regulatory status. Ther. Innov. Regul. Sci. 54, 658–666 (2020). [DOI] [PubMed] [Google Scholar]
- 7. Borysowski, J. & Górski, A. Ethics framework for treatment use of investigational drugs. BMC Med. Ethics 21, 116 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borysowski, J. & Górski, A. Compassionate use of unauthorized drugs: legal regulations and ethical challenges. Eur. J. Intern. Med. 65, 12–16 (2019). [DOI] [PubMed] [Google Scholar]
- 9. Saint‐Raymond, A. et al. Remdesivir emergency approvals: a comparison of the U.S., Japanese, and EU systems. Expert. Rev. Clin. Pharmacol. 13, 1095–1101 (2020). [DOI] [PubMed] [Google Scholar]
- 10. Aliu, P. , Sarp, S. & Fitzsimmons, P. Increasing use of compassionate use/managed access channels to obtain medicines for use in COVID‐19. Clin. Pharmacol. Ther. 110, 26–28 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichols, E. Expanding Access to Investigational Therapies for HIV Infection and AIDS. March 12–13, 1990 Conference Summary (National Academies Press (US), Washington, DC, 1991). [PubMed] [Google Scholar]
- 12. Rahbari, M. & Rahbari, N.N. Compassionate use of medicinal products in Europe: current status and perspectives. Bull. World Health Organ. 89(3), 163(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balasubramanian, G. , Morampudi, S. , Chhabra, P. , Gowda, A. & Zomorodi, B. An overview of compassionate use programs in the European Union member states. Intractable Rare Dis. Res 5, 244–254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarow, J.P. , Lurie, P. , Ikenberry, S.C. & Lemery, S. Overview of FDA's expanded access program for investigational drugs. Overview of FDA's expanded access Program for Investigational Drugs. Ther. Innov. Regul. Sci. 51, 177–179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puthumana, J. , Miller, J.E. , Kim, J. & Ross, J.S. Availability of investigational medicines through the US Food and Drug Administration's expanded access and compassionate use programs. JAMA Netw. Open 1, e180283 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsuyuki, K. , Yano, K. , Watanabe, N. , Aruga, A. & Yamato, M. Compassionate use of drugs and medical devices in the United States, the European Union and Japan. Regen. Ther. 4, 18–26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viale, P.H. The federal “right to try” act: an answer to new treatments during terminal illness? J. Adv. Pract. Oncol. 8, 334–336 (2017). [PMC free article] [PubMed] [Google Scholar]
- 18. Holbein, M.E. , Berglund, J.P. , Weatherwax, K. , Gerber, D.E. & Adamo, J.E. Access to investigational drugs: FDA expanded access programs or “right‐to‐try” legislation? Clin. Transl. Sci. 8, 526–532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ministry of Health, Labour and Welfare . Expanded access clinical trials from humanitarian perspective <https://www.mhlw.go.jp/web/t_doc?dataId=00tc1562&dataType=1&pageNo=1> (in Japanese) (2016). Accessed February 21, 2022.
- 20. Ministry of Health, Labour and Welfare . List of drugs and/or pharmaceutical companies requested for development due to high medical needs based on the results of review by the Investigational Committee on Medically Necessary Unapproved Drugs and Off‐Label Use Drugs <https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iyakuhin/kaihatsuyousei/index.html> (in Japanese) (2010). Accessed February 21, 2022.
- 21. Katayama, A. Patient‐proposed healthcare services and compassionate use program. From the perspective of the system operation. J. Health Care Soc 28, 37–48 (2018). [Google Scholar]
- 22. Pharmaceuticals and Medical Devices Agency, Japan . Department of PMDA in charge of new drugs, medical devices and regenerative medicine products <https://www.pmda.go.jp/files/000205048.pdf> (in Japanese) (2020). Accessed February 21, 2022.
- 23. Ministry of Internal affairs and communications, Japan . Significance of the Japan Standard Commodity Classification (revised in June 1990), revision policy and outline <https://www.soumu.go.jp/toukei_toukatsu/index/seido/syouhin/gaiyou.htm> (in Japanese) (2020). Accessed February 21, 2022.
- 24. World Health Organization . International statistical classification of diseases and related health problems (ICD) <https://www.who.int/classifications/classification‐of‐diseases> (2022). Accessed February 21, 2022.
- 25. McKee, A.E. , Markon, A.O. , Chan‐Tack, K.M. & Lurie, P. How often are drugs made available under the Food and Drug Administration's expanded access process approved? J. Clin. Pharmacol 57(Suppl 10), S136–S142 (2017). [DOI] [PubMed] [Google Scholar]
- 26. Ministry of Health, Labour and Welfare . Handling of designation of drugs for rare diseases <https://www.pmda.go.jp/files/000236893.pdf> (in Japanese) (2020). Accessed February 21, 2022.
- 27. von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 4, e296 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujiwara, Y. et al. Efficacy and safety of osimertinib in a Japanese compassionate use program. Jpn. J. Clin. Oncol. 47, 625–629 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Nishikori, M. et al. An expanded‐access clinical study of thiotepa (DSP‐1958) high‐dose chemotherapy before autologous hematopoietic stem cell transplantation in patients with malignant lymphoma. Int. J. Hematol. 115, 391–398 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller, J.E. , Ross, J.S. , Moch, K.I. & Caplan, A.L. Characterizing expanded access and compassionate use programs for experimental drugs. BMC. Res. Notes 10, 350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gerasimov, E. , Donoghue, M. , Bilenker, J. , Watt, T. , Goodman, N. & Laetsch, T.W. Before it's too late: multistakeholder perspectives on compassionate access to investigational drugs for pediatric patients with cancer. Am. Soc. Clin. Oncol. Educ. Book Annual Meeting 40, 1–10 (2020). [DOI] [PubMed] [Google Scholar]
- 32. Teraoka, A. , Tsutani, K. Compassionate use of drugs – what is and what isn’t. Jpn. Pharmacol. Ther. 40, 831–840 (in Japanese) (2012). [Google Scholar]
- 33. Grein, J. et al. Compassionate use of Remdesivir for patients with severe Covid‐19. N. Engl. J. Med. 382, 2327–2336 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalil, A.C. Treating COVID‐19‐off‐label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 323, 1897–1898 (2020). [DOI] [PubMed] [Google Scholar]
- 35. Borysowski, J. & Górski, A. ClinicalTrials.gov as a source of information about expanded access programs: cohort study. J. Med. Internet Res. 23, e26890 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gerasimov, E. , Donoghue, M. , Bilenker, J. , Watt, T. , Goodman, N. & Laetsch, T.W. Before it's too late: 29.It's too late: multistakeholder perspectives on compassionate access to investigational drugs for pediatric patients with cancer. Am. Soc. Clin. Oncol. Educ. Book 40, 1–10 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


