Inadequate ethnic diversity in clinical trials, restrictive eligibility criteria, delayed or absent prescribing guidance for specific populations, and access lags impacting global health are under active discussion across academic, industry, and regulatory sectors. Outreach efforts, regulatory guidelines, and pragmatic trials are bridging these gaps. Transformative progress will require cross‐stakeholder commitment to responsibly embrace diversity in intrinsic and extrinsic factors in clinical development, enabled by quantitative translational sciences, a Totality of Evidence mindset, purpose, and trust.
In 2019, the US Pharmacopeia and the Massachusetts Institute of Technology Center for Collective Intelligence launched Trust CoLab, an online platform that convened over 100 global leaders in science and medicine. The purpose was to address the question of what developments will shape people’s health between 2020 and 2040, and how will trust be critical in making sure these developments help people everywhere live longer and healthier. The results (https://www.usp.org/200‐anniversary/trust‐or‐consequences) point to a potentially critical role of diversity and inclusion in the trajectory of future global health. For example, slower diffusion of medical advances in poorer regions, questions regarding global applicability of research data, and the impact of demographic shifts, migration, and urbanization (e.g., adoption of Western diets in developing countries) were discussed. Of note, some future scenario narratives implied a highly diverse ecosystem for drug development and health care, pointing to the importance of rethinking our approaches to intrinsic and extrinsic factors as clinical pharmacologists.
There is growing social purpose to expand enrollment in clinical trials to provide access as well as to gather more representative and generalizable results. Clinical pharmacologists have an opportunity to use all the tools of the trade to safely enroll more diverse populations of study participants as well as precisely and accurately manage the resulting heteroscedasticity to transform the resulting data to generalizable knowledge. Herein, we offer a call to action for clinical pharmacologists to rethink their approach to intrinsic and extrinsic factors and enable transformative progress in enhancing diversity and inclusion in drug development.
A core focus of clinical pharmacology in drug development has been to evaluate the effects of intrinsic and extrinsic factors. Historically, we evaluated benefit/risk with pristine control of heterogeneity, relying on focused “special population” studies for intrinsic/extrinsic factors not represented elsewhere in drug development. However, with steadily increasing confidence in the fidelity of predictive translational tools, we are seeing a steady evolution of the paradigm. We are increasingly using population pharmacology models in lieu of dedicated clinical pharmacology studies in a predict‐learn‐confirm‐apply paradigm with a Totality of Evidence mindset. With confidence in quantitative translational frameworks increasing, it opens the door to rethink how we consider population heterogeneity in drug development, to not miss opportunities to enable more diverse and accessible clinical trials. The tools and methodologies are already in use and only need refocusing through the diversity and inclusion lens. For example, we are witnessing growing applications of forward and reverse translational modeling and simulation in precision medicine development—an application we refer to as model‐informed precision medicine (MIPM). MIPM is envisioned as the application of quantitative pharmacology models to generate hypotheses and/or substantiate evidence regarding the contribution of patient‐specific factors (e.g., genetic variation resulting in overexpression of the molecular target of a drug) to the efficacy and/or safety of therapeutics, thereby enhancing precision medicine development. Enablers for MIPM range from quantitative systems pharmacology to disease trajectory models integrating artificial intelligence (AI)/machine learning into pharmacometrics. 1 For enhancing diversity and inclusion in clinical development, the same enablers that drive MIPM apply, with a focus on understanding the impact of diversity in intrinsic and extrinsic factors on disease outcomes and response to therapeutic interventions, to scientifically guide appropriately inclusive development.
A recent analysis of participant diversity in phase III trials supporting the US Food and Drug Administration (FDA) approval of solid tumor anticancer drugs between January 1, 2006, and June 30, 2020 concluded that Black patients and women were under‐represented relative to national reference cancer epidemiology statistics. 2 In randomized controlled trials in rheumatoid arthritis, under‐representation of men (compared to age‐adjusted prevalence of rheumatoid arthritis in men nationally) and of minority racial/ethnic groups, as well as exclusion of elderly with varying upper age limits in over 40% of trials have been discussed as factors that may impact generalizability of results. 3
Efforts to broaden eligibility criteria in clinical trials are on the rise, recognizing that the rationale for exclusion criteria may not be scientifically justified. Progress toward more inclusive clinical development has been enabled in recent years by the efforts of organizations like the American Society of Clinical Oncology, Friends of Cancer Research, and the FDA, with position papers and regulatory guidelines encouraging rational expansion of eligibility criteria—an opportunity for clinical pharmacology. 4 Liu et al. observed substantial heterogeneity in eligibility criteria between advanced non‐small cell lung cancer trials, even among trials of related checkpoint inhibitor biotherapeutics. 5 Using AI methods to learn from real‐world data (RWD) in the Flatiron Health Electronic Health Records‐derived database, they revealed the lack of meaningful impact of many widely used laboratory exclusion criteria on survival outcomes. Clinical trial simulations of more inclusive alternative designs demonstrated in silico that broadening of eligibility criteria could have led to more than doubling the pool of eligible patients, with more women and more patients > 75 years of age, without impacting the hazard ratio for survival benefit or treatment withdrawals due to adverse events. 5 This example highlights the opportunity for data‐driven clinical trial designs to promote diversity and inclusion without compromising benefit/risk, by leveraging RWD and advanced analytics. We posit that the principles and lessons learned from broadening eligibility criteria in oncology trials applying principles of clinical pharmacology are broadly applicable across therapeutic areas.
Unless clinical safety concerns necessitate more restrictive criteria (e.g., owing to risks of nephrotoxicity), inclusion of patients with mild or moderate renal insufficiency (i.e., estimated glomerular filtration rate ≥ 30 mL/min) should be scientifically supported for drugs with expected minimal renal elimination. Population pharmacokinetic and exposure‐response modeling should underwrite appropriate dosing. Such an approach builds clinical experience during development, increasing representativeness of the clinical trial dataset to ultimately inform dosing and risk management in clinical practice. The same is true for drug‐drug interactions where exclusion criteria should be rationally informed by knowledge of drug metabolism/disposition properties and therapeutic index considerations, leveraging physiologically‐based pharmacokinetic (PBPK) models. If a metabolic inhibitor or inducer is expected to increase or decrease systemic exposure of an investigational agent, it should, in principle, be feasible to include patients requiring such co‐administered agents at appropriately reduced or increased dosage, respectively, analogous to what the envisioned prescribing information may suggest. Exposure‐matched dosing in phase II/ III trials to account for actionable sources of heterogeneity in intrinsic or extrinsic factors is not a new concept. Administration of higher doses of investigational agents to patients requiring enzyme‐inducing anti‐epileptic drugs is commonplace in neuro‐oncology trials. The principles equally apply for pediatric‐inclusive development when disease similarity across the age continuum is a reasonable assumption. Quantitative assessment of the reasonableness of assuming disease similarity is an opportunity for both mechanistic systems pharmacology and population disease trajectory models and will require commitment to data sharing and precompetitive collaboration for timely progress. Confidence in proposed posology for highly vulnerable populations (e.g., neonates) in such inclusive trials can be enhanced using quantitative translational frameworks like PBPK models and adaptive safety lead‐in designs ahead of large‐scale expansion.
The International Conference on Harmonization (ICH) E17 guideline provides for ethnic population‐specific dosage in multiregional clinical trials, when needed to account for differences in dose‐exposure relationships. This provision is not widely appreciated, leading to misconceptions that actionable ethnic differences necessitating an alternate dosage should automatically translate to excluding a region (e.g., Asia) in phase III trials. Where appropriate, exposure‐matched regional dosing designs should be considered and discussed with global health authorities as valid options to maintain regional and ethnic diversity and ultimately decrease access lag without compromising robustness of substantiation of evidence, provided consistency in disease‐related intrinsic/extrinsic factors can be reasonably assumed. In this regard, with increasing access to annotated patient‐level longitudinal datasets from clinical trials and RWD, covariate analyses in disease trajectory models are valuable quantitative translational enablers to ascertain consistency in disease‐related intrinsic and extrinsic factors across ethnicities and regions. 6
With exponential increases in our ability to harness multidimensional data both cross‐sectionally and longitudinally, our appreciation of diversity in intrinsic and extrinsic factors has exponentially increased. The current space of intrinsic and extrinsic factors extends well beyond those depicted in the seminal “donut plot” that has served as a beacon for clinical pharmacologists in drug development. Vangay et al. performed a multigenerational and longitudinal assessment of gut microbiome diversity in the context of East to West immigration (Thailand to the United States) and discovered loss in microbiome diversity upon immigration. Bacteroides strains replaced Prevotella, with changes in the microbiome beginning within 6–9 months of US residence and continuing for decades. 7 Thinking back to Trust CoLab, where migration and urbanization were envisioned, and to the role of the gut microbiome in disease pathophysiology and therapeutics (e.g., inflammatory bowel disease and anticancer immunotherapy), these findings have implications for covariate analyses in exposure‐response models. Coupled with advances connecting clinical trial data to RWD through tokenization, opportunities for longitudinal disease progression modeling for understanding the impact of spatiotemporal diversity on outcomes are tantalizing.
Cirrincione and Huang have reviewed the current state of clinical pharmacology knowledge as relevant to pharmacotherapy of the transgender population. 8 Data to inform rational dosing of therapeutics is limited, given the lack of sufficient experience with calculating fundamental parameters like creatinine clearance or ideal body weight, or translation of risks for drug‐drug interactions from the general population. Being a population anticipated to grow over time, we offer a call to action for the PBPK research community to address the needs of the transgender male and female populations building upon prior successes in creating population PBPK frameworks for physiologically complex populations, such as pregnancy and neonates. Translational technologies like Organs‐On‐Chips coupled with quantitative systems pharmacology models may additionally offer avenues to interrogate the impact of dynamic changes in hormones during gender‐affirming treatments on responses to pharmacologic modulation. Poorer survival after diagnosis with prostate cancer has been described in transgender women compared with cisgender men. 9 Considering recent progress in development of a prostate‐on‐a‐chip platform, 10 potential applications to problems of prostate health in transgender women deserve further consideration to help inform development of evidence‐based screening and treatment guidelines.
Human diversity and our ability to deeply characterize its multidimensional and spatiotemporal richness (outer band ofFigure 1 ) are continually increasing. A patient‐centric approach to considering diversity in intrinsic and extrinsic factors in appropriately inclusive clinical development strategies and evidence synthesis is an opportunity for clinical pharmacologists equipped with today’s translational tools (middle band of Figure 1 ) in ways that were not possible in the past. Progress will require cross‐stakeholder trust in a Totality of Evidence approach with a growth mindset and openness to move away from established paradigms (base of Figure 1 ). This will ultimately enable trust in and timely access to evidence‐based medicine for one and all (inner circle of Figure 1 ).
Figure 1.
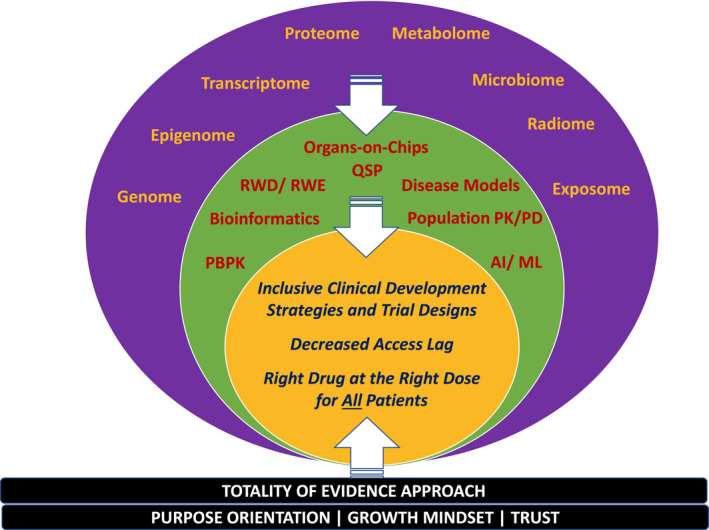
Advances in biomarker technologies and data science have increased our ability to characterize human diversity at multiple levels of intrinsic and extrinsic factors ranging from the genome to the exposome (outer band). These data provide rich substrate for quantitative integration and emulation using emerging quantitative translational tools (middle band). Understanding the impact of diversity in intrinsic and extrinsic factors on disease outcomes and response to therapeutic interventions will provide scientific guidance for appropriately inclusive clinical development, thereby decreasing access lag and advancing therapeutics with the right drug at the right dose for all patients (inner circle). Progress will require purpose orientation with patient centricity, a growth mindset, and cross‐stakeholder trust in a Totality of Evidence approach. In a Totality of Evidence approach, 6 evidence is substantiated through the confidence gained from consistency across multiple approaches and data sources integrated in a mechanism‐informed manner through modeling and simulation. AI, artificial intelligence; ML, machine learning; PBPK, physiologically‐based pharmacokinetics; PK/PD, pharmacokinetics/pharmacodynamics; QSP, quantitative systems pharmacology; RWD, real‐world data; RWE, real‐world evidence.
FUNDING
No funding was received for this paper.
CONFLICT OF INTEREST
K.V. and L.J.B. are employees of EMD Serono Research & Development Institute, Inc., a business of Merck KGaA, and have declared no competing interests for this work.
DISCLAIMER
As an Associate Editor of Clinical Pharmacology and Therapeutics, Karthik Venkatakrishnan was not involved in the review or decision process for this paper.
References
- 1. Terranova, N. , Venkatakrishnan, K. & Benincosa, L.J. Application of machine learning in translational medicine: current status and future opportunities. AAPS J. 23, 74 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yekedüz, E. et al. Assessing population diversity in phase III trials of cancer drugs supporting Food and Drug Administration approval in solid tumors. Int. J. Cancer 149, 1455‐1462 (2021). [DOI] [PubMed] [Google Scholar]
- 3. Strait, A. et al. Demographic characteristics of participants in rheumatoid arthritis randomized clinical trials: a systematic review. JAMA Netw. Open 2, e1914745 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lichtman, S.M. et al. Modernizing clinical trial eligibility criteria: Recommendations of the American society of clinical oncology‐friends of cancer research organ dysfunction, prior or concurrent malignancy, and comorbidities working group. J. Clin. Oncol. 35, 3753–3759 (2017). [DOI] [PubMed] [Google Scholar]
- 5. Liu, R. et al. Evaluating eligibility criteria of oncology trials using real‐world data and AI. Nature 592, 629–633 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venkatakrishnan, K. & Cook, J. Driving access to medicines with a totality of evidence mindset: an opportunity for clinical pharmacology. Clin. Pharmacol. Ther. 103, 373–375 (2018). [DOI] [PubMed] [Google Scholar]
- 7. Vangay, P. et al. US immigration westernizes the human gut microbiome. Cell 175, 962–972 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cirrincione, L.R. & Huang, K.J. Sex and gender differences in clinical pharmacology: implications for transgender medicine. Clin. Pharmacol. Ther. 110, 897–908 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson, S.S. et al. Cancer stage, treatment, and survival among transgender patients in the United States. J. Natl. Cancer Inst. 113, 1221–1227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang, L. et al. Human stroma and epithelium co‐culture in a microfluidic model of a human prostate gland. Biomicrofluidics 13, 64116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


