Abstract
Background
Gemcitabine and cisplatin has limited benefit as treatment for advanced biliary tract cancer (BTC). The addition of an anti‐programmed death receptor (PD‐1)/PD‐ligand (L1) antibody to either systemic chemotherapy or anti‐cytotoxic T‐lymphocyte‐associated protein 4 (CTLA4) antibody has shown benefit in multiple solid tumors.
Methods
In this phase 2 trial, patients 18 years or older with advanced BTC without prior systemic therapy and Eastern Cooperative Oncology Group Performance Status 0–1 were randomized across six academic centers. Patients in Arm A received nivolumab (360 mg) on day 1 along with gemcitabine and cisplatin on days 1 and 8 every 3 weeks for 6 months followed by nivolumab (240 mg) every 2 weeks. Patients in Arm B received nivolumab (240 mg) every 2 weeks and ipilimumab (1 mg/kg) every 6 weeks.
Results
Of 75 randomized patients, 68 received therapy (Arm A = 35, Arm B = 33); 51.5% women with a median age of 62.5 years. The observed primary outcome of 6‐month progression‐free survival (PFS) rates in the evaluable population was 59.4% in Arm A and 21.2% in Arm B. The median PFS and overall survival (OS) in Arm A were 6.6 and 10.6 months, and in Arm B 3.9 and 8.2 months, respectively, in patients who received any treatment. The most common treatment‐related grade 3 or higher hematologic adverse event was neutropenia in 34.3% (Arm A) and nonhematologic adverse events were fatigue (8.6% Arm A) and elevated transaminases (9.1% Arm B).
Conclusions
The addition of nivolumab to chemotherapy or ipilimumab did not improve 6‐month PFS. Although median OS was less than 12 months in both arms, the high OS rate at 2 years in Arm A suggests benefit in a small cohort of patients.
Keywords: chemo immunotherapybiliary cancer, cholangiocarcinoma, immune checkpoint blockade, immunotherapy
Short abstract
Immune checkpoint inhibition alone or in combination with chemotherapy as first‐line therapy does not appear to improve efficacy when compared to chemotherapy alone for patients with advanced biliary cancer in the United States. However, at least one third of the patients were alive at 2 years in the chemoimmunotherapy arm, and additional studies are ongoing to investigate this result, and importantly, evaluate biomarkers predictive for benefit from this treatment regimen.
INTRODUCTION
Patients with advanced biliary tract cancer (BTC) typically receive gemcitabine and cisplatin chemotherapy based on the results of the phase 3, ABC‐02 trial that reported a progression‐free survival (PFS) rate of 59% at 6 months and a median overall survival (OS) of 11.7 months for patients treated with this combination. 1 Efforts to improve outcome with chemotherapy have more recently included evaluation of three drug chemotherapy combinations. A large randomized phase 2 trial evaluating the modified FOLFIRINOX regimen failed to supplant gemcitabine and cisplatin as standard therapy. 2 A National Clinical Trials Network phase 3 trial evaluating the addition of nab‐paclitaxel to gemcitabine and cisplatin based on the promising data from a phase 2 trial has completed accrual. 3 Regardless of that result, there remains a significant need to improve on the modest benefit from standard chemotherapy.
Single‐agent immune checkpoint blockade (ICB) with an anti‐programmed death receptor (PD‐1) or ligand (PD‐L1) antibody in patients with advanced BTC has shown limited benefit after progression on chemotherapy with response rates of 3%–11% and a median PFS rate of 1.4–3.6 months. 4 , 5 Anti‐PD1/PD‐L1 agents have been successfully incorporated into first‐line therapy in multiple malignancies, often combined with chemotherapy or other immune checkpoint inhibitors, such as cytotoxic T‐lymphocyte‐associated protein 4 (CTLA4), to significantly improve outcome in melanoma, 6 gastroesophageal, 7 , 8 non–small cell lung, 9 and hepatocellular cancers. 10 Based on these data, we hypothesized an enhanced benefit of ICB as first‐line treatment with anti‐PD1 treatment in combination with chemotherapy or as dual ICB.
Here, we present results of our multicenter, phase 2 trial in which patients with treatment‐naive, advanced unresectable BTC were randomized to receive anti‐PD1 nivolumab in combination with gemcitabine and cisplatin or anti‐CTLA4 antibody, ipilimumab.
MATERIALS AND METHODS
Study design
This was a multi‐institutional, phase 2 clinical trial (BilT‐01) for patients with advanced biliary cancer and no prior systemic therapy. Planned enrollment was 64 evaluable patients randomized equally to arm A (gemcitabine, cisplatin, and nivolumab) or arm B (nivolumab and ipilimumab). The protocol was approved by the institutional review board at each site. All patients provided written informed consent before enrollment. The study was conducted in accordance with the Declaration of Helsinki and with the Good Clinical Practice Guidelines of the International Conference on Harmonization. Participating study sites and the University of Michigan Data and Safety Monitoring Committee reviewed the safety data (ClinicalTrials.gov identifier NCT03101566).
Outcomes
The primary study end point was defined as the PFS proportion at 6 months following initiation of study treatment. Secondary outcomes included evaluation of best objective response rate (ORR), median PFS, OS, and incidence of adverse events. Patients were replaced for evaluation of the primary end point if they did not receive any protocol therapy, withdrew consent, or were unable to continue treatment before first response assessment. All secondary outcomes of median PFS, median OS, ORR, and adverse events are reported in all patients who received any protocol treatment. Patients were allowed to continue beyond progression per immune‐related response evaluation criteria in solid tumors (irRECIST) criteria if they had stable performance status, tolerated study drug(s), investigator determined potential clinical benefit, and continued progression was not expected to lead to a serious disease‐related complication. 11 Exploratory outcomes, including genomic and transcriptomic analysis of the tumor and its immune microenvironment, will be reported in a future publication.
Patient eligibility
Key patient eligibility criteria included age 18 years or older; pathologic confirmation of biliary cancer (including gallbladder cancer, intrahepatic cholangiocarcinoma, and extrahepatic cholangiocarcinoma), locally advanced or metastatic stage not eligible for resection, transplantation, or ablative therapy; no prior systemic therapy for advanced cancer; radiographically measurable disease per RECIST (version 1.1); Eastern Cooperative Oncology Group Performance Status score of 0–1; and Child‐Pugh class A. Prior liver resection, radiation, or liver‐directed therapies were permitted. Patients with autoimmune diseases or on chronic immunosuppressive medications, including steroids, were not eligible.
Investigational treatment
Patients on Arm A received intravenous gemcitabine (1000 mg/m2), cisplatin (25 mg/m2) on days 1 and 8 followed by nivolumab (360 mg) on day 1 every 21 days. After 6 months, patients transitioned to nivolumab (240 mg) maintenance therapy every 2 weeks. Patients on Arm B received nivolumab (240 mg) every 2 weeks followed by ipilimumab (1 mg/kg) every 6 weeks. Patients were allowed to continue therapy for a total period of 2 years in the absence of progression or unacceptable toxicity.
Assessment and study end points
Patients underwent imaging (computed tomography or magnetic resonance imaging) for response assessment every 8 weeks. Best ORR was determined as per the combined RECIST v1.1 and immune‐related RECIST criteria as evaluated by board‐certified radiologists at each participating site. The PFS was calculated from the date of the first study treatment to either the date of documented disease progression or death from any cause, whichever occurred first. The OS was defined as the time from date of treatment until death or censored at last patient contact. All toxicities related to initial study treatment through 100 days following the last dose of the study treatment were reported and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Only laboratory abnormalities that required protocol treatment to be modified or treatment held were required to be reported.
Statistical analysis
The trial was designed to test the estimated 6‐month PFS proportion of 80% for each study arm (alternative hypothesis) compared to a historical control of 59% (null hypothesis). The trial would have 80% power using a one‐sided test with 5% type I error to test the alternative hypothesis. We sized the trial to accrue 32 evaluable patients for the primary end point per arm.
The distributions for PFS and OS were estimated using the product‐limit method of Kaplan and Meier and reported as median and 75th percentiles with 95% confidence intervals (CI) per study arm. Univariate analysis was performed using the log‐rank test, and best ORR was summarized by number and percentage with exact binomial 95% CIs. Descriptive statistics were used for all clinical and demographic data and adverse events. For the final analysis, the data cutoff date was January 19, 2022 at which time 21 of 68 patients who received any treatment were reported to be alive. Statistical analyses were completed using SAS (SAS Institute Inc, version 9.4).
RESULTS
Patients were recruited across six sites in the United States (University of Michigan, University of Texas Southwestern, Emory University, Northwestern University, University of Washington/Seattle Care Alliance, and University of Wisconsin). The study randomized 75 eligible patients between September 2017 and June 2019, and 68 patients received treatment (Arm A: 35 and Arm B: 33) and were evaluable for secondary outcomes. Three patients on Arm A withdrew consent before first response evaluation for study treatment unrelated to toxicity and were not eligible for primary end point analysis per study protocol (Fig. 1). In the 68 patients who received any study treatment, 51.5% were women and median age was 62.5 (range, 20–80) years. The majority of patients had intrahepatic cholangiocarcinoma (61.8%) and metastatic disease (89.7%). Arm A had a significantly higher proportion of patients with prior surgery as compared to Arm B; all other baseline characteristics were similar between study arms (Table 1).
FIGURE 1.
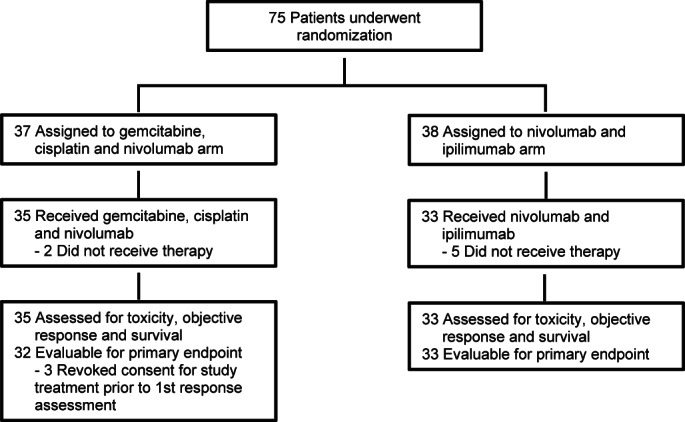
Patient disposition.
TABLE 1.
Baseline characteristics of treated population
| Variable | Total (N = 68) | Arm A (N = 35) | Arm B (N = 33) | p |
|---|---|---|---|---|
| Age, years | ||||
| Mean, median | 61.0, 62.5 | 60.4, 62.0 | 61.6, 65.0 | .63c |
| Range | 20–80 | 20–80 | 34–78 | |
| Gender, No. (%) | ||||
| Female | 35 (51.5) | 16 (45.7) | 19 (57.6) | .34a |
| Male | 33 (48.5) | 19 (54.3) | 14 (42.4) | |
| Disease extent, No. (%) | ||||
| Locally advanced | 7 (10.3) | 3 (8.6) | 4 (12.1) | .63a |
| Metastatic | 61 (89.7) | 32 (91.4) | 29 (87.9) | |
| Tumor location, No. (%) | ||||
| Gallbladder | 14 (20.6) | 7 (20.0) | 7 (21.2) | |
| Intrahepatic | 42 (61.8) | 21 (60.0) | 21 (63.6) | .94b |
| Extrahepatic, distal | 9 (13.2) | 5 (14.3) | 4 (12.1) | |
| Extrahepatic, hilar | 3 (4.4) | 2 (5.7) | 1 (3.0) | |
| Race, No. (%) | ||||
| Caucasian | 56 (82.4) | 31 (88.6) | 25 (75.7) | |
| African American | 8 (11.8) | 4 (11.4) | 4 (12.1) | .25b |
| Asian | 2 (2.9) | 2 (6.1) | ||
| Multiple/not reported | 2 (2.9) | 2 (6.1) | ||
| Ethnicity, No. (%) | ||||
| Hispanic or Latino | 7 (10.3) | 6 (17.1) | 1 (3.0) | |
| Not Hispanic | 60 (88.2) | 29 (82.9) | 31 (94.0) | .10b , d |
| Not reported | 1 (1.5) | 1 (3.0) | ||
| ECOG performance status, No. (%) | ||||
| 0 | 19 (27.9) | 8 (22.9) | 11 (33.3) | .34a |
| 1 | 49 (72.1) | 27 (77.1) | 22 (66.7) | |
| CA 19–9 at baseline, U/ml | ||||
| Median | 211g | 130 | 453g | .54e |
| IQR | 25.0–2040 | 21.1–2418 | 36.0–2040 | |
| Prior therapy reported, No. (%) | ||||
| Surgery | 18 (26.5) | 13 (37.1) | 5 (15.2) | .040a |
| Embolization or ablation | 8 (11.8) | 4 (11.4) | 4 (12.1) | .99b |
| Radiation | 7 (10.3) | 3 (8.6) | 4 (12.1) | .71b |
| Chemotherapyf | 9 (13.2) | 6 (17.1) | 3 (9.1) | .33b |
| Any | 22 (32.4) | 14 (40.0) | 8 (24.2) | .17a |
| None | 46 (67.6) | 21 (60.0) | 25 (75.8) |
Abbreviations: CA 19–9, carbohydrate antigen 19–9; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range (25th–75th percentile).
Chi‐square test.
Fisher exact test when cell frequencies below 5.
t‐test.
Test excludes missing/not reported category.
Wilcoxon rank‐sum test.
For localized disease as perioperative/adjuvant therapy.
A total of 31 of 33 patients of Arm B had baseline values for CA19‐9, two patients did not.
Efficacy
Patients evaluable for the primary end point of estimated 6‐month PFS proportion (N = 65) received a median of 6.6 (range, 1.1–22.6) months and 2.7 (range, 0.7–25.2) months of treatment in Arms A and B, respectively. The median follow‐up was 32.2 and 31.5 months for Arms A and B calculated using reverse censoring method of the product‐limit estimate, respectively. The observed 6‐month PFS in Arm A was 59.4% (95% CI, 40.5–74.0) and in Arm B was 21.2% (95% CI, 9.4–36.3), and neither arm met the alternative hypothesis.
In the population that received any treatment (N = 68), the median PFS was 6.6 months (95% CI, 3.4–7.7) in Arm A and 3.9 (95% CI, 2.3–4.5) months in Arm B (Fig. 2A). The 75th percentile for PFS was 9.0 (95% CI, 7.3–11.2) months in Arm A, and 5.4 (95% CI, 4.4–8.2) months in Arm B. PFS was not significantly better in Arm A when compared to Arm B (p = .077). The median OS in Arm A was estimated at 10.6 (95% CI, 6.4–24.5) months and for Arm B was 8.2 (95% CI, 5.8–16.9) months (p = .61; Fig. 2B). The 75th percentile for OS is not yet estimable. The best ORR in Arms A and B were 22.9% (95% CI, 10.4–40.1) and 3.0% (95% CI, 0.1–15.8%), respectively. Median response duration was 4.3 months in Arm A (range, 1.8–18.5 months) with three patients maintaining response for 11.3, 15.6, and 18.5 months. The single response in Arm B was short lived (1.9 months). On Arm A, seven patients continued therapy beyond progression per immune‐related RECIST criteria for a mean of 73 days (range, 30–166), and on Arm B, six patients for a mean of 73 days (range, 29–180).
FIGURE 2.
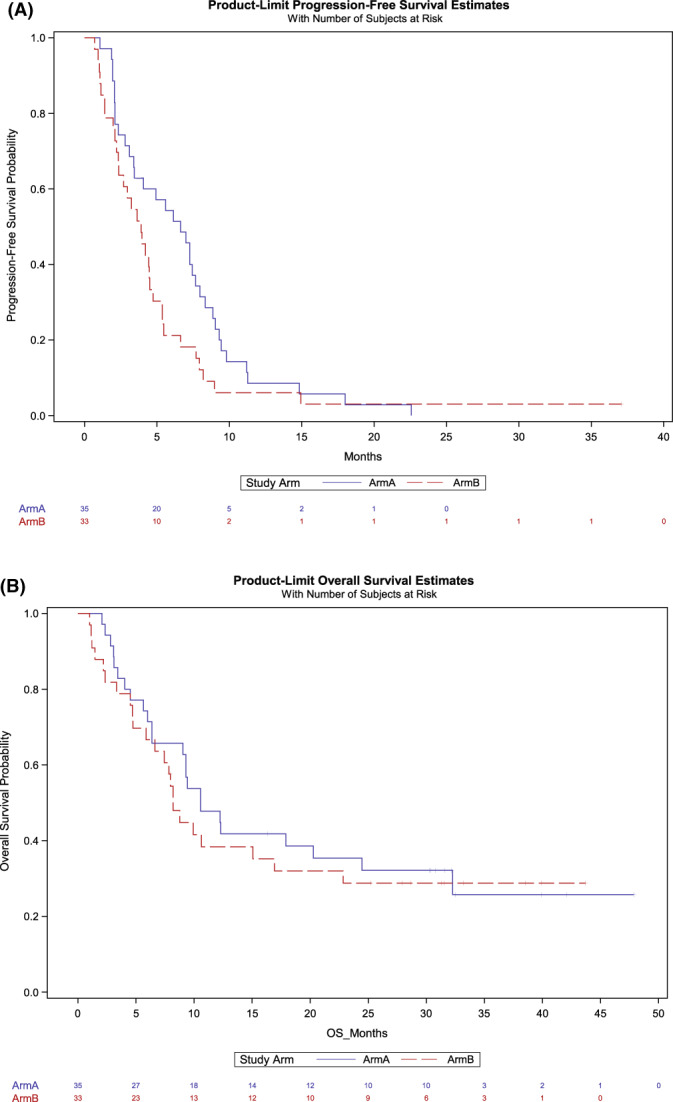
Kaplan–Meier analyses of (A) progression‐free survival and (B) overall survival.
At 12 and 24 months, 47.8% and 35.4% of the patients were alive in Arm A, and 38.4% and 28.8% in Arm B. At the time of data cutoff, 11 (31.4%) patients in Arm A and 10 (30.3%) patients in Arm B were still alive.
Safety
Serious adverse events were reported for 62.9% (95% CI, 44.9–78.5) of patients in Arm A and 36.4% (95% CI, 20.4–54.9) in Arm B (p = .05). Additionally, the median duration of treatment in Arm A was significantly longer compared to Arm B (6.1 vs. 2.7 months; p = .01). Treatment‐related serious adverse events were reported in 13 patients of which 10 events were designated as related to immunotherapy, including diarrhea/colitis (N = 4), hepatitis (N = 3), adrenal insufficiency (N = 1), encephalitis (N = 1), and vomiting (N = 1). Adverse events led to treatment discontinuation for two patients in Arm A and four patients in Arm B.
A total of 54 (79.4%) patients experienced a treatment‐related adverse event (TRAE) of at least grade 1 of which 31 (88.6%) patients were in Arm A and 23 (69.7%) in Arm B. Overall, 34 (50%) patients experienced a grade 3 or higher TRAE (Table 2). In Arm A, the most common grade 3 or higher hematologic and nonhematologic adverse events were neutropenia (34.3%) and fatigue (8.6%), respectively. In Arm B, there were no grade 3 or higher hematologic adverse events, and the most common nonhematologic adverse events were elevated transaminase (alanine or aspartate) levels (9.1% in Arm B). Immune‐related adverse events were not separately identified in the database.
TABLE 2.
Treatment‐related adverse events per patient at grade 3 or higher a
| Event | No. (%) | |
|---|---|---|
| Arm A (N = 35) | Arm B (N = 33) | |
| All hematologic AEs | ||
| Neutropenia | 12 (34.3) | 0 |
| Anemia | 8 (22.9) | 0 |
| Leukopenia | 4 (11.4) | 0 |
| Thrombocytopenia | 3 (8.6) | 0 |
| Lymphopenia | 2 (5.7) | 0 |
| Nonhematologic AEs in ≥5% patients | ||
| Fatigue | 3 (8.6) | 2 (6.1) |
| Elevated aspartate aminotransferase | 2 (5.7) | 3 (9.1) |
| Elevated alanine aminotransferase | 2 (5.7) | 3 (9.1) |
| Diarrhea | 0 | 2 (6.1) |
| Colitis | 0 | 2 (6.1) |
| Nausea | 2 (5.7) | 0 |
Abbreviation: AEs; adverse events.
Only the laboratory abnormalities that required protocol treatment to be modified or treatment to be rendered were required to be reported.
DISCUSSION
This phase 2 multicenter trial randomized patients with advanced biliary cancer to first‐line systemic therapy with nivolumab and combination chemotherapy or dual checkpoint inhibitor treatment with nivolumab and ipilimumab. The observed PFS rates at 6 months of 59.4% and 21.2% in Arms A and B are insufficient to reject the null hypothesis and do not appear to represent an improvement over standard therapy. Although Arm B was clearly inferior to the historical control, Arm A was similar in efficacy to standard treatment with gemcitabine and cisplatin and there was no obvious concern for increased toxicity or differences in secondary outcomes with the addition of nivolumab to chemotherapy, including median survival or response rate.
Of interest, the observed OS rate at 2 years was 35.4% in Arm A with 11 patients alive at the time of data cutoff. In contrast, the likelihood of survival at 2 years with chemotherapy alone is estimated to be between 15%–22%. 1 , 12 , 13 More recently, the results of the phase 3 TOPAZ‐1 trial reported a 2‐year OS rate of 24.9% in the investigational arm of gemcitabine, cisplatin, and durvalumab compared to 10.4% in the gemcitabine, cisplatin, and placebo arm. 14 This improvement in the tail of the survival curve has been previously described in literature across multiple cancers 15 and recognized by the value framework score by the American Society of Clinical Oncology, 16 highlighting a potential role of immunotherapy in biliary cancer in this subset. The increase in OS at 2 years in our trial is certainly limited by the small number of patients in the chemoimmunotherapy arm and may be due to selection bias introduced by trial participation at academic centers. Furthermore, patients with prolonged survival may also reflect the potential benefit from novel targeted therapies post‐trial, such as FGFR2 rearrangements and IDH1 mutations.
A phase 1 trial of nivolumab in combination with gemcitabine and cisplatin in 30 Japanese patients with advanced BTC and no prior systemic treatment reported a median PFS and OS of 4.2 and 15.4 months, respectively. 17 Additionally, a phase 2 trial of durvalumab with gemcitabine and cisplatin in a similar patient population in South Korea reported a median PFS and OS of 11.0 and 18.1 months, respectively, with a robust ORR of 73.4%. 18 Based on these encouraging data, there are ongoing multinational phase 3 randomized trials with gemcitabine and cisplatin, with or without anti‐PD1/PD‐L1 antibody, in patients with advanced biliary cancer. 14 , 19 The results of the phase 3 TOPAZ‐1 trial reported a significant improvement in median OS to 12.8 months in the durvalumab containing arm as compared to 11.5 months with chemotherapy alone (p = .021). Interestingly, the subgroup analysis of OS showed the improvement in survival was limited to Asia versus rest of the world, and to the Asian race versus non‐Asian. 14 Our current trial with chemoimmunotherapy and dual immunotherapy is the first to be reported in advanced BTC in a US population. The apparent differences in the reported efficacy between trials is concerning for benefit limited to a subset of patients. It is plausible that the benefit of anti‐PD1/PD‐L1 checkpoint inhibition is restricted to biliary cancers driven by chronic inflammation as compared to those related to FGFR2 translocations, IDH1/2 mutations, or as yet unknown genomic, immune microenvironment, or geographic differences in patient populations. Biomarker analyses, including genomic and transcriptomic analyses of the tumor and its immune microenvironment from our trial is pending, and similar analyses from other trials are needed to better identify patients with biliary cancer most likely to benefit from chemoimmunotherapy.
Limitations
This randomized study is limited by the size of patient population as well as comparison of each arm to historical control. This trial, however, was a multi‐institutional study conducted at six academic centers by experienced investigators. The trial completed accrual in 22 months and enrolled patients with no prior systemic therapy for advanced disease. It included unselected patients without pre‐determined PD‐L1 or combined prognostic score, or known mismatch repair, microsatellite analysis, or DNA damage repair mutations.
In conclusion, this study found that whereas chemoimmunotherapy and dual immunotherapy in patients with advanced biliary cancer was generally well tolerated, neither treatment combination demonstrated an improvement in PFS rate, median PFS, OS, or response rate. Nevertheless, at least one third of the patients were alive at 2 years in the chemoimmunotherapy arm, and additional studies are ongoing to investigate this result, and importantly, evaluate biomarkers predictive for benefit from this treatment regimen.
AUTHOR CONTRIBUTIONS
Vaibhav Sahai: Conceptualization, data collection, analysis and interpretation of results, and draft manuscript preparation. Kent A. Griffith: Analysis, interpretation of results and draft manuscript preparation. Muhammad S. Beg: Data collection and interpretation of results. Walid L. Shaib: Analysis and interpretation of results. Devalingam Mahalingam: Data collection and interpretation of results. David B. Zhen: Data collection and interpretation of results. Dustin A. Deming: Data collection and interpretation of results. Mark M. Zalupski: Conceptualization, data collection, analysis and interpretation of results, and draft manuscript preparation. All authors have reviewed the results and approved the final version of the manuscript.
Funding
Bristol‐Myers Squibb; University of Michigan Rogel Cancer Center, Grant/Award Number: P30CA046592.
CONFLICTS OF INTEREST
Vaibhav Sahai reports institutional grant funding from Agios, Bristol‐Myers Squibb, Celgene, Clovis, Exelixis, Fibrogen, Incyte, Ipsen, Medimmune, Merck, and Rafael; and consultant fees from AstraZeneca, GlaxoSmithKline, Histosonics, Incyte, QED and Rafael. Kent A. Griffith reports institutional grant funding from Bristol‐Myers Squibb, AstraZeneca, Merch Serono, Five Prime Therapeutics, MedImmune, Genentech, Immunesensor, and Tolero Pharmaceuticals; and consultant fees from Ipsen, Array BioPharm, AstraZeneca, Cancer Commons, Legend Biotech, and Foundation Medicine. Walid L. Shaib reports institutional grant funding from GlaxoSmithKline, Lexicon, Tesaro, Eli Lilly; and consultant fees from Ipsen, Lexicon, Mylan, Bristol‐Myers Squibb, and BluePrint Therapeutics. Devalingam Mahalingam reports institutional grant funding from Amgen, Merck, and Oncolytics; and consultant fees from Qurient, Oncoone, Bristol‐Myers Squibb, Eisai and Exelixis. David B. Zhen reports institutional grant funding from Merck, SeaGen, Daiichi‐Sankyo, and AstraZeneca. Dustin A. Deming reports institutional grant funding from Merck, Arcus, Bristol‐Myers Squibb, Aadi, Takeda, and AstraZeneca; and advisory board member for Eli Lilly, Seattle Genetics, Bayer, and Promega. Mark M. Zalupski reports institutional grant funding from AstraZeneca, MedImmune and Seattle Genetics. The other author made no disclosures.
Supporting information
Appendix
ACKNOWLEDGMENTS
The investigational study drugs nivolumab and ipilimumab were supplied by Bristol‐Myers Squibb. The authors are immensely grateful to the patients and their families for their participation and all the investigators and research staff for enrolling patients. The trial was supported by Bristol‐Myers Squibb and University of Michigan Rogel Cancer Center (P30CA046592).
The trial registration is ClinicalTrials.gov identifier NCT03101566.
REFERENCES
- 1. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273‐1281. [DOI] [PubMed] [Google Scholar]
- 2. Phelip JM, Desrame J, Edeline J, et al. Modified FOLFIRINOX versus CISGEM chemotherapy for patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): A randomized phase II study. J Clin Oncol. 2022;40(3):262‐271. [DOI] [PubMed] [Google Scholar]
- 3. Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, cisplatin, and nab‐paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim RD, Chung V, Alese OB, et al. A phase 2 multi‐institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6(6):888‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piha‐Paul SA, Oh DY, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE‐158 and KEYNOTE‐028 studies. Int J Cancer. 2020;147(8):2190‐2198. [DOI] [PubMed] [Google Scholar]
- 6. Larkin J, Chiarion‐Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous‐cell carcinoma. N Engl J Med. 2022;386(5):449‐462. [DOI] [PubMed] [Google Scholar]
- 8. Janjigian YY, Shitara K, Moehler M, et al. First‐line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro‐oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open‐label, phase 3 trial. Lancet. 2021;398(10294):27‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uprety D. Chemoimmunotherapy for stage IV non‐small‐cell lung cancer. Lancet Oncol. 2019;20(9):e466. [DOI] [PubMed] [Google Scholar]
- 10. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894‐1905. [DOI] [PubMed] [Google Scholar]
- 11. Nishino M, Giobbie‐Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune‐related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936‐3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malka D, Fartoux L, Rousseau V, et al. Gemcitabine and oxaliplatin (GEMOX) alone or in combination with cetuximab as first‐line treatment for advanced biliary cancer: final analysis of a randomized phase II trial (BINGO). ASCO Meeting Abstracts. 2012;30(suppl 15):4032. [Google Scholar]
- 13. Sahai V, Catalano PJ, Zalupski MM, et al. Nab‐paclitaxel and gemcitabine as first‐line treatment of advanced or metastatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4(12):1707‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh D‐Y, He AR, Qin S, et al. A phase 3 randomized, double‐blind, placebo‐controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ‐1. J Clin Oncol. 2022;40(suppl 4):378. [Google Scholar]
- 15. Finn RS, Ryoo B‐Y, Merle P, et al. Pembrolizumab as second‐line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE‐240: a randomized, double‐blind, phase III trial. J Clin Oncol. 2020;38(3):193‐202. [DOI] [PubMed] [Google Scholar]
- 16. Cherny NI, Vries EGE, Dafni U, et al. Comparative assessment of clinical benefit using the ESMO‐Magnitude of Clinical Benefit Scale Version 1.1 and the ASCO Value Framework Net Health Benefit Score. J Clin Oncol. 2019;37(4):336‐349. [DOI] [PubMed] [Google Scholar]
- 17. Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non‐randomised, multicentre, open‐label, phase 1 study. Lancet Gastroenterol Hepatol. 2019;4(8):611‐621. [DOI] [PubMed] [Google Scholar]
- 18. Oh D‐Y, Lee K‐H, Lee D‐W, et al. Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo‐naïve advanced biliary tract cancer (aBTC). J Clin Oncol. 2020;38(suppl 15):4520. [Google Scholar]
- 19. Finn RS, Kelley RK, Furuse J, et al. Abstract CT283: KEYNOTE‐966: a randomized, double‐blind, placebo‐controlled, phase 3 study of pembrolizumab in combination with gemcitabine and cisplatin for the treatment of advanced biliary tract carcinoma. Cancer Res. 2020;80(suppl 16):CT283. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix


