Abstract
Introduction
In light of the lack of an agreed international standard for how to conduct cost-effectiveness analyses (CEAs), including cost–utility analyses (CUAs) from a societal perspective, there is uncertainty regarding to what extent the inclusion of productivity losses/gains in economic evaluations can affect cost-effectiveness results and subsequently decisions on whether to recommend new health technologies. To investigate this, we conducted a systematic review of CEAs and CUAs of drug-based therapies for a set of chronic immune-mediated disorders to understand how cost elements and calculation methods related to productivity losses/gains are used, examine the impact on the incremental cost-effectiveness ratio (ICER) of including productivity costs, and explore factors that affect the inclusion of productivity loss.
Methods
Databases (MEDLINE® In-process, MEDLINE, Embase and Cochrane Library) were searched from January 2010 to October 2020 by two independent reviewers for all CEAs and CUAs in adults with any of the following conditions: ankylosing spondylitis, chronic idiopathic urticaria, Crohn’s disease, fibromyalgia, juvenile idiopathic arthritis, psoriasis, rheumatoid arthritis, systemic lupus erythematosus and ulcerative colitis. Relevant study data were extracted and evidence was synthesized for both qualitative and quantitative analysis. Productivity cost elements including absenteeism, presenteeism, unemployment/early retirement, premature mortality and informal care were extracted, along with the method used to determine them. A multivariate analysis was performed to identify factors associated with the inclusion of productivity loss.
Results
Our searches identified 5016 records, culminating in 198 unique studies from 234 publications following screening. Most of the studies investigated rheumatoid arthritis (37.0%) or psoriasis (32.0%). The majority were CUAs, with some including both a CEA and a CUA (73.0%). Most studies used a payer perspective only (28.5%) or a societal perspective only (21.0%). Of the 49 studies incorporating productivity losses/gains, 42 reported the type of cost element used; all of these used patient absenteeism, either alone or in addition with other elements. Only 16 studies reported the method used to value productivity changes, of which eight used a human capital approach, four used a friction cost approach and four used both approaches. Twenty-eight of the 49 studies (57.1%) reported inclusion of productivity losses/gains as contributing to more favourable cost-effectiveness outcomes and ICERs, while 12 (24.5%) reported no substantial impact. On the basis of a multivariate analysis, rheumatoid arthritis as the target disease had a statistically significant association with the inclusion of productivity loss compared with psoriasis and inflammatory bowel disease.
Conclusions
The results of our review suggest that incorporating productivity cost elements may positively affect cost-effectiveness outcomes in evaluations of therapeutics for immune-mediated disorders. Our work highlights the continued need for clarity when reporting how CEAs and CUAs in this disease area are conducted, in order to better inform healthcare decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02321-z.
Keywords: Cost-effectiveness analysis, Cost-utility analysis, Productivity loss, Systematic literature review, Rheumatoid arthritis, Psoriasis, Indirect cost, Health technology assessment
Key Summary Points
| There are differences regarding to what extent productivity losses/gains are included in CEAs including CUAs. |
| Most of the CEAs/CUAs in immune-mediated disorders identified were conducted in rheumatoid arthritis or psoriasis. Most of the studies that included productivity loss were conducted in the Netherlands, followed by the US and Sweden. |
| For diseases where the impact of productivity loss is already widely reported, such as rheumatoid arthritis, many CEAs incorporate productivity loss. |
| For CEAs of drugs for immune-mediated disorders that incorporate productivity losses and gains into the analysis, the results are typically favourable with respect to the cost-effectiveness of the drug being analysed. |
Introduction
The continued innovation and expansion of the number of available medical technologies coupled with the scarcity of healthcare resources has led to the growth of health technology assessment (HTA) to guide resource allocation and inform policy decision-making [1, 2]. With the support of organizations such as the World Health Organization (WHO), the International Network of Agencies for Health Technology Assessment (INAHTA) and Health Technology Assessment international (HTAi), more countries now appraise technologies in terms of their value for money as well as their potential clinical impact [2–6]. A key component of many HTA systems is cost-effectiveness analysis (CEA), in which the change in health outcomes and the costs associated with the introduction of treatments are estimated. A specific kind of CEA, cost–utility analysis (CUA), determines the gain or loss of quality-adjusted life years (QALYs) that results from introducing a medical intervention and the increase (or decrease) in associated costs [7].
The way in which CEAs and CUAs are performed varies according to the analytical perspective taken. Countries often have their own guidelines and/or guidance for conducting CEAs and CUAs, and CEAs conducted as part of HTA tend to differ as a result. In Australia, Canada, France, Japan and the United Kingdom (UK), the guidelines recommend carrying out analyses from healthcare or public payer perspectives [8–12], with the focus being on costs directly attributable to the introduction of an intervention such as treatment, administration, hospitalization and outpatient costs, as well as costs of national healthcare and insurance programmes. Countries such as Denmark, the Netherlands and Sweden recommend analyses that take a societal perspective, which includes both direct and indirect (e.g. productivity loss) costs—that is, they aim to account for the impact of a disease on the wider society [13–15]. Such a perspective can also be recommended in Australia, Canada, France, the UK and the United States (US), albeit as a secondary analysis to the baseline healthcare or public payer perspective analysis [8–10, 12, 16]. Countries that recommend a societal perspective in HTA have adopted a variety of approaches to conducting economic analyses such as CEA and CUA. Parameters often used include absenteeism, which refers to the productivity lost as a result of taking leave because of illness, and presenteeism, where productivity is lost while at work as a result of illness [17]. Two methods often specified in HTA guidelines to calculate the value of productivity costs include (1) the human capital approach, in which the amount of time lost is multiplied by the amount that would have been paid; and (2) the friction cost approach, where the amount of time taken to find and train a replacement in the patient’s absence is multiplied by the patient’s adjusted gross wage [7, 9, 11, 14, 18, 19]. When it comes to CEAs and CUAs conducted on the basis of academic research, there are rarely distinct guidelines for each country; rather, they are often conducted with reference to published books and checklists [7, 20, 21].
As shown by this variation, the decision on whether and, if so, how to account for productivity effects and other indirect costs in economic evaluations has been the topic of much debate and remains a contentious issue [7, 22, 23]. Despite this, there is a lack of clear understanding of how incorporating such parameters affects the output of these analyses—specifically, the incremental cost-effectiveness ratio (ICER). Nor is there clarity on the reasoning behind the choice of perspectives taken in many countries’ healthcare systems. Some studies have suggested that taking productivity losses/gains into account leads to ICER values either greater or smaller than if they were left out [24–26], suggesting that the choice of whether to include indirect costs can have an impact on healthcare decision-making.
To investigate the effect of including productivity losses and gains on the outcomes of economic evaluations, we previously conducted a systematic literature review (SLR) of all CEAs and CUAs of drug-based treatments that incorporated indirect costs [27]. We found that generally, CEAs and CUAs in which productivity losses were considered led to more favourable ICERs for the treatment of interest than those that did not include these cost elements. Our exhaustive review of the use of productivity losses/gains allowed for a detailed analysis of the various cost elements used across different disease areas. However, while a large number of studies were identified, the quality of reporting in the studies had a bearing on its findings. A review of economic evaluations in a specific disease area, coupled with an investigation of those evaluations that account for indirect costs compared with those using only direct costs (i.e. conducted from a payer perspective only), could provide more insight into the potential for productivity losses/gains in CEAs to lead to lower or higher ICERs.
In our previous review, we found that immunology was the most common focus of CEAs of drug-based treatments involving indirect costs. Approximately one in four (26%) of the included articles assessed immunology, the highest proportion [27]. Rheumatoid arthritis, the disease most often reported among these studies, is a debilitating condition that causes pain in the joints of the fingers and toes, interfering with daily life and often leading to work loss due to attacks or ‘flares’ (sudden onset of symptoms) [28]. We thus considered immunology to be a suitable focus for our investigation and comparative evaluation to further explore the impact on the ICER of including indirect costs.
Thus, we sought to systematically review the literature to (1) identify the characteristics of CEAs and CUAs conducted for immune-mediated disorders and the proportion that have included productivity loss; (2) ascertain the cost elements related to productivity losses/gains included in the CEAs and CUAs and the calculation methods used to determine those elements; and (3) explore factors that may affect the inclusion of productivity loss for CEAs and CUAs of immune-mediated disorders.
Methods
SLR Approach
This SLR was conducted according to the international guidelines laid out by the Centre for Reviews and Dissemination (CRD) [29], the PRISMA Group [30], and the Cochrane Handbook [31]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Definition of Terms
The definitions of indirect costs and productivity losses used were in line with multiple guidelines [10, 18]. Indirect costs were defined as the valuation of resources not directly consumed by the care provided but rendered unavailable because of the patient’s poor health state or premature death. Productivity losses were defined as losses incurred as a result of a total stoppage or partial reduction of the productive activity of the population analysed (including absenteeism and presenteeism), whether this activity is paid or unpaid.
Search Methodology
A search strategy was devised by a team of experienced systematic reviewers to identify all available evidence relevant to our objective. The strategy comprised three sets of terms: a set to search for the type of economic evaluation (i.e. CEA and CUA), and a set of search terms for focussing on drugs of interest (e.g. biologics, Janus kinase inhibitors [JAKi]), and a set to search terms for the relevant diseases and/or populations of interest. The following nine conditions were chosen: ankylosing spondylitis, chronic idiopathic urticaria, Crohn’s disease, fibromyalgia, juvenile idiopathic arthritis, psoriasis, rheumatoid arthritis, systemic lupus erythematosus and ulcerative colitis. Search terms were chosen to cover a number of indexing and free-text terms to ensure that a high proportion of the relevant articles were captured. Table 1 presents the inclusion and exclusion criteria PICOS (population, intervention, comparator, outcomes, study design) elements used. In order to maximize consistency and comparability with the findings, we included only studies targeting adult patients; assumptions were made where there was a mixed population or a lack of clarity on whether studies included people below 18 (see Table 1). Evaluations were included if they involved pharmacological therapies as either the intervention or a comparator. Studies were included if they reported the results of a CEA or CUA; cost–benefit and cost-minimization analyses were excluded since few countries recommend this approach for the economic evaluation of new therapeutic interventions [4]. The results of official HTA submissions to the countries themselves were considered out of scope for this study. The searches were restricted to English-language full-text articles published from January 2010 onwards, or English-language conference abstracts published from 2018 onwards, up to 26 October, 2020. The keywords were combined using Boolean operators to create the search strings listed in Appendix 1 of the Supplementary Material.
Table 1.
Eligibility criteria including PICOS elements
| Category | Details |
|---|---|
| Population |
Adult patients (ages ≥ 18 years old)a with at least one of the following disorders: Ankylosing spondylitis Chronic idiopathic urticaria (including chronic spontaneous urticaria) Crohn’s disease Fibromyalgia Juvenile idiopathic arthritis Psoriasis Rheumatoid arthritis Systemic lupus erythematosus Ulcerative colitis |
| Interventions/comparators | Any drug treatment |
| Study types |
Full economic evaluations: CEAs and CUAs If a study could estimate an ICER (i.e. the study described incremental costs per incremental QALY or life years or cost per response), the study was included |
| Outcomes |
Descriptive differences (e.g. publication year, country, type of analysis, model used, time horizon) Productivity loss elements and approaches Impact of including productivity costs on ICER |
| Language | English only |
| Country | No limits |
| Publication types |
Full-text articles only While there are conference posters and articles that may include cost data, the final data were considered to be uncertain/unverifiable if the full-text article was not available; therefore, only those with full-text articles available were included |
| Time-limits |
2010 to 2020 for full-text articles 2018 to 2020 for the conference abstracts |
CEA cost-effectiveness analysis, CUA cost-utility analysis, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life year
aIf it was unclear in a study whether adults or children were assessed, then the study was included on the basis of the assumption that all the indications examined are more prevalent in adults, especially when it comes to economic studies. Moreover, if a study included both adult and paediatric patients, it was included if subgroup data for adult patients were reported. In a second instance, if adult patients constituted ≥ 80% of the total population, then the study was included and data were extracted for the complete study population
Sources
The following databases were searched: MEDLINE® In-process (via PubMed.gov), MEDLINE and Embase (via Embase.com), and the Cochrane Library (via Cochrane). Searches were also conducted for abstracts of the following conferences: International Society for Pharmacoeconomics and Outcomes Research (ISPOR), and the European Association of Hospital Pharmacists (EAHP). As per the CRD and Cochrane guidelines, the bibliographies of included studies were checked, and relevant systematic reviews and unique studies not identified from the electronic database searching were hand-searched.
Screening and Study Identification
Following the initial searches, records were first added to a library in the reference manager software EndNote™. A de-duplication step was carried out to remove duplicate records, and the records were exported into a worksheet in Microsoft Excel®. The selected study had to provide evidence for one or more of the outcomes of interest in order to be included. Studies presented in languages other than English were excluded from the analysis.
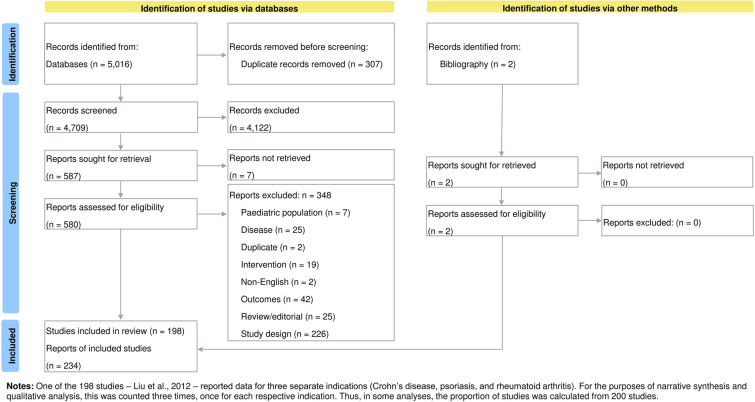
Primary screening was conducted by two reviewers. Each reviewer independently assessed each record’s title and abstract against basic study selection criteria (population, intervention, study design) to decide on whether to exclude the study. Secondary screening of potentially relevant articles was carried out by two reviewers by first obtaining the full-text article, then reviewing against each of the eligibility criteria including PICOS elements (see Table 1). Any uncertainty regarding the inclusion of studies during the primary or secondary screening was resolved by a third reviewer. All articles included or excluded and the reasons behind these decisions are summarized in the PRISMA flow diagram in Fig. 1.
Fig. 1.
Flow diagram depicting search results and selection of studies for analysis
Data Extraction
A standardized evidence data extraction shell was developed in Excel and populated with the information extracted from the included studies. Data were extracted by one reviewer into the tables, and a second reviewer checked and validated key outcomes data (such as model inputs, structure, ICERs, perspective and time horizon) by conducting an independent internal data check scheme. Where multiple publications were identified for the same patient population and setting and reported data for the same cost year and interventions, these studies were linked and extracted together. A list of data extracted is provided in Appendix 2 of the Supplementary Material.
Assessment of Quality of Studies
The reporting quality of the included economic evaluation studies was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2013 guidelines from ISPOR [32]. The CHEERS checklist consists of 24 items across six sections: (1) title and abstract; (2) introduction; (3) methods; (4) results; (5) discussion; and (6) other (e.g. source of funding, conflict of interest). One reviewer rated each item of the checklist as either ‘fully satisfied’, ‘partially satisfied’, ‘not satisfied’ or ‘not applicable’, and a second reviewer validated each rating. The quality assessment was performed in parallel with the data extraction.
Synthesis of Evidence
Following extraction of data using Excel-based forms, evidence was synthesized for qualitative analysis using appropriate graphs and tables. Descriptive study characteristics such as country, disease area, study sponsor, type of study, study design and analysis perspective were recorded as reported from the data extractions. Countries were grouped according to region classifications given by the World Bank Group [33].
Types of productivity cost elements included were determined on the basis of a description of the costs included in the study. Cost elements included absenteeism, presenteeism, unemployment/early retirement, premature mortality and informal care. The approach taken to determine productivity loss/gain was also extracted for each study.
As per the previous work, the effect on the ICER of including productivity costs was split into four categories, depending on the impact reported in the studies: ‘more favourable’, ‘no substantial impact’, ‘less favourable’, and ‘not reported’ [27]. Studies were considered to report a ‘more favourable’ impact if including indirect costs resulted in a lower ICER or an increase in cost savings; where no figures were provided, the authors’ interpretation was used as evidence for reporting a ‘more favourable’ impact. Similarly, studies were labelled as having a ‘less favourable’ impact if inclusion led to a higher ICER or a decrease in cost savings. Studies were labelled as having ‘no substantial impact’ if there was no change in the ICER with the inclusion of productivity costs based on specific figures provided, or if their inclusion was reported by the authors as having no substantial impact where no specific figures were provided. For studies that did not mention the impact of productivity costs inclusion, this was recorded as ‘not reported’.
Statistical Analysis
Univariate and multivariate logistic regression models were performed to identify the factors that affect the inclusion (yes or no) of productivity loss for CEAs/CUAs in immune-mediated disorders. Factors such as disease area, study sponsor, type of study, type of model, publication year, intervention(s) and geographical region were included as explanatory variables. These variables were redefined on the basis of the study characteristics. In terms of disease area, ulcerative colitis and Crohn’s disease were grouped as ‘inflammatory bowel disease’, and diseases other than rheumatoid arthritis, psoriasis and inflammatory bowel disease were grouped as ‘others’. In terms of region, regions other than North America, Europe and Asia (using categories provided by the Ministry of Foreign Affairs of Japan [34]) were grouped as ‘others’. Similarly, Markov and semi-Markov model types were combined as ‘Markov’, and interventions other than biologics and/or JAKi were grouped as ‘others’. Full details are available in Appendix 3 of the Supplementary Material. Odds ratios (ORs), their 95% confidence intervals (CIs) and p values were calculated in the models. The statistical analysis was performed using R version 4.0.3, and a p value of less than 0.05 was considered statistically significant.
Results
Study Selection
Figure 1 presents the PRISMA flow diagram. Our searches identified 5016 records from the included databases. Following removal of duplicates, 4709 records remained. Screening against inclusion and exclusion criteria led to 4122 records being excluded, and seven records could not be retrieved. The remaining 580 reports were assessed against the inclusion/exclusion criteria, of which 348 records were excluded. Two additional reports were identified from bibliographic searches and passed the screening step. Thus, we identified and extracted data from 198 unique economic evaluation studies from 234 publications. A single study reported data for three indications (Crohn’s disease, psoriasis and rheumatoid arthritis) [35]. Analyses by disease were conducted on the basis of 200 reports as the denominator.
Characteristics of Included Studies
The characteristics of the included studies are given in Table 2. Most of the studies investigated rheumatoid arthritis (74; 37.0%) or psoriasis (64; 32.0%). No studies investigating juvenile idiopathic arthritis were included in this review; this disease is most commonly studied in children [36], so none of the identified studies met the eligibility criteria with respect to age of participants. Of the included studies, 157 (78.5%) were model-based studies and 43 (21.5%) were economic evaluations that were run alongside trials. Approximately one-third (21 studies out of 64; 32.8%) of the psoriasis studies were evaluations alongside trials, compared with only 20.3% (15 out of 74) of rheumatoid arthritis studies and 16.7% (three out of 18) of Crohn’s disease studies. None of the studies in ulcerative colitis were economic evaluations run with trials, and all 27 were based on models. The majority of the economic evaluations were CUAs (137; 68.5%).
Table 2.
Characteristics of included studies
| Disease | Total | Rheumatoid arthritisa | Psoriasisa | Ulcerative colitis | Crohn’s diseasea | Ankylosing spondylitis | Chronic idiopathic urticaria | Systemic lupus erythematosus | Fibromyalgia | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | 200 (100%) | 74 (37.0%) | 64 (32.0%) | 27 (13.5%) | 18 (9.0%) | 9 (4.5%) | 2 (1.0%) | 1 (0.5%) | 5 (2.5%) | |
| Study type | Evaluation alongside trial | 43 (21.5%) | 15 (7.5%) | 21 (10.5%) | 0 (0.0%) | 3 (1.5%) | 2 (1.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) |
| Model-based | 157 (78.5%) | 59 (29.5%) | 43 (21.5%) | 27 (13.5%) | 15 (7.5%) | 7 (3.5%) | 2 (1.0%) | 1 (0.5%) | 3 (1.5%) | |
| Economic analysis | CEA | 54 (27.0%) | 13 (6.5%) | 32 (16.0%) | 3 (1.5%) | 3 (1.5%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) |
| CUA | 137 (68.5%) | 58 (29.0%) | 31 (15.5%) | 23 (11.5%) | 15 (7.5%) | 7 (3.5%) | 2 (1.0%) | 1 (0.5%) | 0 (0.0%) | |
| CEA + CUA | 9 (4.5%) | 3 (1.5%) | 1 (0.5%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 3 (1.5%) | |
| Sponsor | Pharmaceutical company | 124 (62.0%) | 44 (22.0%) | 49 (24.5%) | 13 (6.5%) | 6 (3.0%) | 6 (3.0%) | 2 (1.0%) | 1 (0.5%) | 3 (1.5%) |
| Non-pharmaceutical company | 45 (22.5%) | 18 (9.0%) | 6 (3.0%) | 8 (4.0%) | 8 (4.0%) | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) | |
| No funding | 6 (3.0%) | 3 (1.5%) | 0 (0.0%) | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Not reported | 25 (12.5%) | 9 (4.5%) | 9 (5.0%) | 3 (1.5%) | 4 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Model type | Markov | 88 (44.0%) | 30 (15.0%) | 23 (11.5%) | 18 (9.0%) | 11 (5.5%) | 1 (0.5%) | 2 (1.0%) | 0 (0.0%) | 3 (1.5%) |
| Semi-Markov | 11 (5.5%) | 1 (0.5%) | 4 (2.0%) | 3 (1.5%) | 0 (0.0%) | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Decision analytic/decision tree | 22 (11.0%) | 4 (2.0%) | 9 (4.5%) | 5 (2.5%) | 4 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Simulation model | 33 (16.5%) | 22 (11.0%) | 6 (3.0%) | 1 (0.5%) | 0 (0.0%) | 3 (1.5%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | |
| Non-modelling study | 43 (21.5%) | 15 (7.5%) | 21 (10.5%) | 0 (0.0%) | 3 (1.5%) | 2 (1.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) | |
| Not reported | 3 (1.5%) | 2 (1.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Time horizon | ≤ 5 years | 79 (39.5%) | 21 (10.5%) | 24 (12.0%) | 12 (6.0%) | 16 (8.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 5 (2.5%) |
| 6–10 years | 17 (8.5%) | 4 (2.0%) | 8 (4.0%) | 4 (2.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | |
| 11–30 years | 8 (4.0%) | 2 (1.0%) | 0 (0.0%) | 3 (1.5%) | 0 (0.0%) | 2 (1.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | |
| ≥ 31 years | 8 (4.0%) | 3 (1.5%) | 4 (2.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Lifetime | 59 (29.5%) | 33 (16.5%) | 11 (5.5%) | 8 (4.0%) | 2 (1.0%) | 4 (2.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | |
| Not reported | 29 (14.5%) | 11 (5.5%) | 17 (8.5%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Publication year | 2010–2011 | 22 (11.0%) | 10 (5.0%) | 6 (3.0%) | 1 (0.5%) | 1 (0.5%) | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) |
| 2012–2013 | 31 (15.5%) | 11 (5.5%) | 9 (4.5%) | 3 (1.5%) | 6 (3.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) | |
| 2014–2015 | 22 (11.0%) | 12 (6.0%) | 4 (2.0%) | 2 (1.0%) | 1 (0.5%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 1 (0.5%) | |
| 2016–2017 | 38 (19.0%) | 16 (8.0%) | 10 (5.0%) | 8 (4.0%) | 1 (0.5%) | 1 (0.5%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | |
| 2018–2019 | 55 (27.5%) | 14 (7.0%) | 23 (11.5%) | 7 (3.5%) | 6 (3.0%) | 4 (2.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | |
| 2020 | 32 (16.0%) | 11 (5.5%) | 12 (6.0%) | 6 (3.0%) | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Perspective of analysis | Payer | 57 (28.5%) | 21 (10.5%) | 15 (7.5%) | 11 (5.5%) | 5 (2.5%) | 4 (2.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) |
| Societal | 42 (21.0%) | 26 (13.0%) | 4 (2.0%) | 1 (0.5%) | 5 (2.5%) | 2 (1.0%) | 2 (1.0%) | 0 (0.0%) | 2 (1.0%) | |
| Third party | 12 (6.0%) | 4 (2.0%) | 2 (1.0%) | 3 (1.5%) | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Payer + societal | 10 (5.0%) | 5 (2.5%) | 1 (0.5%) | 2 (1.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | |
| Other perspectiveb | 49 (24.5%) | 13 (6.5%) | 23 (11.5%) | 7 (3.5%) | 2 (1.0%) | 2 (1.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) | |
| Not reported | 30 (15.0%) | 5 (2.5%) | 19 (9.5%) | 3 (1.5%) | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Interventions and comparators | Biologics | 121 (60.5%) | 36 (18.0%) | 49 (24.5%) | 15 (7.5%) | 15 (7.5%) | 6 (3.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| JAKi | 11 (5.5%) | 6 (3.0%) | 1 (0.5%) | 4 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Othersc | 29 (14.5%) | 4 (2.0%) | 13 (6.5%) | 3 (1.5%) | 2 (1.0%) | 2 (1.0%) | 0 (0.0%) | 0 (0.0%) | 5 (2.5%) | |
| Biologics + others | 34 (17.0%) | 25 (12.5%) | 1 (0.5%) | 3 (1.5%) | 1 (0.5%) | 1 (0.5%) | 2 (1.0%) | 1 (0.5%) | 0 (0.0%) | |
| JAKi + others | 2 (1.0%) | 2 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Biologics + JAKi | 2 (1.0%) | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Biologics + JAKi + others | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
CEA cost-effectiveness analysis, CUA cost–utility analysis, JAKi Janus kinase inhibitor
aA single study reported data for three indications: Crohn’s disease, psoriasis, and rheumatoid arthritis [35]
bPerspectives other than “payer”, “societal” and “third party” are listed as “other perspective”
cInterventions other than biologics and JAKi are listed as “others”
The majority of the studies were sponsored by pharmaceutical companies (124; 62.0%). The most common disease of focus for the pharmaceutical-sponsored studies was psoriasis, while the most common focus of the non-pharmaceutical-sponsored studies was rheumatoid arthritis. Forty-three (21.5%) studies did not involve economic modelling, of which nearly half (48.8%) were in psoriasis. Of the 157 studies that included modelling, the most common model type was Markov model, both in total (88 out of 157; 56.1%) and for each disease individually.
The most common time horizon used in analyses was 5 years or less, reported for 79 studies (39.5%), followed by lifetime time horizon (59; 29.5%). Whereas for many of the conditions the most common time horizon was 5 years or less, the majority of the evaluations assessing rheumatoid arthritis and ankylosing spondylitis used lifetime time horizons.
The perspective of the economic evaluation was reported in 170 (85.0%) of the 200 studies. The most commonly taken approach was a payer perspective (57; 28.5%), followed by societal perspective (42; 21.0%). Over half (26) of the 42 studies taking a societal perspective were in rheumatoid arthritis. Of the 30 studies that did not report perspective, most (19) of these were in psoriasis.
Most of the included studies investigated the use of biologics (158 studies; 79.0%). The most commonly studied biologics were adalimumab (60 studies out of 158; 38.0%), infliximab (58; 36.7%) and etanercept (51; 32.3%). Sixteen studies out of 200 (8.0%) investigated tofacitinib and/or baricitinib, two small-molecule JAKi. Of the 16 studies of JAKi, nine were in rheumatoid arthritis, six were in ulcerative colitis and one was in psoriasis.
The following details are available in Appendix 4 of the Supplementary Material. Just under one-third of the studies did not report discount rates (61; 30.5%), and for 17 studies (8.5%), no discount rate was used. The cost year used in many of the studies was 2016–2020 (64; 32.0%); 55 studies (27.5%) used a 2011–2015 cost year, 42 (21.0%) studies used a 2006–2010 cost year, and 39 (19.5%) studies did not report the cost year used. The majority of the studies were conducted in either the US (48; 24.0%) or the UK (30; 15.0%). Compared with the other countries, psoriasis was disproportionately represented in the US, with 23 of the 48 studies in this region (47.9%) assessing this disease.
Table 3 presents details of the studies that included productivity losses as part of the analysis. Forty-nine studies were identified that considered productivity loss, the majority of which investigated rheumatoid arthritis (28 studies out of 74 total studies in rheumatoid arthritis; 37.8%) followed by psoriasis (five out of 64; 7.8%), Crohn’s disease (four out of 18; 22.2%) and ulcerative colitis (three out of 27; 11.1%). Most of the studies that included productivity loss were conducted in the Netherlands (11 out of 15; 73.3%) followed by the US (nine out of 48; 18.8%), Sweden (seven out of 10; 70.0%), the UK (three out of 30; 10.0%), Spain (three out of 12; 25.0%), Germany (three out of nine; 33.3%), Japan (three out of nine; 33.3%) and Poland (three out of six; 50.0%).
Table 3.
Characteristics of studies reporting inclusion of productivity loss
| Disease | Total | Rheumatoid arthritisa | Psoriasisa | Ulcerative colitis | Crohn’s diseasea | Ankylosing spondylitis | Chronic idiopathic urticaria | Systemic lupus erythematosus | Fibromyalgia | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | 200 (100%) | 74 (100%) | 64 (100%) | 27 (100%) | 18 (100%) | 9 (100%) | 2 (100%) | 1 (100%) | 5 (100%) | |
| Studies reporting productivity loss (percentage of number of studies for each disease) | 49 (24.5%) | 28 (37.8%) | 5 (7.8%) | 3 (11.1%) | 4 (22.2%) | 3 (33.3%) | 2 (100%) | 1 (100%) | 3 (60.0%) | |
| Geographical region | ||||||||||
| North America | US | 9 (4.5%) | 6 (8.1%) | 1 (1.6%) | 0 (0.0%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) |
| Latin America and the Caribbean | Colombia | 1 (0.5%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Europe | Finland | 2 (1.0%) | 2 (2.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Germany | 3 (1.5%) | 1 (1.4%) | 1 (1.6%) | 0 (0.0%) | 0 (0.0%) | 1 (11.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Italy | 2 (1.0%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100%) | 0 (0.0%) | |
| Netherlands | 11 (5.5%) | 6 (8.1%) | 1 (1.6%) | 1 (3.7%) | 0 (0.0%) | 2 (22.2%) | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | |
| Poland | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 2 (7.4%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Serbia | 2 (1.0%) | 2 (2.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Spain | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (40.0%) | |
| Sweden | 7 (3.5%) | 5 (6.8%) | 1 (1.6%) | 0 (0.0%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| UK | 3 (1.5%) | 2 (2.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | |
| East Asia and Pacific | Japan | 3 (1.5%) | 2 (2.7%) | 1 (1.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Study type | Evaluation alongside trial | 9 (4.5%) | 6 (8.1%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (40.0%) |
| Model-based | 40 (20.0%) | 22 (29.7%) | 5 (7.8%) | 3 (11.1%) | 3 (16.7%) | 3 (33.3%) | 2 (100%) | 1 (100%) | 1 (20.0%) | |
| Economic analysis | CEA | 4 (2.0%) | 0 (0.0%) | 2 (3.1%) | 0 (0.0%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) |
| CUA | 42 (21.0%) | 28 (37.8%) | 3 (4.7%) | 2 (7.4%) | 3 (16.7%) | 3 (33.3%) | 2 (100%) | 1 (100%) | 0 (0.0%) | |
| CEA + CUA | 3 (1.5%) | 0 (0.0%) | 0 (0.0%) | 1 (3.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (40.0%) | |
| Model type | Markov | 28 (14.0%) | 14 (18.9%) | 5 (7.8%) | 3 (11.1%) | 3 (16.7%) | 0 (0.0%) | 2 (100%) | 0 (0.0%) | 1 (20.0%) |
| Decision analytic/ tree | 1 (0.5%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Simulation model | 11 (5.5%) | 7 (9.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (33.3%) | 0 (0.0%) | 1 (100%) | 0 (0.0%) | |
| Non-modelling study | 8 (4.0%) | 5 (6.8%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (40.0%) | |
| Not reported | 1 (0.5%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Sponsor | Pharmaceutical company | 29 (14.5%) | 17 (23.0%) | 4 (6.3%) | 1 (3.7%) | 1 (5.6%) | 2 (22.2%) | 2 (100%) | 1 (100%) | 1 (20.0%) |
| Non-pharmaceutical company | 17 (8.5%) | 9 (12.2%) | 0 (0.0%) | 2 (7.4%) | 3 (16.7%) | 1 (11.1%) | 0 (0.0%) | 0 (0.0%) | 2 (40.0%) | |
| Not reported | 3 (1.5%) | 2 (2.7%) | 1 (1.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Perspective of analysis | Societal | 36 (18.0%) | 23 (31.1%) | 3 (4.7%) | 1 (3.7%) | 3 (16.7%) | 2 (22.2%) | 2 (100%) | 0 (0.0%) | 2 (40.0%) |
| Payer + societal | 10 (5.0%) | 5 (6.8%) | 1 (1.6%) | 2 (7.4%) | 0 (0.0%) | 1 (11.1%) | 0 (0.0%) | 1 (100%) | 0 (0.0%) | |
| Othersb | 3 (1.5%) | 0 (0.0%) | 1 (1.6%) | 0 (0.0%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | |
| Interventions and comparators | Biologics | 17 (8.5%) | 10 (13.5%) | 3 (4.7%) | 0 (0.0%) | 4 (22.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| JAKi | 1 (0.5%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Othersc | 9 (4.5%) | 2 (2.7%) | 1 (1.6%) | 1 (3.7%) | 0 (0.0%) | 2 (22.2%) | 0 (0.0%) | 0 (0.0%) | 3 (60.0%) | |
| Biologics + others | 21 (10.5%) | 14 (18.9%) | 1 (1.6%) | 2 (7.4%) | 0 (0.0%) | 1 (11.1%) | 2 (100%) | 1 (100%) | 0 (0.0%) | |
| JAKi + others | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Biologics + JAKi | 1 (0.5%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
CEA cost-effectiveness analysis, CUA cost–utility analysis, JAKi Janus kinase inhibitor
aA single study reported data for three indications: Crohn’s Disease, psoriasis, and rheumatoid arthritis [35]
bPerspective other than “payer”, “societal” and “third party” are listed as “other perspective”
cInterventions other than biologics and JAKi are listed as “others”
The most commonly used approach was CUA only (42 out of 49; 85.7%), and the most common model type was Markov (28 out of 49; 57.1%). The majority of the studies were funded by pharmaceutical companies (29 out of 49; 59.2%) and employed a societal perspective only (36 out of 49; 73.5%). In terms of interventions, most of the studies investigated biologics, including biologics + others (38 out of 49; 77.6%).
Analysis of Productivity Loss Elements and Approaches
Table 4 presents the data for the studies regarding the productivity loss/gain elements used in the analysis and the approaches taken to determining these.
Table 4.
Productivity loss cost elements used and approaches taken by the included studies
| Total | Rheumatoid arthritis | Psoriasis | Ulcerative colitis | Crohn’s disease | Ankylosing spondylitis | Chronic idiopathic urticaria | Systemic lupus erythematosus | Fibromyalgia | |
|---|---|---|---|---|---|---|---|---|---|
| Studies reporting productivity loss | 49 (100%) | 28 (100%) | 5 (100%) | 3 (100%) | 4 (100%) | 3 (100%) | 2 (100%) | 1 (100%) | 3 (100%) |
| Productivity loss cost elements (percentage of number of studies for each disease) | |||||||||
| Absenteeism only (patients) | 9 (18.4%) | 5 (17.9%) | 1 (20.0%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (66.7%) |
| Absenteeism only (caregivers) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Absenteeism only (patients + caregivers) | 3 (6.1%) | 1 (3.6%) | 0 (0.0%) | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) |
| Presenteeism only (patients) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Absenteeism + presenteeism (patients) | 4 (8.2%) | 1 (3.6%) | 1 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (100%) | 0 (0.0%) | 0 (0.0%) |
| Absenteeism + presenteeism (caregivers) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Absenteeism (patients and caregivers) + presenteeism (patients) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Absenteeism + others (patients)a | 26 (53.1%) | 16 (57.1%) | 2 (40.0%) | 2 (66.7%) | 2 (50.0%) | 3 (100%) | 0 (0.0%) | 1 (100%) | 0 (0.0%) |
| Elements not reported (patients) | 7 (14.3%) | 5 (17.9%) | 1 (20.0%) | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Approach (percentage of number of studies for each disease) | |||||||||
| Human capital only | 8 (16.3%) | 2 (7.1%) | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 1 (33.3%) | 1 (50.0%) | 1 (100%) | 2 (66.7%) |
| Friction cost only | 4 (8.2%) | 4 (14.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Human capital + friction cost | 4 (8.2%) | 2 (7.1%) | 1 (20.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Not reported | 33 (67.3%) | 20 (71.4%) | 4 (80.0%) | 2 (66.7%) | 4 (100%) | 1 (33.3%) | 1 (50.0%) | 0 (0.0%) | 1 (33.3%) |
aOthers includes travel expenses, out-of-pocket costs, losses due to leaving or switching jobs, unemployment, early retirement, and informal care
Of the 49 studies that included productivity losses/gains in the analysis, seven (14.3%) did not clearly state the productivity cost elements that were incorporated into the modelling. The remaining 42 (85.7%) all reported absenteeism as a cost element used. Twelve studies out of 49 (24.5%) modelled absenteeism as the only productivity loss element, while four (8.2%) modelled absenteeism plus presenteeism and 26 (53.1%) modelled absenteeism plus other cost elements (such as travel expenses, out-of-pocket costs, losses due to leaving or switching jobs, unemployment, early retirement and informal care). None of the studies modelled presenteeism as the only productivity cost element. Three studies included caregiver information in the productivity loss calculation.
Of the studies that included productivity changes, the method used to value these was only explicitly reported in approximately one-third (16 out of 49). Twelve of these 16 studies (75.0%) used a human capital approach either alone or with friction cost, while four (25.0%) used a friction cost approach alone.
Qualitative Analysis of Impact of Including Productivity Costs on ICER
Table 5 provides the results of the studies that reported the impact of including productivity losses/gains on the ICER. Of the 49 studies that incorporated productivity loss/gain elements in the analysis, 28 (57.1%) reported inclusion of productivity elements as contributing to more favourable cost outcomes and ICERs, while 12 (24.5%) reported no substantial impact. Nine (18.4%) studies did not report information regarding the potential impact on the ICER. None of the studies reported inclusion to contribute to less favourable outcomes.
Table 5.
Studies reporting the impact of productivity loss/gain inclusion on the ICER
| Total | Rheumatoid arthritis | Psoriasis | Ulcerative colitis | Crohn’s disease | Ankylosing spondylitis | Chronic idiopathic urticaria | Systemic lupus erythematosus | Fibromyalgia | |
|---|---|---|---|---|---|---|---|---|---|
| Studies reporting productivity loss | 49 (100%) | 28 (100%) | 5 (100%) | 3 (100%) | 4 (100%) | 3 (100%) | 2 (100%) | 1 (100%) | 3 (100%) |
| Reported impact of inclusion of productivity losses/gains on the ICER (percentage of number of studies for each disease) | |||||||||
| More favourable | 28 (57.1%) | 12 (42.9%) | 3 (60.0%) | 3 (100%) | 3 (75.0%) | 3 (100%) | 2 (100%) | 1 (100%) | 1 (33.3%) |
| No substantial impact | 12 (24.5%) | 8 (28.6%) | 2 (40.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (66.7%) |
| Less favourable | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Not reported | 9 (18.4%) | 8 (28.6%) | 0 (0.0%) | 0 (0.0%) | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Univariate and Multivariate Logistic Regression Analysis of Factors that Affect Inclusion of Productivity Loss
Table 6 presents the result of the multivariate logistic regression analysis. With a multivariate analysis with all factors included, whether or not the focus of the study was rheumatoid arthritis had a statistically significant impact on the inclusion of productivity loss compared with psoriasis (OR 0.16 [95% CI 0.05, 0.56], p = 0.004) and inflammatory bowel disease (OR 0.23 [95% CI 0.07, 0.73], p = 0.013), but a statistically significant association was not observed for all other diseases that were grouped (OR 1.23 [95% CI 0.32, 4.70], p = 0.764). In terms of the publication year, the study being published between 2014 and 2017 was strongly associated with the inclusion of productivity loss compared with the study publication year being from 2010 to 2013 (OR 2.87 [95% CI 1.04, 7.92], p = 0.041) with a multivariate analysis. Using a univariate analysis, the study type being a CUA and a CEA + CUA had a strong association with the inclusion of productivity loss compared with the CEA-only studies (CUA: OR 5.53 [95% CI 1.87, 16.29], p = 0.002; CEA + CUA: OR 6.25 [95% CI 1.12, 34.90], p = 0.037). However, no statistically significant association with inclusion of productivity loss was observed on the basis of the study type with a multivariate analysis (CUA: OR 3.76 [95% CI 0.90, 15.71], p = 0.070; CEA + CUA: OR 1.62 [95% CI 0.18, 14.95], p = 0.670). Similarly, the use of a Markov model was significantly associated with inclusion of productivity loss compared with the use of a decision analytic/decision tree model in the univariate analysis (OR 0.12 [95% CI 0.02, 0.94], p = 0.044), but this association was not observed in the multivariate analysis (OR 0.20 [95% CI 0.02, 1.80], p = 0.150).
Table 6.
Factors that affect the inclusion of productivity loss
| Variable | Univariate model | Multivariate model | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Disease area | ||||
| Rheumatoid arthritis | 1.00 (reference) | – | 1.00 (reference) | – |
| Psoriasis | 0.14 (0.05–0.39) | 0.000 | 0.16 (0.05–0.56) | 0.004 |
| Inflammatory bowel diseasea | 0.30 (0.12–0.77) | 0.012 | 0.23 (0.07–0.73) | 0.013 |
| Others | 1.85 (0.64–5.35) | 0.257 | 1.23 (0.32–4.70) | 0.764 |
| Publication year | ||||
| 2010–2013 | 1.00 (reference) | – | 1.00 (reference) | – |
| 2014–2017 | 2.55 (1.10–5.90) | 0.029 | 2.87 (1.04–7.92) | 0.041 |
| 2018–2020 | 0.73 (0.30–1.76) | 0.486 | 0.93 (0.33–2.63) | 0.886 |
| Region | ||||
| North America | 1.00 (reference) | – | 1.00 (reference) | – |
| Europe | 2.56 (1.13–5.80) | 0.025 | 1.22 (0.47–3.19) | 0.679 |
| Asia | 0.64 (0.16–2.58) | 0.530 | 0.22 (0.04–1.07) | 0.061 |
| Others | 0.57 (0.06–5.05) | 0.612 | 0.35 (0.03–3.61) | 0.377 |
| Economic analysis | ||||
| CEA | 1.00 (reference) | – | 1.00 (reference) | – |
| CUA | 5.53 (1.87–16.29) | 0.002 | 3.76 (0.90–15.71) | 0.070 |
| CEA + CUA | 6.25 (1.12–34.90) | 0.037 | 1.62 (0.18–14.95) | 0.670 |
| Study sponsor | ||||
| Pharmaceutical company | 1.00 (reference) | – | 1.00 (reference) | – |
| Non-pharmaceutical company and no funding | 1.64 (0.80–3.35) | 0.176 | 1.17 (0.47–2.93) | 0.740 |
| Not reported | 0.45 (0.12–1.60) | 0.216 | 0.51 (0.11–2.37) | 0.394 |
| Model type | ||||
| Markov | 1.00 (reference) | – | 1.00 (reference) | – |
| Decision analytic/decision tree | 0.12 (0.02–0.94) | 0.044 | 0.20 (0.02–1.80) | 0.150 |
| Simulation model | 1.27 (0.54–2.95) | 0.582 | 0.60 (0.21–1.73) | 0.346 |
| Non-modelling study | 0.58 (0.24–1.40) | 0.226 | 0.84 (0.21–3.31) | 0.805 |
| Not reported | 1.27 (0.11–14.55) | 0.849 | 0.84 (0.04–18.76) | 0.912 |
| Intervention(s) | ||||
| Biologics and/or JAKi | 1.00 (reference) | – | 1.00 (reference) | – |
| Others | 1.47 (0.62–3.49) | 0.378 | 2.27 (0.68–7.55) | 0.182 |
CI confidence interval, CEA cost-effectiveness analysis, CUA cost–utility analysis, JAKi Janus kinase inhibitor
aInflammatory bowel disease is a combination of ulcerative colitis and Crohn’s disease
Quality Assessment of Studies
Most of the included studies were of good quality in terms of title (98.5%), abstract (99.5%), background and objectives (97.5%) and incremental costs and outcomes (95.0%). However, the studies varied in quality with respect to methods, results and disclosure of conflicts of interest. Nearly 30% of studies did not report on the discount rate used, 23.2% of studies failed to report the type of economic model used, and 49.0% of studies did not satisfactorily report the analytical methods used. Full results of the quality assessment are available in Appendix 5 of the Supplementary Material.
Discussion
Evaluation of health technologies based on their value for money as well as their safety and efficacy is a crucial and increasingly common component of modern healthcare systems [1, 2, 4–7]. In the current work, we sought to identify all CEAs and CUAs of drug-based therapies for nine chronic immune-mediated disorders and review their characteristics; whether and how they included productivity losses/gains; the impact of productivity cost inclusion on the evaluation outcomes; and whether any factors affected the inclusion of productivity changes in these CEAs and CUAs. We found that (1) 49 of the 200 identified CEAs and CUAs of immune-mediated disorders considered productivity losses/gains; (2) the majority of studies that included productivity changes reported that inclusion led to a more favourable ICER; and (3) factors that significantly affect whether a study considers productivity changes include choice of disease and publication year.
Our review uncovered some trends in the literature. The majority of CEAs/CUAs identified were conducted in the US (48) and the UK (30), with the third, fourth and fifth most common countries being the Netherlands (15), Spain (12) and Sweden (10), respectively, all of which are all defined as high-income economies by the World Bank [33]. A considerable number of the 49 studies that included productivity changes were in the Netherlands (11) and Sweden (seven), an unsurprising result given the preference of these countries’ health systems for taking a societal perspective in evaluations. Overall, there was a trend towards more recent evaluations, with the results suggesting that the number of immunology evaluations is steadily increasing. The majority of the evaluations took a payer perspective, though we also identified a considerable number that used a societal perspective. In many cases, studies included combinations of perspectives e.g. that of the specific country’s healthcare system, plus societal. This may reflect the widespread variation in the ways CEAs and CUAs are conducted and in the definition of the analysis perspectives themselves. Out of the studies, 15% did not clearly report the perspective of evaluation. In particular, approximately 30% (19 out of 64) of the identified studies of psoriasis did not report information on the perspective. This is possibly because the majority of these studies were non-modelling-based evaluations in which cost-effectiveness was assessed alongside a clinical study.
Although our searches did not identify any SLRs that covered CEAs for a broad range of immune-mediated disorders, we did identify SLRs of CEAs for individual immune-mediated disorders with one study each for rheumatoid arthritis [37], inflammatory bowel disease [38], psoriatic arthritis [39], and psoriasis [40]. The review of rheumatoid arthritis CEAs [37] examined the cost-effectiveness of treat-to-target strategies, while the review of inflammatory bowel disease [38] examined therapeutic drug monitoring and did not mention productivity losses. In the other two studies [39, 40], the results included findings from previous studies that included productivity losses in CEA; here, the authors pointed out that including productivity losses in CEA would affect the results of the analysis, but they did not compare the results of CEAs that included productivity losses with those that did not. In this review, almost a quarter of the immunology economic evaluations identified considered productivity losses as part of the analysis (49 out of 200). Of these, most gave the productivity elements used, with the majority of these studies reporting absenteeism as a cost element in the analysis, either by itself or in addition to other cost elements including presenteeism, unemployment, early retirement, informal care, and travel and out-of-pocket costs. While the majority of the studies that incorporated productivity changes were in rheumatoid arthritis, our results showed that the type of cost elements used varied depending on the disorder investigated in the study. None of the studies modelled presenteeism alone, possibly because of the difficulty in measuring lost efficiency at work [41]. Our previous reviews also found that economic evaluations rarely consider costs based on decreases in productivity while at work [27, 42]. Following the COVID-19 pandemic, it has been suggested that the importance of presenteeism may increase with the potential permanent shift in some countries and business sectors to homeworking, emphasizing its effect on societies [43]. Some studies may also simply assume that patients attend work even when experiencing severe symptoms. Furthermore, the productivity of caregivers was reported to be considered in just three of the 200 studies identified, despite the potential importance of caregiver wellbeing and productivity in healthcare decision-making. Indeed, the findings of a recent review suggest that informal caregiving places a burden on caregivers and considerably affects performance at work [44].
While the human capital method was found to be used most often, only 16 studies clearly stated the method for estimating productivity costs. As discussed previously, while many countries specify which is the preferred method of determining these costs, only a few give clear reasons why, reflecting the lack of understanding in this area [27]. Previous reviews have found that the choice of method for calculating productivity losses significantly affects the output of economic analyses in rheumatoid arthritis, and therefore potentially healthcare decision-making [45]. A key barrier to investigating this further is the lack of clear data and the differing requirements across countries. Should more studies be transparent and explicitly state the method of estimating productivity costs, a better understanding of this murky area could be achieved—a necessary step towards obtaining a clearer guideline for conducting economic evaluations from a societal perspective.
The identified evidence base suggests that including productivity elements in an economic evaluation leads to generally more favourable outcomes in terms of cost-effectiveness. Forty of the 49 identified studies that used productivity losses/gains in their evaluations reported the impact of their inclusion on the outcomes. In almost one-third of cases, the consideration of productivity losses could have altered the final decision on whether to reimburse expensive drugs. This finding is unsurprising, given that a new drug in diseases such as psoriasis, arthritis or Crohn’s disease is likely being appraised owing to its considerable effectiveness and ability to alleviate the symptom burden, thus also reducing the amount of work loss seen with these disorders [46–48].
All of the studies that included productivity loss for ankylosing spondylitis, chronic idiopathic urticaria, systemic lupus erythematosus and ulcerative colitis reported a favourable impact on the ICER. Of the 28 CEAs/CUAs that considered productivity changes in evaluations of rheumatoid arthritis, 12 reported a favourable impact on the ICER. This is in line with the results of an SLR investigating indirect costs, which found that productivity costs for rheumatoid arthritis can be up to half of the total costs [49]. Similarly, three of the five psoriasis studies that incorporated productivity losses indicated that this inclusion led to a lower ICER.
Twelve of the studies reported that including productivity effects had little or no impact on the ICER. As explicitly stated in many of these studies, this may have been a consequence of such costs being a very small fraction of the overall costs. For example, in Brown et al., the additional costs in the secondary societal analysis were approximately 10% or less of the overall costs [50]. Alternatively, it could be that the impact of inclusion was similar for both arms of the study, as was the case in the study by Sochi et al. Here, the costs for the societal perspective were $15,000–17,000 higher than the payer perspective costs, for each of the treatment arms, and the resulting incremental cost–utility ratios did not change between the perspectives [51]. While none of the identified studies reported a less favourable cost-effectiveness outcome resulting from inclusion of productivity changes, it may be the case that this is the result of non-reporting bias in the literature; the authors/sponsors of some studies could have found a negative effect of inclusion on the ICER and therefore omitted this from the final analysis [31].
From the investigation of factors that are associated with the likelihood of inclusion of productivity changes, we found that studies were significantly less likely to consider productivity losses/gains if they assessed psoriasis or inflammatory bowel disease than if they assessed rheumatoid arthritis. This reflects the comparatively high proportion of rheumatoid arthritis studies that reported productivity loss. In addition, a strong association was found between the publication years being from 2014 to 2017 and inclusion of productivity costs, compared with the publication year from 2010 to 2013, suggesting an increase in the number of evaluations incorporating productivity changes during that period.
Rheumatoid arthritis is a chronic condition that interferes with daily life [28] and has been reported to have an impact on indirect costs and productivity losses. Direct costs of rheumatoid arthritis account for 25% to just above 50% of the total costs, but indirect costs account for 26–75% [52]. Indirect costs have also been shown to increase as patients’ functional disability increases, but were dominated by productivity losses [53]. In terms of work disability, 28% of patients with early rheumatoid arthritis have been reported to experience work disability [54], with rates ranging from 50% to 90% as the duration increases [55]. Hence, the impact of rheumatoid arthritis on productivity loss was probably widely known from the period of 2014 to 2017, and many studies had included productivity loss. For the publication year, it may also have been affected by changes in interventions of interest. Of the 16 studies of JAKi as interventions, 14 studies were published after 2018. Nine out of 14 studies were sponsored by pharmaceutical companies, and only one of these nine studies took a societal perspective. With the launch of tofacitinib in 2013 [56] and the approval of baricitinib in 2017 [57], the perspective chosen for CEAs/CUAs may have shifted from a focus on biologics and using a societal perspective to a focus on not only biologics but also JAKi using a perspective other than a societal perspective (e.g. a payer perspective).
To our knowledge, the review is the first of its kind to focus on productivity losses/gains in economic evaluations of chronic immune-mediated disorders. When considering these results, the strengths and limitations of our work should be kept in mind. Our searches encompassed three key databases, relevant conference archives and hand-searching of key bibliographies; this range of sources is considered to comprehensively cover the health economics literature. While the size of the evidence base allowed us to examine the variation in approaches and cost elements, with respect to reporting of methods and results, the quality of the studies was generally low. This aligns with the findings of our previous review [27]. For some studies, the ICERs were not presented separately according to inclusion or exclusion of productivity costs, preventing any quantitative examination of the effect of including these in the model. Also, in a considerable number of studies, the approach to determining costs was not sufficiently described. Although policy-based HTA dossiers were not included in this review, if the SLR were conducted including HTA dossiers, it may be possible to examine the impact of including productivity losses/gains in CEA on willingness to pay across countries. This makes it difficult to draw strong conclusions and illustrates the need to investigate this further. The results of the multivariate analysis are only relevant for the model used. In the multivariate analysis, we included as many variables as possible, including those that were thought to be relevant to the inclusion of productivity losses; that were available from the extracted studies; and that could be computed. However, there are limitations to the use of multivariate analysis, as some relevant variables other than those used in the model may have been excluded. As the included studies were conducted before 2022, these were assessed against the previous 2013 CHEERS checklist as opposed to the updated 2022 checklist; the results of the quality assessment may have differed if the updated checklist was used. Future economic evaluations should use the 2022 version of CHEERS, as it has been designed to apply to a broader range of interventions and appraisal types.
Conclusions
The results of our review support the idea that the inclusion of productivity losses or gains in CEAs and CUAs of chronic immune-mediated disorders typically leads to more favourable cost-effectiveness for the intervention of focus. Moreover, inclusion of productivity costs is positively associated with the disease area being rheumatoid arthritis and the publication year being between 2014 and 2017. Future research should also consistently follow established guidelines (e.g. the CHEERS 2022 statement) by reporting all relevant cost components as well as the methods of measurement, to ensure the impact of productivity loss/gain inclusion can be better investigated.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by Pfizer Japan Inc. Pfizer Japan Inc. also funded the journal’s Rapid Service Fee and Open Access Fee.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support were provided by INTAGE Healthcare Inc. and Lumanity, who were funded by Pfizer Japan Inc. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Akira Yuasa, Naohiro Yonemoto, and Shunya Ikeda were responsible for the study conceptualization. Akira Yuasa, Kazumasa Kamei, Toshiaki Murofushi, Michael LoPresti, Ankush Taneja, and Jake Horgan were responsible for overseeing the data extraction and reporting of the results. All of the authors contributed to the review, revision, and finalization of the final manuscript.
Disclosures
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Akira Yuasa, Naohiro Yonemoto, and Kazumasa Kamei are full-time employees of Pfizer Japan Inc. Akira Yuasa, Naohiro Yonemoto, and Kazumasa Kamei hold stocks and stock options from Pfizer Inc. Toshiaki Murofushi and Michael LoPresti are full-time employees of INTAGE Healthcare Inc., and Ankush Taneja and Jake Horgan are full-time employees of Lumanity, both of which received funding from Pfizer Japan Inc. Shunya Ikeda declares no conflicts of interest associated with this manuscript.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
- 1.Mitton C, Seixas BV, Peacock S, Burgess M, Bryan S. Health technology assessment as part of a broader process for priority setting and resource allocation. Appl Health Econ Health Policy. 2019;17(5):573–576. doi: 10.1007/s40258-019-00488-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Institutionalizing health technology assessment mechanisms: a how to guide. Geneva: World Health Organization; 2021. [Google Scholar]
- 3.Bertram MY, Lauer JA, Stenberg K, Edejer TTT. Methods for the economic evaluation of health care interventions for priority setting in the health system: an update from WHO CHOICE. Int J Health Policy Manag. 2021;10:673–677. doi: 10.34172/ijhpm.2020.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Pharmacoeconomic guidelines around the world. 2021. https://tools.ispor.org/peguidelines/. Accessed 24 Nov 2021.
- 5.Tantivess S, Chalkidou K, Tritasavit N, Teerawattananon Y. Health technology assessment capacity development in low- and middle-income countries: experiences from the international units of HITAP and NICE [version 1; peer review: 2 approved] F1000Res. 2017 doi: 10.12688/f1000research.13180.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Health Technology Assessment and Health Benefit Package Survey 2020/2021. 2021. https://www.who.int/teams/health-systems-governance-and-financing/economic-analysis/health-technology-assessment-and-benefit-package-design/survey-homepage. Accessed 24 Jan 2022.
- 7.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4. Oxford: Oxford University Press; 2015. [Google Scholar]
- 8.Australian Government Department of Health. Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee. Version 5.0. 2016. https://pbac.pbs.gov.au/. Accessed 26 Jan 2022.
- 9.Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 4th Edition. Version 1.0. 2017. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed 26 Jan 2022.
- 10.Haute Autorité de santé (HAS). Choices in methods for economic evaluation. 2020. https://www.has-sante.fr/jcms/r_1499251/en/choices-in-methods-for-economic-evaluation. Accessed 26 Jan 2022.
- 11.Center for Outcomes Research and Economic Evaluation for Health-National Institute of Public Health of Japan. Guideline for preparing cost-effectiveness evaluation to the central social insurance medical council. Version 2.0. 2019. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed 26 Jan 2022.
- 12.National Institute for Health and Care Excellence (NICE). NICE health technology evaluations: the manual. Process and methods [PMG36]. 2022. https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation. Accessed 01 Feb 2022.
- 13.Lægemiddelstyrelsen. Guideline on health economic analyses of medicine [In Danish]. 2018. https://laegemiddelstyrelsen.dk/en/reimbursement/general-reimbursement/application/health-economic-analyses-in-reimbursement-applications/. Accessed 26 Jan 2022.
- 14.Zorginstituut Nederland. Guideline for economic evaluations in healthcare. 2016. https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare. Accessed 26 Jan 2022.
- 15.Tandvårds- och läkemedelsförmånsverket. Health economics. Updated: 16 June 2020. 2020. https://www.tlv.se/in-english/medicines/health-economics.html. Accessed 26 Jan 2022.
- 16.Academy of Managed Care Pharmacy (AMCP). AMCP format for formulary submissions – guidance on submission of pre-approval and post-approval clinical and economic information and evidence, Version 4.1. 2020. https://www.amcp.org/Resource-Center/format-formulary-submissions/AMCP-Format-for-Formulary-Submissions-4.1. Accessed 24 Jan 2022.
- 17.Krol M, Brouwer W. How to estimate productivity costs in economic evaluations. Pharmacoeconomics. 2014;32(4):335–344. doi: 10.1007/s40273-014-0132-3. [DOI] [PubMed] [Google Scholar]
- 18.Riewpaiboon A. Measurement of costs for health economic evaluation. J Med Assoc Thai. 2014;97(Suppl 5):S17–26. [PubMed] [Google Scholar]
- 19.Birnbaum H. Friction-cost method as an alternative to the human-capital approach in calculating indirect costs. Pharmacoeconomics. 2005;23(2):103–104. doi: 10.2165/00019053-200523020-00001. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258. doi: 10.1001/jama.1996.03540150055031. [DOI] [PubMed] [Google Scholar]
- 21.Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Int J Technol Assess Health Care. 2022;38(1):e13. doi: 10.1017/s0266462321001732. [DOI] [PubMed] [Google Scholar]
- 22.Lensberg BR, Drummond MF, Danchenko N, Despiégel N, François C. Challenges in measuring and valuing productivity costs, and their relevance in mood disorders. Clinicoecon Outcomes Res. 2013;5:565–573. doi: 10.2147/CEOR.S44866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johannesson M, Jonsson B, Jonsson L, Kobelt G, Zethraeus N. Why should economic evaluations of medical innovations have a societal perspective? OHE Briefing. 2009. https://www.ohe.org/publications/why-should-economic-evaluations-medical-innovations-have-societal-perspective. Accessed 27 Jan 2022.
- 24.Krol M, Papenburg J, Koopmanschap M, Brouwer W. Do productivity costs matter? The impact of including productivity costs on the incremental costs of interventions targeted at depressive disorders. Pharmacoeconomics. 2011;29(7):601–619. doi: 10.2165/11539970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Krol M, Papenburg J, Tan SS, Brouwer W, Hakkaart L. A noticeable difference? Productivity costs related to paid and unpaid work in economic evaluations on expensive drugs. Eur J Health Econ. 2016;17(4):391–402. doi: 10.1007/s10198-015-0685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duevel JA, Hasemann L, Peña-Longobardo LM, et al. Considering the societal perspective in economic evaluations: a systematic review in the case of depression. Health Econ Rev. 2020;10(1):32. doi: 10.1186/s13561-020-00288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuasa A, Yonemoto N, LoPresti M, Ikeda S. Use of productivity loss/gain in cost-effectiveness analyses for drugs: a systematic review. Pharmacoeconomics. 2021;39(1):81–97. doi: 10.1007/s40273-020-00986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker-Bone K, Farrow S. Rheumatoid arthritis. BMJ Clin Evid. 2007;2007:1124. [PMC free article] [PubMed] [Google Scholar]
- 29.Centre for Reviews and Dissemination (University of York). CRD’s guidance for undertaking reviews in health care. 2009. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed 27 Jan 2022.
- 30.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Updated: February 2021. 2021. https://training.cochrane.org/handbook. Accessed 23 Nov 2021.
- 32.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMC Med. 2013;11(1):80. doi: 10.1186/1741-7015-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The World Bank Group. World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 03 Feb 2022.
- 34.Ministry of Foreign Affairs of Japan. 2022. https://www.mofa.go.jp/region/index.html. Accessed 18 May 2022.
- 35.Liu Y, Wu EQ, Bensimon AG, et al. Cost per responder associated with biologic therapies for Crohn’s disease, psoriasis, and rheumatoid arthritis. Adv Ther. 2012;29(7):620–634. doi: 10.1007/s12325-012-0035-7. [DOI] [PubMed] [Google Scholar]
- 36.Martini A, Lovell DJ, Albani S, et al. Juvenile idiopathic arthritis. Nat Rev Dis Primers. 2022;8(1):5. doi: 10.1038/s41572-021-00332-8. [DOI] [PubMed] [Google Scholar]
- 37.Hock ES, Martyn-St James M, Wailoo A, et al. Treat-to-target strategies in rheumatoid arthritis: a systematic review and cost-effectiveness analysis. SN Compr Clin Med. 2021;3(3):838–854. doi: 10.1007/s42399-021-00727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao J, Jiang X, You JHS. A systematic review on cost-effectiveness analyses of therapeutic drug monitoring for patients with inflammatory bowel disease: from immunosuppressive to anti-TNF therapy. Inflamm Bowel Dis. 2021;27(2):275–282. doi: 10.1093/ibd/izaa073. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Islam N, Ma C, Anis AH. Systematic review of cost-effectiveness analyses of treatments for psoriasis. Pharmacoeconomics. 2015;33(4):327–340. doi: 10.1007/s40273-014-0244-9. [DOI] [PubMed] [Google Scholar]
- 40.D'Angiolella LS, Cortesi PA, Lafranconi A, et al. Cost and cost effectiveness of treatments for psoriatic arthritis: a systematic literature review. Pharmacoeconomics. 2018;36(5):567–589. doi: 10.1007/s40273-018-0618-5. [DOI] [PubMed] [Google Scholar]
- 41.Kigozi J, Jowett S, Lewis M, Barton P, Coast J. Estimating productivity costs using the friction cost approach in practice: a systematic review. Eur J Health Econ. 2016;17(1):31–44. doi: 10.1007/s10198-014-0652-y. [DOI] [PubMed] [Google Scholar]
- 42.Yuasa A, Yonemoto N, LoPresti M, Ikeda S. Productivity loss/gain in cost-effectiveness analyses for vaccines: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2021;21(2):235–245. doi: 10.1080/14737167.2021.1881484. [DOI] [PubMed] [Google Scholar]
- 43.Brouwer W, Huls S, Sajjad A, Kanters T, Roijen LH-V, van Exel J. In absence of absenteeism: some thoughts on productivity costs in economic evaluations in a post-corona era. Pharmacoeconomics. 2022;40(1):7–11. doi: 10.1007/s40273-021-01117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martsolf GR, Kandrack R, Rodakowski J, et al. Work performance among informal caregivers: a review of the literature. J Aging Health. 2020;32(9):1017–1028. doi: 10.1177/0898264319895374. [DOI] [PubMed] [Google Scholar]
- 45.Filipovic I, Walker D, Forster F, Curry AS. Quantifying the economic burden of productivity loss in rheumatoid arthritis. Rheumatology (Oxford) 2011;50(6):1083–1090. doi: 10.1093/rheumatology/keq399. [DOI] [PubMed] [Google Scholar]
- 46.Everhov ÅH, Khalili H, Askling J, et al. Sick leave and disability pension in prevalent patients with Crohn’s disease. J Crohns Colitis. 2018;12(12):1418–1428. doi: 10.1093/ecco-jcc/jjy123. [DOI] [PubMed] [Google Scholar]
- 47.Khalili H, Everhov ÅH, Halfvarson J, et al. Healthcare use, work loss and total costs in incident and prevalent Crohn's disease and ulcerative colitis: results from a nationwide study in Sweden. Aliment Pharmacol Ther. 2020;52(4):655–668. doi: 10.1111/apt.15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orbai AM, Reddy SM, Dennis N, et al. Work absenteeism and disability associated with psoriasis and psoriatic arthritis in the USA—a retrospective study of claims data from 2009 to 2020. Clin Rheumatol. 2021;40(12):4933–4942. doi: 10.1007/s10067-021-05839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batko B, Rolska-Wójcik P, Władysiuk M. Indirect costs of rheumatoid arthritis depending on type of treatment—a systematic literature review. Int J Environ Res Public Health. 2019;16(16):2966. doi: 10.3390/ijerph16162966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown S, Everett CC, Naraghi K, et al. Alternative tumour necrosis factor inhibitors (TNFi) or abatacept or rituximab following failure of initial TNFi in rheumatoid arthritis: the SWITCH RCT. Health Technol Assess. 2018;22(34):1–280. doi: 10.3310/hta22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soini EJ, Hallinen TA, Puolakka K, Vihervaara V, Kauppi MJ. Cost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritis. J Med Econ. 2012;15(2):340–351. doi: 10.3111/13696998.2011.649327. [DOI] [PubMed] [Google Scholar]
- 52.Rat AC, Boissier MC. Rheumatoid arthritis: direct and indirect costs. Jt Bone Spine. 2004;71(6):518–524. doi: 10.1016/j.jbspin.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Kobelt G, Jönsson L, Lindgren P, Young A, Eberhardt K. Modeling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2310–2319. doi: 10.1002/art.10471. [DOI] [PubMed] [Google Scholar]
- 54.Eberhardt K, Larsson BM, Nived K, Lindqvist E. Work disability in rheumatoid arthritis–development over 15 years and evaluation of predictive factors over time. J Rheumatol. 2007;34(3):481–487. [PubMed] [Google Scholar]
- 55.Lacaille D. Arthritis and employment research: where are we? Where do we need to go? J Rheumatol Suppl. 2005;72:42–45. [PubMed] [Google Scholar]
- 56.Brooks M. FDA approves tofacitinib for rheumatoid arthritis. 2012. https://www.medscape.com/viewarticle/774015. Accessed 18 May 2022. [DOI] [PubMed]
- 57.Markham A. Baricitinib: first global approval. Drugs. 2017;77(6):697–704. doi: 10.1007/s40265-017-0723-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).