Abstract
Eleven biologic drugs are currently approved for psoriasis management. Real‐life studies are needed to guide clinicians in choosing a tailored‐tail therapy. The aim of our retrospective study is to indirectly compare the efficacy and safety of ixekizumab and brodalumab in psoriasis patients. A single‐centre real‐life retrospective study was performed enrolling moderate‐to‐severe psoriatic patients under biologic treatment with ixekizumab or brodalumab. For each patient, clinical and demographic data were collected and the effectiveness and safety of brodalumab and ixekizumab treatment were evaluated at weeks 4, 12, and 24. Psoriasis Area Severity Index (PASI) and Body Surface Area (BSA) were used for psoriasis severity. A total of 139 patients were included in the study: 98(70.5%) and 41(29.5%) patients received ixekizumab and brodalumab, respectively. Mean PASI and BSA significantly reduced at each follow up for both ixekizumab and brodalumab groups. Even if ixekizumab reached higher rates of PASI90 and PASI100 than brodalumab (PASI90: 43.8% vs. 39.0% PASI100: 20.4% vs. 17.1% at week4 and PASI90: 83.6% vs. 75.6% PASI100: 71.5% vs. 60.9% at week24), these results were not statistically significant. Adverse events, mainly mild, were registered in 25.5% of ixekizumab and 26.8% of brodalumab group, respectively. Discontinuation rate was higher for brodalumab (17.1% vs. 9.1%), without statistical significance. Our study showed comparable efficacy and safety for ixekizumab and brodalumab.
Keywords: anti‐IL‐17, biologics, brodalumab, comparison, ixekizumab, psoriasis, real life setting, real world evidence
1. INTRODUCTION
Psoriasis is a chronic inflammatory skin disease, affecting 1%–3% of global population. 1 , 2 Plaque psoriasis accounts for 90% of clinical manifestations, presenting as sharply demarcated, erythematous plaques covered by silvery scales. 3 Several comorbidities can be associated with psoriasis; particularly psoriatic arthritis (PsA), cardiometabolic diseases, and depression, strongly impacting on patients Quality of Life. 4 , 5 Even if psoriasis pathogenesis is not completely understood, several studies suggested a key role of the interleukin (IL)23/IL17 axis in the development of psoriatic manifestations. 6 Biologic drugs, targeting these cytokines represented one of the most important research advantages in psoriasis management. 7 Indeed, biologic drugs showed encouraging results in severe forms of both psoriasis and PsA, confirming clinical trials results also in real world settings. 8 , 9 , 10 , 11 In this context, the development of anti‐IL17, resulted in the switching of treatment goals from PASI75 to PASI90 or PASI100 due their high efficacy profile. 12 , 13 Among existing anti‐IL17, ixekizumab and brodalumab appear to be able to reach a faster clinical response if compared with other biologics. Indeed, among all biologics for psoriasis, brodalumab showed the shortest time to 25% of patients to achieved PASI90 (3.5 weeks) followed by ixekizumab (4.1 weeks). 14
Brodalumab, a fully human immunoglobulin G2 (IgG2) antiIL‐17RA monoclonal anti‐body, acts by binding the human IL17RA, leading to a disruption in the IL‐17 pathway by blocking the activity of 17A, IL‐17A/F heterodimer, IL‐17F, and 17E (IL‐25). 15 , 16 It has been recently approved (2017) by the European Medicines Agency (EMA) and the U.S. Food and Drug administration (FDA) for the treatment of moderate‐to‐severe plaque psoriasis in adult patients while its use in PsA is still off‐label. 17 , 18 The efficacy and safety of brodalumab have been confirmed in three randomized, double‐blind, phase 3, placebo‐controlled clinical trials (AMAGINE‐1, AMAGINE‐2 and AMAGINE‐3), which showed its promising safety and efficacy profiles, and its superiority to ustekinumab. 19 , 20 , 21 Moreover, different real‐life experiences confirmed the promising results of these trials. 22 , 23 , 24
Ixekizumab is a humanized monoclonal IgG‐4 antibody, specifically binding IL‐17A, which demonstrated strong efficacy in treating moderate‐to‐severe psoriasis. 25 Ixekizumab has been approved by EMA for moderate‐to‐severe plaque psoriasis, pediatric plaque psoriasis, psoriatic arthritis and axial spondylarthritis, and by FDA for moderate‐to‐severe plaque psoriasis and active PsA. 26 , 27 The effectiveness and safety of ixekizumab in moderate‐to‐severe psoriasis has been showed by three randomized, double‐blinded, phase‐3 trials (UNCOVER‐1, UNCOVER‐2 and UNCOVER‐3), 27 , 28 and also confirmed by several real‐world studies. 29 , 30 , 31 Clinical trials also showed ixekizumab superiority respect to etanercept, ustekinumab, and adalimumab. 32 , 33 , 34 , 35 , 36 , 37 However, to date real world studies comparing ixekizumab and brodalumab efficacy and safety profiles in order to highlight eventual differences are still lacking. Real life studies are needed to assess treatment outcomes in daily clinical practice, referring to more complicated patients, which are usually excluded from clinical trials. 38 , 39 They are fundamental to gain additional data to guide treatment choice for psoriasis patients among the jungle of different biologic drugs. Herein, we performed a retrospective cohort study using real‐world data to indirectly compare the efficacy and safety of ixekizumab and brodalumab in psoriasis patients.
2. MATERIALS AND METHODS
A single‐centre real life retrospective study was performed enrolling moderate‐to‐severe psoriatic patients under biologic treatment with ixekizumab or brodalumab for their disease, attending the Psoriasis Care Center of Dermatology at the University Federico II of Naples, from November 1,2019 to December 1, 2021, to indirectly compare effectiveness and safety of these two biologic drugs. Inclusion criteria were: (i) diagnosis of moderate‐to‐severe plaque psoriasis by a dermatologist, (body surface area [BSA] ≥ 5 and/or psoriasis activity severity index [PASI] ≥ 7); (ii) psoriasis duration >1 year; (iii) treatment with brodalumab and ixekizumab. Patients were treated with standard dose of brodalumab (210 mg administered by subcutaneous injection at weeks 0, 1, and 2 followed by 210 mg every 2 weeks) or ixekizumab (two injections of 80 mg subcutaneously at Week 0, followed by 80 mg every 2 weeks up to Week 12, and then every 4 weeks). At baseline, for each patient the following data were collected: (i) personal and demographic data; (ii) psoriasis duration and presence of psoriatic arthritis; (iii) comorbidities; (iv) previous treatments for psoriasis; (v) psoriasis severity through BSA and PASI, routine blood tests (blood count with formula, transaminases, creatinine, azotaemia, glycaemia, erythrocyte sedimentation rate, C reactive protein, total cholesterol and triglycerides levels, protein electrophoresis) and adverse events (AEs) were assessed at every follow‐up visit. The effectiveness of brodalumab and ixekizumab treatment were evaluated at weeks 4, 12, and 24 in terms of mean percentage change from baseline and percentage of patients with a PASI reduction ≥75% (PASI 75), ≥90% (PASI 90) and 100% (PASI 100). Lack of PASI 75 response after 12 weeks was considered primary inefficacy, while loss of PASI 75 response in patients who had previously achieved PASI 75 response at week 12 as a secondary loss of efficacy. Treatment‐emergent AEs, physical examinations and laboratory monitoring were used to evaluate the safety of the treatment whereas efficacy data were analyzed using a last observation carried forward method, where if a patient dropped out of the study the last available value was ‘carried forward’ until the end of the treatment. The present study was conducted respecting the Declaration of Helsinki, and all patients signed an informed consent before starting the study.
2.1. Statistical analysis
Demographic and clinical variables were analyzed through descriptive statistics. Data were presented as number and proportion of patients for categorical variables and as mean ± standard deviation in case of continuous ones. Student's t‐test and Chi‐square test were used to assess the statistical significance of the quantitative and qualitative characteristics differences of the cohort of patients treated with brodalumab and ixekizumab. A p value of <0.05 was statistically significant. The last observation carried forward (LOCF) method was used at each time point. All statistical analyses were performed using GraphPad‐Prism 4.0 (GraphPad Software Inc.).
3. RESULTS
A total of 139 patients were included in the study: 98 (70.5%) received ixekizumab, while 41 (29.5%) patients received brodalumab. Ixekizumab group comprised 67 males (68.3%) and 32 females (31.7%) with a mean age of 54.4 ± 14.3 years while brodalumab group was composed of 24 males (58.8%) and 17 females (41.2%) with a mean age of 48.3 ± 16.1 years (Table 1). Ixekizumab and brodalumab groups were comparable for sex, psoriasis severity, comorbidities, and previous systemic treatment except for mean age and mean psoriasis duration (p < 0.01) which resulted higher for ixekizumab group. (Table 1) Each patient of both study groups had been previously treated with at least one conventional systemic treatment, while previous biological treatment failure was reported in more than half of patients both groups. (Table 1) Demographic data, previous conventional and biological treatments for the two groups were resumed in Table 1.
TABLE 1.
Demographic data and clinical outcomes of patients treated with ixekizumab and brodalumab.
| Treatment groups | Ixekizumab | Brodalumab | p |
|---|---|---|---|
| Number of patients | 98 | 41 | ns |
| Sex | |||
| Male | 67 (68.3%) | 24 (58.8%) | ns |
| Female | 32 (31.7%) | 17 (41.2%) | ns |
| Mean age (years) | 54.4 ± 14.3 | 48.3 ± 16.1 | p < 0.01 |
| Mean duration of psoriasis (years) | 22.8 ± 11.9 | 19.6 ± 7.8 | p < 0.01 |
| Psoriatic arthritis | 41.8% (n = 41) | 36.5% (n = 15) | ns |
| Comorbidities | |||
| Hypertension | 42.8% (n = 42) | 36.5% (n = 15) | ns |
| Dyslipidaemia | 35.7% (n = 35) | 24.4% (n = 10) | ns |
| Diabetes | 16.3% (n = 16) | 7.3% (n = 3) | ns |
| Cardiopathy | 11.2% (n = 11) | 14.6% (n = 6) | ns |
| Previous conventional systemic treatments | |||
| Cyclosporine | 39.8% (n = 39) | 36.5% (n = 15) | ns |
| Acitretin | 27.5% (n = 27) | 43.9% (n = 18) | ns |
| Methotrexate | 47.9% (n = 47) | 48.8% (n = 20) | ns |
| Nb‐UVB phototherapy | 16.3% (n = 16) | 4.8% (n = 2) | ns |
| Previous biologic treatments | |||
| Anti TNF | 52.1% (n = 51) | 56.1% (n = 23) | ns |
| Etanercept | 18.3% (n = 18) | 24.4% (n = 10) | ns |
| Adalimumab | 27.5% (n = 27) | 29.2% (n = 12) | ns |
| Infliximab | 5.1% (n = 5) | 4.8% (n = 2) | ns |
| Certolizumab | 23.4% (n = 23) | 9.7% (n = 4) | ns |
| Golimumab | 5.1% (n = 5) | 9.7% (n = 4) | ns |
| Anti‐IL‐23/23 | 22.4% (n = 22) | 31.7% (n = 13) | ns |
| Ustekinumab | 22.4% (n = 22) | 31.7% (n = 13) | ns |
| Anti‐IL‐17 | 27.5% (n = 27) | 21.9% (n = 15) | ns |
| Secukinumab | 27.5% (n = 27) | 21.9% (n = 9) | ns |
| Ixekizumab | 0% (n = 0) | 14.6% (n = 6) | ns |
| Brodalumab | 0% (n = 0) | 0% (n = 0) | ns |
| Adverse events | |||
| Pharyngitis | 8.1% (n = 8) | 9.7% (n = 4) | ns |
| Flu‐like illness | 6.1% (n = 6) | 4.8% (n = 2) | ns |
| Headache | 5.1% (n = 5) | 7.3% (n = 3) | ns |
| Myalgia/arthralgia | 0% (n = 0) | 2.4% (n = 1) | ns |
| Candidiasis | 6.1% (n = 6) | 2.4% (n = 1) | ns |
| Discontinuation rate (at last follow up visit) | 9.1% (n = 9) | 17.1% (n = 7) | ns |
| Baseline | |||
| Mean PASI | 18.4 ± 7.2 | 16.2 ± 5.4 | ns |
| Mean BSA | 36.7 ± 13.6 | 31.4 ± 14.6 | ns |
| Week 4 | |||
| Mean PASI | 4.8 ± 3.7 | 5.1 ± 3.6 | ns |
| Mean BSA | 15.7 ± 8.9 | 16.9 ± 6.9 | ns |
| PASI90 | 43.8% (n = 43) | 39.0% (n = 16) | ns |
| PASI100 | 20.4% (n = 20) | 17.1% (n = 7) | ns |
| Week 12 | |||
| Mean PASI | 3.2 ± 0.9 | 4.3 ± 0.8 | ns |
| Mean BSA | 5.4 ± 2.9 | 6.1 ± 1.6 | ns |
| PASI90 | 74.5% (n = 73) | 68.2% (n = 28) | ns |
| PASI100 | 44.8% (n = 44) | 41.4% (n = 17) | ns |
| Week 24 | |||
| Mean PASI | 0.9 ± 0.6 | 1.3 ± 0.4 | ns |
| Mean BSA | 2.2 ± 1.4 | 3.5 ± 1.2 | ns |
| PASI90 | 83.6% (n = 82) | 75.6% (n = 31) | ns |
| PASI100 | 71.5% (n = 70) | 60.9% (n = 25) | ns |
Abbreviations: BSA, body surface area; PASI: psoriasis area severity index.
Mean PASI and BSA significantly reduced at each follow up for both ixekizumab and brodalumab groups. Particularly, in ixekizumab group, mean PASI score reduced from 18.4 ± 7.2 at baseline to 4.8 ± 3.7 at Week 4 (p < 0.001), to 3.2 ± 0.9 at Week 12 (p < 0.001), and up to 0.9 ± 0.6 at Week 24 (p < 0.001). As regards brodalumab group, mean PASI decreased from 16.2 ± 5.4 at baseline, to 5.1 ± 3.6 at Week 4 (p < 0.001), to 4.3 ± 0.8 at Week 12 (p < 0.001), and up to 1.3 ± 0.4 at Week 24 (p < 0.001). BSA showed a similar trend for both groups (Table 1). No significant difference in mean PASI and BSA were observed between the treatments at all follow‐ups. No significant differences were found in the percentage of patients achieving absolute PASI ≤1, ≤3, and ≤5 between brodalumab and ixekizumab group. Ixekizumab group reached higher rates of PASI90 and PASI100 than brodalumab at each follow‐up, however, none of these differences resulted statistically significant: (PASI90: 43.8% vs 39.0% PASI100: 20.4% vs. 17.1% at week 4, PASI90: 74.5% vs. 68.2%PASI100: 44.8% vs. 41.4% at week 12, and PASI90: 83.6% vs. 75.6% PASI100: 71.5% vs. 60.9% at week 24) (Table 1) (Figure 1A,B).
FIGURE 1.
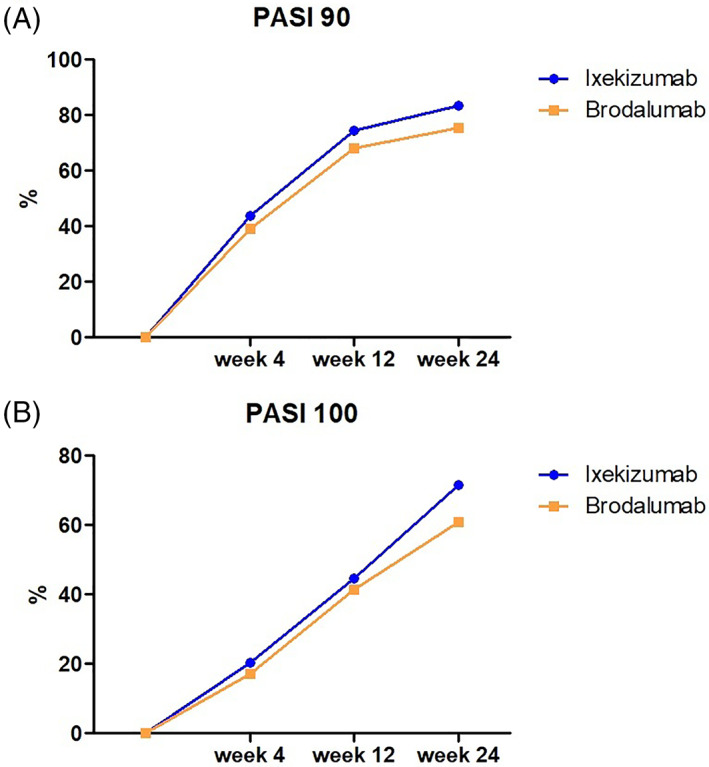
PASI90 (A) and PASI100 (B) responses in ixekizumab and brodalumab groups
Reported mild blood tests alterations were not clinically relevant and did not significantly differ between the two groups (16.3%, n = 16 in ixekizumab group, and 17.1%, n = 7 in brodalumab group). Registered AEs included pharyngitis (8.1%), headache (5.1%), candidiasis infection (6.1%), and flu‐like illness (6.1%) for ixekizumab; in brodalumab group they were represented by pharyngitis (9.7%), flu‐like illness (4.8%), headache (7.3%), myalgia/arthralgia (2.4%), and candidiasis infection (2.4%). No significant differences were registered for ixekizumab and brodalumab. None of these AEs required treatment discontinuations, except for one patient in the ixekizumab group who discontinued treatment due to a recurrent oral candidiasis, early improved after ixekizumab discontinuation. No cases of serious AEs, injection site reaction, malignancy, cardiovascular events were reported in both groups.
Discontinuation rate resulted lower for ixekizumab than brodalumab (9.1% vs. 17.1%) although this data did not reach statistical significance. Among ixekizumab group: five patients discontinued due to primary inefficacy, three patients due to loss of efficacy, and one patient due to AE (recurrent oral Candidiasis). Among Brodalumab group: three patients discontinued due to primary inefficacy, four patients due to loss of efficacy, while no patients discontinued due to any AEs. No significant differences were found among discontinuations between the two groups. Demographic data, clinical data, and treatment outcomes, including mean PASI and BSA, PASI90 and PASI100 responses, and reported AEs, were resumed in Table 1.
4. DISCUSSION
Psoriasis pathogenesis is complex and multifactorial, resulting from a combination of environmental, genetic, and immunologic factors. 40 Even if the exact mechanism behind psoriasis lesions is not completely understood, recent major research advantages revealed IL‐23/Th17 axis as the key immune pathway in psoriasis pathogenesis, with the proinflammatory cytokine IL‐17A as its primary effector. 41 IL‐17A and IL17F represent the most studied subtypes of the IL‐17 family. IL‐17A exists as a homodimer or as a heterodimer with IL‐17F, binding to an IL‐17 receptor that is comprised of IL‐17RA and IL‐17RC, resulting in the activation of multiple and complex pathways. IL17A physio‐logically plays a key role in mucocutaneous defense against extracellular pathogens, such as Candida Albicans. 42 However, an upregulation of IL‐17A response has been found in in autoimmune and inflammatory disease, such as psoriasis. 43 Particularly, a dysregulation of this pathway induces the over‐expression of various pro‐inflammatory cytokines, leading to the recruitment of granulocytes, and resulting in the cycle of inflammation and proliferation that is typically observed in psoriasis lesions. 42 , 43 , 44 , 45 The understanding of the important role of IL17 in psoriasis pathogenesis resulted in the development of targeted biologic therapies. 41 Currently, there are three FDA ap‐provedanti‐IL‐17 pathway: secukinumab, ixekizumab, and brodalumab. 41 Ixekizumab safety and efficacy profiles in psoriatic patients have been evaluated in three clinical trials (UNCOVER‐1, UNCOVER‐2, and UNCOVER‐3). Particularly, the UNCOVER‐1 trial evaluated ixekizumab compared to placebo, 46 while UNCOVER‐2 and UNCOVER‐3 compared ixekizumab to placebo and etanercept. 29 Furthermore, IXORA‐S, a head‐to‐head randomized clinical trial, evaluated ixekizumab efficacy compared to ustekinumab. 34 In all these clinical trials ixekizumab showed promising results in terms of both safety and efficacy profiles, when compared with both placebo and other biological agents. Particularly, ixekizumab resulted superior to placebo in the UN‐COVER‐1 trial (PASI75 reached in 82.6% and 3.9 respectively), superior to placebo and etanercept in UNCOVER‐2 (PASI75 achieved in 77.5%, 2.4% and 41.6% respectively) and UNCOVER‐3 trials (PASI75 achieved in 84.2%, 7.3% and 53.4% respectively), 29 , 46 and superior to ustekinumab in IXORA‐S (PASI90 achieved in 72.8% and 42.9% respectively). 34 Clinical trials also showed ixekizumab efficacy in long term evaluation, and in the treatment of difficult to treat psoriasis like nails, scalp, palmoplantar, genital, and pustular psoriasis. 47 , 48 , 49 , 50 , 51 Promising trials' results were evaluated also by real life setting studies, which confirmed trials results and showed interesting results reached with ixekizumab treatment also in terms of PASI90 and PASI100 responses. 31 , 52 Furthermore, real life studies compared ixekizumab with secukinumab are both highly effective in short‐ and long‐term treatment of psoriasis, even though few differences exist concerning speed of action and long‐term effectiveness, with a faster response in term of achieving PASI75 and PASI90 for ixekizumab. 54 , 55 Brodalumab efficacy and safety profiles were evaluated in three randomized double blinded controlled trials (AMAGINE‐1, AMAGINE‐2 and AMAGINE‐3). These trials showed the superiority of brodalumab in achieving PASI75 respect to placebo in AMAGINE‐1 (PASI75 achieved in 83.3%, 2.7% respectively), AMAGINE‐2 (PASI75 86% and 8% respectively) and AMAGINE‐3 trials (PASI75 85% and 6%, respectively). Brodalumab resulted superior to ustekinumab in achieving PASI100 response at week 12 in AMAGINE‐2 (PASI100 achieved in 44% and 22% respectively) and AMAGINE‐3 (PASI100 achieved in 37% and 19% respectively). 53 , 54 Due to its more recent availability respect to ixekizumab there are fewer data from real life studies. However, available data from recent published real‐life studies confirmed the efficacy and safety profiles showed by trials also in multifailure patients. 22 , 23 , 24 , 55 As regards safety profiles, both ixekizumab and brodalumab resulted safe treatments. Indeed, for both treatments, the most registered AEs were: nasopharyngitis, upper respiratory tract infection, and injection‐site reaction. Moreover, mild or moderate Candida infections resulted higher for both treatments if compared to placebo and other biological agents. Ixekizumab and brodalumab appears as the most rapid biologic drugs available for psoriasis 14 being associated with highest clinical outcomes in both short and long term. To date real life comparison between those drugs are lacking. Real life data are important because these studies usually refers to patients which are excluded by the rigid inclusion and exclusion criteria of clinical trials, such as patients affected by multiple comorbidities, patients suffering from several chronic infections, such as latent tuberculosis infection, as well as patients in different conditions than the ideal ones during trials, such as during the ongoing COVID‐19 pandemic era. 56 , 57 , 58 , 59 Our study confirmed the high efficacy of both ixekizumab and brodalumab treatments, showing significant PASI90 and PASI100 responses at each follow‐up (p < 0.001) reaching PASI 100 in 71.5% and 60.9% respectively at week 24. Comparing the two treatment groups, no significant differences were found in demographic data, except for the mean age and mean psoriasis duration, which resulted significantly higher for ixekizumab group. Regarding clinical outcomes, both treatments resulted highly effective on psoriasis, showing comparable high rates of PASI90 and PASI100 response at each follow‐up, with a rapid onset of action for both groups. Ixekizumab group reached higher rates of PASI90 and PASI100 than brodalumab at each follow‐up, however, none of these differences resulted statistically significant (PASI90: 43.8% vs. 39.0% PASI100: 20.4% vs. 17.1% at week 4, PASI90: 74.5% vs. 68.2%PASI100: 44.8% vs. 41.4% at week 12, and PASI90: 83.6% vs. 75.6% PASI100: 71.5% vs. 60.9% at week 24). Similarly, discontinuation rate resulted lower for ixekizumab than brodalumab (9.1% vs. 17.1%), without being statistically significant.
Our results also confirmed ixekizumab and brodalumab safety profiles, showing comparable rates of AEs. Particularly, reported AEs resulted comparable between ixekizumab and brodalumab: pharyngitis (8.1% vs. 9.7%), headache (5.1% vs. 7.3%), and flu‐like illness (6.1% vs. 4.8%). Candidiasis infection rate resulted higher in the ixekizumab group (6.1% vs. 2.4%) albeit not reaching statistical significance. All of these were mild infection, which rapidly improved after local or systemic treatments. None of these AEs required treatment discontinuations, except for one patient in the ixekizumab group who discontinued treatment due to a mild but recurrent oral candidiasis, early improved after ixekizumab discontinuation. No cases of serious AEs, injection site reaction, malignancy, cardiovascular events were reported in both groups.
4.1. Limitations
Major limitations of our study were the retrospective nature of the study, a relatively small sample of the study population, and a different sample size between ixekizumab and brodalumab groups, due to their different availability in routine clinical practice in our region, Campania, Italy.
5. CONCLUSION
Our study confirmed the high efficacy and safety profiles for both ixekizumab and brodalumab, which resulted to be rapid and highly efficacious treatments for the management of moderate to severe psoriasis with comparable outcomes. Moreover, even if our study highlighted some differences between the two groups in terms of PASI90 and PASI100 responses (with higher responses achieved for ixekizumab), in terms of AEs (higher rates of mild Candida infection reported for ixekizumab), and discontinuation rate (higher for brodalumab: 17.1% vs. 9.1%), none of these differences resulted significant. According to our data, this is the first study evaluating ixekizumab and brodalumab in real life settings, however, more data are needed to confirm our results, with a larger study population to better evaluate any differences among ixekizumab and brodalumab.
AUTHOR CONTRIBUTIONS
Megna Matteo: conceptualization, validation, visualization, writing‐original draft preparation, writing—review & editing. Potestio Luca: Data curation, investigation, methodology, visualization, writing‐original draft preparation. Camela Elisa: Data curation, investigation, methodology, visualization, writing‐original draft preparation. Fabbrocini Gabriella: conceptualization, validation, visualization, writing‐review & editing, supervision. Ruggiero Angelo: Data curation, investigation, methodology, visualization, writing‐original draft preparation. All authors read and approved the final version of the manuscript.
CONFLICT OF INTEREST
Gabriella Fabbrocini acted as a speaker or consultant for Abbvie, Amgen, Eli Lilly, Janssen, Leo‐Pharma, Almyrall, Novartis, and UCB. MatteoMegna acted as a speaker or consultant for Abbvie, Eli Lilly, Janssen, Leo‐Pharma, and Novartis. None of the contributing authors have any conflict of interest, including specific financial interests of relationships and affiliation relevant to the subject matter or discussed materials in the manuscript.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement.
Megna M, Potestio L, Camela E, Fabbrocini G, Ruggiero A. Ixekizumab and brodalumab indirect comparison in the treatment of moderate to severe psoriasis: Results from an Italian single‐center retrospective study in a real‐life setting. Dermatologic Therapy. 2022;35(9):e15667. doi: 10.1111/dth.15667
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruggiero A, Fabbrocini G. Anti‐interleukin‐23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real‐world practice. Clin Exp Dermatol. 2021;47:561‐567. doi: 10.1111/ced.14979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945‐1960. [DOI] [PubMed] [Google Scholar]
- 4. Golbari NM, Porter ML. Current guidelines for psoriasis treatment: a work in progress. Cutis. 2018;101(3S):10‐12. [PubMed] [Google Scholar]
- 5. Ruggiero A, Fabbrocini G. Guselkumab and risankizumab for psoriasis: a 44‐week indirect real‐life comparison. J Am Acad Dermatol. 2021;85(4):1028‐1030. [DOI] [PubMed] [Google Scholar]
- 6. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263‐271. [DOI] [PubMed] [Google Scholar]
- 7. Carrascosa JM, Del‐Alcazar E. New therapies versus first‐generation biologic drugs in psoriasis: a review of adverse events and their management. Expert Rev Clin Immunol. 2018. Apr;14(4):259‐273. [DOI] [PubMed] [Google Scholar]
- 8. Megna M, Potestio L, Ruggiero A, Camela E, Fabbrocini G. Risankizumab treatment in psoriasis patients who failed anti‐IL17: a 52‐week real‐life study. Dermatol Ther. 2022;19:e15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Megna M, Fabbrocini G, Cinelli E, Camela E, Ruggiero A. Guselkumab in moderate to severe psoriasis in routine clinical care: an Italian 44‐week real‐life experience. J Dermatolog Treat. 2022;33(2):1074‐1078. [DOI] [PubMed] [Google Scholar]
- 10. Megna M, Ocampo‐Garza SS. New‐onset psoriatic arthritis under biologics in psoriasis patients: an increasing challenge? Biomedicines. 2021. Oct;9(10):1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruggiero A, Fabbrocini G, Cinelli E, Megna M. Real world practice indirect comparison between guselkumab and risankizumab: results from an Italian retrospective study. Dermatol Ther. 2022;35(1):e15214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ten Bergen LL, Petrovic A. The TNF/IL‐23/IL‐17 axis‐head‐to‐head trials comparing different biologics in psoriasis treatment. Scand J Immunol. 2020;92(4):e12946. [DOI] [PubMed] [Google Scholar]
- 13. Farahnik B, Beroukhim K. Brodalumab for the treatment of psoriasis: a review of phase III trials. Dermatol Ther. 2016;6(2):111‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egeberg A, Andersen YMF. Systematic review on rapidity of onset of action for interleukin‐17 and interleukin‐23 inhibitors for psoriasis. J Eur Acad Dermatol Venereol. 2020;34(1):39‐46. [DOI] [PubMed] [Google Scholar]
- 15. Russell CB, Rand H. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti‐IL‐17 receptor monoclonal antibody. J Immunol. 2014;192(8):3828‐3836. [DOI] [PubMed] [Google Scholar]
- 16. Galluzzo M, D'adamio S. Brodalumab for the treatment of psoriasis. Expert Rev Clin Immunol. 2016;12(12):1255‐1271. [DOI] [PubMed] [Google Scholar]
- 17. Kyntheum® . Summary of product characteristics. Accessed March 01, 2022. https://www.ema.europa.eu/en/documents/product-information/kyntheum-epar-product-information_en.pdf
- 18. Blair HA. Brodalumab: a review in moderate to severe plaque psoriasis. Drugs. 2018;78(4):495‐504. [DOI] [PubMed] [Google Scholar]
- 19. Papp KA, Reich K. A prospective phase III, randomized, double‐blind, placebo‐controlled study of brodalumab in patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol. 2016;175(2):273‐286. [DOI] [PubMed] [Google Scholar]
- 20. Lebwohl M, Strober B. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318‐1328. [DOI] [PubMed] [Google Scholar]
- 21. Puig L, Lebwohl M. Long‐term efficacy and safety of brodalumab in the treatment of psoriasis: 120‐week results from the active comparator e controlled phase 3 AMAGINE‐2 trial. J Am Acad Dermatol. 2020;82(2):352‐359. [DOI] [PubMed] [Google Scholar]
- 22. Bettencourt MS. Real‐world clinical experience with the IL‐17 receptor a antagonist brodalumab. J Drugs Dermatol. 2020;19(2):132‐136. [DOI] [PubMed] [Google Scholar]
- 23. Ruiz‐Villaverde R, Galàn‐Gutierrez M. Brodalumab: short‐term effectiveness and safety in real clinical practice. International Journal of Dermatology. 2020;59:e340. [DOI] [PubMed] [Google Scholar]
- 24. Megna M, Cinelli E, Gallo L, Camela E, Ruggiero A, Fabbrocini G. Risankizumab in real life: preliminary results of efficacy and safety in psoriasis during a 16‐week period. Arch Dermatol Res. 2022;314(6):619‐623. [DOI] [PubMed] [Google Scholar]
- 25. Megna M, Ruggiero A, Di Guida A, Patrì A, Fabbrocini G, Marasca C. Ixekizumab: an efficacious treatment for both psoriasis and hidradenitis suppurativa. Dermatol Ther. 2020;33(4):e13756. [DOI] [PubMed] [Google Scholar]
- 26. Taltz® . Summary of product characteristics. Accessed March 01, 2022. https://www.ema.europa.eu/en/documents/product-information/taltz-epar-product-information_en.pdf
- 27. Craig S, Warren RB. Ixekizumab for the treatment of psoriasis: up‐to‐date. Expert OpinBiolTher. 2020;20(6):549‐557. [DOI] [PubMed] [Google Scholar]
- 28. Leonardi C, Reich K. Efficacy and safety of Ixekizumab through 5 years in moderate‐to‐severe psoriasis: long‐term results from the UNCOVER‐1 and UNCOVER‐2 phase‐3 randomized controlled trials. Dermatol Ther. 2020;10(3):431‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mba AB, Faad MGL. Long‐term efficacy and safety of ixekizumab: a 5‐year analysis of the UNCOVER‐3 randomized controlled trial. J Am Dermatol. 2020;85(2):360‐368. [DOI] [PubMed] [Google Scholar]
- 30. Megna M, Potestio L, Ruggiero A, Camela E, Fabbrocini G. Guselkumab is efficacious and safe in psoriasis patients who failed anti‐IL17: a 52‐week real‐life study. J Dermatolog Treat. 2022;7:1‐5. [DOI] [PubMed] [Google Scholar]
- 31. Magdaleno‐Tapial J, Carmena‐Ramòn R. Efficacy and safety of Ixekizumab in a real‐life practice: a retrospective Bicentric study. ActasDermosifiliogr. 2019;110(7):585‐589. [DOI] [PubMed] [Google Scholar]
- 32. Megna M, Cinelli E. Efficacy and safety of ixekizumab in a group of 16 elderly patients with psoriasis over a 1‐year period. J Eur Acad Dermatol Venereol. 2020;34:e152‐e153. [DOI] [PubMed] [Google Scholar]
- 33. Griffiths CEM, Reich K. Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541‐551. [DOI] [PubMed] [Google Scholar]
- 34. Reich K, Pinter A. Comparison of ixekizumab with ustekinumab in moderate‐to‐severe psoriasis: 24‐week results from IXORA‐S, a phase III study. Br J Dermatol. 2017;177(4):1014‐1023. [DOI] [PubMed] [Google Scholar]
- 35. Blauvelt A, Shi N. Comparison of real‐world treatment patterns among patients with psoriasis prescribed ixekizumab or secukinumab. J Am Acad Dermatol. 2020;82(4):927‐935. [DOI] [PubMed] [Google Scholar]
- 36. Blauvelt A, Papp K. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182(6):1348‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blauvelt A, Shi N. Comparison of real‐world treatment patterns among psoriasis patients treated with Ixekizumab or Adalimumab. Patient Prefer Adherence. 2020;14:517‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruggiero A, Fabbrocini G, Cinelli E, Megna M. Efficacy and safety of guselkumab in psoriasis patients who failed ustekinumab and/or anti‐interleukin‐17 treatment: a real‐life 52‐week retrospective study. Dermatol Ther. 2021;34(1):e14673. [DOI] [PubMed] [Google Scholar]
- 39. Megna M, Ruggiero A, Camela E, Fabbrocini G, Marasca C. A case of erythrodermic psoriasis successfully treated with guselkumab. Dermatol Ther. 2020;33(2):e13238. [DOI] [PubMed] [Google Scholar]
- 40. Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin N Am. 2015;41(4):665‐675. [DOI] [PubMed] [Google Scholar]
- 41. Langrish CL, Chen Y. IL‐23 drives a pathogenic T cellpopulation that induces autoimmune inflammation. J Exp Med. 2005;201(2):233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conti HR, Gaffen SL. IL‐17‐mediated immunity to the opportunistic fungal pathogen Candida albicans . J Immunol. 2015;195(3):780‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu S, Qian Y. IL‐17/IL‐17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci. 2012;122(11):487‐511. [DOI] [PubMed] [Google Scholar]
- 44. Miossec P, Kolls JK. Targeting IL‐17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763‐776. [DOI] [PubMed] [Google Scholar]
- 45. Ly K, Smith MP. Anti IL‐17 in psoriasis. Expert Rev Clin Immunol. 2019;15(11):1185‐1194. [DOI] [PubMed] [Google Scholar]
- 46. Gordon KB, Blauvelt A. Phase 3 trials of Ixekizumab in moderate‐to‐severe plaque psoriasis. N Engl J Med. 2016;375(4):345‐356. [DOI] [PubMed] [Google Scholar]
- 47. Leonardi C, Maari C. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three‐year results from the UNCOVER‐3 study. J Am Acad Dermatol. 2018;79(5):824‐830. [DOI] [PubMed] [Google Scholar]
- 48. Reich K, Leonardi C. Sustained response with ixekizumab treatment of moderate‐to‐severe psoriasis with scalp involvement: results from three phase 3 trials (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). J Dermatolog Treat. 2017;28(4):282‐287. [DOI] [PubMed] [Google Scholar]
- 49. Zachariae C, Gordon K. Efficacy and safety of ixekizumab over 4 years of open‐label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79(2):294‐301. [DOI] [PubMed] [Google Scholar]
- 50. Menter A, Warren RB. Efficacy of ixekizumab compared to etanercept and placebo in patients with moderate‐to‐severe plaque psoriasis and nonpustular palmoplantar involvement: results from three phase 3 trials (UNCOVER‐1, UNCOVER‐2 and UNCOVER‐3). J Eur Acad Dermatol Venereol. 2017;31(10):1686‐1692. [DOI] [PubMed] [Google Scholar]
- 51. Ryan C, Menter A. Efficacy and safety of ixekizumab in a randomized, double‐blinded, placebo‐controlled phase IIIb study of patients with moderate‐to‐severe genital psoriasis. Br J Dermatol. 2018;179(4):844‐852. [DOI] [PubMed] [Google Scholar]
- 52. Calianno G, Esposito M. Ixekizumab improves disease severity, clinical symptoms and quality of life in patients with genital psoriasis: a 24‐week real‐life experience. Dermatol Ther. 2021;34(4):e14993. [DOI] [PubMed] [Google Scholar]
- 53. Caldarola G, Mariani M. Comparison of short‐ and long‐term effectiveness of ixekizumab and secukinumab in real‐world practice. Expert Opin Biol Ther. 2021;21(2):279‐286. [DOI] [PubMed] [Google Scholar]
- 54. Cariti C, Dapavo P. Comparison of Secukinumab and Ixekizumab in psoriasis: a real‐life cohort study on the efficacy and drug survival of 445 patients. J Eur Acad Dermatol Venereol. 2022;36(3):e233‐e235. [DOI] [PubMed] [Google Scholar]
- 55. Galluzzo M, Caldarola G. Use of brodalumab for the treatment of chronic plaque psoriasis: a one‐year real‐life study in the Lazio region. Italy Expert Opin Biol Ther. 2021;21(9):1299‐1310. [DOI] [PubMed] [Google Scholar]
- 56. Marasca C, Ruggiero A, Megna M, Annunziata MC, Fabbrocini G. Biologics for patients affected by hidradenitis suppurativa in the COVID‐19 era: data from a referral center of southern Italy. J Dermatolog Treat. 2022;33(1):592. [DOI] [PubMed] [Google Scholar]
- 57. Napolitano M, Patruno C, Ruggiero A, Nocerino M, Fabbrocini G. Safety of dupilumab in atopic patients during COVID‐19 outbreak. J Dermatolog Treat. 2022;33(1):600‐601. [DOI] [PubMed] [Google Scholar]
- 58. Villani A, Megna M, Scalvenzi M, Fabbrocini G, Ruggiero A. Teledermatology and chronic skin diseases: real life experience in a southern Italian dermatologic Centre. Dermatol Ther. 2020;33(6):e13839. [DOI] [PubMed] [Google Scholar]
- 59. Megna M, Potestio L, Gallo L, Caiazzo G, Ruggiero A, Fabbrocini G. Reply to "Psoriasis exacerbation after COVID‐19 vaccination: report of 14 cases from a single centre" by Sotiriou E et al. J Eur Acad Dermatol Venereol. 2022;36(1):e11‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


