Abstract
DegP is a periplasmic protease that is a member of both the ςE and Cpx extracytoplasmic stress regulons of Escherichia coli and is essential for viability at temperatures above 42°C. [U-14C]acetate labeling experiments demonstrated that phospholipids were degraded in degP mutants at elevated temperatures. In addition, chloramphenicol acetyltransferase, β-lactamase, and β-galactosidase assays as well as sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis indicated that large amounts of cellular proteins are released from degP cells at the nonpermissive temperature. A mutation in pldA, which encodes outer membrane phospholipase A (OMPLA), was found to rescue degP cells from the temperature-sensitive phenotype. pldA degP mutants had a normal plating efficiency at 42°C, displayed increased viability at 44°C, showed no degradation of phospholipids, and released far lower amounts of cellular protein to culture supernatants. degP and pldA degP mutants containing chromosomal lacZ fusions to Cpx and ςE regulon promoters indicated that both regulons were activated in the pldA mutants. The overexpression of the envelope lipoprotein, NlpE, which induces the Cpx regulon, was also found to suppress the temperature-sensitive phenotype of degP mutants but did not prevent the degradation of phospholipids. These results suggest that the absence of OMPLA corrects the degP temperature-sensitive phenotype by inducing the Cpx and ςE regulons rather than by inactivating the phospholipase per se.
Extracytoplasmic stress, such as that caused by heat shock or the overproduction of outer membrane proteins in Escherichia coli, is believed to be caused by the accumulation and aggregation of denatured and misfolded proteins in the membranes and periplasm. Under these conditions the Cpx two-component signal transduction pathway and the alternative sigma factor ςE direct the synthesis of several proteins that are involved in the degradation and refolding of these denatured and misfolded periplasmic proteins, leading to alleviation of the stress (for a review, see reference 50).
The rpoE gene encoding ςE is essential for the viability of cells at all temperatures (22). ςE is known to direct the transcription of degP (htrA), fkpA, rpoE, rpoH, and many others (12, 14, 15, 26, 34). DegP is a protease/chaperone that digests abnormal proteins in the periplasm and has been demonstrated to be necessary for cell viability at temperatures of 42°C and above (33, 35, 55, 57, 58, 59), and FkpA is a peptidyl prolyl cis/trans isomerase (28, 41). RseA, RseB, and RseC are involved in regulating the transcription of genes in the ςE regulon (21, 42). Under heat shock conditions or upon overexpression of outer membrane proteins, denatured and misfolded proteins in the periplasm are sensed by RseA and/or RseB (38). ςE is then released by the cytoplasmic domain, allowing it to direct transcription of the genes in the ςE regulon.
The Cpx two-component signal transduction system directs the transcription of degP, ppiA, ppiD, dsbA, cpxP, and cpxRA and perhaps other genes not yet identified (12, 13, 14, 16, 46). PpiA is another periplasmic peptidyl prolyl cis/trans isomerase (36), and DsbA is a disulfide oxidoreductase involved in the oxidation of disulfide bonds occurring in periplasmic proteins (2). The expression of the Cpx regulon is regulated by CpxA and CpxR (24, 62). CpxA is an inner membrane protein that functions as a histidine kinase, while CpxR is the cytoplasmic response regulator (48, 49). CpxA detects various types of extracytoplasmic stress, such as the overexpression of the lipoprotein NlpE, upon which it phosphorylates CpxR, which then activates transcription of genes in the regulon (55). The Cpx pathway is also activated by the overexpression of P pilus subunits in the absence of the P pilus chaperone PapD (30, 32).
Outer membrane phospholipase A (OMPLA) is located at adhesion sites between the inner and outer membranes of E. coli (3). The crystal structure of OMPLA has revealed that the protein consists of a 12-stranded, antiparallel β barrel, with the active site located on the outer leaflet of the outer membrane (54). The enzyme hydrolyzes phospholipids at one of the fatty acid ester linkages, creating lysophospholipids (1, 51). Under normal growth conditions, pldA mutants have no observable phenotype (1, 23), suggesting that OMPLA is not necessary for normal cell growth. The phospholipase is activated, however, by elevated temperatures (17), colicin release (7, 37, 47), phage infections (10), EDTA treatment (27), spheroplast formation (45), and membrane-perturbing peptides (29, 63).
It has been hypothesized that OMPLA activation is induced by perturbation of the membrane. Activation of the enzyme requires calcium (60) and its dimerization (19, 20) and may require the presence of phospholipids in the outer leaflet of the outer membrane (18, 44, 53). In normally growing cells, OMPLA is in the monomeric form and the outer leaflet is believed to be composed entirely of lipopolysaccharides. Perturbation of the membrane may release lipopolysaccharides from the outer leaflet, which would be replaced by phospholipids from the inner leaflet, creating areas of phospholipid symmetry within the outer membrane (18, 44, 53). The presence of phospholipids in the outer leaflet could then induce OMPLA dimerization and activation. If this hypothesis is correct, OMPLA may be responsible for maintaining the asymmetry of the outer membrane by removing phospholipids from the outer leaflet.
The purpose of this research was to examine the role of OMPLA in extracytoplasmic stress responses. A pldA mutation was found to rescue degP temperature sensitivity and to reduce the amount of periplasmic and cytoplasmic proteins released into the supernatant, in addition to preventing the accumulation of lysophospholipids at elevated temperatures. However, analysis of lacZ fusions to Cpx and to ςE-regulated genes also indicated that the outer membrane phospholipase plays an important role in the state or structure of the membranes of normally growing cells, since both extracytoplasmic stress regulons were activated in its absence. The overexpression of NlpE, which induces the Cpx response (55), was also found to rescue degP temperature sensitivity but did not eliminate the accumulation of lysophospholipids at elevated temperatures. The results thus suggest that it is the extracytoplasmic stress response activation rather than the lack of OMPLA activity per se that rescues the degP pldA mutants at the nonpermissive temperature.
MATERIALS AND METHODS
Media, antibiotics, and growth conditions.
All strains were grown at 30°C in Luria-Bertani (LB) broth unless otherwise stated, and minimal media was made as described (40). When necessary, the media was supplemented with ampicillin (100 μg/ml), kanamycin (100 μg/ml), or 5-fluorocytosine (10 μg/ml). The o-nitrophenyl-β-d-galactoside (ONPG) used for β-galactosidase assays was purchased from Sigma.
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Bacterial strains | ||
| W3110 | IN(rrnD-rrnE)1 rph-1 | 31 |
| CBM | W3110 pldA1 | 8 |
| GL93 | CBM degP::kan | This study |
| GL94 | W3110 degP::kan | This study |
| GL101 | W3110 Δ(argF-lac)U169 | This study |
| GL102 | CBM Δ(argF-lac)U169 | This study |
| GL103 | GL93 Δ(argF-lac)U169 | This study |
| GL104 | GL94 Δ(argF-lac)U169 | This study |
| MC4100 | araD Δ(argF-lac)U169 rpsL150 (Strr) relA1 flbB5301 deoC1 ptsF25 rbsR | 6 |
| JMR312 | MC4100 λRS88(porfA-dsbA-lacZ) | This study |
| JMR314 | MC4100 λRS88(fkpA-lacZ) | This study |
| JMR333 | MC4100 λRS88(degP-lacZ) | This study |
| GL111 | GL101 λRS88(porfA-dsbA-lacZ) | This study |
| GL112 | GL101 λRS88(fkpA-lacZ) | This study |
| GL113 | GL101 λRS88(degP-lacZ) | This study |
| GL121 | GL102 λRS88(porfA-dsbA-lacZ) | This study |
| GL122 | GL102 λRS88(fkpA-lacZ) | This study |
| GL123 | GL102 λRS88(degP-lacZ) | This study |
| GL131 | GL103 λRS88(porfA-dsbA-lacZ) | This study |
| GL132 | GL103 λRS88(fkpA-lacZ) | This study |
| GL133 | GL103 λRS88(degP-lacZ) | This study |
| GL141 | GL104 λRS88(porfA-dsbA-lacZ) | This study |
| GL142 | GL104 λRS88(fkpA-lacZ) | This study |
| GL143 | GL104 λRS88(degP-lacZ) | This study |
| Plasmids | ||
| pBR322 | Apr Tcr | 5 |
| pBR328 | Apr Cmr | 56 |
| pLD404 | pBR322 nlpE | 55 |
Construction of lacZ fusions.
Bacteriophage P1vir, grown on MC4100, was used to transduce Δ(argF-lac)U169 to W3110, CBM, GL93, and GL94, creating GL101, GL102, GL103, and GL104, respectively. Transductants were selected for ΔcodBA on minimal media containing 5-fluorocytosine (4, 43) and were then streaked on minimal medium containing lactose to verify Δlac. lacZ fusions were transduced into each Δlac derivative of W3110 by using P1vir grown on JMR312, JMR314, or JMR333. The transductants were selected on minimal media containing lactose.
Measurement of colony-forming ability.
Strains were grown at 30°C in LB broth (using a New Brunswick G76 water bath shaker) until an optical density at 600 nm (OD600) of ∼1.2 was reached. Each culture was then diluted and plated on LB agar in triplicate and counted after overnight incubation to determine the viability of each strain at each plating temperature.
Measurement of viability after heat shock.
Cultures were grown overnight in LB broth and were then subcultured (1:50) into LB broth and were incubated at 30°C for ∼2 h, after which the cultures were transferred to 44°C. At the time of transfer and at various times thereafter, the OD600 for each culture was measured and samples of each culture were diluted and plated as described above. The agar plate contents were then incubated at 30°C overnight, and the colonies on the plates were counted.
Enzyme assays.
For β-galactosidase assays of lacZ fusion strains, cultures were grown overnight and then subcultured 1:50 and grown to mid-log phase at the indicated temperatures. The assays were performed on 1 ml of bacterial cell culture as previously described (52) by using 20 μl of chloroform and 10 μl of 0.1% sodium dodecyl sulfate (SDS) to permeabilize the cells. Assay mixtures contained ONPG at a concentration of 10 mg/ml, and the change in OD420 was recorded and used to determine β-galactosidase activity, which was expressed as units (nanomoles/minute)/milliliter/OD600. For assays of leakage of cellular proteins, freshly saturated cultures grown at the indicated temperatures were centrifuged for 10 min at 8,000 × g to separate cells from culture supernatants. A cell extract was then prepared by resuspending the cells in the original culture volume of fresh media and rupturing them by two passes through a French pressure cell (Aminco) at a pressure of 16,000 lbs/in2. β-Galactosidase assays were carried out on the extracts and culture supernatants as described above, except that chloroform and SDS were not used. β-Lactamase activity was determined using nitrocefin as the substrate as previously described (61) and was expressed in units (nanomoles/minute)/milliliter/OD600. The chloramphenicol acetyltransferase (CAT) protein was quantitated as nanograms/milliliter/OD600 using an immunoassay as provided and described by Roche Molecular Biochemicals.
Labeling experiments.
Cells were prelabeled by overnight growth in LB broth containing 10 μCi of [U-14C]acetate (57 mCi/mmol) per ml. The cells were then diluted to an OD600 of 0.2 in fresh medium and were grown for a further 4 h at the indicated temperatures. The cultures were then sampled, and their lipids were extracted exactly as described previously (7) and were separated by thin-layer chromatography (TLC) using Whatman PE SIL G/UV plates. After TLC, the labeled lipids were located by autoradiography.
General methods.
For analysis of proteins released to the culture medium during incubation at various temperatures, samples of 1 ml of each bacterial culture were centrifuged for 1 min at 15,000 × g. The supernatants of each sample were then precipitated at a final concentration of 5% trichloroacetic acid. The precipitated protein was centrifuged and then dissolved in 20 μl of electrophoresis sample buffer. The samples were analyzed by electrophoresis on 10% acrylamide SDS-polyacrylamide gel electrophoresis (PAGE) gels and stained with Coomassie blue.
RESULTS
The temperature-sensitive phenotype of degP mutants is suppressed by a pldA mutation.
Although the E. coli protease DegP was originally identified on the basis of its degradation of unstable membrane fusion proteins (58), degP cells are also temperature sensitive for growth. In fact the gene was also identified as htrA in a collection of Tn10 insertion mutants unable to grow at 42°C (33). In this study, the ability of a pldA mutation to correct the degP temperature-sensitive phenotype at nonpermissive temperatures was first observed by comparison of the plating efficiencies of W3110 and its pldA, pldA degP, and degP derivatives incubated at 30, 37, 42, and 44°C on LB agar (Table 2). As demonstrated previously, the degP mutant had decreased colony-forming ability at elevated temperatures with a plating efficiency, relative to that at 30°C, of 30% at 42°C and <0.001% at 44°C. The degP colonies that did form at 42°C were much smaller than those which formed at 30 and 37°C. The introduction of a pldA mutation to the degP mutant resulted in a substantial rescue of its viability at elevated temperatures. The plating efficiency of the pldA degP mutant was 100% when incubated at 42°C and was 55% when incubated at 44°C. The W3110 pldA degP colonies which formed at both elevated temperatures were also smaller than those that formed at 30 and 37°C but were much larger than those of the degP cells.
TABLE 2.
Plating efficiencies of W3110 derivatives grown at various temperatures
| Strain | No. of CFU/ml at various temps (°C)
|
|||
|---|---|---|---|---|
| 30 | 37 | 42 | 44 | |
| W3110 | 8.6 (±0.4) × 108 | 1.1 (±0.1) × 109 | 1.23 (±0.14) × 109 | 1.19 (±0.05) × 109 |
| W3110 pldA | 1.0 (±0.1) × 109 | 1.45 (±0.12) × 109 | 1.07 (±0.10) × 109 | 1.32 (±0.11) × 109 |
| W3110 pldA degP | 1.2 (±0.2) × 109 | 1.2 (±0.2) × 109 | 1.32 (±0.15) × 109 | 6.6 (±1.4) × 108 |
| W3110 degP | 1.1 (±0.1) × 109 | 1.22 (±0.10) × 109 | 3.7 (±2.1) × 108 | <1.0 × 104 |
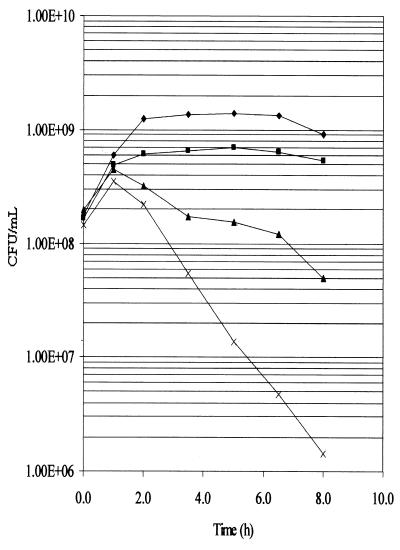
In order to more specifically address the ability of the various mutants to withstand elevated temperatures, the cells were grown to mid-log phase at 30°C and were then shifted to 44°C for various periods of time followed by plating at 30°C (Fig. 1). The viability of the pldA degP mutant, measured after incubation for 6 and 8 h at 44°C, was ∼25 and ∼35-fold greater, respectively, than that of the degP mutant. In addition to the decrease in viability at the elevated temperature, the OD600 for the W3110 degP cultures began to decrease after 2 h of incubation at 44°C, while that of the W3110, W3110 pldA, and W3110 pldA degP cultures either decreased very little or not at all during the 8-h incubation period (data not shown).
FIG. 1.
Survival curves of W3110 derivatives at 44°C. W3110 (⧫), W3110 pldA (■), W3110 pldA degP (▴), and W3110 degP (x) were incubated to an OD600 of ∼0.5 at 30°C in LB broth and were then shifted to 44°C at 0 h and were incubated for another 8 h. At the indicated time points, the cultures were diluted and plated at 30°C.
A pldA mutation suppresses release of cellular protein by degP mutant cells.
It has been shown previously that a mutation in the lpp gene, encoding the major lipoprotein, also rescues the temperature-sensitive phenotype of degP mutants, and it was hypothesized that this was due to increased leakage of heat-denatured proteins from the periplasm (59). The viability assays, however, suggested that the temperature sensitivity of degP mutants is suppressed by a pldA mutation, which would be expected to decrease rather than increase damage to cellular membranes. In addition, the drop in OD of the degP cells suggested that these mutants may release cellular material or even lyse at elevated temperatures. Culture supernatants of wild-type cells as well as the degP and pldA degP mutants were therefore examined for release of cellular protein at 30, 37, and 41°C (which allows an essentially normal growth curve for degP cells). SDS-PAGE analysis demonstrated that the W3110 degP cells released protein into the culture supernatant even when grown at 37°C and released much greater amounts when grown at 41°C (Fig. 2). In contrast, the W3110 pldA degP cells released almost undetectable amounts of protein at 30 and 37°C and only small amounts when grown at 41°C. As expected, control W3110 cells released essentially no protein when incubated at any of the three temperatures.
FIG. 2.
Supernatant proteins from W3110, W3110 degP, and W3110 pldA degP. Cultures were grown at 30, 37, and 41°C, and supernatant proteins were analyzed by SDS-PAGE and stained with Coomassie blue. Protein standards are located in the far left lane.
Assays of the cytoplasmic enzymes β-galactosidase and CAT, as well as of the periplasmic enzyme β-lactamase, were performed on pBR328 transformants of the three strains in order to quantify the release of these representative proteins from the two cellular compartments (Table 3). At 37°C, significant amounts of β-lactamase and CAT and small amounts of β-galactosidase were present in the supernatant of W3110 degP cultures, and at 41°C, large amounts of all three enzymes had been released from the cells. In contrast, the culture supernatant of W3110 pldA degP cells grown at 37°C contained very little of any of the three enzymes, and much less of each enzyme was released when the cells were grown at 41°C. However, at 41°C the double mutant still released β-lactamase and CAT, indicating that although protein release is largely prevented by the presence in the degP cells of the pldA mutation, damage to the envelope is not completely eradicated.
TABLE 3.
Release of cytoplasmic and periplasmic enzymes by W3110-derivative cells at various temperatures
| Temp (°C) | Enzyme | Amt of enzyme assayedc (%) per strain in cells or supernatant
|
|||||
|---|---|---|---|---|---|---|---|
| W3110
|
W3110 degP
|
W3110 pldA degP
|
|||||
| Cells | Supernatant | Cells | Supernatant | Cells | Supernatant | ||
| 37 | β-Gala | 2,909 (100) | 3 (0) | 2,877 (97) | 80 (3) | 1,848 (100) | 3 (0) |
| CAT | 2,098 (98) | 40 (2) | 4,595 (86) | 747 (14) | 2,370 (100) | 8 (0) | |
| β-Lacb | 280 (98) | 5 (2) | 317 (84) | 62 (16) | 191 (99) | 2 (1) | |
| 41 | β-Gal | 2,740 (100) | 7 (0) | 2,756 (84) | 544 (16) | 1,569 (94) | 92 (6) |
| CAT | 8,668 (100) | 39 (0) | 14,267 (74) | 4971 (26) | 2,773 (86) | 465 (14) | |
| β-Lac | 181 (98) | 3 (2) | 141 (40) | 213 (60) | 79 (77) | 24 (23) | |
β-Gal, β-galactosidase.
β-Lac, β-lactamase.
The amount of β-galactosidase and β-lactamase present is expressed in units/milliliter/OD600. The amount of CAT present is expressed in nanograms/milliliter/OD600. The OD600 is taken from the original culture.
Cellular phospholipids are degraded in degP mutants incubated at elevated temperatures.
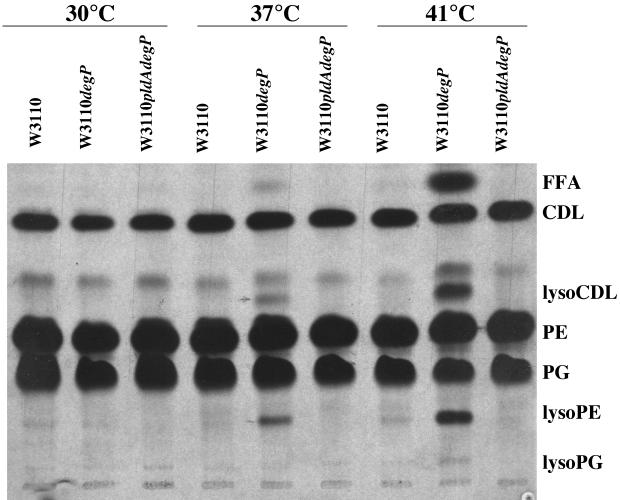
The release of proteins into the supernatant by W3110 degP cells indicated that the permeability barrier of the inner and outer membranes was being compromised at the elevated temperatures, and rescue by the pldA mutation suggested that phospholipid degradation by OMPLA was at least partially responsible for this. This was examined further by growing W3110, W3110 pldA degP, and W3110 degP cells at 30, 37, and 41°C in media containing [U-14C]acetate in order to label the cellular lipids. The lipids were then extracted and separated on TLC plates as described in Materials and Methods. Lysophospholipids were not detected in the lipids of W3110 cells which had been grown at any of the three temperatures (Fig. 3), confirming previous results that the phospholipase is largely inactive during normal growth (1). However, lysophospholipids, including lysophosphatidylethanolamine, at least one lysophospholipid form of cardiolipin, and traces of lysophosphatidylglycerol were present in W3110 degP cells grown at temperatures of 37°C and above, with larger amounts being present at 41 than at 37°C. As expected, no phospholipid degradation was observed in the W3110 pldA degP cells.
FIG. 3.
Radiolabeled phospholipids from W3110, W3110 degP, and W3110 pldA degP cultures. [14C]acetate-labeled cultures were grown at the indicated temperatures, and radiolabeled phospholipids were separated on a TLC plate and autoradiographed. The positions of free fatty acids (FFA), cardiolipin (CDL), lysocardiolipin (lysoCDL), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), lysophosphatidylethanolamine (lysoPE), and lysophosphatidylglycerol (lysoPG) are indicated.
A pldA mutation increases expression of Cpx and ςE regulons.
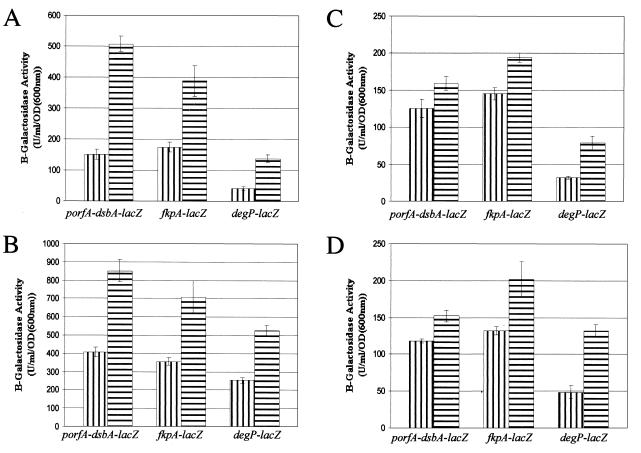
The DegP protease is unique in being regulated as a member of both the Cpx and ςE extracytoplasmic stress regulons, which may respond to different forms of stress in the cell envelope. If the activation of OMPLA at elevated temperatures was a significant part of the stress that kills degP cells, as suggested by the rescue of those cells by the introduction of the pldA mutation, then the pldA mutation should result in lower levels of stress at elevated temperatures than are experienced by bacteria containing OMPLA. The expression of the Cpx and ςE regulons was therefore monitored in cells with the various degP and/or pldA mutant backgrounds by measuring the transcriptional activity of dsbA (Cpx regulon), fkpA (ςE regulon), and degP (Cpx and ςE regulon) using lacZ fusions. GL141 [W3110 degP λRS88(porfA-dsbA-lacZ)], GL142 [W3110 degP λRS88(fkpA-lacZ)], and GL143 [W3110 degP λRS88(degP-lacZ)], as well as the pldA derivative of each strain (GL131, GL132, and GL133, respectively), were incubated at 30 and 41°C, and assays of β-galactosidase activity were performed to determine expression levels of the two regulons. Contrary to expectations, the presence of the pldA mutation resulted in a stimulation of the transcription of all three fusions at both 30 and 41°C (Fig. 4A and B). Similar results were also seen in W3110 and W3110 pldA derivatives containing the lacZ fusions (Fig. 4C and D), except that transcription of all three fusions was lower in the wild type and the pldA mutants than in the degP and pldA degP mutants, respectively. The results thus show that despite removing the possibility of phospholipid degradation, the pldA mutation increased the level of stress experienced by the cells at all temperatures.
FIG. 4.
β-Galactosidase activities of W3110 derivatives. (A and B) degP (vertically striped bars) and pldA degP (horizontally striped bars) mutants containing a porfA-dsbA-lacZ, fkpA-lacZ, or degP-lacZ chromosomal fusion were grown in LB broth at either 30°C (A) or 41°C (B), and their β-galactosidase activities were assayed. (C and D) Wild-type (vertically striped bars) and pldA (horizontally striped bars) mutants containing a porfA-dsbA-lacZ, fkpA-lacZ, or degP-lacZ chromosomal fusion were grown in LB broth at either 30°C (C) or 41°C (D), and their β-galactosidase activities were assayed.
Overproduction of NlpE also increases viability of degP mutants but does not prevent degradation of phospholipids.
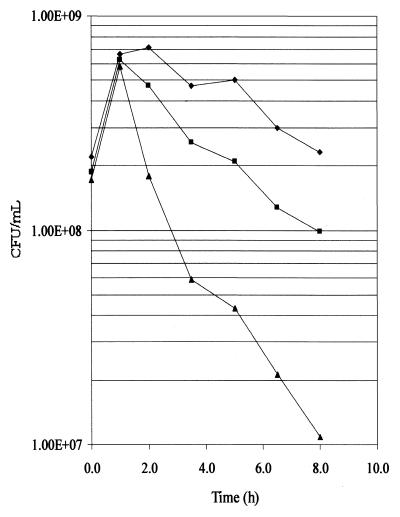
Overproduction of the outer membrane lipoprotein NlpE has previously been shown to induce the Cpx regulon (55). If the pldA mutation rescues degP cells by hyperinducing extracytoplasmic stress responses, as suggested by the fusion expression analysis, then overexpression of NlpE should also suppress the temperature-sensitive phenotype of degP cells. W3110 degP cells containing either the plasmid pLD404 (which constitutively overproduces NlpE) (55) or pBR322 were shifted to 44°C and then plated at 30°C. As shown in Fig. 5, the cells overexpressing NlpE were less sensitive to incubation at the elevated temperature, so that after 8 h at 44°C, the viability was ∼10-fold higher than for the cells containing pBR322. However, W3110 degP(pLD404) cells remained more sensitive to the elevated temperature than W3110 (pLD404) cells, suggesting that as for the pldA mutation, the suppression of the temperature-sensitive phenotype was not complete. The overexpression of NlpE also greatly reduced the leakage of the cellular proteins from the degP mutants, as shown in Table 4 for the release of β-galactosidase and β-lactamase and as observed in trichloroacetic acid precipitates of culture supernatants (data not shown).
FIG. 5.
Survival curves of W3110 derivatives overexpressing NIpE. W3110(pLD404 [overexpresses nlpE]) (⧫), W3110 degP(pLD404) (■), and W3110 degP(pBR322) (▴) were incubated to an OD600 of ∼0.5 at 30°C and were then shifted to 44°C at 0 h and were incubated for another 8 h. At the indicated time points, the cultures were diluted and plated at 30°C.
TABLE 4.
Release of cytoplasmic and periplasmic enzymes by degP cells overproducing NlpE at various temperatures
| Temp (°C) | Enzymea | Amt of enzyme assayedb (%) per strain in cells or supernatants
|
|||
|---|---|---|---|---|---|
| W3110 degP(pBR322)
|
W3110 degP(pLD404)
|
||||
| Cells | Supernatant | Cells | Supernatant | ||
| 37 | β-Gal | 2,817 (92) | 245 (8) | 2,553 (98) | 46 (2) |
| β-Lac | 835 (77) | 251 (23) | 1,563 (93) | 123 (7) | |
| 41 | β-Gal | 2,810 (82) | 599 (18) | 2,104 (94) | 142 (6) |
| β-Lac | 280 (30) | 662 (70) | 829 (77) | 253 (23) | |
β-Gal, β-galactosidase; β-Lac, β-lactamase.
The amount of β-galactosidase and β-lactamase present is expressed in units/milliliter/OD600. The OD600 is taken from the original culture.
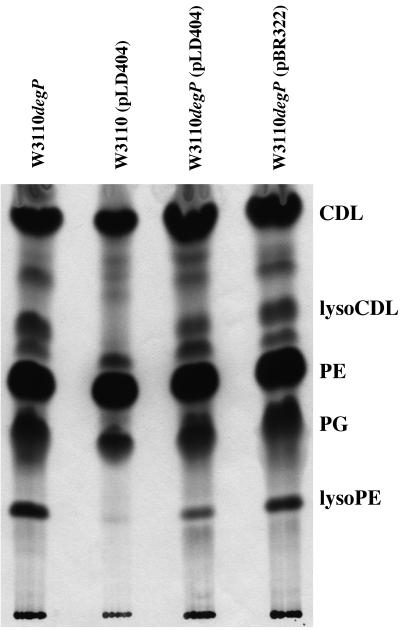
The lipids of these cells were also labeled with [U-14C]acetate and extracted as described above. Lysophospholipids were not present in W3110(pLD404) but were present in W3110 degP cells containing pLD404 or pBR322 (Fig. 6). Therefore, overexpression of NlpE did not prevent the activation of the phospholipase in the degP cells, although W3110 degP(pLD404) cells had fewer lysophospholipids present than did W3110 degP(pBR322) cells.
FIG. 6.
Radiolabeled phospholipids from W3110 degP, W3110(pLD404), W3110 degP(pLD404), and W3110 degP(pBR322) cultures. [14C]acetate-labeled cultures were grown at 44°C, and radiolabeled phospholipids were separated on a TLC plate and autoradiographed. The positions of cardiolipin (CDL), lysocardiolipin (lysoCDL), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), and lysophosphatidylethanolamine (lysoPE) are indicated.
DISCUSSION
It has previously been demonstrated that the periplasmic protease DegP is essential for cell viability at temperatures above 42°C (33, 59). The protease, which can also act as a chaperone, appears to be responsible for refolding or hydrolyzing misfolded proteins that accumulate in the periplasm during heat shock (57). In degP mutants these denatured proteins presumably continue to accumulate until the temperature is reduced or the cell dies, although it is not yet clear what constitutes the lethal event.
In the studies that we report here, it was found that a pldA mutation restores both the plating efficiency at high temperatures and the ability of the degP cells to withstand extended incubation at a temperature of 44°C. In another report of suppression of the temperature-sensitive phenotype of degP cells, it was found that cells which also contained an lpp mutation had an apparently normal plating efficiency (59). Cells deficient in the major lipoprotein have been shown to be leaky for periplasmic proteins, and it was thus hypothesized that the rescue of the degP cells was afforded by the release of the accumulating misfolded and denatured proteins through the lesions in the envelope caused by the lpp mutation. When we examined the supernatant of wild-type and degP cells, however, we found that cells containing the degP mutation released much more protein than did wild-type cells. Furthermore, the pldA mutation greatly reduced the amount of protein released by the degP cells incubated at elevated temperatures. This indicates that, at least for the pldA mutation, the suppression of the degP temperature-sensitive phenotype is not due to the release of misfolded or denatured periplasmic proteins from the cell.
The presence of lysophospholipids in degP mutant cells incubated at elevated temperatures indicates that OMPLA is activated at these temperatures and hydrolyzes phospholipids of the membranes. The suppression of the temperature sensitivity of the degP mutants by the pldA mutation might be an indication that OMPLA activation is an important contributor to the stress conditions being experienced by the degP cells, perhaps by altering the structure of the membrane environment and causing misfolding or misincorporation of membrane proteins. If this were the case, the pldA mutation would be expected to reduce the amount of stress experienced by the degP mutant cells and to lead to a reduction in the activity of the ςE and Cpx extracytoplasmic stress responses. However, as shown in Fig. 4, the pldA mutation was found to induce the Cpx and ςE stress responses rather than reduce them at all temperatures. This suggests that the pldA mutation may suppress the degP temperature-sensitive phenotype by increasing the amounts of the proteins in the cell that are involved in relieving extracytoplasmic stress, which would act to reduce the number of misfolded and denatured proteins accumulating in the envelope.
Experiments involving the overexpression of NlpE were used to further examine the involvement of extracytoplasmic stress responses in the rescue of the degP temperature-sensitive phenotype. The overexpression of NlpE was previously found to rescue E. coli cells from other lethal stress, such as that caused by the induction of a lamB-lacZ-phoA tripartite fusion, by inducing the Cpx response (55). Furthermore, cpxA∗ mutants, which upregulate the Cpx response, were found to suppress the lethal phenotype caused by the LamB-LacZ-PhoA tripartite fusion (14). We found that degP cells were also rescued at elevated temperatures and released less protein from the cells when they contained the pLD404 plasmid resulting in overexpression of NlpE. This suggests that the induction of the Cpx response caused by the NlpE overexpression also serves to partially compensate for the absence of DegP in these cells. These results thus support the hypothesis that a pldA mutation suppresses the degP temperature-sensitive phenotype primarily by inducing the Cpx and ςE responses.
Previous studies have indicated that under normal growth conditions, there is no observable phenotype for pldA mutations in an otherwise wild-type E. coli (1). However, our findings may have a bearing on other phenomena which have been observed in pldA cells and have therefore been attributed to the lack of phospholipase activity. For example, a pldA mutation was found to reduce colonization of the gastric mucosa of mice by Helicobacter pylori (25). This was suggested to be due to a decreased ability to penetrate the mucous layer in the absence of the phospholipase. It is also possible, however, that it is due to harmful effects on the structure of the cell envelope in pldA cells, and at least in E. coli, evidence for such an effect is provided by the induction of the stress responses that occurs in the absence of OMPLA. It has also been demonstrated that the release of a number of colicins requires the presence of a functioning OMPLA in the cell (7, 37, 47). It was further shown that lysophospholipids had accumulated in the moribund colicin-producing cells, and it was thus concluded that activation of the phospholipase and consequent membrane damage was an important part of the mechanism by which the colicin lysis proteins cause the release of the colicins from the producing cells. These interpretations bear reexamination, however, in light of the finding that the absence of the OMPLA causes induction of both extracytoplasmic stress regulons. Given that the colicin A lysis protein has been shown to be a substrate of the DegP protease (9), it would seem possible that the induction of DegP and of other stress-relieving proteins of the Cpx and ςE regulons plays a major role in the decrease in colicin release in the absence of OMPLA. If this interpretation is correct, then hyperinduction of the extracytoplasmic stress regulons by other means such as the overproduction of NlpE should also reduce the level of colicin secretion.
Our results also add to recent studies which suggest that stimuli other than denaturing proteins may form part of the inducing signal for extracytoplasmic stress responses. Danese et al. (11) found that both the Cpx and ςE stress responses were activated by accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate in E. coli membranes. In addition, an E. coli mutant lacking phospatidylethanolamine was shown to express high levels of DegP and displayed other phenotypic effects which could be attributed to activation of the Cpx signal transduction pathway (39). It is not yet clear whether it is alterations in the lipid components of the membranes or the denaturation of membrane proteins which may occur as a result of these alterations that induces the stress responses. In either case, however, these studies together indicate that membrane lipids play a critical role in the state of the extracytoplasmic compartments of the cell.
In summary, a pldA mutation was found to suppress the temperature-sensitive phenotype of degP mutants. In addition to preventing the degradation of lipids that occurs in degP cells at high temperatures, the mutation strongly reduced the amounts of cytoplasmic and periplasmic proteins that are released from degP cells even at 37°C, a temperature at which phospholipid degradation is minimal and at which the plating efficiency and growth of the cells are not markedly affected. However, in spite of the increased viability and evidence of reduced cellular damage in the pldA mutants, the analyses of Cpx- and ςE-dependent gene expression in pldA cells indicated that both extracytoplasmic stress regulons were induced in the absence of OMPLA. This suggests that the rescue of the degP mutants in the absence of OMPLA may be due more to the induction of stress responses than to the lack of phospholipase activity. Consistent with this hypothesis, we found that overexpression of NlpE, which induces a stress response as well, also rescues the degP mutants and reduces protein leakage from the cells, without preventing the activation of the phospholipase. Further studies are required to determine the reason why the loss of OMPLA, despite causing no discernible phenotype in laboratory-grown cultures, causes activation of extracytoplasmic stress responses.
ACKNOWLEDGMENTS
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes for Health Research to S.P.H.
We thank R. A. Kelln for helpful discussions.
REFERENCES
- 1.Audet A, Nantel G, Proulx P. Phospholipase A activity in growing Escherichia coli cells. Biochim Biophys Acta. 1974;348:334–343. doi: 10.1016/0005-2760(74)90213-6. [DOI] [PubMed] [Google Scholar]
- 2.Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 3.Bayer M H, Costello G P, Bayer M E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982;149:758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck C F, Ingraham J L, Neuhard J, Tomassen E. Metabolism of pyrimidine nucleotides by Salmonella typhimurium. J Bacteriol. 1972;110:219–228. doi: 10.1128/jb.110.1.219-228.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 6.Casadaban M J. Transposition and fusion of lac genes to selected promoters in Escherichia coli using bacteriophages lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 7.Cavard D, Baty D, Howard S P, Verheij H M, Lazdunski C. Lipoprotein nature of the colicin A lysis protein; effect of amino acid substitutions at the site of modification and processing. J Bacteriol. 1987;169:2187–2194. doi: 10.1128/jb.169.5.2187-2194.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavard D, Howard S P, Lazdunski C. Functioning of the colicin A lysis protein is affected by Triton X-100, divalent cations and EDTA. J Gen Microbiol. 1989;135:1715–1726. doi: 10.1099/00221287-135-6-1715. [DOI] [PubMed] [Google Scholar]
- 9.Cavard D, Lazdunski C, Howard S P. The acylated precursor form of the colicin A lysis protein is a natural substrate of the DegP protease. J Bacteriol. 1989;171:6316–6322. doi: 10.1128/jb.171.11.6316-6322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronan J E J, Wulff D L. A role for phospholipid hydrolysis in the lysis of Escherichia coli infected with bacteriophage T4. Virology. 1969;38:241–246. doi: 10.1016/0042-6822(69)90365-1. [DOI] [PubMed] [Google Scholar]
- 11.Danese P N, Oliver G R, Barr K, Bowman G D, Rick P D, Silhavy T J. Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J Bacteriol. 1998;180:5875–5884. doi: 10.1128/jb.180.22.5875-5884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danese P N, Silhavy T J. The ςE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 13.Danese P N, Silhavy T J. CpxP, a stress-combative member of the Cpx regulon. J Bacteriol. 1998;180:831–839. doi: 10.1128/jb.180.4.831-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danese P N, Snyder W B, Cosma C L, Davis L J, Silhavy T J. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 15.Dartigalongue C, Missiakas D, Raina S. Characterization of the Escherichia coli ςE regulon. J Biol Chem. 2001;276:20866–20875. doi: 10.1074/jbc.M100464200. [DOI] [PubMed] [Google Scholar]
- 16.Dartigalongue C, Raina S. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase of outer membrane proteins in Escherichia coli. EMBO J. 1998;17:3968–3980. doi: 10.1093/emboj/17.14.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Geus P, van Die I, Bergmans H, Tommassen J, de Haas G. Molecular cloning of pldA, the structural gene for outer membrane phospholipase of E. coli K12. Mol Gen Genet. 1983;190:150–155. doi: 10.1007/BF00330338. [DOI] [PubMed] [Google Scholar]
- 18.Dekker N. Outer-membrane phospholipase A: known structure, unknown biological function. Mol Microbiol. 2000;35:711–717. doi: 10.1046/j.1365-2958.2000.01775.x. [DOI] [PubMed] [Google Scholar]
- 19.Dekker N, Tommassen J, Lustig A, Rosenbusch J P, Verheij H M. Dimerization regulates the enzymatic activity of outer membrane phospholipase A. J Biol Chem. 1997;272:3179–3184. doi: 10.1074/jbc.272.6.3179. [DOI] [PubMed] [Google Scholar]
- 20.Dekker N, Verheij H M, Tommassen J. Bacteriocin release protein triggers dimerization of outer membrane phospholipase A in vivo. J Bacteriol. 1999;181:3281–3283. doi: 10.1128/jb.181.10.3281-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Las Penas A, Connolly L, Gross C A. The ςE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of ςE. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 22.De Las Penas A, Connolly L, Gross C A. ςE is an essential sigma factor in Escherichia coli. J Bacteriol. 1997;179:6862–6864. doi: 10.1128/jb.179.21.6862-6864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doi O, Nojima S. Nature of Escherichia coli mutants deficient in detergent-resistant and/or detergent-sensitive phospholipase A. J Biochem. 1976;80:1247–1258. doi: 10.1093/oxfordjournals.jbchem.a131396. [DOI] [PubMed] [Google Scholar]
- 24.Dong J S, Iuchi S, Kwan H S, Lu Z, Lin E C C. The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene. 1993;136:227–230. doi: 10.1016/0378-1119(93)90469-j. [DOI] [PubMed] [Google Scholar]
- 25.Dorrell N, Martino M C, Stabler R A, Ward S J, Zhang Z W, McColm A A, Farthing M J, Wren B W. Characterization of Helicobacter pylori PldA, a phospholipase with a role in colonization of the gastric mucosa. Gastroenterology. 1999;117:1098–1104. doi: 10.1016/s0016-5085(99)70394-x. [DOI] [PubMed] [Google Scholar]
- 26.Erickson J W, Gross C A. Identification of the ςE subunit of Escherichia coli RNA polymerase: a second alternate ς factor involved in high-temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 27.Hardaway K L, Buller C S. Effect of ethylenediaminetetraacetate on phospholipids and outer membrane function in Escherichia coli. J Bacteriol. 1979;137:62–68. doi: 10.1128/jb.137.1.62-68.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horne S M, Young K D. Escherichia coli and other species of the Enterobacteriaceae encode a protein similar to the family of Mip-like FK506-binding proteins. Arch Microbiol. 1995;163:357–365. doi: 10.1007/BF00404209. [DOI] [PubMed] [Google Scholar]
- 29.Horrevoets A J G, Francke C, Verheij H M, de Haas G H. Activation of reconstituted Escherichia coli outer membrane phospholipase A by membrane perturbing peptides results in an increased reactivity towards affinity label hexadecanesulfonyl fluoride. Eur J Biochem. 1991;198:255–261. doi: 10.1111/j.1432-1033.1991.tb16009.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacob-Dubuisson F, Pinkner J, Xu X, Striker R, Padmanhaban A, Hultgren S J. PapD chaperone function in pilus biogenesis depends on oxidant and chaperone-like activities of DsbA. Proc Natl Acad Sci USA. 1994;91:11552–11556. doi: 10.1073/pnas.91.24.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen K F. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J Bacteriol. 1993;175:3401–3407. doi: 10.1128/jb.175.11.3401-3407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones C H, Danese P N, Pinkner J S, Silhavy T J, Hultgren S J. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 1997;21:6394–6406. doi: 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipinska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for growth only at elevated temperatures. J Bacteriol. 1989;171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipinska B, Sharma S, Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a ς32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988;16:10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipinska B, Zylicz M, Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990;172:1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Walsh C T. Peptidl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci USA. 1990;87:4028–4032. doi: 10.1073/pnas.87.11.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luirinik J, van der Sande C, Tommassen J, Veltkamp E, de Graaf F K, Oudega B. Effects of divalent cations and of phospholipase A activity on excretion of cloacin DF13 and lysis of host cells. J Gen Microbiol. 1986;132:825–834. doi: 10.1099/00221287-132-3-825. [DOI] [PubMed] [Google Scholar]
- 38.Mecsas J, Rouviere P E, Erickson J W, Donohue T J, Gross C A. The activity of ςE, an Escherichia coli heat-inducible ς-factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 39.Mileykovskaya E, Dowhan W. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J Bacteriol. 1997;179:1029–1034. doi: 10.1128/jb.179.4.1029-1034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. [Google Scholar]
- 41.Missiakas D, Betton J-M, Raina S. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol Microbiol. 1996;21:871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- 42.Missiakas D, Mayer M P, Lemaire M, Georgopoulos C, Raina S. Modulation of the Escherichia coli ςE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol Microbiol. 1997;24:355–371. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 43.Neuhard J, Ingraham J. Mutants of Salmonella typhimurium requiring cytidine for growth. J Bacteriol. 1968;95:2431–2433. doi: 10.1128/jb.95.6.2431-2433.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patriarca P, Beckerdite S, Elsbach P. Phospholipases and phospholipid turnover in Escherichia coli spheroplasts. Biochim Biophys Acta. 1972;260:593–600. doi: 10.1016/0005-2760(72)90008-2. [DOI] [PubMed] [Google Scholar]
- 46.Pogliano J, Lynch A S, Belin D, Lin E C C, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 47.Pugsley A P, Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984;3:2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raivio T L, Popkin D L, Silhavy T J. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol. 1999;181:5263–5272. doi: 10.1128/jb.181.17.5263-5272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raivio T L, Silhavy T J. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raivio T L, Silhavy T J. The ςE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol. 1999;2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 51.Scandella C J, Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971;10:4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- 52.Slauch J M, Garrett S, Jackson D E, Silhavy T J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988;170:439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snijder H J, Dijkstra B W. Bacterial phospholipase A: structure and function of an integral membrane phospholipase. Biochim Biophys Acta. 2000;1488:91–101. doi: 10.1016/s1388-1981(00)00113-x. [DOI] [PubMed] [Google Scholar]
- 54.Snijder H J, Ubarretxena-Belandia I, Blaauw M, Kalk K H, Verheij H M, Egmond M R, Dekker N, Dijkstra B W. Structural evidence for dimerization-regulated activation of an integral membrane phospholipase. Nature. 1999;401:717–721. doi: 10.1038/44890. [DOI] [PubMed] [Google Scholar]
- 55.Snyder W B, Davis L J B, Danese P N, Cosma C L, Silhavy T J. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol. 1995;177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soberon X, Covarrubias L, Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980;9:287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- 57.Spiess C, Bell A, Ehrmann M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell. 1999;97:339–347. doi: 10.1016/s0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- 58.Strauch K L, Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci USA. 1988;85:1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strauch K L, Johnson K, Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ubarretxena-Belandia I, Boots J-W P, Verheij H M, Dekker N. Role of cofactor calcium in the activation of outer membrane phospholipase A. Biochemistry. 1998;37:16011–16018. doi: 10.1021/bi9814181. [DOI] [PubMed] [Google Scholar]
- 61.Virden R, Tan A K, Fink A L. Cryoenzymology of staphylococcal β-lactamase: trapping a serine-70-linked acyl-enzyme. Biochemistry. 1990;29:145–153. doi: 10.1021/bi00453a018. [DOI] [PubMed] [Google Scholar]
- 62.Weber R F, Silverman P J. The Cpx proteins of Escherichia coli K-12: structure of the CpxA polypeptide as an inner membrane component. J Mol Biol. 1988;203:467–476. doi: 10.1016/0022-2836(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 63.Weiss J, Beckerdite-Quagliata S, Elsbach P. Determinants of the action of phospholipases A on the envelope phospholipids of Escherichia coli. J Biol Chem. 1979;254:11010–11014. [PubMed] [Google Scholar]