Summary
Background
Tetralogy of Fallot (TOF) is the leading cyanotic congenital heart disease. We commenced open-heart surgery at the Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife, Nigeria in 2016.
Objectives
To review the incidence, pattern, management and treatment outcomes of TOF at the OAUTHC.
Methods
A retrospective audit was undertaken of hospital records, including echocardiograms of patients with TOF seen from January 2016 to February 2020 at the Paediatric Cardiology Unit, OAUTHC.
Results
Seventy-two patients (37 boys and 35 girls) aged 0.17–22 years had TOF. Thirty-three (45.8%) had surgery; 31 (93.9%) corrective surgery and two (6.1%) a modified Blalock–Taussig shunt. Complications following surgery included cardiac dysfunction, post-transfusion malaria, pulmonary regurgitation, pericardial effusion and death (15%). Thirty-nine (54.2%) patients had conservative medical management. Complications included polycythaemia and thrombotic stroke, and 14 (35.9%) patients died.
Conclusions
TOF is associated with significant morbidity and mortality in developing countries. Early and safe corrective surgery is desirable.
Keywords: congenital heart disease, tetralogy of Fallot, echocardiography, surgical repair, surgical outcome, conservative approach outcome, follow-up results
Congenital heart defects are the most common form of congenital abnormalities identified in humans, occurring in approximately one in every 125 live births.1 Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease (CHD) beyond the neonatal age group.2
The earliest account of TOF was first given in 1871 by Dane Niels Stensen, a Danish physician.3 However, it was Etienne- Louis Arthur Fallot in 1888 who first described in precise detail the four cardinal features that characterise this condition.2,3 He identified TOF basically as a single abnormality affecting the main pulmonary artery (PA) and the sub-pulmonary infundibulum, resulting in pulmonary stenosis/right ventricular outflow tract obstruction (RVOTO), ventricular septal defect (VSD), overriding of the aorta, and right ventricular hypertrophy.2,3
The degree of the RVOTO is highly variable and progressive. The specific terminologies used by Fallot were ‘la maladie bleue’ (the blue disease) or ‘cyanose cardiaque’ (cardiac cyanosis).3,4 Maude Abbott, a paediatric cardiologist of Montreal, Canada, coined the term ‘tetralogy of Fallot’ in 1924.3
TOF occurs in approximately one in 3 000 live births and constitutes seven to 10% of all CHD.5,6 It occurs with equal frequency in boys and girls, as well as in all races and ethnic groups.7
The aetiology is unknown, however, it has been associated with conditions such as untreated maternal diabetes mellitus, ingestion of retinoic acid in the first trimester of pregnancy, uncontrolled maternal phenylketonuria and trimethadione use in pregnancy.7,8 It has also been linked with chromosomal anomalies such as trisomies 21, 18 and 13, microdeletions of chromosome 22 and Alagille syndrome.6,8,9 However, approximately 80% of children with TOF are non-syndromic.8,10
TOF patients develop RVOTO and this determines the clinical severity of the disease.6,7 The severity of symptoms depends on the degree of branch and peripheral pulmonary artery stenosis, which may be a determinant factor for survival after surgical repair and subsequent quality of life and longevity.6,7 Cyanosis is often not present at birth, but with increasing hypertrophy of the right ventricular outflow tract and growth, cyanosis becomes recognisable within the first year of life.7,9,11 Hypercyanotic spells are particularly a problem in children under the age of two years. Severe spells may result in unconsciousness, convulsions or death.7,12
Older children have dusky skin, grey sclera, engorged veins, marked clubbing of the fingers and toes and easy fatigue.13-15 Dyspnoea occurs on exertion and the children characteristically squat for relief of dyspnoea.13,16 Polycythaemia occurs secondarily and is often well tolerated.13,16 However, haematocrit above 65% may result in sludging within the microcirculation due to increased viscosity of the blood. Isovolumic partial exchange is indicated for symptomatic treatment.14,16
Chest radiographs classically show a boot-shaped but normalsized heart with an upturned apex (coeur en sabot) in most cases of TOF.12,15 More than a quarter of patients with TOF have a right-sided aortic arch.14,15,17 Pulmonary vascularity is usually diminished bilaterally.7, 14,15
Two-dimensional echocardiography is the mainstay for the diagnosis and follow up of TOF.13-15,17 Cardiac magnetic resonance imaging (CMRI) or a cardiac computerised tomography scan (CCT) may be required to provide further relevant information for diagnosis and surgical intervention.14,15,17 Cardiac catheterisation may be required for further assessment of the haemodynamic status and for the identification of anomalies of the coronary artery that are difficult to detect with echocardiography and other non-invasive means.14,15,18
The goal of medical management is to delay surgery for some months and to avert hypercyanotic spells.15,19 Use of beta-blockers, such as propranolol, is preferred.13,18 Adequate hydration is also advised to prevent dehydration, which can trigger hypercyanotic spells as a result of the release of catecholamines.13,14
In 1945, the first palliative surgery for TOF was done and named after Alfred Blalock and Helen Taussig.18 The modified Blalock–Taussig (mBT) shunt consists of placing a prosthetic tube between the subclavian and pulmonary artery on the same side.13
The predictable management of TOF is surgical correction.13-15 The goal of surgery is directed at relieving the right ventricular outflow obstruction and closing the VSD.19 Relief of the RVOTO may be associated with varying degrees of pulmonary regurgitation, with all patients requiring lifelong follow up to allow for identification of the need for further surgery or intervention.17,19
In the developing world, the diagnosis of TOF is often made quite late or not at all. A large proportion of the children present for the first time with complications arising from the disease. Care of patients with TOF at the OAUTHC was limited to medical palliative care until the advent of open-heart surgery in 2016. This study reviews the incidence, pattern, management and treatment outcomes of TOF at the OAUTHC, Ile-Ife, Nigeria.
Methods
This study was a retrospective audit of the treatment and outcomes of all consecutive patients with TOF, seen over a period of four years and two months (January 2016 to February 2020) at the OAUTHC, Ile-Ife, Nigeria. Hospital records including pre- and postoperative echocardiograms and followup notes were reviewed. These patients all had a transthoracic echocardiographic diagnosis of TOF.
Data reviewed included socio-demographic and clinical details such venous haematocrit, plain chest radiographs and transthoracic echocardiograms. Echocardiography was done using the Esaote™ MyLab30GoldR cardiovascular echocardiography machine (serial number 08538) with colour-coded Doppler echocardiography. The machine is fitted with paediatric transducers (2.5–8 MHz), which were used for the study.
Each child had two-dimensional echocardiography, during which the diagnosis of TOF was made based on the presence of the four components of the disease. CCT and right-sided cardiac catheterisation were ordered in instances where detailed echocardiography studies could not be obtained or were inconclusive. Patients with polycythaemia, defined by a haematocrit value of 65% and above, were managed with partial exchange transfusion. Patients who had suffered complications of the disease were managed symptomatically.
Surgical management in our patients was either by mBT shunt or corrective surgery. The mBT shunt was reserved for two neonates with severe RVOTO. A trans-sternal approach was employed and a 4-mm polytetrafluoroethylene graft was sutured between the innominate and right pulmonary artery to improve pulmonary blood flow. Thirty-one patients had corrective surgery. The goal was to relieve the RVOTO and close the VSD.
In the theatre, general anaesthesia was administered with non-invasive and invasive monitoring. Following sternal division, cardiopulmonary bypass was established via aortobicaval cannulation. The heart was arrested and the surgery proceeded with moderate hypothermia. Obstructing bands in the RVOT were resected via the right atrium and in some cases, the right ventricle. The pulmonary arteries were augmented with a glutaraldehyde-treated pericardial patch. Some children with significantly hypoplastic annulus required a trans-annular glutaraldehyde-treated pericardial patch. The VSD was closed with a Dacron™ patch.
The patients were subsequently weaned off cardiopulmonary bypass and were transferred to a seven-bed intensive care unit (ICU) at the conclusion of surgery. Mechanical ventilation was continued for a couple of hours as stipulated in our unit’s protocol.
The patients were managed in a general ICU by an experienced paediatric intensivist in conjunction with a team of anaesthesiologist and ICU nurses. All patients had immediate post-operative and follow-up echocardiography performed daily for the first five days and again at one, three, six and 12 weeks following surgery. All echocardiograms were done and reviewed by at least two paediatric cardiologists.
Statistical analysis
Data were transcribed and entered in a secure personal computer, stored and analysed using the Windows version 25.0 of the Software, Statistical Programme for Service Solutions (IBM-SPSS Incorporated, Illinois, Chicago, USA). Appropriate descriptive and inferential statistics were used to analyse the variables. Continuous variables were summarised and presented using the mean and standard deviation (SD) while categorical variables were summarised and presented using proportions and percentages.
Results
A total of 72 children with TOF were managed within the period of the study. They were aged 0.17 to 22 years. The mean age of the patients was 4.7 ± 4.0 years. There were 37 males and 35 females, giving a male-to-female ratio of 1:0.95.
Their treatment intervention and outcomes are shown in Table 1. Thirty-one patients (43.0%) had corrective surgery while two (2.8%) underwent modified mBT shunt, a form of conservative approach. The last 39 (54.2%) patients had palliative medical care.
Table 1. Treatment intervention and outcome.
| Outcome, n (%) | |||
| Intervention | Alive | Dead | Total |
| Corrective surgery | 28 (90.3) | 3 (9.7) | 31 (43.0) |
| Blalock-Taussig shunt | 0 (0.00) | 2 (100) | 2 (2.8) |
| Palliative care | 30 (76.9) | 9 (23.1) | 39 (54.2) |
| Total | 58 (80.6) | 14 (19.4) | 72 (100) |
Of the 31 patients who had corrective surgery, 28 (90.3%) were successful while three patients (9.7%) died following the procedure. The two patients who underwent the mBT shunt died; the first died intra-operatively following a cardiac arrest and the second within 48 hours of surgery. The total mortality rate following surgical intervention was therefore five (15.2%). Among those who had palliative care, 30 (76.9%) were alive while nine had (23.1%) died at the time of this report. A higher mortality rate was therefore recorded among the children who had palliative care (23.1%) compared to those who had corrective surgery (9.7%). The overall mortality rate among the patients was 19.4%.
Fig. 1 shows the frequency of immediate postoperative complications in the patients who underwent corrective surgery. The most common immediate post-operative complications were malaria (80.6%), arrhythmias (22.6%) and cardiac dysfunction (19.4%).
Fig. 1.
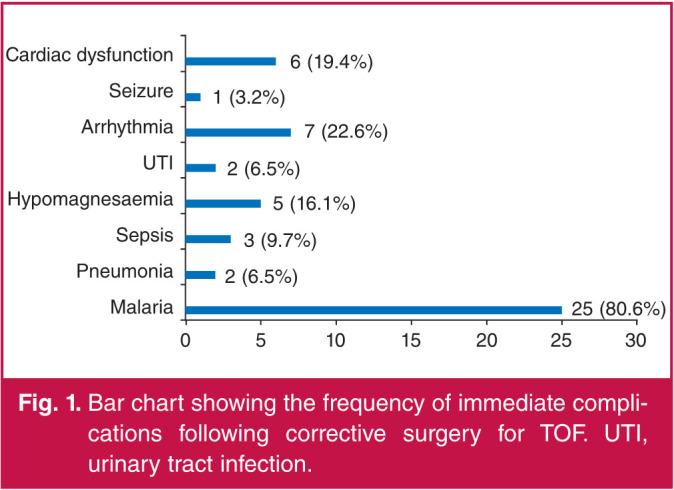
Bar chart showing the frequency of immediate complications following corrective surgery for TOF. UTI, urinary tract infection.
Echocardiographic findings following corrective surgery are shown in Fig. 2. Fifteen (48.8%) patients had free pulmonary regurgitation, nine (29.9%) had residual pulmonary stenosis with a peak gradient greater than 30 mmHg, five (16.1) had mild aortic incompetence, two (6.5%) patients were found to have previously unseen VSD, 18 (58.1%) had pleural effusion, which was the most common complication, 15 (48.4%) patients had pericardial effusion and four (12.9) had right ventricular dysfunction.
Fig. 2.
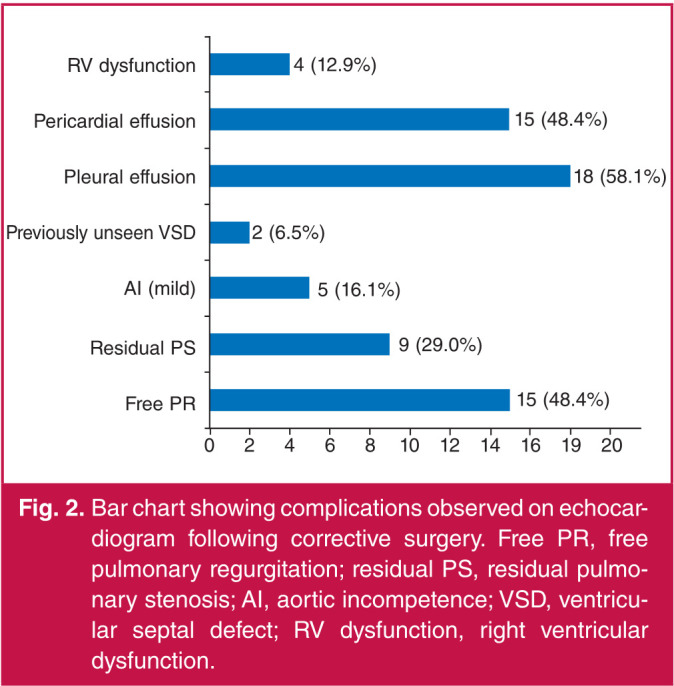
Bar chart showing complications observed on echocardiogram following corrective surgery. Free PR, free pulmonary regurgitation; residual PS, residual pulmonary stenosis; AI, aortic incompetence; VSD, ventricular septal defect; RV dysfunction, right ventricular dysfunction.
Table 2 shows the complications that were noted among the patients who had only palliative care. Thirty-eight (97.4%) of them had polycythaemia, for which they had partial exchange transfusion with normal saline whenever their haematocrit was 65% or above, 16 (41.0%) had right coronary cusp prolapse, 10 (25.6%) patients were found to have right ventricular dysfunction and eight (20.5) had aortic incompetence. The other complications observed are as shown in the table.
Table 2. Complications in patients who had palliative medical care.
| Complications | Number | Percentage |
| Death | 9 | 23.1 |
| Kyphoscoliosis | 2 | 5.1 |
| Polycythaemia | 38 | 97.4 |
| Pneumonia | 8 | 20.5 |
| Stroke | 8 | 20.5 |
| Cerebral abscess | 4 | 10.3 |
| Infective endocarditis | 1 | 2.7 |
| Pica | 2 | 5.1 |
| Right ventricular dysfunction | 10 | 25.6 |
| Right coronary cusp prolapse | 16 | 41.0 |
| Aortic incompetence | 8 | 20.5 |
Discussion
TOF is a complex and treatable cyanotic CHD in the vast majority of patients. In this audit, less than half of the patients had corrective surgery and the average age at surgery was 5.13 years (range 1.1–17 years). This is unlike what occurs in developed nations of the world where corrective surgery is done in infancy.
The ideal age for corrective surgery of TOF has been a subject of debate for some decades.2,20 Early intervention with corrective surgery within the first four to six months of life, and earlier in symptomatic neonates is now advocated.2,20 There are several reasons why experts advocate early corrective surgery of TOF.20,21 Early corrective surgery results in better preservation of the function of the left ventricle and eradicates cyanosis and hypoxaemia, which improves exercise capacity.15,17 Also, it achieves a reduction of the right ventricular pressure overload and significantly reduces secondary injury to the heart, lungs and central nervous system.20,22 Currently, most centres favour corrective surgery in infancy.20-22
It is widely accepted that the outcome following early corrective surgery of TOF in infancy is superior to corrective surgery later in life.20-22 In a systematic review of 143 articles comprising 21 427 patients, Romeo et al.22 studied the outcome after corrective surgery of TOF. They concluded that corrective surgery of TOF in infancy was associated with a low mortality rate and good long-term survival, even though most patients required intensive follow up all through life.
None of our patients had had corrective surgery in infancy, the youngest and oldest of our patients were older than one year and 17 years, respectively. Advanced age at surgery has been linked with a higher mortality rate and this has been attributed to prolonged hypoxia and right ventricular hypertension.23 In Nigeria today, the National Health Insurance Scheme does not cover the cost of open-heart surgery, so patients have to pay out of pocket. Even though the cost of surgery is subsidised, especially when compared with the cost in developed nations, surgery is still out of reach for most patients.
Among the 29 patients who had corrective surgery, three died, resulting in a mortality rate of 9.7% in this group. They had arrhythmias and cardiac dysfunction following surgery. Reports from several studies have shown that corrective surgery can be done with a relatively low mortality rate.18,20 In a cohort of 819 consecutive patients with TOF, Chiu et al.23 reported an early mortality rate of 3.3%. This is far lower than the value obtained in our study. They studied a larger population of patients and this could account for the disparity in mortality rates observed. Azari et al.21 from Iran reported an early mortality rate of 12.2% among 74 children who had corrective surgery of TOF. This mortality rate is similar to that obtained in our study.
Corrective surgery for TOF is associated with excellent long-term results and a survival rate greater than 90%.23 However, there are recognised long-term electrical and anatomical complications that may occur.22-25 We have been able to monitor our patients only for a maximum of four years (range two months to four years). Our outcome thus far has been quite good. All our patients have recovered from the immediate postoperative complications, except for those who still have free pulmonary regurgitation and pulmonic stenosis. None of our patients has required re-operation.
The most common complication we observed among our patients postoperatively was malaria and this was due to the use of blood and blood products during the period of surgery. Malaria is endemic in Nigeria. Globally, malaria is one of the most frequent transfusion-transmitted infections.26
Woolsey in 1911 gave the first description of transfusion-transmitted malaria (TTM).26, 27 TTM is an unintended infection with Plasmodium species resulting from the transfusion of whole blood or any component of blood from a donor infected with malaria to a recipient.26,27 Malaria transmitted by blood transfusion has potentially grave consequences, as infection with Plasmodium falciparum may progress rapidly and become fatal.27,28 In endemic countries such as Nigeria, a significant proportion of donors are potentially infected with malaria parasites and donated blood is not routinely screened for malaria parasites, unlike what is practised in non-malaria endemic regions.27,28
All our patients who developed malaria following surgery made an excellent recovery following treatment with routine anti-malaria medications and none suffered neurological complications of malaria. Most of the patients had fever clinically and infection was confirmed by the evidence of the malaria parasite in a peripheral blood film.
Arrhythmia was observed in 22.6% of our patients following surgery. Arrhythmia following cardiac surgery causes haemodynamic imbalance and often requires treatment. It increases the propensity for mortality.23 Postoperative arrhythmia has been classified as early (occurring within the first two postoperative days) and late. Azari and colleagues25 reported arrhythmias as the most common postoperative complication in the children they studied, occurring in 28.4% of the children.25 Other immediate complications noted among these children were pneumonia sepsis, hypomagnesaemia, urinary tract infection and seizure.
Among the immediate postoperative complications observed on echocardiography in our patients, pleural and pericardial effusion along with free pulmonary regurgitation were the most common, each occurring with a prevalence of 58.1, 48.4 and 48.4%, respectively. The patients with pleural effusion had the effusions drained without sequelae. The pericardial effusions were not life-threatening and resolved spontaneously over time. This was monitored using echocardiography. The group of children with free pulmonary regurgitation had a trans-annular patch without valve replacement during corrective surgery.
Pulmonary regurgitation is a common sequela following corrective surgery for TOF and may be well tolerated for many years after surgery.29 Relief of the obstruction to the RVOT in TOF often impairs the integrity of the pulmonary valve, which results in pulmonary regurgitation. About a decade after repair, 40 to 85% of patients have moderate to severe pulmonary regurgitation.2 Over time, it results in progressive dilation of the right ventricle and biventricular dysfunction, with progressively worsening intolerance to exercise, heart failure, tachyarrhythmia, and later sudden death.2,29 Pulmonary regurgitation very often requires surgical intervention.29
More than half of our patients (54.2%) did not have surgery and were on palliative medical care. The mortality rate among these patients was more than twice the rate among the children who had corrective surgery. The cause of death included right heart failure, infective endocarditis and cerebrovascular accidents. The most common complication occurring in all but one of the patients was polycythaemia (haematocrit ≥ 65%). This was managed by partial exchange transfusion with normal saline. The highest recorded haematocrit among these children was 84%. The procedure was done as often as it was required to minimise the risk for stroke. Unfortunately, among this group, one-fifth of the children suffered a stroke, with only partial recovery in most of them. A tenth of these patients developed cerebral abscesses.
Right ventricular dysfunction was noted in a quarter of the patients. This was identified by echocardiography. Further investigations such as CMRI are yet to be done for these patients because of the cost implications of such investigation. More than a third of these patients had right coronary cusp prolapse, half of whom had concomitant aortic regurgitation.
Corrective surgery for TOF significantly reduces morbidity and mortality among children with TOF. Corrective surgery in infancy is desirable but is not readily accessible due to the cost of the procedure in this part of the world. Many patients find the cost of surgery prohibitive. In most cases, parents enlist the assistance of families and friends and even strangers (through an online appeal for funds) in the pursuit of surgical intervention for their children.
Conclusion
Nigeria is the most populous nation on the African continent with a population of over 200 million and a huge burden of CHD. Annually 70 000 babies with CHD are born in Nigeria.30 The current healthcare infrastructure in Nigeria is unable to cope with the care and treatment of this large number of children with CHD,30 thus denying these innocent children a chance at life. We seize this opportunity to implore the Federal Ministry of Health in Nigeria to fund the treatment of all patients with CHD. In addition, we appeal to the Ministry to establish centres of excellence in paediatric cardiology in the six geo-political zones of the country where children with CHD can access quality care and treatment at no cost and without delay.
References
- 1.Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine (Baltimore) 2020;99(23):e20593. doi: 10.1097/MD.0000000000020593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Ven JPG, van den Bosch E, Bogers AJCC, Helbing WA. Current outcomes and treatment of tetralogy of Fallot. F1000Res. 2019;8:F1000 Faculty Rev 1530. doi: 10.12688/f1000research.17174.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz-Frias J, Guillaume M. Tetralogy of Fallot. [Updated 2020 Nov 20]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513288/ [PubMed] [Google Scholar]
- 4.Khan SM, Drury NE, Stickley J, Barron DJ, Brawn WJ, Jones TJ, Anderson RH, Crucean A. Tetralogy of Fallot: morphological variations and implications for surgical repair. Eur J Cardiothorac Surg. 2019;56(1):101–109. doi: 10.1093/ejcts/ezy474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llamosas-Falcon L, Bermejo-Sanchez E, Sanchez-Diaz G, Villaverde- Hueso A, Posada de la Paz M, Alonso-Ferreira V. Tetralogy of Fallot in Spain: A nationwide registry-based mortality study across 36 years. Orphanet J Rare Dis. 2019;14(1):79. doi: 10.1186/s13023-019-1056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rugonyi S. Genetic and flow anomalies in congenital heart disease. AIMS Genet. 2016;3(3):157–166. doi: 10.3934/genet.2016.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien P, Marshall AC. Tetralogy of Fallot. Circulation. 2014;130:e26–29. doi: 10.1161/CIRCULATIONAHA.113.005547. [DOI] [PubMed] [Google Scholar]
- 8.Jernigan EG, Strassle PD, Stebbins RC, Meyer RE, Nelson JS. Effect of concomitant birth defects and genetic anomalies on infant mortality in tetralogy of Fallot. Birth Defects Res. 2017;109(14):1154–1165. doi: 10.1002/bdr2.1057. [DOI] [PubMed] [Google Scholar]
- 9.Animasahun BA, Madise-Wobo AD, Falase BA, Omokhodion SI. The burden of Fallot’s tetralogy among Nigerian children. CardioVasc Diagn Ther. 2016;6(5):453–458. doi: 10.21037/cdt.2016.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page DJ, Miossec MJ, Williams SG, Fotiou E, Monaghan RM, Cordell HJ. et al. Deleterious genetic variants in NOTCH1 are a major contributor to the incidence of non-syndromic tetralogy of Fallot. BioRxiv. April 2018. [Google Scholar]
- 11.Hayes-Lattin M, Salmi D. Educational Case: Tetralogy of Fallot and a review of the most common forms of congenital heart disease . Acad Pathol. 2020;7(2):237428952093409. doi: 10.1177/2374289520934094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson R, Ross O, Griksaitis MJ. Tetralogy of Fallot. BJA Educ. 2019;19(11):362–369. doi: 10.1016/j.bjae.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein D. Nelson Textbook of Paediatrics. 21st edn. Philadelphia: Elsevier Saunders: 2020. Cyanotic congenital heart lesions: Tetralogy of Fallot. In: Kliegman RM, St. Geme JW, Blum NJ, Shah SS, Tasker RC, Wilson KM, Behrman RE (eds).. pp. 9451–9466. [Google Scholar]
- 14.Park MK. Park’s The Paediatric Cardiology Handbook. 5th edn. Philadelphia: Elsevier Saunders: 2016. . Chapter 9, Cyanotic congenital heart defects: 133–188. [Google Scholar]
- 15.Agarwala B. Tetralogy of Fallot. J Cardiol. 2017;1(2):000107. [Google Scholar]
- 16.Carano N, Tchana B. Letter to the Editor: An equivalent posture to squatting is seen in an unoperated adult with tetralogy of Fallot. Cardiol Young. 2008;18(6):644. doi: 10.1017/S1047951108003144. [DOI] [PubMed] [Google Scholar]
- 17.Gunduz E, Gorgel A, Dursun R, Durgun HM, Cil H, Icer M. et al. A case of uncorrected tetralogy of Fallot undiagnosed until adulthood and presenting with polycythaemia. Cardiol Res. 2014;5(6):198–200. doi: 10.14740/cr374e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedair R, Iriart X. Educational series in congenital heart disease: Tetralogy of Fallot: Diagnosis to long-term follow-up. Echo Res Pract. 2019;6(1):R9–R23. doi: 10.1530/ERP-18-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanous E, Mogyorosy G. Does the prophylactic and therapeutic use of beta-blockers in preoperative patients with tetralogy of Fallot significantly prevent and treat the occurrence of cyanotic spells? Interact Cardiovasc Thorac Surg. 2017;25(4):647–650. doi: 10.1093/icvts/ivx135. [DOI] [PubMed] [Google Scholar]
- 20.Mainwaring RD, Hanley FL. Tetralogy of Fallot repair: How I teach it. Ann Thorac Surg. 2016;102(6):1776–1781. doi: 10.1016/j.athoracsur.2016.09.111. [DOI] [PubMed] [Google Scholar]
- 21.Rahmath MRK, Boudjemline Y. Tetralogy of Fallot will be treated interventionally within two decades. Pediatr Cardiol. 2020;41:539–545. doi: 10.1007/s00246-020-02297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romeo JL, Etnel JR, Takkenberg JJ, van de Woestijne P, Bogers AJ, Mokhles MM. et al. Outcome after surgical repair of tetralogy of Fallot: A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2020;159(1):220–236. doi: 10.1016/j.jtcvs.2019.08.127. [DOI] [PubMed] [Google Scholar]
- 23.Chiu SN, Wang JK, Chen HC, Lin MT, Wu ET, Chen CA. et al. Longterm survival and unnatural deaths of patients with repaired tetralogy of Fallot in an Asian cohort. Circ Cardiovasc Qual Outcomes. 2012;5:120–125. doi: 10.1161/CIRCOUTCOMES.111.963603. [DOI] [PubMed] [Google Scholar]
- 24.Sun G, Wang X, Chen J, Ma R, Li F, Chen L. et al. Primary repair of tetralogy of Fallot in infants: transatrial/transpulmonary or transventricular approach. Asian J Surg. 2013;36(4):137–143. doi: 10.1016/j.asjsur.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Azari A, Nezafati M, Bigdelu L. Early post-operative mortality of total correction of tetralogy of Fallot. J Cardiothoracic Med. 2017;5(4):222–225. [Google Scholar]
- 26.Ahmadpour E, Foroutan-Rad M, Majidiani H, Moghaddam SM, Hatam-Nahavandi K, Hosseini SA. et al. Transfusion-transmitted malaria: A systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(7):283. doi: 10.1093/ofid/ofz283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faruk JA. Blood transfusion malaria: A literature review. Ann Niger Med. 2016;10(2):49–57. [Google Scholar]
- 28.Mangano VD, Perandin F, Tiberti N. et al. Risk of transfusion-transmitted malaria: evaluation of commercial ELISA kits for the detection of anti-Plasmodium antibodies in candidate blood donors. Malaria J. 2019;18(17) doi: 10.1186/s12936-019-2650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senthilnathan S, Dragulescu A, Mertens L. Pulmonary regurgitation after tetralogy of Fallot repair: A diagnostic and therapeutic challenge. J Cardiovasc Echogr. 2013;23(1):1–9. doi: 10.4103/2211-4122.117975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekure EN, Sadoh WE, Bode-Thomas F, Yilgwan CS, Orogade AA, Animasahun AB. et al. Audit of availability and distribution of paediatric cardiology services and facilities in Nigeria. Cardiovasc J Afr. 2017;28(1):54–59. doi: 10.5830/CVJA-2016-057. [DOI] [PMC free article] [PubMed] [Google Scholar]


