Abstract
BACKGROUND
Factor XIa inhibitors for the prevention and treatment of venous and arterial thromboembolism may be more effective and result in less bleeding than conventional anticoagulants. Additional data are needed regarding the efficacy and safety of milvexian, an oral factor XIa inhibitor.
METHODS
In this parallel-group, phase 2 trial, we randomly assigned 1242 patients undergoing knee arthroplasty to receive one of seven postoperative regimens of milvexian (25 mg, 50 mg, 100 mg, or 200 mg twice daily or 25 mg, 50 mg, or 200 mg once daily) or enoxaparin (40 mg once daily). The primary efficacy outcome was venous thromboembolism (which was a composite of asymptomatic deep-vein thrombosis, confirmed symptomatic venous thromboembolism, or death from any cause). The principal safety outcome was bleeding.
RESULTS
Among the patients receiving milvexian twice daily, venous thromboembolism developed in 27 of 129 (21%) taking 25 mg, in 14 of 124 (11%) taking 50 mg, in 12 of 134 (9%) taking 100 mg, and in 10 of 131 (8%) taking 200 mg. Among those receiving milvexian once daily, venous thromboembolism developed in 7 of 28 (25%) taking 25 mg, in 30 of 127 (24%) taking 50 mg, and in 8 of 123 (7%) taking 200 mg, as compared with 54 of 252 patients (21%) taking enoxaparin. The dose–response relationship with twice-daily milvexian was significant (one-sided P<0.001), and the 12% incidence of venous thromboembolism with twice-daily milvexian was significantly lower than the prespecified benchmark of 30% (one-sided P<0.001). Bleeding of any severity occurred in 38 of 923 patients (4%) taking milvexian and in 12 of 296 patients (4%) taking enoxaparin; major or clinically relevant nonmajor bleeding occurred in 1% and 2%, respectively; and serious adverse events were reported in 2% and 4%, respectively.
CONCLUSIONS
Postoperative factor XIa inhibition with oral milvexian in patients undergoing knee arthroplasty was effective for the prevention of venous thromboembolism and was associated with a low risk of bleeding. (Funded by Bristol Myers Squibb and Janssen Research and Development; AXIOMATIC-TKR ClinicalTrials.gov number, NCT03891524.)
Oral anticoagulants are a mainstay for the prevention and treatment of venous and arterial thromboembolism. Although direct oral anticoagulants have replaced vitamin K antagonists for many indications, bleeding remains the major side effect. Fear of bleeding contributes to the underuse of anticoagulants in eligible patients with atrial fibrillation and to the inappropriate use of low-dose direct oral anticoagulant regimens.1,2 Therefore, the need for safer oral anticoagulants persists.
Factor XI is a promising target for the development of safer anticoagulants because it is an important driver of thrombus growth but plays a subsidiary part in hemostasis.3 Thus, patients with congenital factor XI deficiency are at lower risk for venous thromboembolism and ischemic stroke than those with normal factor XI levels but rarely have spontaneous bleeding.4–6
The development of new anticoagulants usually starts with dose-finding studies involving patients undergoing elective knee arthroplasty, because efficacy can be objectively and efficiently assessed by using venography to determine the incidence of deep-vein thrombosis after surgery. In such patients, preoperative subcutaneous administration of an antisense oligonucleotide that reduces factor XI levels or postoperative factor XI inhibition with intravenous abelacimab, a factor XI–directed antibody, was superior to enoxaparin for the prevention of venous thromboembolism.7,8
Milvexian is a selective factor XIa inhibitor that is rapidly absorbed after oral administration and has a half-life of approximately 12 hours.9 In this proof-of-principle phase 2 trial (Antithrombotic Treatment with Factor XIa Inhibition to Optimize Management of Acute Thromboembolic Events in Total Knee Replacement [AXIOMATIC-TKR]), we compared the efficacy and safety of milvexian and enoxaparin in patients undergoing elective knee arthroplasty.
METHODS
TRIAL DESIGN AND OVERSIGHT
We used a randomized, parallel-group, adaptive design with blinded outcome adjudication to compare milvexian with enoxaparin. The trial was open label for treatment assignment to milvexian or enoxaparin, but patients and observers (trial personnel and assessors of outcomes) were unaware of the milvexian dose regimen.
An operations committee and a steering committee were responsible for the design and oversight of the trial, in collaboration with the sponsors (Bristol Myers Squibb and Janssen Research and Development). The sponsors were responsible for data collection, maintenance, and analysis. The institutional review board at each participating center approved the protocol, which is available with the full text of this article at NEJM.org. All the patients provided written informed consent.
The members of the steering committee were unaware of the treatment assignments. The operations committee, whose members were aware of the treatment assignments, periodically reviewed trial outcomes and adverse events for safety oversight and, with guidance from predefined criteria for insufficient efficacy or excessive bleeding, made recommendations to the steering committee to continue the trial unchanged or to discontinue or add milvexian dose regimens.
An independent clinical-events committee whose members were unaware of the treatment assignments adjudicated all venograms for the presence and extent of deep-vein thrombosis, all suspected episodes of symptomatic venous thromboembolism or bleeding, and all deaths. The first, second, and last authors wrote the first draft of the manuscript with subsequent input from the other authors. No one who is not an author contributed to the writing of the manuscript. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol.
PATIENTS
Patients undergoing elective unilateral total knee arthroplasty were eligible if they were 50 years of age or older, had a medically stable condition, and were appropriate candidates for anticoagulant prophylaxis. The main exclusion criteria were contraindications to enoxaparin (e.g., creatinine clearance, <30 ml per minute), a history of severe hepatic impairment or previous venous thromboembolism, the use of long-term antithrombotic therapy other than aspirin (≤100 mg per day), or the inability to undergo venography. The full list of inclusion and exclusion criteria is provided in the protocol.
RANDOMIZATION AND TRIAL TREATMENT
An adaptive design was used to improve patient recruitment for the dose–response evaluation. Initially, eligible patients were randomly assigned, in a 1:1:1:1:1:1:2 ratio, to one of seven parallel treatment groups, which included four twice-daily milvexian regimens (25 mg, 50 mg, 100 mg, or 200 mg), two once-daily milvexian regimens (25 mg or 200 mg), and enoxaparin (40 mg once daily), respectively. Randomization was performed postoperatively with the use of a centralized interactive Web-based response system and was stratified according to the geographic region of the trial centers. Patients in the milvexian groups were asked to take a total of four capsules (active drug or matching placebo) per day, two capsules in the morning and two capsules in the evening. In the enoxaparin group, the drug was administered subcutaneously at a dose of 40 mg once daily. Trial medication was started 12 to 24 hours after surgery. A single dose of subcutaneous enoxaparin was allowed the evening before surgery according to the local standard of care. Treatment with milvexian or enoxaparin was given for 10 to 14 days after surgery.
The operations committee performed one ad hoc and one prespecified interim analysis. As a result of the ad hoc analysis, which occurred when 252 patients had undergone randomization, enrollment into the group receiving 25 mg of milvexian once daily was stopped because the point estimate for the incidence of venous thromboembolism was 25%, which met the prespecified criteria for insufficient efficacy. A regimen of 50 mg of milvexian once daily was then added to gain more information about the efficacy and safety of once-daily as compared with twice-daily milvexian dosing. On the basis of the results of the prespecified interim analysis, which was undertaken when at least 50 patients with a venogram that could be evaluated or with a confirmed symptomatic event were enrolled in each of the twice-daily milvexian groups, the randomization ratio was modified to enable recruitment of approximately 150 patients into the group receiving 50 mg of milvexian once daily.
TRIAL OUTCOMES
The primary efficacy outcome was venous thromboembolism, which was a composite of asymptomatic deep-vein thrombosis (detected by mandatory unilateral venography performed 10 to 14 days after surgery), confirmed symptomatic venous thromboembolism (symptomatic deep-vein thrombosis of the leg or nonfatal pulmonary embolism), or death from any cause. Unilateral venography that is performed only on the operated leg detects more than 90% of deep-vein thromboses in patients undergoing unilateral knee arthroplasty and reduces discomfort and the risk of allergic reactions or kidney damage from the contrast material.7,10
The major secondary efficacy outcomes were proximal deep-vein thrombosis (symptomatic or asymptomatic), distal deep-vein thrombosis (symptomatic or asymptomatic), nonfatal pulmonary embolism, and death. An exploratory efficacy outcome was the extent of venous thrombosis on venography, which was assessed by the adjudication committee with the use of predefined categories.
The principal safety outcome was bleeding of any severity, which was defined as the composite of major bleeding, clinically relevant nonmajor bleeding, and minimal bleeding. Secondary safety outcomes were major bleeding, clinically relevant nonmajor bleeding, clinically relevant bleeding (defined as the composite of major bleeding and clinically relevant nonmajor bleeding), and minimal bleeding. Bleeding was classified as major if it was overt and was associated with a decrease in the hemoglobin level of 2 g per deciliter or more or resulted in transfusion of 2 or more units of blood with a temporal association within 24 to 48 hours of the bleeding episode, or if it occurred in a critical area or organ or contributed to death. Bleeding at the surgical site was defined as major only if it warranted intervention; caused hemodynamic instability; or caused hemarthrosis that delayed mobilization or wound healing and resulted in prolonged hospitalization or deep wound infection. Overt bleeding that did not meet the criteria for major bleeding, but that warranted medical examination or intervention or had clinical consequences, was classified as clinically relevant nonmajor bleeding. Bleeding that did not meet the criteria for major or clinically relevant nonmajor bleeding was classified as minimal bleeding.11
SURVEILLANCE AND FOLLOW-UP
Patients were evaluated preoperatively within 30 days before surgery, at the time of randomization, and after surgery on day 1, days 4 and 7 (if patients were still hospitalized), days 10 to 14, and at 6 weeks (±10 days). Patients were instructed to report symptoms suggestive of venous thromboembolism or bleeding.
LABORATORY MEASUREMENTS
The activated partial-thromboplastin time and prothrombin time were measured in a central laboratory with the use of Actin FS and Innovin, respectively (Siemens Healthcare). The activated partial-thromboplastin time ratio and prothrombin time ratio for each patient were calculated by dividing postoperative values by those measured preoperatively.
STATISTICAL ANALYSIS
The primary hypothesis was that milvexian would reduce the risk of venous thromboembolism during the 10-to-14-day treatment period, thereby establishing proof of efficacy. The criteria for proof of efficacy were defined a priori as either a significant dose–response trend with the twice-daily milvexian regimens or an incidence of venous thromboembolism with the combined twice-daily milvexian regimens that was significantly lower than 30%. A postoperative incidence of venous thromboembolism of 30% was chosen as a conservative estimate of the incidence without thromboprophylaxis in patients undergoing knee arthroplasty.10 The primary efficacy outcome focused on twice-daily milvexian regimens because with a half-life of approximately 12 hours, milvexian is suitable for twice-daily dosing. A prespecified multiplicity correction was applied for the testing of the two components of the primary hypothesis; the family-wise error rate was controlled at a one-sided alpha level of 0.1, with a one-sided alpha level of 0.05 assigned to each component, to have more than 99% power to declare proof of efficacy.
The planned sample was 900 patients, with an option to increase the sample to approximately 1200 patients depending on the results of the interim analysis or the percentage of patients with venograms that could not be evaluated. We calculated that the trial would have at least 99% power to show proof of efficacy with a one-sided alpha level of 5% if 80% or more of enrolled patients could be evaluated for efficacy and if the incidence of venous thromboembolism ranged from 18% with the lowest twice-daily regimen of milvexian to 11% with the highest twice-daily regimen. It was also prespecified that the efficacy of the highest-dose regimen of milvexian with acceptable safety would be compared with that of enoxaparin. This comparison was estimated to have at least 90% power with a one-sided alpha level of 5%.
The primary analysis for efficacy was performed in the modified intention-to-treat population, which included all the patients who had received at least one dose of a trial medication and had a venogram within the prespecified time window that could be evaluated, a documented symptomatic venous thromboembolic event, or a fatal event. Because deep-vein thrombosis after knee arthroplasty is often asymptomatic, the confirming or ruling out of thrombosis requires a venogram that can be evaluated. The incidence of venous thromboembolism in the combined groups receiving milvexian twice daily was compared with the 30% benchmark with the use of the exact binomial test. Evidence for a dose–response trend with the twice-daily milvexian regimens was evaluated with the multiple comparison procedures and modeling (MCP-Mod) framework with the use of prespecified models (see the statistical analysis plan, available with the protocol at NEJM.org).12 For analysis of the major secondary efficacy outcomes, the Cochran–Mantel–Haenszel method with trial region as a stratification factor was used to calculate the risk ratio and the corresponding confidence interval for each milvexian group relative to the enoxaparin group, and two-sided testing was used to compare individual milvexian dose regimens with enoxaparin.
Analysis of safety outcomes was performed in the safety population, which included all the patients who had undergone randomization and had received at least one dose of a trial medication; the time for analysis included the on-treatment period plus 2 days. For each bleeding outcome, the incidence in each milvexian group was compared with that in the enoxaparin group with the same methods used for evaluation of the secondary efficacy outcomes. The Kaplan–Meier method was used to assess the time to the first occurrence of any bleeding with milvexian or enoxaparin.
RESULTS
PATIENTS
From June 27, 2019, through February 25, 2021, a total of 1242 patients underwent randomization at 118 centers in 18 countries. The analysis populations are provided in Figure 1; all the patients were followed for 30 days. The characteristics of the patients at baseline were similar across the trial groups (Table 1). Treatment adherence as assessed by counting pills or enoxaparin syringes was 80% or higher in 95% of randomly assigned patients, and 75% of randomly assigned patients were 100% adherent.
Figure 1. Enrollment, Randomization, and Populations for Analyses.
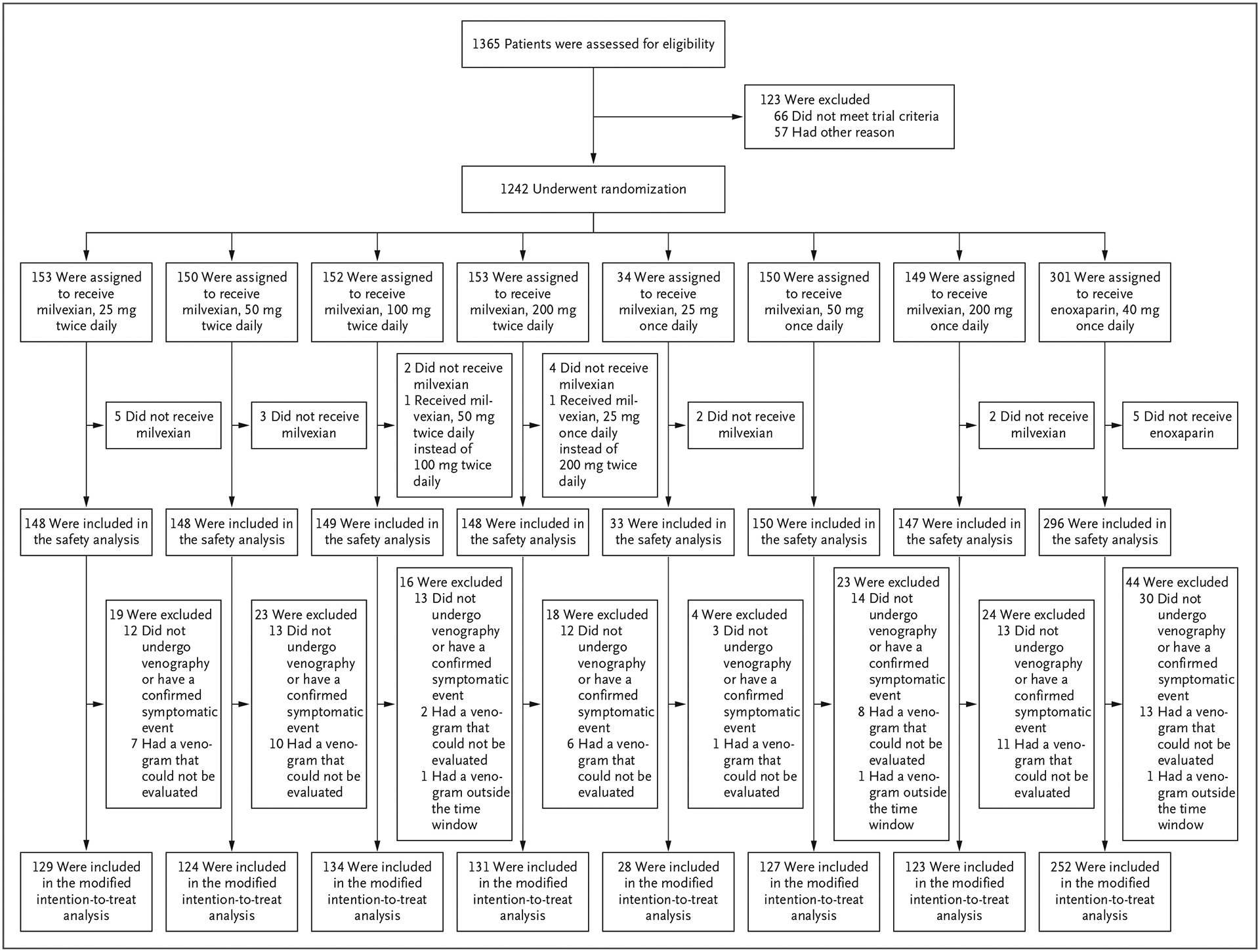
Enrollment in the group receiving 25 mg of milvexian once daily was stopped early by the trial operations committee after an ad hoc analysis showed insufficient efficacy. A regimen of 50 mg of milvexian once daily was then added to the trial regimens. One patient who was assigned to receive 100 mg of milvexian twice daily received 50 mg twice daily, and another patient who was assigned to receive 200 mg of milvexian twice daily received 25 mg once daily. For the safety analyses, these patients were included in the actual treatment group, whereas for the efficacy analyses, they were included in the planned treatment group.
Table 1.
Demographic and Clinical Characteristics of the Patients.*
| Characteristic | Milvexian Twice Daily | Milvexian Once Daily | Enoxaparin† | |||||
|---|---|---|---|---|---|---|---|---|
| 25 mg | 50 mg | 100 mg | 200 mg | 25 mg | 50 mg | 200 mg | ||
| Modified intention-to-treat population | ||||||||
| No. of patients | 129 | 124 | 134 | 131 | 28 | 127 | 123 | 252 |
| Age — yr | ||||||||
| Median | 69 | 68 | 67 | 69 | 67 | 68 | 68 | 68 |
| Range | 51–87 | 51–87 | 50–86 | 50–86 | 55–77 | 50–88 | 50–88 | 50–90 |
| Female sex — no. (%) | 92 (71) | 89 (72) | 88 (66) | 89 (68) | 18 (64) | 92 (72) | 88 (72) | 171 (68) |
| Race — no. (%)‡ | ||||||||
| White | 112 (87) | 107 (86) | 117 (87) | 116 (89) | 28 (100) | 103 (81) | 105 (85) | 216 (86) |
| Asian | 16 (12) | 15 (12) | 14 (10) | 14 (11) | 0 | 21 (17) | 15 (12) | 29 (12) |
| Black | 1 (1) | 1 (1) | 3 (2) | 1 (1) | 0 | 0 | 0 | 3 (1) |
| Other | 0 | 1 (1) | 0 | 0 | 0 | 0 | 3 (2) | 2 (1) |
| Weight — kg | ||||||||
| Median | 83 | 79 | 85 | 84 | 82 | 80 | 82 | 81 |
| Range | 40–150 | 44–150 | 48–130 | 42–132 | 56–134 | 48–181 | 48–129 | 41–146 |
| Baseline creatinine clearance — ml/min§ | ||||||||
| Median | 86 | 92 | 90 | 94 | 95 | 88 | 91 | 92 |
| Interquartile range | 71–109 | 75–113 | 70–118 | 70–114 | 72–127 | 70–105 | 73–115 | 72–114 |
| Preoperative enoxaparin — no. (%) | 8 (6) | 9 (7) | 8 (6) | 7 (5) | 2 (7) | 10 (8) | 11 (9) | 28 (11) |
| Type of anesthesia — no. (%)¶ | ||||||||
| General | 29 (22) | 25 (20) | 31 (23) | 34 (26) | 5 (18) | 29 (23) | 26 (21) | 57 (23) |
| Spinal | 99 (77) | 99 (80) | 102 (76) | 96 (73) | 23 (82) | 101 (80) | 99 (80) | 194 (77) |
| Epidural | 4 (3) | 5 (4) | 5 (4) | 4 (3) | 0 | 6 (5) | 3 (2) | 7 (3) |
| Regional | 20 (16) | 33 (27) | 42 (31) | 33 (25) | 6 (21) | 33 (26) | 29 (24) | 58 (23) |
| Other | 2 (2) | 4 (3) | 3 (2) | 4 (3) | 0 | 1 (1) | 4 (3) | 9 (4) |
| Duration of surgery — hr | ||||||||
| Median | 1.3 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Range | 0.5–3.5 | 0.7–3.1 | 0.5–6.6 | 0.6–2.8 | 0.8–2.8 | 0.6–3.3 | 0.7–3.5 | 0.4–3.5 |
| Tourniquet use — no. (%) | 89 (69) | 88 (71) | 98 (73) | 84 (64) | 20 (71) | 93 (73) | 84 (68) | 188 (75) |
| Duration of tourniquet use — hr | ||||||||
| Median | 1.2 | 1.1 | 1.2 | 1.1 | 1.3 | 1.2 | 1.3 | 1.2 |
| Range | 0.3–15 | 0.2–23 | 0.3–10 | 0.4–10 | 0.2–19 | 0.3–20 | 0.3–15 | 0.2–15 |
| Time after surgery to ambulation — days | ||||||||
| Median | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Range | 0–3 | 0–3 | 0–4 | 0–4 | 0–4 | 0–4 | 0–6 | 0–4 |
| Baseline factor XI clotting activity — %∥ | ||||||||
| Median | 109 | 107 | 104 | 110 | 107 | 100 | 102 | 114 |
| Interquartile range | 94–127 | 91–119 | 93–119 | 95–124 | 89–116 | 87–117 | 87–113 | 100–131 |
| Baseline activated partial-thromboplastin time — sec** | ||||||||
| Median | 26 | 27 | 26 | 26 | 26 | 26 | 26 | 26 |
| Interquartile range | 24–28 | 25–29 | 25–28 | 24–28 | 25–28 | 25–29 | 25–28 | 25–28 |
| Safety population | ||||||||
| No. of patients | 148 | 148 | 149 | 148 | 33 | 150 | 147 | 296 |
| Time after surgery to milvexian or enoxaparin administration — hr | ||||||||
| Median | 22 | 22 | 22 | 22 | 22 | 22 | 23 | 22 |
| Range | 13–26 | 13–26 | 13–25 | 12–26 | 13–25 | 13–26 | 14–26 | 7–34 |
| Duration of milvexian or enoxaparin treatment — days | ||||||||
| Median | 12 | 12 | 12 | 12 | 12 | 13 | 12 | 12 |
| Range | 1–16 | 2–16 | 4–17 | 4–16 | 9–14 | 6–16 | 1–16 | 1–15 |
The modified intention-to-treat population included all the patients who had received at least one dose of a trial medication and had a venogram within the prespecified time window that could be evaluated, a documented symptomatic venous thromboembolic event, or a fatal event. The safety population included all the patients who had received at least one dose of a trial medication. Percentages may not total 100 because of rounding.
Enoxaparin was administered subcutaneously at a dose of 40 mg once daily.
Race was reported by the patient. Three patients in the group receiving 50 mg of milvexian once daily and two in the enoxaparin group chose not to report information on race.
Creatinine clearance values were calculated with the use of the Cockcroft and Gault equation.
Patients may have undergone more than one type of anesthesia.
The normal range for factor XI clotting activity is 60 to 150%.
The normal range for the activated partial-thromboplastin time is 22 to 29 seconds.
EFFICACY
Venograms that could be evaluated were obtained in 1047 of 1219 patients (86%) who received a trial medication (Fig. 1); 127 of 923 patients (14%) in the milvexian groups and 45 of 296 patients (15%) in the enoxaparin group did not undergo venography, had venograms that could not be evaluated, or had venograms outside the prespecified time window.
Efficacy outcomes are provided in Table 2. Among the patients receiving milvexian twice daily, venous thromboembolism (primary efficacy outcome) developed in 27 of 129 (21%) taking 25 mg, in 14 of 124 (11%) taking 50 mg, in 12 of 134 (9%) taking 100 mg, and in 10 of 131 (8%) taking 200 mg (total daily milvexian doses of 50 mg, 100 mg, 200 mg, and 400 mg, respectively). These findings are consistent with a significant dose–response relationship (one-sided P<0.001). Venous thromboembolism developed in 63 of 518 patients (12%) given twice-daily milvexian, an incidence significantly lower than the prespecified benchmark of 30% (one-sided P<0.001). Thus, both proof-of-efficacy criteria were met.
Table 2.
Efficacy Outcomes.*
| Outcome | Milvexian Twice Daily | Milvexian Once Daily | Enoxaparin (N = 252) | |||||
|---|---|---|---|---|---|---|---|---|
| 25 mg (N = 129) | 50 mg (N = 124) | 100 mg (N = 134) | 200 mg (N = 131) | 25 mg (N = 28) | 50 mg (N = 127) | 200 mg (N = 123) | ||
| Primary efficacy outcome: venous thromboembolism † | ||||||||
| Any event — no. (%) | 27 (21) | 14 (11) | 12 (9) | 10 (8) | 7 (25) | 30 (24) | 8 (7) | 54 (21) |
| Relative risk vs. enoxaparin (95% CI) | 0.97 (0.65–1.45) | 0.53 (0.31–0.90) | 0.42 (0.23–0.76) | 0.37 (0.19–0.69) | 1.00 (0.51–1.97) | 1.15 (0.78–1.70) | 0.30 (0.15–0.62) | — |
| Components of the primary efficacy outcome — no.‡ | ||||||||
| Death from any cause | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Nonfatal pulmonary embolism | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Symptomatic distal deep-vein thrombosis | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| Asymptomatic proximal deep-vein thrombosis | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 2 |
| Asymptomatic distal deep-vein thrombosis | 26 | 13 | 9 | 10 | 7 | 26 | 8 | 50 |
| Extent of deep-vein thrombosis on venography — no. | ||||||||
| Confluent distal into proximal | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 1 |
| Isolated proximal | ||||||||
| Large: ≥10 cm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Small: <10 cm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Isolated distal | ||||||||
| Extensive: ≥2 veins | 9 | 5 | 1 | 2 | 5 | 9 | 1 | 20 |
| Limited: <2 veins | 17 | 8 | 9 | 8 | 2 | 18 | 7 | 30 |
Efficacy outcomes were assessed in the modified intention-to-treat population, which included all the patients who had received at least one dose of a trial medication and had a venogram within the prespecified time window that could be evaluated, a documented symptomatic venous thromboembolic event, or a fatal event. CI denotes confidence interval.
Venous thromboembolism was a composite of asymptomatic deep-vein thrombosis (detected by mandatory unilateral venography performed 10 to 14 days after surgery), confirmed symptomatic venous thromboembolism (symptomatic deep-vein thrombosis of the leg or nonfatal pulmonary embolism), or death from any cause.
There were no cases of symptomatic proximal deep-vein thrombosis.
Among the patients receiving milvexian once daily, venous thromboembolism developed in 7 of 28 (25%) taking 25 mg, in 30 of 127 (24%) taking 50 mg, and in 8 of 123 (7%) taking 200 mg; these findings are also consistent with a significant dose–response relationship (one-sided P<0.001). In the enoxaparin group, venous thromboembolism developed in 54 of 252 patients (21%). The per-protocol analysis yielded similar efficacy results (Table S1 in the Supplementary Appendix).
One patient in the enoxaparin group died by suicide 1 day after randomization; no deaths from pulmonary embolism occurred. Three patients had symptomatic nonfatal pulmonary embolism (one in the enoxaparin group and two in the milvexian groups). The extent of thrombosis with milvexian and enoxaparin is shown in Table 2.
BLEEDING
The bleeding outcomes are provided in Table 3. The principal safety outcome of bleeding of any severity occurred in 38 of 923 patients (4%) given milvexian and in 12 of 296 patients (4%) given enoxaparin. Most bleeding episodes were in the minimal category and involved the surgical site. No major bleeding episodes were seen with milvexian, and one occurred with enoxaparin. The incidence of clinically relevant bleeding (the composite of major bleeding and clinically relevant nonmajor bleeding) was 1% with milvexian and 2% with enoxaparin.
Table 3.
Safety Outcomes.*
| Outcome | Milvexian Twice Daily | Milvexian Once Daily | Enoxaparin (N = 296) | |||||
|---|---|---|---|---|---|---|---|---|
| 25 mg (N = 148) | 50 mg (N = 148) | 100 mg (N = 149) | 200 mg (N = 148) | 25 mg (N = 33) | 50 mg (N = 150) | 200 mg (N = 147) | ||
| Any bleeding — no. (%) | 2 (1) | 7 (5) | 7 (5) | 5 (3) | 0 | 8 (5) | 9 (6) | 12 (4) |
| Relative risk vs. enoxaparin (95% CI) | 0.33 (0.08–1.43) | 1.15 (0.47–2.82) | 1.14 (0.47–2.80) | 0.81 (0.29–2.24) | 0 (NA) | 1.17 (0.50–2.72) | 1.51 (0.66–3.43) | — |
| Major bleeding or clinically relevant nonmajor bleeding — no. (%) | 0 | 2 (1) | 1 (1) | 1 (1) | 0 | 2 (1) | 1 (1) | 5 (2) |
| Relative risk vs. enoxaparin (95% CI) | 0 (NA) | 0.79 (0.16–3.96) | 0.39 (0.05–3.30) | 0.39 (0.05–3.28) | 0 (NA) | 0.68 (0.14–3.39) | 0.40 (0.05–3.34) | — |
| Major bleeding — no. (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1)† |
| Clinically relevant nonmajor bleeding — no. (%) | 0 | 2 (1) | 1 (1) | 1 (1) | 0 | 2 (1) | 1 (1) | 4 (1) |
| Serious adverse event — no. (%) | 5 (3) | 5 (3) | 5 (3) | 2 (1) | 1 (3) | 2 (1) | 2 (1) | 11 (4) |
| At least one adverse event — no. (%) | 56 (38) | 67 (45) | 51 (34) | 54 (36) | 7 (21) | 58 (39) | 65 (44) | 113 (38) |
| Adverse event leading to discontinuation of treatment — no. (%) | 2 (1) | 7 (5) | 2 (1) | 4 (3) | 0 | 4 (3) | 6 (4) | 8 (3) |
Safety outcomes were assessed in the safety population, which included all the patients who had received at least one dose of a trial medication; the time for analysis included the period during which a trial medication was administered plus 2 days. NA denotes not applicable.
The patient had a spontaneous subdural hematoma with decreased level of consciousness.
OTHER SAFETY OUTCOMES
Adverse events with treatment were reported in 358 of 923 patients (39%) given milvexian and in 113 of 296 patients (38%) given enoxaparin, whereas serious adverse events were reported in 2% and 4%, respectively (Table 3 and Tables S2 and S3). Median hemoglobin levels after surgery were similar with milvexian and enoxaparin (Fig. S1).
PHARMACODYNAMIC AND PHARMACOKINETIC MEASURES
Milvexian increased the activated partial-thromboplastin time ratio in a dose-dependent manner, whereas enoxaparin had no apparent effect; no evidence of a dose-dependent increase in bleeding was noted with milvexian (Fig. 2). Neither milvexian nor enoxaparin increased the prothrombin time ratio (Fig. S2).
Figure 2. Activated Partial-Thromboplastin Time (aPTT) Ratios and Bleeding Incidences.
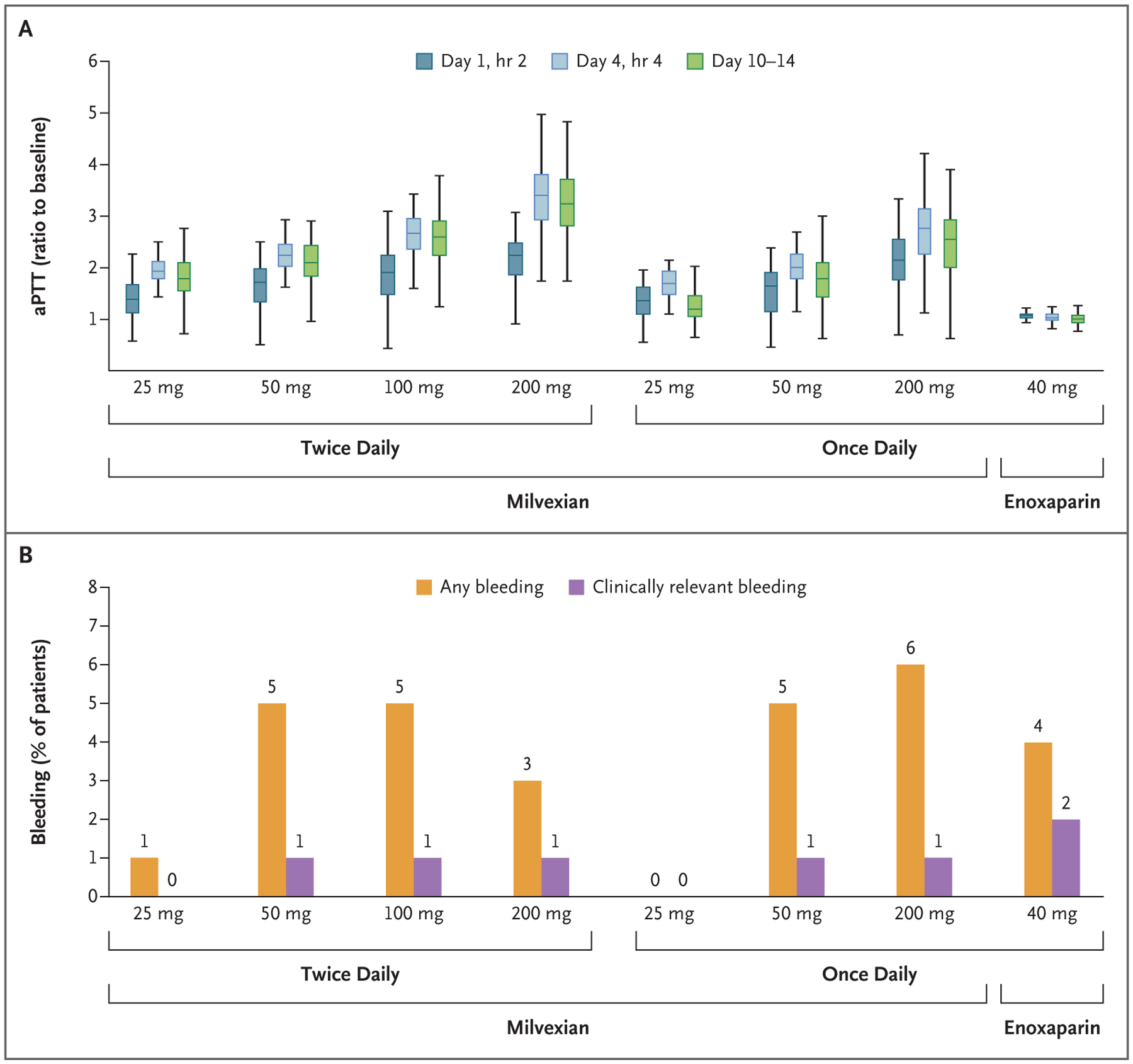
Panel A shows box plots of aPTT ratios with the various doses of milvexian and with enoxaparin. The median is indicated by the horizontal line in the box; the top and bottom of the box indicate the upper and lower limits, respectively, of the interquartile range; and the vertical lines above and below the box indicate the maximum and minimum values, respectively. Panel B shows the incidences of any bleeding and clinically relevant bleeding (defined as the composite of major bleeding and clinically relevant nonmajor bleeding) according to trial group.
DISCUSSION
This trial provides proof of principle that milvexian is an effective antithrombotic agent. Milvexian significantly reduced the incidence of venous thromboembolism after elective knee arthroplasty in a dose-dependent manner with both twice-daily and once-daily regimens. Furthermore, the incidence of venous thromboembolism was significantly lower with daily milvexian doses of 100 mg or more than that with enoxaparin. Although the incidence of any bleeding was 4% with both milvexian and enoxaparin, the incidence of the composite of major bleeding and clinically relevant nonmajor bleeding was low across total daily doses of milvexian that ranged from 25 to 400 mg. Therefore, postoperative factor XIa inhibition with milvexian was effective for preventing venous thromboembolism and was associated with a low risk of clinically relevant bleeding.
The lower incidence of venous thromboembolism and the tendency for less extensive thrombosis with higher doses of milvexian than with enoxaparin highlight the role of factor XI in the pathogenesis of venous thrombosis after surgery. This concept is supported by the observation that an antisense oligonucleotide that reduces factor XI levels and abelacimab, an antibody that binds factor XI and prevents its activation, also are more effective than enoxaparin for thromboprophylaxis after knee arthroplasty.7,8 However, the antisense oligonucleotide and abelacimab require parenteral administration, whereas milvexian is given orally.
The findings with milvexian contrast with those with osocimab, an antibody that inhibits factor XIa. When administered as a single intravenous injection 12 to 24 hours after surgery, osocimab was noninferior but not superior to enoxaparin for the prevention of venous thromboembolism after knee arthroplasty.13 These divergent results may reflect differences in the extent of factor XIa inhibition. Alternatively, as a small molecule, milvexian may gain better access to factor XIa generated at sites of thrombus formation. Regardless of mechanism, the current data indicate that postoperative factor XIa inhibition with milvexian provides effective thromboprophylaxis against venous thromboembolism. Additional studies are needed to determine the efficacy of milvexian for the prevention of arterial thrombosis.
Milvexian treatment increased the activated partial-thromboplastin time ratio, a finding consistent with selective inhibition of factor XIa. Despite the dose-dependent increase in the activated partial-thromboplastin time ratio, no dose–response relationship was seen with respect to bleeding, a finding consistent with the poor correlation between the extent of prolongation of the activated partial-thromboplastin time and the propensity for bleeding in patients with congenital factor XI deficiency.4 This dissociation probably reflects the fact that factor XI activation is essential for clot formation in the activated partial-thromboplastin time assay but is mostly dispensable for hemostasis.3
Some methodologic aspects of our trial require comment. First, the strength of our conclusion regarding the low incidence of clinically relevant bleeding with milvexian is limited by the modest sample. Further studies are needed to confirm the safety of milvexian alone or in combination with antiplatelet drugs. Second, the trial was open label with respect to assignment to milvexian or enoxaparin. However, to minimize bias, the trial was blinded with respect to assignment to a milvexian regimen, and all outcomes were adjudicated by a committee whose members were unaware of treatment assignments. Third, the inclusion of minimal bleeding in the principal safety outcome ensured that all suspected bleeding events were documented and centrally adjudicated. With the open-label design, however, a potential for bias in favor of enoxaparin existed because investigators might be more vigilant for bleeding with an experimental agent than with an agent that has been used for years. Because of stricter definitions, the potential for bias was lessened in the assessment of clinically relevant bleeding, the outcome most often used for safety assessment in this patient population.7,8,13
In our trial, oral milvexian reduced the risk of postoperative thromboembolism among patients undergoing knee arthroplasty in a dose-dependent manner without increasing the risk of bleeding as compared with enoxaparin. Further studies are needed to determine whether oral anticoagulants that target factor XIa can dissociate thrombosis from hemostasis.
Supplementary Material
Acknowledgments
Supported by Bristol Myers Squibb and Janssen Research and Development.
Footnotes
REFERENCES
- 1.Steinberg BA, Gao H, Shrader P, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J 2017; 194: 132–40. [DOI] [PubMed] [Google Scholar]
- 2.Sanghai S, Wong C, Wang Z, et al. Rates of potentially inappropriate dosing of direct-acting oral anticoagulants and associations with geriatric conditions among older patients with atrial fibrillation: the SAGE-AF study. J Am Heart Assoc 2020; 9(6): e014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu C, Hutt E, Bloomfield DM, Gailani D, Weitz JI. Factor XI inhibition to uncouple thrombosis from hemostasis: JACC review topic of the week. J Am Coll Cardiol 2021; 78: 625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duga S, Salomon O. Congenital factor XI deficiency: an update. Semin Thromb Hemost 2013; 39: 621–31. [DOI] [PubMed] [Google Scholar]
- 5.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost 2011; 105: 269–73. [DOI] [PubMed] [Google Scholar]
- 6.Preis M, Hirsch J, Kotler A, et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood 2017; 129: 1210–5. [DOI] [PubMed] [Google Scholar]
- 7.Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 2015; 372: 232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhamme P, Yi BA, Segers A, et al. Abelacimab for prevention of venous thromboembolism. N Engl J Med 2021; 385: 609–17. [DOI] [PubMed] [Google Scholar]
- 9.Perera V, Abelian G, Li D, et al. Single-dose pharmacokinetics of BMS-986177/JNJ-70033093 in participants with mild or moderate hepatic impairment compared to healthy participants. Eur Heart J 2020; 41: Suppl 2 (https://academic.oup.com/eurheartj/article/41/Supplement_2/ehaa946.3371/6005220). [Google Scholar]
- 10.Fuji T, Fujita S, Tachibana S, Kawai Y. A dose-ranging study evaluating the oral factor Xa inhibitor edoxaban for the prevention of venous thromboembolism in patients undergoing total knee arthroplasty. J Thromb Haemost 2010; 8: 2458–68. [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010; 8: 202–4. [DOI] [PubMed] [Google Scholar]
- 12.Pinheiro J, Bornkamp B, Glimm E, Bretz F. Model-based dose finding under model uncertainty using general parametric models. Stat Med 2014; 33: 1646–61. [DOI] [PubMed] [Google Scholar]
- 13.Weitz JI, Bauersachs R, Becker B, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA 2020; 323: 130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


