Abstract
Aim
The aim of the study was to measure serum levels of molecular markers of inflammation in patients undergoing non‐surgical root canal retreatment (Re‐RCT) and periapical surgery (PS) for the treatment of apical periodontitis and to establish if such levels are influenced by the size of apical radiolucencies at baseline and by the treatment outcome.
Methodology
A total of 115 participants were recruited (n = 50 Controls, n = 35 Re‐RCT, n = 30 PS). Preoperative periapical radiographs and cone beam CT (CBCT) scans of teeth were taken. Blood was collected from treatment groups at baseline, 3‐, 6‐, and 12‐month post‐treatment and from controls at baseline and 12 months. Serum levels of IL‐1β, IL‐6, IL‐8, TNF‐α, Pentraxin 3, ICAM‐1, VCAM‐1, hs‐CRP, FGF‐23, MMP‐2, MMP‐8, MMP‐9, C3 and ADMA were analysed using multiplex immunoassay and enzyme‐linked immunosorbent assay. Different time points within the same group were compared using Wilcoxon signed‐rank test, and differences between groups were analysed using the Mann–Whitney test. Non‐linear association between different factors was assessed using Spearman's correlation.
Results
Preoperative serum levels of FGF‐23, IL‐1β, hs‐CRP and ADMA were significantly higher in the diseased groups compared with controls (p < .001; p = .008; p < .001; p = .013, respectively). The preoperative size of the radiolucency was associated with increased levels of FGF‐23, IL‐1β and IL‐6. At 3‐months following treatment, IL‐1β, IL‐8, hs‐CRP, C3, MMP‐2 and MMP‐9 levels increased compared with baseline in treatment groups. IL‐1β and IL‐8 further increased at 6 months, whereas FGF‐23, hs‐CRP, C3, MMP2 and MMP‐9 decreased. One‐year post‐treatment, FGF‐23, pentraxin‐3 and ADMA were significantly reduced below baseline levels. At the 1‐year review, CBCT revealed that 25.9% of treated cases completely healed, while 63% were healing, and 11.1% failed. Treatment outcome was found to be influenced by preoperative levels of ADMA and IL‐8 levels at 6 months.
Conclusions
Both symptomatic and asymptomatic apical periodontitis (AP) can contribute to increased levels of molecular markers of inflammation. A further transient inflammatory markers rise after root canal retreatment and apical surgery were demonstrated. Successful endodontic treatment and periapical surgery result in a long‐term reduction in inflammatory marker levels.
Keywords: apical periodontitis, cardiovascular disease, inflammatory markers, multiplex luminex immunoassay, non‐surgical root canal retreatment, periapical surgery
INTRODUCTION
Apical periodontitis (AP) is a state of inflammation affecting the periapical area of teeth with a global prevalence of 52% at individual level and 5% at tooth level (Tiburcio‐Machado et al. (2020). It can contribute to a persistent low‐grade systemic inflammation which might impact patient's systemic health (Georgiou et al., 2019).
Several studies have attempted with contradicting results, to associate AP with systemic conditions such as diabetes and cardiovascular disease (CVD) (Berlin‐Broner et al., 2017; Jimenez‐Sanchez et al., 2020; Perez‐Losada et al., 2020). CVDs are the leading cause of death worldwide (WHO, 2011) and comprises a number of conditions including cerebrovascular disease, coronary heart diseases, congenital heart disease, peripheral arterial diseases, deep vein thrombosis and pulmonary embolism (World Health Organization, 2017). Most CVDs are caused by the development of endothelial dysfunction followed by the formation of atherosclerotic plaques comprising of lipids and connective tissue along with inflammatory, endothelial and smooth muscle cells, affecting the intima, media and adventitia layers of large‐ and medium‐calibre arteries (Milutinović et al., 2020; Wolf & Ley, 2019).
Elevated levels of inflammatory bioactive molecules following dental surgical treatments can induce a systemic inflammatory response leading to a transient increased risk of cardiovascular events (Chen et al., 2018; Graziani et al., 2017; Minassian et al., 2010)Further, signs of endothelial dysfunction were detected in otherwise healthy young adults with apical periodontitis (Bergandi et al., 2019; Cotti et al., 2011) and a reduction in inflammation and endothelial dysfunction was demonstrated after root canal treatments (Bergandi et al., 2019; Poornima et al., 2021). In a recent literature review a weak association was found between CVDs and AP (Jakovljevic et al., 2020). The authors concluded that further, well‐designed, longitudinal clinical studies with long‐term follow‐ups were required to establish the impact of AP on systemic health.
Therefore, the aim of this longitudinal cohort study was to measure the levels of inflammatory markers (IL‐1β, IL‐6, IL‐8, tumour necrosis factor [TNF]‐α, high sensitive [hs]‐CRP, E‐selectin, pentraxin‐3, ADMA, vascular cell adhesion molecule [VCAM]‐1, intercellular adhesion molecule [ICAM]‐1, C3, matrix metalloprotease [MMP]‐2, MMP‐8, MMP‐9) in patients with AP undergoing non‐surgical root canal re‐treatment (Re‐RCT) and periapical surgery (PS) at baseline and 3, 6, 12 months post‐treatment. These findings were correlated with clinical signs and symptoms, size of the lesion and outcome of treatment using both periapical radiographs and cone beam computed tomography images (CBCT).
MATERIALS AND METHODS
Sample size calculation
The sample size was calculated based on ADMA levels (Cotti et al., 2015). Assuming a mean value of 0.65 (SD 0.1) in controls and of 0.74 (SD 0.15) in the treatment groups with 80% power at 5% level of significance, the required sample size was found to be 19 patients per group.
Patient recruitment
Patients undergoing Re‐RCT and PS referred to the post‐graduate Endodontic consultation clinic at Guy's Dental Institute were approached for participating in the research. The participants were divided into three groups (Re‐RCT, PS and controls) according to the inclusion and exclusion criteria summarized in Table 1. The control group included healthy participants without any chronic inflammatory condition, no history of any surgery in the past 6 months, no antibiotics taken 3 months before sample collection, healthy periodontium and no teeth with AP or history of root canal treatment.
TABLE 1.
Inclusion and exclusion criteria
| Inclusion | Exclusion |
|---|---|
| >18 years old | Smokers |
| Pregnant women | |
| Teeth with periodontal pockets>4 mm/endodontic—periodontal lesion | |
| Root canal retreatment cases | Patients with chronic inflammatory condition (Asthma, Inflammatory bowel diseases, Irritable bowel syndrome, Chronic peptic ulcer, Rheumatoid arthritis, Ulcerative colitis, Liver diseases, Crohn's disease, Sinusitis, Active hepatitis, Autoimmune diseases, Tuberculosis, Renal diseases, or Cancer). |
| Teeth requiring periapical surgery | Patients on medication altering bone metabolism |
| Unrestorable teeth | |
| Antibiotics in last 3 months | |
| Surgical procedure in last 6 months |
Clinical examination
At the consultation appointment, patient's presenting complaint along with detailed medical, dental and social histories were recorded. Extra‐ and intra‐oral examinations were carried out including basic periodontal examination (BPE). The periapical health was investigated by palpation, percussion, sensibility testing along with periapical radiographs using a beam aiming device x‐ray unit operating at 65 kV and 7 mAs (Heliodent; Sirona) and a photostimulable phosphor plates (Digora Optime, Soredex). Small volume (40 mm3) CBCT scans were also taken (3D Accuitomo F170; J Morita Manufacturing) with exposure parameters set at 90 kV and 5.0 mAs of the tooth in question. Participants' height, weight and waist circumference were recorded. Patients were allowed to rest for 15 min before measuring the blood pressure using an upper arm blood pressure monitor (A&D Medical), two readings were taken and an average was recorded (Chobanian et al., 2003).
At the treatment appointment, patients were asked to have an overnight fast and HbA1C [A1cNow + (BHR Pharmaceuticals Ltd.); total cholesterol (TC), high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), triglycerides (TRG) and TC/HDL levels were measured using CardioChek PA blood analyser (BHR Pharmaceuticals Ltd.) with testing strips (CHO + HDL strips, BHR Pharmaceuticals Ltd.).
For healthy controls, medical and dental histories were recorded along with full mouth examination, BPE, full mouth panoramic radiograph, height, weight, waist circumference, blood pressure and levels of HbA1C, TC, triglycerides, HDL, LDL and TC/HDL.
Root canal retreatment procedure
Blood samples were collected using a safety blood collection set (Greiner) in a BD Vacutainer SST II Advance 8.5 ml tube (Becton Dickinson). All samples were transported on ice in a cool bag to the lab and were processed to obtain aliquots of the serum, which were stored at −80°C for further analysis.
All treatments were performed in a single visit, under local anaesthesia and using a dental operating microscope (3‐step‐entree; Global). Isolation of the tooth to be treated was undertaken using a rubber dam and light‐cured gingival barrier (OpalDam; Ultradent). After caries removal, the tooth was built up with a direct composite restoration (SDR flow+; Dentsply Sirona) to facilitate rubber dam isolation.
Coronal access was achieved followed by removal of root canal filling using Gates Glidden burs, Headstrom files (H‐files), Flexofiles (Dentsply Sirona) and rotary instruments. Determination of working length was achieved using an electronic apex locator (Root‐ZX; J. Morita Corp.) and confirmed with a digital periapical radiograph. A crown‐down approach using rotary instruments (ProTaper® Gold; Dentsply Sirona) at 300 RPM and a maximum torque of 4 N to a size F2 master apical size was used for root canal mechanical preparation, whenever possible. The canals were disinfected with 2.5% NaOCl throughout the treatment, delivered using a side‐vented 27‐gauge needle. Final irrigation with 2.5% NaOCl agitated using the Endoactivator system (Dentsply Sirona), for 30 s followed by sterile saline solution, and 17% EDTA (Pulpdent). After a final rinse with saline, the root canals were dried with sterile paper points (Dentsply Sirona). The master Gutta‐percha points were disinfected for 3 min in 2.5% NaOCl before canal filling. The canals were filled with Gutta‐percha (Dentsply Sirona) and resin‐based root canal sealer (AH Plus; Dentsply Sirona) using a warm compaction technique with endodontic heat source unit (System B; EIE‐Analytic Technology) and obturation gun (B&L BioTech).
Access cavities were restored with flowable hybrid composite (CORECEM™; RTD). A final radiograph was taken after placement of the restoration. Teeth were then referred to the patient's general dentist for a cuspal coverage if indicated.
Periapical surgery treatment
Blood samples were taken preoperatively as described for Re‐RCT group. A Surgical flap was raised to expose the tooth and the periapical lesion. The periapical micro‐surgery was carried out with the aid of dental operating microscope (3 step entree; Global). After carrying out osteotomy, excavation of the lesion and root end resection; a 3 mm retro‐preparation was undertaken using a diamond‐coated ultrasonic tip (SATELEC®, Acteon). The prepared canal was filled using MTA (Dentsply Sirona), and the incision was sutured using 5–0 Ethilon sutures (Ethicon) which were removed 1 week after treatment.
Control group samples
From the control group blood samples were collected using the same protocols described above at baseline and 1 year.
Review appointments
Patients were reviewed at 3‐month (3 M), 6‐month (6 M) and 1‐year (1 Yr) post‐treatment. Detailed medical history including COVID‐19 status and vaccination status were taken. Dental history and clinical examination were recorded. Blood samples were taken in each review appointment for biomarker analysis.
Measurements of HbA1C (A1cNow + [BHR Pharmaceuticals]; TC, HDL, LDL, TRG and TC/HDL using CardioChek PA blood analyser and CHO + HDL strips [BHR Pharmaceuticals Ltd.]) were undertaken at the 1 year review appointment. Periapical healing was assessed at 1 year using long cone periapical radiographs along and where possible CBCT scan.
Blood samples processing
The blood samples collected in the BD Vacutainer SST II Advance 8.5 ml tube (Becton Dickinson) were centrifuged for 10 min at a speed of 2000 g and a temperature of 4°C using Eppendorf centrifuge 5810R (Eppendorf) to separate the serum from the blood. The serum was also stored in a −80°C freezer in an aliquot of 0.5 ml in a 1.5 microcentrifuge tube (Fisher Scientific). The serum was analysed using the Magnetic Assay human premixed multi‐analyte kit (R&D systems, Bio‐techne) according to manufactures instructions for identification of inflammatory mediators levels (FGF‐23, IL‐1β, IL‐6, IL‐8, hs‐CRP, pentraxin 3, TNF‐α, MMP2, MMP8, MMP9, E‐selectin, VCAM‐1 and ICAM‐1) using the Bio‐Rad Bio‐Plex 200 analysers (Bio‐rad). Levels of C3 and ADMA were detected using enzyme‐linked immunosorbent assay (ELISA) and iMark microplate absorbance reader (Bio‐Rad).
Radiographic outcome analysis
Periapical radiographs and CBCT scans were assessed by two experienced endodontic specialists. The outcome scores were recorded after consensus agreement between both examiners using a six‐point classification (Patel et al., 2012).
Statistical analysis
Statistical analyses were undertaken using IBM® SPSS® (Version 15.0). Normality was tested using histogram, Kolmogorov–Smirnov and Shapiro–Wilk's tests. As the data were not normally distributed, Wilcoxon signed‐rank test was used to compare the difference in distributions between different time points within the same group. For multiple comparison, p‐values were corrected by Bonferroni's criteria. Moreover, Mann–Whitney test was used to identify the significant difference between two groups. Level of significance was set to p < .05 and 95% confidence interval was generated using one sample t‐test.
Due to the lack of normality in biomarker levels, the values were transformed to a logarithmic scale and the difference between Median of the Control and the Disease groups was calculated using two‐sample t‐test. Linear associations with other continuous variables were tested using Pearson's correlations and for other non‐linear associations Spearman's correlation was used. Furthermore, regression models were conducted to estimate beta coefficients involved in those significant correlations. Multiple regression models were also conducted to control the potential confounding effect of some variables at T0 (HbA1c, Triglycerides, HDL, BMI, waist circumference, age and gender) on the primary relationships. Level of significance was set to p < .05.
Inter‐examiner reliability for both PA and CBCT was determined by the linearly weighted Kappa's index to assess the concordance.
RESULTS
One hundred and fifteen participants were recruited for the study, including 65 AP patients and 50 controls. Out of 65 AP patients (24 males, 41 females; mean age: 43.3 [24–75]), 45 patients had symptoms while 20 patients were asymptomatic. Overall, 35 patients had root canal retreatment and 30 patients had periapical surgery. The control group consisted of 50 participants (18 males, 32 females; mean age: 32.4 [19–51]).
The control group patients had a smaller waist circumference and BMI than AP patients at baseline. Median levels of HbA1C, TC, LDL, TRG, TC/HDL, WC and BMI were higher in the treatment group as compared to the control group with statistically significant differences in levels of HbA1C (p = .0005), TRG (p = .0035), LDL (p = .0096), WC (p = .033) and BMI (p = .031) (Table 2, Figure 1).
TABLE 2.
Descriptive data of control and treatment groups
| Controls | Pre‐treatment | 1‐year post‐treatment |
p‐Value Control vs. pre‐treatment |
|
|---|---|---|---|---|
| Gender |
Male n = 18 Female n = 32 |
Male n = 24 Female n = 41 |
||
| Age | 32.4 (19–51) | 43.3 (24–75) | ||
| Height (cm) | 166.50 ± 9.04 | 169.60 ± 9.70 | ||
| Weight (kg) | 67.73 ± 11.00 | 75.36 ± 14.99 | ||
| BMI (kg/m2) | 24.39 ± 3.23 | 26.14 ± 4.40 | p = .031 | |
| Waist circumference (cm) | 79.14 ± 10.19 | 82.5 ± 11.10 | p = .178 | |
| Systolic blood pressure (mmHg) | 120 (110–135) | 121 (100–159) | ||
| Diastolic blood pressure (mmHg) | 75 (61–87) | 78 (54–115) | ||
| HbA1C (%) | 4.30 (4.0–7.4) | 4.9 (4.30–6.10) | 4.95 (4.0–7.70) | p = .0005 |
| Total cholesterol (mmol/L) | 5.32 (2.62–8.39) | 5.95 (2.59–10.21) | 5.28 (2.59–8.88) | p = .054 |
| HDL (mmol/L) | 1.91 (0.98–2.99) | 1.93 (1.02–3.11) | 1.55 (1.03–3.11) | p = .934 |
| LDL (mmol/L) | 2.74 (1.0–5.07) | 3.47 (0.77–6.35) | 2.82 (0.70–6.18) | p = .0096 |
| Triglycerides (mmol/L) | 0.84 (0.57–5.65) | 1.04 (0.57–2.56) | 1.29 (0.57–5.65) | p = .0035 |
| TC/HDL (mmol/L) | 2.65 (1.50–4.90) | 3.0 (1.66–5.30) | 3.45 (1.64–5.90) | |
Note: The values are given as Mean ± Standard Deviation; Median (Minimum–Maximum).
FIGURE 1.
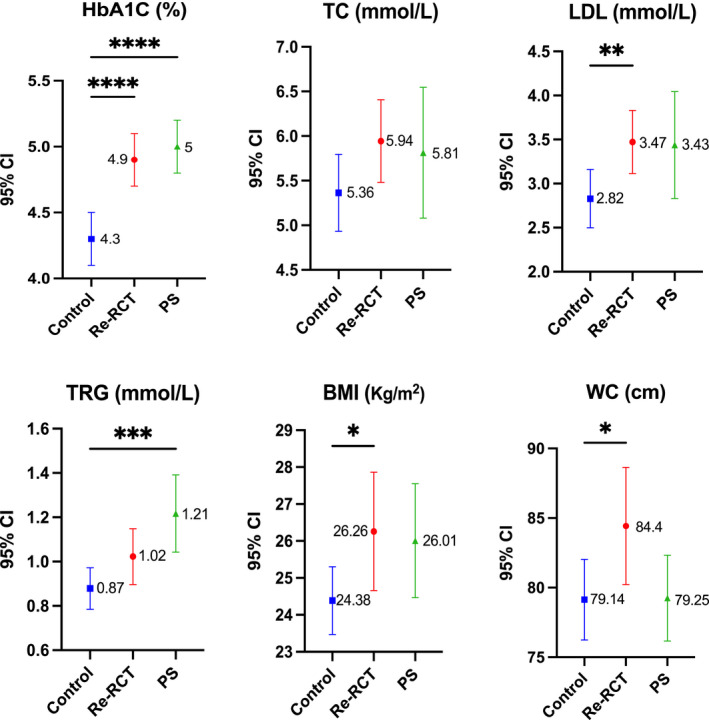
Mean and 95% confidence interval values for HbA1c, TC, LDL, TRG, WC and BMI as measured at pre‐treatment time point in control, non‐surgical root canal re‐treatment (Re‐RCT) and periapical surgery (PS); *(p < .05); **(p < .01); ***(p < .001), ****(p < .0001). LDL, low‐density lipoprotein; TC, total cholesterol; TRG, triglycerides.
Treatment outcome
Out of 65 patients, 40 patients were reviewed at 3 months. This number decreased to 37 at 6 months. However, 1‐year post‐treatment 50 patients were recalled. Based on PA radiographs, 38% (n = 19) of the cases were completely healed, 54% (n = 27) were healing, and 8% (n = 4) failed at 1 year. Based on CBCT, 25.9% (n = 7) had completely healed, while 63% (n = 17) were still in the healing phase, and 11.1% (n = 3) failed.
Linear Kappa (κ) index of inter‐examiner reliability of the outcome revealed a substantial agreement between readings of examiners for both PA and CBCT (0.84 [0.69–0.98], 0.76 [90.57–0.95]), respectively.
Inflammatory markers
Preoperative levels
Preoperative levels of FGF‐23, IL‐1β, hs‐CRP and ADMA were significantly higher (p < .001; p = .008; p < .001; p = .013) in the diseased group than in controls (Table 3). However, when splitting the diseased groups, FGF‐23 (p < .001) and hs‐CRP (p < .001) were significantly higher in Re‐RCT group than in controls, while FGF‐23 (p < .001), IL‐1β (p = .014) and hs‐CRP (p < .001) were significantly higher in PS group than in controls, while ADMA (p = .016) and C3 (p = .035) were significantly lower in PS group.
TABLE 3.
Comparison between levels of inflammatory markers in control and diseased groups at baseline
| Control | Diseased | p‐Value | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (Min–Max) | Mean (SD) | Median (Min–Max) | ||
| FGF‐23 | 30.35 ± 28.3 |
22.18 (12.32–165.71) |
172.17 ± 542.78 |
51.52 (21.57–3675.88) |
<.001*** |
| IL‐1β | 11.19 ± 5.52 |
10.17 (1.8–23.88) |
17.53 ± 14.32 |
12.82 (1.47–87.87) |
.008** |
| IL‐8 | 14.36 ± 13.25 |
9.6 (1.51–78.03) |
18.35 ± 24.21 |
9.63 (3.29–160.36) |
.763 |
| Pentraxin 3 | 2174.95 ± 1486.43 |
1540.72 (294.4–6271.35) |
1843.61 ± 1671.74 |
1306.78 (99.2–8838.34) |
.065 |
| TNF‐α | 12.44 ± 4.14 |
11.59 (5.25–23.69) |
18.37 ± 22.66 |
12.39 (3.54–163.01) |
.377 |
| ICAM‐1 | 374323.2 ± 315784.91 |
281437.59 (1539.82–1565849.39) |
408464.5 ± 283934.05 |
364848.32 (600.76–1288559.5) |
.253 |
| IL‐6 | 5.75 ± 2.4 |
5.49 (1.61–13.93) |
7.06 ± 7.93 |
5.41 (1.49–54.76) |
.870 |
| MMP‐8 | 3091.03 ± 2841.94 |
2294.95 (517.74–12419.27) |
2752.02 ± 2425.22 |
2141.28 (180.5–14879.36) |
.756 |
| E‐Selectin | 27079.82 ± 10500.63 |
26084.29 (7751.53–49362.5) |
26154.19 ± 13726.45 |
22124.63 (7508.67–60580.21) |
.343 |
| VCAM‐1 | 914641.64 ± 341820.08 |
862611.07 (461660.23–2 290 000) |
705054.43 ± 289750.99 |
646518.65 (131680.4–1 614 700) |
.001** |
| hs‐CRP | 31141.72 ± 29060.64 |
32509.37 (5228.6–212312.1) |
78287.56 ± 87056.44 |
53443.49 (1330.48–470330.34) |
<.001*** |
| MMP‐2 | 19 362 ± 8474.86 |
17398.56 (1919.27–38586.4) |
16724.39 ± 6487.86 |
14968.64 (7465.01–40088.94) |
.066 |
| MMP‐9 | 14167.84 ± 11145.15 |
9334.19 (2551.94–43993.9) |
16647.96 ± 11737.83 |
14642.85 (2529.1–51066.38) |
.208 |
| C3 | 1124.13 ± 599.46 |
869.72 (424.48–2679.57) |
909.51 ± 642.48 |
712.92 (13.38–2365.9) |
.067 |
| ADMA | 4541.08 ± 2024.78 |
4943.07 (536.95–8043.56) |
39284.02 ± 131064.5 |
6874.14 (104.19–606040.85) |
.013* |
Note: The values are given as Mean ± Standard Deviation; Median (Minimum–Maximum); p Value—Difference between Median of Control and Disease group using Mann–Whitney test; *(p < .05); **(p < .01); ***(p < .001), ****(p < .0001).
After adjusting the data by other CVD predictors/covariate including HbA1c, TRG, HDL, BMI, age and gender; the differences between apical periodontitis group and controls were still significant for FGF‐23, VCAM‐1 and hs‐CRP ([p = .026], [p < .001], [p = .043], respectively) but not for ADMA at baseline. (Tables S1–S4).
Age, gender and ethnicity
Males had significantly higher levels of IL‐1β (p = .02), TNF‐α (p = .011), ICAM‐1 (p = .013), IL‐6 (p = .007) and e‐selectin (p < .001) than females. Age and ethnicity had no effect on the levels of biomarkers at baseline.
Effect of size of the radiolucency on levels of Inflammatory markers at baseline
An increase by 1 mm of the size of the lesion was associated with an increase in serum levels of FGF‐23, IL‐1β and IL‐6 by 6.2% (p = .33), 6.7% (p = .005) and 5.2% (p = .005), respectively (Figure 2a–c).
FIGURE 2.
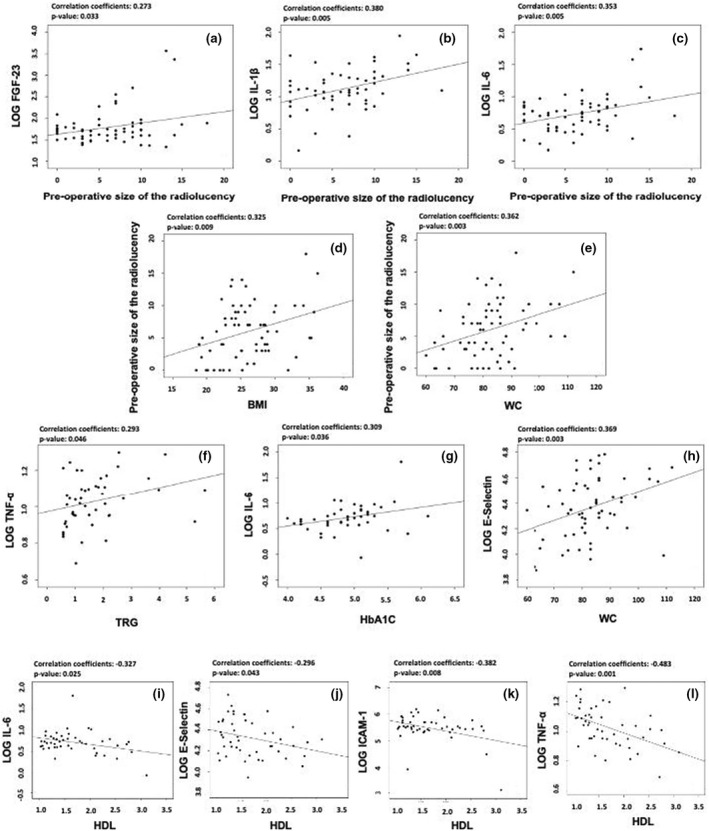
Correlation plots; (a, b, c) correlations between the preoperative size of the lesion with levels of FGF‐23, IL‐1β and IL‐6; (d, e) correlations between BMI and waist circumference with the preoperative size of the lesion; (f) correlation between 1 Yr triglycerides levels with levels of TNF‐α; (g) correlation between 1 Yr HbA1c levels with levels of IL‐6; (h) correlation between waist circumference with E‐selectin levels; (i, j, k, l) correlation between 1 Yr HDL levels with levels of IL‐6, E‐selectin, ICAM‐1 and TNF‐α.
Effect of BMI and waist circumference on the size of the radiolucency at baseline
An increase in BMI (kg/m2) and WC (cm) was associated with an increase in the size of the radiolucency by 0.31 mm (p = .009) and 0.14 mm (p = .003), respectively (Figure 2d,e).
3‐Month post‐treatment
At 3‐month (3 M) post‐treatment, in both the Re‐RCT and PS groups, levels of IL‐1β, IL‐8, hs‐CRP, C3, MMP‐2 and MMP‐9 increased when compared to levels before treatment at T0. In the Re‐RCT group, compared with levels at T0, MMP‐2, MMP‐9 and C3 were significantly increased at 3 M (p = .042; p = .03; p = .018 respectively), whereas in the PS group, hs‐CRP and C3 were significantly increased at 3 M (p = .007; p = .006, respectively) (Figure 3).
FIGURE 3.
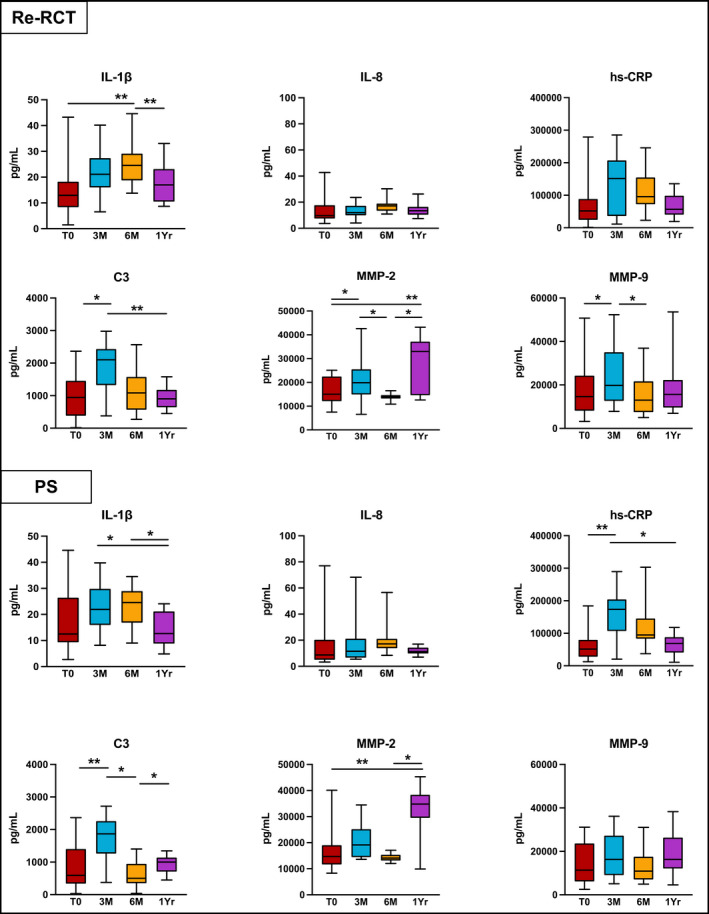
Inflammatory markers' levels that increased 3‐months after non‐surgical root canal retreatment (Re‐RCT) and periapical surgery (PS). p‐Value—difference between Medians was compared using Wilcoxon Signed‐Rank test; (T0) before treatment; (3 M) 3‐month review; (6 M) 6‐month review; (1 Yr) 1‐year review; *(p < .05); **(p < .01).
In the Re‐RCT group, TNF‐α was significantly decreased at 3 M (p = .03). PS patients also showed a significant reduction in their levels of TNF‐α (p = .043), Pentraxin‐3 (p = .012), VCAM‐1 (p = .019), and ADMA (p = .023) at 3 M (Figure 4).
FIGURE 4.
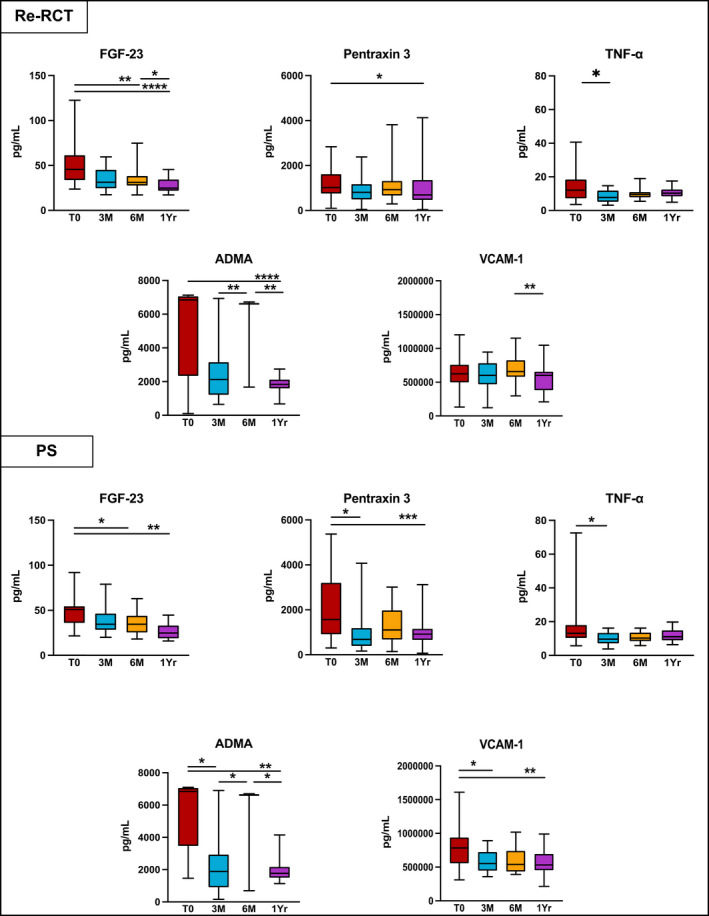
Inflammatory markers' levels which reduced after non‐surgical root canal retreatment (Re‐RCT) and periapical surgery (PS). p Value—difference between Medians was compared using Wilcoxon Signed‐Rank test; (T0) before treatment; (3 M) 3‐month review; (6 M) 6‐month review; (1 Yr) 1‐year review; *(p < .05); **(p < .01); ***(p < .001), ****(p < .0001).
6‐Month post‐treatment
At 6‐month (6 M) post‐treatment in both treatment groups, the levels of IL‐1β and IL‐8 further increased compared with 3 M. There was a significant rise in levels of IL‐1β between T0 and 6 M in the Re‐RCT group (p = .005). ADMA was also significantly increased in both the Re‐RCT and PS groups between 3 M and 6 M (p = .008, p = .046), respectively (Figures 3 and 4).
Compared with T0, a significant reduction at 6 M was seen in the levels of FGF‐23 in both the Re‐RCT and PS groups, (p = .002; p = .028), respectively. In the Re‐RCT group from 3 M to 6 M, MMP‐2 and MMP‐9 were significantly reduced (p = .029; p = .027), whereas in the PS group the levels of C3 were significantly decreased (p = .046) in this time period (Figures 3 and 4).
1‐Year post‐treatment
Generally, the levels of FGF‐23, pentraxin‐3, ADMA and VCAM‐1 at 1‐year (1 Yr) post‐treatment were reduced below the levels recorded at T0. The Re‐RCT group showed a significant reduction at 1 Yr compared with T0 in the levels of FGF‐23 (p < .001), Pentraxin‐3 (p = .013), and ADMA (p < .001); whereas in the PS group, levels of FGF‐23, pentraxin‐3, VCAM‐1 and ADMA were significantly reduced at 1 Yr (p = .001; p = .003, p = .004; p = .003, respectively). Furthermore, after the initial 3 M rise, the levels of IL‐1β, IL‐8, hs‐CRP and C3 returned close to the pre‐treatment values at T0 in both treatment groups. However, levels of MMP‐2 and MMP‐9 increased again between 6 M and 1 Yr with a significant increase in MMP‐2 levels between T0 and 1 Yr in both the Re‐RCT and PS groups (p = .004; p = .007, respectively) (Figures 3 and 4).
Apical periodontitis patients also showed higher medians of reduction (or lower medians of increments) between T0 and 1 Yr for most of the inflammatory markers, compared with controls (Table 4).
TABLE 4.
Median of differences in inflammatory markers' levels (1 Yr – T0) between control and diseased groups
| Control | Diseased | p‐Value | |
|---|---|---|---|
|
Median (Min–Max) |
Median (Min–Max) |
||
| FGF‐23 |
9.73 (−84.85–43.30) |
−23.25 (−2290.83–0.75) |
<.001*** |
| IL‐1β |
12.27 (−2.94–29.86) |
2.33 (−21.81–23.05) |
<.001*** |
| IL‐8 |
13.20 (−53.86–30.68) |
1.81 (−60.01–43.26) |
.018* |
| Pentraxin 3 |
−712.95 (−4423.19–848.30) |
−506.44 (−5713.71–3045.42) |
.842 |
| TNF‐α |
6.58 (−4.55–19.66) |
−2.40 (−56.28–6.68) |
<.001*** |
| ICAM‐1 |
157298.40 (−15679.31–744264.41) |
−7930.73 (−309938.38–565303.47) |
.001** |
| IL‐6 |
0.70 (−3.66–9.87) |
0.13 (−43.56–58.50) |
.066 |
| MMP‐8 |
70.92 (−10128.77–8016.13) |
95.33 (−11193.18–6584.93) |
.850 |
| E‐Selectin |
11115.80 (−16010.17–80732.12) |
−3784.97 (−25636.14–25294.13) |
.001** |
| VCAM‐1 |
132848.12 (−586423.55–1621437.33) |
−124444.23 (−920491.86–287123.56) |
.034* |
| hs‐CRP |
34698.61 (−4064.71–109538.16) |
8421.95 (−334896.38–95955.55) |
.013* |
| MMP‐2 |
13046.33 (525.72–21849.70) |
14494.33 (−9878.80–31734.41) |
.491 |
| MMP‐9 |
2561.71 (−23992.76–28933.22) |
3025.84 (−39642.44–39106.00) |
.861 |
| C3 |
−111.26 (−1317.21–314.15) |
41.33 (−1024.83–1144.36) |
.179 |
| ADMA |
−2738.97 (−5206.02–3112.71) |
−5142.16 (−604309.77–2051.32) |
.008** |
Note: The values are given as Median (Minimum–Maximum); p Value—Difference between Median of Control and Disease group using Mann–Whitney test; *(p < .05); **(p < .01); ***(p < .001), ****(p < .0001).
Effect TRG, HbA1C, HDL and waist circumference on the levels of inflammatory markers at 1 yr
Indicators of metabolic syndrome such as TRG, HbA1c, HDL and WC all significantly correlated with the levels of specific biomarkers. An additional single unit of TRG correlates with an increase in the 1 Yr levels of TNF‐α by 7.9% (p = .046) (Figure 2f). Furthermore, HbA1c levels were positively correlated to the 1 Yr levels of IL‐6 (p = .019) (Figure 2g). Additionally, one extra cm in waist circumference influenced the levels of e‐selectin by 1.9% (p = .003) (Figure 2h).
High‐density lipoprotein inversly correlated with the levels of IL‐6, e‐selectin, ICAM‐1 and TNF‐α. An additional unit of HDL correlated with a 30.2% reduction in IL‐6 (p = .025), 19.5% reduction in e‐selectin (p = .043), 55.6% reduction in ICAM‐1 (p = .008), and a 23.8% reduction in TNF‐α (p = .001) (Figure 2i–l).
Association between inflammatory markers variation and treatment outcome
The low number of failures (n = 4 and n = 3 using PA and CBCT, respectively) did not allow statistical comparison between the pre‐ and post‐treatment levels of inflammatory mediators. However, the difference between pre‐ and post‐treatment levels of FGF‐23, pentraxin‐3 and ADMA in healing and healed cases were broadly similar. Using PA outcome, FGF‐23 and ADMA was significantly reduced in the healing (p = .001, p < .001 respectively) and healed (p < .001, p = .004 respectively) groups at 1‐year post‐treatment compared with pre‐treatment levels. A significant reduction was also seen in the levels of pentraxin 3 (p < .001), and VCAM‐1 (p = .002) in the healing group at 1 year post‐treatment compared with pre‐treatment levels. Similarly, when utilizing CBCT outcome, levels of FGF‐23, pentraxin 3, VCAM‐1 and ADMA were significantly reduced in the healing group (p = .001; p = .001; p = .015; and p = .013, respectively).
When correlating the outcome with the levels of inflammatory markers, increased ADMA baseline levels caused a significant reduction in the proportion of successful outcomes (p = .024). PA and CBCT outcomes were also significantly correlated with the levels of IL‐8 at 6 M (p = .001, p = .002).
DISCUSSION
In this longitudinal cohort study patients with AP showed an increased systemic inflammatory burden compared with controls. In particular, IL‐1β, hs‐CRP and FGF‐23 were significantly higher in AP patients than controls. Furthermore, higher levels of IL‐1β and FGF‐23 were found in patients with larger apical radiolucencies. These findings were consistent with other studies which found raised levels of ADMA, hs‐CRP, IL‐1β, IL‐6, MMP‐8 and E‐selectin in AP patients compared with controls (Cotti et al., 2011; Garrido et al., 2019). It is therefore evident that AP increases the systemic inflammatory burden.
The levels of IL‐1β, IL‐8, CPR, C3, MMP‐2 and MMP‐9 increased 3 to 6 months after treatment. One‐year post‐treatment, the levels of FGF‐23, pentraxin‐3 and ADMA were significantly reduced below baseline levels. Invasive dental procedures induce a transient increase of inflammatory markers' levels with potential risk of vascular events (Habbab et al., 2019) especially in vulnerable patients. However, successful endodontic treatment does have a long‐term benefit on the levels on inflammatory mediators and endothelial dysfuntion markers which might outweigh the short‐lived adverse effect. In fact, it has been demonstrated that an endothelial dysfunction improvement is associated with the reduction in inflammatory mediators following primary root canal treatment (Bergandi et al., 2019; Cotti et al., 2011).
To our knowledge, this is the first study in which the reduction in inflammation biomarkers has been associated with root canal retreatments and periapical surgery.
This is also the first study in which the variation of inflammatory marker levels between baseline and 1 yr in the treated patients has been compared with that of a control group of healthy subjects. The comparison with the control group allowed us to demonstrate that the variations of the levels of biomarkers detected in patients treated for apical periodontitis were different from the normal variations detected in healthy subjects which may depend on other sources of inflammation. Even though COVID‐19 lockdown restrictions caused a reduction in the recall rate at 6 M (n = 37); at 1‐year post‐treatment, the recall rate reached above 76% (n = 50).
The proinflammtory cytokine IL‐1β can induce the secretion of the acute phase protein CRP from hepatocytes (Sproston & Ashworth, 2018). Increased CRP levels have consistently been associated with increased risk of myocardial infarction, stroke and peripheral artery disease (Lagrand et al., 1999). Its elevated levels are also linked with increased levels of FGF‐23 (Munoz Mendoza et al., 2017) which is emerging as a novel biomarker of CVD risk (Batra et al., 2016; Vázquez‐Sánchez et al., 2021). High FGF‐23 levels can affect both heart and blood vessels and are associated with cardiac dysfunction, subclinical atherosclerosis, hypertension, left ventricular hypertrophy and a higher mortality rate (Vázquez‐Sánchez et al., 2021). Elevated levels of ADMA, an endogenous nitric oxide synthase inhibitor (Böger, 2004), can affect vascular tone resulting in vasoconstriction and promote vascular inflammation, thereby playing an essential role in endothelial dysfunction; it is therefore regarded as a marker for early development of CVDs. Since raised levels of these inflammatory markers associated with AP can potentiate systemic inflammation resulting in atherosclerosis and CVD risk, this study highlights the need for timely endodontic treatment for AP, whether symptomatic or asymptomatic, to remove the source of chronic systemic inflammatory burden and benefit patient health.
IL‐1β induces other cytokines and chemokines such as IL‐8 (Dinarello, 1988). This explains the rise seen in both IL‐1β and IL‐8 levels at 3 and 6 months. IL‐8 is a chemokine which plays a role in RANKL‐related osteoblastic/osteoclastic activity for bone remodelling. It also induces neutrophil migration for clearing of infection (Gomes & Herrera, 2018). Hence, the increased levels of IL‐1β and IL‐8 found after root canal treatment were associated with both the removal of infection and progression towards periapical healing. IL‐1β also stimulates endothelial cells to release Selectins and MMPs; and hepatocytes to release CRP (Cheng et al., 2010; Sproston & Ashworth, 2018). The gelatinases, MMP‐2 and MMP‐9, have a role in the degeneration of extracellular matrix, tissue repair and remodelling by modulating cytokines/chemokines (Page‐McCaw et al., 2007). Both MMPs degrade IL‐1β which explains the inverse relation between MMPs and interleukins in our findings (Ito et al., 1996), where IL‐1β and IL‐8 were decreased 1‐year post‐treatment while conversely MMPs increased from 6 months to 1 year for bone remodelling. The human complement component C3 keeps this cascade active, clearing pathogens and debris and promoting tissue regeneration (Ricklin et al., 2016). In our study, levels of C3 increased 3 months after treatment but then gradually decreased by 6 months and further by 1 year. In a recent systematic review and meta‐analysis, Georgiou et al. (2019) also found that levels of C3 was significantly lower after root canal treatment and healing of AP.
When we assessed the radiographic outcome of treated case, most of the cases were in the healed and healing category with only few failed cases.
To minimize the confounding factors, patients included in our study group had neither chronic systemic inflammatory conditions nor known risk factors for CVD development. When we compared the baseline values of patients' metabolic syndrome (MetS) factors—a known risk for CVD development (National Cholesterol Education Program Adult Treatment Panel III, 2001), including waist circumference/BMI, triglycerides, HDL, HbA1C and blood pressure, we found that both treatments groups had higher values of these factors compared with the control group at baseline. However, these values were still within the normal range according to the National Cholesterol Education Program (Adult Treatment Panel III) (2001). Irrespective of these findings, when the data were adjusted for HbA1C, TRG, HDL, BMI, age and gender, the apical periodontitis group at baseline still had significantly higher levels of FGF23, IL‐1β and hs‐CRP relative to controls.
There is some evidence suggesting that AP correlates with higher HbA1C levels—one of the criteria for MetS that can contribute to diabetic metabolic dysregulation (Arya et al., 2017; González Navarro et al., 2017; Segura‐Egea et al., 2012; Segura‐Egea et al., 2015). Furthermore, studies have shown that patients with diabetes mellitus have a higher prevalence of AP and greater size of periapical lesion (Britto et al., 2003; Fouad & Burleson, 2003; Segura‐Egea et al., 2005; Segura‐Egea et al., 2012). The findings of our study suggest that either AP potentiates these metabolic syndrome factor levels or patients who have higher metabolic syndrome factors are more susceptible to the development of AP due altered immune responses.
When we correlated the inflammatory markers with metabolic syndrome factors, we found that postoperative HbA1C was positively correlated with levels of IL‐6. This was consistent with findings of Al‐Shukaili et al. (2013). Triglycerides also showed positive correlation with TNF‐α, an important biomarker in the development of atherosclerosis through promotion of adhesion molecule expression on endothelial cells, recruitment and activation of inflammatory cells, and initiation of the inflammatory cascade inside the arterial wall (Skoog et al., 2002). TNF‐α has been found to directly interfere with the metabolic pathway of triglycerides and cholesterol (Memon et al., 1993), supporting our finding that an increase of TNF‐α is associated with increased levels of triglycerides. Furthermore, waist circumference was found to be significantly correlated with increased levels of E‐selectin. Several health risks are related to an increase of waist circumference. In a systematic review, Darsini et al. (2020) found that hypertension, diabetes mellitus and high cholesterol levels were associated with increased waist circumference (Darsini et al., 2020). In our study, it was also found that increases in BMI and waist circumference are positively correlated with the size of the radiolucencies. It is therefore possible that metabolic syndrome can somehow interfere with the inflammation processes which lead to the development of apical periodontitis. However, higher levels of HDL (known to help in the hepatic removal of other types of cholesterol from the bloodstream) was inversely associated with levels of IL‐6, ICAM‐1, E‐selectin and TNF‐α, in agreement with the findings of Zuliani et al. (2007) and Muñoz‐Vega et al. (2018).
CONCLUSION
Apical periodontitis contributes to the increased levels of systemic inflammatory markers and potentially increases the risk of chronic systemic inflammatory conditions such as atherosclerosis and CVDs. The transient increase of inflammatory markers' levels after both root canal retreatment and apical surgery potentially might increase the risk of vascular events especially in vulnerable patients. However, successful endodontic treatment does have a long‐term benefit on vascular and systemic health which is likely to outweigh the short‐lived adverse effect.
AUTHOR CONTRIBUTIONS
A. Bakhsh contributed to conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, validation, visualization, writing—original draft preparation and writing—review and editing; D. Moyes contributed to conceptualization, methodology, resources, supervision and writing—review and editing; G. Proctor contributed to supervision, resources and writing—review and editing; F. Mannocci contributed to conceptualization, methodology, resources, supervision and writing—review and editing, S. Niazi contributed to conceptualization, methodology, data curation, funding acquisition, investigation, project administration, supervision, resources, validation, visualization, writing—original draft preparation and writing—review and editing.
CONFLICT OF INTEREST
The authors deny any conflicts of interest related to this study.
ETHICS STATEMENT
The protocol of the study was approved by the London‐Hampstead Research Ethics Committee (IRAS project ID 207795) and by the London‐Riverside Research Ethics Committee (REC reference: 20/LO/0024).The participants were given a detailed verbal and written information regarding the purpose of the study, and written consent was obtained in accordance with the Declaration of Helsinki.
Supporting information
Table S1–S4
ACKNOWLEDGEMENT
We would like to thank Juan Luis Gómez Martínez for his help in statistical analysis of the data.
Bakhsh, A. , Moyes, D. , Proctor, G. , Mannocci, F. & Niazi, S.A. (2022) The impact of apical periodontitis, non‐surgical root canal retreatment and periapical surgery on serum inflammatory biomarkers. International Endodontic Journal, 55, 923–937. Available from: 10.1111/iej.13786
Funding information
The study is funded by British Endodontic Society (Grant for Research Work) and European Society of Endodontology (Annual Research Grant). Sadia Ambreen Niazi is the principal grant holder of these grants. Abdulaziz Bakhsh is a PhD student funded by a scholarship from Umm Al‐Qura University, Makkah, Saudi Arabia. Grant number: 4360144104.
DATA AVAILABILITY STATEMENT
Data available on request from the authors
REFERENCES
- Al‐Shukaili, A. , Al‐Ghafri, S. , Al‐Marhoobi, S. , Al‐Abri, S. , Al‐Lawati, J. & Al‐Maskari, M. (2013) Analysis of inflammatory mediators in type 2 diabetes patients. International Journal of Endocrinology, 2013, 976810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya, S. , Duhan, J. , Tewari, S. , Sangwan, P. , Ghalaut, V. & Aggarwal, S. (2017) Healing of apical periodontitis after nonsurgical treatment in patients with type 2 diabetes. Journal of Endodontics, 43(10), 1623–1627. [DOI] [PubMed] [Google Scholar]
- Batra, J. , Buttar, R.S. , Kaur, P. , Kreimerman, J. & Melamed, M.L. (2016) FGF‐23 and cardiovascular disease: review of literature. Current Opinion in Endocrinology, Diabetes, and Obesity, 23(6), 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergandi, L. , Giuggia, B. , Alovisi, M. , Comba, A. , Silvagno, F. , Maule, M. et al. (2019) Endothelial dysfunction marker variation in young adults with chronic apical periodontitis before and after endodontic treatment. Journal of Endodontics, 45, 500–506. [DOI] [PubMed] [Google Scholar]
- Berlin‐Broner, Y. , Febbraio, M. & Levin, L. (2017) Apical periodontitis and atherosclerosis: is there a link? Review of the literature and potential mechanism of linkage. Quintessence International, 48(7), 527–534. [DOI] [PubMed] [Google Scholar]
- Böger, R.H. (2004) Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the "L‐arginine paradox" and acts as a novel cardiovascular risk factor. The Journal of Nutrition, 134(10 Suppl), 2842S–2847S; discussion 2853S. [DOI] [PubMed] [Google Scholar]
- Britto, L.R. , Katz, J. , Guelmann, M. & Heft, M. (2003) Periradicular radiographic assessment in diabetic and control individuals. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 96(4), 449–452. [DOI] [PubMed] [Google Scholar]
- Chen, T.T. , D'Aiuto, F. , Yeh, Y.C. , Lai, M.S. , Chien, K.L. & Tu, Y.K. (2018) Risk of myocardial infarction and ischemic stroke after dental treatments. Journal of Dental Research, 98, 157–163. [DOI] [PubMed] [Google Scholar]
- Cheng, C.Y. , Kuo, C.T. , Lin, C.C. , Hsieh, H.L. & Yang, C.M. (2010) IL‐1beta induces expression of matrix metalloproteinase‐9 and cell migration via a c‐Src‐dependent, growth factor receptor transactivation in A549 cells. British Journal of Pharmacology, 160(7), 1595–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian, A.V. , Bakris, G.L. , Black, H.R. , Cushman, W.C. , Green, L.A. , Izzo, J.L., Jr. et al. (2003) Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension, 42(6), 1206–1252. [DOI] [PubMed] [Google Scholar]
- Cotti, E. , Dessi, C. , Piras, A. , Flore, G. , Deidda, M. , Madeddu, C. et al. (2011) Association of endodontic infection with detection of an initial lesion to the cardiovascular system. Journal of Endodontics, 37(12), 1624–1629. [DOI] [PubMed] [Google Scholar]
- Cotti, E. , Zedda, A. , Deidda, M. , Piras, A. , Flore, G. , Ideo, F. et al. (2015) Endodontic infection and endothelial dysfunction are associated with different mechanisms in men and women. Journal of Endodontics, 41(5), 594–600. [DOI] [PubMed] [Google Scholar]
- Darsini, D. , Hamidah, H. , Notobroto, H.B. & Cahyono, E.A. (2020) Health risks associated with high waist circumference: a systematic review. Journal of Public Health Research, 9(2), 1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello, C.A. (1988) Biology of interleukin 1. The FASEB Journal, 2(2), 108–115. [PubMed] [Google Scholar]
- Fouad, A.F. & Burleson, J. (2003) The effect of diabetes mellitus on endodontic treatment outcome: data from an electronic patient record. Journal of the American Dental Association (1939), 134(1), 43–51; quiz 117–118. [DOI] [PubMed] [Google Scholar]
- Garrido, M. , Cardenas, A.M. , Astorga, J. , Quinlan, F. , Valdés, M. , Chaparro, A. et al. (2019) Elevated systemic inflammatory burden and cardiovascular risk in young adults with endodontic apical lesions. Journal of Endodontics, 45(2), 111–115. [DOI] [PubMed] [Google Scholar]
- Georgiou, A.C. , Crielaard, W. , Armenis, I. , de Vries, R. & van der Waal, S.V. (2019) Apical periodontitis is associated with elevated concentrations of inflammatory mediators in peripheral blood: a systematic review and meta‐analysis. Journal of Endodontics, 45(11), 1279–1295.e3. [DOI] [PubMed] [Google Scholar]
- Gomes, B. & Herrera, D.R. (2018) Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Brazilian Oral Research, 32(suppl 1), e69. [DOI] [PubMed] [Google Scholar]
- González Navarro, B. , Pintó Sala, X. & Jané Salas, E. (2017) Relationship between cardiovascular disease and dental pathology. Systematic review. Medicina Clínica, 149(5), 211–216. [DOI] [PubMed] [Google Scholar]
- Graziani, F. , D'Aiuto, F. , Gennai, S. , Petrini, M. , Nisi, M. , Cirigliano, N. et al. (2017) Systemic inflammation after third molar removal: a case‐control study. Journal of Dental Research, 96(13), 1505–1512. [DOI] [PubMed] [Google Scholar]
- Habbab, K.M. , D'Aiuto, F. , Habbab, M.A. & Porter, S.R. (2019) Molecular markers relevant to myocardial injury following dental extraction in patients with or without coronary artery disease. BDJ Open, 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, A. , Mukaiyama, A. , Itoh, Y. , Nagase, H. , Thøgersen, I.B. , Enghild, J.J. et al. (1996) Degradation of interleukin 1β by matrix metalloproteinases. Journal of Biological Chemistry, 271(25), 14657–14660. [DOI] [PubMed] [Google Scholar]
- Jakovljevic, A. , Duncan, H.F. , Nagendrababu, V. , Jacimovic, J. , Milasin, J. & Dummer, P.M.H. (2020) Association between cardiovascular diseases and apical periodontitis: an umbrella review. International Endodontic Journal, 53(10), 1374–1386. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Sanchez, M.C. , Cabanillas‐Balsera, D. , Areal‐Quecuty, V. , Velasco‐Ortega, E. , Martin‐Gonzalez, J. & Segura‐Egea, J.J. (2020) Cardiovascular diseases and apical periodontitis: association not always implies causality. Medicina Oral, Patología Oral y Cirugía Bucal, 25(5), e652–e659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrand, W.K. , Visser, C.A. , Hermens, W.T. , Niessen, H.W.M. , Verheugt, F.W.A. , Wolbink, G.J. et al. (1999) C‐reactive protein as a cardiovascular risk factor. Circulation, 100(1), 96–102. [DOI] [PubMed] [Google Scholar]
- Memon, R.A. , Grunfeld, C. , Moser, A.H. & Feingold, K.R. (1993) Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology, 132(5), 2246–2253. [DOI] [PubMed] [Google Scholar]
- Milutinović, A. , Šuput, D. & Zorc‐Pleskovič, R. (2020) Pathogenesis of atherosclerosis in the tunica intima, media, and adventitia of coronary arteries: an updated review. Bosnian Journal of Basic Medical Sciences, 20(1), 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian, C. , D'Aiuto, F. , Hingorani, A.D. & Smeeth, L. (2010) Invasive dental treatment and risk for vascular events: a self‐controlled case series. Annals of Internal Medicine, 153(8), 499–506. [DOI] [PubMed] [Google Scholar]
- Munoz Mendoza, J. , Isakova, T. , Cai, X. , Bayes, L.Y. , Faul, C. , Scialla, J.J. et al. (2017) Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney International, 91(3), 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz‐Vega, M. , Massó, F. , Páez, A. , Vargas‐Alarcón, G. , Coral‐Vázquez, R. , Mas‐Oliva, J. et al. (2018) HDL‐mediated lipid influx to endothelial cells contributes to regulating intercellular adhesion molecule (ICAM)‐1 expression and eNOS phosphorylation. International Journal of Molecular Sciences, 19(11), 3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cholesterol Education Program (Adult Treatment Panel III) . (2001) Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA, 285(19), 2486–2497. [DOI] [PubMed] [Google Scholar]
- Page‐McCaw, A. , Ewald, A.J. & Werb, Z. (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nature Reviews. Molecular Cell Biology, 8(3), 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S. , Wilson, R. , Dawood, A. , Foschi, F. & Mannocci, F. (2012) The detection of periapical pathosis using digital periapical radiography and cone beam computed tomography – part 2: a 1‐year post‐treatment follow‐up. International Endodontic Journal, 45(8), 711–723. [DOI] [PubMed] [Google Scholar]
- Perez‐Losada, F.L. , Estrugo‐Devesa, A. , Castellanos‐Cosano, L. , Segura‐Egea, J.J. , Lopez‐Lopez, J. & Velasco‐Ortega, E. (2020) Apical periodontitis and diabetes mellitus type 2: a systematic review and meta‐analysis. Journal of Clinical Medicine, 9(2), 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poornima, L. , Ravishankar, P. , Abbott, P.V. , Subbiya, A. & PradeepKumar, A.R. (2021) Impact of root canal treatment on high‐sensitivity C‐reactive protein levels in systemically healthy adults with apical periodontitis – a preliminary prospective, longitudinal interventional study. International Endodontic Journal, 54(4), 501–508. [DOI] [PubMed] [Google Scholar]
- Ricklin, D. , Reis, E.S. , Mastellos, D.C. , Gros, P. & Lambris, J.D. (2016) Complement component C3 – The “Swiss Army Knife” of innate immunity and host defense. Immunological Reviews, 274(1), 33–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura‐Egea, J.J. , Castellanos‐Cosano, L. , Machuca, G. , López‐López, J. , Martín‐González, J. , Velasco‐Ortega, E. et al. (2012) Diabetes mellitus, periapical inflammation and endodontic treatment outcome. Medicina Oral, Patología Oral y Cirugía Bucal, 17(2), e356–e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura‐Egea, J.J. , Jimenez‐Pinzon, A. , Rios‐Santos, J.V. , Velasco‐Ortega, E. , Cisneros‐Cabello, R. & Poyato‐Ferrera, M. (2005) High prevalence of apical periodontitis amongst type 2 diabetic patients. International Endodontic Journal, 38(8), 564–569. [DOI] [PubMed] [Google Scholar]
- Segura‐Egea, J.J. , Martín‐González, J. & Castellanos‐Cosano, L. (2015) Endodontic medicine: connections between apical periodontitis and systemic diseases. International Endodontic Journal, 48(10), 933–951. [DOI] [PubMed] [Google Scholar]
- Skoog, T. , Dichtl, W. , Boquist, S. , Skoglund‐Andersson, C. , Karpe, F. , Tang, R. et al. (2002) Plasma tumour necrosis factor‐alpha and early carotid atherosclerosis in healthy middle‐aged men. European Heart Journal, 23(5), 376–383. [DOI] [PubMed] [Google Scholar]
- Sproston, N.R. & Ashworth, J.J. (2018) Role of C‐reactive protein at sites of inflammation and infection. Frontiers in Immunology, 9, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio‐Machado, C.S. , Michelon, C. , Zanatta, F.B. , Gomes, M.S. , Marin, J.A. & Bier, C.A. (2020) The global prevalence of apical periodontitis: a systematic review and meta‐analysis. International Endodontic Journal, 54, 712–735. [DOI] [PubMed] [Google Scholar]
- Vázquez‐Sánchez, S. , Poveda, J. , Navarro‐García, J.A. , González‐Lafuente, L. , Rodríguez‐Sánchez, E. , Ruilope, L.M. et al. (2021) An overview of FGF‐23 as a novel candidate biomarker of cardiovascular risk. Frontiers in Physiology, 12, 632260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2011) Global atlas on cardiovascular disease prevention and control policies, strategies and interventions. In: Federation WWH, ed.
- Wolf, D. & Ley, K. (2019) Immunity and inflammation in atherosclerosis. Circulation Research, 124(2), 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2017) Cardiovascular diseases (CVDs). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Zuliani, G. , Volpato, S. , Blè, A. , Bandinelli, S. , Corsi, A.M. , Lauretani, F. et al. (2007) High interleukin‐6 plasma levels are associated with low HDL‐C levels in community‐dwelling older adults: the InChianti study. Atherosclerosis, 192(2), 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4
Data Availability Statement
Data available on request from the authors


