Abstract
Objectives
The purpose of this analysis was to compare target‐lesion failure (TLF) of a permanent polymer zotarolimus‐eluting stent (PP‐ZES) versus a polymer‐free amphilimus‐eluting stent (PF‐AES) in diabetics.
Background
The improvement of outcomes with new‐generation drug‐eluting stent as seen in the general population is less pronounced among diabetics. The PF‐AES introduces an elution‐technology with potential enhanced performance in diabetics.
Methods
In this subanalysis of the ReCre8 trial, patients were randomized to either a PP‐ZES or PF‐AES after stratification for diabetes and troponin status. The primary device‐oriented endpoint was TLF, a composite of cardiac death, target‐vessel myocardial infarction and target‐lesion revascularization.
Results
In the ReCre8 trial, 304 (20%) patients were diabetic and 96 (6%) had insulin‐dependent diabetes mellitus. There was no statistically significant difference between the two study arms regarding the primary endpoint (PP‐ZES 7.2% vs. PF‐AES 4.0%; p = .21), although the composite of net adverse clinical events was higher in the PP‐ZES arm (15.7 vs. 8.0%; p = .035). Stent thrombosis was low in both groups with no cases in the PP‐ZES arm and 1 case in the PF‐AES arm (p = .32). Regarding insulin‐treated diabetics, TLF was higher in the PP‐ZES arm (14.9 vs. 2.1%; p = .022).
Conclusions
Diabetics could potentially benefit from a dedicated stent, releasing sirolimus with a lipophilic carrier (amphilimus‐formulation). Future trials should confirm the potential benefit of a PF‐AES in this population.
Keywords: percutaneous coronary intervention, drug‐eluting stents, diabetes mellitus
Abbreviations
- DAPT
dual antiplatelet therapy
- DES
drug‐eluting stent
- IDDM
insulin‐dependent diabetes mellitus
- NACE
net adverse clinical events
- PCI
percutaneous coronary intervention
- PF‐AES
polymer‐free amphilimus‐eluting stent
- PP‐ZES
permanent polymer zotarolimus‐eluting stent
- TLF
target‐lesion failure
1. INTRODUCTION
Patients with diabetes mellitus are more susceptible to vascular disease, including coronary artery disease, due to endothelial dysfunction, inflammation and thrombosis. 1 , 2 As the global prevalence of diabetes mellitus is expected to grow with over 200 million people between 2015 and 2040, 3 this subgroup of coronary artery disease patients becomes more important and requires a special focus in contemporary stent studies. Moreover, it is well‐documented that diabetic patients have a higher risk of adverse outcomes following percutaneous coronary intervention (PCI) especially with regard to reinterventions. 4 , 5 Despite improved outcomes since the development of new‐generation drug‐eluting stents (DES), 6 , 7 patients with diabetes mellitus remain at high risk of adverse events following PCI. 8
Among the diabetic population, insulin‐dependent diabetes mellitus (IDDM) patients represent a subgroup of patients with the highest chance of adverse outcomes after PCI. 9 , 10 Previous studies have shown a two‐ to four‐fold increase in event rates (such as target‐lesion failure [TLF], cardiac death and revascularization) in IDDM patients as compared to non‐diabetics. 5 , 11
The polymer‐free amphilimus‐eluting stent (PF‐AES) was designed with abluminal reservoirs releasing amphilimus – a combination of sirolimus and long‐chain fatty acids. This provides a potential benefit for patients with diabetes mellitus as diabetic cells overexpress membrane fatty acid transporters, increasing fatty acid uptake 12 and therefore the uptake of the effectual drug. This may improve outcomes in diabetic patients as diabetic cells are relatively resistant to the anti‐restenotic drugs used in contemporary stents (e.g., sirolimus). 13
The RESERVOIR trial 14 compared efficacy of the PF‐AES and an everolimus‐eluting stent in patients with a history of diabetes. Regarding the primary endpoint of neointimal volume obstruction, noninferior efficacy of the PF‐AES was demonstrated with 12 versus 16% obstruction in the everolimus‐eluting arm. Similarly, in‐stent late loss was lower in the PF‐AES arm and a larger minimal lumen diameter was seen at follow‐up, suggesting a beneficial effect of the PF‐AES in this diabetic subgroup.
The aim of this subanalysis of the ReCre8 trial 15 was to evaluate 12 months post‐discharge clinical outcomes after implantation of either a permanent polymer zotarolimus‐eluting stent (PP‐ZES) or PF‐AES in diabetic patients according to enrollment stratification.
2. MATERIALS AND METHODS
2.1. Study design and population
The ReCre8 trial 15 was a physician‐initiated, prospective, randomized, multicenter trial that compared a PP‐ZES (Resolute Integrity, Medtronic Vascular, Santa Rosa, CA) to a PF‐AES (Cre8, Alvimedica, Istanbul, Turkey) in an all‐comers population requiring PCI. Patients were included across three European PCI centers; University Medical Center Utrecht and Zuyderland Medical Center Heerlen, the Netherlands and the National Institute of Cardiac Surgery and Interventional Cardiology Luxembourg, Luxembourg. The ReCre8 trial was approved by the Medical Research Ethics Committee Utrecht as well as the institutional review boards of each participating center. Written informed consent was obtained from all study participants. The ReCre8 trial was registered at clinicaltrials.gov (NCT02328898).
The study design has been previously reported. 16 In brief, patients were stratified for diabetes mellitus and troponin status after which block‐randomization assigned patients in a 1:1 ratio to receive either a PP‐ZES or PF‐AES. Allocation toward the diabetic stratum occurred by a review of current drug use and medical history at randomization. Patients were treated with either one month (troponin negative patients) or 12 months (troponin positive patients) of dual antiplatelet therapy (DAPT). The ReCre8 trial had an all‐comers design with few criteria restricting inclusion. Patients were eligible for inclusion if they were over 18 years of age and there were clinical symptoms of ischemia present requiring patients to undergo PCI with implantation of a DES in a lesion with a reference vessel diameter of 2.5–4.5 mm. This substudy of the ReCre8 trial reports results from a subgroup of patients with a history of diabetes after 12 months follow‐up.
2.2. Study endpoints
The device‐oriented primary endpoint of TLF was composed of cardiac death, target‐vessel myocardial infarction, and target‐lesion revascularization. The patient‐oriented secondary endpoint of net adverse clinical events (NACE) was a composite of all‐cause death, any myocardial infarction, any unplanned revascularization, stroke, and major bleeding. Additionally, all components of the composite endpoints were analyzed separately. The endpoints that were analyzed in this subanalysis were similar to the endpoints in the main publication. Clinical endpoints were defined according to the Academic Research Consortium. 17 Endpoint definitions were previously described. 15
2.3. Statistical analysis
Distributions of dichotomous variables are reported as counts and percentages and were compared between groups using Chi‐square or Fisher exact test. Continuous variables are described as mean ± standard deviation and compared using the Independent group Student's t test or Wilcoxon Rank Sum test. Kaplan Meier time‐to‐event estimates for the primary endpoint, secondary endpoint, and individual events were compared using log‐rank test. Time‐to–first‐event was defined as the number of days between primary PCI and occurrence of any component of the primary or secondary endpoint. All analyses were performed on post‐discharge events. Follow‐up was censored at 12 months. Differences were considered statistically significant at a 2‐tailed p‐value of .05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Figures were generated using GraphPad Prism version 8.3 (GraphPad, Inc., San Diego, CA).
3. RESULTS
3.1. Baseline and procedural characteristics
From the total of 1,491 patients included in the ReCre8 trial, 304 patients with diabetes mellitus were included in this analysis. Baseline characteristics were similar between the PP‐ZES and PF‐AES arm (Table 1). Based on the American College of Cardiology/American Heart Association criteria, 18 58% presented with at least one complex lesion. Over half of the diabetic population was treated with only one month of DAPT as they presented with troponin negative disease. Compliance with the prescribed use of DAPT and aspirin was high and did not differ between the study arms (Table S1).
Table 1.
Baseline characteristics
| Overall | PP‐ZES | PF‐AES | p‐value | |
|---|---|---|---|---|
| (n = 304) | (n = 153) | (n = 151) | ||
| Clinical characteristics | ||||
| Age (years) | 66.5 ± 9.7 | 67.1 ± 9.6 | 65.9 ± 9.9 | .28 |
| Male sex | 224 (73.7) | 113 (73.9) | 111 (73.5) | .95 |
| Body mass index (kg/m2) | 29.1 ± 4.7 | 29.0 ± 4.6 | 29.2 ± 4.8 | .75 |
| Hypertension | 217 (71.4) | 111 (72.5) | 106 (70.2) | .53 |
| Hypercholesterolemia | 182 (59.9) | 89 (58.2) | 93 (61.6) | .66 |
| Insulin‐treated diabetes mellitus | 96 (31.6) | 47 (30.7) | 49 (32.5) | .75 |
| Current smoker | 65 (21.4) | 28 (18.3) | 37 (24.5) | .22 |
| Family history of cardiovascular disease | 104 (34.2) | 51 (33.3) | 53 (35.1) | .95 |
| Renal insufficiency, eGFR<60 a | 55 (18.1) | 31 (20.3) | 24 (15.9) | .35 |
| Relevant medical history | ||||
| Previous MI | 82 (27.0) | 42 (27.5) | 40 (26.5) | .98 |
| Previous PCI | 86 (28.3) | 50 (32.7) | 36 (23.8) | .087 |
| Previous CABG | 39 (12.8) | 23 (15.0) | 16 (10.6) | .25 |
| Clinical presentation | ||||
| Stable angina | 140 (46.1) | 71 (46.4) | 69 (45.7) | .90 |
| Acute coronary syndrome | 128 (42.1) | 67 (43.8) | 61 (40.4) | .55 |
| Coronary anatomy | ||||
| Left main | 7 (2.3) | 4 (2.6) | 3 (2.0) | 1.00 |
| Left anterior descending artery | 154 (50.7) | 73 (47.7) | 81 (53.6) | .30 |
| Left circumflex artery | 123 (40.5) | 64 (41.8) | 59 (39.1) | .62 |
| Right coronary artery | 158 (52.0) | 80 (52.3) | 78 (51.7) | .91 |
| Arterial bypass graft | 6 (2.0) | 4 (2.6) | 2 (1.3) | .68 |
| Venous bypass graft | 11 (3.6) | 7 (4.6) | 4 (2.6) | .54 |
| Lesion characteristics | ||||
| ≥1 complex lesion b | 177 (58.2) | 90 (58.8) | 87 (57.6) | .83 |
| 1 bifurcation lesion | 66 (21.7) | 31 (20.3) | 35 (23.2) | .54 |
| ≥1 chronic total occlusion | 27 (8.9) | 12 (7.8) | 15 (9.9) | .52 |
| ≥1 small vessel (RVD < 2.75 mm) | 110 (36.2) | 54 (35.3) | 56 (37.1) | .74 |
| Procedural characteristics | ||||
| Radial approach | 269 (88.5) | 135 (88.2) | 134 (88.7) | .71 |
| Number of diseased coronary vessels | ||||
| 1 | 160 (52.6) | 83 (54.2) | 77 (51.0) | .92 |
| 2 | 95 (31.3) | 45 (29.4) | 50 (33.1) | |
| ≥3 | 49 (16.1) | 25 (16.3) | 24 (15.9) | |
Note: Data are n (%) or means ± SD.
Abbreviations: CABG, coronary artery bypass grafting; eGFR, estimated Glomerular filtration rate; MI, myocardial infarction; NSTEMI, non‐ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PF‐AES, polymer‐free amphilimus‐eluting stents; PP‐ZES, permanent polymer zotarolimus‐eluting stents; RVD, reference vessel diameter; STEMI, ST‐segment elevation myocardial infarction.
Renal insufficiency was defined as an estimated glomerular filtration rate of less than 60 ml per min per 1 × 73 m2.
Complex lesions were defined as lesion classification type B2 or C according to the American College of Cardiology/American Heart Association.
Procedural characteristics were comparable between the PP‐ZES and PF‐AES arm (Table 2). On average, 1.30 stents per lesion and 1.91 stents per patient were implanted. Procedural success was similar at 98.2 and 98.6%.
Table 2.
Lesion and procedural characteristics
| Diabetic population | ||||
|---|---|---|---|---|
| Overall | PP‐ZES | PF‐AES | p‐value | |
| (n = 447) | (n = 225) | (n = 222) | ||
| Procedural characteristics | ||||
| No. of stents per lesion | 1.30 ± 0.6 | 1.31 ± 0.7 | 1.29 ± 0.6 | .81 |
| No. of stents per patient | 1.91 ± 1.3 | 1.92 ± 1.2 | 1.90 ± 1.3 | .88 |
| Total stent length, mm | 51.1 ± 21.8 | 50.7 ± 19.7 | 51.5 ± 24.3 | .98 |
| Stent diameter, mm | 2.96 ± 0.4 | 2.96 ± 0.4 | 2.96 ± 0.4 | .86 |
| Multi overlapping stents | 87 (19.5) | 43 (19.1) | 44 (19.8) | .87 |
| Lesion complexity | ||||
| ACC/AHA class A | 55 (12.3) | 27 (12.0) | 28 (12.6) | .87 |
| ACC/AHA class B1 | 148 (33.1) | 78 (34.7) | 70 (31.5) | |
| ACC/AHA class B2 | 105 (23.5) | 53 (23.6) | 52 (23.4) | |
| ACC/AHA class C | 138 (30.9) | 66 (29.3) | 72 (32.4) | |
| GP IIb/IIIa antagonist use | 33 (7.4) | 13 (5.8) | 20 (9.0) | .21 |
| Procedural success | 440 (98.4) | 221 (98.2) | 219 (98.6) | .68 |
Note: Data are n (%) or means ± SD.
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; GP IIb/IIIA, glycoprotein IIb/IIIa; PF‐AES, polymer‐free amphilimus‐eluting stents; PP‐ZES, permanent polymer zotarolimus‐eluting stents.
3.2. Clinical outcomes in PP‐ZES versus PF‐AES
Post‐discharge clinical outcomes after 12 months are shown in Table 3. The primary endpoint of TLF did not statistically differ between the two study arms (PP‐ZES 7.2% vs. PF‐AES 4.0%; p = .21), as shown in Figure 1. The secondary endpoint of NACE occurred more frequently in the PP‐ZES group (15.7 vs. 8.0%; p = .035), mostly driven by a higher (cardiac) death rate. There were no significant differences among the separate components of the endpoints.
Table 3.
Post‐discharge clinical outcomes at 12 months
| Diabetic patients | Insulin‐dependent diabetic patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | PP‐ZES | PF‐AES | p‐value | Overall | PP‐ZES | PF‐AES | p‐value | |
| (n = 303) | (n = 153) | (n = 150) | (n = 95) | (n = 47) | (n = 48) | |||
| TLF a | 17 (5.6) | 11 (7.2) | 6 (4.0) | .21 | 8 (8.4) | 7 (14.9) | 1 (2.1) | .022 |
| NACE b | 36 (11.9) | 24 (15.7) | 12 (8.0) | .035 | 18 (18.9) | 14 (29.8) | 4 (8.3) | .009 |
| All‐cause death | 13 (4.3) | 9 (5.9) | 4 (2.7) | .16 | 8 (8.4) | 7 (14.9) | 1 (2.1) | .024 |
| Cardiac death | 9 (3.0) | 7 (4.6) | 2 (1.3) | .094 | 6 (6.3) | 5 (10.6) | 1 (2.1) | .080 |
| Any MI | 3 (1.0) | 0 (0.0) | 3 (2.0) | .084 | 2 (2.1) | 0 (0.0) | 2 (4.2) | .17 |
| TV‐MI | 1 (0.3) | 0 (0.0) | 1 (0.7) | .32 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ‐ |
| Stent thrombosis c | 1 (0.3) | 0 (0.0) | 1 (0.7) | .32 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ‐ |
| Late (31d to 12 m) | 1 (0.3) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ‐ | |
| Any unplanned revascularisation | 16 (5.3) | 10 (6.5) | 6 (4.0) | .29 | 5 (5.3) | 4 (8.5) | 1 (2.1) | .14 |
| TLR | 7 (2.3) | 4 (2.6) | 3 (2.0) | .69 | 2 (2.1) | 2 (4.3) | 0 (0.0) | .14 |
| Stroke | 3 (1.0) | 3 (2.0) | 0 (0.0) | .080 | 3 (3.2) | 3 (6.4) | 0 (0.0) | .063 |
| Major bleeding | 6 (2.0) | 5 (3.3) | 1 (0.7) | .10 | 3 (3.2) | 2 (4.3) | 1 (2.1) | .55 |
Note: Data are n (%).
Abbreviations: MI, myocardial infarction; NACE, net adverse clinical events; PF‐AES, polymer‐free amphilimus‐eluting stents; PP‐ZES, permanent polymer zotarolimus‐eluting stents; TLF, target‐lesion failure; TLR, target‐lesion revascularisation; TV‐MI, target‐vessel myocardial infarction.
TLF was defined as a composite of cardiac death, target‐vessel myocardial infarction and target‐lesion revascularisation.
NACE was defined as all‐cause death, any myocardial infarction, any unplanned revascularisation, stroke and major bleeding.
Definite or probable stent thrombosis.
Figure 1.
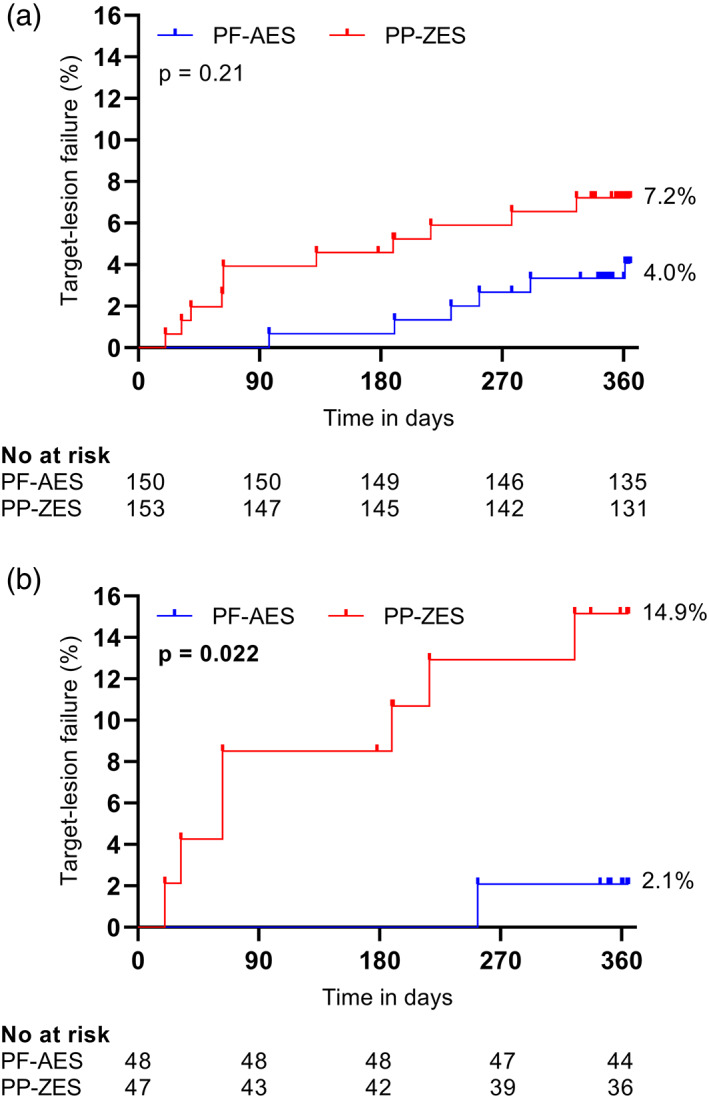
Kaplan–Meier time‐to‐event estimates for TLF compared using log‐rank test. (a) TLF for PP‐ZES and PF‐AES in diabetic patients. (b) TLF for PP‐ZES and PF‐AES in insulin‐dependent diabetic patients. TLF was defined as a composite of cardiac death, target‐vessel myocardial infarction and target‐lesion revascularization. PF‐AES, polymer‐free amphilimus‐eluting stents; PP‐ZES, permanent polymer zotarolimus‐eluting stents; TLF, target‐lesion failure [Color figure can be viewed at wileyonlinelibrary.com]
A total of nine cardiac deaths occurred in the first year following stent implantation. In the PP‐ZES arm, there were two cases of end‐stage heart failure, two unsuccessful resuscitations without a known cause, one unsuccessful resuscitation with major bleeding while on DAPT, and two unknown causes of death. The two cases of cardiac death in the PF‐AES arm were one case of sudden, unwitnessed death and a patient with cardiac decompensation and subsequent asystole.
One case of stent thrombosis (0.7%) occurred in the PF‐AES arm with no cases in the PP‐ZES arm. This case of stent thrombosis occurred in a troponin negative patient 290 days after stent implantation.
Results for the subgroup of IDDM patients are shown in Table 3. Among IDDM patients, TLF was more frequent in patients treated with the PP‐ZES as compared to the PF‐AES (14.9 vs. 2.1%; p = .022). Similarly, there was a higher incidence of NACE among patients in the PP‐ZES arm (29.8 vs. 8.3%; p = .009). The event rate for the individual endpoint of all‐cause death was higher in the PP‐ZES arm (14.9 vs. 2.1%; p = .024).
4. DISCUSSION
The main findings of this pre‐specified subanalysis of the ReCre8 trial are (a) there was no statistically significant difference in TLF between the PP‐ZES arm and the PF‐AES arm; (b) patients treated with the PF‐AES had a lower NACE rate in the diabetic population and the insulin‐dependent diabetic population; and (c) a lower rate of TLF and all‐cause death was observed in patients treated with PF‐AES in a population of insulin‐dependent diabetics.
Patients in this trial reflect a real‐world population of patients undergoing PCI due to its all‐comers design without exclusion criteria for clinical presentation and only one restrictive angiographic criterion.
In the main publication, 15 no significant differences were observed between the two study stents in the overall group of patients undergoing PCI. In the current analysis of a subgroup of diabetic patients, evaluation of differences between the two stent types favored PF‐AES in the diabetic population regarding the endpoint of NACE. Among IDDM patients, evaluation of outcomes favored PF‐AES regarding TLF, NACE, and all‐cause death. The sole case of stent thrombosis in our trial after 290 days was observed in the PF‐AES arm. As this patient presented with troponin negative disease, DAPT duration was one month. Considering this event occurred over eight months after DAPT was discontinued, it does not appear to be linked to early DAPT cessation.
With TLF rates of 7.2% in the PP‐ZES arm and 4.0% in the PF‐AES arm at 12 months follow‐up, our findings were comparable to rates reported in other trials. The ASTUTE registry 19 found a TLF rate of 4.9% 12 months after PF‐AES implantation in a diabetic population. When compared to our results, the Investig8 registry 20 found higher event rates for TLF, target‐lesion revascularization, myocardial infarction, and cardiovascular death in their diabetic population following PF‐AES implantation. These results were assessed at a longer follow‐up duration of 18 months which may explain higher event rates. However, time‐to‐event curves show no new target‐lesion revascularization events after 360 days and only few TLFs in the last six months of follow‐up.
Regarding the PP‐ZES, the BIONICS randomized trial 8 reported higher rates of TLF, myocardial infarction, and target‐lesion revascularization as compared to event rates in our study. Remarkably, none of the patients treated with the PP‐ZES in our diabetic population had a post‐discharge myocardial infarction within 12 months following PCI. In the PF‐AES arm, three cases of post‐discharge myocardial infarction occurred (2.0%). Of the three cases, one (0.7%) was a target‐vessel myocardial infarction indicating TLF. As this difference was not statistically significant, and the ReCre8 trial was not powered for evaluation of subgroups or events occurring at low rates, this is not indicative of any difference between the two study stents.
Similar to the BIONICS trial, patients in the SORT‐OUT III substudy 21 that were implanted with the PP‐ZES had a myocardial infarction rate of 4.7% after 18 months. However, with the exception of cardiac death all evaluated endpoints were notably higher when compared to our event rates, a finding that is unlikely to be due to extended follow‐up duration alone. As compared to the SORT‐OUT III substudy, the higher rate of cardiac death among our patients may in part be attributable to the great difference in clinical presentation with a ST‐segment elevation myocardial infarction among the diabetic population of the SORT‐OUT III (3–7%) and the ReCre8 trial (17%).
A notable finding in our clinical outcomes is the large proportion of revascularizations in a non‐target lesion. From all revascularizations at 12 months follow‐up, 56% in the diabetic population and 60% in the insulin‐dependent diabetic population was not caused by restenosis of the culprit lesion. This is in line with previous studies regarding the impact of disease progression in diabetic patients following PCI. A single‐center study including diabetic patients undergoing multivessel PCI 22 reported that from 21 repeat revascularizations, 12 (57%) were – at least in part – caused by disease progression. Similarly, a study including patients with diabetes after implantation of at least one DES 23 reported that disease progression contributed to 53% of repeat revascularizations. This shows that we need to attend to secondary prevention measures, specifically in this high‐risk population.
In our analyses, the difference in event rates between the diabetic and IDDM population was greater among patients treated with the PP‐ZES. At 12 months follow‐up, the rates of both TLF (7.2 vs. 14.9%) and NACE (15.7 vs. 29.8%) were twice as high in the IDDM population as compared to the entire group of diabetics. Interestingly, this finding was not seen in the population treated with the PF‐AES: NACE was similar at 8% and TLF was even lower in the IDDM population (4.0 vs. 2.1%). This might suggest that the absence of a polymer and the lipophilic (amphilimus) drug carrier is especially beneficial in IDDM patients.
One of the aspects in which our study differs from most contemporary stent studies is the duration of DAPT. Current guidelines by the European Society of Cardiology and European Association for Cardio‐Thoracic Surgery 24 recommend treating patients with six months of DAPT after elective PCI in stable coronary artery disease. With prior studies on the PF‐AES 25 in mind, patients in the ReCre8 trial with troponin negative disease were treated with one month of DAPT. 15 In this subanalysis, over half of our diabetic population consisted of patients with troponin negative disease. In addition to the previously described events, stent thrombosis was low at 0.3% and stroke occurred in 1% of diabetic patients. Major bleeding occurred in 2.0% of patients.
A recently published study design for the SUGAR trial 26 may shed an interesting new light on the current analysis. In a diabetic population, this trial compares the use of improved adaptations of the PP‐ZES and PF‐AES used in this trial. An all‐comers diabetic population undergoing PCI will be randomized to either the Resolute Onyx (successor of the PP‐ZES) or the Cre8 EVO (successor of the PF‐AES). The trial recommends treatment with three to 6 months of DAPT in stable patients and 12 months in patients with an acute coronary syndrome. An estimated 1,164 patients will be followed up to two years and trial completion is expected in the end of 2023.
5. LIMITATIONS
This study has several limitations. First, as this report was a subanalysis of the ReCre8 trial, this analysis was not powered to detect differences between the different subgroups and therefore outcomes are considered to be hypothesis‐generating. As a result, some of the findings may rely on chance. Second, patients were stratified for diabetes based on drug use and medical history at randomization. No additional information was collected for type of diabetes or HbA1c. Therefore, there is a possibility of crossover bias. Lastly, treating physicians and patients were not blinded for the allocated treatment arm. Since the endpoints were defined according to international standards and were rigorously adjudicated by a blinded, independent clinical event committee, we do not expect that the lack of a double‐blind design changed our findings.
6. CONCLUSION
Based on the results of this subanalysis of the ReCre8 trial, diabetic patients could potentially benefit from a dedicated polymer‐free stent design releasing sirolimus formulated with a lipophilic carrier (amphilimus formulation). Future randomized controlled trials should confirm the potential benefit of a PF‐AES in this specific patient population.
7. IMPACT ON DAILY PRACTICE
Diabetic patients are at a higher risk of ischemic events, especially reintervention, after PCI. Based on this pre‐specified subanalysis of the ReCre8 trial, diabetic patients and particularly IDDM patients could potentially benefit from a dedicated polymer‐free stent design with a specific lipophilic (amphilimus) drug carrier.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Data S1. Supplementary Information.
ACKNOWLEDGEMENTS
The authors thank all contributing research nurses, technicians and personnel who participated in the successful execution of this study. The authors express special thanks to Yvonne Breuer, Manager, Department of Research and Development and Astrid Links, Data Manager, University Medical Center Utrecht, and all physicians involved in the execution of this study.
van Hemert ND, Rozemeijer R, Voskuil M, et al. Clinical outcomes after permanent polymer or polymer‐free stent implantation in patients with diabetes mellitus: The ReCre8 diabetes substudy. Catheter Cardiovasc Interv. 2022;366–372. 10.1002/ccd.29685
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
REFERENCES
- 1. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570‐2581. [DOI] [PubMed] [Google Scholar]
- 2. Richardt G, Maillard L, Nazzaro MS, et al. Polymer‐free drug‐coated coronary stents in diabetic patients at high bleeding risk: a pre‐specified sub‐study of the LEADERS FREE trial. Clin Res Cardiol. 2019;108:31‐38. [DOI] [PubMed] [Google Scholar]
- 3. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. [DOI] [PubMed] [Google Scholar]
- 4. Lee TT, Feinberg L, Baim DS, et al. Effect of diabetes mellitus on five‐year clinical outcomes after single‐vessel coronary stenting (a pooled analysis of coronary stent clinical trials). Am J Cardiol. 2006;98:718‐721. [DOI] [PubMed] [Google Scholar]
- 5. Kalkman DN, Woudstra P, den Heijer P, et al. One year clinical outcomes in patients with insulin‐treated diabetes mellitus and non‐insulin‐treated diabetes mellitus compared to non‐diabetics after deployment of the bio‐engineered COMBO stent. Int J Cardiol. 2017;226:60‐64. [DOI] [PubMed] [Google Scholar]
- 6. Stefanini GG, Holmes DRJ. Drug‐eluting coronary‐artery stents. N Engl J Med. 2013;368:254‐265. [DOI] [PubMed] [Google Scholar]
- 7. Dangas GD, Serruys PW, Kereiakes DJ, et al. Meta‐analysis of everolimus‐eluting versus paclitaxel‐eluting stents in coronary artery disease: final 3‐year results of the SPIRIT clinical trials program (clinical evaluation of the Xience V Everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. 2013;6:914‐922. [DOI] [PubMed] [Google Scholar]
- 8. Konigstein M, Ben‐Yehuda O, Smits PC, et al. Outcomes among diabetic patients undergoing percutaneous coronary intervention with contemporary drug‐eluting stents: analysis from the BIONICS randomized trial. JACC Cardiovasc Interv. 2018;11:2467‐2476. [DOI] [PubMed] [Google Scholar]
- 9. Dangas GD, Farkouh ME, Sleeper LA, et al. Long‐term outcome of PCI versus CABG in insulin and non‐insulin‐treated diabetic patients: results from the FREEDOM trial. J Am Coll Cardiol. 2014;64:1189‐1197. [DOI] [PubMed] [Google Scholar]
- 10. Pepe M, Sardella G, Stefanini GG, et al. Impact of insulin‐treated and noninsulin‐treated diabetes mellitus in all‐comer patients undergoing percutaneous coronary interventions with polymer‐free Biolimus‐eluting stent (from the RUDI‐FREE registry). Am J Cardiol. 2019;124:1518‐1527. [DOI] [PubMed] [Google Scholar]
- 11. Pi S‐H, Rhee T‐M, Lee JM, et al. Outcomes in patients with diabetes mellitus according to insulin treatment after percutaneous coronary intervention in the second‐generation drug‐eluting stent era. Am J Cardiol. 2018;121:1505‐1511. [DOI] [PubMed] [Google Scholar]
- 12. Glatz JFC, Luiken JJFP, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367‐417. [DOI] [PubMed] [Google Scholar]
- 13. Woods TC. Dysregulation of the mammalian target of rapamycin and P27Kip1 promotes intimal hyperplasia in diabetes mellitus. Pharmaceuticals. 2013;6:716‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romaguera R, Gómez‐Hospital JA, Gomez‐Lara J, et al. A randomized comparison of reservoir‐based polymer‐free Amphilimus‐eluting stents versus Everolimus‐eluting stents with durable polymer in patients with diabetes mellitus the RESERVOIR clinical trial. JACC Cardiovasc Interv. 2016;9:42‐50. [DOI] [PubMed] [Google Scholar]
- 15. Rozemeijer R, Stein M, Voskuil M, et al. Randomized all‐comers evaluation of a permanent polymer Zotarolimus‐eluting stent versus a polymer‐free Amphilimus‐eluting stent. Circulation. 2019;139:67‐77. [DOI] [PubMed] [Google Scholar]
- 16. Rozemeijer R, Stein M, Frambach P, et al. Rationale and design of amphilimus sirolimus‐eluting stents versus zotarolimus‐eluting stents in all‐comers requiring percutaneous coronary intervention (ReCre8): a multicenter randomized clinical trial. Catheter Cardiovasc Interv. 2018;91:410‐416. [DOI] [PubMed] [Google Scholar]
- 17. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344‐2351. [DOI] [PubMed] [Google Scholar]
- 18. Ellis SG, Vandormael MG, Cowley MJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease: implications for patient selection. Circulation. 1990;82:1193‐1202. [DOI] [PubMed] [Google Scholar]
- 19. Colombo A, Godino C, Donahue M, et al. One‐year clinical outcome of amphilimus polymer‐free drug‐eluting stent in diabetes mellitus patients insight from the ASTUTE registry (AmphilimuS Italian mUlticenTre rEgistry). Int J Cardiol. 2016;214:113‐120. [DOI] [PubMed] [Google Scholar]
- 20. Sardella G, Stella P, Chiarito M, et al. Clinical outcomes with reservoir‐based polymer‐free amphilimus‐eluting stents in real‐world patients according to diabetes mellitus and complexity: the INVESTIG8 registry. Catheter Cardiovasc Interv. 2018;91:884‐891. [DOI] [PubMed] [Google Scholar]
- 21. Maeng M, Jensen LO, Tilsted HH, et al. Outcome of sirolimus‐eluting versus zotarolimus‐eluting coronary stent implantation in patients with and without diabetes mellitus (a SORT OUT III substudy). Am J Cardiol. 2011;108:1232‐1237. [DOI] [PubMed] [Google Scholar]
- 22. Loutfi M, Mulvihill NT, Boccalatte M, Farah B, Fajadet J, Marco J. Impact of restenosis and disease progression on clinical outcome after multivessel stenting in diabetic patients. Catheter Cardiovasc Interv. 2003;58:451‐454. [DOI] [PubMed] [Google Scholar]
- 23. Tousek P, Pavei A, Oreglia J, et al. Impact of atherosclerotic disease progression on mid‐term clinical outcome in diabetic patients in the drug‐eluting stent era. EuroIntervention. 2009;4:588‐592. [DOI] [PubMed] [Google Scholar]
- 24. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardio‐Thoracic Surg. 2018;53:34‐78. [DOI] [PubMed] [Google Scholar]
- 25. Prati F, Romagnoli E, Valgimigli M, et al. Randomized comparison between 3‐month Cre8 des vs. 1‐month vision/Multilink8 BMS neointimal coverage assessed by OCT evaluation: the DEMONSTRATE study. Int J Cardiol. 2014;176:904‐909. [DOI] [PubMed] [Google Scholar]
- 26. Romaguera R, Salinas P, Brugaletta S, et al. Second‐generation drug‐eluting stents in diabetes (SUGAR) trial: rationale and study design. Am Heart J. 2020;222:174‐182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary Information.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.


