Abstract
Background
Uncovering the neural mechanisms that underlie symptoms of attention deficit hyperactivity disorder (ADHD) requires studying brain development prior to the emergence of behavioural difficulties. One new approach to this is prospective studies of infants with an elevated likelihood of developing ADHD.
Methods
We used a prospective design to examine an oscillatory electroencephalography profile that has been widely studied in both children and adults with ADHD – the balance between lower and higher frequencies operationalised as the theta–beta ratio (TBR). In the present study, we examined TBR in 136 10‐month‐old infants (72 male and 64 female) with/without an elevated likelihood of developing ADHD and/or a comparison disorder (Autism Spectrum Disorder; ASD).
Results
Infants with a first‐degree relative with ADHD demonstrated lower TBR than infants without a first‐degree relative with ADHD. Further, lower TBR at 10 months was positively associated with temperament dimensions conceptually related to ADHD at 2 years. TBR was not altered in infants with a family history of ASD.
Conclusions
This is the first demonstration that alterations in TBR are present prior to behavioural symptoms of ADHD. However, these alterations manifest differently than those sometimes observed in older children with an ADHD diagnosis. Importantly, altered TBR was not seen in infants at elevated likelihood of developing ASD, suggesting a degree of specificity to ADHD. Taken together, these findings demonstrate that there are brain changes associated with a family history of ADHD observable in the first year of life.
Keywords: Attention deficit hyperactivity disorder, theta–beta ratio, infancy, autism spectrum disorder, electroencephalography
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a neurodevelopmental condition, with an estimated worldwide prevalence of 3%–5% (Polanczyk, Salum, Sugaya, Caye, & Rohde, 2015). Characterised by symptoms of inattention, hyperactivity and impulsivity, ADHD can negatively impact life expectancy and quality of life (Faraone et al., 2021). In the UK, ADHD is typically diagnosed in middle childhood through a combination of clinical interviews and observer‐reports. However, the genetic factors that predispose an individual towards this highly heritable condition likely act predominately prenatally, affecting brain development for years before the consolidation of the full clinical phenotype (Faraone & Larsson, 2019). Identifying the brain changes that precede the onset of behavioural symptoms could help with earlier identification of individuals who require additional support, and could provide useful outcome measures for early interventions.
Identifying early brain changes can be accomplished by studying the early development of individuals with a high likelihood of later developing ADHD. ADHD is known to be heritable, with increased prevalence in individuals who have a sibling or a parent with the condition (Miller et al., 2019), with both genetic and environmental factors (pre‐ and postnatal) contributing to familial transmission (Thapar et al., 2013). Prospective longitudinal studies follow infants with a family history of ADHD (and who are therefore at elevated likelihood of developing later symptoms) across development. This allows the opportunity to investigate potential early markers of the condition prior to the onset of symptoms. A small number of prospective studies have found differences in attention, activity levels and motor skills in the first years of life in infants with a family history of ADHD, compared to those without (Auerbach, Atzaba‐Poria, Berger, & Landau, 2004; Auerbach et al., 2008; Begum Ali, Charman, Johnson, & Jones, 2020; Miller et al., 2020). However, these studies have largely focused on observational measures of behaviour (measured via parent report or during observations of toy play). Neural differences may emerge prior to overt behaviour in infants who later develop the condition, and could be critical to understanding the mechanisms underlying the later emergence of symptoms.
Electroencephalography (EEG) is a strong candidate method for investigating early neurocognitive markers of ADHD, given its high temporal resolution and suitability for infants and young children. Indeed, previous research suggests that EEG can be used to detect neural phenotypes (including increased absolute and relative theta power) that may be related to ADHD liability in infants (Shephard, Fatori, et al., 2019). Alterations in theta power have long been a target of interest in ADHD. For example, in 2013, the US Food and Drug Administration (FDA) supported the use of the balance between theta and higher frequency power (theta/beta power ratio or TBR, measured using EEG at the central midline of the scalp, Cz) as a measure to provide additional information in the diagnostic assessment process for ADHD (Food & Drug Administration, 2013). Although effect sizes have declined with time, meta‐analyses show that a subset of children with ADHD show differences in TBR and its use as a prognostic indicator has been suggested (Arns, Conners, & Kraemer, 2013). In some studies where TBR did not differ, diagnostic differences in theta power remained (e.g. Kiiski et al., 2020). However, previous work on TBR/theta power have primarily focused on adults and older children who already have a diagnosis of ADHD, contributing to the difficulty in disentangling primary changes in brain activity from secondary effects of ascertainment, treatments and lifestyle changes.
Here, we used EEG to investigate theta power and the theta/beta ratio in 10‐month‐old infants with a family history of ADHD. To examine specificity to family history of ADHD, we also included a comparison condition (ASD). Research on TBR in relation to the core features of ASD is sparse. A recent study conducted by Isaev et al. (2020) found a negative association between average look duration and TBR during the viewing of social video stimuli in autistic children (compared to a positive association in typically developing children), but did not look at group differences in TBR. Previous work has identified reduced theta power in children with ASD+ADHD relative to those with ADHD only (Shephard et al., 2018), the opposite profile to the increased theta power and reduced higher frequency power commonly observed in ADHD (Arns et al., 2013). Thus, we anticipated that the inclusion of infants with a family history of ASD would be an appropriate test of specificity.
We selected this age range because previous studies have indicated that behavioural differences in infants at elevated likelihood of ADHD may begin to emerge from 12 months of age (Hatch et al., 2020); indeed, we recently did not find behavioural differences at 10 months in the present cohort of infants (Goodwin et al., 2021). We were interested in whether a potential neural marker could be detected prior to the presence of observable behaviours related to the ADHD phenotype. In adults and children, TBR is typically measured during resting state, such as during eyes closed or eye open conditions; such resting state EEG paradigms are not appropriate for infants, who cannot follow verbal instructions to sit still or to close their eyes. To keep infants calm and engaged during EEG data collection, infants attended to a set of naturalistic videos suitable for continuous EEG analysis (Jones, Venema, Lowy, Earl, & Webb, 2015). We investigated relative theta (2–5Hz)/low beta ratios (9–14Hz) both over all channels recorded, and specifically at Cz. Of note, we selected 2–5Hz as it has been recently established as the infant‐appropriate theta band definition (Saby & Marshall, 2012; Xie, Mallin, & Richards, 2018). We focus on the lower beta band to minimise potential effects of muscle contamination. Where effects of family history of ADHD were detected, we examined consistency across other definitions of beta (i.e. high beta, 14–20 Hz) and across power in theta and beta bands examined separately.
Longitudinally, we investigated whether TBR at 10 months associated with observable, dimensional measures of temperament at 2 years of age. Temperament refers to stable, constitutionally based individual differences that have been linked to a predisposition for later psychopathology (Kostyrka‐Allchorne, Wass, & Sonuga‐Barke, 2020). We measured the three primary temperamental domains (surgency, effortful control and negative affect) with well‐validated parent‐report measures of toddler temperament (Putnam, Gartstein, & Rothbart, 2006) that show stability across development (Putnam, Rothbart, & Gartstein, 2008) and that associate with later ADHD traits in mid‐childhood (Shephard, Bedford, et al., 2019) and adolescence (Einziger et al., 2018). It was expected that the group at elevated likelihood of ADHD would demonstrate a different TBR profile, and that TBR values in infancy would associate with later ADHD‐like temperament traits. However, given evidence that infant manifestations of cognitive traits of the related neurodevelopmental condition (ASD) can show the opposite pattern to manifestations in childhood (e.g. Jones & Klin, 2013), we did not make a specific prediction as to the direction of these associations.
Methods
Participants and ethical considerations
Participants were recruited for a longitudinal study running from 2013 to 2019 (see Appendix S1.1 for full details). Informed written consent was provided by the parent(s) prior to the commencement of the study. Ethical approval was granted by the National Research Ethics Service and the Research Ethics Committee of the Department of Psychological Sciences, Birkbeck, University of London. Participant families were reimbursed expenses for travel, subsistence and overnight stay if required. Infants were given a certificate and t‐shirt after each visit.
Infants either had a first degree relative with a community clinical diagnosis of ASD (ASD‐L), a first degree relative with community clinical diagnosis or probable ADHD (confirmed with Conners questionnaires; ADHD‐L), or no first‐degree relatives with either diagnosis (TL; Appendix S5). For analysis, each infant in the study was assigned a status for familial likelihood of ASD and ADHD separately (see Appendix S2.1 and Table S1 for further details). This approach allowed us to test the effect of familial likelihood of ASD, familial likelihood of ADHD, and their interaction (see Figure 1a). Our final sample included 93 infants with No ADHD‐L (TL + ASD‐L only) and 43 infants with ADHD‐L (ADHD‐L only + ASD+ADHD‐L).
Figure 1.
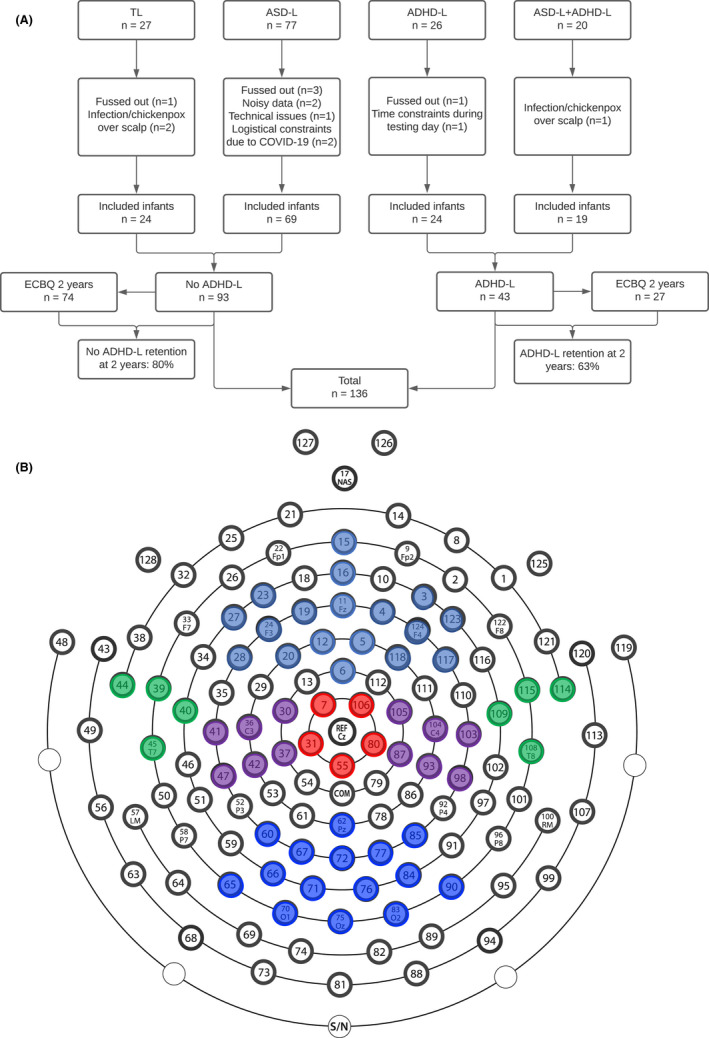
Figure detailing 10 months EEG task and 2 year ECBQ questionnaire attrition (Panel A) and electrode clusters used in analyses over Frontal (pale blue), Parietal (purple), Posterior (blue), Temporal (green) and Cz regions (red). (Panel B). Whole Head analyses involve electrode numbers 1–124
EEG collection
Electroencephalography was recorded continuously using the EGI NetAmps 400 amplifier and EGI (Philips Neuro, Oregon, USA) 128‐electrode Hydrocel Sensor Net, online referenced to Cz at 500Hz. Infants were seated on their parents/caregiver’s lap, 60cm from a screen displaying naturalistic Social (women singing) or Nonsocial (toys moving) dynamic videos (see Appendix S2.2) designed to produce calm attention (Jones et al., 2015) for up to a total of 3 minutes per condition and interspersed through a longer EEG session. All testing took place in a sound attenuated and electrically shielded room (see Appendix S2.3; Appendix S2.3.1).
EEG processing
Electroencephalography was bandpass filtered (0.1–100 Hz), and 1‐second segmented. Data were manually artefact rejected in NetStation 4.5; segments with excessive artefact (e.g. gross motor movement and eye blinks), where infants were not looking at the video or with >25 noisy channels were manually excluded. Infants with fewer than 10 artefact‐free trials in either condition were excluded (see Table 1). Noisy channels were interpolated from neighbouring channels using spline interpolation. 1‐second nonoverlapping segments were referenced to the average reference, imported into Matlab, detrended and subjected to a fast Fourier transform (FFT); with a 1 Hz resolution; values were extracted from 1 to 20 Hz. Power values were logged and averaged across artefact‐free segments and frequency ranges: theta (2–5 Hz) and low beta (9–14 Hz), within a priori topographical groups of electrodes (Figure 1b). Relative power was computed as each band divided by total spectral power from 1 to 20 Hz (see Figure S1). Relative power was computed as each band divided by total spectral power from 1 to 20 Hz (see Figure S1). Previous research has suggested relative power to be more robust than absolute power in developmental populations due to bone thickness, skull resistance and impedances (Benninger, Matthis, & Scheffner, 1984). Further, relative power has greater test–retest reliability, is less prone to artefacts and is more sensitive to changes in the frequency composition across age (Clarke, Barry, McCarthy, & Selikowitz, 2001; Govindan et al., 2017; John et al., 1980). Finally, theta–beta ratio (TBR) was calculated from relative power in the theta/beta band.
Table 1.
Range, mean (and SD) of participant demographics and trial numbers (presented and retained after artefact rejection) for those included in the EEG analysis. Results from one‐way ANOVA testing group differences
| ASD‐L | ADHD‐L | ASD+ADHD‐L | TL | p | |
|---|---|---|---|---|---|
| n | 69 | 24 | 19 | 24 | |
| Age in days (SD) |
287–357 318.99 (15.01) |
278–384 326.08 (27.98) |
300–354 319 (14.71) |
294–358 322.42 (16.48) |
.38 |
| Sex (%female) | 36m, 33f (48%) |
13m, 11f (46%) |
11m, 8f (42%) |
13m, 11f (46%) |
.95 |
| Mullen Composite Score (SD) c |
50–136 87.68 (15.33) |
61–128 85.54 (16.12) |
59–107 82.32 (12.17) |
58–114 88.71 (12.88) |
.46 |
| Maternal education Secondary/Tertiary frequency (%Tertiary) | 19/48 (70%) | 6/18 (75%) | 10/8 (42%) | 2/20 (83%) | .01 b |
| Presented trials | |||||
| Nonsocial (SD) |
50–237 a 153.38 (47.32) |
60–179 170.58 (25.09) |
50–232 a 156.63 (47.31) |
60–212 a 166.08 (30.99) |
.28 |
| Social (SD) |
60–179 170.58 (25.09) |
60–179 171.96 (24.52) |
32–234 a 164.16 (53.08) |
60–213 a 169.67 (30.25) |
.5 |
| Retained trials | |||||
| Nonsocial (SD) |
22–170 98.48 (40.41) |
36–171 100.83 (29.47) |
21–146 91.06 (47.31) |
24–172 103.58 (49.52) |
.77 |
| Social (SD) |
13–169 104.99 (46) |
26–160 110.42 (33.05) |
12–173 110.26 (44.68) |
10–167 101.71 (48.97) |
.89 |
Some infants were presented with a further video in each condition due to technical difficulties (n = 3 TL, n = 4 ASD‐L, n = 1 ASD+ADHD‐L).
Chi square tests demonstrated significant associations between Group and Maternal Education [χ2 (3) = 10.73, p = .01], however control analyses including Maternal Education as a covariate remained substantively the same as analyses presented in the main text.
MSEL population average composite score: M = 100, SD = 15. The composite score for the current sample is lower, further details for this somewhat lower score is given in Appendix S2.4.1
Behavioural measures
We used a number of behavioural measures (see Appendix S2.4). For example, developmental level was assessed for descriptive purposes at 10 months using the Mullen Scales of Early Learning (Mullen, 1995), a standardised measure of developmental ability (see Appendix S2.4.1 for administration details). At 24 months, temperament was measured with the Effortful Control, Surgency and Negative Affect subscales of the parent‐report Early Childhood Behavioural Questionnaire (Appendix S2.4.2).
Analysis strategy
Analyses used generalised estimating equations (GEEs) with compound symmetry and maximum likelihood estimates. The relevant EEG metric was the dependent variable and models included fixed factors of Sex (male, female), Condition (Social, Nonsocial), Likelihood (ASD‐L, ADHD‐L; with the interaction term of ASD‐L*ADHD‐L) and Region (Frontal, Parietal, Temporal, Posterior and Cz; with the interaction term Region*ADHD‐L). Models were repeated with age in days, highest level of maternal education and number of artefact‐free segments as covariates (Appendix S3.3; Table S5), accounting for outliers (Appendix S3.4) or restricted to our ADHD‐only and TL groups (Appendix S3.5–S3.5.2; Table S6); results were substantively the same. We used bivariate correlations for associations with 24‐month trait measures. To demonstrate specificity to the theta band, we repeated our analyses within the alpha band, with analyses demonstrating no effect of ADHD likelihood (see Appendix S3.6). Finally, we conducted a further analysis that showed that our findings generalised to different definitions of theta (4–6 Hz; Shephard, Fatori, et al., 2019; Appendix S3.7).
Results
TBR
Infants with an elevated likelihood of ADHD showed a lower TBR than those with a lower likelihood of ADHD [F(1, 136) = 4.21, p =.042, ηp 2 = .03; Figure 2]. There was no significant effect of an elevated likelihood of ASD [F(1, 136) = .93, p = .335, ηp 2 = .007] or an interaction of ASD*ADHD elevated likelihood [F(1, 136) = .006, p = .939, ηp 2 = 0]; Figure 3.
Figure 2.
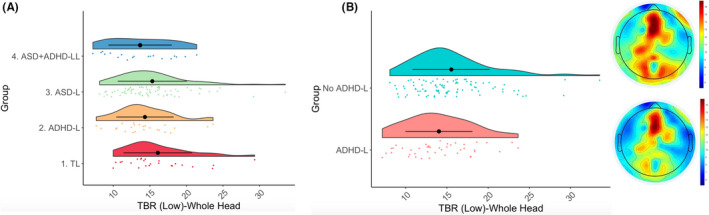
Raincloud plots showing TBR‐Low (collapsed across Condition) over the whole head in our four groups (Panel A) and between our ADHD‐L and No ADHD‐L groups (Panel B), with corresponding topographic plots
Figure 3.
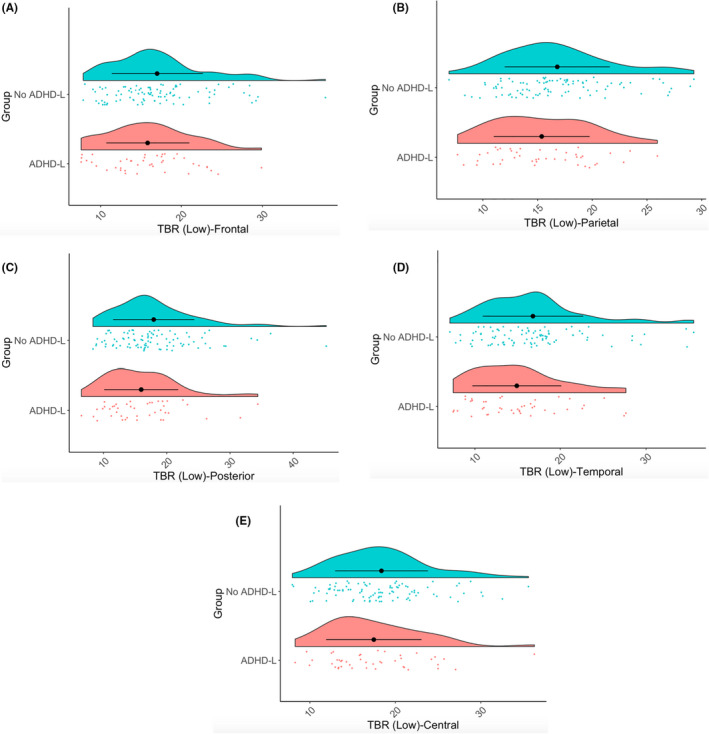
Raincloud plots showing TBR‐Low over frontal (Panel A), parietal (Panel B), posterior (Panel C), temporal (Panel D) and central (Panel E) regions for ADHD‐L and No ADHD‐L participants
As expected (Jones et al., 2015), TBR was greater in the Social versus Nonsocial condition [F(1, 1207) = 17.46, p < .001, ηp 2 = .01; Appendix S3.1; Table S2]. There was a main effect of Region [F(4, 1204) = 48.88, p < .001, ηp 2 = .12; Figure 3], but no interaction of Region and ADHD likelihood [F(4, 1204) = .87, p = .484, ηp 2 = .003], indicating that those with an elevated likelihood of ADHD showed lower TBR across the scalp. Male infants demonstrated higher TBR [F(1, 136) = 4.86, p = .029, ηp 2 = .03]. In an additional GEE model, the interaction of Sex and ADHD‐L did not have any significant influence [F(1, 136) = .2, p = .65, ηp 2 = .001].
Underlying frequency bands
Theta
Theta power was lower in infants with an elevated likelihood of ADHD F(1, 136) = 5.25, p = .023, ηp 2 = .04] across all areas of the brain [F(4, 1205) = 41.93, p < .001, ηp 2 = .12], and lower for the Nonsocial versus Social video [F(1, 1208) = 9.99, p = .002, ηp 2 = .008]. There was no effect of an elevated likelihood of ASD [F(1, 136) = 2.39, p = .125, ηp 2 = .02], nor an interaction of ASD*ADHD‐L [F(1, 136) = 1.11, p = .295, ηp 2 = .008]. There was no effect of Sex [F(1, 136) = .69, p = .408, ηp 2 = .005].
Low beta
Beta power was higher in infants with an elevated likelihood of ADHD [F(1, 143) = 4.32, p = .04, ηp 2 = .03] over all brain regions [F(4, 1150) = 85.87, p < .001, ηp 2 = .23]. Beta power was higher in the Nonsocial condition [F(1, 1152) = 13.04, p < .001, ηp 2 = .01] and higher in females than males [F(1, 143) = 4.86, p = .029, ηp 2 = .03]. There was no effect of an elevated likelihood of ASD [F(1, 143) = .87, p = .353, ηp 2 = .006] or interaction effect [ASD*ADHD; F(1, 143) = .02, p = .888, ηp 2 = .0]. A broadly similar pattern was also found with a higher frequency band definition of beta (Appendix S3.2–3.2.2).
Relationship with temperament
We examined the relationship between TBR‐Low (the TBR in the lower beta band; 9–14 Hz) over the Whole Head collapsed across experimental condition (Social/Nonsocial); see Appendix S3.2.3 for similar patterns with TBR‐High (TBR in the higher theta band; 15–19 Hz). Further, we restricted these correlational analyses to participants with an elevated likelihood of ADHD (to avoid confounding dimensional associations with the group effect already identified; see Appendix S3.2.4 and Table S4 for correlations showing no relationship between TBR and temperament in our No ADHD‐L sample).
Results showed that lower TBR‐Low was associated with greater Surgency [r(25) = −.43, p = .03] at 24 months, but not Effortful Control [r(27) = .12, p = .54] or Negative Affect [r(27) = .10, p = .36]. Exploring associations between TBR‐Low and Surgency components, we found lower levels of TBR‐Low were associated with greater Impulsivity [r(24) = −.5, p = .01] and greater Sociability [r(23) = −.53, p = .01; Figure 4], but not Activity Level, High Intensity Pleasure or Positive Anticipation (all ps > .2; alpha level reduced to p = .01 for multiple correlations; Table S3).
Figure 4.
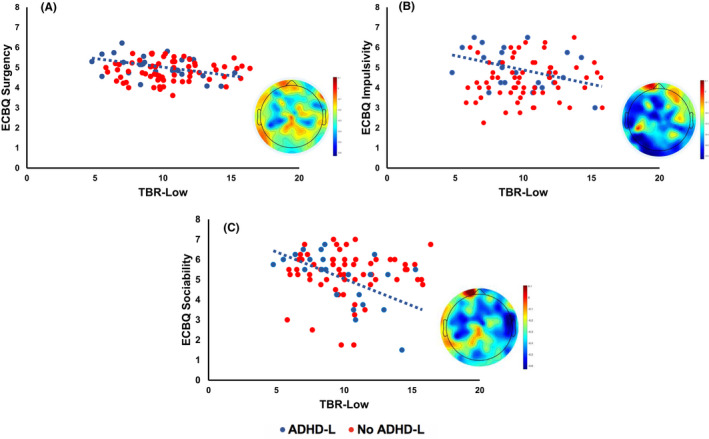
Scatter graphs showing TBR‐Low over the whole head and ECBQ Surgency (Panel A), Impulsivity (Panel B) and Sociability (Panel C) scores in the ADHD‐L group (blue). The No ADHD‐L group is shown in red for comparison. Topographic plots depict the correlation between TBR‐Low and ECBQ scores for the ADHD‐L group
Discussion
We show changes in oscillatory brain activity in 10‐month‐old infants with a family history of ADHD that relate to later individual differences in temperamental impulsivity and sociability. This effect was related to changes in both the theta and beta bands (both lower and upper) and their relative balance (TBR). Further, alterations in TBR were associated with later ADHD‐related traits at 24 months (temperamental surgency, and particularly its constituent subdomains of impulsivity and sociability). The direction of the effect was the opposite to that observed in older children and adults – in infants a family history of ADHD we saw a lower theta/beta ratio, rather than the higher ratio observed more often in older cohorts. These results identify a neural mechanism in infancy that may relate to later temperamental traits often associated with ADHD and further work to examine whether alterations in TBR associate later in childhood to ADHD traits may be warranted.
Increased TBR in children is predominantly driven by greater theta power (Barry, Clarke, Johnstone, McCarthy, & Selikowitz, 2009) and we also found strong effects on the theta band in infancy. In typically developing infants, theta activity has been associated with attention, cognitive control and working memory (Orekhova, Stroganova, Posikera, & Elam, 2006) and task‐dependent dynamic changes in theta power have been linked to learning, memory and attention (Begus & Bonawitz, 2020; Jones et al., 2020). This raises the possibility that the observed infant changes in TBR may reflect the early emergence of cognitive control difficulties in our cohort. Indeed, it has been proposed that in adulthood the theta‐beta ratio reflects cognitive demand and attentional control (e.g. van Son et al., 2019). However, it is important to note that we did not see any relations between TBR and effortful control (a parent‐report measure that includes measures of attentional control) in the current study. Rather, we observed relations with temperamental surgency and impulsivity. Interestingly, a recent neurofeedback study demonstrated that modulations in theta/beta ratio may reduce impulsivity through alterations in medial frontal inhibitory control (Bluschke, Broschwitz, Kohl, Roessner, & Beste, 2016). Others have proposed that ADHD‐related impulsivity and surgency may rather be underpinned by dysregulation in emotional states (e.g. Martel, 2009) and related to altered physiological reactivity (Karalunas, Gustafsson, Fair, Musser, & Nigg, 2019). Further work with direct measures of attention, cognition and psychophysiology in infancy could help unpack the psychological mechanisms that relate to the neural changes we observe. Indeed, future work could also examine the link between TBR and early emerging cognitive control, in the context of the current task, in more traditional resting state paradigms and also in tasks that are designed to manipulate endogenous attention.
Of note, our results differ from a recent study that found higher theta power over posterior regions in 6‐month‐old infants with mothers demonstrating higher levels of ADHD symptoms (Shephard, Fatori, et al., 2019). When examining the current study with the same parameters (4–6Hz posterior region), we confirmed our finding of lower theta in our cohort. The age difference between the studies may be relevant (6 vs. 10 months); further longitudinal analysis will be required to examine this possibility. Alternatively, whilst both studies presented infants with videos, the content of the stimuli were quite different (e.g. abstract shapes used by Shephard, Fatori, et al., 2019 vs. spinning toys and singing ladies in the current study). Although our findings did not interact with the Social versus Nonsocial context of recording, it may be that variation in stimulus materials may contribute to cross‐study differences.
The observation that lower (rather than higher) TBR relates both to familial likelihood of ADHD and later ADHD‐related traits is also different from the most common direction of effect in older children (Arns et al., 2013). In general, over development there are decreases in power across all frequency bands that are most pronounced in the lower frequencies (Uhlhaas, Roux, Rodriguez, Rotarska‐Jagiela, & Singer, 2010). Thus, a lower theta–beta ratio could be viewed as an index of relatively accelerated development. In the related literature on infants with later ASD, there are other reports of developmental reversals from an initial pattern of apparently greater maturity to relatively less maturity than controls. For example, infants with later ASD also look more at the eyes of a caregiver at 2 months relative to typically developing infants, but less at the eyes by 36 months (Jones & Klin, 2013). Such patterns favour models in which it is the speed and nature of developmental trajectories that are altered in neurodevelopmental disorders, rather than static or modular views of particular atypicalities. Longitudinal investigation is required to test this possibility.
An alternative explanation is that the direction of our effect reflects differences in the contexts in which EEG is recorded. In older populations, theta/beta ratio is typically measured during ‘resting state’, where participants look at a fixation cross or close their eyes and sit still for a prolonged period. Although referred to as ‘rest’, this may in fact require significant behavioural control in individuals with ADHD. Indeed, individuals with ADHD show an attenuation of the typical increase in theta from baseline to task (Skirrow et al., 2015). This potentially indicates a greater exertion of attentional control during a baseline or waiting period (Hsu, Broyd, Helps, Benikos, & Sonuga‐Barke, 2013; Loo et al., 2009). In comparison, when testing infants, explicit instructions to sit still cannot be used. Thus, as discussed previously, we measured TBR during passive viewing of relatively engaging naturalistic videos. These may have captured the infant’s attention without the need for excessive behavioural control, and thus we may see the more ‘natural’ lower levels of theta/beta ratio in infancy. Of note, one advantage is that this design is also less likely to generate results contaminated by motion artefact (Appendix S4).
Whilst early studies of children with ADHD consistently reported a higher theta/beta ratio and higher theta power relative to controls, some more recent investigations have actually observed lower theta power, in line with our data (e.g. during eyes closed rest Giertuga et al., 2017). Indeed, the direction of effects of TBR studies appear to be reversing over time in the field, with more recent (compared to earlier) studies showing comparable levels of TBR across groups, as a result of increases in TBR in the control group (Arns et al., 2013). The underlying processes remain unclear, but may include inclusion/exclusion criteria, publication bias and decreases in sleep duration over the years (Arns et al., 2013b). Our work may reignite interest in TBR by demonstrating that atypicalities in this measure of brain activity are present prior to diagnosis of ADHD. Indeed, Arns et al. (2013) note that one particularly promising use of TBR may be as a prognostic, rather than a diagnostic, marker. Further, our results do suggest some specificity of the TBR in that we only see this altered neural response in infants with an elevated likelihood of ADHD, and not ASD. We also observed relations with ADHD‐relevant temperamental traits (Surgency, Impulsivity and Sociability; Karalunas et al., 2019; Kerner auch Koerner, Gust, & Petermann, 2018; Sullivan et al., 2015). Indeed, infants with an elevated likelihood of ADHD demonstrate increased levels of Impulsivity from as early as 12 months (Miller et al., 2020). Importantly, Surgency is also related to ADHD genetic risk, suggesting shared familial mechanisms (Nigg et al., 2020); Sociability has been related to externalising problems, which has a greater occurrence rate in ADHD populations (e.g. Gartstein et al., 2012; Nigg, Goldsmith, & Sachek, 2004). Thus, the temperamental relations we observe may meaningfully relate to ADHD‐relevant individual differences, though further replication is required. Further, longitudinal investigation will be required to determine whether the oscillatory differences observed relate to later ADHD symptoms once they become measurable. Future work should also investigate other EEG measures putatively relevant to ADHD such as absolute and relative neural power, alpha asymmetry, functional connectivity and sensory gating (see Table S7).
It is important to note that, by the 2‐year time point, there was a differential attrition rate, with fewer families in our ADHD likelihood group able to attend an in‐person lab visit. Further, a proportion of our sample of infants with a family history of ADHD also had a family history of co‐occurring ASD. However, we view this as a strength of our study design, as given the fact that there were no interactions of ASD likelihood, the current study was able to determine that altered TBR was present in only those with a family history of ADHD.
Summary
In a prospective study, we show that changes in brain signals observed in children with ADHD may be present in infancy. Specifically, we show a lower theta‐beta ratio in infants with a first degree relative with ADHD. Theta‐beta ratio at 10 months of age was associated with later ADHD‐related temperamental traits at 2 years of age. Further work will be required to determine whether the signatures we observe map onto a later ADHD diagnosis, how they change with age, and whether or not they are only present in a subgroup of individuals with later ADHD diagnosis. Understanding the neural mechanisms in infancy that underlie the emergence of later ADHD‐related traits could be an important step in identifying appropriate targets or outcome measures for early interventions implemented during a period of heightened plasticity (Goodwin et al., 2016; Jones, Dawson, Kelly, Estes, & Webb, 2017).
Disclaimer
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.) Any views expressed are those of the author(s) and not necessarily those of the funders. [Correction added on 30 March 2022, after first online publication: The disclaimer has been added in this version.]
Key points.
Previous research has shown atypical theta–beta ratios in ADHD populations (both in childhood and adulthood). This research has been conducted on populations subsequent to a diagnosis of ADHD.
The current study demonstrates atypicalities in theta‐beta ratios prior to a diagnosis of ADHD (e.g. in 10‐month‐old infants at an elevated likelihood of ADHD).
Lower theta–beta ratios were specific to our ADHD sample and not seen in our elevated likelihood ASD sample.
Theta–beta ratios in infancy were related to ADHD‐relevant temperamental traits at 2 years.
Theta–beta ratios could be used as a prognostic, rather than diagnostic, marker of ADHD.
Supporting information
Appendix S1. Relative power figure.
Appendix S2. Methods.
Appendix S3. Results.
Appendix S4. Motion.
Appendix S5. Medical and Psychiatric History interview.
Acknowledgements
This research was supported by awards from the Medical Research Council (MR/K021389/1; M.H.J., T.C.) and (MR/T003057/1; E.J.H.J., M.H.J., T.C.), MQ (MQ14PP_83, M.H.J., E.J.H.J., T.C.). Further, this work was also supported by the EU‐AIMS and AIMS‐2‐TRIALS programmes funded by the Innovative Medicines Initiative (IMI) Joint Undertaking Grant Nos. 115300 (M.H.J., T.C.) and No. 777394 (M.H.J., E.J.H.J. and T.C.; European Union’s FP7 and Horizon 2020, respectively). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme, with in‐kind contributions from the European Federation of Pharmaceutical Industries and Associations (EFPIA) companies and funding from Autism Speaks, Autistica and SFARI.
The authors would like to thank the researchers who helped with data collection and recruitment; Kim Davies, Janice Fernandes, Marian Greensmith, and Natalie Vaz. The authors would also like to thank the placement student (Zoë Freeman) who helped in data collection. Finally, the authors would like to warmly thank all the parents and infants that took part in this study. The STAARS team consists of: Mary Agyapong, Tessel Bazelmans, Leila Dafner, Mutluhan Ersoy, Laurel Fish, Teodora Gliga, Rianne Haartsen, Alexandra Hendry, Rebecca Holman, Sarah Kalwarowsky, Anna Kolesnik, Laura Pirazzoli and Chloë Taylor. T.C. has served as a paid consultant to F. Hoffmann‐La Roche Ltd and Servier. He has received royalties from Sage Publications and Guildford Publications.
The remaining authors have declared that they have no competing or potential conflicts of interest.
Conflict of interest statement: See Acknowledgements for full disclosures.
References
- Arns, M. , Conners, C.K. , & Kraemer, H.C. (2013). A decade of EEG theta/beta ratio research in ADHD: A meta‐analysis. Journal of Attention Disorders, 17, 374–383. [DOI] [PubMed] [Google Scholar]
- Auerbach, J.G. , Atzaba‐Poria, N. , Berger, A. , & Landau, R. (2004). Emerging developmental pathways to ADHD: Possible path markers in early infancy. Neural Plasticity, 11, 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach, J.G. , Berger, A. , Atzaba‐Poria, N. , Arbelle, S. , Cypin, N. , Friedman, A. , & Landau, R. (2008). Temperament at 7, 12, and 25 months in children at familial risk for ADHD. Infant and Child Development, 17, 321–338. [Google Scholar]
- Barry, R.J. , Clarke, A.R. , Johnstone, S.J. , McCarthy, R. , & Selikowitz, M. (2009). Electroencephalogram θ/β ratio and arousal in attention‐deficit/hyperactivity disorder: Evidence of independent processes. Biological Psychiatry, 66, 398–401. [DOI] [PubMed] [Google Scholar]
- Begum Ali, J. , Charman, T. , Johnson, M.H. , & Jones, E.J. , & BASIS/STAARS Team (2020). Early motor differences in infants at elevated likelihood of autism spectrum disorder and/or attention deficit hyperactivity disorder. Journal of Autism and Developmental Disorders, 50, 4367–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begus, K. , & Bonawitz, E. (2020). The rhythm of learning: Theta oscillations as an index of active learning in infancy. Developmental Cognitive Neuroscience, 45, 100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger, C. , Matthis, P. , & Scheffner, D. (1984). EEG development of healthy boys and girls. Results of a longitudinal study. Electroencephalography and Clinical Neurophysiology, 57, 1–12. [DOI] [PubMed] [Google Scholar]
- Bluschke, A. , Broschwitz, F. , Kohl, S. , Roessner, V. , & Beste, C. (2016). The neuronal mechanisms underlying improvement of impulsivity in ADHD by theta/beta neurofeedback. Scientific Reports, 6, 31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, A.R. , Barry, R.J. , McCarthy, R. , & Selikowitz, M. (2001). Age and sex effects in the EEG: Development of the normal child. Clinical Neurophysiology, 112, 806–814. [DOI] [PubMed] [Google Scholar]
- Einziger, T. , Levi, L. , Zilberman‐Hayun, Y. , Auerbach, J.G. , Atzaba‐Poria, N. , Arbelle, S. , & Berger, A. (2018). Predicting ADHD symptoms in adolescence from early childhood temperament traits. Journal of Abnormal Child Psychology, 46, 265–276. [DOI] [PubMed] [Google Scholar]
- Faraone, S.V. , Banaschewski, T. , Coghill, D. , Zheng, Y.I. , Biederman, J. , Bellgrove, M.A. , … & Wang, Y. (2021). The World Federation of ADHD International Consensus Statement: 208 Evidence‐based conclusions about the disorder. Neuroscience & Biobehavioral Reviews, 128, 789–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone, S.V. , & Larsson, H. (2019). Genetics of attention deficit hyperactivity disorder. Molecular Psychiatry, 24, 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2013). De novo classification request for neuropsychiatric EEG‐based assessment aid for ADHD (NEBA) system. K112711. [Google Scholar]
- Gartstein, M.A. , Putnam, S. , Rothbart, M.K. , Gartstein, M.A. , Putnam, S.P. , & Rothbart, M.K. (2012). Etiology of preschool behavior problems: Contributions of temperament attributes in early childhood. Infant Mental Health Journal, 33, 197–211. [DOI] [PubMed] [Google Scholar]
- Giertuga, K. , Zakrzewska, M.Z. , Bielecki, M. , Racicka‐Pawlukiewicz, E. , Kossut, M. , & Cybulska‐Klosowicz, A. (2017). Age‐related changes in resting‐state EEG activity in attention deficit/hyperactivity disorder: A cross‐sectional study. Frontiers in Human Neuroscience, 11, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, A. , Hendry, A. , Mason, L. , Bazelmans, T. , Begum Ali, J. , Pasco, G. , … & Johnson, M. H. (2021). Behavioural Measures of Infant Activity but Not Attention Associate with Later Preschool ADHD Traits. Brain sciences, 11(5), 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, A. , Salomone, S. , Bolton, P. , Charman, T. , Jones, E.J.H. , Pickles, A. , … & Johnson, M.H. (2016). Attention training for infants at familial risk of ADHD (INTERSTAARS): Study protocol for a randomised controlled trial. Trials, 17, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan, R.B. , Massaro, A. , Vezina, G. , Tsuchida, T. , Cristante, C. , & Du Plessis, A. (2017). Does relative or absolute EEG power have prognostic value in HIE setting? Clinical Neurophysiology, 128, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch, B. , Iosif, A.‐M. , Chuang, A. , de la Paz, L. , Ozonoff, S. , & Miller, M. (2020). Longitudinal differences in response to name among infants developing ASD and risk for ADHD. Journal of Autism and Developmental Disorders, 51, 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, C.‐F. , Broyd, S.J. , Helps, S.K. , Benikos, N. , & Sonuga‐Barke, E.J.S. (2013). “Can waiting awaken the resting brain?” A comparison of waiting‐ and cognitive task‐induced attenuation of very low frequency neural oscillations. Brain Research, 1524, 34–43. [DOI] [PubMed] [Google Scholar]
- Isaev, D.Y. , Major, S. , Murias, M. , Carpenter, K.L. , Carlson, D. , Sapiro, G. , & Dawson, G. (2020). Relative average look duration and its association with neurophysiological activity in young children with autism spectrum disorder. Scientific Reports, 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, E.R. , Ahn, H. , Prichep, L. , Trepetin, M. , Brown, D. , & Kaye, H. (1980). Developmental equations for the electroencephalogram. Science, 210, 1255–1258. [DOI] [PubMed] [Google Scholar]
- Jones, E.J.H. , Dawson, G. , Kelly, J. , Estes, A. , & Webb, S.J. (2017). Parent‐delivered early intervention in infants at risk for ASD: Effects on electrophysiological and habituation measures of social attention. Autism Research, 10, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E.J.H. , Goodwin, A. , Orekhova, E. , Charman, T. , Dawson, G. , Webb, S.J. , & Johnson, M.H. (2020). Infant EEG theta modulation predicts childhood intelligence. Scientific Reports, 10, 11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E.J.H. , Venema, K. , Lowy, R. , Earl, R.K. , & Webb, S.J. (2015). Developmental changes in infant brain activity during naturalistic social experiences. Developmental Psychobiology, 57, 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, W. , & Klin, A. (2013). Attention to eyes is present but in decline in 2–6‐month‐old infants later diagnosed with autism. Nature, 504, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas, S.L. , Gustafsson, H.C. , Fair, D. , Musser, E.D. , & Nigg, J.T. (2019). Do we need an irritable subtype of ADHD? Replication and extension of a promising temperament profile approach to ADHD subtyping. Psychological Assessment, 31, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner auch Koerner, J. , Gust, N. , & Petermann, F. (2018). Developing ADHD in preschool: Testing the dual pathway model of temperament. Applied Neuropsychology: Child, 7, 366–373. [DOI] [PubMed] [Google Scholar]
- Kiiski, H. , Bennett, M. , Rueda‐Delgado, L.M. , Farina, F.R. , Knight, R. , Boyle, R. , … & Whelan, R. (2020). EEG spectral power, but not theta/beta ratio, is a neuromarker for adult ADHD. European Journal of Neuroscience, 51, 2095–2109. [DOI] [PubMed] [Google Scholar]
- Kostyrka‐Allchorne, K. , Wass, S.V. , & Sonuga‐Barke, E.J.S. (2020). Research Review: Do parent ratings of infant negative emotionality and self‐regulation predict psychopathology in childhood and adolescence? A systematic review and meta‐analysis of prospective longitudinal studies. Journal of Child Psychology and Psychiatry, 61, 401–416. [DOI] [PubMed] [Google Scholar]
- Loo, S.K. , Hale, T.S. , Macion, J. , Hanada, G. , McGough, J.J. , McCracken, J.T. , & Smalley, S.L. (2009). Cortical activity patterns in ADHD during arousal, activation and sustained attention. Neuropsychologia, 47, 2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel, M.M. (2009). Research Review: A new perspective on attention‐deficit/hyperactivity disorder: emotion dysregulation and trait models. Journal of Child Psychology and Psychiatry, 50, 1042–1051. [DOI] [PubMed] [Google Scholar]
- Miller, M. , Iosif, A.‐M. , Bell, L.J. , Farquhar‐Leicester, A. , Hatch, B. , Hill, A. , … Ozonoff, S. (2020). Can familial risk for ADHD be detected in the first two years of life? Journal of Clinical Child & Adolescent Psychology, 50, 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. , Musser, E.D. , Young, G.S. , Olson, B. , Steiner, R.D. , & Nigg, J.T. (2019). Sibling recurrence risk and cross‐aggregation of attention‐deficit/hyperactivity disorder and autism spectrum disorder. JAMA Pediatrics, 173, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, E.M. (1995). Mullen scales of early learning. Circle Pines, MN: AGS. [Google Scholar]
- Nigg, J. , Goldsmith, H. , & Sachek, J. (2004). Temperament and attention deficit hyperactivity disorder: The development of a multiple pathway model. Journal of Clinical Child and Adolescent Psychology, 53, 42–53. [DOI] [PubMed] [Google Scholar]
- Nigg, J.T. , Karalunas, S.L. , Gustafsson, H.C. , Bhatt, P. , Ryabinin, P. , Mooney, M.A. , … & Wilmot, B. (2020). Evaluating chronic emotional dysregulation and irritability in relation to ADHD and depression genetic risk in children with ADHD. Journal of Child Psychology and Psychiatry, 61, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova, E.V. , Stroganova, T.A. , Posikera, I.N. , & Elam, M. (2006). EEG theta rhythm in infants and preschool children. Clinical Neurophysiology, 117, 1047–1062. [DOI] [PubMed] [Google Scholar]
- Polanczyk, G.V. , Salum, G.A. , Sugaya, L.S. , Caye, A. , & Rohde, L.A. (2015). Annual Research Review: A meta‐analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of Child Psychology and Psychiatry, 56, 345–365. [DOI] [PubMed] [Google Scholar]
- Putnam, S.P. , Gartstein, M.A. , & Rothbart, M.K. (2006). Measurement of fine‐grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behavior and Development, 29, 386–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam, S.P. , Rothbart, M.K. , & Gartstein, M.A. (2008). Homotypic and heterotypic continuity of fine‐grained temperament during infancy, toddlerhood, and early childhood. Infant and Child Development, 17, 387–405. [Google Scholar]
- Saby, J.N. , & Marshall, P.J. (2012). The utility of EEG band power analysis in the study of infancy and early childhood. Developmental Neuropsychology, 37, 253–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard, E. , Bedford, R. , Milosavljevic, B. , Gliga, T. , Jones, E.J.H. , Pickles, A. , … & The BASIS Team (2019). Early developmental pathways to childhood symptoms of attention‐deficit hyperactivity disorder, anxiety and autism spectrum disorder. Journal of Child Psychology and Psychiatry, 60, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard, E. , Fatori, D. , Mauro, L.R. , de Medeiros Filho, M.V. , Hoexter, M.Q. , Chiesa, A.M. , … & Polanczyk, G.V. (2019). Effects of maternal psychopathology and education level on neurocognitive development in infants of adolescent mothers living in poverty in Brazil. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4, 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard, E. , Tye, C. , Ashwood, K.L. , Azadi, B. , Asherson, P. , Bolton, P.F. , & McLoughlin, G. (2018). Resting‐state neurophysiological activity patterns in young people with ASD, ADHD, and ASD + ADHD. Journal of Autism and Developmental Disorders, 48, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow, C. , McLoughlin, G. , Banaschewski, T. , Brandeis, D. , Kuntsi, J. , & Asherson, P. (2015). Normalisation of frontal theta activity following methylphenidate treatment in adult attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology, 25, 85–94. [DOI] [PubMed] [Google Scholar]
- Sullivan, E.L. , Holton, K.F. , Nousen, E.K. , Barling, A.N. , Sullivan, C.A. , Propper, C.B. , & Nigg, J.T. (2015). Early identification of ADHD risk via infant temperament and emotion regulation: A pilot study. Journal of Child Psychology and Psychiatry, 56, 949–957. [DOI] [PubMed] [Google Scholar]
- Thapar, A. , Cooper, M. , Eyre, O. & Langley, K. (2013). Practitioner review: What have we learnt about the causes of ADHD? Journal of Child Psychology and Psychiatry, 54, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas, P.J. , Roux, F. , Rodriguez, E. , Rotarska‐Jagiela, A. , & Singer, W. (2010). Neural synchrony and the development of cortical networks. Trends in Cognitive Sciences, 14, 72–80. [DOI] [PubMed] [Google Scholar]
- van Son, D. , De Blasio, F.M. , Fogarty, J.S. , Angelidis, A. , Barry, R.J. , & Putman, P. (2019). Frontal EEG theta/beta ratio during mind wandering episodes. Biological Psychology, 140, 19–27. [DOI] [PubMed] [Google Scholar]
- Xie, W. , Mallin, B.M. , & Richards, J.E. (2018). Development of infant sustained attention and its relation to EEG oscillations: An EEG and cortical source analysis study. Developmental Science, 21, e12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Relative power figure.
Appendix S2. Methods.
Appendix S3. Results.
Appendix S4. Motion.
Appendix S5. Medical and Psychiatric History interview.


