Abstract
Schwannomatosis comprises a group of hereditary tumor predisposition syndromes characterized by, usually benign, multiple nerve sheath tumors, which frequently cause severe pain that does not typically respond to drug treatments. The most common schwannomatosis‐associated gene is NF2, but SMARCB1 and LZTR1 are also associated. There are still many cases in which no pathogenic variants (PVs) have been identified, suggesting the existence of as yet unidentified genetic risk factors. In this study, we performed extended genetic screening of 75 unrelated schwannomatosis patients without identified germline PVs in NF2, LZTR1, or SMARCB1. Screening of the coding region of DGCR8, COQ6, CDKN2A, and CDKN2B was carried out, based on previous reports that point to these genes as potential candidate genes for schwannomatosis. Deletions or duplications in CDKN2A, CDKN2B, and adjacent chromosome 9 region were assessed by multiplex ligation‐dependent probe amplification analysis. Sequencing analysis of a patient with multiple schwannomas and melanomas identified a novel duplication in the coding region of CDKN2A, disrupting both p14ARF and p16INK4a. Our results suggest that none of these genes are major contributors to schwannomatosis risk but the possibility remains that they may have a role in more complex mechanisms for tumor predisposition.
Keywords: candidate genes, CDKN2A, CDKN2B, COQ6, DGCR8, schwannomatosis screening
1. INTRODUCTION
Schwannomatosis comprises a group of autosomal dominant tumor predisposition syndromes characterized by the development of multiple schwannomas. The most common form is associated with the NF2 gene, but at least two further genetically distinct forms exist. Causative variants for non‐NF2‐related schwannomatosis have been primarily identified in two genes; SMARCB1 (SWI/SNF‐related, matrix‐associated, actin dependent regulator of chromatin, subfamily b, member 1) and LZTR1 (leucine zipper like transcription regulator 1), both located in the chromosome 22q region although these variants only account for 30%−40% of sporadic cases and 70%−80% of familial cases (Evans et al., 2018; Hulsebos et al., 2007; Kehrer‐Sawatzki et al., 2017; Piotrowski et al., 2014). In addition, the majority of non‐NF2‐related schwannomatosis cases are sporadic (MacCollin et al., 2005), suggesting the existence of novel schwannomatosis variants and/or genes.
Previous studies have proposed a role for additional genes in the pathogenesis of schwannomatosis. Whole exome sequencing (WES) analysis of 10 Korean sporadic schwannomatosis patients, identified 26 variants of which 13 were predicted to be pathogenic from in silico analysis. One of these potentially pathogenic variants (PVs) was a missense change (NM_000077.4:c.85C>A; p.Ala29Ser) located in exon 1 of the cyclin dependent kinase inhibitor 2A (CDKN2A) gene, in the chromosome 9p21.3 region (Min et al., 2020).
CDKN2A encodes two proteins, p16INK4a and the alternatively translated p14ARF, both of which have a role in tumor suppression, through regulation of Rb and p53 pathways (Quelle et al., 1995; Zhang et al., 1998a,b). Loss of function of both CDKN2A and its tandemly linked gene CDKN2B, which encodes p15INK4b, another regulator of the Rb pathway (Hannon & Beach, 1994), have been implicated in a variety of cancers from central nervous system (CNS) tumors, including schwannomas (Ali et al., 2021; Almeida et al., 2008; Cancer Genome Atlas Research, 2008; S. Zhang et al., 1996) pancreatic cancer, renal cancer, and melanoma (Goldstein et al., 2006; Jafri et al., 2015; McNeal et al., 2015; Patel et al., 2020; Tu et al., 2018). Indeed, CDKN2A is one of the main susceptibility genes for familial melanoma with both point mutations and gene deletions implicated in pathogenesis (Goldstein & Tucker, 1997; Hussussian et al., 1994; Kamb et al., 1994; Pollock et al., 1998; Whiteman et al., 1997). In addition, a splicing variant in CDKN2A (NM_000077.4:c.151–1G>C), responsible for loss of p16INK4a and p14ARF has been reported in a number of families affected by multiple neoplasms, including nerve sheath tumors and melanomas (Petronzelli et al., 2001; Prowse et al., 2003; Sargen et al., 2016). Notably in the most recent of these reports, Sargen et al. (2016) observed that a number of nerve sheath tumors, across affected family members carrying the CDKN2A variant, presented features consistent with both schwannoma as well as neurofibroma histopathology.
Other proposed candidate genes for schwannomatosis include the coenzyme Q6, monooxygenase (COQ6) gene, and DGCR8 microprocessor complex subunit (DGCR8). There has been one report of a constitutional missense variant in exon 6 of COQ6 (NM_182476.2: c.622G>C; p.Asp208His) segregating with disease in a schwannomatosis affected family (K. Zhang et al., 2014). More recently, a study identified a germline variant in exon 7 of DGCR8 (NM_022720.6:c.1552G>A; p.Glu518Lys) in all affected members of a family with both euthyroid multinodular goiter (MNG) and schwannomatosis (Rivera et al., 2020). This variant, which was predicted to be pathogenic by a number of algorithms and by in silico models, was subsequently characterized to determine its role in disruption of micro RNA biogenesis. Furthermore, a recent analysis of 13 schwannomas from patients affected by schwannomatosis and MNG identified the p.Glu518Lys pathogenic variant in DGCR8 as the only germline pathogenic variant in four of these tumors (Nogué et al., 2022). All 13 tumors were found to have loss of heterozygosity (LOH) in the chromosome 22q region containing DGCR8, LZTR1, SMARCB1, and NF2. For 5/13 tumors, all from the same individual, no other germline PVs in other schwannomatosis genes were identified and no somatic NF2 variants were identified in 4/5 tumors resected from this patient. The authors propose a new model for schwannoma formation in which the inactivating mutation in DGCR8 constitutes the first hit, whereas loss of the second DGCR8 allele, along with LZTR1, SMARCB1, and NF2 constitute hits 2, 3, 4, and 5. For some of these tumor, a sixth somatic hit was also seen in NF2 in the remaining 22q allele.
The purpose of this study was to assess the contribution of variants in COQ6, DGCR8, CDKN2A, and CDKN2B to pathogenesis in a group of patients whose clinical features are consistent with schwannomatosis diagnosis, but for whom routine genetic analysis failed to identify PVs in the known schwannomatosis genes; NF2, SMARCB1, and LZTR1. These individuals were also negative for germline chromosome 22q11.2 deletions, to confirm schwannomatosis diagnosis.
2. MATERIALS AND METHODS
DNA extracted from lymphocytes of 77 schwannomatosis patients from 75 schwannomatosis families, from the local register at the Manchester Center for Genomic Medicine was used for analysis. Demographic data for our cohort is summarized in Table 1. All patients included in the study met current clinical diagnostic criteria for schwannomatosis (Evans et al., 2018). These patients had also previously undergone routine genetic screening from which no PVs in NF2, SMARCB1, or LZTR1 were identified. Routine analysis for schwannomatosis consists of screening of the coding region of NF2, SMARCB1, and LZTR1, including 15 base pairs of intronic region at each side of exon−intron boundaries as well as part of the untranslated regions where PVs are known to occur. Forty‐two patient samples in our cohort were received in or after 2013 and have been screened using next generation sequencing (NGS) with a mean coverage of ×1000 for NF2, optimized for detection of mosaicism to a level of 5%. NGS analysis was also carried out at a read depth of ×350 for SMARCB1 and LZTR1 on 46/77 and 52/77 patients, respectively. For patients for whom NF2 screening was carried out by Sanger sequencing only (34/77 individuals), one or more tumor samples were analyzed when available (10/34). Clinical genetic testing techniques used for screening of index cases are summarized in Supporting Information: Table S1. Genetic testing of two anatomically distinct tumor samples ruled out a diagnosis of mosaic NF2 for 2 of these patients. A summary of molecular testing of these tumors is presented in Supporting Information: Table S2. The remaining 32/35 whose samples were collected before 2013, and did not undergo NGS screening for NF2 variants, were classified as schwannomatosis patients based on current clinical diagnostic criteria (Evans et al., 2018), but mosaic NF2 has not been excluded genetically. A summary of clinical details and results from clinical genetic testing for patients in our cohort is provided in Supporting Information: Table S2.
Table 1.
Summary of demographics for cohort of schwannomatosis patients
| % of total | |||||
|---|---|---|---|---|---|
| Age at the time of genetic screening | |||||
| Male | Female | Familial | Sporadic | 0−30 | >30 |
| 57 | 43 | 12 | 88 | 19 | 81 |
The presence of copy number variants is also routinely assessed through multiplex ligation‐dependent probe amplification (MLPA) analysis of NF2 (probe‐set P044; MRC Holland), SMARCB1 (probe‐set P258; MRC Holland), and LZTR1 (probe‐set P455; MRC Holland). Finally, LOH is assessed in tumor samples, when available, with NF2 intragenic and flanking polymorphic microsatellite markers. Ethical approval for the study was obtained from the North West—Greater Manchester Central Research Ethics Committee (reference 10/H1008/74). Research based sample screening and analysis were carried out under ethics approval (reference 10/H1008/74) obtained from the North West 7–Greater Manchester Central Research Ethics Committee. Patient data from large clinical databases was anonymized for this study.
Primers were designed to target flanking sites at each side of exons for regions of COQ6 (NM_182476.2), DGCR8 (NM_022720.6), CDKN2A (NM_000077.4), and CDKN2B (NM_004936.3) and are listed in Supporting Information: Table S3. In addition, two intronic regions of SMARCB1 known to harbor PVs for schwannomatosis (Piotrowski et al., 2021; Smith et al., 2020) were also screened. Sanger sequencing of amplicons was then carried out using BigDye™ Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific) and an ABI 3100 automated sequencer (Applied Biosystems).
MLPA was performed for 70 of the 77 samples (for which DNA was available), using the SALSA MLPA kit, P419 probemix (MRC‐Holland) probe set from MRC Holland. Briefly, 100 ng DNA was used for the hybridization, ligation, and amplification of exon probes for control and test samples according to the manufacturer's instructions and analyzed on an ABI 3100 automated sequencer (Applied Biosystems).
In silico analysis was performed for all variants identified in our cohort. Potential pathogenicity of missense variants was assessed using REVEL v4.2 (Ioannidis et al., 2016) and BayesDel v4.2 (Feng, 2017). Nonsense and intronic variants were assessed using CADD v1.4 (Rentzsch et al., 2019). Maximum credible population allele frequency values (Whiffin et al., 2017) and, when applicable, constrain metrics from gnomAD v2.1.1 (Karczewski et al., 2020) and DECIPHER v11.9 (Firth et al., 2009) were also used to aid in classification of variants according to the American College of Medical Genetics and Genomics (ACMG) guidelines (Richards et al., 2015; Tavtigian et al., 2018) and the Association for Clinical Genomic Science (ACGS) best practice guidelines for variant classification in rare disease (Ellard et al., 2020). Potential splicing effects of variants were assessed using SpliceAI (Jaganathan et al., 2019), as well as MaxEntScan (Yeo & Burge, 2004), GeneSplicer (Pertea et al., 2001), NNSPLICE (Reese et al., 1997), EX‐SKIP (Raponi et al., 2011), and SpliceSiteFinder‐Like (Shapiro & Senapathy, 1987) as implemented in Alamut® Visual software.
3. RESULTS
No pathogenic or likely PVs were identified in DGCR8, COQ6, or CDKN2B. Bidirectional sequencing revealed a heterozygous single nucleotide duplication in exon 2 of CDKN2A (NG_007485.1:g.28291dup) in DNA from a patient with five nerve sheath tumors (two were considered hybrid tumors with high schwann cell content but some neurofibroma features). As the patient had no clinical features of NF1 and no vestibular schwannomas she was considered to have presumed schwannomatosis. Molecular analysis was carried out in one of the two independent hybrid tumors, found no PVs in NF2, SMARCB1, or LZTR1 and no evidence for LOH for NF2 markers. This patient also had family history of melanoma (affected paternal grandfather and uncle) and previously presented with two melanomas, which were a malignant melanoma Clark stage 3 and a superficial spreading melanoma in situ. The duplication identified in this patient disrupted both isoforms of CDKN2A, which code for p16INK4a (NM_000077.4:c.158dup; p.Met53fs) and p14ARF (NM_058195.3:c.201dup; p.Asp68Ter), respectively. This is similar to a previous report of a splicing variant in CDKN2A (NM_000077.4:c.151–1G>C) that resulted in inactivation of both gene isoforms (Sargen et al., 2016) and which was observed in DNA samples from three members of a family affected by melanoma and multiple nerve sheath tumors, some of which showed overlapping schwannoma and neurofibroma features.
The variant we identified in CDKN2A has not been reported previously but it is located in a highly conserved region and has been classified as pathogenic based on ACMG and ACGS guidelines (Figure 1). To investigate this duplication in schwannomatosis, we screened the coding sequence of CDKN2A in one schwannoma sample from this same patient. The NM_000077.4:c.158dup/NM_058195.3:c.201dup was also present in heterozygous form. No other variants were identified, except previously reported polymorphisms. Previous analysis of this tumor sample revealed no evidence of 22q involvement. The variant was inherited from her unaffected father. Her paternal grandfather and uncle both had a history of melanoma; however DNA was not available from these family members for molecular testing.
Figure 1.
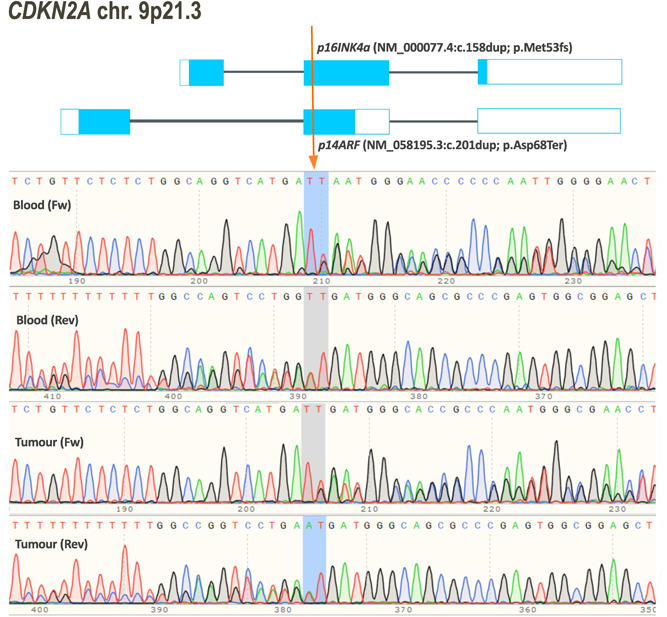
Duplication in CDKN2A in a schwannomatosis patient also diagnosed with melanoma. Schematic representation of both isoforms of CDKN2A, indicating the position of a single base pair (T) duplication that results in a frame shift for both proteins, p16INK4a (NM_000077.4:c.158dup; p.Met53fs) and p14ARF (NM_058195.3:c.201dup; p.Asp68Ter). The duplication was identified by bidirectional sequencing of a lymphocyte derived DNA sample from a schwannomatosis patient who was also diagnosed with melanoma. Additionally, a schwannoma sample was available for sequencing analysis, which confirmed the presence of this variant in the tumor.
MLPA analysis was carried out in 70 people for whom high quality DNA was available. No copy number abnormalities were detected in CDKN2A or CDKN2B for any of the samples analyzed. A reproducible decrease in signal of a single control probe for the transmembrane channel like 1 (TMC1) gene on chromosome 9q21.13, was seen in a patient with thyroid cancer and schwannomatosis. Sequencing of the probe region found no known polymorphisms, suggesting the possibility of hemizygosity. Mutations in the TMC1 gene have been associated with congenital and progressive hearing loss (Kurima et al., 2002) but no links to thyroid disorders or predisposition to nervous system tumors have been established. However, the significance of this result remains unclear. Previous analysis of schwannoma DNA identified LOH on chromosome 22, but subsequent MLPA analysis for CDKN2A failed for this sample.
4. DISCUSSION
The predominant model of inheritance for familial non‐NF2‐related schwannomatosis is a dominant model, similar to that of NF1 and NF2‐related schwannomatosis. However, whilst routine genetic analysis is able to identify around 92%−95% of variants responsible for familial cases for NF1 and NF2‐related schwannomatosis, the proportion of familial non‐NF2‐related schwannomatosis cases that can be attributed to known variants is much lower (70%−80%). This proportion is even lower for sporadic schwannomatosis cases (30%−40%) (Evans et al., 2018; Kehrer‐Sawatzki et al., 2017). The genetic architecture of non‐NF2‐related schwannomatosis appears to be more complex than that of NF1 and NF2‐related schwannomatosis with constitutional SMARCB1 PVs contributing in much higher proportion to familial schwannomatosis than they do to sporadic cases, whereas germline LZTR1 PVs seem to contribute similarly to familial and sporadic cases (Evans et al., 2018). This has prompted increasing efforts to identify novel PVs for schwannomatosis, some of which might turn out to be located in hitherto undiscovered schwannomatosis loci.
Many of these efforts have benefitted from advances in technologies for genomic analysis. WES of DNA from both sporadic and familial schwannomatosis samples have implicated a number of genes and variants of interest (K. Zhang et al., 2014; Min et al., 2020; Rivera et al., 2020). However, the extent to which these variants can be considered causative for schwannomatosis has proved to be harder to determine. In some cases, such as the recently reported germline variant in DGCR8 (NM_022720.7:c.1552G>A; p.Glu518Lys), functional characterization has provided strong evidence of the pathogenic nature of the variant. Additionally, clinical features of individuals carrying this variant prompted the authors to conclude that the variant might define a novel syndrome, characterized by the cooccurrence of schwannomatosis and familial MNG. Of note, only one of the schwannomatosis patients in our cohort had a thyroid related comorbidity, which was not classified as familial and no DGCR8 variant was identified in this patient.
In contrast to DGCR8, evidence supporting the involvement of variants in CDKN2A and COQ6 in schwannomatosis pathogenesis is less conclusive. Previous variants reported as potentially associated with schwannomatosis for these genes (K. Zhang et al., 2014; Min et al., 2020) have not been fully characterized, so the mechanisms through which they might contribute to disease remain unclear.
In the case of COQ6, there is need for more exhaustive functional analysis to establish a plausible mechanism through which dysfunction of COQ6 might lead to schwannomatosis, particularly in the absence of evidence for bi‐allelic inactivation of this gene in tumor tissue from affected members of the family in which the variant was originally identified. Indeed, questions have been raised about the role of this variant as a causative variant for schwannomatosis (Trevisson et al., 2015), since there were other variants that were identified by the same study that originally reported the COQ6 variant, but that were not deemed of interest by the authors. To date, no other studies have identified COQ6 variants in schwannomatosis patients, including our present study.
A link between malignant melanoma and nervous system tumors has been established by a number of studies. Particularly, germline whole gene deletions of CDKN2A and CDKN2B are known to be associated with familial syndromes predisposing to malignant melanoma as well as other nervous system tumors, including meningioma, astrocytoma, and schwannoma (Azizi et al., 1995; Bahuau et al., 1997, 1998; Chan et al., 2017; Kaufman et al., 1993). At the somatic level, inactivation of CDKN2A and CDKN2B has been identified as an important feature in a number of tumors, most notably melanomas and tumors in the CNS (Boström et al., 2001; Casula et al., 2019; Ghasimi et al., 2016; Gonzalez‐Zulueta et al., 1995; McNeal et al., 2015; Rousseau et al., 2003). Interestingly, loss of CDKN2 proteins in meningiomas has been established as an important consideration for tumor classification and, in some cases, a determinant of tumor progression (Goutagny et al., 2010; Suppiah et al., 2019). This raises the possibility for a more prominent role of variants in CDKN2A and CDKN2B genes as modulators of clinical phenotypes. The role of CDKN2A dysfunction in the pathology of schwannomatosis has not been established but some clues may emerge from the study of the mechanisms involved in transformation of neurofibromas into malignant peripheral nerve sheath tumors (MPNSTs) resulting from bi‐allelic inactivation of CDKN2A (Chaney et al., 2020; Magallón‐Lorenz et al., 2021; Nielsen et al., 1999). These mechanisms appear to be relevant not only to MPNSTs tumor progression but also to multiple malignant and benign tumor predisposition (Sargen et al., 2016). It is therefore possible that the in CDKN2A we report here might be contributing to a similar complex syndrome.
The absence of germline PVs in DGCR8, COQ6, or CDKN2B within a group of clinically well characterized schwannomatosis patients suggests that none of these genes is likely to be a major contributor to schwannomatosis pathogenesis on its own, although the possibility remains that they may have a role in complex clinical phenotypes. There is also a possibility that at least a proportion of the schwannomatosis cases that remain genetically unexplained might be caused by a variant within NF2, SMARCB1, or LZTR1 that has been missed by routine diagnostic methods. This could help explain the fact that previous studies using whole genome sequencing of schwannomatosis patients have either been unable to identify novel candidate genes (Hutter et al., 2014) or have reported potentially PVs in a number of loci, which have not been validated (K. Zhang et al., 2014; Min et al., 2020). Indeed a deep intronic PV, NM_003073.5:c.795+1498C>T, leading to the inclusion of a cryptic exon and a truncated product, has previously been reported in intron 6 of SMARCB1 (Smith et al., 2020). In addition, a recent deep massive parallel sequencing study of 35 schwannomatosis cases (Piotrowski et al., 2021) reported two novel deep intronic variants in intron 4 of SMARCB1 (NM_003073.5:c.500+883T>G and NM_003073.5:c.500+887G>A). Both of these variants were further characterized by means of RNA analysis and demonstrated to result in retention of part of intron 4 and a truncated transcript. We have screened these intronic regions in our cohort and found that none of our patients is a carrier for any of these three variants. The variants in intron 4 were covered by our clinical NGS panel, but deep‐intronic regions are not typically scrutinized for diagnostic purposes. The intron 6 variant was not captured by the panel. Furthermore, limitations of some of the techniques used in the past for genetic molecular testing might mean that for some individuals, low level mosaic variants in NF2 have been missed. This in turn may result in misdiagnosis of mosaic NF2 cases as non‐NF2‐related schwannomatosis, particularly for cases where there is limited availability of tumor tissue (Evans et al., 2018; Kehrer‐Sawatzki et al., 2018). The current use of high read depth NGS analysis in clinical genetic testing has improved the rate of detection for mosaic PVs in NF2 (Contini et al., 2015; Evans et al., 2020; Louvrier et al., 2018), however reanalysis of patient samples is not always possible. Future efforts to find novel PVs for schwannomatosis might be greatly aided by a similar approach to the one used by Piotrowski and colleagues, which involved deep sequencing of the full gene region of NF2, SMARCB1 and LZTR1, which will help detect noncoding PVs that are difficult to identify by standard NGS panels or WES.
In addition, there is accumulating evidence for PVs contributing to schwannomatosis combined with other conditions has been set by the case for families affected by more than one condition in which a particular variant is observed to cosegregate with disease, such as the previously described variant in DGCR8 (Rivera et al., 2020) or for families affected by CDKN2A‐associated melanoma, who also have an increased risk for other cancers present (Goldstein et al., 2006). This is also the case for people affected by melanoma along with nervous system tumors due to deletion of both CDKN2A and CDKN2B, with suggestions that inactivation of CDKN2A/B genes might be responsible for the melanoma phenotype, while loss of adjacent genes might contribute to the development of other neoplasms (Chan et al., 2017). The presence of schwannomas in our melanoma patient and the retention of the single base duplication in CDKN2A in tumor DNA suggest that inactivation of p14ARF and p16ink4a, may be enough for schwannoma formation. The presence of additional factors contributing to risk for both conditions has also been explored in previous studies. One intriguing possibility is the potential effect of noncoding elements on the observed phenotype. One of these elements, a long noncoding RNA (CDKN2B‐AS1) spanning the two exons of CDKN2B, was discovered by a study aimed to determine the size of a 9p21 deletion in a large family affected by melanoma‐astrocytoma syndrome (Pasmant et al., 2007). The authors suggest, p14ARF and CDKN2B‐AS1 might share a promoter, a fact supported by their discovery of a significant correlation in transcript levels of CDKN2B‐AS1 with those of p14ARF, p16INK4a, and CDKN2B in healthy tissue as well as breast tumor samples and NF1‐associated tumor samples.
Exploration of the possible interactions of potential candidate genes for schwannomatosis with the known causative genes, might provide some insight into the type and location of novel PVs in cases where no variants in SMARCB1, LZTR1, or NF2 have been identified. Furthermore, functional characterization of known causative variants for schwannomatosis will undoubtedly advance our understanding of potentially new mechanisms of disease in schwannomatosis, particularly for variants located in noncoding regions of both SMARCB1 and LZTR1. This in turn might lead to elucidation of important correlations of these genes with other loci, and ultimately to an increased ability for accurate diagnosis and classification of schwannomatosis cases based on their clinical and molecular features.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGEMENTS
This study was supported by USAMRAA CDMRP Neurofibromatosis Research Program, Investigator‐Initiated Research Award (W81XWH1910334). D. G. E. and M. J. S. are also supported by the Manchester National Institute for Health Research (NIHR) Biomedical Research Center (IS‐BRC‐1215‐20007).
Perez‐Becerril, C. , Wallace, A. J. , Schlecht, H. , Bowers, N. L. , Smith, P. T. , Gokhale, C. , Eaton, H. , Charlton, C. , Robinson, R. , Charlton, R. S. , Evans, D. G. , & Smith, M. J. (2022). Screening of potential novel candidate genes in schwannomatosis patients. Human Mutation, 43, 1368–1376. 10.1002/humu.24424
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
An entry has been made for the CDKN2A NM_000077.4:c.158dup variant in the LOVD database and can be found here: https://databases.lovd.nl/shared/variants/0000839293#00004876
REFERENCES
- Ali, M. W. , Patro, C. , Devall, M. , Dampier, C. H. , Plummer, S. J. , Kuscu, C. , Adli, M. , Lai, R. K. , & Casey, G. (2021). A functional variant on 9p21.3 related to glioma risk affects enhancer activity and modulates expression of CDKN2B‐AS1. Human Mutation, 42, 1208–1214. 10.1002/humu.24244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, L. O. , Custódio, A. C. , Araújo, J. J. , Rey, J. A. , Almeida, J. R. , Santos, M. J. , Clara, C. A. , & Casartelli, C. (2008). Mutational analysis of genes p14ARF, p15INK4b, p16INK4a, and PTEN in human nervous system tumors. Genetics and Molecular Research, 7, 451–459. 10.4238/vol7-2gmr445 [DOI] [PubMed] [Google Scholar]
- Azizi, E. , Friedman, J. , Pavlotsky, F. , Iscovich, J. , Bornstein, A. , Shafir, R. , & Nass, D. (1995). Familial cutaneous malignant melanoma and tumors of the nervous system. A hereditary cancer syndrome. Cancer, 76, 1571–1578. [DOI] [PubMed] [Google Scholar]
- Bahuau, M. , Vidaud, D. , Jenkins, R. B. , Bièche, I. , Kimmel, D. W. , Assouline, B. , Vidaud, M. , Smith, J. S. , Alderete, B. , Cayuela, J. M. , Harpey, J. P. , Caille, B. , & Vidaud, M. (1998). Germ‐line deletion involving the INK4 locus in familial proneness to melanoma and nervous system tumors. Cancer Research, 58, 2298–2303. [PubMed] [Google Scholar]
- Bahuau, M. , Vidaud, D. , Kujas, M. , Palangié, A. , Assouline, B. , Chaignaud‐Lebreton, M. , Caille, B. , Prieur, M. , Vidaud, M. , Harpey, J. P. , Lafourcade, J. , & Caille, B. (1997). Familial aggregation of malignant melanoma/dysplastic naevi and tumours of the nervous system: An original syndrome of tumour proneness. Annales de Genetique, 40, 78–91. [PubMed] [Google Scholar]
- Boström, J. , Meyer‐Puttlitz, B. , Wolter, M. , Blaschke, B. , Weber, R. G. , Lichter, P. ,… Reifenberger, G. (2001). Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. American Journal of Pathology, 159, 661–669. 10.1016/s0002-9440(10)61737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research, N . (2008). Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature, 455, 1061–1068. 10.1038/nature07385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula, M. , Paliogiannis, P. , Ayala, F. , De Giorgi, V. , Stanganelli, I. , Mandalà, M. , Colombino, M. , Manca, A. , Sini, M. C. , Caracò, C. , Ascierto, P. A. , Satta, R. R. , Lissia, A. , Cossu, A. , & Palmieri, G. , Melanoma Unit of Sassari (MUS), Italian Melonama Intergroup (IMI) . (2019). Germline and somatic mutations in patients with multiple primary melanomas: A next generation sequencing study. BMC Cancer, 19(1), 772. 10.1186/s12885-019-5984-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, A. K. , Han, S. J. , Choy, W. , Beleford, D. , Aghi, M. K. , Berger, M. S. , … Solomon, D. A. (2017). Familial melanoma‐astrocytoma syndrome: Synchronous diffuse astrocytoma and pleomorphic xanthoastrocytoma in a patient with germline CDKN2A/B deletion and a significant family history. Clinical Neuropathology, 36, 213–221. 10.5414/np301022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney, K. E. , Perrino, M. R. , Kershner, L. J. , Patel, A. V. , Wu, J. , Choi, K. , … Ratner, N. (2020). Cdkn2a loss in a model of neurofibroma demonstrates stepwise tumor progression to atypical neurofibroma and MPNST. Cancer Research, 80, 4720–4730. 10.1158/0008-5472.Can-19-1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini, E. , Paganini, I. , Sestini, R. , Candita, L. , Capone, G. L. , Barbetti, L. , Papi, L. , Falconi, S. , Frusconi, S. , Giotti, I. , Giuliani, C. , Torricelli, F. , Benelli, M. , & Papi, L. (2015). A systematic assessment of accuracy in detecting somatic mosaic variants by deep amplicon sequencing: application to NF2 gene. PLoS One, 10, e0129099. 10.1371/journal.pone.0129099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECIPHER. , authors. https://www.deciphergenomics.org/
- Ellard, S. , L Baple, E. , Callaway, A. , Berry, I. , Forrester, N. , Turnbull, C. , & McMullan, D. J. (2020). ACGS best practice guidelines for variant classification in rare disease 2020. Association for Clinical Genomic Science. https://www.acgs.uk.com/quality/best-practice-guidelines/#VariantGuidelines
- Ensembl., authors https://www.ensembl.org/index.html
- Evans, D. G. , Bowers, N. L. , Tobi, S. , Hartley, C. , Wallace, A. J. , King, A. T. , Smith, M. J. , Lloyd, S. , Rutherford, S. A. , Hammerbeck‐Ward, C. , Pathmanaban, O. N. , Freeman, S. R. , Ealing, J. , Kellett, M. , Laitt, R. , Thomas, O. , Halliday, D. , Ferner, R. , Taylor, A. , … Smith, M. J. (2018). Schwannomatosis: A genetic and epidemiological study. Journal of Neurology, Neurosurgery and Psychiatry, 89, 1215–1219. 10.1136/jnnp-2018-318538 [DOI] [PubMed] [Google Scholar]
- Evans, D. G. , Hartley, C. L. , Smith, P. T. , King, A. T. , Bowers, N. L. , Tobi, S. , Smith, M. J. , Wallace, A. J. , Perry, M. , Anup, R. , Lloyd, S. , Rutherford, S. A. , Hammerbeck‐Ward, C. , Pathmanaban, O. N. , Stapleton, E. , Freeman, S. R. , Kellett, M. , Halliday, D. , Parry, A. , … Smith, M. J. (2020). Incidence of mosaicism in 1055 de novo NF2 cases: Much higher than previous estimates with high utility of next‐generation sequencing. Genetics in Medicine, 22, 53–59. 10.1038/s41436-019-0598-7 [DOI] [PubMed] [Google Scholar]
- Feng, B. J. (2017). PERCH: A unified framework for disease gene prioritization. Human Mutation, 38, 243–251. 10.1002/humu.23158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth, H. V. , Richards, S. M. , Bevan, A. P. , Clayton, S. , Corpas, M. , Rajan, D. , Carter, N. P. , Van Vooren, S. , Moreau, Y. , Pettett, R. M. , & Carter, N. P. (2009). DECIPHER: database of chromosomal imbalance and phenotype in humans using ensembl resources. The American Journal of Human Genetics, 84, 524–533. 10.1016/j.ajhg.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frequency Filter. , authors. http://cardiodb.org/allelefrequencyapp/
- Ghasimi, S. , Wibom, C. , Dahlin, A. M. , Brännström, T. , Golovleva, I. , Andersson, U. , & Melin, B. (2016). Genetic risk variants in the CDKN2A/B, RTEL1 and EGFR genes are associated with somatic biomarkers in glioma. Journal of Neuro‐Oncology, 127, 483–492. 10.1007/s11060-016-2066-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- gnomAD. , authors. https://gnomad.broadinstitute.org/
- Goldstein, A. M. , Chan, M. , Harland, M. , Gillanders, E. M. , Hayward, N. K. , Avril, M. F. , … Yakobson, E. (2006). High‐risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL. Cancer Research, 66, 9818–9828. 10.1158/0008-5472.Can-06-0494 [DOI] [PubMed] [Google Scholar]
- Goldstein, A. M. , & Tucker, M. A. (1997). Screening for CDKN2A mutations in hereditary melanoma. Journal of the National Cancer Institute, 89, 676–678. 10.1093/jnci/89.10.676 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Zulueta, M. , Bender, C. M. , Yang, A. S. , Nguyen, T. , Beart, R. W. , Van Tornout, J. M. , & Jones, P. A. (1995). Methylation of the 5' CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Research, 55, 4531–4535. [PubMed] [Google Scholar]
- Goutagny, S. , Yang, H. W. , Zucman‐Rossi, J. , Chan, J. , Dreyfuss, J. M. , Park, P. J. , … Kalamarides, M. (2010). Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clinical Cancer Research, 16, 4155–4164. 10.1158/1078-0432.Ccr-10-0891 [DOI] [PubMed] [Google Scholar]
- Hannon, G. J. , & Beach, D. (1994). pl5INK4B is a potentia effector of TGF‐β‐induced cell cycle arrest. Nature, 371, 257–261. 10.1038/371257a0 [DOI] [PubMed] [Google Scholar]
- Hulsebos, T. J. , Plomp, A. S. , Wolterman, R. A. , Robanus‐Maandag, E. C. , Baas, F. , & Wesseling, P. (2007). Germline mutation of INI1/SMARCB1 in familial schwannomatosis. American Journal of Human Genetics, 80, 805–810. 10.1086/513207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussussian, C. J. , Struewing, J. P. , Goldstein, A. M. , Higgins, P. A. , Ally, D. S. , Sheahan, M. D. , Dracopoli, N. C. , Clark WH, J. r , Tucker, M. A. , & Dracopoli, N. C. (1994). Germline p16 mutations in familial melanoma. Nature Genetics, 8, 15–21. 10.1038/ng0994-15 [DOI] [PubMed] [Google Scholar]
- Hutter, S. , Piro, R. M. , Reuss, D. E. , Hovestadt, V. , Sahm, F. , Farschtschi, S. , Mautner, V. F. , Kehrer‐Sawatzki, H. , Wolf, S. , Lichter, P. , von Deimling, A. , Schuhmann, M. U. , Pfister, S. M. , Jones, D. T. , & Mautner, V. F. (2014). Whole exome sequencing reveals that the majority of schwannomatosis cases remain unexplained after excluding SMARCB1 and LZTR1 germline variants. Acta Neuropathologica, 128, 449–452. 10.1007/s00401-014-1311-1 [DOI] [PubMed] [Google Scholar]
- Ioannidis, N. M. , Rothstein, J. H. , Pejaver, V. , Middha, S. , McDonnell, S. K. , Baheti, S. , Sieh, W. , Musolf, A. , Li, Q. , Holzinger, E. , Karyadi, D. , Cannon‐Albright, L. A. , Teerlink, C. C. , Stanford, J. L. , Isaacs, W. B. , Xu, J. , Cooney, K. A. , Lange, E. M. , Schleutker, J. , … Sieh, W. (2016). REVEL: An ensemble method for predicting the pathogenicity of rare missense variants. American Journal of Human Genetics, 99, 877–885. 10.1016/j.ajhg.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri, M. , Wake, N. C. , Ascher, D. B. , Pires, D. E. , Gentle, D. , Morris, M. R. , … Maher, E. R. (2015). Germline mutations in the CDKN2B tumor suppressor gene predispose to renal cell carcinoma. Cancer Discovery, 5, 723–729. 10.1158/2159-8290.Cd-14-1096 [DOI] [PubMed] [Google Scholar]
- Jaganathan, K. , Kyriazopoulou Panagiotopoulou, S. , McRae, J. F. , Darbandi, S. F. , Knowles, D. , Li, Y. I. , Kosmicki, J. A. , Arbelaez, J. , Cui, W. , Schwartz, G. B. , Chow, E. D. , Kanterakis, E. , Gao, H. , Kia, A. , Batzoglou, S. , Sanders, S. J. , & Farh, K. K. (2019). Predicting splicing from primary sequence with deep learning. Cell, 176(3), 535–548. 10.1016/j.cell.2018.12.015 [DOI] [PubMed] [Google Scholar]
- Kamb, A. , Shattuck‐Eidens, D. , Eeles, R. , Liu, Q. , Gruis, N. A. , Ding, W. , Ding, W. , Hussey, C. , Tran, T. , Miki, Y. , & Weaver‐Feldhaus, J. (1994). Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nature Genetics, 8, 23–26. 10.1038/ng0994-22 [DOI] [PubMed] [Google Scholar]
- Karczewski, K. J. , Francioli, L. C. , Tiao, G. , Cummings, B. B. , Alföldi, J. , Wang, Q. , MacArthur, D. G. , Collins, R. L. , Laricchia, K. M. , Ganna, A. , Birnbaum, D. P. , Gauthier, L. D. , Brand, H. , Solomonson, M. , Watts, N. A. , Rhodes, D. , Singer‐Berk, M. , England, E. M. , Seaby, E. G. , … MacArthur, D. G. (2020). The mutational constraint spectrum quantified from variation in 141,456 humans. Nature, 581, 434–443. 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, D. K. , Kimmel, D. W. , Parisi, J. E. , & Michels, V. V. (1993). A familial syndrome with cutaneous malignant melanoma and cerebral astrocytoma. Neurology, 43, 1728–1731. 10.1212/wnl.43.9.1728 [DOI] [PubMed] [Google Scholar]
- Kehrer‐Sawatzki, H. , Farschtschi, S. , Mautner, V. F. , & Cooper, D. N. (2017). The molecular pathogenesis of schwannomatosis, a paradigm for the co‐involvement of multiple tumour suppressor genes in tumorigenesis. Human Genetics, 136, 129–148. 10.1007/s00439-016-1753-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer‐Sawatzki, H. , Kluwe, L. , Friedrich, R. E. , Summerer, A. , Schäfer, E. , Wahlländer, U. , Mautner, V. F. , Matthies, C. , Gugel, I. , Farschtschi, S. , Hagel, C. , Cooper, D. N. , & Mautner, V. F. (2018). Phenotypic and genotypic overlap between mosaic NF2 and schwannomatosis in patients with multiple non‐intradermal schwannomas. Human Genetics, 137, 543–552. 10.1007/s00439-018-1909-9 [DOI] [PubMed] [Google Scholar]
- Kurima, K. , Peters, L. M. , Yang, Y. , Riazuddin, S. , Ahmed, Z. M. , Naz, S. , Griffith, A. J. , Arnaud, D. , Drury, S. , Mo, J. , Makishima, T. , Ghosh, M. , Menon, P. S. , Deshmukh, D. , Oddoux, C. , Ostrer, H. , Khan, S. , Riazuddin, S. , Deininger, P. L. , … Griffith, A. J. (2002). Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair‐cell function. Nature Genetics, 30, 277–284. 10.1038/ng842 [DOI] [PubMed] [Google Scholar]
- Louvrier, C. , Pasmant, E. , Briand‐Suleau, A. , Cohen, J. , Nitschké, P. , Nectoux, J. , Parfait, B. , Orhant, L. , Zordan, C. , Goizet, C. , Goutagny, S. , Lallemand, D. , Vidaud, M. , Vidaud, D. , Kalamarides, M. , & Parfait, B. (2018). Targeted next‐generation sequencing for differential diagnosis of neurofibromatosis type 2, schwannomatosis, and meningiomatosis. Neuro‐Oncology, 20, 917–929. 10.1093/neuonc/noy009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCollin, M. , Chiocca, E. A. , Evans, D. G. , Friedman, J. M. , Horvitz, R. , Jaramillo, D. , Roach, E. S. , Lev, M. , Mautner, V. F. , Niimura, M. , Plotkin, S. R. , Sang, C. N. , Stemmer‐Rachamimov, A. , & Roach, E. S. (2005). Diagnostic criteria for schwannomatosis. Neurology, 64, 1838–1845. 10.1212/01.WNL.0000163982.78900.AD [DOI] [PubMed] [Google Scholar]
- Magallón‐Lorenz, M. , Fernández‐Rodríguez, J. , Terribas, E. , Creus‐Batchiller, E. , Romagosa, C. , Estival, A. , Gel, B. , Perez Sidelnikova, D. , Salvador, H. , Villanueva, A. , Blanco, I. , Carrió, M. , Lázaro, C. , Serra, E. , & Gel, B. (2021). Chromosomal translocations inactivating CDKN2A support a single path for malignant peripheral nerve sheath tumor initiation. Human Genetics, 140, 1241–1252. 10.1007/s00439-021-02296-x [DOI] [PubMed] [Google Scholar]
- McNeal, A. S. , Liu, K. , Nakhate, V. , Natale, C. A. , Duperret, E. K. , Capell, B. C. , & Ridky, T. W. (2015). CDKN2B loss promotes progression from benign melanocytic nevus to melanoma. Cancer Discovery, 5, 1072–1085. 10.1158/2159-8290.Cd-15-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, B. J. , Kang, Y. K. , Chung, Y. G. , Seo, M. E. , Chang, K. B. , & Joo, M. W. (2020). Germline mutations for novel candidate predisposition genes in sporadic schwannomatosis. Clinical Orthopaedics and Related Research, 478, 2442–2450. 10.1097/corr.0000000000001239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, G. P. , Stemmer‐Rachamimov, A. O. , Ino, Y. , Møller, M. B. , Rosenberg, A. E. , & Louis, D. N. (1999). Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. The American Journal of Pathology, 155, 1879–1884. 10.1016/S0002-9440(10)65507-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogué, C. , Chong, A. S. , Grau, E. , Han, H. , Dorca, E. , Roca, C. , Rivera, B. , Mosquera, J. L. , Lázaro, C. , Foulkes, W. D. , Brunet, J. , & Rivera, B. (2022). DGCR8 and the six hit, three‐step model of schwannomatosis. Acta Neuropathologica, 143, 115–117. 10.1007/s00401-021-02387-z [DOI] [PubMed] [Google Scholar]
- Pasmant, E. , Laurendeau, I. , Héron, D. , Vidaud, M. , Vidaud, D. , & Bièche, I. (2007). Characterization of a germ‐line deletion, including the entire INK4/ARF locus, in a melanoma‐neural system tumor family: Identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Research, 67, 3963–3969. 10.1158/0008-5472.Can-06-2004 [DOI] [PubMed] [Google Scholar]
- Patel, S. , Wilkinson, C. J. , & Sviderskaya, E. V. (2020). Loss of both CDKN2A and CDKN2B allows for centrosome overduplication in melanoma. Journal of Investigative Dermatology, 140, 1837–1846. 10.1016/j.jid.2020.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea, M. , Lin, X. , & Salzberg, S. L. (2001). GeneSplicer: A new computational method for splice site prediction. Nucleic Acids Research, 29, 1185–1190. 10.1093/nar/29.5.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronzelli, F. , Sollima, D. , Coppola, G. , Martini‐Neri, M. E. , Neri, G. , & Genuardi, M. (2001). CDKN2A germline splicing mutation affecting both p16(ink4) and p14(arf) RNA processing in a melanoma/neurofibroma kindred. Genes, Chromosomes & Cancer, 31, 398–401. 10.1002/gcc.1159 [DOI] [PubMed] [Google Scholar]
- Piotrowski, A. , Koczkowska, M. , Poplawski, A. B. , Bartoszewski, R. , Króliczewski, J. , Mieczkowska, A. , Messiaen, L. M. , Gomes, A. , Crowley, M. R. , Crossman, D. K. , Chen, Y. , Lao, P. , Serra, E. , Llach, M. C. , Castellanos, E. , & Messiaen, L. M. (2021). Targeted massively parallel sequencing of candidate regions on chromosome 22q predisposing to multiple schwannomas: An analysis of 51 individuals in a single‐center experience. Human Mutation, 43, 74–84. 10.1002/humu.24294 [DOI] [PubMed] [Google Scholar]
- Piotrowski, A. , Xie, J. , Liu, Y. F. , Poplawski, A. B. , Gomes, A. R. , Madanecki, P. , Messiaen, L. M. , Fu, C. , Crowley, M. R. , Crossman, D. K. , Armstrong, L. , Babovic‐Vuksanovic, D. , Bergner, A. , Blakeley, J. O. , Blumenthal, A. L. , Daniels, M. S. , Feit, H. , Gardner, K. , Hurst, S. , … Messiaen, L. M. (2014). Germline loss‐of‐function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nature Genetics, 46, 182–187. 10.1038/ng.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, P. M. , Spurr, N. , Bishop, T. , Newton‐Bishop, J. , Gruis, N. , van der Velden, P. A. , … Hayward, N. K. (1998). Haplotype analysis of two recurrent CDKN2A mutations in 10 melanoma families: Evidence for common founders and independent mutations. Human Mutation, 11, 424–431. [DOI] [PubMed] [Google Scholar]
- Prowse, A. H. , Schultz, D. C. , Guo, S. , Vanderveer, L. , Dangel, J. , Bove, B. , Cairns, P. , Daly, M. , & Godwin, A. K. (2003). Identification of a splice acceptor site mutation in p16INK4A/p14ARF within a breast cancer, melanoma, neurofibroma prone kindred. Journal of Medical Genetics, 40, e102. 10.1136/jmg.40.8.e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle, D. E. , Zindy, F. , Ashmun, R. A. , & Sherr, C. J. (1995). Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell, 83, 993–1000. 10.1016/0092-8674(95)90214-7 [DOI] [PubMed] [Google Scholar]
- Raponi, M. , Kralovicova, J. , Copson, E. , Divina, P. , Eccles, D. , Johnson, P. , Vorechovsky, I. , Baralle, D. , & Vorechovsky, I. (2011). Prediction of single‐nucleotide substitutions that result in exon skipping: Identification of a splicing silencer in BRCA1 exon 6. Human Mutation, 32, 436–444. 10.1002/humu.21458 [DOI] [PubMed] [Google Scholar]
- Reese, M. G. , Eeckman, F. H. , Kulp, D. , & Haussler, D. (1997). Improved splice site detection in Genie. Journal of Computational Biology, 4, 311–323. 10.1089/cmb.1997.4.311 [DOI] [PubMed] [Google Scholar]
- Rentzsch, P. , Witten, D. , Cooper, G. M. , Shendure, J. , & Kircher, M. (2019). CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Research, 47, D886–d894. 10.1093/nar/gky1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, S. , Aziz, N. , Bale, S. , Bick, D. , Das, S. , Gastier‐Foster, J. , Committee, A. L. Q. A. , Grody, W. W. , Hegde, M. , Lyon, E. , Spector, E. , Voelkerding, K. , Rehm, H. L. , & ACMG Laboratory Quality Assurance, C. (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine, 17, 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera, B. , Nadaf, J. , Fahiminiya, S. , Apellaniz‐Ruiz, M. , Saskin, A. , Chong, A. S. , Foulkes, W. D. , Sharma, S. , Wagener, R. , Revil, T. , Condello, V. , Harra, Z. , Hamel, N. , Sabbaghian, N. , Muchantef, K. , Thomas, C. , de Kock, L. , Hébert‐Blouin, M. N. , Bassenden, A. V. , … Foulkes, W. D. (2020). DGCR8 microprocessor defect characterizes familial multinodular goiter with schwannomatosis. Journal of Clinical Investigation, 130, 1479–1490. 10.1172/JCI130206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau, E. , Ruchoux, M. M. , Scaravilli, F. , Chapon, F. , Vinchon, M. , De Smet, C. , Vikkula, M. , Godfraind, C. , & Vikkula, M. (2003). CDKN2A, CDKN2B and p14ARF are frequently and differentially methylated in ependymal tumours. Neuropathology and Applied Neurobiology, 29, 574–583. 10.1046/j.0305-1846.2003.00505.x [DOI] [PubMed] [Google Scholar]
- Sargen, M. R. , Merrill, S. L. , Chu, E. Y. , & Nathanson, K. L. (2016). CDKN2A mutations with p14 loss predisposing to multiple nerve sheath tumours, melanoma, dysplastic naevi and internal malignancies: A case series and review of the literature. British Journal of Dermatology, 175, 785–789. 10.1111/bjd.14485 [DOI] [PubMed] [Google Scholar]
- Shapiro, M. B. , & Senapathy, P. (1987). RNA splice junctions of different classes of eukaryotes: Sequence statistics and functional implications in gene expression. Nucleic Acids Research, 15, 7155–7174. 10.1093/nar/15.17.7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. J. , Bowers, N. L. , Banks, C. , Coates‐Brown, R. , Morris, K. A. , Ewans, L. , Evans, D. G. R. , Wilson, M. , Pinner, J. , Bhaskar, S. S. , Cammarata‐Scalisi, F. , Wallace, A. J. , & Evans, D. (2020). A deep intronic SMARCB1 variant associated with schwannomatosis. Clinical Genetics, 97, 376–377. 10.1111/cge.13637 [DOI] [PubMed] [Google Scholar]
- SpliceAI. , authors. https://spliceailookup.broadinstitute.org/
- Suppiah, S. , Nassiri, F. , Bi, W. L. , Dunn, I. F. , Hanemann, C. O. , Horbinski, C. M. , Hashizume, R. , James, C. D. , Mawrin, C. , Noushmehr, H. , Perry, A. , Sahm, F. , Sloan, A. , Von Deimling, A. , Wen, P. Y. , Aldape, K. , & Zadeh, G. , International Consortium on Meningiomas . (2019). Molecular and translational advances in meningiomas. Neuro‐Oncology, 21, i4–i17. 10.1093/neuonc/noy178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavtigian, S. V. , Greenblatt, M. S. , Harrison, S. M. , Nussbaum, R. L. , Prabhu, S. A. , Boucher, K. M. , & Biesecker, L. G. (2018). Modeling the ACMG/AMP variant classification guidelines as a Bayesian classification framework. Genetics in Medicine, 20, 1054–1060. 10.1038/gim.2017.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisson, E. , Clementi, M. , & Salviati, L. (2015). Is there a link between COQ6 and schwannomatosis? Genetics in Medicine, 17, 312–313. 10.1038/gim.2014.211 [DOI] [PubMed] [Google Scholar]
- Tu, Q. , Hao, J. , Zhou, X. , Yan, L. , Dai, H. , Sun, B. , Zhao, X. , Yang, D. , An, S. , Lv, L. , Jiao, B. , Chen, C. , Lai, R. , Shi, P. , & Zhao, X. (2018). CDKN2B deletion is essential for pancreatic cancer development instead of unmeaningful co‐deletion due to juxtaposition to CDKN2A. Oncogene, 37, 128–138. 10.1038/onc.2017.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiffin, N. , Minikel, E. , Walsh, R. , O'donnell‐Luria, A. H. , Karczewski, K. , Ing, A. Y. , Ware, J. S. , Barton, P. , Funke, B. , Cook, S. A. , MacArthur, D. , & Ware, J. S. (2017). Using high‐resolution variant frequencies to empower clinical genome interpretation. Genetics in Medicine, 19, 1151–1158. 10.1038/gim.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman, D. C. , Milligan, A. , Welch, J. , Green, A. C. , & Hayward, N. K. (1997). Germline CDKN2A mutations in childhood melanoma. JNCI: Journal of the National Cancer Institute, 89, 1460. 10.1093/jnci/89.19.1460 [DOI] [PubMed] [Google Scholar]
- Yeo, G. , & Burge, C. B. (2004). Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. Journal of Computational Biology, 11, 377–394. 10.1089/1066527041410418 [DOI] [PubMed] [Google Scholar]
- Zhang, K. , Lin, J.‐W. , Wang, J. , Wu, X. , Gao, H. , Hsieh, Y.‐C. , Yen, Y. , Hwu, P. , Liu, Y. R. , Su, L. , Chiou, H. Y. , Wang, D. , Yuan, Y. C. , Whang‐Peng, J. , Chiu, W. T. , & Yen, Y. (2014). A germline missense mutation in COQ6 is associated with susceptibility to familial schwannomatosis. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 16, 787–792. 10.1038/gim.2014.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Endo, S. , Koga, H. , Ichikawa, T. , Feng, X. , Onda, K. , Kumanishi, T. , Washiyama, K. , & Kumanishi, T. (1996). A comparative study of glioma cell lines for p16, p15, p53 and p21 gene alterations. Japanese Journal of Cancer Research, 87, 900–907. 10.1111/j.1349-7006.1996.tb02118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Xiong, Y. , & Yarbrough, W. G. (1998a). ARF promotes MDM2 degradation and stabilizes p53: ARF‐INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell, 92, 725–734. 10.1016/S0092-8674(00)81401-4 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Xiong, Y. , & Yarbrough, W. G. (1998b). ARF promotes MDM2 degradation and stabilizes p53: ARF‐INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell, 92, 725–734. 10.1016/s0092-8674(00)81401-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
An entry has been made for the CDKN2A NM_000077.4:c.158dup variant in the LOVD database and can be found here: https://databases.lovd.nl/shared/variants/0000839293#00004876


