Abstract
Mobocertinib is an oral tyrosine kinase inhibitor approved for treatment of patients with locally advanced or metastatic non‐small cell lung cancer (mNSCLC) with epidermal growth factor receptor gene (EGFR) exon 20 insertion (ex20ins) mutations previously treated with platinum‐based chemotherapy. These exposure–response analyses assessed potential relationships between exposure and efficacy or safety outcomes in platinum‐pretreated patients with EGFRex20ins‐positive mNSCLC who received mobocertinib 160 mg once daily (q.d.) in a pivotal phase I/II study. A statistically significant relationship between the independent review committee‐assessed objective response rate and molar sum exposure to mobocertinib and its active metabolites (AP32960 and AP32914) was not discernable using a longitudinal model of clinical response driven by normalized dynamic molar sum exposure or a static model of best clinical response based on time‐averaged molar sum exposure. However, the longitudinal model suggested a trend for decreased probability of response with the change in mobocertinib molar sum exposure between the 160‐ and 120‐mg doses (odds ratio: 0.78; 95% confidence interval: 0.55–1.10; P = 0.156). Time‐averaged molar sum exposure was a significant predictor of the rate of grade ≥ 3 treatment‐emergent adverse events (AEs). Taken together, these exposure–efficacy and exposure–safety results support a favorable benefit‐risk profile for the approved mobocertinib 160‐mg q.d. dose and dose modification guidelines for patients experiencing AEs.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The recommended dose of mobocertinib (160 mg once daily [q.d.]) demonstrated deep and durable clinical responses in patients with EGFR exon 20 insertion‐positive non‐small cell lung cancer.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Do exposure–response relationships for efficacy and safety support selection of the 160‐mg dose of mobocertinib?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Molar sum exposure to mobocertinib and its active metabolites was not a significant predictor of clinical response rates, indicating consistent efficacy benefit across the range of exposures achieved with the 160‐mg dose. Exposure significantly correlated with the overall rate of grade ≥ 3 adverse events, but not with rates of individual AEs of clinical interest (e.g., diarrhea, rash).
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ These analyses provided clinical pharmacology support for the benefit‐risk profile associated with the approved mobocertinib 160‐mg q.d. dosing regimen.
Mobocertinib is a first‐in‐class, oral, irreversible tyrosine kinase inhibitor (TKI) designed to selectively target in‐frame epidermal growth factor receptor gene (EGFR) exon 20 insertion (ex20ins) mutations found in patients with non‐small cell lung cancer (NSCLC). 1 The selective inhibitory activity of mobocertinib against activating EGFR mutations, including EGFRex20ins and other EGFR mutations (exon 19 deletions and L858R), with or without the T790M resistance mutation, was first demonstrated in preclinical studies. 1 The recommended phase II dose of mobocertinib, 160 mg q.d., demonstrated durable clinical responses in patients with EGFRex20ins‐positive metastatic NSCLC (mNSCLC) in the first‐in‐human dose‐escalation, expansion, and extension (EXCLAIM) phase I/II trial (ClinicalTrials.gov NCT02716116). 2 Among 114 platinum‐pretreated patients with EGFRex20ins‐positive mNSCLC treated with mobocertinib 160 mg q.d. in the dose‐escalation, expansion, and extension parts of the phase I/II study, the confirmed objective response rate (ORR) was 35% per investigators and 28% per independent review committee (IRC), with median follow‐up of 14.2 months (data cutoff: November 1, 2020). 3 The median duration of IRC‐assessed responses was 17.5 months, the median IRC‐assessed progression‐free survival was 7.3 months, and the median overall survival was 24.0 months. 3 In September 2021, the US Food and Drug Administration (FDA) granted accelerated approval to mobocertinib for treatment of adult patients with locally advanced or mNSCLC with EGFRex20ins mutations, as detected by an FDA‐approved test, whose disease has progressed on or after platinum‐based chemotherapy. 4
The safety profile of mobocertinib was characterized by manageable gastrointestinal and cutaneous adverse events (AEs), consistent with the known profile for EGFR TKIs. 3 The most common (> 20%) any‐grade treatment‐related AEs were diarrhea (91%), rash (45%), paronychia (38%), decreased appetite (35%), nausea (34%), dry skin (31%), vomiting (30%), elevated blood creatinine (25%), stomatitis (24%), and pruritus (21%). 3 The only grade ≥ 3 treatment‐related AE reported in > 10% of patients was diarrhea (21%). 3 Of note, QTc interval prolongation was observed in 11% of patients in the platinum‐pretreated patients cohort (N = 114). 3
Mobocertinib is metabolized by cytochrome P450 (CYP) 3A to two active metabolites, AP32960 and AP32914, which have potency similar to that of mobocertinib for inhibiting EGFR. 1 The metabolites AP32960 and AP32914 account for 36% and 4% of the combined molar sum area under the plasma concentration–time curve (AUC), respectively. 4 Mobocertinib, AP32960, and AP32914 inhibited and induced CYP3A in vitro (data on file; Takeda Development Center Americas, Inc.). Furthermore, repeat dosing of mobocertinib 160 mg q.d. in patients with mNSCLC was associated with lower than expected accumulation based on its plasma elimination half‐life and the 24‐hour dosing interval, suggesting autoinduction of metabolism by mobocertinib, likely via induction of CYP3A. 5 Exposures following treatment with mobocertinib were expressed as molar sum based on the sum of mobocertinib, AP32960, and AP32914 concentrations in molar units. 6 Co‐administration of mobocertinib with itraconazole, a strong CYP3A inhibitor, increased the molar sum AUC from time 0 to infinity (AUC0–∞) of mobocertinib, AP32960, and AP32914 by 527%, whereas co‐administration with rifampin, a strong CYP3A inducer, reduced the molar sum AUC0–∞ by 95%. 5 Food‐effect studies showed no clinically meaningful differences in molar sum AUC0–∞ when mobocertinib was co‐administered with a low‐fat meal or high‐fat meal compared with fasting conditions. 4 , 6
A population pharmacokinetic (PK) model for plasma concentrations of mobocertinib, AP32960, and AP32914 was developed based on data from two phase I studies in healthy adults and two phase I/II studies in patients with mNSCLC, including the dose‐escalation, expansion, and extension parts of the phase I/II study. The PK of mobocertinib, AP32960, and AP32914 was well‐characterized by a joint model, which described the PK of mobocertinib, AP32960, and AP32914. 7 The PK of mobocertinib and the PK of AP32960 were described by a two‐compartment PK model, whereas a one‐compartment model was used to describe the PK of AP32914. An enzyme compartment with drug‐dependent stimulation of enzyme production (i.e., auto‐induction) was included to describe the observed nonlinear PK of the three moieties. Model‐based simulations demonstrated that age, body weight, race, sex, creatinine clearance, estimated glomerular filtration rate, alanine aminotransferase, aspartate aminotransferase, bilirubin, and albumin did not have clinically meaningful effects on the systemic exposures of mobocertinib, AP32960, and AP32914 in patients with mNSCLC, suggesting that dose adjustment is not required based on these covariates. 7
The objective of this exposure–response analysis was to assess potential relationships between exposure to mobocertinib and its active metabolites (AP32960 and AP32914) with clinical ORRs, rates of selected AEs, and time to first dose reduction in patients with EGFRex20ins‐positive mNSCLC previously treated with platinum‐based chemotherapy who received mobocertinib 160 mg q.d. utilizing data from the phase I/II study.
METHODS
Study design and patient population
All efficacy and safety data contributing to the analysis were from a multicohort, three‐part, open‐label multinational phase I/II clinical trial (ClinicalTrials.gov NCT02716116) of mobocertinib in adult patients with advanced mNSCLC refractory to standard therapies. 2 , 3 Study methodology has been previously published. 2 Patients received oral mobocertinib (5–180 mg/day) in a 3 + 3 design in the dose escalation‐phase (part 1) and the recommended phase II dose of mobocertinib (160 mg q.d.) in the expansion (part 2) and extension phases (part 3). Dose delays or reductions were allowed for AEs. The first dose reduction was to mobocertinib 120 mg q.d., followed by a reduction to 80 mg q.d. if needed. After dose reduction, patients continued treatment at the reduced dose. The blood sampling schedule for PK assessments is provided in Table S1 . The protocol was approved by appropriate local review boards or ethics committees. The population considered for exposure–response analyses included patients with EGFRex20ins‐positive mNSCLC previously treated with platinum‐based chemotherapy who received mobocertinib 160 mg q.d. in the dose‐escalation part, expansion cohort 1, or the EXCLAIM extension cohort, and had both PK and relevant efficacy or safety data available. The data cutoff date for the exposure–response analyses was May 29, 2020.
Data set construction was performed using SAS (version 9.4; SAS Institute, Cary, NC). Exposure–response analyses and simulations were performed using R (versions 3.6.3 and 4.0.2; R Core Team, Vienna, Austria).
Exposure–efficacy analyses
Relationships between systemic exposure and IRC‐assessed clinical response (partial response [PR] or better vs. stable disease or worse) were characterized using logistic regression models. The exposure metrics evaluated (normalized dynamic molar sum exposure and time‐averaged molar sum exposure) were derived based on individual estimated parameter values predicted by the population PK model and available dosing information, and therefore accounted for dose modifications and interruptions for individual patients. Other exposure metrics that did not account for dose modifications and interruptions were not considered in the exposure–response analyses.
First, a repeated‐measures logistic regression model was used to describe longitudinal response assessments (i.e., probability of a response of PR or better at a given visit for an individual patient) as a function of normalized dynamic molar sum exposure. Normalized dynamic molar sum exposure was defined as the total AUC for predicted molar sum concentrations of mobocertinib (Cmobo), AP32960 (C960), and AP32914 (C914) in the time interval from the previous response assessment (t prev) to the time of the current response assessment (t curr) based on predictions from the population PK model taking into account dose reductions and dose interruptions. Normalized dynamic exposure was derived using the form:
The logit (log‐odds) of the predicted probability of response was calculated using the following equation:
and were scalar and vector parameters that represented the baseline logit and the effect of the predictor, (time‐dependent exposure or baseline covariates) on the logit, respectively, and was a random effect implemented to nonparametrically describe the correlation between repeated measurements.
Second, a binary logistic regression model was evaluated for its ability to describe the relationship between time‐averaged molar sum exposure and the probability of achieving a best response of confirmed PR or better (i.e., confirmed ORR) vs. stable disease or worse. Time‐averaged molar sum exposure was defined as the molar sum exposure for mobocertinib, AP32960, and AP32914 based on administered doses from day 1 of treatment (i.e., first dose) up to the time of a single (i.e., best) efficacy response per patient, divided by the total time to the event (t curr), calculated as:
The predicted concentration‐time courses used to derive time‐averaged exposure accounted for dose reductions and interruptions.
Response probability was defined similarly to in the longitudinal response model described above, except that it did not include the terms for the random effect and time (t).
For each exposure–efficacy model, a base model was developed to assess the relationship between molar sum exposure and the probability of clinical response. If exposure was not identified as a statistically significant predictor of response at the level of P = 0.05, additional covariate analysis was not performed, and the final model was identical to the base model.
The predictions of the repeated‐measures logistic regression model were graphically compared with the observed data. First, observed responses were summarized to a probability of response within each patient. Second, in order to facilitate comparison and account for correlations in the observed data, assessments were aggregated by patient by deriving the within‐patient probability of response. The mean of normalized dynamic molar sum exposure was calculated within each patient. Patient‐specific probabilities were then summarized by plotting the mean of patient‐specific response probabilities vs. the mean normalized dynamic molar sum exposure for quartiles of the observed data.
The predictions of the binary logistic regression model were graphically compared with the observed data by showing the probability of observed and predicted response for each quartile of time‐averaged exposure.
Exposure–safety analyses
Models were developed to describe the relationship between time‐averaged molar sum exposure and grade ≥ 3 treatment‐emergent adverse events (TEAEs), grade ≥ 3 treatment‐related TEAEs, and treatment‐emergent serious adverse events based on a binary logistic regression model similar to the model of clinical response, and selected clinically relevant AEs (diarrhea, nausea, paronychia, rash, stomatitis, and vomiting) based on proportional odds logistic regression models. For each patient, the dependent variable was the highest reported grade of the AE (or no AE) occurring after initiation of mobocertinib treatment and up to 30 days after the last dose of mobocertinib. If an AE occurred more than once for a patient, then the time to the first occurrence of the highest grade of the AE was used. The relationship between time‐averaged molar sum exposure and the probability of developing an AE was estimated by the following proportional odds logistic regression model:
was a scalar parameter that represented the baseline logit for an AE of severity grade or lower and was a vector parameter that represented the effect of the predictor, (i.e., time‐averaged molar sum exposure) on the logit. If exposure was not identified as a statistically significant predictor of an AE (at P = 0.05), additional covariate analysis was not performed, and the final model was identical to the base model. Predicted AE exposure–response curves with 95% confidence bounds and observed AE rates with 95% confidence intervals (CIs) within time‐averaged exposure quartiles were plotted for all exposure–safety analyses.
Exposure–dose reduction analysis
A time‐to‐event Cox proportional hazard model was developed to evaluate time‐averaged exposure as a predictor of the time to the first AE‐related mobocertinib dose reduction. The probability of being event‐free (i.e., no dose reduction) up to time t, S(t), was related to the hazard function h(t) using the following equations:
was a nonparametric baseline hazard, the general function, f, represented the effect of time‐averaged exposure as a predictor of dose reductions, and , the coefficient for the effect of the predictor, and , … were potential or known risk factors (covariates), and the coefficients (β) for the corresponding log‐hazard ratios (HRs).
Kaplan–Meier plots of the time to the first mobocertinib dose reduction (i.e., showing the proportion of patients free of dose adjustment) were created and stratified by time‐averaged exposure. If exposure was not identified as a statistically significant predictor of the time to first mobocertinib dose reduction (at P = 0.05), additional covariate analysis was not performed, and the final model was identical to the base model.
Model‐based predictions for dose reduction from 160 to 120 mg q.d. mobocertinib
For efficacy and safety exposure–response models, the impact of exposure on odds ratios (ORs) or HRs for events of interest was estimated for a decrease of exposure corresponding to a change of 753 nM∙h/day, which reflects the population PK model‐predicted change in molar sum exposure for a dose reduction from mobocertinib 160 mg q.d. (3300 nM∙h/day) to mobocertinib 120 mg q.d. (2547 nM∙h/day) at steady‐state.
RESULTS
Exposure–response data set
One hundred fourteen patients with platinum pretreated EGFRex20ins‐positive mNSCLC treated with mobocertinib 160 mg q.d. were included in the exposure–response population for analysis of efficacy and safety outcomes. A summary of demographics and baseline characteristics that were evaluated as covariates in the exposure–response analyses are shown in Table 1 .
Table 1.
Demographics and baseline characteristics of the exposure–response analysis population
| n = 114 | |
|---|---|
| Continuous covariate, mean (range) | |
| Age, years | 59.6 (27–84) |
| Body weight, kg | 66.8 (37.3–118.1) |
| Categorical covariates, n (%) | |
| Sex | |
| Female | 75 (65.8) |
| Male | 39 (34.2) |
| Race | |
| Asian | 68 (59.6) |
| White | 42 (36.8) |
| Black or African American | 3 (2.6) |
| Other | 1 (0.9) |
Exposure–efficacy analyses
Model‐predicted and observed probabilities of longitudinal clinical response of PR or better are plotted against normalized dynamic molar sum exposure in Figure 1a . Modeling of the probability of longitudinal clinical response showed that a decrease in normalized dynamic molar sum exposure corresponding to the change in exposures associated with mobocertinib 160 mg q.d. vs. 120 mg q.d. (i.e., difference of 753 nM∙h/day) was associated with a lower likelihood of IRC‐assessed response that did not reach statistical significance (OR: 0.778, 95% CI: 0.550–1.10, P = 0.156; Table 2 ).
Figure 1.
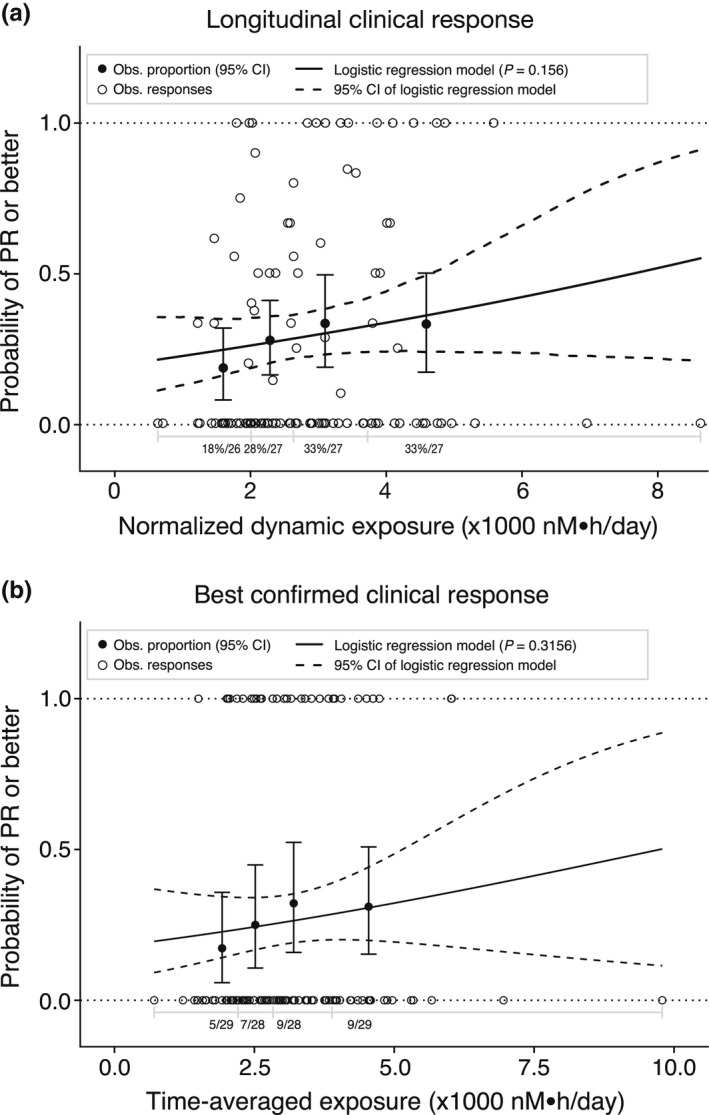
Model‐predicted and observed (Obs.) probabilities of (a) longitudinal clinical response of partial response (PR) or better plotted against normalized dynamic exposure and (b) best confirmed response of PR or better plotted against time‐averaged exposure. Solid (dashed) curves show the model‐predicted mean probability of confirmed PR or better (95% confidence interval [CI]). Closed circles (error bars) show observed mean probability of PR or better (95% CI based on the Pearson–Klopper method in panel b) by exposure quartile. Open circles indicate individual probabilities of PR or better. In a, n%/N is the mean of individual probabilities/total number of patients in each exposure quartile. In b, n/N is the number of patients with PR or better/total number of patients in each quartile. Seven patients without valid longitudinal clinical response assessments were excluded.
Table 2.
Model‐predicted clinical response rates following treatment with mobocertinib 160 mg q.d. and 120 mg q.d.
| Outcome | Predictor | Predicted response rate, % (95% CI) | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| 160 mg q.d. | 120 mg q.d. | ||||
| Longitudinal clinical response (PR or better) | Normalized dynamic molar sum exposure | 30.7 (23.2, 39.4) | 27.9 (21.3, 35.8) | 0.78 (0.55, 1.10) | 0.156 |
| Best confirmed response (PR or better) | Time‐averaged molar sum exposure | 26.7 (19.3, 35.6) | 24.5 (16.9, 34.1) | 0.89 (0.71, 1.12) | 0.316 |
CI, confidence interval; PR, partial response.
Model‐predicted and observed probabilities of best confirmed IRC‐assessed response of PR or better (i.e., confirmed ORR) are plotted against time‐averaged molar sum exposure in Figure 1b . A decrease in time‐averaged molar sum exposure corresponding to the change in exposures between the 160‐mg q.d. vs. 120‐mg q.d. dose was associated with a lower IRC‐assessed response rate that did not reach statistical significance (OR: 0.889, 95% CI: 0.706–1.12, P = 0.3156; Table 2 ).
Exposure–safety analyses
We explored the relationship between time‐averaged molar sum exposure and occurrence of AEs (Table 3 ). Increasing time‐averaged molar sum exposure was significantly (P = 0.004) correlated with increased probability of grade ≥ 3 TEAEs (Figure 2a ). No covariates were found to significantly affect this relationship. The model predicted that a decrease in time‐averaged molar sum exposure corresponding to a dose reduction from 160 mg to 120 mg q.d. mobocertinib would decrease the OR of grade ≥ 3 TEAEs by ~ 30% (OR: 0.701, 95% CI: 0.534–0.92, P = 0.004). No statistically significant relationships were identified between time‐averaged molar sum exposure and any of the AEs of clinical interest evaluated, including diarrhea (P = 0.156; Figure 2b ), nausea (P = 0.230; Figure 2c ), paronychia (P = 0.844; Figure 2d ), rash (P = 0.066; Figure 2e ), stomatitis (P = 0.061; Figure 2f ), and vomiting (P = 0.402; Figure 2g ). The relative contribution of mobocertinib, AP32960, and AP32914 exposures to the molar sum were ~ 61, 35, and 4%, respectively, based on the exposure–response analysis of best confirmed clinical response (IRC‐assessed), rash, and diarrhea. The contributions were similar across exposure quartiles with mobocertinib ranging from 58% to 64%, AP32960 ranging from 31% to 38%, and AP32914 ranging from 4% to 5%, confirming that the molar sum could be credibly considered as a single exposure metric in the exposure–response analyses. Predicted rates of AEs based on typical predicted exposures following treatment with mobocertinib 160 mg q.d. (3300 nM·h/day) vs. 120 mg q.d. (2547 nM·h/day) without dose reductions and dose interruptions, and corresponding ORs are shown in Table S2 .
Table 3.
Incidence of adverse events in the exposure–safety analysis population
| Event, n (%) | n = 114 |
|---|---|
| Grade ≥ 3 TEAEs | 75 (66) |
| Drug‐related grade ≥ 3 TEAEs | 53 (46) |
| Treatment‐emergent SAEs | 52 (46) |
| TEAEs of clinical interest (any grade) | |
| Diarrhea | 105 (92) |
| Nausea | 42 (37) |
| Paronychia | 40 (35) |
| Rash | 66 (58) |
| Stomatitis | 27 (24) |
| Vomiting | 46 (40) |
SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
Figure 2.
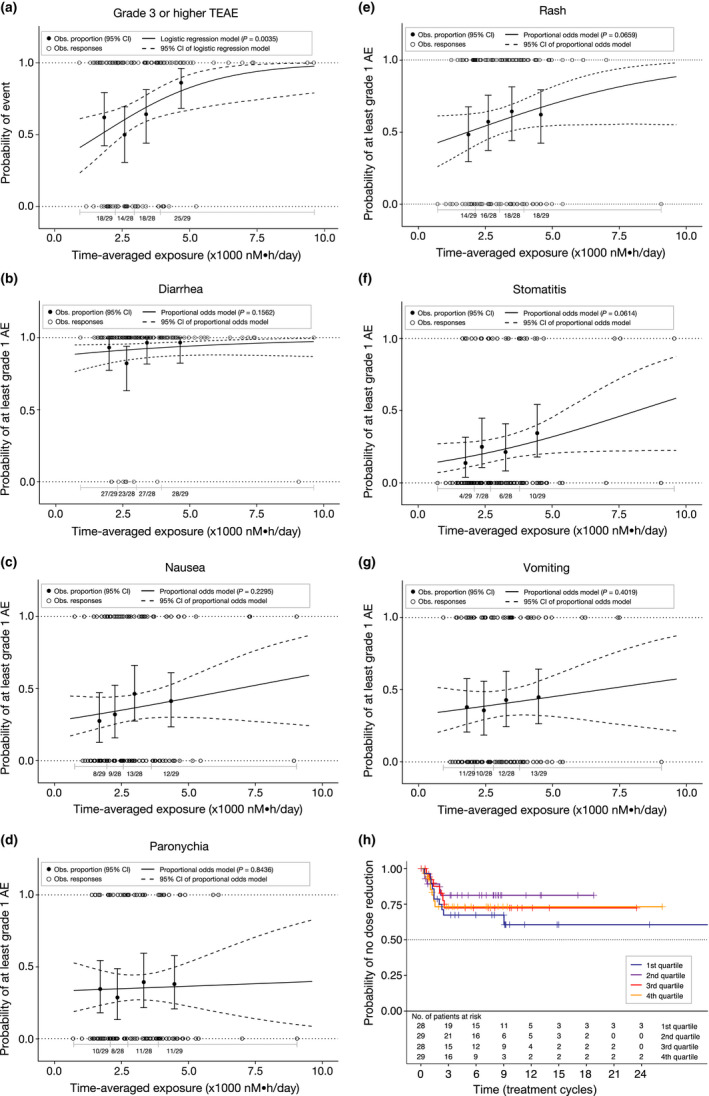
Exposure–safety analyses. Observed (Obs.) and model‐predicted proportions of patients with (a) grade ≥ 3 treatment‐emergent adverse events (TEAEs) and grade ≥ 1 TEAEs of (b) diarrhea, (c) nausea, (d) paronychia, (e) rash, (f) stomatitis, and (g) vomiting. Solid (dashed) curves show model‐predicted probability of the event (95% confidence interval [CI]). Closed circles (error bars) show observed proportion of patients with the TEAE (95% CI based on the Pearson–Klopper method) by exposure quartile. Open circles indicate data from individual patients. n/N is the number of patients with an event/total number of patients in each exposure quartile. P value represents the no exposure effect on the probability of the adverse event (AE). (h) Kaplan–Meier estimates of the time to first mobocertinib dose reduction after cycle 1 day 1 stratified by time‐averaged molar sum exposure quartiles.
Relationship between molar sum exposure and time to first dose reduction
Time‐averaged molar sum exposure was not a significant predictor of the time to first dose reduction in the time‐to‐event model (P = 0.959; Figure 2h ). The estimated HR for time to first dose reduction during treatment with 160 mg q.d. vs. 120 mg q.d. was 0.994 (95% CI: 0.797–1.24).
DISCUSSION
These exposure–response analyses evaluated relationships among mobocertinib exposure, based on the molar sum of exposures to mobocertinib and its active metabolites (AP32960 and AP32914), and clinical efficacy and safety outcomes. Exposure metrics were derived from a previously developed population PK model and efficacy and safety data were obtained from 114 patients with platinum‐pretreated EGFRex20ins‐positive mNSCLC treated with mobocertinib 160 mg q.d. in a phase I/II clinical trial. Mobocertinib 160 mg q.d. was the maximum tolerated dose and recommended phase II dose based on the dose‐escalation portion of the phase I/II study. 2 Both time‐dependent (normalized dynamic molar sum exposure between two response assessments) and static (time‐averaged molar sum exposure) metrics were implemented in modeling of clinical ORRs. Time‐dependent exposure metrics may be more relevant than static metrics for characterizing exposure–response relationships, as they more accurately capture fluctuations in exposure over time caused by dose modifications. 8 , 9 However, in the present analysis, neither exposure metric was identified as a statistically significant predictor of clinical response, suggesting that the efficacy benefit of mobocertinib is consistent across the observed range of molar sum exposures achieved after administration of the 160‐mg q.d. dose. The longitudinal clinical response model describing the relationship between the time‐dependent exposure metric (normalized dynamic molar sum exposure) and probability of response of PR or better at each assessment suggested a trend for decreased likelihood of response between the 160‐ and 120‐mg dose levels (OR: 0.78; 95% CI: 0.55–1.10; P = 0.156). This is consistent with data from patients with mNSCLC and EGFRex20ins mutations in the phase I portion of the study, which demonstrated investigator‐assessed confirmed ORRs of 43% at the 160‐mg starting dose compared with 19% at the 120‐mg starting dose, thereby suggesting that a lower starting dose may adversely affect efficacy. 2 Additionally, among the 114 platinum‐pretreated patients with (n = 29) and without (n = 85) dose reductions due to TEAEs, ORRs per IRC were 21% (6/29; 95% CI: 8–40%) and 31% (26/85; 95% CI: 21–42%), respectively (J.C.‐H. Yang, unpublished data, March 2022).
The lack of a statistically significant exposure–efficacy relationship may be due to only one dose level being evaluated. Furthermore, most patients were able to maintain dosing with 160 mg q.d., potentially resulting in a narrower exposure range available for this analysis. Notably, steady‐state average concentration (Cave) values of mobocertinib, AP32960, and AP32914 following administration of mobocertinib 160 mg q.d. were greater than (for mobocertinib and AP32960) or similar to (for AP32914) the half‐maximal inhibitory concentration values for cellular inhibition of EGFRex20ins mutants with NPG, ASV, NPH, SVD, and FQEA insertions. 1 A similar absence of a relationship between drug exposure and probability of response has been observed with other EGFR TKIs. 10 , 11 , 12 Other efficacy outcomes, such as progression‐free survival and overall survival, may correlate with systemic exposures in the absence of an ORR‐exposure relationship. 9 However, these end points were not evaluated in the present analysis as these data were immature at the time of the analysis cutoff used for the exposure–response analyses.
The exposure–safety analyses identified statistically significant relationships between time‐averaged molar sum exposure and the probability of grade ≥ 3 TEAEs, with higher exposures increasing the probability of experiencing a grade ≥ 3 event. The model predicted that a dose reduction from 160 to 120 mg would decrease the odds of experiencing grade ≥ 3 TEAEs by ~ 30%. In contrast, no statistically significant relationships were identified between time‐averaged molar sum exposure and any individual AEs of clinical interest, including diarrhea, nausea, paronychia, rash, stomatitis, and vomiting. However, the models predicted that dose reduction from 160 to 120 mg would result in ORs for each AE that were < 1, indicating a general trend toward lower probability of AEs with lower exposure. These trends approached statistical significance for the AEs of rash (OR: 0.82; P = 0.066) and stomatitis (OR: 0.83; P = 0.061). Of note, concentrations of mobocertinib and its metabolites in the gastrointestinal tract were not available, which precluded any additional analyses relating local concentrations to the gastrointestinal AEs of nausea, vomiting, and diarrhea. In addition, evaluation of the relationship between exposure and QTc interval prolongation was not explored in this analysis; however, categorical electrocardiogram outlier analysis by mobocertinib dose did not identify any readily apparent trend (Table S3 ). Statistically significant relationships between drug exposure and AEs typical of EGFR inhibition (e.g., diarrhea, rash) have been reported for other EGFR TKIs. 10 , 11 , 13 , 14 The tolerability of mobocertinib during extended treatment was supported by the small percentage (25%) of patients with a dose reduction and time to event modeling demonstrating that molar sum exposure was not a significant predictor of the time to first mobocertinib dose reduction.
CONCLUSIONS
Exposure–efficacy modeling indicated that molar sum exposure of mobocertinib and its active metabolites is not a significant predictor of efficacy based on the analysis of longitudinal clinical responses and overall best confirmed response. Thus, the clinical efficacy benefit of mobocertinib appears to be consistent across the range of systemic exposures (AUC) achieved with the starting dose of 160 mg q.d. The longitudinal clinical response model suggested a decreased likelihood of response between the 160‐ and 120‐mg dose levels. Increased time‐averaged molar sum exposure of mobocertinib significantly correlated with the probability of grade ≥ 3 TEAEs. Exposure–safety modeling identified no statistically significant relationships between molar sum exposure and the occurrence of AEs of diarrhea, nausea, paronychia, rash, stomatitis, and vomiting; however, a decrease in molar sum exposure corresponding to a dose reduction from 160 to 120 mg was associated with a trend toward lower probability of these AEs. Taken together, these exposure–response results support a favorable benefit‐risk profile of the recommended dosing regimen of mobocertinib (160 mg q.d.) for patients with mNSCLC.
FUNDING
This study is sponsored by Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
CONFLICT OF INTEREST
N.G.: Employment (Takeda); A.L.: Employee of Certara, a consulting firm under contract with Takeda; H.W.: Employee of Certara, a consulting firm under contract with Takeda; P.M.D.: Employee of Certara, a consulting firm under contract with Takeda; S.Z.: Employment (Takeda); M.J.H.: Employment (Takeda); J.L.: Employment (Takeda); M.M.: Employment (Takeda).
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. N.G., P.M.D., S.Z., and M.J.H. designed the research. A.L., H.W., P.M.D., and S.Z. analyzed the data.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families, and their caregivers; the study investigators and their team members at each study site; and colleagues from Millennium Pharmaceuticals, Inc., Cambridge, MA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. Professional medical writing assistance was provided by Lela Creutz, PhD, and Amy Zannikos, PharmD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by Millennium Pharmaceuticals, Inc. Teodor G. Paunescu, PhD, (Takeda Pharmaceuticals U.S.A., Inc.) is acknowledged for editorial assistance.
DATA AVAILABILITY STATEMENT
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results of the completed study, will be made available after the publication of the final study results within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after de‐identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
- 1. Gonzalvez, F. et al. Mobocertinib (TAK‐788): a targeted inhibitor of EGFR exon 20 insertion mutants in non–small cell lung cancer. Cancer Discov. 11, 1672–1687 (2021). [DOI] [PubMed] [Google Scholar]
- 2. Riely, G.J. et al. Activity and safety of mobocertinib (TAK‐788) in previously treated non–small cell lung cancer with EGFR exon 20 insertion mutations from a phase 1/2 trial. Cancer Discov. 11, 1688–1699 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou, C. et al. Treatment outcomes and safety of mobocertinib in platinum‐pretreated patients with EGFR exon 20 insertion–positive metastatic non–small cell lung cancer: a phase 1/2 open‐label nonrandomized clinical trial. JAMA Oncol. 7, e214761 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Exkivity (mobocertinib) package insert. Takeda Pharmaceuticals America Inc, Lexington, MA: (2021). [Google Scholar]
- 5. Zhang, S. et al. Effects of itraconazole and rifampin on the pharmacokinetics of mobocertinib (TAK‐788), an oral epidermal growth factor receptor inhibitor, in healthy volunteers. Clin. Pharmacol. Drug Dev. 10, 1044–1053 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang, S. et al. Single‐dose pharmacokinetics and tolerability of the oral epidermal growth factor receptor inhibitor mobocertinib (TAK‐788) in healthy volunteers: low‐fat meal effect and relative bioavailability of 2 capsule products. Clin. Pharmacol. Drug Dev. 10, 1028–1043 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta, N. , Pierillas, P.B. , Hanley, M.J. , Zhang, S. & Diderichsen, P.M. Population pharmacokinetics of mobocertinib in healthy volunteers and patients with non–small cell lung cancer. CPT: Pharmacometrics Syst. Pharmacol. 11, 731–744(2022). 10.1002/psp4.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau, Y.Y. , Gu, W. , Ho, Y.Y. , Hong, Y. , Zhang, X. & Urban, P. Application of time‐dependent modeling for the exposure–efficacy analysis of ceritinib in untreated ALK‐rearranged advanced NSCLC patients. Cancer Chemother. Pharmacol. 84, 501–511 (2019). [DOI] [PubMed] [Google Scholar]
- 9. Gupta, N. et al. Brigatinib dose rationale in anaplastic lymphoma kinase‐positive non‐small cell lung cancer: exposure–response analyses of pivotal ALTA study. CPT Pharmacometrics Syst. Pharmacol. 9, 718–730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown, K. et al. Population pharmacokinetics and exposure–response of osimertinib in patients with non‐small cell lung cancer. Br. J. Clin. Pharmacol. 83, 1216–1226 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukudo, M. et al. Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non‐small cell lung cancer. Clin. Pharmacokinet. 52, 593–609 (2013). [DOI] [PubMed] [Google Scholar]
- 12. Wind, S. , Schnell, D. , Ebner, T. , Freiwald, M. & Stopfer, P. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin. Pharmacokinet. 56, 235–250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li, J. et al. CYP3A phenotyping approach to predict systemic exposure to EGFR tyrosine kinase inhibitors. J. Natl. Cancer Inst. 98, 1714–1723 (2006). [DOI] [PubMed] [Google Scholar]
- 14. Niebecker, R. , Maas, H. , Staab, A. , Freiwald, M. & Karlsson, M.O. Modeling exposure‐driven adverse event time courses in oncology exemplified by afatinib. CPT Pharmacometrics Syst. Pharmacol. 8, 230–239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results of the completed study, will be made available after the publication of the final study results within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after de‐identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.


