Abstract
Patient: Male, 34-year-old
Final Diagnosis: Gastric sarcoidosis
Symptoms: Abdominal pain • nausea • vomiting • weight loss
Medication: —
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology • General and Internal Medicine
Objective:
Rare disease
Background:
Sarcoidosis is an inflammatory condition with multisystem involvement of unknown etiology that is characterized by noncaseating granulomas. Gastrointestinal (GI) involvement of sarcoidosis is not commonly seen in patients with extrapulmonary disease but can result in luminal narrowing, ulceration, and, less commonly, bleeding and obstruction. Patients that present with symptomatic gastric sarcoidosis are extremely rare. Definitive diagnosis can be challenging due to the need for endoscopic biopsy, which may not be performed if the diagnosis is not considered. Biopsy may be falsely negative due to the patchy mucosal involvement of this disease.
Case Report:
This case describes a 38-year-old mixed-race man who presented to the Emergency Department with GI symptoms including nausea, vomiting, and abdominal pain, which persisted after being recently discharged from an outside hospital. The patient had a known history of multisystem sarcoid including pulmonary and neurosarcoidosis, and was maintained on immunosuppressive therapy. The patient underwent upper endoscopy with biopsy confirming a new diagnosis of gastric sarcoidosis.
Conclusions:
There is an important role for early endoscopy in the diagnosis of patients with symptomatic gastric sarcoidosis to facilitate early treatment initiation and escalation or titration of immunosuppressive therapy, especially in patients with a known history of sarcoidosis with extrapulmonary involvement. The described endoscopic appearance of gastric sarcoidosis is variable in the published literature; endoscopic biopsy is therefore essential to diagnosing this disease. This type of disease progression should be considered in all sarcoid patients with persistent GI symptoms that do not resolve with conservative management, including those who are already on established immunosuppressive therapy.
Keywords: Endoscopy, Gastrointestinal Diseases, Sarcoidosis
Background
Sarcoidosis is a rare inflammatory condition with multisystem involvement of unknown etiology. It is characterized by noncaseating granulomas, which are collections of inflammatory cells usually seen in the lung, skin, and lymph nodes. Sarcoidosis involves the pulmonary system in over 90% of cases [1–3]. Extrapulmonary involvement carries significant morbidity and is underdiagnosed. Sarcoidosis can affect organs in a patchwork fashion. The reported frequency of GI involvement is 0.1–3.4% [4] and the frequency of symptomatic involvement of the GI tract is less than 1% [5]. The inflammation and fibrosis caused by sarcoidosis can result in luminal narrowing, ulceration, or diminished peristalsis. Rarely, patients present with more acute symptoms, such as bleeding or bowel obstruction [6]. In patients with a previously established diagnosis of sarcoidosis, particularly extrapulmonary sarcoidosis, it is essential to consider new or worsening localized symptoms as progression of disease, and to perform an appropriate diagnostic evaluation. We describe a case of a patient on treatment with immunosuppressive therapy for pulmonary and neurosarcoidosis who had worsening GI symptoms requiring endoscopic evaluation and biopsy, confirming gastric sarcoid.
Case Report
A 38-year-old mixed-race man presented to the Emergency Department with intractable nausea, vomiting, and abdominal pain for 1 month. He had a past medical history of bipolar disorder, anemia, multisystem sarcoidosis, pulmonary embolism, chronic hypoxic respiratory failure on home oxygen, and steroid-induced avascular necrosis of bilateral femoral heads and left humeral head, previously treated mycobacterium avium complex and tuberculosis infections. During the month prior to admission, the patient was only able to tolerate a liquid diet due to vomiting, resulting in a weight loss of 3.6 kg. He denied blood or bile in his vomitus. His abdominal pain was diffuse but mostly localized to the lower abdomen and epigastrium. He denied any dysphagia or change in bowel habit.
The patient’s sarcoidosis was first suspected in 2018 when a retroperitoneal lymph node biopsy returned positive for noncaseating granulomas. This was performed in the context of significant weight loss, fevers, and night sweats. Computed tomography (CT) and magnetic resonance imaging during multiple subsequent hospital and clinic presentations showed pulmonary and leptomeningeal findings suspicious for sarcoidosis. Noncaseating granulomas were confirmed by bronchial biopsy and L2 spinous process biopsy in 2020. The patient was treated with a prolonged course of steroids and was subsequently transitioned to methotrexate (Figures 1, 2).
Figure 1.
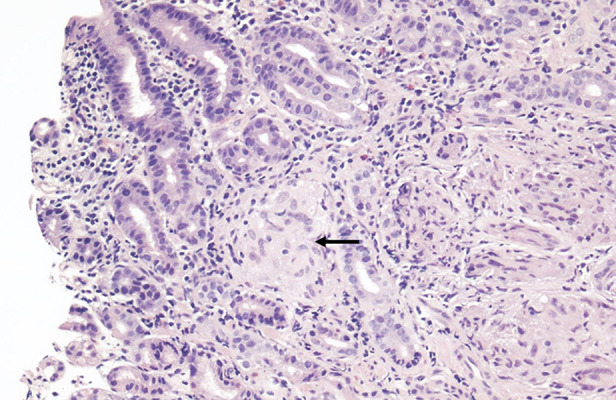
Gastric oxyntic-type mucosa, notable for the presence of noncaseating granulomas (black arrow) in the lamina propria. These granulomas are well formed, and consist of epithelioid cells and giant cells. There is a background of focally active chronic gastritis (H. pylori-negative by immunohistochemistry), and reactive epithelial changes.
Figure 2.
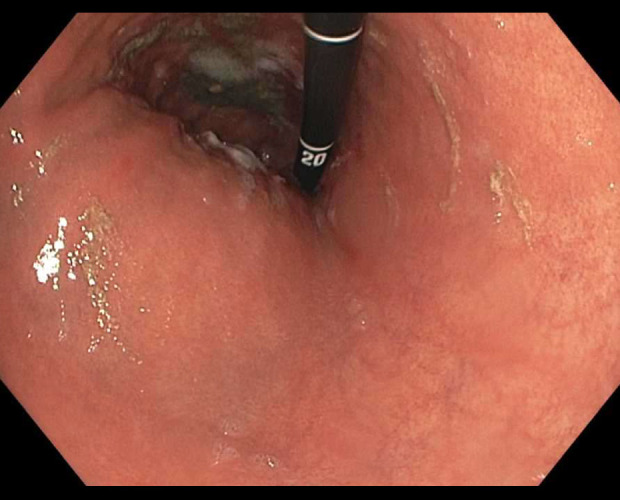
Gastric body with focal erosions during endoscopic evaluation.
The patient had recently presented to an outside hospital with the same gastrointestinal symptoms. He underwent a barium swallow there, which showed normal esophageal motility and a small hiatal hernia with mild reflux. A gastric emptying study was attempted but terminated due to the patient vomiting. The patient’s symptoms were attributed to a recently increased dose of methotrexate and the dosage was then decreased.
After presenting to our institution, he continued to experience nausea, vomiting, and abdominal pain after treatment with anti-emetics, intravenous fluids, pain medication, and a modified diet. A CT of the abdomen and pelvis was performed, which was unremarkable. The patient then underwent an upper gastrointestinal barium swallow series, which was aborted due to the patient vomiting. An esophagogastroduodenoscopy was then performed, which showed a few dispersed erosions in the gastric body, which were biopsied, and otherwise normal appearances of the esophagus, stomach, and upper duodenum. His nausea and vomiting improved and he was able to tolerate a regular diet. The patient was discharged with follow-up pending biopsy results. Biopsy of the erosions later revealed noncaseating granulomas focally present in the lamina propria of the gastric oxyntic mucosa, with focally active mild chronic gastritis.
The patient reported a significant negative impact on his daily life secondary to his sarcoidosis. His chronic hypoxic respiratory failure and oxygen dependence limited his exercise tolerance, social activities, and employment opportunities despite his young age. He also experienced pain, discomfort, and limited range of motion due to the avascular necrosis of his hips and shoulder secondary to prolonged high doses of steroids. He is largely homebound as a result and requires assistance with most of his activities of daily living. His abdominal pain, vomiting, and weight loss led to further deterioration in his quality of life and the new diagnosis of gastric involvement despite being on treatment took a considerable toll on the patient.
Following discharge, the patient attended a rheumatology out-patient clinic and was initiated on adalimumab in addition to methotrexate. He had repeat echocardiography, showing new left ventricular dysfunction concerning for cardiac sarcoidosis with negative cardiac PET imaging. Review of records 6 months following discharge revealed continued hospital and clinic presentations with nausea, vomiting, and abdominal pain.
Discussion
Gastric sarcoidosis is a very rare clinical manifestation seen in patients with extrapulmonary involvement, which requires endoscopic biopsy for confirmation of diagnosis. A nationwide case–control study of 25 French cases of gastrointestinal sarcoidosis in 2016 observed that macroscopic lesions could involve any part of the digestive tract, including the esophagus (9%), stomach (78%), duodenum (9%), colon (25%), and rectum (19%). The majority of patients with digestive tract involvement were of Afro-Caribbean origin [7] Interestingly, this agrees with the results of the ACCESS study (A Case Control Etiologic Study of Sarcoidosis), in which extrathoracic involvement was more common in Black than White subjects (P<0.001). [8]
The most common GI symptoms reported in other case reports are abdominal pain, nausea, vomiting, and weight loss [5,9,10], as seen in this patient. These symptoms are non-specific and therefore difficult to distinguish from other GI pathologies such as malignancy, gastroparesis, gastroesophageal reflux disease, and irritable bowel syndrome.
This patient’s endoscopic evaluation revealed only a few benign appearing scattered erosions in the gastric body. The remainder of the examination was largely unremarkable. A review of 44 well-documented cases of gastric sarcoidosis published from 1955 to 2008 showed a significant variability in endoscopic findings, with an ulcerative picture being most common (43%), but with 14% of these patients having normal mucosa [10]. Granulomatous infiltration is often patchy and may be missed on biopsy. If the inflammation is confined to the submucosa and deeper layers of the stomach, superficial endoscopic biopsies may miss the diagnosis. If the infiltration is diffuse, endos-copy may reveal an appearance similar to linitis plastica [11].
A careful pathology examination is needed in incident cases, as noncaseating, non-necrotizing granulomas can also be seen in atypical tuberculosis, Whipple’s disease, Crohn’s disease, carcinomatosis, and fungal infections, among others [12,13]. Histiocytic markers such as CD68 are usually strongly reactive in sarcoid granulomas. Pan-cytokeratin staining, which is seen in malignancy, should be negative. Staining the pathology for microorganisms can help rule out suspected infectious causes. It is important to discuss the case with the pathologist to have an appropriate evaluation of the biopsy specimen performed. Immunohistochemical staining can be useful but is not always pathognomonic [13].
Sarcoidosis patients with worsening and persisting GI symptoms should be considered for early endoscopic evaluation and biopsy to avoid delay in diagnosis and treatment. A high index of suspicion is particularly recommended in patients of Afro-Caribbean race who have pre-existing extrapulmonary sarcoidosis and who failed non-invasive testing and treatment. Obtaining random biopsies in these cases should be considered even in areas where the mucosa appears normal. As seen in the initial course of this patient’s symptoms, the cause of his nausea and vomiting was incorrectly attributed to high methotrexate dosing and the dose was reduced.
Current accepted methods of treatment of sarcoidosis are based on only small trials and expert consensus. Published treatment algorithms provide recommendations for the initiation dosage and taper schedule of steroids, but there is significant variability in this. At present, there are no site-specific treatment algorithms for extrapulmonary involvement. Methotrexate, azathioprine, leflunomide, and mycophenolate are the most commonly utilized steroid-sparing agents. Overall, trials to determine the ideal dosing and length of therapy are limited by the rarity and heterogeneity of the disease, as well as lack of standardized outcome measures [14]. The patient in this report was transitioned to methotrexate after developing debilitating avascular necrosis on steroid therapy, but experienced disease progression on methotrexate; therefore, adalimumab was added as a third-line agent. Biologic therapy including tumor necrosis factor inhibitors (eg, infliximab and adalimumab) can be considered in patients that are unable to tolerate other treatment options or have refractory disease. Studies on both infliximab and adalimumab have shown significant clinical improvement for treatment-refractory sarcoidosis [15,16].
There are no standardized criteria to monitor patient’s symptoms and clinical response following the commencement of therapy. We noted the patient continued to be symptomatic after commencing adalimumab but it is unclear whether the frequency, severity, or nature of his symptoms have objectively changed. Suggested approaches include patient journaling to follow the time to toleration of diet and percentage of tolerated diet, as well as the frequency of abdominal pain episodes with use of an abdominal pain scale [6].
The findings of this case study are informative to clinicians managing sarcoid patients in considering further evaluation of new systemic symptoms possibly representing disease progression, and in considering the need for immunosuppressive medication titration or alternative therapy.
Conclusions
Patients with pulmonary and extrapulmonary sarcoidosis remain susceptible to disease progression even when on high-dose immunosuppressive therapy. Involvement of the gastrointestinal tract is rare but possible. Patients with sarcoidosis who present with persistent gastrointestinal symptoms should be evaluated with endoscopy and biopsy. The aim of diagnosing gastrointestinal sarcoidosis is to adjust therapy to prevent disease progression and complications and potentially improve quality of life.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Lynch JP, 3rd, Kazerooni EA, Gay SE. Pulmonary sarcoidosis. Clin Chest Med. 1997;18(4):755–85. doi: 10.1016/s0272-5231(05)70417-2. [DOI] [PubMed] [Google Scholar]
- 2.Ungprasert P, Carmona EM, Utz JP, et al. Epidemiology of sarcoidosis 1946–2013. Mayo Clin Proc. 2016;91(2):183–88. doi: 10.1016/j.mayocp.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belperio JA, Shaikh F, Abtin FG, et al. Diagnosis and treatment of pulmonary sarcoidosis: A review. JAMA. 2022;327(9):856–67. doi: 10.1001/jama.2022.1570. [DOI] [PubMed] [Google Scholar]
- 4.Al-Kofahi K, Korsten P, Ascoli C, et al. Management of extrapulmonary sarcoidosis: Challenges and solutions. Ther Clin Risk Manag. 2016;12:1623–34. doi: 10.2147/TCRM.S74476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeds JS, McAlindon ME, Lorenz E, et al. Gastric sarcoidosis mimicking irritable bowel syndrome – cause not association? World J Gastroenterol. 2006;12(29):4754–56. doi: 10.3748/wjg.v12.i29.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadpara N, Greenwald HS, Parkman HP. Treatment of a gastrointestinal sarcoidosis flare: A multidisciplinary approach for a multisystem disease. Am J Case Rep. 2021;22:e932494. doi: 10.12659/AJCR.932494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghrenassia E, Mekinian A, Chapelon-Albric C, et al. Digestive-tract sarcoidosis: French nationwide case-control study of 25 cases. Medicine (Baltimore) 2016;95(29):e4279. doi: 10.1097/MD.0000000000004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–89. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 9.Chinitz MA, Brandt LJ, Frank MS, et al. Symptomatic sarcoidosis of the stomach. Dig Dis Sci [Internet] 1985;30(7):682–88. doi: 10.1007/BF01308419. [DOI] [PubMed] [Google Scholar]
- 10.Afshar K, BoydKing A, Sharma OP, Shigemitsu H. Gastric sarcoidosis and review of the literature. J Natl Med Assoc. 2010;102(5):419–22. doi: 10.1016/s0027-9684(15)30577-0. [DOI] [PubMed] [Google Scholar]
- 11.Liang DB, Price JC, Ahmed H, et al. Gastric sarcoidosis: Case report and literature review. J Natl Med Assoc. 2010;102(4):348–51. doi: 10.1016/s0027-9684(15)30608-8. [DOI] [PubMed] [Google Scholar]
- 12.Mehta AC, Ali SR. Mnemonic for the differential diagnosis of non-caseating granulomas. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34(2):200–7. doi: 10.36141/svdld.v34i2.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tana C, Donatiello I, Caputo A, et al. Clinical features, histopathology and differential diagnosis of sarcoidosis. Cells. 2021;11(1):59. doi: 10.3390/cells11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerke AK. Treatment of sarcoidosis: A multidisciplinary approach. Front Immunol. 2020;11:545413. doi: 10.3389/fimmu.2020.545413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baughman RP, Lower EE. Infliximab for refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18(1):70–74. [PubMed] [Google Scholar]
- 16.Crommelin HA, van der Burg LM, Vorselaars ADM, et al. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir Med. 2016;115:72–77. doi: 10.1016/j.rmed.2016.04.011. [DOI] [PubMed] [Google Scholar]


