Abstract
Objective
Cross‐sectional studies demonstrate that catecholamine stimulation of fat cell lipolysis is blunted in obesity. We investigated whether this defect persists after substantial weight loss has been induced by metabolic surgery, and whether it is related to the outcome.
Design/Methods
Patients with obesity not able to successfully reduce body weight by conventional means (n = 126) were investigated before and 5 years after Roux‐en‐Y gastric bypass surgery (RYGB). They were compared with propensity‐score matched subjects selected from a control group (n = 1017), and with the entire group after adjustment for age, sex, body mass index (BMI), fat cell volume and other clinical parameters. Catecholamine‐stimulated lipolysis (glycerol release) was investigated in isolated fat cells using noradrenaline (natural hormone) or isoprenaline (synthetic beta‐adrenoceptor agonist).
Results
Following RYGB, BMI was reduced from 39.9 (37.5–43.5) (median and interquartile range) to 29.5 (26.7–31.9) kg/m2 (p < 0.0001). The post‐RYGB patients had about 50% lower lipolysis rates compared with the matched and total series of controls (p < 0.0005). Nordrenaline activation of lipolysis at baseline was associated with the RYGB effect; those with high lipolysis activation (upper tertile) lost 30%–45% more in body weight, BMI or fat mass than those with low (bottom tertile) initial lipolysis activation (p < 0.0007).
Conclusion
Patients with obesity requiring metabolic surgery have impaired ability of catecholamines to stimulate lipolysis, which remains despite long‐term normalization of body weight by RYGB. Furthermore, preoperative variations in the ability of catecholamines to activate lipolysis may predict the long‐term reduction in body weight and fat mass.
Keywords: adipocytes, catecholamines, glycerol, Roux‐en‐Y gastric bypass
Introduction
Obesity shortens life expectancy and is associated with multiple comorbidities [1, 2]. Factors directly related to energy metabolism may be causally involved [3]. Genetic studies suggest that besides central nervous system appetite‐regulating processes, peripheral metabolic regulators may also contribute to interindividual variations in body mass index (BMI) [4]. In particular, long‐term studies suggest that impaired ability of fat cells to mobilize lipids (lipolysis)—which constitutes a key process in supplying energy sources to the metabolism—could facilitate the development of excess body fat [5].
Lipolysis is under hormonal control by insulin, catecholamines and natriuretic peptides [6]. The decreased ability of catecholamines to activate lipolysis in obesity was first demonstrated in vivo [7] and subsequently in fat cells [8]. Numerous cross‐sectional studies in vivo and in vitro have confirmed this lipolytic catecholamine resistance in obesity [6, 9]. If lipolysis is not adequately increased by catecholamines, lipid turnover could shift towards fat accumulation and promote adipose tissue expansion [10]. Recent longitudinal studies of fat cell lipid turnover support this idea [11]. These studies demonstrate a decrease over time in turnover mainly due to a decreased lipolysis rate. If this is not compensated by a decreased incorporation of lipids into fat cells (i.e., less caloric intake), body weight increases over time. Furthermore, persons who do not have obesity but have a family history thereof display impaired catecholamine action in fat cells [12], and disturbances in fat cell lipolysis predict future weight gain [5, 13]. Also, children with obesity have decreased lipolytic action of catecholamines [14]. Taken together, available data suggest that impaired catecholamine‐stimulated fat cell lipolysis contributes to obesity development and to the difficulties in long‐term reduction of body weight with lifestyle interventions. A yet unanswered question is if the lipolysis defect remains following long‐term body‐weight reduction. If so, it might contribute to the difficulties in maintaining the lower body weight. Previous studies before and after weight loss are short term and therefore do not contribute to our understanding of the role of lipolytic regulation in conditions with and without excess fat mass. There is a continuous change in lipolysis and expression of adipose genes related to lipid metabolism during initial weight loss and subsequent weight regain [15, 16, 17]. Therefore, earlier studies—conducted when the patients were still in or just after an energy‐deficit phase—do not adequately address the question [18, 19]. We previously measured the overall lipolytic activity in pieces of subcutaneous adipose tissue obtained before and 2 as well as 5 years after metabolic surgery for obesity [17]. As expected, lipolysis was normalized at 2 years, but it increased to preoperative levels 5 years after surgery. Whether this means that catecholamine‐induced lipolysis is also abnormal long term following metabolic surgery is presently unknown. Another unanswered question is whether variations in the impairment of lipolysis are associated with the outcome of metabolic surgery.
In the present study, we addressed the above‐mentioned questions, that is, whether hormone‐induced fat cell lipolysis is persistently altered in patients with obesity after weight reduction and whether lipolysis prior to surgery is associated with long‐term loss of body weight. We considered subjects with obesity referred to metabolic (bariatric) surgery a suitable study group. Such patients were investigated before and 5 years after Roux‐en‐Y gastric bypass surgery (RYGB), when only slight weight regain had occurred. They were compared with propensity‐score matched (PSM) patients from a large nonselected control group with a broad range in BMI, in which none had undergone weight‐loss treatments with metabolic surgery at the time of examination. We also related fat cell lipolysis in RYGB patients at baseline with changes in body weight, BMI and fat mass after 5 years.
Methods
Participants
Patients and controls were recruited from the Stockholm County area (Sweden) and investigated at one centre (Karolinska University Hospital, Huddinge). The RYGB patients (n = 126) were referred for surgery due to long‐term failure to successfully reduce body weight by conventional approaches including diet, physical exercise and/or medication. In two studies (NCT01785134 and NCT01727245) carried out from 2006 through 2019 using almost identical protocols, participants were investigated before and 5 years after surgery (flow chart in Fig. 1). In the first study, the indications for RYGB included BMI ≥35 kg/m2. The second study also included three patients with a preoperative BMI of 32–34 kg/m2 who had additional indications for metabolic surgery (diabetes or unsatisfactory response to prior bariatric surgery). In both studies, patients were weight stable before surgery and were not treated with any low‐calorie diet preoperatively. Patients with type 1 diabetes were excluded. Briefly, a total of 277 patients were screened; 234 patients underwent baseline examination, whereof 126 were re‐examined after 5 years. At baseline, seven patients (5.6%) had type 2 diabetes treated with diet, metformin or sulphonylureas—but not insulin, incretins or thiazolidinediones—and no subject had diabetes at 5 years. A nonselected control group consisted of 1017 persons above 18 years of age examined at the laboratory between 1987 and 2019. Controls (see all clinical data in Table S1) displayed a large interindividual variation in BMI and had not undergone any metabolic surgery at the time of the examination of lipolysis. Obesity was defined as BMI ≥30 kg/m2. None of the controls had type 1 diabetes or any other severe chronic disorders, except for type 2 diabetes (n = 99, 9.7%), which was treated as for the RYGB patients. None of the patients or controls had severe chronic disease. For additional comparisons, we also investigated the 103 patients with obesity included in the RYGB studies, but who were lost to follow‐up before the 5‐year post‐surgery visit (RYGB noncompleters). At examination, all the patients and controls were weight stable (<± 1 kg change during the last 3 months) according to self report. The regional board of ethics of Stockholm, Sweden, approved the studies (record number for Study 1: 2005/1141‐32, approved 8 December 2005 and for Study 2: 2011/1002‐31/1 approved 12 September 2011). After detailed explanation of the purpose and nature of the study, each participant provided oral and written informed consent.
Fig. 1.
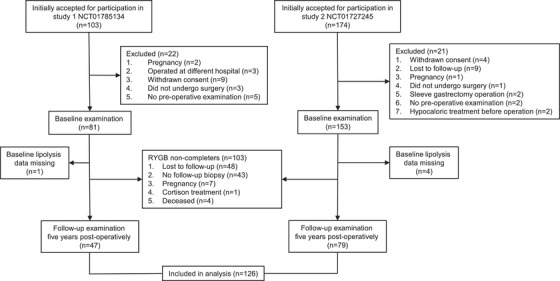
Patient inclusion process. Flow chart for recruiting and investigating patients in the two Roux‐en‐Y gastric bypass (RYGB) studies.
Procedures
The individuals were examined in the morning after an overnight fast. Height, weight and circumferences of the hip and waist were measured. Physical activity was assessed using a questionnaire and graded 1–4, as described in detail [17]. A venous plasma sample was obtained for routine clinical chemistry measurements. Overall insulin sensitivity was measured as the homeostasis model assessment (HOMA) [20] using the formula serum‐fasting insulin (mU/L) times fasting plasma glucose (mmol/L) divided by 22.5. Blood pressure was measured in the supine position after a 15 min rest. Body fat was measured by bioimpedance. Finally, an abdominal subcutaneous fat biopsy was obtained by needle aspiration in the paraumbilical area. The adipose tissue experiments have repeatedly been described and discussed in detail [8, 12, 15–17, 21] and were performed by the same technical staff throughout the study. In brief, isolated fat cells were prepared and incubated in vitro for 2 h without (basal) or with increasing concentrations of either noradrenaline or isoprenaline (synthetic nonselective beta‐adrenoceptor agonist). At the end of the incubation, medium glycerol concentrations (a measure of lipolysis) were determined. During lipolysis, glycerol and fatty acids are formed as the end product of hydrolysis of acylglycerols. Fatty acid release does not accurately reflect lipolysis because a substantial amount is re‐esterified by fat cells into new acylglycerols. Glycerol, on the other hand, is not reutilized by fat cells in any quantitatively important way. There is some incomplete lipolysis to diacylglycerol during catecholamine stimulation. However, this slight underestimation of lipolysis is not important, as the formation of diacylglycerol and the production of glycerol during stimulated lipolysis are strongly correlated [22]. The lipolytic response to catecholamines was determined as glycerol release at the maximum effective agonist concentration. On a separate aliquot of fat cells, the diameter of 100 cells was measured and mean fat cell volume and weight were determined by established formulas, as previously described [17, 18, 19]. Because there is no consensus on how to express lipolysis, we used the two most common modes: per lipid weight or per number of fat cells. These modes of expression were considered as physiologically relevant because they represent lipid mobilization per unit of fat mass and fat cell, respectively. As determination of fat cell volume and number share the same initial formulas, the rate of lipolysis per number of cells is dependent on the prevailing fat cell volume, unlike lipolysis per lipid weight.
Statistical methods
Descriptive values in text and tables are given as mean ± Standard Deviation (SD) or as median with interquartile range (25th–75th), and in the figures as box and whisker plots (5th –95th percentiles) or scatter plots. A 95% confidence interval is shown for estimates provided by regression analyses. As most values were not normally distributed, we used nonparametric tests when two groups of variables were compared (paired sign test Spearman's rank correlation or the Wilcoxon test and Pearson's chi‐square test). We used PSM to address and minimize potential selection bias and differences between the 5‐year post‐RYGB patients and controls. In brief, a logistic regression model was fitted for the patient status (patient vs. control), whereas pairing was implemented with a 1:2 ratio, the nearest neighbour method and a 0.2 width of caliper [23]. In PSM, we adjusted for the following factors known to influence lipolysis: age [24], sex [25] and BMI [9]. Fat cell volume also influences lipolysis when expressed per cell [26]. This factor was not included in PSM because we expected from earlier studies [17] that the volume would decrease to below normal range in post‐RYGB patients at 5 years post surgery. PSM groups were summarized using descriptive statistics, and covariate balance after matching was confirmed by using standardized mean differences [27]. The selection of factors for the matchings was determined by acquisition of the maximum possible number of RYGB patients and standardized mean difference <0.1. As the matching on fat cell volume between post‐RYGB and controls would adversely affect covariate balance, we evaluated the impact of fat cell volume on variations in lipolysis between post‐RYGB and controls using multiple regression. The models also included the PSM parameters mentioned above plus additional cofactors mentioned in the table that were considered to potentially influence lipolysis. The robust sandwich estimator was used to estimate standard errors; two‐sided p‐values were reported, and a p‐value <0.05 was considered significant. Analyses were performed using Stata version 15 (StataCorp, College Station, TX, USA), R version 3.6.1 (The R Foundation for Statistical Computing Platform) and JMP version 14.1.0 (SAS Institute Inc, Cary, NC, USA).
Results
Because more than 95% of patients in the two RYGB studies were recruited using the same inclusion criteria, and all were investigated in an identical fashion, we combined them in the analysis (Fig. 1). Body weight was recorded before and at 1, 2 and 5 years after RYGB at the routine clinical check‐ups. The values (mean ± SD, in kilograms) were 113 ± 16, 79 ± 14, 77 ± 14 and 83 ± 16, respectively (p < 0.0001 for each comparison). Thus, at the 5‐year follow‐up, patients were, at a group level, not in a catabolic state. From the control group, we first selected all subjects without obesity (nonobese) and compared them with RYGB completers before and 5 years after surgery as well as RYGB noncompleters (i.e., lost to follow‐up, see Methods). Clinical data are displayed in Table S2 for RYGB completers and nonobese controls, and in Table S3 for RYGB noncompleters. As expected, at baseline both RYGB groups showed the well‐known alterations in anthropometric measures and cardiometabolic profile, and the RYGB noncompleters had similar phenotypes at baseline as RYGB completers except being somewhat younger and more insulin resistant (higher HOMA values). All abnormal parameters improved 5 years after RYGB. Median BMI was reduced from 39.9 kg/m2 to 29.5 kg/m2. In other words, on average, the patients transformed from severe (Class 2) obesity to a nonobese state.
Figure 2 shows the fat cell lipolysis data for the three groups described above. When expressed per lipid weight (Fig. 2a–c), basal (spontaneous) and stimulated lipolysis rates at baseline were lower in RYGB noncompleters and completers than in nonobese controls. In the post‐RYGB state, basal lipolysis increased to similar levels as in nonobese controls (p = 0.095). Lipolysis stimulated with noradrenaline was similar before and 5 years after RYGB, but increased slightly with isoprenaline at 5 years. However, both measures significantly remained about 50% lower than in the nonobese control group. In other words, impaired stimulated lipolysis expressed per lipid mass remained despite long‐term body‐weight reduction. Similar results were obtained when women were analysed separately (data not shown).
Fig. 2.
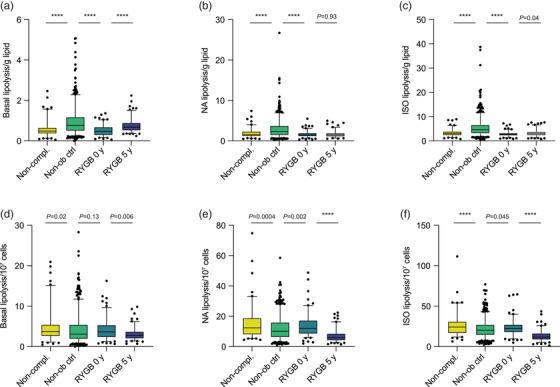
Findings with lipolysis. Lipolysis is expressed as μmoles of glycerol release per 2 h. (a–c) Lipolysis per gram of lipids. (d–f) Lipolysis per 107 fat cells. Fat cells were incubated in the basal state (a,d) with noradrenaline (NA, b,e) or isoprenaline (ISO, c,f). Results before and 5 years after Roux‐en‐Y gastric bypass (RYGB) were compared by paired sign test. Control persons were compared with RYGB patients who did not return for 5‐year re‐examination (noncompleters) and with RYGB completers before surgery using the Wilcoxon test.
When lipolysis was expressed per number of fat cells (Fig. 2d–f), values for noradrenaline and isoprenaline were higher in RYGB noncompleters and completers at baseline in comparison to nonobese controls. Basal and stimulated lipolysis per cell decreased significantly 5 years after RYGB operation to levels below those for nonobese controls (p = 0.035 for basal and <0.0001 for stimulated lipolysis).
The next step was to elucidate whether lipolysis in post‐RYGB patients was normalized or not. Therefore, it was necessary to correct for imbalances between subjects undergoing RYGB versus the nonselected controls in multiple factors that impact lipolysis. Among these, age, sex, BMI and/or body fat distribution (waist‐to‐hip ratio, WHR) are the most established to affect lipolysis (see Methods). To control for this, we compared the PSM control subjects to the post‐RYGB subjects. This resulted in adequately balanced cohorts (Fig. 3). Data (Table 1) showed that noradrenaline‐ and isoprenaline‐stimulated lipolysis in the post‐RYGB group was about 50% lower than among the PSM controls, irrespective of whether lipolysis was expressed per lipid weight or per number of fat cells. However, fat cell size was smaller in post‐RYGB patients than in PSM controls (Table 1), which could contribute to the between‐group differences in lipolysis per cell. Many of the clinical parameters were significantly more favourable in patients than in PSM controls.
Fig. 3.
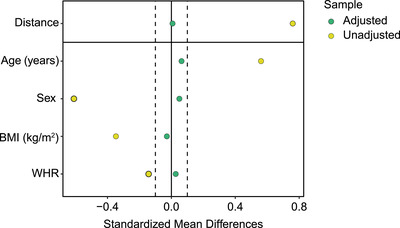
Propensity‐score matching. Control subjects were matched in the ratio 2:1 to Roux‐en‐Y gastric bypass patients, 5 years after surgery. The graph shows the covariate balance by standardized mean differences before (unadjusted—yellow dots) and after (adjusted—green dots) propensity‐score matching for the parameters listed on the left of the graph.
Table 1.
Propensity‐score matching (PSM) before and after Roux‐en‐Y gastric bypass (RYGB) surgery. Control subjects were propensity‐score matched 2:1, to patients before (pre‐RYGB) and 5 years after (post‐RYGB) RYGB. Data are presented as median and 25% interquartile range. P‐values were calculated by Wilcoxon's nonparametric test for continuous parameters and Pearson's chi square test for categorical parameters. Measures highlighted in italics were included for the PSM. Lipolysis is μmoles of glycerol release per 2 h
| Measure | PSM control, n = 244 | Post‐RYGB, n = 122 | p‐Value |
|---|---|---|---|
| Age (years) | 48.0 (41.0–56.0) | 49.0 (42.0–55.0) | 0.59 |
| Sex | 0.62 | ||
| Women | 218 (89.3%) | 107 (87.7%) | |
| Men | 26 (10.7%) | 15 (12.3%) | |
| Body mass index (kg/m2) | 27.65 (24.35–34.95) | 29.55 (26.70–31.70) | 0.20 |
| Waist‐to‐hip ratio | 0.93 (0.87–0.98) | 0.92 (0.88–0.97) | 0.92 |
| Body fat (kg) | 30 (25–43) | 30 (25–36) | 0.14 |
| Fat cell volume (nanolitres) | 0.64 (0.50–0.80) | 0.43 (0.36–0.58) | <0.0001 |
| ISO lipolysis/g lipid | 3.98 (2.71–5.49) | 2,98 (2.29–3.76) | <0.0001 |
| ISO lipolysis/107 fat cells | 22.44 (15.1–31.2) | 11.8 (8.57–16.7) | <0.0001 |
| Basal lipolysis/g lipid | 0.72 (0.48–1.29) | 0.67 (0.52–0.89) | 0.16 |
| Basal lipolysis/107 fat cells | 4.18 (2.19–8.07) | 2.83 (1.99–3.77) | <0.0001 |
| NA lipolysis/g lipid | 2.13 (1.31–3.40) | 1.52 (1.10–1.95) | <0.0001 |
| NA lipolysis/107 fat cells | 12.42 (6.61–20.48) | 5.98 (4.27–8.61) | <0.0001 |
| Diabetes (yes/no) | 28/216 | 0/122 | <0.0001 |
| Hyperlipidemia (yes/no) | 13/231 | 1/121 | 0.07 |
| Hypertension (yes/no) | 36/208 | 24/98 | 0.29 |
| Physical activity (score) | 2 (1–2) | 2 (2–2) | 0.08 |
| P‐glucose (mmol/L) | 5.3 (4.9–6.0) | 5.0 (4.8–5.4) | 0.0002 |
| P‐cholesterol (mmol/L) | 5.1 (4.4–5.9) | 4.2 (3.8–4.9) | <0.0001 |
| P‐HDL‐cholesterol (mmol/L) | 1.3 (1.1–1.6) | 1.6 (1.3–1.8) | <0.0001 |
| P‐triglycerides (mmol/L) | 1.2 (0.80–1.65) | 0.89 (0.66–1.2) | <0.0001 |
| HOMA (units) | 1.8 (1.2–3.3) | 0.2 (0.1–1.0) | <0.0001 |
Abbreviations: HDL, high‐density lipoprotein; HOMA, homeostasis model assessment of insulin sensitivity; ISO, isoprenaline; NA, noradrenaline; P, fasting plasma.
To further analyze lipolysis in the post‐RYGB state, we compared—using multiple regression—RYGB patients at 5 years with all control subjects for various factors that are known or might influence lipolysis, including fat cell volume. The comprehensive model included patient‐group (RYGB vs. controls) BMI, fat cell volume, sex, age, physical activity, waist‐to‐hip ratio, HOMA and diagnosis/treatment of type 2 diabetes/hyperlipidemia or hypertension. In this model, the subject group did contribute significantly to the variations in basal lipolysis (Table S4). However, being a control or post‐RYGB patient contributed significantly (p < 0.0005) to variations in isoprenaline‐ (Table 2) and noradrenaline‐stimulated (Table 3) lipolysis irrespective of whether it was expressed per cell or lipid weight. Thus, a low rate of lipolysis 5 years after RYGB is not secondary to any cofactors influencing lipolysis. Besides the subject group, fat cell volume, age and HOMA were significant regressors for all forms of stimulated lipolysis depicted in Tables 2 and 3.
Table 2.
Influence of different factors on isoprenaline‐induced lipolysis in control subjects and RYGB patients at 5‐year follow‐up
| Lipolysis per lipid weight | Lipolysis per fat cell number | |||||||
|---|---|---|---|---|---|---|---|---|
| β‐coefficient | SE | p‐value | 95% CI | β‐coefficient | SE | p‐value | 95% CI | |
| Control or RYGB | −1.6 | 0.27 | <0.0001 | −2.2–1.1 | −6.0 | 1.3 | <0.00001 | −8.5–3.5 |
| Fat cell volume | −3.6 | 0.53 | <0.0001 | −4.7–2.6 | 22.2 | 2.6 | <0.0001 | 17.2–27.3 |
| Body mass index | −0.001 | 0.017 | 0.94 | −0.03–0.03 | 0.12 | 0.08 | 0.13 | −0.04–0.28 |
| Age | −0.047 | 0.007 | <0.0001 | −0.06–0.03 | −0.16 | 0.04 | <0.0001 | −0.23–0.09 |
| Sex | 0.53 | 0.22 | 0.014 | 0.11–0.96 | 1.5 | 1.1 | 0.14 | −0.52–3.60 |
| Waist‐to‐hip ratio | −1.74 | 1.31 | 0.18 | −4.3–0.8 | −9.4 | 6.4 | 0.14 | −21.9–3.1 |
| HOMA | 0.066 | 0.03 | 0.054 | −0.001–0.13 | 0.34 | 0.17 | 0.045 | 0.008–0.66 |
| Physical activity | −0.13 | 0.13 | 0.31 | −0.38–0.12 | −0.71 | 0.62 | 0.25 | −1.9–0.51 |
| Type 2 diabetes | −0.23 | 0.31 | 0.47 | −0.83–0.38 | −1.4 | 1.5 | 0.34 | −4.4–1.5 |
| Hyperlipidemia | −0.19 | 0.32 | 0.55 | −0.83–0.44 | 0.63 | 1.6 | 0.69 | −2.5–3.7 |
| Hypertension | −0.19 | 0.25 | 0.45 | −0.68–0.30 | −1.1 | 1.2 | 0.37 | −3.4–1.3 |
Note: A multiple regression model was built for factors known to or that might influence the rate of fat cell lipolysis. Controls are all subjects who had not been subjected to weight reduction surgery at the time of investigation. They were compared with RYGB patients investigated 5 years after surgery.
Note: n = 897 and 896 for lipolysis/lipid weight and/cell, respectively. r2 for the model is 0.20 and 0.32 for lipolysis/lipid weight and/cell, respectively.
Abbreviations: CI, confidence interval; HOMA, homeostasis model assessment of insulin sensitivity; RYGB, Roux‐en‐Y gastric bypass; SE, standard error.
Table 3.
Influence of different factors on noradrenaline‐induced lipolysis in control subjects and RYGB patients at 5‐year follow‐up
| Lipolysis per lipid weight | Lipolysis per fat cell number | |||||||
|---|---|---|---|---|---|---|---|---|
| β‐coefficient | SE | p‐value | 95% CI | β‐coefficient | SE | p‐value | 95% CI | |
| Control or RYGB | −0.95 | 0.17 | <0.0001 | −1.3–0.6 | −3.5 | −5.4 | 0.0004 | −5.4–1.6 |
| Fat cell volume | −1.7 | 0.35 | <0.0001 | −2.4–1.0 | 14.8 | 10.9 | <0.001 | 10.9–18.6 |
| Body mass index | 0.003 | 0.011 | 0.82 | −0.02–0.02 | 0.10 | −0.0017 | 0.092 | −0.02–0.23 |
| Age | −0.044 | 0.005 | <0.0001 | −0.05–0.03 | −0.20 | −0.25 | <0.0001 | −0.25–0.15 |
| Sex | 0.095 | 0.14 | 0.51 | −0.19–0.38 | 0.61 | −0.95 | 0.44 | −0.95–2.18 |
| Waist‐to‐hip ratio | 0.46 | 0.87 | 0.60 | −1.2–2.2 | −0.23 | −9.8 | 0.96 | −9.8–9.3 |
| HOMA | 0.058 | 0.023 | 0.01 | 0.014–0.103 | 0.35 | 0.104 | 0.006 | 0.10–0.60 |
| Physical activity | −0.21 | 0.085 | 0.01 | −0.38 to −0.05 | −1.21 | −2.1 | 0.011 | −2.1–0.3 |
| Type 2 diabetes | 0.056 | 0.203 | 0.78 | −0.34–0.45 | 0.016 | −2.2 | 0.99 | −2.2–2.2 |
| Hyperlipidemia | −0.067 | 0.212 | 0.75 | −0.48–0.35 | 0.58 | −1.7 | 0.62 | −1.7–2.9 |
| Hypertension | −0.070 | 0.163 | 0.67 | −0.39–0.25 | −0.58 | −2.4 | 0.53 | −2.4–1.2 |
Note: A multiple regression model was built for factors know to or that might influence the rate of fat cell lipolysis. n = 894 and 893 for lipolysis/lipid weight and/cell, respectively. r2 for the model is 0.18 and 0.35 for lipolysis/lipid weight and/cell, respectively. See legend to Table 2 for details.
Abbreviations: CI, confidence interval; SE, standard error.
We finally examined whether lipolysis at baseline was related to the outcome of RYGB. This was not the case when absolute rates per lipid weight or per fat cell number were used (values not shown). However, when lipolysis was expressed as noradrenaline stimulated divided by basal lipolysis, a clear relationship was observed (Fig. 4). The activation of basal rate of lipolysis by noradrenaline was significantly and positively correlated with changes in body weight, BMI or total fat mass between 0 and 5 years (Fig. 4a–c). This relationship was apparent when patients belonging to the highest and lowest tertile of noradrenaline/basal lipolysis at baseline were compared (Fig. 4d–f). On average, those with high initial lipolysis activation lost approximately 30% more body weight or BMI and about 45% more body fat than those with low activation of lipolysis.
Fig. 4.
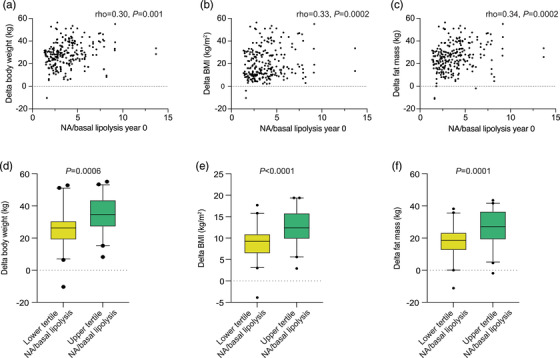
Prediction of Roux‐en‐Y gastric bypass outcome from initial lipolytic activity. Noradrenaline/basal lipolysis at baseline was compared with the difference between values at baseline minus at 5 years (delta) for body weight (a,d), body mass index (b,e) or fat mass (c,f). Spearman's rank correlation or the Wilcoxon test was used for statistical analysis.
Discussion
Obesity is a heterogenous condition with regard to comorbidities and adipose tissue function [28, 29]. Herein we shed new light on the impact of long‐term weight reduction on fat cell lipolysis in subjects who undergo metabolic surgery because of otherwise treatment‐resistant obesity. It is unexpectedly demonstrated that patients with this form of obesity have a lipolysis defect that persists after long‐term marked weight reduction to, on a group basis, a nonobese state. In addition, we show for the first time that initial lipolysis activity is associated with loss of body weight/body fat following RYGB.
Catecholamines markedly activate fat cell lipolysis. This effect was altered in the expected way before surgery in RYGB patients, and it was decreased by about 50% in RYGB patients 5 years after surgery. In addition, the latter was independent of expression by lipid weight or by number of fat cells in adipose tissue. Almost half of the body's total pool of fat cells has been shown to be renewed after 5 years due to cell turnover [30]. Thus, the new fat cells developed in the post‐obese state seem to ‘inherit’ the same lipolytic defect as was present in the obese state.
In previous lipolysis studies, patients who underwent RYGB were examined up to 2 years after surgery [18, 19], when they may still have been close to the catabolic weight reduction phase [31]. A negative energy balance might induce protective ‘antistarvation’ mechanisms involving reduced lipolysis and reduced energy expenditure, which might thereby mask defects, evident only after weight stabilization or regain [16]. Following metabolic surgery, body weight often starts relapsing after 3–5 years, reaching a new state with either neutral or slightly positive energy balance [31]. At the time of re‐examination, many of the RYGB patients in our study were clearly in this slight anabolic state.
Important factors influencing adipose tissue function are BMI, sex, age and fat cell size [24, 25, 26]. Our findings of differences between post‐obese RYGB patients and controls with regard to catecholamine‐induced lipolysis were independent of these factors. Access to a large control group allowed us to select individuals who were matched to RYGB patients before and after surgery as well as RYGB patients who were not re‐examined after 5 years (noncompleters). This implied the use of different control subjects for the three comparisons, but the differences in lipolysis remained statistically significant in all analyses. Moreover, we obtained similar results with both synthetic beta‐adrenoceptor and endogenous catecholamine agonists, suggesting that the lipolysis defects are pathophysiologically relevant. We did not use fat cell size in the PSM as we anticipated it to be reduced below the normal size 5 years after surgery, making it unlikely to be matched on that variable with controls [17]. It was, however, included in a comprehensive multiple regression analysis confirming the impact of fat cell size on lipolysis [9, 26]. Importantly, the multivariate analysis (Tables 2 and 3) demonstrated that the decreased rate of catecholamine‐induced lipolysis per cell number or lipid weight was independent of all examined cofactors.
Whether RYGB patients have a specific lipolysis defect that is not present in other forms of obesity is currently not known. Although a large number of subjects with obesity were included in the control group, we lack sufficient information regarding their obesity history to be able to create a valid comparison group of subjects with obesity who had never used any form of body‐weight reduction therapy prior to investigation at our laboratory. In any case, RYGB completers are likely to be representative of other patients referred to metabolic surgery given the findings in the noncompleting RYGB patients. The latter had similar lipolysis alterations as RYGB completers before surgery when compared with control subjects. Furthermore, the baseline characteristics of completers and noncompleters were very similar.
The mechanisms behind the lipolysis defect in the investigated form of obesity is currently not known. It is possible that patients referred to and selected for RYGB may be a subset with a particularly reduced lipolytic capacity possibly related to the failures of the preceding obesity treatments. It may be an early factor or an imprinting phenomenon that emerges during the build‐up of excess fat mass. A support for the former notion is the previous finding that low rates of catecholamine‐stimulated lipolysis in vivo or in vitro are associated with future body‐weight gain [5]. It could also be genetic. However, our recent genome‐wide association study showed rather weak relationships between genetic variations and lipolysis [32], suggesting that a possible genetic contribution is most likely polygenic. It is, however, not likely that the defect is secondary to prior attempts to decrease body weight. Short‐term modest weight reduction is associated with increased lipolysis rates, which is opposite to our findings [33].
The basal rate of lipolysis is altered in obesity [6], which this study confirmed. However, the importance of basal lipolysis for obesity is not clear, and the findings herein were less consistent than for lipolysis stimulated by catecholamines.
The observed resistance to catecholamine‐induced lipolysis may above all be a marker and predictor, rather than a cause, of long‐term poor response to RYGB. On the other hand, it is possible that the persistence of lipolytic defect is important for long‐term body‐weight reduction after metabolic surgery. If fat cells cannot adequately mobilize fatty acids for subsequent oxidation, this may facilitate net lipid accumulation in fat cells, as discussed [11]. Clearly, this hypothesis must be validated by prospective controlled studies. It appears that initial lipolysis activity is of clinical importance for the outcome of metabolic surgery. A high initial ability for noradrenaline to accelerate basal (spontaneous) fat cell lipolysis at baseline was associated with a much better reduction over time in body weight, BMI and fat mass in comparison with a low effect. It should be stressed that body‐weight loss depends on the energy balance. We cannot exclude that the observed lipolysis defect above all reflects decreased fatty acid oxidation or increased lipid partitioning in nonadipose tissues. Nonetheless, a well‐functioning regulation of lipid turnover involving lipolysis seems to be needed for a healthy energy balance
Despite low rates of lipolysis, many clinical parameters were more favourable in the post‐obese than in the control state, confirming our previous 5‐year study of RYGB [17]. The reason for this discrepancy is not clear, and calls for further investigations. One can speculate that subjects with obesity referred to metabolic surgery may be healthier in some aspects than other people with obesity.
This study has some limitations. It was much more difficult to recruit men than women, and we lack sufficient statistical power to determine possible gender differences. On the other hand, the findings with lipolysis are true for women as judged by a separate analysis of this sex. We investigated abdominal subcutaneous adipose tissue, which displays a stronger association with cardiometabolic disorders than gluteal/femoral adipose tissue [34]. Consequently, lipolysis in other adipose sites could be differently influenced by obesity/weight reduction. For practical and ethical reasons, it was impossible to simultaneously study these regional aspects. Although unlikely, other surgical approaches than RYGB may influence lipolysis differently. We investigated lipolysis in vitro because in vivo methods are cumbersome and resource intensive. However, catecholamine lipolysis in vivo and in vitro correlate with each other [35]. Catecholamines have other actions in fat cells than lipolysis, such as on glucose metabolism and gene expression. The role of these effects in obesity is currently unknown. We did not examine the additional important hormone regulators of lipolysis, insulin and natriuretic peptides. The latter hormone was not considered to be important until after we started the present study. Recent studies in vivo on a subgroup of the presently studied patients show normal or increased suppression of circulating glycerol and fatty acid release during euglycemic hyperinsulinaemic clamp in the post‐obese state [36, 37]. This suggests that the insulin resistance of lipolysis action is not present in our patients after RYGB. Finally, our study was not population based. However, such a selection approach is not practical because the random population had to be selected at the start of the study, which was about 35 years ago. Such a population is not representative for the more recent recruitment because of continuous changes in the occurrence of obesity.
Based on the results, we propose the following novel model for fat cell lipolysis in patients with obesity subjected to metabolic surgery. At baseline, they display a similar defect in catecholamine‐induced lipolysis in comparison with nonobese subjects, as demonstrated numerous times before. Surprisingly, the defect persists long term after successful body‐weight reduction. Those with marked ability to activate lipolysis by catecholamine stimulation have a better possibility of reducing body weight and fat mass than those with a less effective catecholamine function. The possible causal relationship between lipolysis and changes in body weight as well as fat mass needs to be further examined in prospective studies.
In summary, subjects with obesity who undergo metabolic surgery have markedly lower catecholamine‐induced fat cell lipid mobilization, which persists for a long time after major reduction in body weight. Furthermore, the ability of catecholamines to activate lipolysis at baseline predicts the amount of weight loss following metabolic surgery.
Conflict of interest
None of the authors have any conflict of interest to declare.
Author contributions
P. A., M. R. and T. I. A. S. designed the study and analysed data and interpreted the results together with D. P. A. A. T. recruited the patients and supervised the RYGB. M. I. K. was responsible for the propensity‐score matching of subjects. E. N. and E. S. provided register data. P. A. and M. R. wrote the first version of the paper. All authors contributed to further writing and approved the final version.
Supporting information
Table S1: Clinical data for the whole control group
Table S2: Clinical data for non‐obese subjects and RYGB patients examined before and 5 years after surgery
Table S3: Baseline clinical data for non‐completers (RYGB patients not returning for a 5‐year follow‐up)
Table S4: Influence of different factors on basal lipolysis in control subjects and RYGB patients at 5‐year follow‐up
Acknowledgements
This study was supported by the Strategic Diabetes Research Program at Karolinska Institutet, the Swedish Research Council (CIMED, ERC‐SyG SPHERES 856404), the Novo Nordisk Foundation (including the Tripartite Immuno‐metabolism Consortium grant NNF15CC0018486), the MSAM Consortium (grant NNF15SA0018346), the MeRIAD Consortium (grant 0064142), the Swedish Diabetes Foundation, the Knut and Alice Wallenberg Foundation (including Wallenberg Clinical Scholar to M. R.), the Erling–Persson Family Foundation and the Stockholm County Council. We thank Professor Torben Martinussen and PhD Lars Ängquist at the University of Copenhagen for statistical advice.
Rydén M, Andersson DP, Kotopouli MI, Stenberg E, Näslund E, Thorell A, et al. Lipolysis defect in people with obesity who undergo metabolic surgery. J Intern Med 2022;292:667–678.
Contributor Information
Mikael Rydén, Email: mikael.ryden@ki.se.
Peter Arner, Email: peter.arner@ki.se.
References
- 1. NCDRF Collaboration . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–93. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, et al. Obesity pathogenesis: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:267–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rohde K, Keller M, la Cour Poulsen L, Bluher M, Kovacs P, Bottcher Y. Genetics and epigenetics in obesity. Metabolism. 2019;92:37–50. [DOI] [PubMed] [Google Scholar]
- 5. Arner P, Andersson DP, Backdahl J, Dahlman I, Ryden M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metabolism. 2018;28:45–54.e3. [DOI] [PubMed] [Google Scholar]
- 6. Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–66. [DOI] [PubMed] [Google Scholar]
- 7. Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989;83:1168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reynisdottir S, Wahrenberg H, Carlstrom K, Rossner S, Arner P. Catecholamine resistance in fat cells of women with upper‐body obesity due to decreased expression of beta 2‐adrenoceptors. Diabetologia. 1994;37:428–35. [DOI] [PubMed] [Google Scholar]
- 9. Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–82. [DOI] [PubMed] [Google Scholar]
- 10. Nishizawa H, Shimomura I. Fat cell lipolysis and future weight gain. J Diabetes Investig. 2019;10:221–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arner P, Bernard S, Appelsved L, Fu KY, Andersson DP, Salehpour M, et al. Adipose lipid turnover and long‐term changes in body weight. Nat Med. 2019;25:1385–9. [DOI] [PubMed] [Google Scholar]
- 12. Hellstrom L, Langin D, Reynisdottir S, Dauzats M, Arner P. Adipocyte lipolysis in normal weight subjects with obesity among first‐degree relatives. Diabetologia. 1996;39:921–8. [DOI] [PubMed] [Google Scholar]
- 13. Frankl J, Piaggi P, Foley JE, Krakoff J, Votruba SB. In vitro lipolysis is associated with whole‐body lipid oxidation and weight gain in humans. Obesity (Silver Spring). 2017;25:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bougneres P, Stunff CL, Pecqueur C, Pinglier E, Adnot P, Ricquier D. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J Clin Invest. 1997;99:2568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mai K, Li L, Wiegand S, Brachs M, Leupelt V, Ernert A, et al. An integrated understanding of the molecular mechanisms of how adipose tissue metabolism affects long‐term body weight maintenance. Diabetes. 2019;68:57–65. [DOI] [PubMed] [Google Scholar]
- 16. van Baak MA, Mariman ECM. Mechanisms of weight regain after weight loss—the role of adipose tissue. Nat Rev Endocrinol. 2019;15:274–87. [DOI] [PubMed] [Google Scholar]
- 17. Hoffstedt J, Andersson DP, Eriksson Hogling D, Theorell J, Näslund E, Thorell A, et al. Long‐term protective changes in adipose tissue after gastric bypass. Diabetes Care. 2017;40:77–84. [DOI] [PubMed] [Google Scholar]
- 18. Lofgren P, Hoffstedt J, Ryden M, Thorne A, Holm C, Wahrenberg H, et al. Major gender differences in the lipolytic capacity of abdominal subcutaneous fat cells in obesity observed before and after long‐term weight reduction. J Clin Endocrinol Metab. 2002;87:764–71. [DOI] [PubMed] [Google Scholar]
- 19. Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia. 2005;48:2334–42. [DOI] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 21. Arner P, Andersson DP, Backdahl J, Dahlman I, Ryden M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metab. 2018;28:45–54.e3. [DOI] [PubMed] [Google Scholar]
- 22. Arner P, Liljeqvist L, Ostman J. Metabolism of mono‐ and diacylglycerols in subcutaneous adipose tissue of obese and normal‐weight subjects. Acta Med Scand. 1976;200:187–94. [DOI] [PubMed] [Google Scholar]
- 23. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One‐to‐many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):69–80. [DOI] [PubMed] [Google Scholar]
- 24. Mancuso P, Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front Endocrinol (Lausanne). 2019;10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ. 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stenkula KG, Erlanson‐Albertsson C. Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol. 2018;315:R284–95. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neeland IJ, Poirier P, Despres JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137:1391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwok KH, Lam KS, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med. 2016;48:e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7. [DOI] [PubMed] [Google Scholar]
- 31. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. [DOI] [PubMed] [Google Scholar]
- 32. Kulyte A, Lundback V, Lindgren CM, Luan J, Lotta LA, Langenberg C, et al. Genome‐wide association study of adipocyte lipolysis in the GENetics of adipocyte lipolysis (GENiAL) cohort. Mol Metab. 2020;34:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739–46. [DOI] [PubMed] [Google Scholar]
- 34. Karpe F, Pinnick KE. Biology of upper‐body and lower‐body adipose tissue–link to whole‐body phenotypes. Nat Rev Endocrinol. 2015;11:90–100. [DOI] [PubMed] [Google Scholar]
- 35. Kolehmainen M, Ohisalo JJ, Kaartinen JM, Tuononen V, Pääkkönen M, Poikolainen E, et al. Concordance of in vivo microdialysis and in vitro techniques in the studies of adipose tissue metabolism. Int J Obes Relat Metab Disord. 2000;24:1426–32. [DOI] [PubMed] [Google Scholar]
- 36. Mileti E, Kwok KHM, Andersson DP, Mathelier A, Raman A, Bäckdahl J, et al. Human white adipose tissue displays selective insulin resistance in the obese state. Diabetes. 2021;70:1486–97. [DOI] [PubMed] [Google Scholar]
- 37. Kwok KHM, Andersson DP, Ryden M, Arner P. A longitudinal study of the antilipolytic effect of insulin in women following bariatric surgery. Int J Obes (Lond). 2021;45:2675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Clinical data for the whole control group
Table S2: Clinical data for non‐obese subjects and RYGB patients examined before and 5 years after surgery
Table S3: Baseline clinical data for non‐completers (RYGB patients not returning for a 5‐year follow‐up)
Table S4: Influence of different factors on basal lipolysis in control subjects and RYGB patients at 5‐year follow‐up


