Abstract
Purpose
To evaluate and investigate the feasibility of flattening filter‐free (FFF) beam for the whole‐brain radiotherapy (WBRT) with hippocampus sparing.
Methods
Eighteen patients with volumetric‐modulated arc therapy (VMAT) plans in FFF and conventional beam modes were included in this study. The prescribed dose was 30 Gy in 10 fractions. The conformity index (CI), heterogeneity index reported by TPS (HI‐M), and homogeneity index (HI) for planning target volume (PTV) were evaluated. Subsequently, the following parameters for PTV were calculated and compared: D 2%, D 98%; the mean dose, maximum dose, and minimal dose for OARs. Plan modulation index, total MUs, and the gamma index were used to evaluate the plan quality.
Results
HI‐M results were similar for the two techniques (1.239 vs. 1.247, respectively, p = 0.048); FFF beam plans yielded lower D2% compared to FF beam plans (3,416.3 cGy vs. 3,437.2 cGy, p = 0.22), mean dose (3,177.5 cGy vs. 3,195.2 cGy, p = 0.009), and CI (0.884 vs. 0.876, p = 0.001) for PTV. Significant differences were observed between the two beam modes (FF model vs. FFF model) for the maximum dose (1,612.9 cGy vs. 1,470.2 cGy, respectively, p < 0.001), minimum dose (987.6 cGy vs. 898.8 cGy, respectively, p < 0.001), and the mean dose (1144.4 cGy vs. 1047.3 cGy, respectively, p < 0.001) to the hippocampus, and the maximum dose to the eyes (2,792.6 cGy vs. 2,751.3 cGy, respectively, p < 0.001). The average total MUs for FFF‐VMAT plans was significantly greater than FF‐VMAT plans. However, differences for the plan modulation index and the gamma index were negligible.
Conclusion
In comparison with FF beam, the FFF beam mode offers a clear benefit with respect to WBRT with hippocampal sparing.
Keywords: flattening filter‐free, hippocampal sparing, volumetric‐modulated arc therapy, whole‐brain radiotherapy
FFF beam mode can offer a clear benefit with hippocampus sparing.
The average total MUs for FFF‐VMAT plans was more. However, the modulation index and gamma index were different not too much.
FFF beam plans provided the lower HI‐M than FF beam, lower D2%, mean dose and CI for PTV
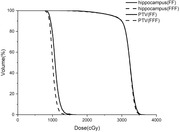
.
1. INTRODUCTION
Whole‐brain radiotherapy (WBRT) is a fundamental treatment for patients with metastases in brain, 1 which is one of the most common brain tumors in adults. 2 However, WBRT imparts side effects including neurocognitive dysfunction. 3 , 4 To prevent such side‐effects from irradiation, modern intensity modulated radiotherapy techniques have been developed and to reduce the hippocampus dose during WBRT. 5 , 6 , 7 , 8 , 9 , 10
The flattening filters equipped in the treatment head of linac were used to generate a relatively uniform dose at a certain depth. However, the flattening filters have become unnecessary in modern treatment techniques, because of the varying fluence patterns needed in these techniques. 11 FFF beams have relatively distinctive dosimetric features, including sharper penumbra, less head scatter, and less peripheral dose, which have shown a lower probability of normal tissue complications. The purpose of this study was to investigate the benefit of FFF beams in the VMAT of hippocampal sparing considering plan quality, modulation index, and plan verification. This is a retrospective paper where all cases have been treated with FF beam mode.
2. MATERIALS AND METHODS
2.1. Contouring and planning
Eighteen patients treated with FF beam model were included. Brain computed tomography (CT; SOMATOM, Siemens Healthcare GmbH) was used to obtain 1.5 mm sliced images of the patients. The Philips scanner (Philips, Cleveland, OH, USA) was used to obtain MR images and the slice thickness was 1.5 mm. The CT and MRI datasets were registered in the Monaco (Elekta AB, Stockholm, Sweden) for target volume contouring.
All of the cases were treated under VersaHD unit (Elekta AB) with Agility head of this study. The 80‐pair interdigitating MLCs in the head have a projected lead width of 5 mm at the isocenter. For each case, the coplanar VMAT with four arcs were optimized in the Monaco TPS. The four full arcs optimized simultaneously and to be delivered in one beam (clock‐counterclockwise‐clock‐counterclockwise). The flattening filter free (FFF) with 6‐MV photon beams energy were selected. In order to ensure confidence in accuracy of dose calculation and delivery, these studies were referred. 12 , 13
The hippocampus was delineated on the MR images according to the RTOG 0933 protocol. The margin of 5 mm was used to treatment plan for the tolerance of setup. The same radiation oncologist delineated the other OARs, including the eyes and lenses.
2.2. Prescription and OAR constraints
The prescription dose was 30 Gy/10F to the PTV. All plans needed at least 90% of PTV covered by 100% of the prescribed dose. The hippocampus dose was constrained according to the protocol of RTOG 0933. The maximal doses limited for the lens and the eyes were 8 and 30 Gy respectively. Both FF‐VMAT and FFF‐VMAT plans were normalized to achieve prescription dose to PTV and limited dose to the OARs.
2.3. Plan evaluation
The conformity index reported by Monaco (CI), heterogeneity index reported by Monaco (HI‐M), and the homogeneity index (HI) for PTV were evaluated. The following dose volume parameters for PTV and OARs were recorded, using PTV D 2% as the “maximum dose,” PTV D98% as the “minimum dose,” by TPS to evaluate the dose “tail.”
The CI formula used by Monaco is defined as:
where TV is the volume of PTV, V RX is the volume of target covered by prescription dose, and V RI is the total volume of the prescription dose. The CI describes how the volume of prescribed dose conforms to the shape and size of the target volume.
The formula of HI‐M by Monaco is:
The HI‐M describes the uniformity of dose within a target volume. Although the D 5% is defined as the dose delivered to the hottest 5% of the tissue. The D 95% is the minimum dose received by 95% of the tissue. The values close to 1 are considered to be optimal.
The HI is defined as:
where D 2% and D 98% is the same as previously defined. D median is the median dose to the PTV. HI is used to describe the uniformity of dose distribution as well; in contrast to HI‐M values, the values close to 0 are considered optimal.
2.4. Plan quality
The complexity of plan, the Gamma index of plan verification, and the total MU were used to evaluate the plan quality. Several studies have given the suggestions of using modulation indices to predict VMAT delivery accuracy. 14 This study used the modulation complexity score for VMAT (MCSv) 15 evaluation, which is a previously suggested modulation index for IMRT. 16
For each plan, the verification plans were generated and evaluated with the ArcCHECK™ (Sun Nuclear Corporation, Melbourne, FL, USA). For the local gamma evaluation, the 3% per 3 mm gamma criteria was used, which is the most commonly used in other sites. 17
2.5. Statistical analysis
Statistical comparisons of the results were performed using Origin, version 2018 (OriginLab Corporation, Northhampton, MA, USA) by paired‐sample t tests. A two‐sided p‐value < 0.05 indicated a statistically significant difference.
3. RESULTS
3.1. Dose for PTV
PTV dose parameters (mean ± SD) for the different beam modes are presented in Table 1. The D 98%, D 2%, D mean, D max, CI, HI‐M, and HI for PTV were also compared. After values were normalized to 90% of PTV covered 100% prescription dose, the two beam modes provided similar D 98%. FFF‐VMAT provided the lower average D 2% (3,416.3 ± 36.17 cGy) and mean dose (3,177.5 ±23.8 cGy) for PTV, and no significant differences in the D max were found between the modes. Although there are some statistically significant significances for PTV, these are not clinically significant. According to the HI‐M formula by TPS, the better target dose heterogeneity would be achieved by FFF beam plans (1.239, p = 0.048). The two modes provided similar CIs (0.876 vs. 0.884). No statistically significant difference in HI was found between the two beam modes. Figure 1 shows average DVHs for the dose to PTV for each of the two beam modes.
TABLE 1.
Dose parameters for PTV
| FF | FFF | p | |
|---|---|---|---|
| PTV, D 98 (cGy) | 2282.4 ± 123.5 | 2283.8 ± 139.8 | 0.028 |
| PTV, D 2 (cGy) | 3437.2 ± 43.6 | 3416.3 ± 36.17 | 0.022 |
| D Mean (cGy) | 3195.2 ± 29.6 | 3177.5 ± 23.8 | 0.009 |
| D Max (cGy) | 3635.7 ± 41.1 | 3623.7 ± 56.1 | 0.138 |
| CI | 0.876 ± 0.015 | 0.884 ± 0.015 | 0.001 |
| HI‐M | 1.247 ± 0.036 | 1.239 ± 0.038 | 0.048 |
| HI | 0.388 ± 0.052 | 0.385 ± 0.047 | 0.661 |
FIGURE 1.
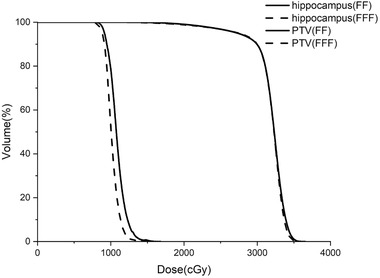
PTV average dose–volume histograms for treatment planning with the two beam modes
3.2. Dose for Hippocampus and other OARs
The dose parameters comparisons for hippocampus are shown in Table 2. Significant differences in D max (1,470.2 ± 136.3 cGy, p < 0.001), D min (898.8 ± 140.5 cGy, p < 0.001), and D mean (1047.3 ± 51.8 cGy, p < 0.001) were observed between the two beam modes . Compared to FF‐VMAT, FFF beam plans yielded lower average maximum doses, minimum doses, and mean doses to the hippocampus. For eyes and lenses, both beam modes met the dose constraints we set. Plans in FFF mode yielded the slightly lower average maximum doses to eyes (p < 0.05). There were no statistically significant differences observed for the dose to lens. Figure 1 shows average DVHs for the dose to hippocampus in the two beam modes.
TABLE 2.
Dose to Hippocampus and other OARs
| FF | FFF | p | |
|---|---|---|---|
| Hippocampus, D max (cGy) | 1612.9 ± 175.3 | 1470.2 ± 136.3 | <0.001 |
| Hippocampus, D min (cGy) | 987.6 ± 189.8 | 898.8 ± 140.5 | <0.001 |
| Hippocampus, D mean (cGy) | 1144.4 ± 81.1 | 1047.3 ± 51.8 | <0.001 |
| Lens, D max (cGy) | 671.2 ± 42.0 | 668.2 ± 43.5 | 0.373 |
| Eyes, D max (cGy) | 2792.6 ± 321.8 | 2751.3 ± 326.3 | 0.031 |
3.3. Plan quality
The plan quality for each plan is compared in Table 3. The FFF‐VMAT plans generated significantly more MUs than the FF plan (1,955.8 vs. 1,641.9, respectively, p < 0.001). No significant differences were observed in the MSCv and Gamma index between the two beam modes.
TABLE 3.
Plan quality
| FF | FFF | p | |
|---|---|---|---|
| MSCv | 0.144 ± 0.014 | 0.141 ± 0.014 | 0.234 |
| Gamma index | 98.7 ± 0.6 | 98.3 ± 0.7 | 0.072 |
| MU | 1641.9 ± 177.2 | 1955.8± 198.6 | <0.001 |
4. DISCUSSION
The two beam modes achieved PTV target coverage, they provided similar D 98% and CI values. The D mean, D 2%, and HI‐M values for FFF VMAT was lower than those with the FF beam plan, but this difference could be negligible. There were no statistically significant differences in the D max and HI between the modes. Thus, the FFF beams yielded a little bit better results in target volume coverage and conformity than the FF beams. The dosimetry advantage for target can be neglected by the FFF mode; these results are similar to those of other studies. 18 , 19 The FFF mode can increase the peripheral dose of the target area with nonuniform tissues, such as SBRT for lung cancer. 20
The development of advanced techniques is increasing the feasibility of hippocampal avoidance during WBRT, resulting in greater memory preservation and quality of life of patients. 21 Lower average maximum, minimum, and mean doses to the hippocampus were achieved by the FFF beams VMAT. Meanwhile, FFF also significantly reduced the eye dose. Other studies have also confirmed the ability to protect OARs in FFF mode. 22 , 23 These can be attributed to the unique dosimetry characteristics of the FFF such as smaller leakage. At the same time, the high‐speed moving blade, a collimator speed of 9 cm/scan, 24 also better utilize the high dose rate characteristics of the FFF mode.
Because the FFF mode requires more MUs for a point in a segment far away from the central axis to achieve the same depth dose as the point in the central, the average total MUs for FFF‐VMAT plans was greater than that of the FF‐VMAT plans. This finding is in line with the results from previous studies. 19 , 25 , 26 However, there was no significant difference observed in the plan quality among the two beam modes. Although our results show that the FFF‐VMAT plan can produce very good protection of the hippocampus, it is important to note that the FFF beams increased the total MUs about 20%.
One limitation of this study is that the experience of the dosimetrist has a greater impact on the quality of the modern intensity‐modulated radiotherapy plan.
5. CONCLUSIONS
From a dosimetric perspective, the FFF beam mode can offer a clear benefit for WBRT with hippocampal sparing when compared to the FF beam plans. The plan verification showed that both of the FF and FFF plans had acceptable results. Therefore, by the results of this study, it is suggested that the use of the FFF beam is feasible and also provides efficacious treatment for WBRT with hippocampal sparing if carefully applied.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
T. Ji designed the study; F. Cai and S. Lu collected the patients’ clinical data and delineated target volume; T. Ji and G. Li optimized and expert reviewed the patients’ treatment plans; T. Ji analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Ji T, Sun L, Cai F, Li G. Comparison between flattening filter‐free (FFF) and flattened photon beam VMAT plans for the whole brain radiotherapy (WBRT) with hippocampus sparing. Asia‐Pac J Clin Oncol. 2022;18:e263–e267. 10.1111/ajco.13624
REFERENCES
- 1. Kong W, Jarvis CR, Sutton DS, Ding K, Mackillop WJ. The use of palliative whole brain radiotherapy in the management of brain metastases. Clin Oncol. 2012;24:e149–e158. [DOI] [PubMed] [Google Scholar]
- 2. Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 3. Shi L, Molina DP, Robbins ME, Wheeler KT, Brunso‐Bechtold JK. Hippocampal Neuron number is unchanged 1 year after fractionated whole‐brain irradiation at middle age. Int J Radiat Oncol Biol Phys. 2008;71:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gondi V, Pugh SL, Tome WA, et al. Preservation of Memory With conformal avoidance of the hippocampal neural stem‐cell compartment during whole‐brain radiotherapy for brain metastases (RTOG 0933): A phase II multi‐institutional trial. J Clin Oncol. 2014;32:3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsu F, Carolan H, Nichol A, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1–3 brain metastases: A feasibility study using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;76:1480‐1485. [DOI] [PubMed] [Google Scholar]
- 6. Kim KS, Wee CW, Seok JY, et al. Hippocampus‐sparing radiotherapy using volumetric modulated arc therapy (VMAT) to the primary brain tumor: The result of dosimetric study and neurocognitive function assessment. Radiat Oncol. 2018;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang B, Li H, Kong F, et al. Dosimetric study and neurocognitive function of hippocampal‐sparing whole‐brain radiotherapy. Technol Cancer Rest. 2017;16:1266–1271. [Google Scholar]
- 8. Pokhrel D, Sood S, McClinton C, et al. Treatment planning strategy for whole‐brain radiotherapy with hippocampal sparing and simultaneous integrated boost for multiple brain metastases using intensity‐modulated arc therapy. Med Dosim. 2016;41:315–322. [DOI] [PubMed] [Google Scholar]
- 9. Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal‐sparing whole‐brain radiotherapy: A “how‐to” technique using helical tomotherapy and linear accelerator‐based intensity‐modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nevelsky A, Ieumwananonthachai N, Person OK, et al. EP‐1396 feasibility of hippocampal‐sparing whole‐brain radiotherapy using elekta equipment. Radiother Oncol. 2012;103:S530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Georg D, Knöös T, McClean B. Current status and future perspective of flattening filter free photon beamsa. Med Phys. 2011;38:1280–1293. [DOI] [PubMed] [Google Scholar]
- 12. Narayanasamy G, Saenz DL, Defoor D, Papanikolaou N, Stathakis S. Dosimetric validation of Monaco treatment planning system on an Elekta VersaHD linear accelerator. J Appl Clin Med Phys. 2017;18:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Narayanasamy G, Saenz D, Cruz W, Ha CS, Papanikolaou N, Stathakis S. Commissioning an Elekta Versa HD linear accelerator. J Appl Clin Med Phys. 2016;17:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiavassa S, Bessieres I, Edouard M, Mathot M, Moignier A. Complexity metrics for IMRT and VMAT plans: a review of current literature and applications. Br J Radiol. 2019;92:20190270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masi L, Doro R, Favuzza V, Cipressi S, Livi L. Impact of plan parameters on the dosimetric accuracy of volumetric modulated arc therapy. Med Phys. 2013;40:71718. [DOI] [PubMed] [Google Scholar]
- 16. McNiven AL, Sharpe MB, Purdie TG. A new metric for assessing IMRT modulation complexity and plan deliverability. Med Phys. 2010;37:505–515. [DOI] [PubMed] [Google Scholar]
- 17. Lang S, Reggiori G, Puxeu VJ, et al. Pretreatment quality assurance of flattening filter free beams on 224 patients for intensity modulated plans: A multicentric study. Med Phys. 2012;39:1351–1356. [DOI] [PubMed] [Google Scholar]
- 18. Zhang W, Lin Z, Yang Z, et al. Evaluation of the dosimetric impact of applying flattening filter‐free beams in intensity‐modulated radiotherapy for early‐stage upper thoracic carcinoma of oesophagus. J Med Radiat Sci. 2015;62:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung JB, Kim JS, Eom KY, et al. Comparison of VMAT‐SABR treatment plans with flattening filter (FF) and flattening filter‐free (FFF) beam for localized prostate cancer. J Appl Clin Med Phys. 2015;16:302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vassiliev ON, Kry SF, Wang HC, Peterson CB, Chang JY, Mohan R. Radiotherapy of lung cancers: fFF beams improve dose coverage at tumor periphery compromised by electronic disequilibrium. Physics in Medicine & Biology. 2018;63:195007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pokhrel D, Sood S, Lominska C, et al. Potential for reduced radiation‐induced toxicity using intensity‐modulated arc therapy for whole‐brain radiotherapy with hippocampal sparing. J Appl Clin Med Phys. 2015;16:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiorentino A, Giaj‐Levra N, Tebano U, et al. Stereotactic ablative radiation therapy for brain metastases with volumetric modulated arc therapy and flattening filter free delivery: feasibility and early clinical results. Radiol Med (Torino). 2017;122:676–682. [DOI] [PubMed] [Google Scholar]
- 23. Rieber J, Tonndorf‐Martini E, Schramm O, et al. Radiosurgery with flattening‐filter‐free techniques in the treatment of brain metastases. Strahlenther Onkol. 2016;192:789–796. [DOI] [PubMed] [Google Scholar]
- 24. Boda‐Heggemann J, Mai S, Fleckenstein J, et al. Flattening‐filter‐free intensity modulated breath‐hold image‐guided SABR (Stereotactic ABlative Radiotherapy) can be applied in a 15‐min treatment slot. Radiother Oncol. 2013;109:505–509. [DOI] [PubMed] [Google Scholar]
- 25. Sun WZ, Chen L, Yang X, Wang B, Deng XW, Huang XY. Comparison of treatment plan quality of VMAT for esophageal carcinoma with: Flattening filter beam versus flattening filter free beam. J Cancer. 2018;9:3263–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Treutwein M, Hipp M, Koelbl O, Dobler B. Volumetric‐modulated arc therapy and intensity‐modulated radiation therapy treatment planning for prostate cancer with flattened beam and flattening filter free linear accelerators. J Appl Clin Med Phys. 2017;18:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]


