Abstract
Background
Line‐field confocal optical coherence tomography (LC‐OCT) is a novel, non‐invasive technique that provides in vivo, high‐resolution images in both vertical and horizontal sections.
Objectives
The aim of the study was to evaluate LC‐OCT imaging in some inflammatory disorders and to correlate the resulting features with histopathology.
Methods
The retrospective study included patients with histopathological confirmed diagnosis of plaque psoriasis, atopic eczema and lichen planus, who were imaged with LC‐OCT before the biopsy. LC‐OCT was performed with the commercially available LC‐OCT device.
Results
A total of 15 adult patients with histopathologically proven plaque psoriasis (N: 5), atopic eczema (N: 5) and lichen planus (N: 5) were included. In all cases, LC‐OCT allowed the in vivo recognition of the main microscopic features of the examined inflammatory skin disease, with a strong correlation with histopathology.
Conclusions
Although future studies on larger series of patients are necessary, LC‐OCT, based on these preliminary findings, may represent a promising tool in inflammatory skin disorders with potential applications including enhanced diagnosis, biopsy guidance, follow‐up and treatment monitoring.
Introduction
Line‐field confocal optical coherence tomography (LC‐OCT) is a novel, non‐invasive tool for in vivo, real‐time skin imaging. It is based on an interferometric technique using a supercontinuum laser source (600–900 nm) and a line scan camera with an imaging speed of 8 frames per second. 1 , 2 , 3 It measures the echo time delay and amplitude of light backscattered from cutaneous microstructures, providing high‐resolution images (axial: 1.1 μm; lateral: 1.3 μm) with a penetration depth of up to 500 μm. 1 , 2 , 3 The user may switch in real time from a histology‐like vertical mode to a confocal‐like horizontal mode, and the integrated software also provides three‐dimensional (3D) skin reconstructions. Thus, LC‐OCT combines the advantages of reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) in terms of resolution, penetration and orientation. 1 , 2 , 3
LC‐OCT allows the recognition and measurement of the different layers of the epidermis and dermis and their structures and findings with cellular level definition, providing a sort of ‘virtual biopsy’ (Fig. 1). In the vertical view (Fig. 1a), the stratum corneum (SC) appears as a hyper‐reflective band clearly distinguished from the underlying viable epidermis; the stratum granulosum/spinosum features a honeycomb pattern composed by roundish/polygonal keratinocytes with hypo‐reflective nuclei; the dermo‐epidermal junction (DEJ) appears as a hypo‐reflective line clearly separating the epidermis from the dermis, with a wavy shape due to the presence of dermal papillae; the dermis displays an overall hyper‐reflective signal due to the presence of collagen/elastic fibres appearing as bright, wavy, linear structures; vessels appear as hypo‐reflective structures. 2 , 4 The same structures may also be observed in the horizontal view (Fig. 1b) and in the 3D reconstruction (Fig. 1c).
Figure 1.
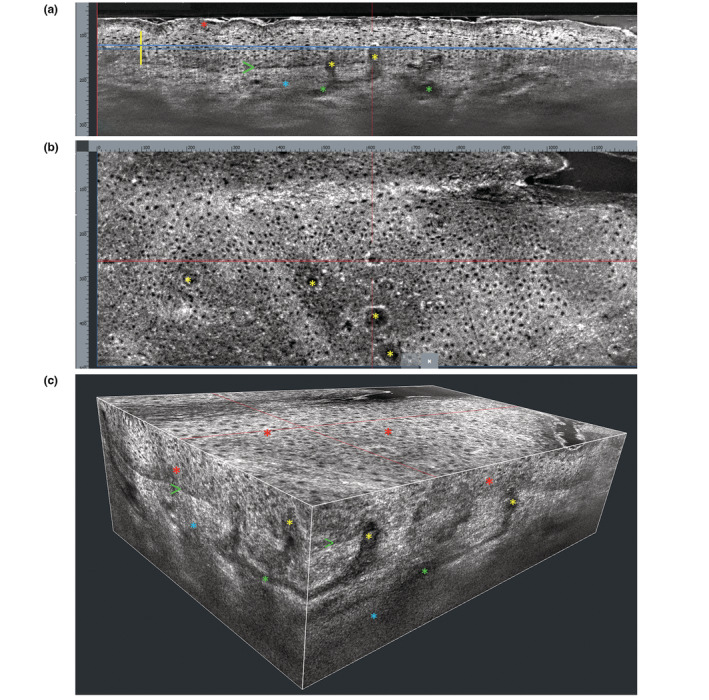
Healthy skin of the forearm. (a) Vertical LC‐OCT section shows hyper‐refractive stratum corneum (red asterisk); stratum granulosum/spinosum showing a honeycomb pattern (yellow vertical line); well‐defined dermo‐epidermal junction (green arrow); superficial/mid‐dermis (blue asterisk) containing canalicular vessels (green asterisks) hypo‐refractive dermal papillae (yellow asterisks). (b) Horizontal LC‐OCT section (taken at the level of the blue line in a) shows a honeycomb epidermal pattern and some hypo‐refractive, roundish dermal papillae (yellow asterisks). (c) 3D LC‐OCT reconstruction shows: stratum granulosum/spinosum with a honeycomb pattern (red asterisks); well‐defined dermo‐epidermal junction (green arrows); hypo‐refractive dermal papillae (yellow asterisks); superficial/mid‐dermis (blue asterisks) containing canalicular vessels (green asterisks).
The usefulness of LC‐OCT has been reported in a variety of skin disorders such as infectious diseases, autoimmune bullous diseases, skin tumours and other conditions. 1 , 3 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 The aim of our study was to evaluate LC‐OCT imaging in some common inflammatory skin disorders, that is plaque psoriasis, atopic eczema and lichen planus and to correlate the resulting features with histopathological findings.
Methods
This retrospective study included patients observed at the Dermatology Clinic of the University of Catania with histopathological confirmed diagnosis of plaque psoriasis, atopic eczema and lichen planus, who were imaged with LC‐OCT before the biopsy. The inclusion criteria were a definite histopathological diagnosis and the availability of good quality LC‐OCT and histopathological images. Exclusion criteria were the use of topical and/or systemic treatments specific for the diseases in the previous 2 and 4 weeks, respectively. LC‐OCT was performed with the commercially available DeepLive™ (DAMAE Medical, Paris, France), which provides images with an axial resolution of 1.1 μm, a lateral resolution of 1.3 μm and a field of view of 1.2 mm × 0.5 mm × 0.5 mm. All patients gave informed consent. The study was performed in accordance with the Declaration of Helsinki.
Results
A total of 15 adult patients (mean age: 34 years, range: 24–61 years) were included in the study: 5 with plaque psoriasis, 5 with atopic eczema and 5 with lichen planus. In all cases, a strong correlation was observed between LC‐OCT and corresponding histopathological images (Figs 2, 3, 4).
Figure 2.
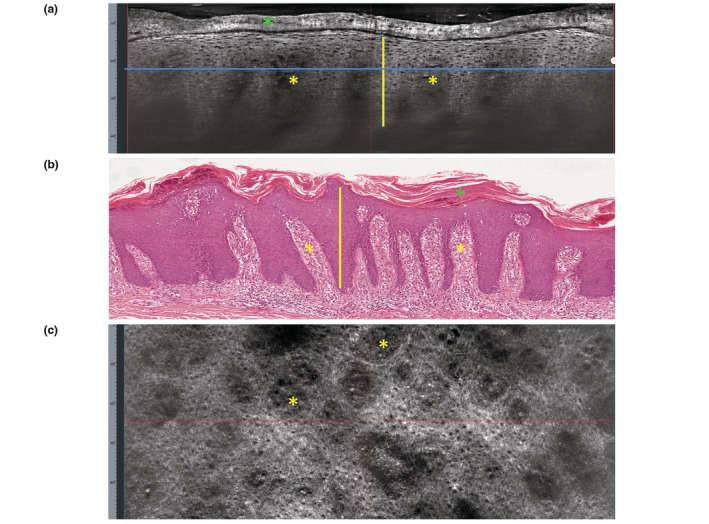
Plaque psoriasis. (a) Vertical LC‐OCT section shows thickening of the stratum corneum (green asterisk); thickening of the viable epidermis with elongation of the rete ridges (yellow vertical line); hypo‐refractive, elongated dermal papillae containing dark canalicular structures (yellow asterisks). (b) Vertical histopathology correlation shows: hyperparakeratosis (green asterisk); acanthosis and elongated rete ridges (yellow line); papillomatosis (yellow asterisks) (H&E, ×100). (c) Horizontal LC‐OCT section (taken at the level of the blue line in a) shows roundish, enlarged, hypo‐refractive dermal papillae (yellow asterisks) containing canalicular, ectatic vessels.
Figure 3.

Atopic eczema. (a) Vertical LC‐OCT section shows thickened and disrupted stratum corneum (green asterisk); in the viable epidermis, a disrupted honeycomb pattern (yellow asterisk) and dark roundish areas containing scattered keratinocytes (yellow arrows). (b) Vertical histopathology correlation shows: superficial sero‐crust (green asterisk), spongiosis (yellow asterisk), intraepidermal vesicles (yellow arrow) and inflammatory cells (H&E, ×100). (c) Horizontal LC‐OCT section (taken at the level of the blue line in a) shows spongiosis (yellow asterisk), vesicles (yellow arrows) and small bright inflammatory cells (blue arrows).
Figure 4.
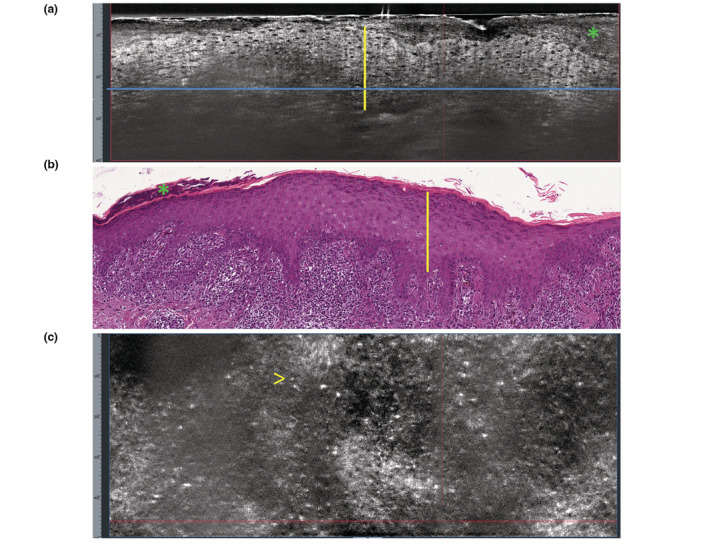
Lichen planus. (a) Vertical LC‐OCT section shows thickening of the stratum corneum (green asterisk); epidermal thickening (yellow vertical line); poorly defined dermo‐epidermal junction (blue horizontal line). (b) Vertical histopathology correlation shows: hyperorthokeratosis (green asterisk); acanthosis with hypergranulosis (yellow vertical line); ‘band‐like’ lymphohistiocytic infiltrate of the dermo‐epidermal junction and rete ridges sawtoothing (H&E, ×100). (c) Horizontal LC‐OCT section at the level of the dermo‐epidermal junction (taken at the level of the blue line in a) shows absence of evident dermal papillae and presence of small, bright inflammatory cells (arrow).
Plaque psoriasis
Patients presented with diffuse erythematous‐desquamative patches. LC‐OCT in the vertical view revealed, in all lesions: thickening of the stratum corneum showing amorphous, highly refractive structures, corresponding to hyperkeratotic scales; thickening of the viable epidermis with elongation of the rete ridges; hypo‐refractive, elongated dermal papillae containing dark canalicular structures corresponding to capillary proliferation and dilatation (Fig. 2a). The papillomatosis is evident especially in comparison with normal skin (Fig. 1). In the horizontal LC‐OCT view, the main feature was represented by the presence, at the dermo‐epidermal junction, of roundish, enlarged hypo‐refractive dermal papillae containing canalicular ectatic vessels that corresponded to the red dots observed with the dermoscopic integrated camera (Fig. 2c).
Atopic eczema
Patients presented with diffuse pruritic, erythematous patches. LC‐OCT in the vertical view revealed, in all lesions: localized areas of thickened and disrupted stratum corneum alternating hypo and hyper‐refractive layers and corresponding to the yellowish serocrusts observed with the dermoscopic integrated camera; normal thickness at the level of the stratum granulosum/spinosum, areas of disrupted honeycomb pattern with widening of intercellular spaces between keratinocytes, due to spongiosis, and dark roundish areas of variable size, containing scattered keratinocytes, corresponding to vesicles. Moreover, small polygonal/round bright inflammatory cells might be detected (Fig. 3a). Similar microscopic features were displayed in the horizontal LC‐OCT view (Fig. 3c).
Lichen planus
Patients presented with multiple, pruritic, lilaceous papules. LC‐OCT in the vertical view revealed, in all cases: thickening of the stratum corneum; thickening of the viable epidermis resulting from the histopathological wedge‐shaped hypergranulosis and corresponding to the Wickham striae observed by the dermoscopic integrated camera; poorly defined DEJ and dermal papillae; dilated dermal vessels (Fig. 4a). In the horizontal LC‐OCT view, the main feature was the presence of multiple small, bright inflammatory cells obscuring the DEJ (Fig. 4c).
Discussion
LC‐OCT appears to be suitable for the in vivo examination of inflammatory skin diseases. With the handheld probe, which works in contact with the skin, different skin areas, including difficult‐to‐access ones (periorificial areas, genitalia and interdigital spaces), may be easily and rapidly explored while live images are displayed on the monitor in real time. During the examination, the operator can quickly switch between horizontal or vertical vision. Also, the dermoscopic integrated camera allows a precise positioning over the area to examine, so to reduce the risks connected to relocation or probe slipping. 3 Once a representative field has been identified, a skin volume of 1200x500x500μm may be recorded in about 60 s and then the integrated software allows a 3D ‘navigation’ within the acquired images and post‐processing procedures such as measurements and contrast enhancement.
In our series of patients, LC‐OCT allowed the in vivo recognition of the main microscopic features of the examined inflammatory skin diseases, with a negligible variability among individuals with the same skin condition and a strong correlation with histopathology (Figs 2b,3b,4b), although the limited penetration depth of LC‐OCT imaging prevented in some cases the evaluation of the entire thickening of the epithelial layers as well as of the length of the dermal papillae. Interestingly, the integrated dermoscopic camera allows a precise correlation between LC‐OCT and dermoscopic findings.
From our results some considerations can be made: the horizontal LC‐OCT images observed in psoriasis, eczema and lichen planus appear to be similar to that obtained by RCM, 15 , 16 , 17 with a slightly lower resolution (1.3 μm vs. 0.8 μm, respectively) and a higher penetration depth (~500 μm vs. ~200 μm). Compared with RCM, LC‐OCT has the great advantage to additionally provide vertical images that allow an immediate view of the different layers of the skin corresponding to the same perspective of conventional histopathology. As regards OCT and dynamic‐OCT, some studies reported their application in inflammatory skin disorders including psoriasis and eczema: although these devices have the advantage of higher depth penetration (up to 1.5 mm), the resolution is lower (3–15 μm) than RCM and LC‐OCT. 18 , 19 Finally, new multimodal systems for cutaneous imaging integrate horizontal high‐resolution RCM with transverse high‐depth OCT in the same device, thus enabling rapid collection of spatially co‐registered RCM‐OCT images 18 , 20 : promising results have been reported for skin cancer, 20 but no studies have been performed in inflammatory disorders so far.
In conclusion, we presented the first study describing the LC‐OCT aspects of some inflammatory skin diseases. Limitations of our study include the small number of enrolled patients and the retrospective design. Although future studies on larger series of patients, also assessing quantitative data, are necessary, LC‐OCT, based on these preliminary findings, may represent a promising tool in inflammatory skin disorders with potential applications including enhanced diagnosis, biopsy guidance, follow‐up and treatment monitoring.
Acknowledgement
Open Access Funding provided by Universita degli Studi di Catania within the CRUI‐CARE Agreement.
Conflict of interest
None declared.
Funding sources
None.
Statement of any prior presentation: None.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Dubois A, Levecq O, Azimani H et al. Line‐field confocal optical coherence tomography for high‐resolution noninvasive imaging of skin tumors. J Biomed 2018; 23: 106007. [DOI] [PubMed] [Google Scholar]
- 2. Ruini C, Schuh S, Sattler E, Welzel J. Line‐field confocal optical coherence tomography‐practical applications in dermatology and comparison with established imaging methods. Skin Res Technol 2021; 27: 340–352. [DOI] [PubMed] [Google Scholar]
- 3. Verzì AE, Micali G, Lacarrubba F. Line‐field confocal optical coherence tomography may enhance monitoring of superficial basal cell carcinoma treated with imiquimod 5% cream: a pilot study. Cancers (Basel) 2021; 13: 4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monnier J, Tognetti L, Miyamoto M et al. In vivo characterization of healthy human skin with a novel, non‐invasive imaging technique: line‐field confocal optical coherence tomography. J Eur Acad Dermatol Venereol 2020; 34: 2914–2921. [DOI] [PubMed] [Google Scholar]
- 5. Tognetti L, Carraro A, Cinotti E et al. Line‐field confocal optical coherence tomography for non‐invasive diagnosis of lichenoid dermatoses of the childhood: a case series. Skin Res Technol 2021; 27: 1178–1181. [DOI] [PubMed] [Google Scholar]
- 6. Cinotti E, Tognetti L, Cartocci A et al. Line‐field confocal optical coherence tomography for actinic keratosis and squamous cell carcinoma: a descriptive study. Clin Exp Dermatol 2021; 46: 1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tognetti L, Cinotti E, Suppa M et al. Line field confocal optical coherence tomography: an adjunctive tool in the diagnosis of autoimmune bullous diseases. J Biophotonics 2021; 14: e202000449. [DOI] [PubMed] [Google Scholar]
- 8. Lacarrubba F, Verzì AE, Puglisi DF, Broggi G, Caltabiano R, Micali G. Line‐field confocal optical coherence tomography of xanthogranuloma: correlation with vertical and horizontal histopathology. J Cutan Pathol 2021; 48: 1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lenoir C, Diet G, Cinotti E et al. Line‐field confocal optical coherence tomography of sebaceous hyperplasia: a case series. J Eur Acad Dermatol Venereol 2021; 35: e509–e511. [DOI] [PubMed] [Google Scholar]
- 10. Lacarrubba F, Verzì AE, Puglisi DF, Micali G. Line‐field confocal optical coherence tomography: a novel, non‐invasive imaging technique for a rapid, in‐vivo diagnosis of herpes infection of the skin. J Eur Acad Dermatol Venereol 2021; 35: e404–e406. [DOI] [PubMed] [Google Scholar]
- 11. Ruini C, Schuh S, Pellacani G, French L, Welzel J, Sattler E. In vivo imaging of Sarcoptes scabiei infestation using line‐field confocal optical coherence tomography. J Eur Acad Dermatol Venereol 2020; 34: e808–e809. [DOI] [PubMed] [Google Scholar]
- 12. Verzì AE, Micali G, Lacarrubba F. Line‐field confocal optical coherence tomography in molluscum contagiosum: a case series. J Eur Acad Dermatol Venereol 2021; 35: e934–e936. [DOI] [PubMed] [Google Scholar]
- 13. Tognetti L, Fiorani D, Cinotti E, Rubegni P. Tridimensional skin imaging in aquagenic keratoderma: virtual histology by line‐field confocal optical coherence tomography. Int J Dermatol 2021; 60: e52–e54. [DOI] [PubMed] [Google Scholar]
- 14. Lenoir C, Cinotti E, Tognetti L et al. Line‐field confocal optical coherence tomography of actinic keratosis: a case series. J Eur Acad Dermatol Venereol 2021; 35: e900–e902. [DOI] [PubMed] [Google Scholar]
- 15. Lacarrubba F, Pellacani G, Gurgone S, Verzì AE, Micali G. Advances in non‐invasive techniques as aids to the diagnosis and monitoring of therapeutic response in plaque psoriasis: a review. Int J Dermatol 2015; 54: 626–634. [DOI] [PubMed] [Google Scholar]
- 16. Ardigo M, Agozzino M, Franceschini C, Lacarrubba F. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin 2016; 34: 487–496. [DOI] [PubMed] [Google Scholar]
- 17. Verzì AE, Lacarrubba F, Caltabiano R, Broggi G, Musumeci ML, Micali G. Reflectance confocal microscopy features of plaque psoriasis overlap with horizontal histopathological sections: a case series. Am J Dermatopathol 2019; 41: 355–357. [DOI] [PubMed] [Google Scholar]
- 18. Manfredini M, Liberati S, Ciardo S et al. Microscopic and functional changes observed with dynamic optical coherence tomography for severe refractory atopic dermatitis treated with dupilumab. Skin Res Technol 2020; 26: 779–787. [DOI] [PubMed] [Google Scholar]
- 19. Csuka EA, Ward SC, Ekelem C, Csuka DA, Ardigò M, Mesinkovska NA. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med 2021; 53: 776–797. [DOI] [PubMed] [Google Scholar]
- 20. Iftimia N, Sahu A, Cordova M et al. The potential utility of integrated reflectance confocal microscopy‐optical coherence tomography for guiding triage and therapy of basal cell carcinomas. J Cancer 2020; 11: 6019–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


