Abstract
Aim
To determine the serum bile acid (BA) response to 75‐g oral glucose in individuals without diabetes, and whether this is attenuated in patients with ‘early’ type 2 diabetes (T2D) and related to the glycaemic response at 2 hours in either group.
Methods
Forty newly diagnosed, treatment‐naïve Han Chinese T2D subjects and 40 age‐, gender‐, and body mass index‐matched controls without T2D ingested a 75‐g glucose drink after an overnight fast. Plasma glucose and serum concentrations of total and individual BAs, fibroblast growth factor‐19 (FGF‐19), total glucagon‐like peptide‐1 (GLP‐1), and insulin, were measured before and 2 hours after oral glucose.
Results
Fasting total BA levels were higher in T2D than control subjects (P < .05). At 2 hours, the BA profile exhibited a shift from baseline in both groups, with increases in conjugated BAs and/or decreases in unconjugated BAs. There were increases in total BA and FGF‐19 levels in control (both P < .05), but not T2D, subjects. Plasma glucose concentrations at 2 hours related inversely to serum total BA levels in control subjects (r = −0.42, P = .006). Total GLP‐1 and the insulin/glucose ratio were increased at 2 hours in both groups, and the magnitude of the increase was greater in control subjects.
Conclusions
The serum BA response to a 75‐g oral glucose load is attenuated in patients with ‘early’ T2D, as is the secretion of FGF‐19 and GLP‐1, while in individuals without T2D it correlates with 2‐hour plasma glucose levels. These observations support a role for BAs in the regulation of postprandial glucose metabolism.
Keywords: bile acids, fibroblast growth factor‐19, glucagon‐like peptide‐1, postprandial glycaemia, type 2 diabetes
1. INTRODUCTION
Bile acids (BAs) are cholesterol‐derived metabolites. In hepatocytes, cholesterol is converted to the primary BAs cholic acid (CA) and chenodeoxycholic acid (CDCA), which are then conjugated with glycine or taurine before secretion into the bile. After meal ingestion, BAs are discharged into the intestine, and the majority are reabsorbed and return to the liver for re‐secretion. A fraction of BAs escape into the large intestine and are metabolized into secondary BAs, such as deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA), for passive absorption or faecal excretion. 1 , 2
BAs have long been regarded solely as ‘intestinal detergents’ to facilitate fat digestion. However, increasing evidence suggests that they are also pivotal signalling molecules, orchestrating glucose and lipid metabolism through activation of the nuclear farnesoid X receptor (FXR) and membrane Takeda G protein receptor 5 (TGR5). 3 , 4 For example, ileal BA resorption (and hence stimulation of FXR in enterocytes) is coupled to the release of fibroblast growth factor 19 (FGF‐19), 1 which reduces hepatic glucose production and increases peripheral glucose disposal, independently of insulin. 5 FGF‐19 also constitutes a negative feedback signal that regulates hepatic BA synthesis. 6 BA‐dependent activation of TGR5 on the basal membranes of enteroendocrine L‐cells has been linked to the secretion of glucagon‐like peptide‐1 (GLP‐1), 7 , 8 which, in turn, regulates glucose metabolism via pleiotropic actions, including glucose‐dependent stimulation of insulin and suppression of glucagon, slowing of gastric emptying, and inhibition of energy intake. 9 , 10 In healthy individuals, intrajejunal administration of taurocholic acid (TCA) has been reported to reduce the glycaemic response to small intestinal glucose infusion, in association with augmented stimulation of GLP‐1. 11 The metabolic benefits of bariatric surgery are often accompanied by substantial increases in intestinal and circulating BAs, 12 , 13 while supplementation with a BA mixture over 28 days was also reported to stimulate GLP‐1 and FGF‐19 secretion and improve glycaemic control in subjects with type 2 diabetes (T2D). 14 Accordingly, interventions that increase intestinal BAs appear desirable for improved metabolic control.
Surprisingly, fasting plasma or serum total BA levels have been reported to be augmented in patients with T2D and/or obesity. 1 , 15 , 16 , 17 By contrast, the postprandial response of serum BAs appears blunted in subjects with obesity. 18 Moreover, faecal BA excretion is also increased in morbid obesity, 19 suggesting that intestinal BA resorption could be impaired, consistent with the observation that the expression of the intestinal BA transporter, apical sodium bile acid co‐transporter, is lower in overweight compared with lean individuals. 20 In a retrospective analysis, plasma concentrations of BAs, particularly unconjugated and glycine‐conjugated DCA and UDCA, both during fasting and following glucose or fat‐containing mixed nutrients, were reported to be elevated in T2D. 21 Of note, that study comprised a small number of subjects with T2D (n = 15), who had a comparatively long diabetes duration (6‐20 years) and suboptimal glycaemic control (mean HbA1c 7.5%), and who were taking medication (e.g. metformin) known to affect BA metablism, 22 and therefore could not allow to uncover any pathophysiological features of serum BAs in ‘early’ T2D. Recently, in a large longitudinal Chinese cohort of healthy subjects, 23 BA species in the baseline fasting serum were analysed to evaluate their association with incident T2D during a median 3‐year follow‐up. 23 Fasting serum levels of unconjugated BAs (CA, CDCA, and DCA) were found to be inversely associated with the risk of T2D, while the opposite was the case with conjugated BAs, including glycocholic acid (GCA), TCA, glycochenodeoxycholic acid (GCDCA), taurochenodeoxycholic acid (TCDCA), and tauroursodeoxycholic acid (TUDCA). However, the impact of the BA response to nutrients on blood glucose concentrations in individuals with and without T2D is not known, and this extends to the blood glucose value at 2 hours after a 75‐g oral glucose load, which is used widely to diagnose diabetes.
The primary aim of the current study was to determine the serum BA response to a 75‐g oral glucose load in patients with ‘early’ T2D in comparison with individuals without T2D. The secondary aim was to determine whether this was related to the glycaemic response at 2 hours in either group.
2. MATERIALS AND METHODS
2.1. Subjects
Forty treatment‐naïve Han Chinese subjects with newly diagnosed T2D (according to the World Health Organisation 1999 criteria) were studied (Table 1). The diagnosis of diabetes had been made at a regular health‐screening visit. A control group of 40 Han Chinese subjects without diabetes, with the proportion of each gender and mean age and body mass index (BMI) matched to the T2D patients, was also studied. No subject was taking any medication known to influence blood glucose, lipids, or BA metabolism, had impaired liver or renal function, or had a history of gastrointestinal disease, including significant upper or lower gastrointestinal symptoms, pancreatitis, or gastrointestinal surgery. The protocol was approved by the Human Research Ethics Committee of Zhongda Hospital, Southeast University, Nanjing, China (approval number: 2016ZDSYLL092‐P01), and the study was conducted in accordance with the Declaration of Helsinki. Subjects provided written informed consent prior to their enrolment in the study.
TABLE 1.
Demographic and biochemical variables in subjects with and without type 2 diabetes (T2D). Data are means ± SEM
| Subjects without T2D (N = 40) | Subjects with T2D (N = 40) | P value | |
|---|---|---|---|
| Sex (male/female) | 11/29 | 12/28 | .80 |
| Age (y) | 56.8 ± 0.9 | 57.1 ± 1.3 | .81 |
| BMI (kg m−2) | 27.5 ± 0.4 | 27.9 ± 0.6 | .60 |
| Waist circumference (cm) | 87.9 ± 1.4 | 90.3 ± 1.6 | .20 |
| HbA1c (%) | 5.4 ± 0.1 | 6.8 ± 0.1 | <.001 |
| Cholesterol (mmol L−1) | 5.3 ± 0.2 | 5.7 ± 0.2 | .15 |
| Triglycerides (mmol L−1) | 1.7 ± 0.2 | 2.1 ± 0.3 | .30 |
| Low‐density lipoprotein (mmol L−1) | 3.1 ± 0.1 | 3.4 ± 0.1 | .06 |
| High‐density lipoprotein (mmol L−1) | 1.6 ± 0.1 | 1.5 ± 0.1 | .20 |
| Plasma glucose (mmol L−1) | |||
| Baseline | 5.2 ± 0.1 | 7.9 ± 0.2 | <.001 |
| 2 h | 5.9 ± 0.2## | 15.0 ± 0.5### | <.001 |
| Serum insulin (mU L−1) | |||
| Baseline | 5.8 ± 0.3 | 8.3 ± 0.6 | .001 |
| 2 h | 25.8 ± 3.5### | 42.3 ± 5.0### | .004 |
| QUICKI | 0.37 ± 0.0029 | 0.33 ± 0.0032 | <.001 |
| SPISE | 5.90 ± 0.16 | 5.71 ± 0.23 | .48 |
| TyG | 8.69 ± 0.08 | 9.23 ± 0.11 | <.001 |
| Insulin/glucose ratio | |||
| Baseline | 1.1 ± 0.1 | 1.1 ± 0.1 | .24 |
| 2 h | 4.0 ± 0.5### | 3.0 ± 0.4### | .08 |
| Serum FGF‐19 (pg ml−1) | |||
| Baseline | 187.8 ± 14.3 | 244.6 ± 24.6 | .046 |
| 2 h | 235.0 ± 21.8# | 276.0 ± 43.5 | .39 |
| Serum total GLP‐1 (pmol L−1) | |||
| Baseline | 31.3 ± 4.0 | 26.5 ± 2.0 | .31 |
| 2 h | 60.7 ± 4.0#### | 47.2 ± 3.6#### | .02 |
Note: Differences in variables between the two groups were compared using unpaired Student's t test, except gender, which used Chi‐square analysis. Differences in measurements between baseline and 2 hours within each group were compared using paired Student's t test. P < .05 was considered significant. #P < .05, ##P < .01, ###P < .001, and #### P < 0.0001 indicate differences between baseline and 2 hours. n = 40 for both groups for all analyses, except n = 30, 28, and 39 for FGF‐19, GLP‐1, and insulin (and related QUICKI), respectively.
Abbreviations: BMI, body mass index; FGF‐19, fibroblast growth factor‐19; GLP‐1, glucagon‐like peptide‐1; QUICKI, quantitative insulin‐sensitivity check index; SPISE, single point insulin sensitivity estimator; TyG, triglycerides‐glucose index.
2.2. Protocol
Subjects were evaluated on a single visit after an overnight fast, at which they underwent a 75‐g oral glucose tolerance test (OGTT). Venous blood was collected into tubes with and without K2EDTA immediately before, and at 2 hours after, oral glucose. Plasma and serum were separated and stored at −80°C for subsequent analyses.
2.3. Sample analysis
Fasting and 2‐hour post‐OGTT plasma glucose (PG) (i.e. fasting plasma glucose [FPG] and 2‐hour PG), serum triglycerides, cholesterol, high‐density lipoproteins (HDL), and low‐density lipoproteins (LDL) were measured using an automated biochemistry analyzer (Synchron LX‐20, Beckman Coulter Inc.). HbA1c was measured using high‐performance liquid chromatography (D‐10 Hemoglobin Analyzer, Bio‐Rad Inc.). Serum insulin (10‐1113, Mercodia), FGF‐19 (DF1800, R&D Systems), and total GLP‐1 (EZGLP1T‐36 K, Millipore) concentrations were measured by ELISA.
Serum BAs were analysed using liquid chromatography‐mass spectrometry. The sample preparation and analysis followed the protocol described previously, 24 with the exception that the samples were injected into a Waters Acquity I Class UPLC‐Xevo G2‐S QTOF (Waters Corp., Milford) platform, which has a higher resolution, but a lower sensitivity, compared with the previous platform (Sciex 4000 QTRAP). Accordingly, the accurate mass of the molecular ion of individual BAs was used for quantitation, and the raw data of only nine major BAs, namely, CA, CDCA, DCA, GCA, GCDCA, GDCA, TCA, TCDCA, and TDCA, were processed with Quanlynx (Waters Corp.), as the concentrations of other BA species were below the limit of detection. Total BA concentrations were calculated as the sum of the nine individual BAs. The R2 values of the calibration curve in the linear range from 0.32 to 1000 μg ml−1 were 0.994, 0.969, 0.990, 0.989, 0.991, 0.989, 0.996, 0.987, and 0.990 for CA, CDCA, DCA, GCA, GCDCA, GDCA, TCA, TCDCA, and TDCA respectively, providing sufficient sensitivity and linearity.
2.4. Calculations
Changes in PG at 2 hours were computed by subtracting FPG from 2‐hour PG. The quantitative insulin‐sensitivity check index (QUICKI) was calculated as 1/[log(fasting insulin [μU × ml−1]) + log(fasting glucose[mg × dl−1]) to estimate hepatic insulin sensitivity. 25 , 26 The single point insulin sensitivity estimator (SPISE) was computed as 600 × HDL‐C(0.185)/(triglycerides (TG)(0.2) × BMI(1.338)) to describe whole‐body insulin sensitivity. 27 Triglycerides‐glucose index (TyG) was calculated as ln [triglycerides (mg × dl−1) × fasting glucose (mg × dl−1)/2] to reflect insulin resistance. 28 The insulin/glucose ratio was also calculated for evaluation of insulin secretion against the prevailing blood glucose concentrations. 29
2.5. Statistical analyses
All data were checked for normal distribution and lognormal distribution using the Anderson–Darling test. Data with a normal distribution were compared using paired (i.e. baseline vs. 2 hours within each group) or unpaired (subjects without vs. with T2D) Student's t test. Data that were not normally distributed were either loge (ln) transformed prior to the use of the paired or unpaired Student's t test, or analysed without ln transformation using the Wilcoxon signed‐rank test (within each group) or Mann–Whitney U test (between groups). The distribution of gender between the two groups was compared using Chi‐square analysis. BA concentrations were found to be lognormally distributed, such that changes in individual and total BA concentrations at 2 hours were computed using both their absolute and ln values. Differences in ln(BA) profiles were evaluated using orthogonal partial least‐squares discrimination analysis (OPLS‐DA). Receiver operating characteristic (ROC) curves were used to illustrate the ability of changes in ln(BA) to identify the metabolic phenotype of the group. Logistic regression analysis was also used to assess the association between the serum BA response to oral glucose and the odds of being diagnosed with T2D. Pearson correlation analysis was used to evaluate the relationships between variables. Normally distributed data are presented as means ± SEM, and non‐normally distributed data as medians and interquartile ranges (25th‐75th percentiles). P less than .05 was considered statistically significant.
The normal distribution and lognormal distribution test, Student's t tests, and ANOVA were conducted with GraphPad Prism 8 (GraphPad Software, LLC., CA), and OPLS‐DA with Simca software, version 14 (MKS Data Analytics Solutions, Umeå). Logistic regression, Pearson correlation, Mann–Whitney U test, and ROC were calculated using IBM SPSS Statistics, version 20 (IBM, New York). Heat maps were drawn with R, version 3.6.3 (R Foundation for Statistical Computing) and the ‘pheatmap’ package.
3. RESULTS
Demographic and biochemical data in the two groups are summarized in Table 1. As anticipated, HbA1c, FPG, and 2‐hour PG were higher in subjects with T2D than in those without T2D (P < .001 each; Table 1). There were no differences in gender, age, BMI, waist circumference, serum cholesterol, triglycerides, LDL, or HDL between the two groups.
3.1. Serum BAs
Fasting serum total BA concentrations were slightly higher in subjects with T2D than in those without T2D (P = .0328) (Figure 1A). At 2 hours, OPLS‐DA revealed a pattern of separation in the combined individual ln(BA) data (i.e. the BA profile) from that of baseline within both groups (Figure 2). However, total BA levels increased from baseline in subjects without T2D (P = .0005), but did not change significantly in subjects with T2D (Figure 1A). In both groups, there was substantial interindividual variation in serum concentrations of each individual BA, with only a tendency for CDCA to be higher in subjects with T2D than in those without T2D at baseline (P = .1 for Figure 1D; P = .034, Figure S1D). At 2 hours, although consistent patterns of a reduction in unconjugated BAs and an increase in conjugated BAs were evident for both groups, only the changes in DCA, GCA, GDCA, and TDCA were significant in subjects without T2D, and changes in CA, CDCA, DCA, GDCA, and TDCA were significant in subjects with T2D (Figure 1C–K). The magnitude of changes in both total and each individual BA levels (except for DCA and TDCA in the ln‐transformed dataset) differed between the two groups (Figure 1B). Comparisons of raw BA data using Wilcoxon signed‐rank test (within each group) or Mann–Whitney U test (between groups) showed essentially similar findings (Figure S1A–K).
FIGURE 1.
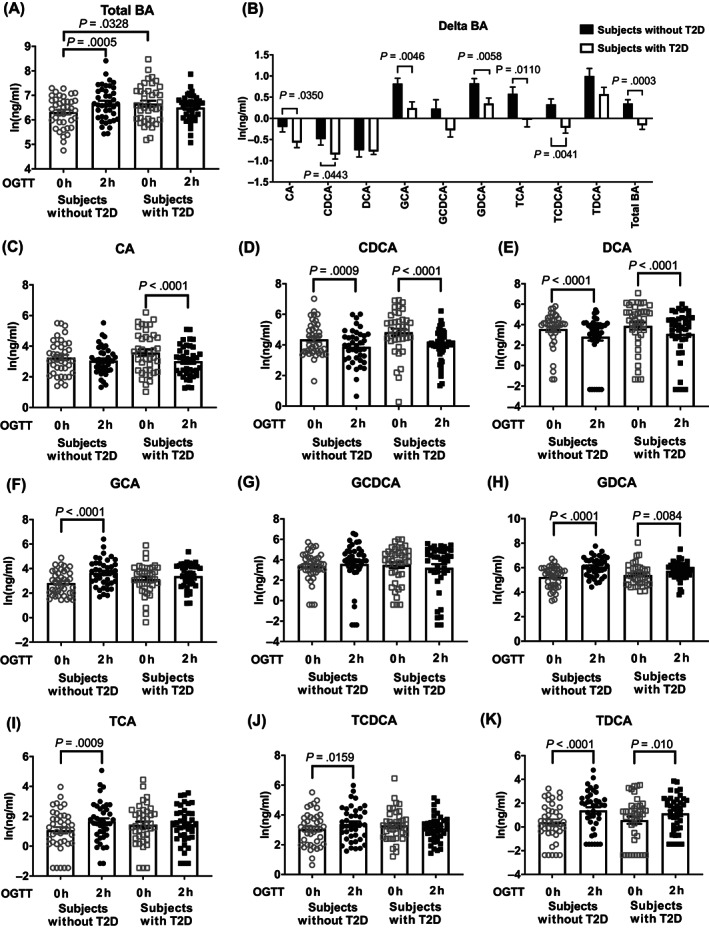
The ln‐transformed serum concentrations of total bile acid (BA) (A), cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), glycocholic acid (GCA), glycochenodeoxycholic acid (GCDCA), glycodeoxycholic acid (GDCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), and taurodeoxycholic acid (TDCA) before and 2 hours after 75‐g oral glucose (C–K), as well as their respective changes following oral glucose (B) (n = 40 for each group). Paired or unpaired t test was used for comparison between the two groups. OGTT, oral glucose tolerance test; T2D, type 2 diabetes
FIGURE 2.
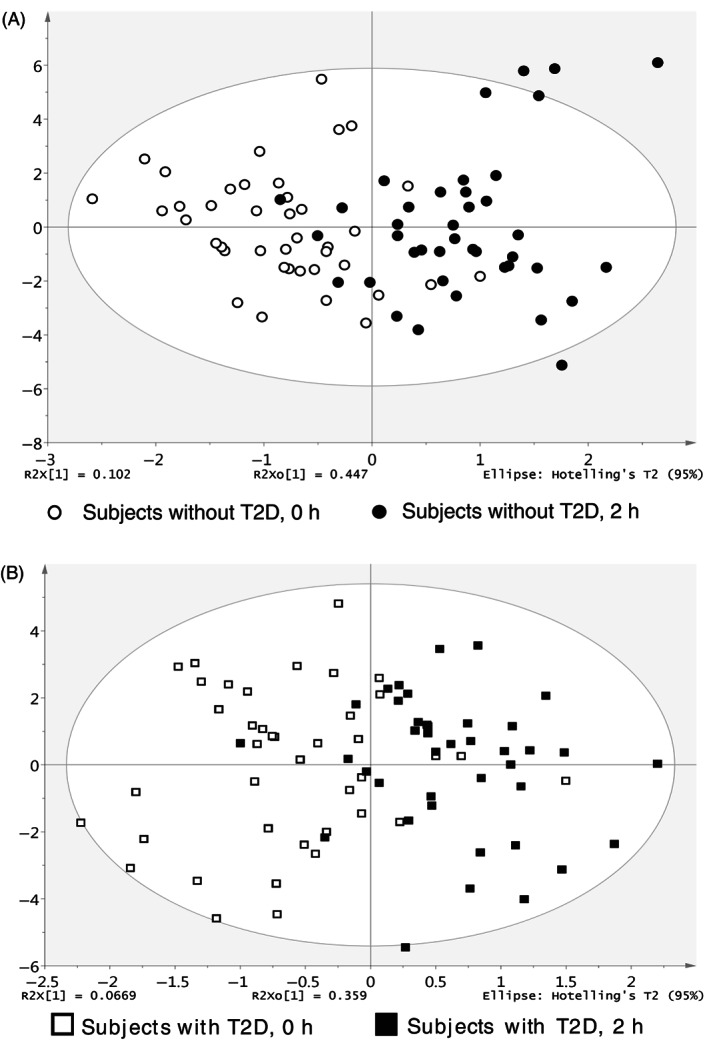
Score scatterplots of orthogonal partial least squares discriminant analysis (OPLS‐DA) of the bile acid (BA) profiles before and 2 hours after oral glucose in subjects without type 2 diabetes (T2D) (A) and with T2D (B) (n = 40 per group). The separations in the score scatterplots in panels A and B reflected the changes in the BA profiles after oral glucose in both subject groups
3.2. Serum total GLP‐1, FGF‐19 and insulin, insulin/glucose ratio, QUICKI, SPISE, and TyG
Fasting serum total GLP‐1 concentrations did not differ between the two groups. At 2 hours, there was an increase in serum total GLP‐1 concentrations in both groups, but the magnitude of this increase was less in subjects with T2D than in those without T2D (P = .039) (Table 1).
Fasting serum FGF‐19 concentrations were slightly higher in subjects with T2D than in those without T2D (P = .046). At 2 hours, serum FGF‐19 increased in subjects without T2D (P = .011), but did not change in those with T2D (Table 1).
Serum insulin concentrations were higher in subjects with T2D than in those without T2D at both fasting and 2 hours (P = .001 and .004, respectively). There was no difference in the fasting insulin/glucose ratio between the two groups, but this ratio tended to be lower at 2 hours in the subjects with T2D (P = .08). QUICKI index was lower, and TyG higher, in subjects with T2D than in those without T2D (P < .001 each), indicating reduced hepatic insulin sensitivity or increased insulin resistance in subjects with T2D. However, the whole‐body insulin sensitivity, as reflected by SPISE, did not differ between the two groups (Table 1).
3.3. Relationships between demographic and biochemical variables and BAs
The relationships of demographic and metabolic variables with total and individual BAs in subjects with and without T2D are summarized in Figure 3. Serum BA levels at baseline and 2 hours in subjects without T2D correlated positively with HDL and negatively with triglycerides, while changes in individual and total BA levels after oral glucose correlated negatively with waist circumference (Figure 3A). However, these relationships were less evident in subjects with T2D (Figure 3B).
FIGURE 3.
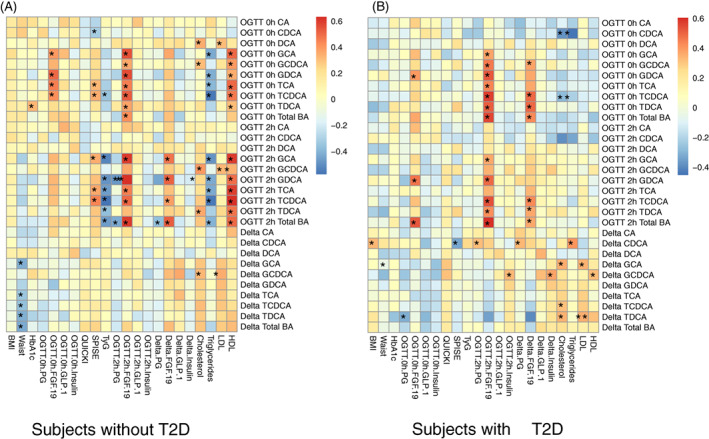
Heatmaps showing Pearson correlations between bile acids (BAs) and demographic and biochemical variables in subjects without type 2 diabetes (T2D) (A) and with T2D (n = 40 per group). Blue colour indicates negative r values, while red colour indicates positive r values. * P < .05 is considered significant correlation. Serum BA levels at baseline and 2 hours in subjects without T2D correlated positively with high‐density lipoproteins (HDL) and negatively with triglycerides, while changes in individual and total BA levels after oral glucose correlated negatively with waist circumference. However, these relationships were less evident in subjects with T2D. In both groups, serum fibroblast growth factor‐19 (FGF‐19) concentrations at 2 hours correlated positively with serum total and conjugated, but not unconjugated, BA levels. By contrast, there were no significant relationships between serum glucagon‐like peptide‐1 (GLP‐1) concentrations and total and individual BA levels at either fasting or 2 hours in either group. There was also a lack of consistent patterns for the relationships between quantitative insulin‐sensitivity check index (QUICKI), or serum insulin, and total or individual BA levels in either group. However, single point insulin sensitivity estimator (SPISE) was related directly to taurocholic acid (TCA) and taurochenodeoxycholic acid (TCDCA) at both fasting and 2 hours in subjects without T2D, but not in those with T2D. Triglycerides‐glucose index (TyG) correlated negatively with five conjugated and total BAs at 2 hours after oral glucose tolerance test (OGTT) in subjects without T2D, but not in those with T2D. The 2‐hour glucose, expressed either as the absolute level or the change from baseline, was related inversely to serum total BA levels at 2 hours in subjects without T2D, but not in those with T2D. BMI, body mass index; LDL, low‐density lipoproteins
In both groups, serum FGF‐19 concentrations at 2 hours correlated positively with serum total and conjugated, but not unconjugated, BA levels. By contrast, there were no significant relationships between serum GLP‐1 concentrations and total and individual BA levels at either fasting or 2 hours in either group. There was also a lack of consistent patterns for the relationships between hepatic insulin sensitivity (QUICKI), or serum insulin levels, and total or individual BAs in either group. However, the whole‐body insulin sensitivity (as assessed by SPISE) was related directly to several conjugated BA levels, particularly TCA and TCDCA, at both fasting and 2 hours in subjects without (but not with) T2D. TyG was related inversely to five individual conjugated (GCA, GDCA, TCA, TDCA, and TCDCA) and total BA levels at 2 hours after OGTT in subjects without (but not with) T2D (Figure 3A,B).
The 2‐hour glucose, expressed either as the absolute level (r = −0.42, P = .006; Figure 4A) or the change from baseline (figure not shown), was related inversely to serum total BA levels at 2 hours in healthy subjects, but not in those with T2D. Consistent with this, the ROC analysis showed that changes in BAs after oral glucose, particularly for total BAs (AUC 0.726), had a reasonable capacity for identifying the metabolic phenotype of the groups (Figure 4B). Furthermore, the logistic regression analysis revealed that an impaired serum BA response to oral glucose was associated with increased odds of being diagnosed with T2D (odds ratio 1.882; 95% confidence interval 1.094‐3.237).
FIGURE 4.
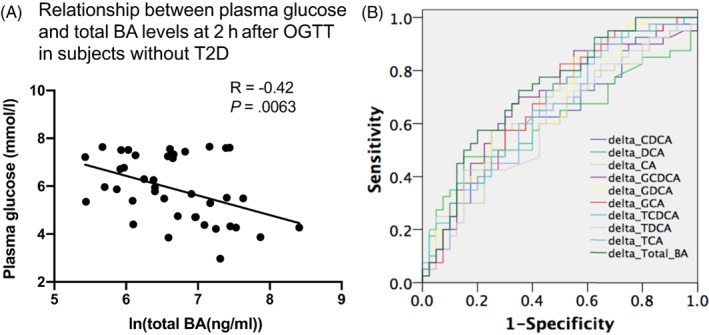
A, Relationship between 2‐hour plasma glucose and serum total bile acid (BA) concentrations in subjects without type 2 diabetes (T2D). B, ROC curves for identification of the healthy and subjects with T2D based on the change of ln BAs from baseline to 2 hours, with area under the curve (AUC) being greatest in the change of ln total BA (AUC = 0.726). CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; OGTT, oral glucose tolerance test; ROC, receiver operating characteristic; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid
4. DISCUSSION
Our study has characterized the effects of a 75‐g oral glucose load on serum BAs in subjects with and without T2D and their impact on glycaemia, FGF‐19, and GLP‐1. The key observations were that (i) in subjects without T2D, serum BAs (predominantly conjugated BAs) increased after oral glucose, and the magnitude of this increase correlated directly with FGF‐19 and inversely with the blood glucose level at 2 hours; and (ii) in subjects with T2D, while fasting serum BAs were higher, there was no significant response to oral glucose, which paralleled changes in FGF‐19 and GLP‐1. These observations, accordingly, support the concept that BAs contribute to postprandial blood glucose homeostasis in health and T2D.
The two groups were matched for gender, age, and BMI to minimize potential confounding effects. Serum cholesterol, triglycerides, LDL, and HDL were also found to be comparable. Importantly, none of the subjects with T2D was taking medication known to affect BA metabolism. Heterogeneity in these factors is probable to have accounted for substantial inconsistencies in previous reports of circulating BA levels. 30 , 31 Indeed, we observed a number of correlations between serum BAs and demographic and biochemical measures in both groups. Although subjects with T2D, compared with those without T2D, exhibited impaired hepatic insulin sensitivity (QUICKI), the whole‐body insulin sensitivity (SPISE) did not differ between the two groups, attesting to the ‘early’ stage of T2D in this group of patients.
As has been reported in subjects with obesity but without diabetes, 15 , 16 , 17 the subjects with T2D exhibited augmented fasting serum BAs, but a lack of any significant response at 2 hours after oral glucose. These observations contrast with those of a retrospective analysis involving healthy individuals and a small group of subjects with T2D, in which both fasting and postprandial serum BA levels were reported to be higher in subjects with T2D. 21 However, in the latter study, subjects had more ‘advanced’ T2D and were taking medication (e.g. metformin) that might have affected BA metabolism. The current study focused on the measurement of serum BAs before and 2 hours after OGTT, given that these time points are widely used for the diagnosis of diabetes. The OPLS‐DA revealed a clear separation of the BA profiles before and 2 hours after OGTT in both groups; there were consistent trends for a reduction in unconjugated BAs and an increase in conjugated BAs, in accord with the conjugation and release of BAs following ingestion of the glucose drink. The augmentation of fasting serum BA levels in subjects with T2D probably reflects an increase in BA synthesis. 3 There is evidence that hyperglycaemia is a major driver of increased expression of CYP7A1, the rate‐limiting enzyme converting cholesterol to the primary BA, CDCA. 32 In support of this concept, fasting serum CDCA was higher in the T2D group. After oral glucose, changes in serum BAs, particularly conjugated BAs (which are the main forms of BAs released into the upper gut), paralleled the rise in FGF‐19 in both groups. Given that FGF‐19 is released primarily in the ileum upon intestinal BA absorption and activation of enterocyte FXR, 33 , 34 and that meal‐related gallbladder emptying (and BA release into the small intestine) is usually intact in subjects with T2D, 35 the blunted BA response to oral glucose in subjects with T2D probably reflects an impairment in intestinal BA resorption.
There is recent evidence that variations in fasting serum BAs may predict the future risk of T2D. 23 However, in the current study, we did not observe any relationship between FPG and serum BAs. Rather, both 2‐hour PG and the increase in PG at hours from baseline were related inversely to serum BA levels in subjects without T2D. Moreover, the logistic regression analysis also revealed that a reduced serum BA response to oral glucose predicted increased odds of being diagnosed with T2D. This suggests that the BA profile after oral glucose or meals may be a better predictor of dysglycaemia, and that measurement of serum BAs during the OGTT, in addition to PG, may complement the assessment of the risk of T2D. In line with this concept, subjects with T2D did not exhibit a significant increase in serum total BAs at 2 hours. Interestingly, intrahepatic cholestasis of pregnancy is associated with augmented glycaemic excursions after meals, 36 and cholecystectomized patients—who lack physiological pulses of BA release into the small intestine—also exhibit an elevated postprandial blood glucose response. 37 Conversely, supplementation with exogenous BAs in both healthy and subjects with T2D has been reported to reduce postprandial glycaemia. 11 , 14 Moreover, bariatric surgery or diversion of bile to the distal gut, leading to augmented luminal and circulating BA concentrations, is associated with improved glycaemic control in subjects with T2D 13 , 38 , 39 and animal models. 40 , 41 These observations support the concept that effective enterohepatic circulation during the postprandial phase is critical to the maintenance of postprandial glucose homeostasis.
The links between serum BA and PG responses to oral glucose may potentially be underpinned by the secretion of FGF‐19 and GLP‐1, both of which exhibit pleiotropic actions in relation to glucose metabolism and were impaired in subjects with T2D. As discussed above, the secretion of FGF‐19 is coupled to intestinal BA signalling via FXR. Our observation of a direct relationship between the rise in serum FGF‐19 and serum BA levels at 2 hours in both groups is consistent with this concept. The blunted GLP‐1 response to oral glucose at 2 hours in subjects with T2D was also in agreement with previous findings reported in screen‐detected T2D. 42 However, the link of GLP‐1, as opposed to FGF‐19, to BAs is less specific, because GLP‐1 secretion, in addition to the effect induced by BAs, may also be affected by the rate of delivery of glucose to, and its transit through, the small intestine, as well as the responsiveness of the small intestine to glucose. 43 Subjects with T2D predictably exhibited impairments in insulin secretion (as assessed by the insulin/glucose ratio) and hepatic insulin sensitivity, which, albeit comparatively modest given the early stage of T2D, would, to some extent, contribute to elevated FPG and 2‐hour PG in this group. In the control group, we also noted direct relationships between SPISE (a surrogate measure of whole‐body insulin sensitivity) and several conjugated BAs, particularly TCA and TCDCA, at both fasting and 2 hours. Further studies are warranted to clarify whether this phenomenon is related directly to BA signalling or indirectly via other pathways.
Several potential limitations should be noted in interpreting our observations. First, we employed a 75‐g glucose solution rather than a mixed meal and measured serum BAs only at baseline and 2 hours. However, this approach aligns with the measurement of PG for the clinical diagnosis of diabetes. Although our OLPS‐DA indicated a profound shift in the BA profile at 2 hours after oral glucose in both groups, circulating BAs usually peak at 30 minutes after oral glucose. 30 Accordingly, more frequent measurements of serum BAs after oral glucose may provide additional insights. Second, BAs are concentrated in the enterohepatic circulation, and peripheral BA concentrations tend to reflect hepatic ‘spillover’, 17 , 30 , 31 rather than nutrient‐mediated release and the luminal load of BAs. Studies that directly compare postprandial luminal BA levels in subjects with and without T2D are indicated. Third, although there is evidence that gallbladder emptying is often unaffected in uncomplicated T2D, assessment of gallbladder emptying would help to delineate the mechanism(s) responsible for the impaired serum BA response to oral glucose in subjects with T2D. Fourth, although male and female subjects exhibited similar patterns of the serum BA responses to oral glucose in either group, the proportion of male and female subjects in both groups was unbalanced, with significant differences in biochemical measures (e.g. total cholesterol levels) between them. Accordingly, future work with larger sample sizes of properly matched male and female subjects is needed to clarify the impact of sex on the outcomes of serum BAs. Fifth, our observations were derived from a small cohort of newly diagnosed, treatment‐naïve T2D subjects with comparatively good glycaemic control; further work in subjects with clearly defined prediabetes and ‘advanced’ T2D would provide insights as to how circulating BAs vary across the spectrum of T2D. Finally, given the exploratory nature of the current study, a sample size calculation was not performed. However, our data were clear‐cut, such that increasing sample size is unlikely to result in substantial changes in our conclusions.
In conclusion, the serum BA response to a 75‐g oral glucose load in ‘early’ T2D is attenuated, as is the secretion of FGF‐19 and GLP‐1, while the BA response in individuals without T2D correlates with PG levels at 2 hours. These observations warrant further studies to investigate the role of BAs in the regulation of postprandial glucose metabolism, including the potential for postprandial BA concentrations to predict the risk of developing T2D, and for BA‐based therapies to have a role in the management of this condition.
CONFLICT OF INTEREST
RLY has received research funding from AstraZeneca and Pfizer and drug supplies from Boehringer Ingelheim and Takeda Pharmaceuticals. KLJ has received research funding from Sanofi and drug supplies from Merck Sharp & Dohme. MH has participated in the advisory boards and/or symposia for Novo Nordisk, Sanofi, Novartis, Eli Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, and AstraZeneca and has received honoraria for this activity. CKR has received research funding from AstraZeneca, Merck Sharp & Dohme, Eli Lilly, Novartis, and Sanofi. TW has received travel support from Novartis and Sanofi and research funding from Novartis and AstraZeneca. None of the other authors has any personal or financial conflict of interest to declare.
AUTHOR CONTRIBUTIONS
XW and CC were involved in data collection and interpretation, statistical analysis, and writing of the manuscript; CX and WH were involved in data analysis and interpretation, and reviewing of the manuscript. RLY, KLJ, MH, and CKR were involved in conception and design of the study, data interpretation, and reviewing of the manuscript. TW and ZS were involved in conception and design of the study, data interpretation, statistical analysis and drafting of the manuscript, and are guarantors of this work.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14683.
Supporting information
Figure S1 Serum concentrations of total BA (A), cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), glycocholic acid (GCA), glycochenodeoxycholic acid (GCDCA), glycodeoxycholic acid (GDCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA) and taurodeoxycholic acid (TDCA) before and 2 h after 75 g oral glucose (C‐K), as well as their respective changes following oral glucose (B) in subjects with and without type 2 diabetes (T2D) (n = 40 for each group). Wilcoxon signed‐rank test was used to compare difference between t = 0 and 2 h within each group. Mann–Whitney U test was used to compare difference between two groups.
ACKNOWLEDGEMENTS
KLJ is supported by a William T. Southcott Research Fellowship. TW is supported by a Mid‐Career Fellowship from The Hospital Research Foundation. The study was supported by the Australian National Health and Medical Research Council (1147333), the National Natural Science Foundation of China (81870561, 82071671), and the National Key R&D Program of China (2016YFC1305700). Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians. [Correction added on 18 May 2022: CAUL funding statement has been added.]
Wang X, Chen C, Xie C, et al. Serum bile acid response to oral glucose is attenuated in patients with early type 2 diabetes and correlates with 2‐hour plasma glucose in individuals without diabetes. Diabetes Obes Metab. 2022;24(6):1132-1142. doi: 10.1111/dom.14683
Xuyi Wang and Chang Chen contributed equally to this manuscript.
Funding information Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians. WOA Institution: The University of Adelaide. Blended DEAL: CAUL 2022.
Contributor Information
Zilin Sun, Email: sunzilin1963@126.com.
Tongzhi Wu, Email: tongzhi.wu@adelaide.edu.au.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding authors on reasonable request.
REFERENCES
- 1. Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling ‐ mechanisms and research needs. Nat Rev Endocrinol. 2019;15(12):701‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia‐Canaveras JC, Donato MT, Castell JV, Lahoz A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC‐MRM‐MS‐validated method. J Lipid Res. 2012;53(10):2231‐2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie C, Huang W, Young RL, et al. Role of bile acids in the regulation of food intake, and their dysregulation in metabolic disease. Nutrients. 2021;13(4):1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiorucci S, Distrutti E, Carino A, Zampella A, Biagioli M. Bile acids and their receptors in metabolic disorders. Prog Lipid Res. 2021;82:101094. [DOI] [PubMed] [Google Scholar]
- 5. Ge X, Yin L, Ma H, Li T, Chiang JY, Zhang Y. Aldo‐keto reductase 1B7 is a target gene of FXR and regulates lipid and glucose homeostasis. J Lipid Res. 2011;52(8):1561‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin DJ, Wang L. Bile acid‐activated receptors: a review on FXR and other nuclear receptors. Handb Exp Pharmacol. 2019;256:51‐72. [DOI] [PubMed] [Google Scholar]
- 7. Thomas C, Gioiello A, Noriega L, et al. TGR5‐mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brighton CA, Rievaj J, Kuhre RE, et al. Bile acids trigger GLP‐1 release predominantly by accessing basolaterally located G protein‐coupled bile acid receptors. Endocrinology. 2015;156(11):3961‐3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xie C, Jones KL, Rayner CK, Wu T. Enteroendocrine hormone secretion and metabolic control: importance of the region of the gut stimulation. Pharmaceutics. 2020;12(9):790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu T, Rayner CK, Horowitz M. Incretins. Handb Exp Pharmacol. 2016;233:137‐171. [DOI] [PubMed] [Google Scholar]
- 11. Wu T, Bound MJ, Standfield SD, Jones KL, Horowitz M, Rayner CK. Effects of taurocholic acid on glycemic, glucagon‐like peptide‐1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab. 2013;98(4):E718‐E722. [DOI] [PubMed] [Google Scholar]
- 12. Albaugh VL, Banan B, Antoun J, et al. Role of bile acids and GLP‐1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2019;156(4):1041‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris LA, Kayser BD, Cefalo C, et al. Biliopancreatic diversion induces greater metabolic improvement than Roux‐en‐Y gastric bypass. Cell Metab. 2019;30(5):855‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calderon G, McRae A, Rievaj J, et al. Ileo‐colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP‐1‐producing enteroendocrine cells in human obesity and diabetes. EBioMedicine. 2020;55:102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haeusler RA, Camastra S, Nannipieri M, et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. J Clin Endocrinol Metab. 2016;101(5):1935‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prinz P, Hofmann T, Ahnis A, et al. Plasma bile acids show a positive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Front Neurosci. 2015;9:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vincent RP, Omar S, Ghozlan S, et al. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50(Pt 4):360‐364. [DOI] [PubMed] [Google Scholar]
- 18. Glicksman C, Pournaras DJ, Wright M, et al. Postprandial plasma bile acid responses in normal weight and obese subjects. Ann Clin Biochem. 2010;47(Pt 5):482‐484. [DOI] [PubMed] [Google Scholar]
- 19. Straniero S, Rosqvist F, Edholm D, et al. Acute caloric restriction counteracts hepatic bile acid and cholesterol deficiency in morbid obesity. J Intern Med. 2017;281(5):507‐517. [DOI] [PubMed] [Google Scholar]
- 20. Renner O, Harsch S, Matysik S, Lutjohann D, Schmitz G, Stange EF. Upregulation of hepatic bile acid synthesis via fibroblast growth factor 19 is defective in gallstone disease but functional in overweight individuals. United European Gastroenterol J. 2014;2(3):216‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sonne DP, van Nierop FS, Kulik W, Soeters MR, Vilsboll T, Knop FK. Postprandial plasma concentrations of individual bile acids and FGF‐19 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2016;101(8):3002‐3009. [DOI] [PubMed] [Google Scholar]
- 22. Sansome DJ, Xie C, Veedfald S, Horowitz M, Rayner CK, Wu T. Mechanism of glucose‐lowering by metformin in type 2 diabetes: role of bile acids. Diabetes Obes Metab. 2020;22(2):141‐148. [DOI] [PubMed] [Google Scholar]
- 23. Lu J, Wang S, Li M, et al. Association of serum bile acids profile and pathway dysregulation with the risk of developing diabetes among normoglycemic chinese adults: findings from the 4C study. Diabetes Care. 2021;44(2):499‐510. [DOI] [PubMed] [Google Scholar]
- 24. Chen C, Hu B, Wu T, et al. Bile acid profiles in diabetic (db/db) mice and their wild type littermates. J Pharm Biomed Anal. 2016;131:473‐481. [DOI] [PubMed] [Google Scholar]
- 25. Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402‐2410. [DOI] [PubMed] [Google Scholar]
- 26. El‐Karaksy HM, El‐Raziky MS, Fouad HM, et al. The value of different insulin resistance indices in assessment of non‐alcoholic fatty liver disease in overweight/obese children. Diabetes Metab Syndr. 2015;9(2):114‐119. [DOI] [PubMed] [Google Scholar]
- 27. Paulmichl K, Hatunic M, Hojlund K, et al. Modification and validation of the triglyceride‐to‐HDL cholesterol ratio as a surrogate of insulin sensitivity in white juveniles and adults without diabetes mellitus: the single point insulin sensitivity estimator (SPISE). Clin Chem. 2016;62(9):1211‐1219. [DOI] [PubMed] [Google Scholar]
- 28. Kang B, Yang Y, Lee EY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes. 2017;41(5):789‐792. [DOI] [PubMed] [Google Scholar]
- 29. Wu T, Ma J, Bound MJ, et al. Effects of sitagliptin on glycemia, incretin hormones, and antropyloroduodenal motility in response to intraduodenal glucose infusion in healthy lean and obese humans and patients with type 2 diabetes treated with or without metformin. Diabetes. 2014;63(8):2776‐2787. [DOI] [PubMed] [Google Scholar]
- 30. Fiamoncini J, Curi R, Daniel H. Metabolism of bile acids in the post‐prandial state. Essays Biochem. 2016;60(5):409‐418. [DOI] [PubMed] [Google Scholar]
- 31. Steiner C, Othman A, Saely CH, et al. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PLoS One. 2011;6(11):e25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li T, Chanda D, Zhang Y, Choi HS, Chiang JY. Glucose stimulates cholesterol 7alpha‐hydroxylase gene transcription in human hepatocytes. J Lipid Res. 2010;51(4):832‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Claudel T, Staels B, Kuipers F. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25(10):2020‐2030. [DOI] [PubMed] [Google Scholar]
- 34. Chavez‐Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(7):1679‐1694. [DOI] [PubMed] [Google Scholar]
- 35. Sonne DP, Rehfeld J, Holst JJ, Vilsboll T, Knop FK. Postprandial gallbladder emptying in patients with type 2 diabetes: potential implications for bile‐induced secretion of glucagon‐like peptide‐1. Eur J Endocrinol. 2014;171(4):407‐419. [DOI] [PubMed] [Google Scholar]
- 36. Martineau MG, Raker C, Dixon PH, et al. The metabolic profile of intrahepatic cholestasis of pregnancy is associated with impaired glucose tolerance, dyslipidemia, and increased fetal growth. Diabetes Care. 2015;38(2):243‐248. [DOI] [PubMed] [Google Scholar]
- 37. Sonne DP, Hare KJ, Martens P, et al. Postprandial gut hormone responses and glucose metabolism in cholecystectomized patients. Am J Physiol Gastrointest Liver Physiol. 2013;304(4):G413‐G419. [DOI] [PubMed] [Google Scholar]
- 38. Zheng X, Chen T, Zhao A, et al. Hyocholic acid species as novel biomarkers for metabolic disorders. Nat Commun. 2021;12(1):1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Nierop FS, de Jonge C, Kulik W, et al. Duodenal‐jejunal lining increases postprandial unconjugated bile acid responses and disrupts the bile acid‐FXR‐FGF19 axis in humans. Metabolism. 2019;93:25‐32. [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Liu T, Wang Y, et al. Comparative effects of bile diversion and duodenal‐jejunal bypass on glucose and lipid metabolism in male diabetic rats. Obes Surg. 2016;26(7):1565‐1575. [DOI] [PubMed] [Google Scholar]
- 41. Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Faerch K, Torekov SS, Vistisen D, et al. GLP‐1 response to oral glucose is reduced in prediabetes, screen‐detected type 2 diabetes, and obesity and influenced by sex: the ADDITION‐PRO study. Diabetes. 2015;64(7):2513‐2525. [DOI] [PubMed] [Google Scholar]
- 43. Xie C, Huang W, Watson LE, et al. Plasma GLP‐1 response to oral and intraduodenal nutrients in health and type 2 diabetes ‐ impact on gastric emptying. J Clin Endocrinol Metab. 2021;dgab828. doi: 10.1210/clinem/dgab828 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Serum concentrations of total BA (A), cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), glycocholic acid (GCA), glycochenodeoxycholic acid (GCDCA), glycodeoxycholic acid (GDCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA) and taurodeoxycholic acid (TDCA) before and 2 h after 75 g oral glucose (C‐K), as well as their respective changes following oral glucose (B) in subjects with and without type 2 diabetes (T2D) (n = 40 for each group). Wilcoxon signed‐rank test was used to compare difference between t = 0 and 2 h within each group. Mann–Whitney U test was used to compare difference between two groups.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding authors on reasonable request.


