Abstract
A preeminent challenge in alkene difunctionalization is the control of regio-, diastereo-, and enantioselectivity. In this Perspective, a Pd/Cu-cooperative catalytic system for alkene arylboration is highlighted that allows for the controlled introduction of substituents. In particular, examples that allowed for divergent reactivity from a single substrate based on the tuning of catalysts and reaction conditions are emphasized.
Keywords: copper, palladium, boron, arylboration, cooperative catalysis
Graphical Abstract
Cooperative catalysis involves the activation of two substrates by two different catalysts that subsequently undergo a reaction to achieve product formation.1 This type of catalysis offers additional opportunities compared to single catalyst systems not only for the possibility of achieving a greater number of transformations in a single step but also due to the potential for each catalytic cycle to be tailored toward selective and divergent product outcomes. For this reason, among others, cooperative catalysis has emerged as a research area of intense interest.1
Alkene difunctionalization is an important strategy for chemical synthesis because molecular complexity can be rapidly generated from simple alkene precursors.2 However, an inherent challenge with alkene difunctionalization is the control of selectivity (regio-, diastereo-, and enantioselectivity) (Scheme 1A). Classical strategies rely on substrate design or directing groups to aid in the control of selectivity.3 However, an ideal scenario would be one in which each group could be programmatically added to the alkene with control of the regio-, diastereo- and enantioselectivity without substrate interference or assistance. Without question, this is a lofty goal in alkene functionalization; however, cooperative catalysis offers the opportunity for the precise introduction of substituents where each catalyst is responsible for a distinct aspect of selectivity (Scheme 1B). In the hypothetical scenario illustrated in Scheme 1B, Cat-1 and Cat-3 control the regioselectivity for the introduction of the first substituent, whereas Cat-2 and Cat-4 allow for stereoselective incorporation of the second group. Properly harnessed, this approach has great promise for starting with a single isomer of substrate to produce a multitude of divergent, difunctionalized analogs.
Scheme 1.
Alkene Functionalization
While many useful methods have been developed to achieve selective alkene difunctionalization, our lab has been interested in alkene carboboration,4 particularly via cooperative catalysis. These reactions are useful because in addition to the generation of a new C–C bond, a synthetically versatile C–B bond is formed.5 In this Perspective, we highlight examples in which Pd/Cu-cooperative catalysis has been used to achieve divergent selectivity from a single starting substrate in alkene arylboration reactions.6,7
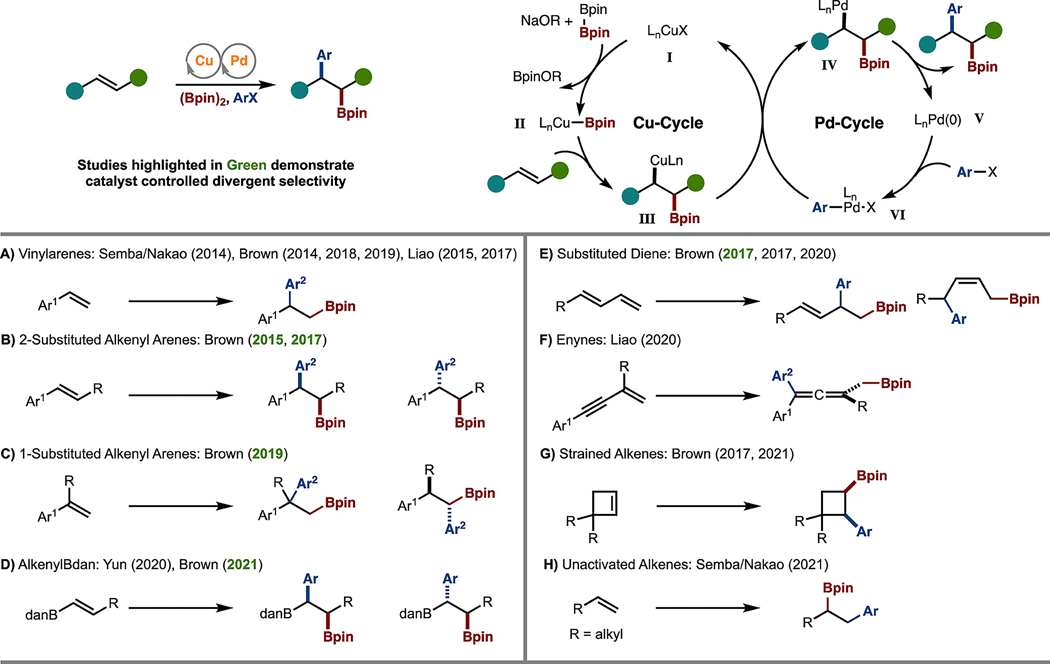
The inspiration and basis for many of the arylboration reactions highlighted in this Perspective originated from a seminal 2006 report from Sadighi and co-workers.8 In this paper, it was described that NHC–Cu–Bpin complexes can undergo syn-addition to alkenes to generate stereodefined alkyl–Cu complexes. In addition, as the reaction generally occurs with activated alkenes, such as styrene, the formation of a benzyl–Cu complex is heavily favored. Since these contributions, many research groups have investigated catalytic processes in which the generated alkyl–Cu complex is captured by an electrophile to generate either protoboration adducts or a myriad of difunctionalized products.4,9
In 2014, Semba and Nakao11 and our lab10 disclosed independent reports for the Pd/Cu-catalyzed arylboration of alkenyl arenes (Scheme 2A). In general, these reactions are proposed to proceed by addition of an in situ generated LCu–Bpin (Scheme 2, catalytic cycle, intermediate II) to an alkene to form an alkyl–Cu intermediate (III), which undergoes Pd-catalyzed cross-coupling (Scheme 2, Pd cycle).
Scheme 2.
Summary of Pd/Cu-Catalyzed Arylborations of Alkenes Developed to Date (2021)
Since the initial report from Semba and Nakao11 and our lab,10 several contributions have been made in this area. With respect to advancing the arylboration of alkenyl arenes, our lab investigated the reactions of 1,2- and 1,1-disubstituted derivatives (Scheme 2B,C).12 In addition, enantioselective variants emerged from our lab and that of Liao.12b–d,13 The concepts learned in these studies have been extended to the reactions of alkenylBdan derivatives (Scheme 2D).14,15 The examples with alkenyl bromides are particularly noteworthy as the products can be useful starting reagents for a double-allylation sequence.15
During the development of alkenyl arene arylboration, the reactions of dienes emerged. For example, our lab reported on the arylboration of 1- and 2-substituted 1,3-dienes (Scheme 2E).16 More recently, Liao et al. developed enantioselective reactions of enynes to generate chiral allenes (Scheme 2F).17
The move away from the reaction of conjugated alkenes has been met with resistance. However, our lab has been able to develop arylboration reactions of strained alkenes such as norbornene12b and cyclobutene18 derivatives (Scheme 2G). While reactions of unactivated alkenes are particularly challenging, Semba et al. have made a notable advance (Scheme 2H).19 While not highlighted in this review, recent developments in Ni catalysis have led to the development of a general process for 1,2-arylboration of unactivated alkenes.20,21
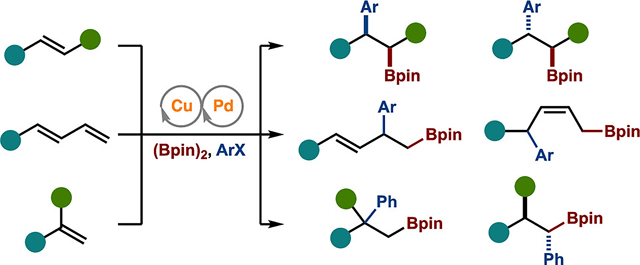
Among all the methods illustrated in Scheme 2, the control of selectivity has been achieved in some context, thus partly fulfilling the project goal outlined in Scheme 1. However, several examples outlined in Scheme 2 (highlighted in green text) have taken steps toward the controlled, divergent introduction of substituents from a conserved starting substrate.
DIASTEREODIVERGENCE
After the publication of the initial report for the arylboration of vinylarenes, our attention then focused toward arylboration of 1,2-disubstituted alkenyl arenes to allow for the synthesis of molecules with two adjacent stereogenic centers.12a Key to development of this type of reaction was identifying a Pd catalyst that selectively allows for either syn- or anti-transmetalation of the stereodefined alkyl–Cu complex. The evaluation of various Pd catalysts led to the finding that [Pd–RuPhos]22 in THF allowed for the synthesis of the syn-diastereomer, whereas [Pd–Pi–Bu3] in toluene led to the synthesis of the anti-diastereomer (Scheme 3A,B). The observed stereodivergence is consistent across a range of substrates. Some of these concepts have been extended to enantioselective and diastereodivergent reactions of alkenyl arenes and alkenylBdan derivatives.12b,15
Scheme 3.
Arylboration of Alkenyl Arenes
Mechanism studies confirmed that the stereodivergence occurs at the transmetalation stage of the reaction (Scheme 3C). More specifically, the alkyl–Cu complex could be generated as an intermediate and then subjected to the Pd-catalyzed cross-coupling conditions. In each case, the expected stereoisomer was observed as the major product. For the reaction promoted by [Pd–RuPhos], a four-centered, stereo-retentive transmetalation is proposed (Scheme 3C, top).23 In the case of the reactions promoted by [Pd–Pi–Bu3], (Bpin)2 and NaOt-amyl were found to be necessary to observe high diastereoselectivity. Therefore, it is proposed that the addition of a Lewis base is necessary for the stereoinvertive transmetalation via an SE2 type process (Scheme 3C, bottom). Similar stereoinvertive SE2 transmetalations invoke Lewis bases to facilitate the process.24 Finally, it should be noted that solvent plays an important role in both transformations; however, its role is unclear.
REGIODIVERGENCE (1,2 vs 1,4)
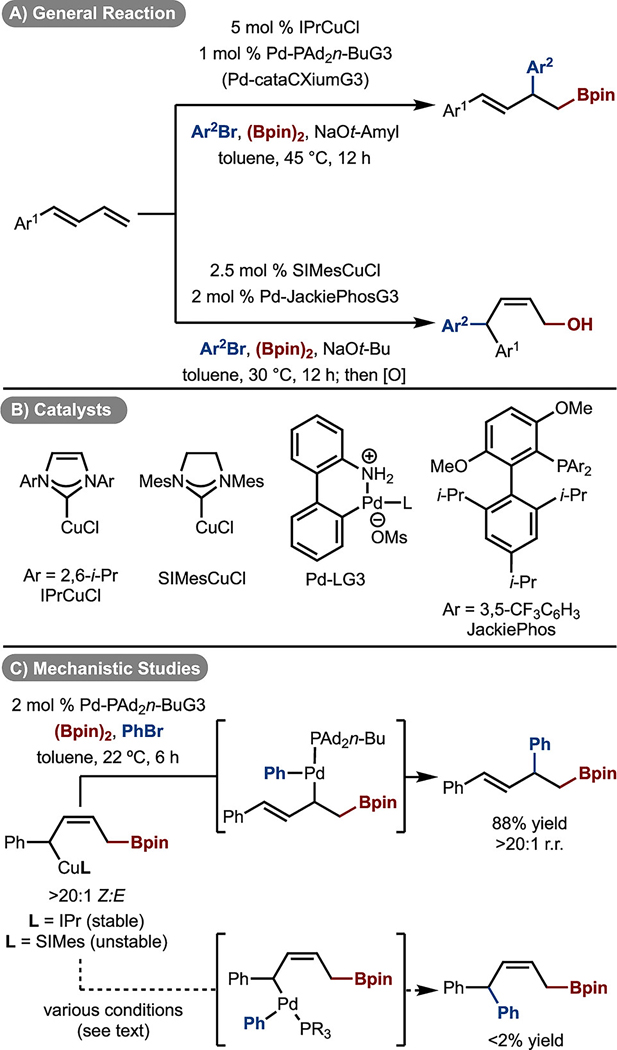
The arylboration of 1,3-dienes has also been extensively explored.16 The development of these reactions is complicated for several reasons: (1) the addition of the NHC–Cu–Bpin complex can generate η1-1,2-, η1-1,4-, or η3-π-allyl–Cu complexes, (2) the transmetalation can occur through either a direct or an allylic process,25 and (3) the reductive elimination can occur at one of the two sites.
With these challenges in mind, our group set out to develop a regioselective arylboration of 1-substituted dienes (Scheme 4).16b From these investigations, it was uncovered that the 1,2-addition product was favored with IPr–CuCl in combination with [Pd–PAd2n-Bu],26 whereas the 1,4-product was formed using SIMes–CuCl in conjunction with [Pd–JackiePhos]22 (Scheme 4A,B).
Scheme 4.
Arylboration of 1,3-Dienes
The mechanistic investigation revealed that the addition of the Bpin unit occurs at the terminal position and the Cu center is located at the 1-position, indicative of a formal 1,4-borylcupration (Scheme 4C). In addition, the Z-alkene was the major product from the reaction. While the allyl–Cu complex was observed with the reaction of both SIMes-CuCl and IPr–CuCl, it was only stable when isolated from the latter. Thus, the treatment of the complex derived from IPr–CuCl to the Pd-catalyzed cross-coupling conditions led to the formation of the observed 1,2-addition product. While an allylic transmetalation is likely, it is not clear at this time whether an open-or closed-transition state is operative. In the case of the complex derived from SIMes–CuCl, while it could be observed by 1H NMR, it proved to be too unstable to carry out the analogous stoichiometric studies. However, given that the observed 1,4-addition product contains a Z-alkene and the generated alkyl–Cu complex is of the same configuration, they are likely connected on the reaction coordinate via the allyl–Pd complex.
REGIODIVERGENCE (1,2 vs 1,1)
The 1,2-arylboration of 1,1-disubstituted alkenyl arenes is challenging due to the formation of a sterically congested C–C bond. Despite this challenge, our lab was able to develop this process to allow for the synthesis of a quaternary carbon.12c During our studies, we uncovered an unexpected 1,1-arylboration reaction and viewed this as a unique opportunity to tailor the system for regiodivergent outcomes. Whereas several Pd catalysts allowed for the synthesis of the 1,2-arylboration product (e.g., [Pd–APhos]),27 the 1,1-arylboration required the use of [Pd–PCy3] (Scheme 5A,B).
Scheme 5.
Arylboration of 1-Substituted Alkenyl Arenes
On the basis of mechanistic studies, the regiodivergence occurs after the formation of the alkyl–Cu complex during the transmetalation with Pd. In the case of the 1,2-arylboration, the generated alkyl–Cu complex undergoes transmetalation and rapid reductive elimination. In the case of the 1,1-arylboration, after transmetalation, the reductive elimination is slow and a β-hydride elimination/reinsertion process becomes competitive (Scheme 5C). It is proposed that the rapid reductive elimination reaction for the 1,2-arylboration occurs due to the steric pressure induced by APhos. In the case of the 1,1-arylboration, mechanistic studies revealed that a phosphine-free Pd complex is likely operative. In the absence of large phosphine-based ligands, reductive elimination is slow and, therefore, β-hydride elimination dominates to provide eventual access to the 1,1-arylboration products.
CONCLUSIONS AND FUTURE PERSPECTIVE
As seen with the highlighted examples, Pd/Cu-catalyzed arylboration reactions can be tuned to achieve divergent reaction outcomes in several different contexts. Despite such successes, many challenges remain within the field: (1) The mechanistic understanding needs to improve. While in most of these Pd/Cu cooperative catalysis systems the basic premise and timing of the steps can be elucidated due to the stability of the alkyl–Cu complexes (allowing for their stoichiometric generation and subsequent study with the Pd catalytic cycle), the delineation of ligand and/or substrate effects is much more difficult. This, in turn, makes it quite difficult to predict and design Pd/Cu systems with a specific outcome in mind (i.e., predicting which exact system will provide a 1,2-arylboration product featuring a stereoinvertive transmetalation).Therefore, to aid in the development of future systems, we think that ligand parametrization and/or multivariate analyses that incorporate substrate effects will prove useful.28 (2) Some of the systems outlined in Scheme 2 result in the formation of one isomer. While undoubtedly important, it would be valuable to be able to tune the catalysts or conditions to achieve divergent reactivity, for example, probing whether a catalyst can be identified to generate the 1,2-arylboration adduct of a 1,3-enyne (see Scheme 2F). (3) Most of the systems that have been identified for divergent reactivity rely on tuning of the Pd catalysts. This is typically because, with activated alkenes, the initial borylcupration occurs along the path that allows for the generation of a stable π-benzyl or π-allyl complex. Additional efforts need to be placed on identifying systems where the Cu catalyst is tuned to achieve catalyst-controlled regioselective borylcupration. For example, in the case of the arylboration of cyclobutenes (Scheme 2G), perhaps a Cu catalyst could be identified that would ultimately result in the formation of the other regioisomer.7 (4) Finally, there is a need to increase substrate scope. While there have been recent advances involving the reaction of unactivated alkenes and heterocyclic motifs, more work needs to be done to expand the utility of this system in these areas. Additionally, secondary or tertiary alkyl electrophiles remain a formidable challenge for Pd/Cu cooperative catalysis. Innovations in the above areas, as well as others, will continue to aid in achieving the goal of selective alkene functionalization and the synthesis of complex molecules from simple substrates.
ACKNOWLEDGMENTS
We thank Indiana University and the NIH (R35GM131755) for financial support.
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acscatal.1c05696
Contributor Information
Stanna K. Dorn, Indiana University, Department of Chemistry, Bloomington, Indiana 47405, United States
M. Kevin Brown, Indiana University, Department of Chemistry, Bloomington, Indiana 47405, United States.
REFERENCES
- (1).(a) Sammis GM; Danjo H; Jacobsen EN Cooperative Dual Catalysis: Application to the Highly Enantioselective Conjugate Cyanation of Unsaturated Imides. J.Am. Chem. Soc 2004, 126, 9928–9929. [DOI] [PubMed] [Google Scholar]; (b) Allen AE; MacMillan DWC Synergistic Catalysis: A Powerful Synthetic Strategy for New Reaction Development. Chem. Sci 2012, 3, 633–658. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Romiti F; del Pozo J; Paioti PHS; Gonsales SA; Li X; Hartrampf FWW; Hoveyda AH Different Strategies for Designing Dual-Catalytic Enantioselective Processes: From Fully Cooperative to Non-Cooperative Systems. J. Am. Chem. Soc 2019, 141 (45), 17952–17961. [DOI] [PubMed] [Google Scholar]; (d) Kim UB; Jung DJ;Jeon HJ; Rathwell K; Lee S Synergistic Dual Transition Metal Catalysis. Chem. Rev 2020, 120, 13382–13433. [DOI] [PubMed] [Google Scholar]
- (2).(a) For representative review, see: Coombs JR; Morken JP Catalytic Enantioselective Functionalization of Unactivated Terminal Alkenes. Angew. Chem., Int. Ed. Engl 2016, 55, 2636–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dhungana RK; Kc S; Basnet P; Giri R Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Record 2018, 18, 1314–1340. [DOI] [PubMed] [Google Scholar]; (c) Derosa J; Apolinar O; Kang T; Tran VT; Engle KM Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci 2020, 11, 4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hoveyda AH; Evans DA; Fu GC Substrate-Directable Chemical Reactions. Chem. Rev 1993, 93, 1307–1370. [Google Scholar]
- (4).(a) For recent reviews, see: Shimizu Y; Kanai M Recent Progress in Copper-Catalyzed Difunctionalization of Unactivated Carboncarbon Multiple Bonds. Tetrahedron Lett 2014, 55, 3727–3737. [Google Scholar]; (b) Semba K; Nakao Y Cross-Coupling Reactions by Cooperative Pd/Cu or Ni/Cu Catalysis Based on the Catalytic Generation of Organocopper Nucleophiles. Tetrahedron 2019, 75, 709–719. [Google Scholar]; (c) Whyte A; Torelli A; Mirabi B; Zhang A; Lautens M Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal 2020, 10, 11578–11622. [Google Scholar]; (d) Liu Z; Gao Y; Zeng T; Engle KM Transition-Metal-Catalyzed 1,2-Carboboration of Alkenes: Strategies, Mechanisms, and Stereocontrol. Isr. J. Chem 2020, 60, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Sandford C; Aggarwal VK Stereospecific Functionalizations and Transformations of Secondary and Tertiary Boronic Esters. Chem. Commun 2017, 53, 5481–5494. [DOI] [PubMed] [Google Scholar]
- (6).(a) Yu S-H; Gong T-J; Fu Y Three-Component Borylallenylation of Alkynes: Access to Densely Boryl-Substituted Ene-allenes. Org. Lett 2020, 22, 2941–2945. [DOI] [PubMed] [Google Scholar]; (b) Mateos J; Rivera-Chao E; Fananas-Mastral M Synergistic Copper/Palladium Catalysis for the Regio- and Stereoselective Synthesis of Borylated Skipped Dienes. ACS Catal 2017, 7, 5340–5344. [Google Scholar]; (c) Mateos J; Fuentes-Vara N; Fra L; Rivera-Chao E; Vázquez-Galñanes N; Chaves-Pouso A; Fananas-Mastral M Transmetalation as Key Step in the Diastereo- and Enantioselective Synergistic Cu/Pd-Catalyzed Allylboration of Alkynes with Racemic Allylic Carbonates. Organometallics 2020, 39, 740–745. [Google Scholar]; (d) Zhuo K-F; Xu W-Y; Gong T-J; Fu Y The Dual-Catalyzed Boryldifluoroallylation of Alkynes: An Efficient Method for the Synthesis of Skipped gem-Difluorodienes. Chem. Commun 2020, 56, 2340–2343. [DOI] [PubMed] [Google Scholar]; (e) Vázquez-Galñanes N; Fananas-Mastral M Stereoselective Synthesis of Borylated 1,3-Dienes by Synergistic Cu/Pd Catalysis. ChemCatChem 2018, 10, 4817–4820. [Google Scholar]; (f) Huang Y; Bergmann AM; Brown MK (Hetero)-arylboration of Alkynes: A Strategy for the Synthesis of α,α-bis(hetero)arylketones. Org. Biomol Chem 2019, 17, 5913–5915. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Lesieur M; Bidal YD; Lazreg F; Nahra F; Cazin CSJ Versatile Relay and Cooperative Palladium(0) N-Heterocyclic Carbene/Copper(I) N-Heterocyclic Carbene Catalysis for the Synthesis of Tri- and Tetrasubstituted Alkenes. ChemCatChem 2015, 7, 2108–2112. [Google Scholar]
- (7).(a) To be clear, divergent selectivity can be achieved with single catalyst systems; however, cooperative catalysis offers additional opportunities for divergent selectivity. For single catalyst systems, see: Zhong C; Kunii S; Kosaka Y; Sawamura M; Ito H Enantioselective Synthesis of Trans-Aryl- and -Heteroaryl-Substituted Cyclopropylboronates by Copper(I)-Catalyzed Reactions of Allylic Phosphates with a Diboron Derivative. J. Am. Chem. Soc 2010, 132, 11440–11442. [DOI] [PubMed] [Google Scholar]; (b) Su W; Gong T-J; Lu X; Zhang Q; Xu M-Y; Yu C-G; Xu Z-Y; Yu H-Z; Xiao B; Fu Y Ligand-Controlled Regiodivergent Copper-Catalyzed Alkylboration of Alkenes. Angew. Chem., Int. Ed 2015, 54, 12957–12961. [DOI] [PubMed] [Google Scholar]
- (8).Laitar DS; Tsui EY; Sadighi JP Copper(l) β-Boroalkyls from Alkene Insertion: Isolation and Rearrangement. Organometallics 2006, 25, 2405–2408. [Google Scholar]
- (9).(a) For a Cu/Pd catalyzed allylboration of alkene, see: Jia T; Cao P; Wang B; Lou Y; Yin X; Wang M; Liao J A Cu/Pd Cooperative Catalysis for Enantioselective Allylboration of Alkenes. J. Am. Chem. Soc 2015, 137, 13760–13763. [DOI] [PubMed] [Google Scholar]; (b) Yuan Y; Wu F; Xu J; Wu X Four-Component Borocarbonylation of Vinylarenes Enabled by Cooperative Cu/Pd Catalysis: Access to B-Boryl Ketones and B-Boryl Vinyl Esters. Angew. Chem. Int. Ed 2020, 59, 17055–17061. [DOI] [PubMed] [Google Scholar]
- (10).Smith KB; Logan KM; You W; Brown MK Alkene Carboboration Enabled by Synergistic Catalysis. Chem.–Eur. J 2014, 20, 12032–12036. [DOI] [PubMed] [Google Scholar]
- (11).Semba K; Nakao Y Arylboration of Alkenes by Cooperative Palladium/Copper Catalysis. J. Am. Chem. Soc 2014, 136, 7567–7570. [DOI] [PubMed] [Google Scholar]
- (12).(a) Logan KM; Smith KB; Brown MK Copper/Palladium Synergistic Catalysis for the Syn- and Anti-Selective Carboboration of Alkenes. Angew. Chem., Int. Ed 2015, 54, 5228–5231. [DOI] [PubMed] [Google Scholar]; (b) Logan KM; Brown MK Catalytic Enantioselective Arylboration of Alkenylarenes. Angew. Chem., Int. Ed 2017, 56, 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bergmann AM; Dorn SK; Smith KB; Logan KM; Brown MK Catalyst-Controlled 1,2- and 1,1-Arylboration of α-Alkyl Alkenyl Arenes. Angew. Chem., Int. Ed 2019, 58, 1719–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Huang Y; Brown MK Synthesis of Bisheteroarylalkanes by Heteroarylboration: Development and Application of a Pyridylidene-Copper Complex. Angew. Chem., Int. Ed 2019, 58, 6048–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Jia T; Cao P; Wang B; Lou Y; Yin X; Wang M; Liao J A Cu/Pd Cooperative Catalysis for Enantioselective Allylboration of Alkenes. J.Am. Chem. Soc 2015, 137, 13760–13763. [DOI] [PubMed] [Google Scholar]; (b) Chen B; Cao P; Yin X; Liao Y; Jiang L; Ye J; Wang M; Liao J Modular Synthesis of Enantioenriched 1,1,2-Triarylethanes by an Enantioselective Arylboration and Cross-Coupling Sequence. ACS Catal 2017, 7, 2425–2429. [Google Scholar]
- (14).Lee H; Lee S; Yun J Pd-Catalyzed Stereospecific Cross-Coupling of Chiral α-Borylalkylcopper Species with Aryl Bromides. ACS Catal 2020, 10, 2069–2073. [Google Scholar]
- (15).Dorn SK; Tharp AE; Brown MK Modular Synthesis of a Versatile Double-Allylation Reagent for Complex Diol Synthesis. Angew. Chem., Int. Ed 2021, 60, 16027–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Smith KB; Brown MK Regioselective Arylboration of Isoprene and Its Derivatives by Pd/Cu Cooperative Catalysis. J. Am. Chem. Soc 2017, 139, 7721–7724. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sardini SR; Brown MK Catalyst Controlled Regiodivergent Arylboration of Dienes. J. Am. Chem. Soc 2017, 139, 9823–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bergmann AM; Sardini SR; Smith KB; Brown MK Regioselective Arylboration of 1,3-Butadiene. Isr. J. Chem 2020, 60, 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Liao Y; Yin X; Wang X; Yu W; Fang D; Hu L; Wang M; Liao J Enantioselective Synthesis of Multisubstituted Allenes by Cooperative Cu/Pd-Catalyzed 1,4-Arylboration of 1,3-Enynes. Angew. Chem., Int. Ed 2020, 59, 1176–1180. [DOI] [PubMed] [Google Scholar]
- (18).Simlandy AK; Lyu M-Y; Brown MK Catalytic Arylboration of Spirocyclic Cyclobutenes: Rapid Access to Highly Substituted Spiro[3.n]Alkanes. ACS Catal 2021, 11, 12815–12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Semba K; Ohtagaki Y; Nakao Y 1,2-Arylboration of Aliphatic Alkenes by Cooperative Palladium/Copper Catalysis. Tetrahedron Lett 2021, 72, 153059–153064. [Google Scholar]
- (20).(a) Logan KM; Sardini SR; White SD; Brown MK Nickel-Catalyzed Stereoselective Arylboration of Unactivated Alkenes. J. Am. Chem. Soc 2018, 140, 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sardini SR; Lambright AL; Trammel GL; Omer HM; Liu P; Brown MK Ni-Catalyzed Arylboration of Unactivated Alkenes: Scope and Mechanistic Studies. J. Am. Chem. Soc 2019, 141, 9391–9400. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen L-A; Lear A; Gao P; Brown MK Nickel-Catalyzed Arylboration of Alkenylarenes: Synthesis of Boron-Substituted Quaternary Carbons and Regiodivergent Reactions. Angew. Chem. Int. Ed 2019, 58, 10956–10960. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lambright AL; Liu Y; Joyner IA; Logan KM; Brown MK Mechanism-Based Design of an Amide-Directed Ni-Catalyzed Arylboration of Cyclopentene Derivatives. Org. Lett 2021, 23, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sardini SR; Brown MK Nickel-Catalyzed Arylboration of Cyclopentene. Org. Synth 2020, 97, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Trammel GL; Kuniyil R; Crook PF; Liu P; Brown MK Nickel-Catalyzed Dearomative Arylboration of Indoles: Regioselective Synthesis of C2- and C3-Borylated Indolines. J. Am. Chem. Soc 2021, 143, 16502–16511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) For a 1,1-arylboration of an alkene, see: Wang W; Ding C; Yin G Catalyst-Controlled Enantioselective 1,1-Arylboration of Unactivated Olefins. Nat. Catal 2020, 3, 951–958. [Google Scholar]; (b) For Ni-catalyzed arylboration of vinylarenes, see: Wang W; Ding C; Pang H; Yin G Nickel-Catalyzed 1,2-Arylboration of Vinylarenes. Org. Lett 2019, 21, 3968–3971. [DOI] [PubMed] [Google Scholar]
- (22).Ingoglia BT; Wagen CC; Buchwald SL Biaryl Monophosphine Ligands in Palladium-Catalyzed C-N Coupling: An Updated User’s Guide. Tetrahedron 2019, 75, 4199–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).(a) Casares JA; Espinet P; Salas G 14-Electron T-Shaped [PdRXL] Complexes: Evidence or Illusion? Mechanistic Consequences for the Stille Reaction and Related Processes. Chem.–Eur. J 2002, 8, 4843–4853. [DOI] [PubMed] [Google Scholar]; (b) Cordovilla C; Bartolomé C; Maríinez-Ilarduya JM; Espinet P The Stille Reaction, 38 Years Later. ACS Catal 2015, 5, 3040–3053. [Google Scholar]
- (24).(a) Lee JCH; McDonald R; Hall DG Enantioselective Preparation and Chemoselective Cross-Coupling of 1,1-Diboron Compounds. Nat. Chem 2011, 3, 894–899. [DOI] [PubMed] [Google Scholar]; (b) Sandrock DL; Jean-Gerard L; Chen C; Dreher SD; Molander GA Stereospecific Cross-Coupling of Secondary Alkyl β-Trifluorobor-atoamides. J. Am. Chem. Soc 2010, 132, 17108–17110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).For example, see: Yang Y; Buchwald SL Ligand-Controlled Palladium-Catalyzed Regiodivergent Suzuki-Miyaura Cross-Coupling of Allylboronates and Aryl Halides. J. Am. Chem. Soc 2013, 135, 10642–10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zapf A; Ehrentraut A; Beller M A New Highly Efficient Catalyst System for the Coupling of Nonactivated and Deactivated Aryl Chlorides with Arylboronic Acids. Angew. Chem. Int. Ed 2000, 39, 4153–4155. [DOI] [PubMed] [Google Scholar]
- (27).Guram AS; King AO; Allen JG; Wang X; Schenkel LB; Chan J; Bunel EE; Faul MM; Larsen RD; Martinelli MJ; Reider PJ New Air-Stable Catalysts for General and Efficient Suzuki-Miyaura Cross-Coupling Reactions of Heteroaryl Chlorides. Org. Lett 2006, 8, 1787–1789. [DOI] [PubMed] [Google Scholar]
- (28).Zhao S; Gensch T; Murray B; Niemeyer ZL; Sigman MS; Biscoe MR Enantiodivergent Pd-Catalyzed C-C Bond Formation Enabled through Ligand Parameterization. Science 2018, 362, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]