Abstract
Background
Abnormal motility patterns in the jejunum can be detected in patients with prominent colonic content, and these abnormalities may be due to either a primary jejunal dysfunction or a reflex distortion. The objective of the present study was to determine the effect of colonic distension on small bowel postprandial motility using high‐resolution manometry.
Methods
Single center, controlled, parallel, randomized, single blind study in healthy subjects testing the effect of colonic filling vs sham infusion on the responses to a meal in 16 healthy subjects. Nutrients were continuously infused in the proximal jejunum (2 Kcal/min) during the 2‐h study period to induce a steady‐state postprandial motor pattern. Jejunal motility was measured by water‐perfused, high‐resolution manometry. After 1 h postprandial recording (basal period), gas was infused during 7.5 min via a rectal tube (720 mL or sham infusion), and jejunal motility was recorded for another hour.
Key Results
Jejunal postprandial motility during the basal period was characterized by two overlapping components: a) continuous segmental activity (non‐propagated or shortly propagated) and b) intercurrent propagated fronts (3.8 ± 1.1 fronts of 2‐5 clustered contractions/h >10 cm propagation). As compared to sham infusion, colonic gas filling: a) inhibited continuous segmental contractile activity (by 17 ± 4%; p = 0.044 vs control group) and b) stimulated intermittent propagated fronts (up to 9.0 ± 2.2 fronts/h; p = 0.017 vs control group).
Conclusions and Inferences
Long retrograde reflexes induced by colonic distension distort the balance between segmental and propagated activity, and may affect the normal response of the jejunum to food ingestion. Jejunal manometry in patients may be artifacted by colonic overload.
Keywords: colonic distension, high‐resolution manometry, postprandial motility, propagated contractions, small bowel motility
Colonic filling with gas stimulated intermittent propagated fronts in the jejunum; hence, mild colonic distension induces long‐distance retrograde reflexes that alter normal postprandial small bowel motility.

1. INTRODUCTION
Intestinal manometry is the current gold standard for the evaluation of patients with suspected small bowel dysmotility. 1 However, patients with suspected dysmotility frequently present colonic distension, and in this case, it becomes uncertain whether manometric abnormalities in the small bowel are related to a primary intestinal disorder, for example, gut neuro‐myopathy, or are secondary to colonic distension, for example, responses to long‐distance retrograde reflexes.
We hypothesized that colonic distension may elicit long‐distance retrograde reflexes targeting the upper gut. Our aim was to determine whether and in what form, colonic distension may affect small bowel motility. Since the dysmotility associated to colonic distension is more evident during the postprandial period, 2 we tested the effect of colonic distension on postprandial motility, experimentally induced by continuous infusion of nutrients directly into the small bowel. In this proof‐of‐concept study, we tested physiologic levels of colonic distension, using an experimental model that has been previously validated in our laboratory. 3 , 4 , 5 The jejunal response to colonic distension was evaluated by means of high‐resolution manometry (HRM) with multiple, closely spaced recording sites, because recent studies showed that HRM identifies motor features undetectable by conventional manometry. 6
2. MATERIAL AND METHODS
2.1. Study design
Single‐center, controlled, parallel, randomized, single blind study in healthy subjects testing the effect of colonic distension on the responses to meal ingestion. Colonic distension was produced by filling the colon with gas. The primary outcome was the effect on postprandial jejunal motility; secondary outcomes were the changes in postprandial sensations, abdominal girth, and vagal tone (Figure 1). Randomization into test group (with rectal gas infusion) and control group (sham infusion) was performed 1:1 by a computer‐generated list. The study protocol was approved by the Ethics Committee of the University Hospital Vall d’Hebron, and all participants gave their written informed consent before enrollment. The study protocol was registered with the ClinicalTrials.gov (ID: NCT05046743). All co‐authors had access to the study data and reviewed and approved the final manuscript.
FIGURE 1.
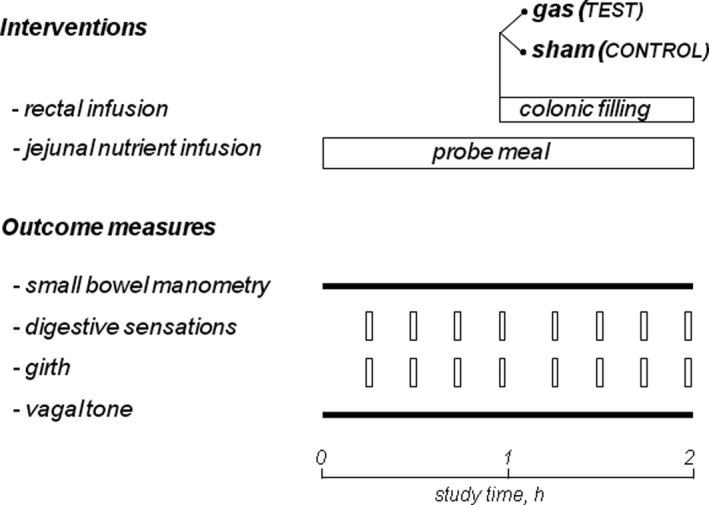
Experimental design. The responses to intestinal nutrients (outcomes) were measured before (basal) and during colonic filling; the effects of gas versus sham filling were compared in a parallel, randomized design in healthy subjects (n = 16)
2.2. Participants
Sixteen healthy, non‐obese subjects without a history of gastrointestinal symptoms were recruited by public advertising. Exclusion criteria were chronic health conditions, use of medications (except sporadic use of NSAIDs and antihistaminics), alcohol abuse and use of recreational drugs. Absence of current digestive symptoms was verified using a standard abdominal symptom questionnaire (no symptom > 2 on a 0–10 scale). Psychological and eating disorders were excluded using the following tests: Hospital Anxiety and Depression scale (HAD), Dutch Eating Behavior Questionnaire (DEBQ—Emotional eating, External eating, Restrained eating), and Physical anhedonia scale (PAS).
2.3. Interventions
2.3.1. Jejunal nutrient infusion
Postprandial condition was induced using a formula composed of 250 ml of a liquid nutrient mixture (Fresubin Protein Energy, Fresenius Kabi, 1.0 kcal/ml) combined with 85 ml of drinkable water. Using an infusion pump (Compat Ella, Nestle) at a rate of 165 ml/h a steady nutrient infusion of 2.0 Kcal/min was achieved. The nutrient infusion was infused into the jejunum via a catheter (1.02 mm inner diameter) attached to the manometric tube (see below). The nutrient infusion was started at the beginning of the experiment and was maintained during the 2‐h study period.
2.3.2. Colonic filling
After 1 h jejunal nutrient infusion, a balloon‐catheter (Foley catheter 20 F; Bard, Barcelona, Spain) was introduced into the rectum and hermetically connected to a volumetric pump (BIG‐3000, Soifer). The intra‐rectal balloon was inflated with 10 ml of water to prevent anal gas leaks. In the test group, 720 ml of a gas mixture was infused into the colon over a 7.5‐min period (at a constant flow rate of 96 ml/min); subsequently the outflow was blocked and colonic gas filling was maintained during 1 h. The gas mixture infused (88% nitrogen, 6.5% carbon dioxide, and 5.5% oxygen, bubbled into water for saturation) mimicked the partial pressures of venous blood gases to minimize diffusion across the intestine–blood barrier. 7 In the control group a sham infusion was performed. At the end of the study the rectal tube was opened to allow colonic venting (Figure 1).
2.4. Outcome measures
2.4.1. Jejunal motor activity
Jejunal motility was measured by high‐resolution manometry, using a technique that has been described before in detail. 6 In brief, a customized 35‐channel perfusion catheter (Mui Scientific) made of silicone (external diameter of 4.7 mm) was used. Perfusion side‐holes (recording sites) were located 58 and 48 cm from the tip of the catheter, to register antral and duodenal contractions, respectively; the following 33 side‐holes were spanned at 1‐cm intervals from 37 to 5 cm from the tip, to measure jejunal contractile activity. The nutrient perfusion tube was attached to the manometric tube with the distal end opening 38 cm from the tip, that is, 1 cm above the first jejunal recording site.
After calibration of the manometric system, the catheter with a metallic guidewire in the central lumen to facilitate localization, was introduced transnasally and placed into the small bowel under fluoroscopic control. After intubation, participants were positioned supine in bed, and the catheter was connected to a low‐compliance manometric system (Solar GI HRM, MMS‐Laborie) and each channel was perfused with distilled water at 0.15 ml/min (total volume infused 315 ml/h). Jejunal motility was continuously registered during the 2‐h study period.
2.4.2. Digestive sensations
Perception scales graded from 0 (not at all) to 6 (very severe) were used to measure: (a) abdominal bloating (defined as fullness/pressure), (b) sensation of abdominal distension (defined as sensation of increase in girth), (c) borborygmi/colicky sensation, (d) abdominal discomfort, and (e) nausea/vomiting; scales graded from −5 to +5 were used to measure: (f) hunger/satiety (from extremely hungry to completely satiated), (g) digestive well‐being (extremely unpleasant to extremely pleasant) and (h) mood (very negative to very positive). Subjects received standard instructions on how to fill‐out the scales to report the sensations perceived over the preceding 15‐min period during the study (Figure 1). This method has been extensively used and validated in detail. 3 , 5 , 8
2.4.3. Changes in girth
Once the participants were positioned in bed (see Procedure below), a non‐ stretch belt was placed over the umbilicus. The overlapping ends of the belts were carefully adjusted by means of two elastic bands so that the belts constantly adapted to the circumference of the abdominal wall. Measurements during the study were directly taken using a metric tape measure attached to the belts. 4 Measurements were taken at 15‐min intervals without manipulation of the belt‐tape assembly.
2.4.4. Vagal tone
A subset of participants (n = 8, four patients from each group) underwent continuous heart rate monitoring during the study to assess changes in heart rate variability. High quality inter‐beat data was recorded during the entire experiment using a Bluetooth heart rate strap (H10, Polar Electro). R‐R intervals and cardiac interbit intervals were obtained. Vagal tone was assessed using the root mean square of the successive differences between normal heartbeats (RMSSD), as described previously. 9
2.5. General procedure
Participants were instructed to follow a diet excluding legumes, vegetables, onion, garlic, nuts, cereals, whole meal bread and fizzy drinks for the 2 days prior to the study; meat, fish, eggs, rice, pasta and/or white bread were permitted, while dairy products, salad, fruit, and alcoholic beverages were prohibited. The evening before the study, they were instructed to eat a light dinner.
The studies were conducted in a quiet isolated room after an 8‐h fast. After intubation, participants were positioned supine in bed, the manometric catheter was connected to the manometric system, and jejunal manometry was continuously recorded throughout the study. After a 10‐min equilibration period, jejunal nutrient infusion was started and maintained until the end of the studies. The responses to jejunal nutrients were measured for 1 h (basal period). Subsequently, the balloon‐catheter was positioned into the rectum, connected to the infusion pump and either gas (in the test group) sham infusion (in the control group) was performed for 7.5 min and anal outflow was blocked. The effect of colonic filling (with gas or sham) on postprandial activity was studied for 1 h (intervention period) (Figure 1).
2.6. Data analysis
Manometric recordings were analyzed (by CM and LA) both visually and using the MMS Database Software v9.5h (MMS‐Laborie), as previously described. 6 The total number of phasic pressure waves (reversible pressure increase >10 mmHg, lasting >2 s and <10 s) was automatically measured by computerized analysis. Propagated fronts (2–5 contractions propagating over at least 10 contiguous recording sites) were visually identified. Their velocity of propagation was measured as the length of propagation (distance between the first and last sensor detecting each propagated event) divided by the duration of propagation (time interval between the onset of the contraction at the first and last sensor). Contractile activity that did not fulfill the criteria of propagated front [non‐propagated or shortly propagated (<10 cm) contractions], that is, segmental activity, was not directly quantified, but was inferred from the total number of contractions. 6 The outcomes were analyzed during the last 45 min of the basal period and the intervention period.
Changes in abdominal girth during the study were referenced to the girth measurement at the beginning of the study, that is, before jejunal nutrient infusion was started. Vagal tone, measured by heart rate variability (HRV), was assessed: (a) at the beginning of the experiments, (b) during the basal period, and (c) during the intervention period (either gas or sham colonic filling). Prior to HRV computation, all recorded data were visually inspected for correctness, and then underwent automatic artifact correction. HRV analysis of the exported data was performed on a computer using a dedicated HRV software (HRV Premium 3.4.2, Kubios Oy).
2.7. Statistical analysis
Primary statistical analysis was performed by comparing the effect of the intervention (differences from basal period) in the gas infusion group (test) versus sham infusion group (control).
Statistical analysis was performed with SPSS Statistics for Windows (V22.0, IBM). Data are presented as mean values ± standard error. Normality of data distribution was evaluated by the Shapiro Wilk test. Comparisons of parametric, normally distributed data were made by Student's t‐test, paired tests for intragroup comparisons and unpaired tests for intergroup comparisons; otherwise, the Wilcoxon signed rank test was used for paired data within groups, and the Mann‐Whitney U test for unpaired data between groups. Differences were considered significant at a p value < 0.05.
3. RESULTS
3.1. Demographics and study flow
Sixteen healthy subjects (6 women and 10 men, 19–44 years age range, body mass index between 18.5 and 28 kg/m2) were randomized into test or control groups. There were no differences in age, gender distribution or body mass index between groups (Table 1). Two participants, one in each group, did not tolerate the study procedure and withdrew from the study before the intervention period. Fourteen subjects completed the study protocol (7 subjects per group) and were included for analysis.
TABLE 1.
Demographics
| Test group | Control group | p value | |
|---|---|---|---|
| Female/male | 3/5 | 3/5 | 0.617 |
| Age, years | 24.0 ± 2.7 | 22.5 ± 0.8 | 0.114 |
| Weight, kg | 69.6±4.3 | 69.5 ± 2.7 | 0.873 |
| BMI, kg/m2 | 22.5 ± 0.6 | 22.6 ± 0.5 | 0.999 |
Abbreviation: BMI, body mass index.
3.2. Postprandial motor activity (basal period)
During the basal period, a postprandial‐type motility pattern was recorded in all subjects, characterized by two overlapping components: a background of segmental activity (non‐propagated or shortly propagated contractions) with intercurrent propagated fronts (3.8 ± 1.1 fronts/h) (see definitions in Data analysis section above; Figures 2 and 3). Most propagated fronts (79%) originated at the proximal recording sites and propagated aborally (at 0.75 ± 0.3 cm/s) throughout the 33 jejunal recording sites (32 cm); the rest originated at different levels and propagated aborally over a mean of 28 ±1 cm (no retrograde propagation was observed). The number of propagated fronts was similar in the proximal and distal recording sites; however, segmental activity was more prominent in the proximal than in the distal part of the jejunum, and this was reflected by a higher number of total contractions (3.1 ± 0.4 vs. 2.2 ± 0.3 contractions/min, respectively; p = 0.001). No differences between test and control groups were detected during the basal period (Figure 3).
FIGURE 2.
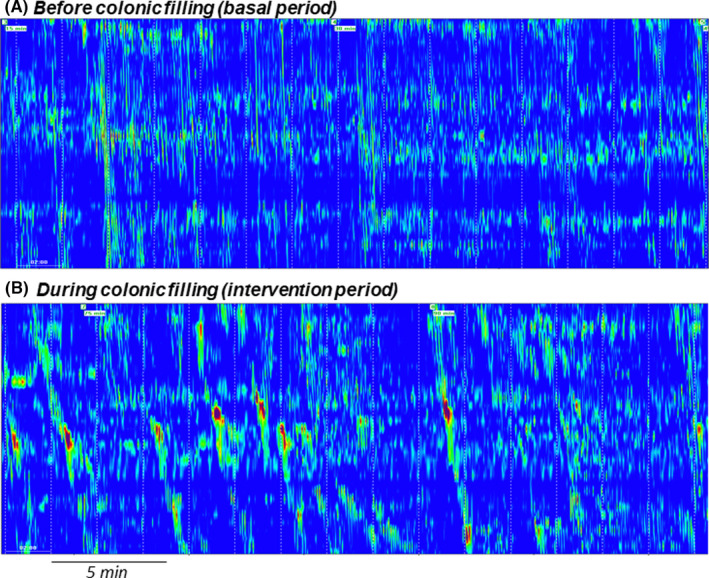
Examples of jejunal high‐resolution manometry in the same subject. Note, stimulation of propagated fronts during colonic filling (B) as compared to basal (A)
FIGURE 3.
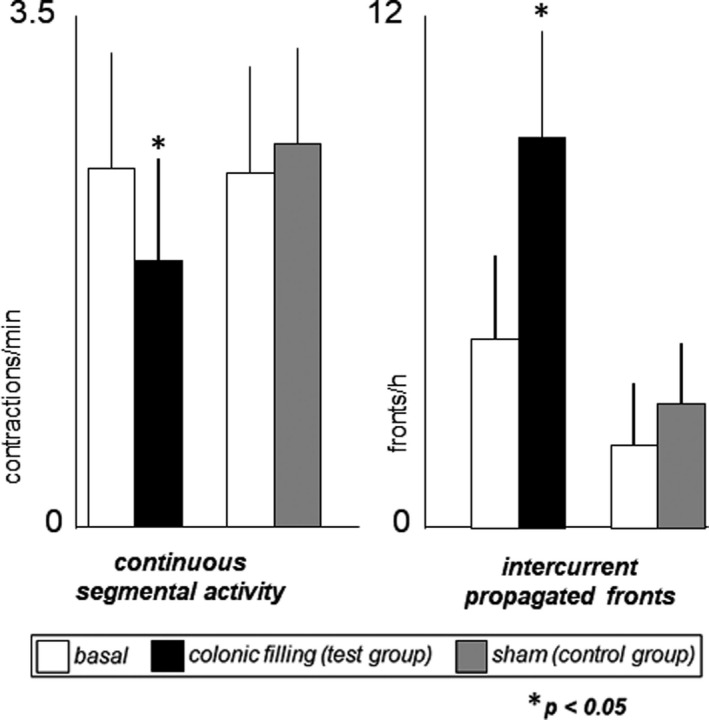
Effect of colonic filling on postprandial small bowel motility. Continuous segmental activity [non‐propagated or shortly propagated (<10 cm) contractions] decreased, and intercurrent propagated (>10 cm) fronts increased during colonic gas filling (test group), whereas sham infusion had no effects (control group)
3.3. Effect of colonic distension on jejunal motility
In the test group, colonic filling with gas produced a significant change in jejunal motility with differential effects on the two components of the postprandial pattern, as follows (Figures 2 and 3). Colonic filling with gas was associated with an increase in the number of propagated fronts (increase by 4.1 ± 1.1 fronts/h; p = 0.027 vs. basal period), without significant differences in the site of origin, velocity or distance of propagation. However, the total number of contractions decreased (by 17 ± 4%; p = 0.033 vs. basal period), reflecting a reduction in segmental activity; no region‐specific differences were detected (total number of contractions decreased by 14 ± 5% in the proximal and 21 ± 7% in the distal part; p = 0.404).
The effect of gas filling on jejunal motility was triggered immediately after gas infusion (3.5 ± 0.4 contractions/min during the 5 min before gas infusion vs. 2.8 ± 0.5 contractions during the 5 min immediately after gas infusion; p < 0.05), and was maintained throughout the whole intervention period (2.2 ± 0.4, 2.3 ± 0.5, 2.2 ± 0.5 contractions/min in the first, second and third 15‐min evaluation periods during gas infusion).
In the control group, sham infusion did not modify jejunal motor activity, neither the number of propagated fronts (change by 0.9 ± 0.5 propagated fronts/h; p = 0.484 vs. basal period) or total number of contractions (10 ± 11% change from basal; p = 0.537 vs basal period) nor (Figure 3). The changes from basal were significantly different between test and control groups, both the number of propagated fronts (p = 0.017) and total contractile activity (p = 0.044).
3.4. Perception of digestive sensations
All subjects tolerated jejunal nutrient infusion during the basal period with perception of mild digestive sensations, similar in control and test groups; to note, participants reported sensation of digestive well‐being and positive mood. In the control group, no changes in digestive postprandial sensations were observed during sham infusion. In the test group, colonic filling with gas induced an increase in perception of digestive sensations (Figure 4), and the effect (delta from basal) was significantly more prominent than that of sham infusion for the sensation of abdominal distension (p = 0.007), fullness (p = 0.009) and discomfort (p = 0.001). These sensations were associated with a significant impairment of digestive well‐being in the test group (p = 0.006 vs. basal), but not in the control group; p = 0.457 vs. basal and p = 0.005 vs. test group); a similar trend (not reaching statistical significance) was observed for mood (Figure 4).
FIGURE 4.
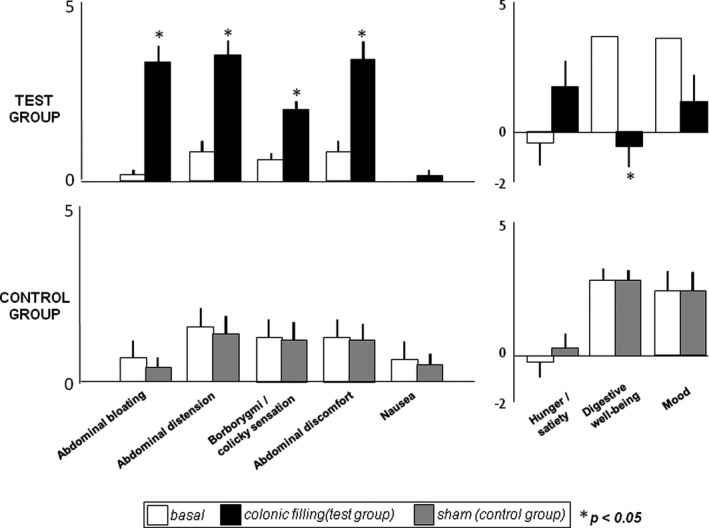
Effect of colonic filling on postprandial digestive sensations. Colonic filling was associated with a significant increase of digestive sensations, and impaired hedonic response (test group), whereas sham infusion had no effects (control group)
3.5. Effect on abdominal girth
During the basal period, jejunal nutrient infusion did not induce girth changes in either the test or control groups. In the test group, colonic gas filling induced a significant increase in girth (by 17 ± 3 mm; p = 0.002 vs. basal), but no change was observed during sham infusion in the control group (change by −1 ± 2 mm; p = 0.356 vs basal period; p = 0.002 vs. test group).
3.6. Effect on vagal tone
Vagal tone during the basal period was similar in the test and control groups, and no changes were detected after gas infusion (RMSSD change by −3.7 ± 2.9 ms; p = 0.498 vs. basal) or after sham infusion RMSSD change by 2.0 ± 2.8 ms; p = 0.251 vs. basal; p = 0.185 vs. gas infusion).
4. DISCUSSION
Our data indicate that relatively mild colonic distension in healthy subjects induces long‐distance retrograde reflexes affecting jejunal motor activity.
In our experimental model, continuous infusion of nutrients directly into the jejunum induced a steady‐state motor activity, without presence of the cycling activity that characterizes the fasting motor pattern. In a recent study using jejunal high‐resolution manometry, we demonstrated that in healthy subjects the postprandial motor pattern induced by a normal meal consists of a combination of continuous segmental activity (i.e., non‐propagated or shortly propagated contractions) and intermittent propagated activity, the latter characterized by single or short bursts of propagated contractions with higher amplitude than non‐propagated activity. 6 The characteristics of the pattern induced by jejunal nutrient infusion in the present study coincide with those of the postprandial pattern after a normal meal. Participants tolerated the nutrient infusion with mild homeostatic sensations, associated to digestive well‐being and positive mood, and without discomfort or changes in girth, a response similar to the sensory experience after a comfort meal. These sensations also remained steady over the 2‐h nutrient infusion period in the control experiments without colonic gas infusion (sham infusion).
Colonic distension was produced by rectal gas infusion. Previous studies with radiolabeled gas using the same experimental model showed a uniform distribution of the gas infused along the colon, as well as an effective ileocolic junction preventing gas reflux into the small bowel. 3 The colonic gas load in the present study induced abdominal sensations, increased girth and reduced digestive well‐being, but did not involve nausea or changes in vagal tone, suggesting that the stimulus did not disturb the physiologic conditions. Indeed, the volume load (720 ml) was half to that previously tested. 5 This colonic stimulus distorted the postprandial motor pattern in the jejunum with differential effects on the two motor components: inhibition of non‐propagated contractile activity and stimulation of propagated fronts.
The functional implications of the jejunal response cannot be as ascertained, but the propagated fronts resemble the motor pattern that develops in the small bowel proximal to a luminal occlusion. In the presence of intestinal obstruction, the proximal small bowel generates a motor pattern characterized by repeat clusters of contractions that propagate caudally, with suppression of the background of segmental contractions, that is, the “minute rhythm.” 10 , 11 Conceivably, this response represents a reactive propulsive pattern to overcome the downstream obstacle. Interestingly, the same pattern was observed in patients with suspected dysmotility, who at the time of the test presented the colon distended by fecal retention. In a subset of these patients, small bowel manometry was repeated after colonic cleansing, and the “minute rhythm” pattern was no longer detected. 2 These non‐controlled observations indicate that colonic distension may elicit obstructive motility patterns in the small bowel. The present study with mild colonic distension supports this conclusion, and suggests that the jejunal response was mediated by long retrograde reflexes, but we cannot ascertain the degree of colonic distension required to induce a full blown dysmotility pattern. The manometric catheter used for this study was specifically designed to measure jejunal activity, and hence, the effect of gas distension on the duodenum (and the duodenal transition zone) was not evaluated. 12
Long‐distance retrograde reflexes are a key mechanism in the physiologic feedback control of gut function, regulating gastric tone, gastric emptying, upper small bowel phasic activity and intestinal tone. 13 , 14 , 15 , 16 It has been shown that experimental colonic distension in humans elicits propagated contractions via a local, peristaltic reflex directly mediated by the enteric nervous system. 17 , 18 , 19 , 20 Colonic stimulation also elicits long‐distance reflexes modulating the activity of other areas of the gut. 21 A recent study in a canine model showed that distension of the colon inhibits small bowel contractions via long‐distance retrograde inhibitory reflexes. 22
Our data may have clinical relevance, since it suggests that colonic overload alters normal postprandial motility and hence, the interpretation of the small bowel manometry. For this reason, it might be recommendable to rule out colonic retention, and if necessary, clean the colon, before investigating small bowel motility.
CONFLICT OF INTEREST
No conflicts of interest.
AUTHOR CONTRIBUTIONS
LA was involved in conduction of studies, data analysis, and manuscript preparation; CM was involved in study design, study management, data analysis and manuscript preparation; DML was involved in data analysis; FA was involved in study design, data interpretation, and manuscript preparation.
Alcalá‐Gonzalez LG, Malagelada C ,Livovsky DM,Azpiroz F. Effect of colonic distension on small bowel motility measured by jejunal high‐resolution manometry. Neurogastroenterology & Motility. 2022;34:e14351. doi: 10.1111/nmo.14351
REFERENCES
- 1. Keller J,Bassotti G,Clarke J, et al. Expert consensus document: advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15(5):291‐308. doi: 10.1038/nrgastro.2018.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malagelada C,Monrroy H,Mego M, et al. Fecal impaction in the colon may induce obstructive patterns in intestinal manometry. Neurogastroenterol Motil. 2019;31(S4):e13671. doi: 10.1111/nmo.13671 [DOI] [PubMed] [Google Scholar]
- 3. Hernando‐Harder AC,Serra J,Azpiroz F, et al. Colonic responses to gas loads in subgroups of patients with abdominal bloating. Am J Gastroenterol. 2010;105(4):876‐882. doi: 10.1038/ajg.2010.75 [DOI] [PubMed] [Google Scholar]
- 4. Tremolaterra F,Villoria A,Azpiroz F,Serra J,Aguadé S,Malagelada JR. Impaired viscerosomatic reflexes and abdominal‐wall dystony associated with bloating. Gastroenterology. 2006;130(4):1062‐1068. doi: 10.1053/j.gastro.2005.12.036 [DOI] [PubMed] [Google Scholar]
- 5. Burri E,Cisternas D,Villoria A, et al. Accommodation of the abdomen to its content: integrated abdomino‐thoracic response. Neurogastroenterol Motil. 2012;24(4):312‐e162. doi: 10.1111/j.1365-2982.2011.01846.x [DOI] [PubMed] [Google Scholar]
- 6. Alcala‐Gonzalez LG,Malagelada C,Galan C,Nieto A,Accarino A,Azpiroz F. Propagation patterns of jejunal motor activity measured by high‐resolution water‐perfused manometry. Neurogastroenterol Motil. 2021;33(12):e14240. 10.1111/nmo.14240 [DOI] [PubMed] [Google Scholar]
- 7. Harder H,Serra J,Azpiroz F,Passas MC,Aguade S,Malagelada JR. Intestinal gas distribution determines abdominal symptoms. Gut. 2003;52(12):1708‐1713. doi: 10.1136/gut.52.12.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monrroy H,Pribic T,Galan C, et al. Meal enjoyment and tolerance in women and men. Nutrients. 2019;11(1):119. doi: 10.3390/nu11010119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monrroy H,Borghi G,Pribic T, et al. Biological response to meal ingestion: gender differences. Nutrients. 2019;11(3):702. doi: 10.3390/nu11030702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Summers RW,Anuras S,Green J. Jejunal manometry patterns in health, partial intestinal obstruction, and pseudoobstruction. Gastroenterology. 1983;85(6):1290‐1300. doi: 10.1016/S0016-5085(83)80009-2 [DOI] [PubMed] [Google Scholar]
- 11. Frank JW,Sarr MG,Camilleri M. Use of gastroduodenal manometry to differentiate mechanical and functional intestinal obstruction: an analysis of clinical outcome. Am J Gastroenterol. 1994;89(3):339‐344. doi: 10.1111/j.1572-0241.1994.tb07858.x [DOI] [PubMed] [Google Scholar]
- 12. Dent J,Deloose E,Dinning P, et al. Manometric demonstration of duodenal/jejunal motor function consistent with the duodenal brake mechanism. Neurogastroenterol Motil. 2020;32(10):5‐7. doi: 10.1111/nmo.13835 [DOI] [PubMed] [Google Scholar]
- 13. Rouillon JM,Azpiroz F,Malagelada JR. Reflex changes in intestinal tone: relationship to perception. Am J Physiol‐Gastrointest Liver Physiol. 2017;261(2):G280‐G286. doi: 10.1152/ajpgi.1991.261.2.g280 [DOI] [PubMed] [Google Scholar]
- 14. Welch IM,Davison PA,Worlding J,Read NW. Effect of ileal infusion of lipid on jejunal motor patterns after a nutrient and nonnutrient meal. Am J Physiol. 1988;255(6 Pt 1):G800‐G806. doi: 10.1152/ajpgi.1988.255.6.G800 [DOI] [PubMed] [Google Scholar]
- 15. Iovino P,Azpiroz F,Domingo E,Malagelada JR. The sympathetic nervous system modulates perception and reflex responses to gut distention in humans. Gastroenterology. 1995;108(3):680‐686. doi: 10.1016/0016-5085(95)90439-5 [DOI] [PubMed] [Google Scholar]
- 16. Spiller RC,Trotman IF,Higgins BE, et al. The ileal brake–inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25(4):365‐374. doi: 10.1136/gut.25.4.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liem O,Van Den Berg MM,Mousa HM, et al. Distention of the colon is associated with initiation of propagated contractions in children. Neurogastroenterol Motil. 2010;22(1):19–23. doi: 10.1111/j.1365-2982.2009.01383.x [DOI] [PubMed] [Google Scholar]
- 18. Pervez M,Ratcliffe E,Parsons SP,Chen JH,Huizinga JD. The cyclic motor patterns in the human colon. Neurogastroenterol Motil. 2020;32(5):e13807. doi: 10.1111/nmo.13807 [DOI] [PubMed] [Google Scholar]
- 19. Milkova N,Parsons SP,Ratcliffe E,Huizinga JD,Chen JH. On the nature of high‐amplitude propagating pressure waves in the human colon. Am J Physiol Gastrointest Liver Physiol. 2020;318(4):G646‐G660. doi: 10.1152/ajpgi.00386.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heitmann PT,Mohd Rosli R,Maslen L, et al. High‐resolution impedance manometry characterizes the functional role of distal colonic motility in gas transit. Neurogastroenterol Motil. 2021;34(1):1–14. doi: 10.1111/nmo.14178 [DOI] [PubMed] [Google Scholar]
- 21. Miller L,Roland BC,Whitson M,Passi M,Cheung M,Vegesna A. Clinical and translational aspects of normal and abnormal motility in the esophagus, small intestine and colon. In:Said H, ed. Physiology of the Gastrointestinal Tract. Academic Press; 2018:485‐516. doi: 10.1016/B978-0-12-809954-4.00022-0 [DOI] [Google Scholar]
- 22. Song J,Yin J,Chen JDZ. Inhibitory effects and sympathetic mechanisms of distension in the distal organs on small bowel motility and slow waves in canine. Cell Biochem Biophys. 2015;73(3):665‐672. doi: 10.1007/s12013-015-0679-4 [DOI] [PubMed] [Google Scholar]


