Abstract
In the general population, low‐grade inflammation has been established as a risk factor for all‐cause mortality. We hypothesized that an inflammatory milieu beyond the time of recovery from the surgical trauma could be associated with increased long‐term mortality in kidney transplant recipients (KTRs). This cohort study included 1044 KTRs. Median follow‐up time post‐engraftment was 10.3 years. Inflammation was assessed 10 weeks after transplantation by different composite inflammation scores based on 21 biomarkers. We constructed an overall inflammation score and five pathway‐specific inflammation scores (fibrogenesis, vascular inflammation, metabolic inflammation, growth/angiogenesis, leukocyte activation). Mortality was assessed with Cox regression models adjusted for traditional risk factors. A total of 312 (29.9%) patients died during the follow‐up period. The hazard ratio (HR) for death was 4.71 (95% CI: 2.85–7.81, p < .001) for patients in the highest quartile of the overall inflammation score and HRs 2.35–2.54 (95% CI: 1.40–3.96, 1.52–4.22, p = .001) for patients in the intermediate groups. The results were persistent when the score was analyzed as a continuous variable (HR 1.046, 95% CI: 1.033–1.056, p < .001). All pathway‐specific analyses showed the same pattern with HRs ranging from 1.19 to 2.70. In conclusion, we found a strong and consistent association between low‐grade systemic inflammation 10 weeks after kidney transplantation and long‐term mortality.
Keywords: donors and donation: donation after circulatory death (DCD), editorial/personal viewpoint, ethics, ethics and public policy, law/legislation, organ perfusion and preservation, organ procurement, organ procurement and allocation, solid organ transplantation
Short abstract
A cohort study consisting of kidney transplant recipients shows a strong and consistent association between subclinical inflammation in the early post‐transplant period and long‐term mortality.
Abbreviations
- AXL6
receptor tyrosine kinase 6
- CatS
Cathepsin S
- CNI
calcineurin inhibitor
- CRP
C‐reactive protein
- CsA
cyclosporine
- CVD
cardiovascular disease
- DGF
delayed graft function
- DM
diabetes mellitus
- ECM
extracellular matrix
- EGFR
estimated glomerular filtration rate
- EPCR
endothelial protein C receptor
- ESRD
end‐stage renal disease
- FGF
fibroblast growth factor
- GAS6
growth arrest specific gene 6
- GDF‐15
growth differentiation factor 15
- HR
hazard ratio
- IGF
insulin‐like growth factor
- IGFBP
insulin‐like growth factor binding protein
- IL
interleukin
- IPTW
inverse probability of treatment weighing
- IQR
interquartile range
- KT
kidney transplantation
- KTRs
kidney transplant recipients
- MIF
macrophage inhibitory factor
- NGAL
neutrophil gelatinase‐associated lipocalin
- OPN
osteopontin
- OR
odds ratio
- PRA
panel reactive antibody
- PTDM
post‐transplant diabetes mellitus
- PTX3
pentraxin 3
- sTNFR1
soluble tumor necrosis factor receptor 1
- TCC
terminal C5b‐9 complement complex
- TNF
tumor necrosis factor
- YKL40
tyrosine (Y), lysine (K), leucine (L)‐40
1. INTRODUCTION
Low‐grade systemic inflammation is established as a risk factor for all‐cause mortality in the general population 1 and is associated with increased risk and severity of several diseases, including cardiovascular disease (CVD) and cancer. 2 , 3 , 4 , 5 In the general population, activity in the innate immune system, as measured by elevated levels of tumor necrosis factor (TNF), interleukin (IL)‐6, and high‐sensitivity c‐reactive protein (CRP), has been associated with increased morbidity and mortality, 1 , 6 , 7 and inflammation is considered an important pathway in the development of atherosclerosis. Other inflammatory pathways such as endothelial dysfunction and vascular inflammation have also been associated with increased mortality. 8 , 9 It is well known that kidney transplant recipients (KTRs) have increased overall mortality, in particular caused by CVD, malignancy, and infectious diseases. 10 Inflammation seems to be of particular importance in the pathogenesis for development of CVD in KTRs. 11 , 12 , 13 Increased IL‐6 and CRP have been associated with cardiovascular events and all‐cause mortality in KTRs. 14 A study performed on the same population as in this study, showed an association between elevated terminal C5b‐9 complement complex (TCC) early after kidney transplantation (KT) and mortality and reduced graft survival. 15 However, data that include several inflammatory pathways describing the complex network of inflammation and the relation to all‐cause mortality in KTRs are scarce.
In end‐stage kidney disease the levels of general inflammatory markers (e.g., TNF, IL‐6, CRP) are increased. It has been reported that some inflammatory biomarkers regress and eventually normalize within the first two months after KT. 16 The surgical procedure per se induces a short transient inflammatory response. It has however been shown that a low‐grade systemic inflammation can persist in a proportion of KTRs. 16 , 17 Such low‐grade inflammation 10 weeks after transplantation has previously been postulated to play a role in the pathogenesis of post‐transplant diabetes mellitus (PTDM) 18 , 19 , 20 which is as an important risk factor for overall mortality after KT. 21 , 22 It has also been suggested that an inflammatory milieu plays a role in vascular calcification by stimulating fibrogenesis 23 , 24 being risk factors for death in chronic kidney disease patients. 25
The main goal of the study was to examine the effect of general systemic inflammation measured 10 weeks after KT on long‐term mortality, and for this purpose we analyzed a wide spectrum of markers reflecting different but overlapping inflammatory pathways. We also constructed composite inflammation scores representing different pathways of subclinical inflammation and investigated their associations with long‐term post‐transplant patient survival.
2. MATERIALS AND METHODS
2.1. Study population and design
The study population has previously been described by Witczak et al. 15 Patients who received a kidney transplant at the Norwegian national transplant center at Oslo University Hospital, Rikshospitalet between April 2007 and October 2012 were eligible for inclusion. In this period, a total of 1349 adult patients (18 years and older) underwent a kidney transplantation. After transplantation all patients were to perform a 10‐weeks surveillance investigation unless they had an ongoing infection, or a recent or ongoing acute rejection. Patients with early graft loss (n = 22) or other clinical reasons for not attending the 10‐weeks investigation (n = 136), as well as 147 patients who were not examined due to reduced lab accessibility in 2011 were excluded from analysis. The remaining 1044 (77.3%) KTRs had valid measurements for most of the specific inflammatory biomarkers and were included in the study, and among these 1001 patients had measures of all the biomarkers included in the overall inflammation score. Survival data were retrieved from the Norwegian Renal Registry on December 23rd, 2020. The study was approved by the Regional Ethics Committee in Norway and was performed in accordance with the Helsinki Declaration.
2.2. Immunosuppressive protocol
For standard immunological risk recipients, immunosuppressive therapy during the study period consisted of induction treatment with methylprednisolone and IL‐2 receptor antibody (basiliximab), and maintenance treatment with glucocorticoids, the cell proliferation inhibitor mycophenolate, and a calcineurin inhibitor (CNI). 15 Patient with known donor specific antibodies at time of transplantation or receiving an ABO‐incompatible transplant were classified as immunological high risk and received intravenous human immunoglobulin and rituximab, in addition to methylprednisolone and basiliximab, as induction therapy. Panel reactive antibody (PRA) positive (>20%) recipients were classified as immunological intermediate risk and received methylprednisolone and anti‐thymocyte globulin as induction treatment. At the beginning of the study period, tacrolimus was preferred to younger patients (<50 years), while cyclosporine (CsA) was prescribed to older patients (>50 years) and to patients with BMI above 30 kg/m2 or with preoperative impaired glucose tolerance. During the study period, the standard regimen was revised, and from January 2011, tacrolimus was preferred for all patients with the exception of patients with impaired glucose tolerance.
CNI trough concentrations for immunological standard‐risk recipients were tacrolimus 3–7 µg/L from day of engraftment, while for CsA the target was initially 200–300 µg/L and then gradually reduced to 75–125 µg/L after 6 months. For high and intermediate immunological risk recipients, initial trough levels were higher and tapered to 6–10 t µg/L (tacrolimus) and 150–250 µg/L (CsA) by 10 weeks after transplantation and throughout the first year. Mycophenolate mofetil 750 mg (540 mg mycophenolate sodium) twice daily was used in combination with tacrolimus and 1000 mg (720 mg) in combination with CsA. Prednisolone was tapered to 10 mg/day by week 5 in standard – risk recipients and by week 6–7 in high or intermediate risk recipients.
2.3. Measurement of inflammatory biomarkers
Blood samples were taken at the follow‐up visit 10 weeks after KT and were stored in a biobank at minus 80°C for research purposes. All analyses in this study were performed on these samples by measurement in plasma or serum by enzyme immunoassays (EIAs) from R&D Systems (Stillwater, MN). Intra‐ and inter‐assay coefficients of variation were <10% for all assays. The inflammatory biomarkers were chosen due to their relevance in post‐transplant complications, in particular metabolic disturbances, and morbidity and mortality in the general population (Table 1). We selected a wide specter of inflammatory biomarkers reflecting several different pathways including fibrogenesis, vascular inflammation and endothelial dysfunction, metabolic inflammation, angiogenesis, and leukocyte activity. The aim was to explore the subtle variation in the inflammatory response in order to identify if any pathways were more relevant in post‐transplant mortality.
TABLE 1.
Overview over the inflammation scores and their corresponding inflammatory and related biomarkers
| Inflammatory biomarkers | Description and functional aspects |
|---|---|
| Overall inflammation score | |
| Growth differentiation factor 15 (GDF−15) | GDF−15 is part of TGF‐beta‐family, and it plays a role in the extracellular matrix regulation. It is expressed in a broad variety of tissues. The molecule is associated with increased cardiovascular stress and inflammation, and it predicts risk of several diseases, including cardiovascular disease and development of CKD. Produced by macrophages and is associated with an inflammatory environment 31 , 32 , 33 |
| CXC chemokine ligand 16 (CXCL16) | CXCL16 also plays a role in the chemotaxis with recruitment of leukocytes to locations with inflammation, in particularly vascular inflammation. Mediator of the atherogenesis development 27 |
| Soluble tumor necrosis factor receptor 1 (sTNFR1) | General marker representing TNF activity. Increases during inflammation. Involved in innate immunity |
| Macrophage inhibitory factor (MIF) | Important regulator of innate immunity and is classified as an inflammatory cytokine. Regulated by several cytokines including TNF and sustains macrophage inflammatory functions. Associated with vascular dysfunction and graft loss 34 |
| Pentraxin 3 (PTX3) | PTX3 is an acute phase protein, it is a marker of activity of the innate immune system and is also in particular related to vascular inflammation. Produced and released by many cell types in response to primary inflammatory signals such as IL−1 and TNF 35 |
| Tyrosine (Y), lysine (K), leucine (L)−40 (YKL40) | YKL40 is associated with inflammation and endothelial dysfunction among patients after kidney transplantation. Connected with overall and cardiovascular mortality in the non‐transplant population 9 , 36 |
| Granulysin | Granulysin is expressed in CD8+ T‐lymphocytes, NK‐cells, and to a lesser extent in CD4+ T‐lymphocytes. It has to main functions; 1) a cytotoxic antimicrobial effect against bacteria, parasites and fungi, and 2) involvement in the migration of immune cells as it is released from damaged immune cells and thus leads to chemotaxis 37 , 38 |
| Insulin‐like growth factor binding protein 1 (IGFBP1) | Regulates IGF−1 bioactivity, glucose homeostasis, and tissue regeneration. Increases during inflammation |
| Periostin | Periostin is associated with ECM remodeling. Associated with renal fibrosis and chronic inflammatory diseases such as asthma, atopic dermatitis etc. 39 |
| Neutrophil gelatinase‐associated lipocalin (NGAL) | Is possibly related to an inflammatory response involved in CVD. It is also used as a biomarker of acute kidney injury 40 |
| Terminal 5b−9complement complex (TCC) | Biomarker for complement system activity. Important part of the innate immune system. Associated with a proinflammatory environment |
| Fibrogenesis | |
| GDF−15 | Described above |
| Syndecan | Involved in fibrosis processing and is associated with renal function. There are reports connecting the molecule to an inflammatory state 41 |
| Osteopontin (OPN) | Involved in calcification and inflammatory processes. Related to several metabolic and vascular outcomes 42 |
| Cathepsin S (CatS) | Associated with IMT in CKD and could be associated with several vascular and metabolic outcomes. Induces CCL2‐expression. Produced in response to inflammatory stimuli 43 |
| Periostin | Described above |
| General/vascular inflammation | |
| CXCL16 | Described above |
| sTNFR1 | Described above |
| PTX3 | Described above |
| TCC | Described above |
| Metabolic inflammation | |
| IGFBP1 | Described above |
| Resistin | Adipokine, could be related to vascular and metabolic outcomes, and is believed to play a regulatory role in several inflammatory diseases 44 |
| Insulin‐like growth factor 1 (IGF−1) | Reduced by inflammation. Insulin sensitivity and vasculoprotective factor |
| Chemerin | Linked to renal function, obesity, glucose tolerance and hyperlipidemia. Have been shown to correlate with an inflammatory state 45 |
| Growth and angiogenesis | |
|
Growth arrest‐specific gene 6 (GAS6) Receptor tyrosine kinase 6 (AXL6) |
Relationship between GAS6 and TAM receptors. Involved in vascular inflammation and several kidney diseases. Can have both pro‐ and anti‐inflammatory effects 46 |
| Endothelial protein C receptor (EPCR) | Enhances anticoagulation by accelerating the activation of protein C to activated protein C and mediates anti‐inflammatory effects 47 |
| Leukocyte activity | |
| MIF | Described above |
| Granulysin | Described above |
| NGAL | Described above |
| YKL−40 | Described above |
| Predictive post‐hoc inflammation score | |
| CXCL16 | Described above |
| GDF−15 | Described above |
| Granulysin | Described above |
| IGFBP1 | Described above |
| Biomarkers only tested individually | |
| Insulin‐like growth factor binding protein 3 (IGFBP3) | IGFBP3 is involved in ECM regulation, and is induced by inflammatory cytokines. In patients with RA, it has been shown that IGFBP3 suppresses the production of proinflammatory cytokines by reducing the NF‐kappa‐B activity. Regulator of IGF‐signaling 48 , 49 |
CKD, chronic kidney disease; CVD, cardiovascular disease; ECM, extracellular matrix remodeling; IMT, interna‐media thickness; IL, interleukin; NK, natural killer; RA, rheumatoid arthritis; TAM, Tyro3, Axl and MER tyrosine receptor kinases; TGF, transforming growth factor; TNF, tumor necrosis factor.
2.4. Inflammation scores
Detailed information about the biomarkers included for each score is presented in Table 1. Seven composite inflammation scores were constructed based on the statistical principles used by Bonaccio et al. for the INFLA‐score in the Moli‐sani study. 1 Deciles were generated for each biomarker. Values within the four highest deciles scored 1 to 4, while the four lowest deciles scored −4 to −1. The fifth and sixth deciles scored 0 points. The scores for each inflammatory biomarker were summed up, and the total score was then divided into quartiles for survival analyses. In addition to an overall inflammation score including 11 biomarkers, we studied five pathway‐specific inflammatory scores representing increased fibrogenesis, general/vascular inflammation, metabolic inflammation, growth/angiogenesis, and leukocyte activation. The inflammation scores were assessed as both categorical and continuous variables. One biomarker, IGFBP3, was only tested as an individual biomarker and was not included in any of the composite scores to avoid covariance bias in the pathway analyses due to its close relationship with IGF‐1. Based on the independent biomarker analyses, we constructed a post‐hoc inflammation score including the biomarkers with the largest effect estimates to test its predictive ability (IGFBP1, CXCL16, GDF‐15, and Granulysin).
2.5. Statistical analyses
All statistical analyses were performed by using IBM SPSS Statistics 27, except of Kaplan‐Meier Plots that were made using StataCorp Stata/SE 16.0. Differences in baseline characteristics between the inflammatory groups were tested by One‐Way ANOVA for continuous data, and Pearsons Chi‐square tests were used for categorical data. Kaplan‐Meier plots were created for the different inflammation scores, and Log‐Rank tests were used to test differences in survival rates. Cox proportional hazard regression analyses were performed to examine the independent association for each inflammatory score with all‐cause mortality. The models were adjusted for age, body mass index, estimated glomerular filtration rate (eGFR), sex, pretransplant diabetes mellitus (DM), PTDM, smoking status, type of CNI, dialysis vintage, deceased/living donor, and immunological risk. Time zero in the survival analyses was date of transplant. The inflammation scores were tested both as continuous and categorical variables, and in the analyses with the categorical variable the group with lowest inflammation score (first quartile) was used as reference value. We also performed analyses on 5‐year mortality. The proportional‐hazards assumption was tested by PH‐Tests. Additional stratified Cox regression analyses were performed for KTRs below and above 65 years of age, for patients with or without DM (both pretransplant DM and PTDM), and for patients with autoimmune/inflammatory vs. non‐inflammatory causes of end‐stage renal disease (ESRD). We also performed Cox models adjusted for delayed graft function (DGF) (only on patients transplanted between 2009–2012). All of the inflammatory biomarkers were tested both independently and all together in the same multivariable model, and the values were included in separate models both as their true and standardized value. The values were standardized by dividing the true value by the standard deviation. We constructed a post‐hoc inflammation score consisting of the four biomarkers with the highest HRs per standard deviation in the analysis involving all biomarkers and tested it in the same Cox regression model. Predictive factors for being in the upper overall inflammation score group (fourth quartile) were explored using multivariable logistic regression models. Inverse probability of treatment weighing (IPTW) analysis, with logarithmic treatment model, was performed to examine the effect of cyclosporine and tacrolimus on the probability of being in the upper quartile of the overall inflammation score.
3. RESULTS
3.1. Study population
Descriptive baseline characteristics are presented in Table 2. The median follow‐up time was 10.3 years (interquartile range [IQR] 8.5 to 11.8 years), and a total of 312 (29.9%) patients died during the study period. Median time to death was 6.4 years (IQR 3.9 to 8.9 years). The sum of the overall inflammation score varied from −39 to 38 (see Methods for score definition). CVD was the cause of death in 100 (32.1%) patients, 82 (26.3%) patients died of infections, and 130 (41.7%) died of malignancy and other causes. The relative frequencies of death cause per inflammation score percentiles are presented in Table 2. During the study period we experienced 409 (39.2%) overall graft losses. Of these, 144 (35.2%) were death‐censored graft loss.
TABLE 2.
Demographic and baseline data according to quartiles of the overall inflammation score
| Quartiles of the overall inflammation score | ||||||
|---|---|---|---|---|---|---|
| All patients | First quartile (−39 to −11) | Second quartile (−10 to −1) | Third quartile (0 to 9) | Fourth quartile (10 to 38) | p‐value | |
| Number (%) | 1044 | 240 (24.0%) | 250 (25.0%) | 258 (25.8%) | 253 (25.3%) | – |
| Age (years) | 52.2 (14.4) | 43.8 (14.3) | 50.7 (13.5) | 54.4 (13.0) | 59.6 (11.9) | <.001 |
| Sex (male) | 718 (68.8%) | 149 (62.0%) | 171 (68.4%) | 186 (72.1%) | 180 (71.4%) | .068 |
| BMI (kg/height2) | 25.4 (6.8) | 24.5 (3.6) | 26.1 (11.5) | 25.6 (4.2) | 25.5 (4.5) | .068 |
| Weight (kg) | 77.0 (15.7) | 73.8 (14.6) | 77.8 (16.0) | 78.2 (15.1) | 78.0 (16.7) | .03 |
| Current smoker (%) | 217 (20.8%) | 40 (16.7%) | 53 (21.3%) | 63 (24.4%) | 49 (19.4%) | .353 |
| Dialysis vintage (months) | 13.3 (15.2) | 10.0 (13.9) | 10.7 (13.7) | 14.2 (15.4) | 18.3 (16.3) | <.001 |
| Deceased donor (%) | 706 (67.6%) | 128 (53.3%) | 151 (60.4%) | 194 (75.2%) | 204 (80.1%) | < .001 |
| Immunological high risk a | 79 (7.6%) | 15 (20.5%) | 21 (28.8%) | 21 (28.8%) | 16 (21.9%) | .686 |
| Delayed graft function b | 59 (8.4%) | 6 (10.2%) | 6 (10.2%) | 15 (25.4%) | 32 (54.2%) | <.001 |
| Prednisolon dose (mg) | 11.2 (4.4) | 11.0 (4.2) | 11.6 (4.7) | 11.3 (4.6) | 11.1 (3.8) | .377 |
| CNI: | <.001 | |||||
| Tacrolimus (%) | 567 (54.3%) | 175 (73.5%) | 140 (56.0%) | 121 (47.5%) | 106 (42.1%) | |
| Cyclosporine (%) | 444 (42.5%) | 57 (23.9%) | 105 (42.0%) | 125 (49.0%) | 140 (55.6%) | |
| Other (%) | 27 (2.6%) | 6 (2.5%) | 5 (2.0%) | 9 (3.5%) | 6 (2.4%) | |
| Cyclosporine conc (μg/l) | 153.2 (57.8) | 130.1 (47.5) | 152.2 (56.5) | 153.5 (59.3) | 163.3 (59.1) | .005 |
| Tacrolimus conc (μg/l) | 7.0 (2.2) | 7.0 (2.3) | 6.9 (2.0) | 7.1 (2.0) | 6.8 (2.4) | .737 |
| Type 1 DM (%) | 99 (9.5%) | 14 (5.8%) | 17 (6.8%) | 37 (14.3%) | 27 (10.7%) | <.001 |
| Type 2 DM (%) | 120 (11.5%) | 16 (6.7%) | 25 (10.0%) | 28 (10.8%) | 47 (18.6%) | <.001 |
| PTDM (%) | 72 (6.9%) | 17 (7.1%) | 8 (3.2%) | 21 (8.1%) | 24 (9.5%) | <.001 |
| Creatinine (umol/l) | 120.5 (39.8) | 101.7 (25.0) | 112.0 (28.7) | 123.0 (36.0) | 144.6 (50.7) | <.001 |
| eGFR (ml/min/1.73m2) | 61.3 (21.2) | 73.9 (18.3) | 64.7 (18.7) | 58.2 (19.5) | 48.6 (20.2) | <.001 |
| Graft loss (%) | 409 (39.2%) | 40 (10.2%) | 79 (20.2%) | 106 (27.0%) | 167 42.6%) | <.001 |
| Death‐censored graft loss (%) | 144 (13.8%) | 22 (16.2%) | 28 (20.6%) | 38 (27.9%) | 48 (35.3%) | .008 |
| Number of deceased (%) | 312 (29.9%) | 20 (6.6%) | 55 (18.2%) | 83 (27.5%) | 144 (47.7%) | <.001 |
| Causes of death: | .025 | |||||
| CVD (%) | 100 (32.1%) | 8 (8.3%) | 15 (15.6%) | 32 (33.3%) | 41 (42.7%) | |
| Infections (%) | 82 (26.3%) | 3 (3.7%) | 11 (13.5%) | 21 (25.9%) | 46 (56.8%) | |
| Other causes (%) | 139 (41.7%) | 9 (7.2%) | 29 (23.2%) | 30 (24.0%) | 57 (45.6%) | |
The numbers are presented as means with belonging standard deviation for continuous variables, and total numbers and percentage for the categorical values. Because of some missing values the numbers do not add up to 100%. Cyclosporine and tacrolimus concentrations are trough levels. eGFR is based on the CKD‐EPI equation. Differences in continuous variables were tested with one‐way ANOVA, and differences in categorical variables were tested with Pearsons Chi‐square tests.
Immunological high risk was defined as one of either: PRA >20%, ABO‐incompatible transplantation, or more than two prior kidney transplants.
Structured data on delayed graft function was only available from 2009 (704 patients).
3.2. Long‐term outcomes
3.2.1. Overall inflammation scores and mortality
Table 3 and Figure 1 illustrate the association between the overall inflammation score and all‐cause mortality adjusted for traditional risk factors. An increased inflammation score was positively associated with mortality, and the risk increased with higher levels of inflammation (second quartile HR: 2.35, third quartile HR: 2.52, fourth quartile HR: 4.67, Table 3, Figure 1). Age, dialysis vintage, current smoking, DM prior to transplantation, and PTDM were also independently associated with increased risk of death, while deceased donor, BMI, sex, eGFR and immunological high risk were not (Table 3). The overall inflammation score was also tested as a continuous variable in the Cox regression model, and it showed a HR of 1.045 (95% CI: 1.033–1.056, p < .001). In the analyses considering five‐year overall mortality, the same pattern was present for both the overall inflammation score as a categorical variable (second quartile HR 3.52, p = .025, third quartile HR 2.61, p = .077, fourth quartile HR 6.02, p < .001), and as a continuous variable (HR 1.046, p < .001).
TABLE 3.
Cox regression model. Association between classical risk factors and the quartiles of overall inflammation scores and all‐cause mortality
| Variables | Hazard ratio | 95% CI for hazard ratio | p‐value |
|---|---|---|---|
| Age (years) | 1.078 | 1.064–1.091 | <.001 |
| BMI (kg/height2) | 0.983 | 0.956–1.012 | .253 |
| Sex (male) | 1.086 | 0.838–1.408 | .532 |
| eGFR (ml/min/1.73m2) | 1.003 | 0.996–1.010 | .398 |
| Dialysis vintage (months) | 1.015 | 1.008–1.022 | <.001 |
| Cyclosporine (yes) | 0.829 | 0.634–1.083 | .168 |
| Deceased donor (yes) | 1.366 | 0.988–1.890 | .059 |
| Current smoker (yes) | 2.265 | 1.738–2.952 | <.001 |
| Pretransplant DM (yes) | 1.383 | 1.039–1.842 | .026 |
| PTDM (yes) | 1.697 | 1.162–2.476 | .006 |
| Immunological high risk a | 1.113 | 0.665–1.863 | .684 |
| Overall inflammation score | |||
| Second quartile | 2.350 | 1.395–3.958 | .001 |
| Third quartile | 2.535 | 1.522–4.222 | <.001 |
| Fourth quartile | 4.713 | 2.845–7.810 | <.001 |
Inflammation score as quartiles, based on values of GDF‐15, CXCL16, sTNFR1, MIF, PTX3, Ykl40, granulysin, IGFBP1, perisotin and NGAL. First quartile of the overall inflammation score as reference value.
Abbreviation: DM, diabetes mellitus.
Immunological high risk was defined as one of either: PRA >20%, ABO‐incompatible transplantation, or more than two prior kidney transplants.
FIGURE 1.
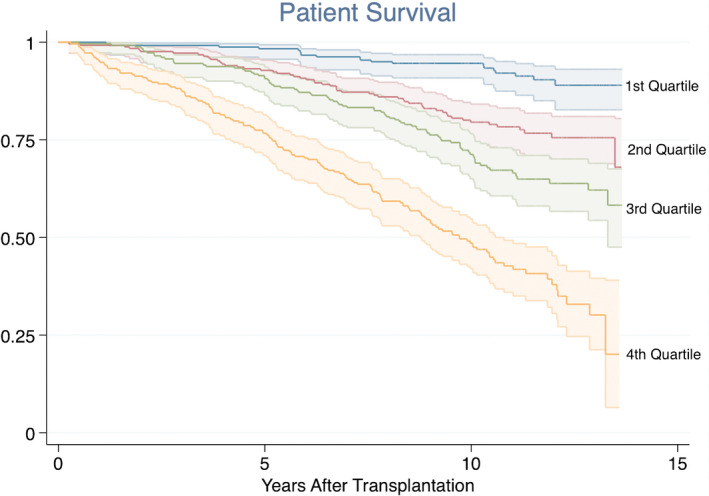
Kaplan‐Meier plots showing the association between the overall inflammation score and all‐cause mortality. Log‐rank test: p < .001
In stratified Cox regression analyses for KTRs <65 years (n = 773), KTRs within the second quartile had a HR of 2.62 (95% CI: 1.35–5.08), the third quartile 2.66 (95% CI: 1.39–5.12), and the fourth quartile 5.17 (95% CI: 2.70–9.90). Among KTRs who were ≥65 years (n = 220), the second quartile had a HR of 2.27 (95% CI: 0.92–5.62), the third quartile 2.33 (95% CI: 0.99–5.52), and the fourth quartile 4.00 (95% CI: 1.69–9.46). Age, dialysis vintage, current smoking, pretransplant DM, and PTDM were associated with increased mortality rate in the <65 group, while pretransplant DM and PTDM were not significantly associated with mortality in the ≥65 group. Stratified Cox regression analyses were also performed for patients with DM (both pretransplant DM and PTDM), and the HR for KTRs within the second quartile was 2.99 (95% CI: 1.30–6.89), in the third quartile the HR was 1.54 (95% CI: 0.68–3.50), and in the fourth quartile the HR was 3.65 (95% CI: 1.64–8.15) These results were similar to the non‐diabetic population. Finally, we performed Cox regression analyses in patients with autoimmune/inflammatory (second quartile HR 2.67, p = .003, third quartile HR 2.45, p = .006, fourth quartile HR 4.40, p < .001) and non‐inflammatory causes of ESRD (second quartile HR 1.75, p = .240, third quartile HR 2.14, p = .087, fourth quartile HR 4.67, p < .001). The analysis adjusted for DGF were nearly identical as the main model (second quartile HR 2.34, third quartile HR 2.55, fourth quartile HR 4.79, p < .001).
3.2.2. Pathway‐specific inflammation scores and mortality
Increased pathway‐specific inflammatory scores were also positively associated with mortality (Table 4 and Figure 2). The strongest associations were found for fibrogenesis (HR 1.77–2.63) and general/vascular inflammation (1.19–2.70). The inflammation scores were also tested as continuous variables: the fibrogenesis score had a HR of 1.052 (95% CI: 1.030–1.075, p < .001), the general/vascular score had a HR of 1.069 (95% CI: 1.048–1.091, p < .001), the metabolic score had a HR of 1.036 (95% CI: 1.011–1.061, p = .005), growth/angiogenesis score had a HR of 1.031 (1.003–1.061, p = .030), and the leukocyte activity score had a HR of 1.049 (95% CI: 1.026–1.072, p < .001).
TABLE 4.
Cox regression analyses showing the associations between the pathway‐specific inflammatory scores and long‐term all‐cause mortality with the first quartile as reference category
| Pathway‐specific inflammation scores | Hazard ratios (95% CI, p‐value) | ||
|---|---|---|---|
| Second quartile | Third quartile | Fourth quartile | |
| Fibrogenesis (n = 948) | 1.78 (1.10–2.85, p = .018) | 1.93 (1.20–3.10, p = .007) | 2.65 (1.65–4.25, p < .001) |
| Vascular/general inflammation (n = 1022) | 1.19 (0.79–1.79, p = .403) | 1.92 (1.30–2.86, p = .001) | 2.70 (1.83–3.99, p < .001) |
| Metabolic inflammation (n = 953) | 1.63 (1.13–2.35, p = .009) | 1.48 (1.04–2.11, p = .031) | 1.64 (1.12–2.40, p = .011) |
| Growth/angiogenesis (n = 1025) | 1.58 (1.13–2.21, p = .008) | 1.25 (0.88–1.76, p = .217) | 1.51 (1.07–2.14, p = .020) |
| Leukocyte activation (n = 1020) | 1.52 (1.04–2.21, p = .029) | 1.69 (1.15–2.48, p = .007) | 2.03 (1.39–2.97, p < .001) |
Multivariable Cox regression model adjusted for sex, age, BMI, eGFR, CNI‐type, current smoking, pretransplant DM and PTDM, dialysis vintage, and immunological risk a category. First quartile as reference value.
Immunological high risk was defined as one of either: PRA >20%, ABO‐incompatible transplantation, or more than two prior kidney transplants.
FIGURE 2.
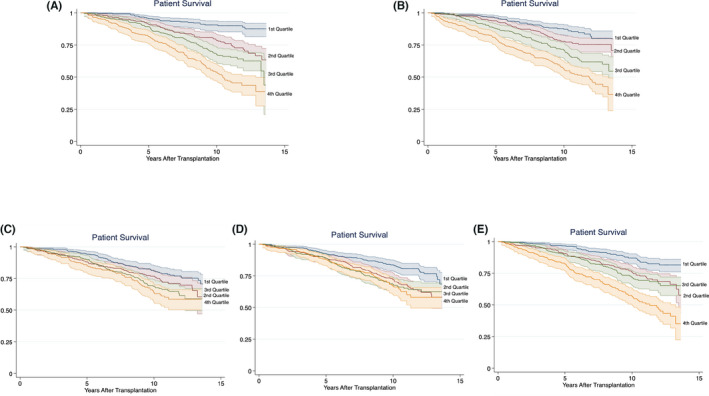
Kaplan‐Meier plot showing the association between pathway‐specific inflammation scores and long‐term all‐cause mortality. (A) Fibrogenesis score and mortality. Log‐rank: p < .001. (B) General/vascular inflammation score and mortality. Log‐rank: p < .001. (C) Metabolic inflammation score and mortality. Log‐rank: p < .001. (D) Growth/angiogenesis score and mortality. Log‐rank: p < .001. (E) Leukocyte activation score and mortality. Log‐rank: p < .001
3.2.3. Independent biomarkers and mortality
Associations between all‐cause mortality and the individual inflammatory biomarkers were tested independently for each biomarker adjusted for traditional risk factors (both alone and together with all the other biomarkers in the same model [Table 5]). Six biomarkers showed a significant positive association in all analyses: Periostin (HR: 1.001, 95% CI: 1.000–1.002), IGFP1 (HR: 1.002, 95% CI: 1.000–1.003), GDF‐15 (HR 1.153, 95% CI: 1.047–1.283), Granulysin (HR: 1.090, 95% CI: 1.012–1.177), PTX3 (HR 1.053, 95% CI: 1.003–1.116) and CXCL16 (HR 2.786 95% CI: 1.542–5.034). None of the biomarkers showed a significant negative association. In the analyses where the biomarkers were tested alone, but adjusted for traditional biomarkers, 14 biomarkers were significantly associated with mortality, and IGFBP3 showed a significant negative association.
TABLE 5.
Cox regression models describing the associations between the individual biomarkers and all‐cause mortality
| Inflammatory biomarker | HR true value | HR standardized value a | p‐value | HR true value | HR standardized value b | p‐value |
|---|---|---|---|---|---|---|
| MIF | 1.019 | 1.123 | .021 | 1.010 | 1.062 | .292 |
| Periostin | 1.002 | 1.192 | .002 | 1.001 | 1.150 | .028 |
| IGFBP1 | 1.003 | 1.279 | <.001 | 1.002 | 1.170 | .011 |
| IGFBP3 | 0.858 | 0.877 | .031 | 0.884 | 0.900 | .159 |
| IGF−1 | 1.000 | 0.927 | .304 | 1.000 | 1.051 | .566 |
| Cathepisin S | 1.009 | 1.054 | .423 | 1.005 | 1.032 | .672 |
| Resistin | 1.007 | 1.087 | .149 | 0.997 | 0.968 | .680 |
| Osteopontin | 1.005 | 1.076 | .236 | 0.997 | 0.952 | .518 |
| AXL6 | 1.030 | 1.087 | .162 | 1.028 | 1.080 | .266 |
| GAS6 | 1.013 | 1.037 | .429 | 0.996 | 0.989 | .882 |
| GDF−15 | 1.214 | 1.297 | <.001 | 1.153 | 1.210 | .006 |
| Granulysin | 1.095 | 1.175 | .003 | 1.090 | 1.165 | .026 |
| PTX3 | 1.061 | 1.166 | <.001 | 1.053 | 1.145 | .049 |
| EPCR | 1.009 | 1.105 | .006 | 1.009 | 1.096 | .218 |
| sTNFR1 | 1.402 | 1.264 | <.001 | 0.977 | 0.984 | .845 |
| Syndecan | 1.028 | 1.120 | .014 | 0.982 | 0.929 | .448 |
| CXCL16 | 4.214 | 1.443 | <.001 | 2.786 | 1.299 | <.001 |
| YKL40 | 1.003 | 1.242 | <.001 | 1.001 | 1.103 | .120 |
| Chemerin | 1.002 | 1.136 | .034 | 1.001 | 1.052 | .481 |
| NGAL | 1.001 | 1.093 | .131 | 1.000 | 1.033 | .688 |
| TCC | 1.477 | 1.143 | .014 | 1.245 | 1.078 | .389 |
Cox regression model adjusted for: age, BMI, sex, dialysis vintage, eGFR, deceased donor, type of CNI, smoking status, pretransplant DM or PTDM, and immunological risk c .
Each biomarker was tested alone the model as both its true and standardized value (adjusted for the variables described above).
All biomarkers were included together in the model as both its true and standardized value (adjusted for the variables described above).
Immunological high risk was defined as one of either: PRA >20%, ABO‐incompatible transplantation, or more than two prior kidney transplants.
These associations were also investigated by converting the true value of each biomarker into standardized values. In these analyses, each increase equivalent of the standard deviation gave these HRs: Periostin HR: 1.150 (95% CI: 1.015–1.302), IGFBP1 HR: 1.170 (95% CI: 1.037–1.320), GDF‐15 HR: 1.210 (95% CI: 1.055–1.388), Granulysin HR: 1.165 (95% CI: 1.018–1.334), PTX3 HR: 1.145 (95% CI: 1.002–1.312), and CXCL16 HR: 1.299 (95% CI: 1.117–1.510).
3.2.4. Predictive model
The association between the post‐hoc inflammation score and long‐term mortality was tested in the same Cox regression model. The same pattern as in the overall inflammation score was present. HRs in the intermediate groups was 2.11 (95% CI: 1.27–3.50) and 3.04 (95% CI: 1.86–4.95) respectively, while the HR in the fourth quartile was 4.57 (95% CI: 2.80–7.48) compared to the first quartile (Figure 3). In the analyses including the post‐hoc inflammation score as a continuous variable, the HR was 1.106 (95% CI: 1.078–1.134, p < .001).
FIGURE 3.
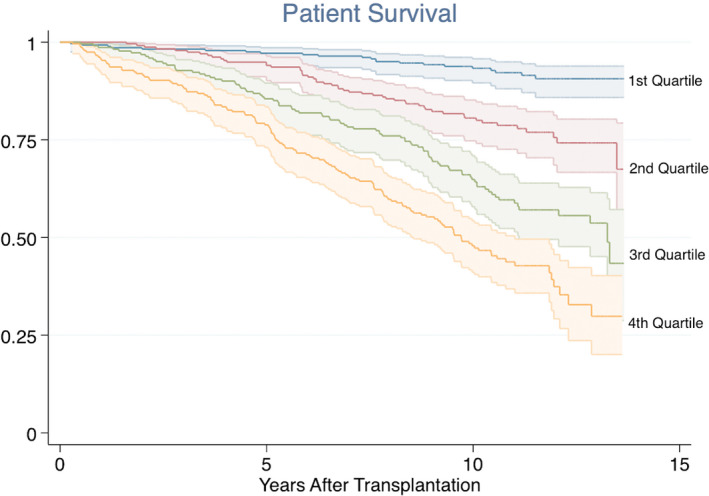
Kaplan‐Meier plot showing the association between the post‐hoc predictive inflammation score and all‐cause mortality. Log‐rank test: p < .001
3.3. Risk factor determination
We performed logistic regression models to evaluate risk factors for being in the upper overall inflammation score group. Age (OR: 1.03, 95% CI: 1.01–1.05), pretransplant DM (OR: 2.48, 95% CI: 1.64–3.75), BMI (OR: 0.96, 95% CI: 0.92–0.99), eGFR (OR: 0.96, 95% CI: 0.95–0.97), and dialysis vintage (OR: 1.02, 95% CI: 1.01–1.03) at baseline showed a significant association, while PTDM, type of CNI, deceased donor, sex, donor age, and smoking status were not significantly associated. Based on the IPTW analysis, the use of cyclosporine compared to tacrolimus did not increase the probability of being in the upper quartile of the overall inflammation score (coefficient 0.19, 95% CI [−0.18 to 0.22], p = .855).
4. DISCUSSION
The mortality rate after KT is higher than what is seen in the general population. Traditional risk factors such as lipid levels, age and kidney function do not explain this discrepancy alone. 11 , 12 In this single center study, we have described a significant association between a subclinical inflammatory environment early after kidney transplantation and all‐cause mortality.
Low‐grade systemic inflammation, assessed by an overall inflammation score consisting of 11 biomarkers, showed a 5‐fold increased risk of mortality for patients in the upper quartile, and a 2‐ to 3‐fold increased risk for patients in the two middle quartiles, compared to patients in the lowest quartile. The same pattern was also present when the score was assessed as a continuous variable. The mortality risks in the two middle quartiles were greater than previously reported risks associated to smoking and PTDM. 22 , 26 Potential underlying specific pathways were associated to mortality and increased fibrogenesis and general/vascular inflammation showed the highest effect estimates. These pathways are clearly relevant in relation to KT and long‐term outcome. Combination of the biomarkers with largest effect estimates in a predictive post‐hoc inflammation score was reflective of the overall inflammation score, highlighting the importance of inflammation per se rather than specific pathways. CVD and infectious diseases were the most common causes of death, but the relative frequencies of death causes were similar in patients in the upper and lower inflammation cohorts throughout.
Our findings suggest that the importance of inflammation among KTRs is significant, both statistically and clinically. The results are in line with observations in the non‐transplant population, and these findings align with results on compliment activation and mortality. 15 Typically, the association between inflammation and mortality has been explored by means of single biomarkers. In our study, a wide spectrum of biomarkers was tested independently and as part of composite scores. In all analyses, the association with long‐term mortality was strong. KTRs comprise a heterogenous group of patients, and their inflammatory state can be difficult to determine by traditional inflammatory markers due to the use of immunosuppressive drugs. At the time of sampling for biomarker analyses, the included patients had no clinical signs of any acute infections or other inflammatory conditions. In spite of this, analyses of inflammatory biomarkers revealed a proportion of patients with subclinical systemic inflammation. There were no differences between cohorts of patients with autoimmune/inflammatory and non‐inflammatory causes of ESRD. By using composite inflammation scores, in particular the overall inflammation score and the predictive post‐hoc inflammation score, it may be possible to describe the effect of inflammation on long‐term mortality in KTRs and thereby identify patients with increased risk. However, prospective studies are needed to confirm these findings.
By looking at the inflammatory biomarkers independently, CXCL16 emerges as a single factor with possible potential as a prognostic marker. CXCL16 plays a role in the recruitment of leukocytes into inflamed tissue, and it is in particular linked to vascular inflammation. 27 In the non‐transplant population, CXCL16 has been described as a risk factor for CVD and mortality following acute cardiovascular events. 28 , 29 CXCL16 has been suggested to be involved in the recruitment of T‐cells to the kidney, potentially involved in the pathogenesis of inflammatory kidney diseases. The present study is however, to the best of our knowledge, the first report of CXCL16 as marker of mortality following KT.
The proportion of patients using tacrolimus appears to be lower among patients in the upper inflammation score groups, whereas the proportion of cyclosporine users increases with increased inflammation scores. There were no significant differences in respective trough levels of tacrolimus and cyclosporine between the groups. One possible explanation could be that tacrolimus has a more pronounced immunosuppressive effect compared to cyclosporine. 30 It should though be kept in mind that our immunosuppressive protocol warranted that patients with increased risk of developing PTDM were more likely to get cyclosporine in this time period. Both type 2 DM and PTDM are associated with an inflammatory milieu. 18 However, in the Cox regression models, CNI type did not influence the results. These analyses also suggests that the main effect of PTDM on mortality is independent of inflammation.
Strengths of the study are that it is a single center study with a uniform follow‐up program. Consequently, even though KTRs in general are a heterogenous group, these patients have received the same short‐ and long‐term post‐transplant follow‐up. Furthermore, the number of patients included in the study is large (>1000), and dropouts are virtually non‐existent. All of the patients measured the biomarkers at a defined time point. By using composite inflammation scores the data are less sensitive to outliers. The most important limitation is the observational study design. Even though we have adjusted for known potential confounders in the analyses, the study is of observational character and residual confounding is most likely present. In addition, complete comorbidity data was not available for all patients. The renal clearance of the biomarkers is not well characterized. We have assumed that these variations do not affect the distributions of biomarkers, but this is a potential limitation, although eGFR was not shown to be a significant explanatory variable in the multivariable analyses.
These findings are associative; however, they could have potential consequences for future follow‐up strategies and studies. The inflammation scores’ predictive abilities on identification of patients at risk could be exploited with for example closer monitoring, more aggressive antihypertensive treatment, and/or lower cholesterol‐ and HbA1c target levels. The results also give insight into why KTRs have high long‐term mortality rates, and this could be targeted in future studies on for instance different immunosuppressive regimes for patients with various grades of inflammation.
In conclusion, our results strongly suggest that an inflammatory environment in the early period after kidney transplantation is an important risk factor for long‐term mortality and an underlying factor for the high mortality rates in KTRs. No specific inflammatory pathway stood out as causative, suggesting that general inflammation is most important.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
ACKNOWLEDGMENTS
The study was financed through a testamentary grant given by the University of Oslo and Telemark Hospital Trust.
Heldal TF, Åsberg A, Ueland T, et al. Inflammation in the early phase after kidney transplantation is associated with increased long‐term all‐cause mortality. Am J Transplant. 2022;22:2016–2027. doi: 10.1111/ajt.17047
DATA AVAILABILITY STATEMENT
The data are available on request from the corresponding author. However, the data are not publicly available due to privacy restrictions.
REFERENCES
- 1. Bonaccio M, Di Castelnuovo A, Pounis G, et al. A score of low‐grade inflammation and risk of mortality: prospective findings from the Moli‐sani study. Haematologica. 2016;101(11):1434‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta‐analyses. BMJ. 2000;321(7255):199‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engström G, Hedblad BO, Stavenow L, et al. Incidence of obesity‐associated cardiovascular disease is related to inflammation‐sensitive plasma proteins: a population‐based cohort study. Arterioscler Thromb Vasc Biol. 2004;24(8):1498‐1502. [DOI] [PubMed] [Google Scholar]
- 4. Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003;169(2):203‐214. [DOI] [PubMed] [Google Scholar]
- 5. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C‐reactive protein, albumin, or leukocyte count with coronary heart disease: meta‐analyses of prospective studies. JAMA. 1998;279(18):1477‐1482. [DOI] [PubMed] [Google Scholar]
- 7. Schnabel RB, Yin X, Larson MG, et al. Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol. 2013;33(7):1728‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Jager J, Dekker JM, Kooy A, et al. Endothelial dysfunction and low‐grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler Thromb Vasc Biol. 2006;26(5):1086‐1093. [DOI] [PubMed] [Google Scholar]
- 9. Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL‐40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol. 2010;143(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 10. Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16(8):1545‐1549. [DOI] [PubMed] [Google Scholar]
- 11. Jardine AG, Gaston RS, Fellstrom BC, Holdaas H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet. 2011;378(9800):1419‐1427. [DOI] [PubMed] [Google Scholar]
- 12. Kiberd B, Panek R. Cardiovascular outcomes in the outpatient kidney transplant clinic: the Framingham risk score revisited. Clin J Am Soc Nephrol. 2008;3(3):822‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ducloux D, Kazory A, Chalopin JM. Predicting coronary heart disease in renal transplant recipients: a prospective study. Kidney Int. 2004;66(1):441‐447. [DOI] [PubMed] [Google Scholar]
- 14. Abedini S, Holme I, März W, et al. Inflammation in renal transplantation. Clin J Am Soc Nephrol. 2009;4(7):1246‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Witczak BJ, Pischke SE, Reisæter AV, et al. Elevated terminal C5b–9 complement complex 10 weeks post kidney transplantation was associated with reduced long‐term patient and kidney graft survival. Front Immunol. 2021;12(4391). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simmons EM, Langone A, Sezer MT, et al. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end‐stage renal disease patients. Transplantation. 2005;79(8):914‐919. [DOI] [PubMed] [Google Scholar]
- 17. Cueto‐Manzano AM, Morales‐Buenrostro LE, González‐Espinoza L, et al. Markers of inflammation before and after renal transplantation. Transplantation. 2005;80(1):47‐51. [DOI] [PubMed] [Google Scholar]
- 18. Heldal TF, Ueland T, Jenssen T, et al. Inflammatory and related biomarkers are associated with post‐transplant diabetes mellitus in kidney recipients: a retrospective study. Transpl Int. 2018;31(5):510‐519. [DOI] [PubMed] [Google Scholar]
- 19. Kim YG, Ihm C‐G, Lee TW, et al. Association of genetic polymorphisms of interleukins with new‐onset diabetes after transplantation in renal transplantation. Transplantation. 2012;93(9):900‐907. [DOI] [PubMed] [Google Scholar]
- 20. Kim JS, Kim SK, Park JY, et al. Significant association between toll‐like receptor gene polymorphisms and posttransplantation diabetes mellitus. Nephron. 2016;133(4):279‐286. [DOI] [PubMed] [Google Scholar]
- 21. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178‐185. [DOI] [PubMed] [Google Scholar]
- 22. Valderhaug TG, Hjelmesæth J, Hartmann A, et al. The association of early post‐transplant glucose levels with long‐term mortality. Diabetologia. 2011;54(6):1341‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Czaya B, Faul C. FGF23 and inflammation‐a vicious coalition in CKD. Kidney Int. 2019;96(4):813‐815. [DOI] [PubMed] [Google Scholar]
- 24. Sotomayor CG, Te Velde‐Keyzer CA, de Borst MH, Navis GJ, Bakker SJL. Lifestyle, inflammation, and vascular calcification in kidney transplant recipients: perspectives on long‐term outcomes. J Clin Med. 2020;9(6):1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seiler S, Heine GH, Fliser D. Clinical relevance of FGF‐23 in chronic kidney disease. Kidney Int Suppl. 2009;114:S34–S42. [DOI] [PubMed] [Google Scholar]
- 26. Opelz G, Döhler B. Influence of current and previous smoking on cancer and mortality after kidney transplantation. Transplantation. 2016;100(1):227‐232. [DOI] [PubMed] [Google Scholar]
- 27. Lehrke M, Millington SC, Lefterova M, et al. CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. J Am Coll Cardiol. 2007;49(4):442‐449. [DOI] [PubMed] [Google Scholar]
- 28. Jansson AM, Aukrust Pål, Ueland T, et al. Soluble CXCL16 predicts long‐term mortality in acute coronary syndromes. Circulation. 2009;119(25):3181‐3188. [DOI] [PubMed] [Google Scholar]
- 29. Ueland T, Smedbakken LM, Hallén J, et al. Soluble CXCL16 and long‐term outcome in acute ischemic stroke. Atherosclerosis. 2012;220(1):244‐249. [DOI] [PubMed] [Google Scholar]
- 30. Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63(7):977‐983. [DOI] [PubMed] [Google Scholar]
- 31. Kempf T, Eden M, Strelau J, et al. The transforming growth factor‐beta superfamily member growth‐differentiation factor‐15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98(3):351‐360. [DOI] [PubMed] [Google Scholar]
- 32. Secchiero P, Corallini F, Gonelli A, et al. Antiangiogenic activity of the MDM2 antagonist nutlin‐3. Circ Res. 2007;100(1):61‐69. [DOI] [PubMed] [Google Scholar]
- 33. Ho JE, Hwang S‐J, Wollert KC, et al. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin Chem. 2013;59(11):1613‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonacina F, Baragetti A, Catapano AL, Norata GD. Long pentraxin 3: experimental and clinical relevance in cardiovascular diseases. Mediators Inflamm. 2013;2013:725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malyszko J, Koc‐Zorawska E, Malyszko J. YKL‐40, a marker of cardiovascular disease and endothelial dysfunction, in kidney transplant recipients. Transplant Proc. 2014;46(8):2651‐2653. [DOI] [PubMed] [Google Scholar]
- 37. Sparrow E, Bodman‐Smith MD. Granulysin: the attractive side of a natural born killer. Immunol Lett. 2020;217:126‐132. [DOI] [PubMed] [Google Scholar]
- 38. Deng A, Chen S, Li Q, Lyu SC, Clayberger C, Krensky AM. Granulysin, a cytolytic molecule, is also a chemoattractant and proinflammatory activator. J Immunol. 2005;174(9):5243‐5248. [DOI] [PubMed] [Google Scholar]
- 39. Izuhara K, Nunomura S, Nanri Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017;74(23):4293‐4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sivalingam Z, Larsen SB, Grove EL, Hvas AM, Kristensen SD, Magnusson NE. Neutrophil gelatinase‐associated lipocalin as a risk marker in cardiovascular disease. Clin Chem Lab Med. 2017;56(1):5‐18. [DOI] [PubMed] [Google Scholar]
- 41. Angsana J, Chen J, Smith S, et al. Syndecan‐1 modulates the motility and resolution responses of macrophages. Arterioscler Thromb Vasc Biol. 2015;35(2):332‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Icer MA, Gezmen‐Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17‐24. [DOI] [PubMed] [Google Scholar]
- 43. Wilkinson RD, Williams R, Scott CJ, Burden RE. Cathepsin S: therapeutic, diagnostic, and prognostic potential. Biol Chem. 2015;396(8):867‐882. [DOI] [PubMed] [Google Scholar]
- 44. Filková M, Haluzík M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin Immunol. 2009;133(2):157‐170. [DOI] [PubMed] [Google Scholar]
- 45. Lehrke M, Becker A, Greif M, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161(2):339‐344. [DOI] [PubMed] [Google Scholar]
- 46. van der Meer JH, van der Poll T, van ‘t Veer C. TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood. 2014;123(16):2460‐2469. [DOI] [PubMed] [Google Scholar]
- 47. Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32(Suppl 1):49‐60. [DOI] [PubMed] [Google Scholar]
- 48. Price WA, Moats‐Staats BM, Stiles AD. Pro‐ and anti‐inflammatory cytokines regulate insulin‐like growth factor binding protein production by fetal rat lung fibroblasts. Am J Respir Cell Mol Biol. 2002;26(3):283‐289. [DOI] [PubMed] [Google Scholar]
- 49. Lee H‐S, Woo SJ, Koh H‐W, et al. Regulation of apoptosis and inflammatory responses by insulin‐like growth factor binding protein 3 in fibroblast‐like synoviocytes and experimental animal models of rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):863‐873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on request from the corresponding author. However, the data are not publicly available due to privacy restrictions.


