Abstract
Some studies suggest a relationship between semen quality and pregnancy rates of assisted reproduction technologies (ART). Others have questioned the utility of semen quality as proxy for fertility in couples attempting to conceive with or without assistance. We aimed to investigate the current body of evidence which correlates semen parameters and clinical pregnancy among couples utilizing ART (i.e. in vitro fertilization [IVF], intracytoplasmic sperm injection [ICSI]) through a systematic review and meta‐analysis of cross‐sectional and retrospective cohort studies. Pooled Odd Ratio (OR) for oligo‐, astheno‐ and teratospermic compared to normospermic number of ART cycles were calculated among. Meta‐regression and sub‐group analysis were implemented to model the contribution of clinical/demographic and laboratory standards differences among the studies. Overall, 17 studies were analysed representing 17,348 cycles were analysed. Pooled OR for impaired sperm concentration, motility and morphology was 1 (95%Confidence Interval [CI]: 0.97–1.03), 0.88 (95%CI: 0.73–1.03) and 0.88 (95%CI: 0.75–1) respectively. Further analysis on sperm morphology showed no differences with regard of IVF versus ICSI (p = 0.14) nor a significant correlation with rising reference thresholds (Coeff: −0.02, p = 0.38). A temporal trend towards a null association between semen parameters and clinical pregnancy was observed over the 20‐year observation period (Coeff: 0.01, p = 0.014). The current analysis found no association between semen quality (as measured by concentration, motility or morphology) and clinical pregnancy rates utilizing ART. Future investigations are necessary to explore the association between semen parameters and other ART outcomes (e.g. fertilization, implantation, birth and perinatal health).
Keywords: ART cycles, assisted reproductive technologies, ICSI, IVF, semen parameters
1. INTRODUCTION
Infertility, defined as attempting to conceive for 12 months without success, affects about 15% of couples. Within these couples, a contributing male factor can be identified in 30%–50% of cases (Del Giudice, Busetto, et al., 2020; Kasman et al., 2020; Thoma et al., 2013; Del Giudice, Kasman, et al., 2020). Assisted reproductive technology (ART) is commonly utilized in overcoming male factor infertility. One of the most common methods of fertilization is intracytoplasmic sperm injection (ICSI), initially used in managing male factor infertility with severely impaired spermatogenesis (Palermo et al., 1992). Over time, ICSI has been employed in treating aetiologies beyond male factors (Hollingsworth et al., 2007; Ola et al., 2001). However, it has been suggested that semen quality may be associated with IVF success given that spermatogenic success may be associated with gamete quality (Farquhar & Marjoribanks, 2018).
Threshold values of semen parameters are conventionally based on the guidelines set by World Health Organization (World Health Organization, 2010) and are used to stratify men into fertile or subfertile categories (Verheyen et al., 1999). While some studies suggest a correlation between semen quality and pregnancy rates, others have questioned the utility of semen quality as proxy for fertility in couples attempting to conceive (Akanji Tijani & Bhattacharya, 2010; Boeri et al., 2021; van der Steeg et al., 2011; van Weert et al., 2004).
While oocyte quality is a major driver of ART outcomes (Del Giudice, Busetto, et al., 2020; Lopes et al., 1998; Sousa & Tesarik, 1994; Tesarik et al., 2002), the relationship between semen quality and ART outcomes also remains uncertain. The aim of the meta‐analysis and systematic review is to investigate the association of semen parameters and ART pregnancy rates.
2. MATERIALS AND METHODS
We followed previously established guidelines in designing this systematic review and meta‐analysis (Stroup et al., 2000). PICO criteria were used to establish a research question in the following fashion: what is the association of impaired semen quality and clinical pregnancy achieved by ART? Additionally, we sought to compare the current evidence within available cohort studies. More specifically, pooled OR were determined for oligo‐, astheno or teratospermic features in comparison with normospermic features among infertile couples undergoing ART.
2.1. Evidence acquisition
A systematic literature review was performed in the Embase, Cochrane and PubMed from the dates of inception to December 2020, without language restriction, in order to identify studies that have investigated sperm parameters and success rates among ART cycles for clinical pregnancy among infertile couples. The reference lists of these studies were also screened for additional relevant articles. We included and critically evaluated original observational retrospective or prospective cohort studies (Level of Evidence: III 1–2). Abstracts, meeting reports and case reports were excluded from the analysis. Search terms included but were not limited to assisted reproductive techniques or technologies or ART AND in vitro fertilization or IVF, AND intracytoplasmic sperm injection or ICSI AND male factor infertility AND semen quality or parameters AND fertility status or fertility impairment AND pregnancy rate or clinical pregnancy; secondary fields: sperm concentration; sperm motility; sperm morphology; normospermic; number ART cycles. Table S1 provides a comprehensive list of search terms used.
2.2. Study selection and inclusion criteria
Data included in the analysis were sourced original articles that examined infertile couples undergoing ART reporting clinical pregnancy outcomes, defined by detectable foetal heartbeat per number of cycles of IVF or ICSI. Only studies reporting descriptive statistics of subjects with oligo‐ or astheno‐ or teratozoospermia compared to control groups were further evaluated. Studies were considered eligible if no significant differences in the male and female demographics was relevant when assessing cases versus controls, and if results presented were controlled for female factors. Articles were excluded if the data on cycles from control group were not assessed or were not available from the article. The titles and abstracts of all articles were independently screened by three authors (FDG, AMK and TC) using predefined inclusion criteria. Three authors (FDG, FB and MLE) then independently examined the full manuscript of the articles to assess if inclusion criteria was met. All investigators agreed upon the decision for final inclusion.
Data were obtained from included studies using a standardized form: origin/year of study (institution and period of enrolment), study population size, mean/median age (± SD/range) of the participants and controls, semen parameters threshold references, sample size (impaired semen quality subgroups – total, impaired semen quality subgroups with clinical pregnancy; control group totals, controls with clinical pregnancy) and reference standards used to determine fertile matched control groups.
2.3. Study quality assessment and statistical analysis
The “Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies,” provided by the National Institute of Health (NIH) was used to assess the risk of bias (RoB). Biases screened for included selection bias, information bias, measurement bias or confounding bias (including cointerventions, differences at baseline in patient characteristics and other factors are presented in Table S2). Studies were rated as poor quality, fair or good, with higher risk of bias leading to poor quality (“‐”) ratings and low risk of bias leading to good quality (“+”) ratings (NIH Guidelines, 2015). Publication bias was tested for by both the determination of a p value using the Deeks’ asymmetry test and the visual assessment of the Deeks’ funnel plot (DerSimonian & Laird, 1986). The potential nature of studies “missed” in our review was explored using the Trim and Fill method (Duval & Tweedie, 2000). Pooled odds ratio (OR) and 95% Confidence Intervals (CI) were used to compare treatments. Evaluation for presence of heterogeneity (Higgins et al., 2003) was done using the following: (1) Higgins I2 test with inconsistency indexes (I 2): 0%–40%, minimal heterogeneity; 30%–60%, moderate heterogeneity; 50%–90%, substantial heterogeneity; and 75%–100%, considerable heterogeneity and (2) Cochran's Q‐test with p < 0.05 suggesting heterogeneity. Pooled OR estimates were calculated using a random‐effects model (Mantel & Haenszel, 1959). We graphically displayed our results as forest plots, with pooled ORs indicating overall odds in favour of infertile couples with or without at least one semen parameter impairment to achieve clinical pregnancy. Subgroup analysis was performed looking at differences in ART techniques (i.e. IVF vs. ICSI). Meta‐regression analysis was performed using available quantitative variables extracted from the studies to model the contribution of possible confounders to the aim of interest as well as to examine further potential sources of heterogeneity. ORs were charted against the following variables: publication year, mean paternal age at baseline and values of reference thresholds for sperm parameters. After generic OR estimates were obtained, they were plotted with the area of the circles proportional to the inverse of the squared standard errors of the studies included. Calculations were performed using Stata version 16.1 (Stata Corporation, College Station, TX, USA), and all tests were two‐sided, with p < 0.05 set as threshold for statistical significance.
3. RESULTS
3.1. Search results
The initial search yielded 256 articles (PubMed: 117; Web of Science: 33; Embase: 16; Cochrane: 9; and Scopus: 81). A total of 142 articles were excluded because they contained overlapping data or were that appeared in multiple databases. Of the remaining 114, 95 were subsequently excluded since they did not examine couple infertility and ART (n = 64), were not reporting information on semen parameters (n = 8), were focused on animal experiments (n = 17) or were editorials or review papers (n = 5). The remaining 20 journal references were then critically analysed and evaluated. After this in‐depth review, a further three did not meet the inclusion criteria due to missing exactable information. The remaining 17 studies were eligible for inclusion in our review (Fig. S1). No study was considered to be seriously flawed as per the “Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies.” There was moderately low risk to performance bias across all included studies. There was no risk of attrition bias due to incomplete outcome data in the included studies. Table S2 and Figure S1 illustrate the Individual RoB as well as visual assessment of the Deeks’ funnel plots.
3.2. Study location, types and sample size cohort
Table 1 summarizes the main findings, patient description and study characteristics of each article. Of the 17 studies included (Kruger et al., 1986; Grow et al., 1994; Nagy et al., 1995; Oehninger et al., 1995; Palermo et al., 1995; Ben‐Chetrit et al., 1995; Mansour et al., 1995; Lundin et al., 1997; Mercan et al., 1998; Osawa et al., 1999; Keegan et al., 2007; Dubey et al. 2007; Capelouto et al., 2018; Schachter‐Safrai et al. Mariappen et al., 2018; Chen et al., 2020; Kasman et al., 2021), eight took place within the United States, two in Europe (Belgium and Sweden), one in South Africa, one in Egypt, one in Japan, one in Israel, one in Australia, one in Canada and one in China (Table 1). Four studies investigated only the association of semen parameters and pregnancy rates in IVF. Ten studies investigated only semen parameters associations pregnancy rates in ICSI, while three studies investigated both techniques. Three papers included were cross‐sectional cohort studies, while the remaining 14 were retrospective cohort studies (Table 1).
TABLE 1.
Demographics and clinical characteristics of the 17 studies included in the systematic review and meta‐analysis
| Author | Year | Country | Study design (Level of Evidence) | Sample size (n. of cycles) | Average age (Years) | Technique | Population Description | Main findings |
|---|---|---|---|---|---|---|---|---|
| Kruger et al | 1986 | South Africa | Cross‐sectional cohort study (III‐2) | 190 | – | IVF | Women with bilateral tubal damage and their partners | Higher fertilization and pregnancy rate in men with normal sperm morphology >14%. |
| Grow et al | 1994 | USA | Retrospective cohort study (III‐2) | 316 | – | IVF |
Patients with previous total failure of fertilization or <7.5 × 105 total motile sperm |
Severe teratozoospermia yields a lower implantation rate and ongoing pregnancy rate. |
| Nagy et al | 1995 | Belgium | Cross‐sectional cohort study (III‐2) | 965 | 34.9 | ICSI | Patients with failed or very low (<10%) fertilization rate in the previous standard IVF cycle(s) or unsuitable sperm parameters for conventional IVF | ICSI can provide high normal fertilization, cleavage. |
| Oehninger et al | 1995 | USA |
Retrospective cohort study (III‐2) |
1163 | 35.4 | ICSI | Couples with male factor infertility associated with total failed fertilization in at least one previous attempt or unsuitable sperm parameters for conventional IVF | None of the sperm parameters of the original sperm analysis correlated with ICSI outcome. |
| Palermo et al | 1995 | USA | Retrospective cohort study (III‐2) | 227 | 36.2 | ICSI | From 29 September 1993 to 14 March 1994, assisted fertilization was in couples with long‐standing infertility | Sperm parameters do not clearly affect the outcome of ICSI. |
| Ben‐Chetrit et al | 1995 | Canada | Retrospective cohort study (III‐2) | 672 | ‐ | IVF | cycles of successful oocyte retrieval performed from 10 August 1992 to 31 December 1993 from couple with male factor infertility | Couples with severe male factor infertility should be considered for standard IVF, as long as adequate total motile sperm can be recovered. |
| Mansour et al | 1995 | Egypt | Retrospective cohort study (III‐2) | 1433 | – | ICSI | ICSI cycles was performed for the treatment of infertility due to male factor with different degrees of severity. |
The fertilization and pregnancy rates are not affected by different semen parameters as long as morphologically well‐shaped live sperms could be used. |
| Lundin et al | 1997 | Sweden | Retrospective cohort study (III‐2) | 622 | 32.8 | IVF +ICSI | Patients who participated in an IVF or ICSI programme during a 2‐year period. | Poor sperm morphology (<5% normal forms) is a factor that results in impaired fertilization. |
| Mercan et al | 1998 | USA | Cross‐sectional cohort study (III‐2) | 715 | 34.9 | ICSI | ICSI cycles and conventional IVF cycles performed between April 1994 and March 1996 | Couples undergoing ICSI with severe male infertility have a reduced fertilization rate than patients undergoing clinical ICSI and IVF with non‐male infertility. |
| Osawa et al | 1999 | Japan | Retrospective cohort study (III‐2) | 398 | 33.9 | IVF +ICSI |
cycles of IVF and ICSI between July 1995 and May 1997. Couples who underwent ICSI had previously failed standard IVF and/or had extremely low‐sperm parameters (<500,000 progressive motile spermatozoa/ml). |
The predominant abnormal form affects the ICSI outcome in the case of <4% normal forms. |
| Keegan et al | 2007 | USA | Retrospective cohort study (III‐2) | 518 | 37.7 | IVF +ICSI |
Couples were selected who experienced their first and/or second IVF cycle between 01 January 2002 and 21 December 2004, with at least one oocyte retrieved per cycle, semen analysis performed, and >2 million motile |
Isolated teratozoospermia generally does not impact on the major indices of IVF. |
| Dubey et al | 2008 | USA | Retrospective cohort study (III‐2) | 52 | 36.7 | IVF | Patients undergoing their first IVF–PGD cycle for idiopathic recurrent pregnancy loss or multiple failed IVF implantations between 01 January 2004 and 30 September 2006 | Results suggest that sperm morphology plays an important role in the outcome of IVF–PGD cycles. |
| Capelouto et al | 2018 | USA | Retrospective cohort study (III‐2) | 845 | 41.3 | ICSI | Patients undergoing IVF cycles from a private fertility clinic between 2008 and 2015. | Neither advancing male age, elevated BMI nor poor sperm quality were associated with outcomes in frozen donor oocyte IVF cycles in this study. |
| Schachter‐ Safrai et al | 2018 | Israel | Retrospective cohort study (III−2) | 332 | 34.8 | ICSI | Included couples after embryo transfer using fertilization by IMSI between January 2008 and May 2017. | IMSI procedure may be more efficient in severe compound sperm pathologies than in patients with one abnormal sperm parameter. |
| Mariappen et al | 2018 | Australia | Retrospective cohort study (III−2) | 1280 | ‐ | ICSI | Patients undergoing IVF treatment cycles from 1 April 2008 to 30 November 2017 conducted at one facility | The outcomes were not significantly influenced by semen parameters or male age with respect to the likelihood of clinical pregnancy or live birth. |
| Chen et al | 2019 | China | Retrospective cohort study (III‐2) | 3155 | 30.2 | ICSI |
Patients undergoing c‐IVF, early rescue ICSI (RICSI) treatment and follow‐up from January 2015 to May 2017 |
Normal sperm morphology rate <4% significantly increased the total fertilization failure rate but did not affect the clinical or neonatal outcomes. |
| Kasman et al | 2020 | USA |
Retrospective cohort study (III‐2) |
4517 | 37.7 | ICSI | Any female undergoing ART from January 2012 to December 2018 who had Available demographic and semen parameters | Sperm motility is associated with pregnancy rates, while other semen parameters are not. |
Abbreviations: ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization.
3.3. Study sample sizes and participant ages
In total, 17,348 cycles were analysed, with individual studies ranging from 190 cycles and 4,517. Mean ages of the men ranged from 30.2 to 41.3 years old. Some of the studies did not report subject ages. Three papers (Ben‐Chetrit et al., 1995; Mansour et al., 1995; Oehninger et al., 1995) included couples presenting with only male factor infertility, while two allowed only inclusion of couples with female factor infertility (Kasman et al., 2021; Kruger et al., 1986). The remaining studies included couples treated for both conditions.
3.4. Teratozoospermia and pregnancy rates
Thirteen studies (Capelouto et al., 2018; Chen et al., 2020; Grow et al., 1994; Keegan et al., 2007; Kruger et al., 1986; Lundin et al., 1997; Mansour et al., 1995; Mariappen et al., 2018; Mercan et al., 1998; Nagy et al., 1995; Oehninger et al., 1995; Osawa et al., 1999; Schachter‐Safrai et al., 2019) included in the cumulative synthesis reported outcomes on the association of teratozoospermia and pregnancy rates with a reference threshold for teratozoospermia which was 4% in eight studies (Capelouto et al., 2018; Chen et al., 2020; Grow et al., 1994; Mariappen et al., 2018; Mercan et al., 1998; Oehninger et al., 1995; Osawa et al., 1999; Schachter‐Safrai et al., 2019), 5% in three (Keegan et al., 2007; Lundin et al., 1997; Mansour et al., 1995) and 14% in two remaining studies (Kruger et al., 1986; Nagy et al., 1995). Odds ratio estimates ranged from 0.08 to 1.41 with OR <1 indicating lower pregnancy with teratospermia while >1 represents higher odds of pregnancy with teratospermia. The pooled OR estimate was equivalent under a fixed‐ or random‐effect model: 0.88 (95%CI: 0.75–1) Figure 1a. There was no evidence to support any meaningful heterogeneity between the studies: Q = 23.4 (d.f. = 12), p = 0.02; I 2 = 48.7% and the findings from the two models were not materially different. Because of this finding, we chose to present random‐effects models for all analyses. No single study was found to significantly affect the heterogeneity statistic when excluded from analysis. Furthermore, the funnel plot suggests that there was no small‐study effect (Egger test, p = 0.78), which supports the absence of publication bias. The “Trim and Fill” method suggests that no “missing” studies need to be included to remove any asymmetry from the funnel plot Figure S2a. At subgroup analysis of ART technique (i.e. IVF vs. IVF/ICSI), there was an association with semen morphology in case of IVF studies (OR, 0.59, 95%CI: 0.25–0.93; lower morphology associated with lower pregnancy rate) but not for ICSI (OR, 0.87, 95%CI: 0.73–1.02) (Figure 1b) in presence of a non‐significant test of groups differences (p = 0.14). On meta‐regression analysis, we found a positive correlation among more recent year of publication and decreasing effect size (Coeff: 0.01, SE: 0.004, p = 0.014) suggesting modern studies showing no association between morphology and pregnancy rate while older studies favoured a negative association (Fig. S3). In contrast, there were no significant trends between baseline men's age, the total number of cycles per study nor the reference threshold for teratozoospermia (Fig. S3).
FIGURE 1.
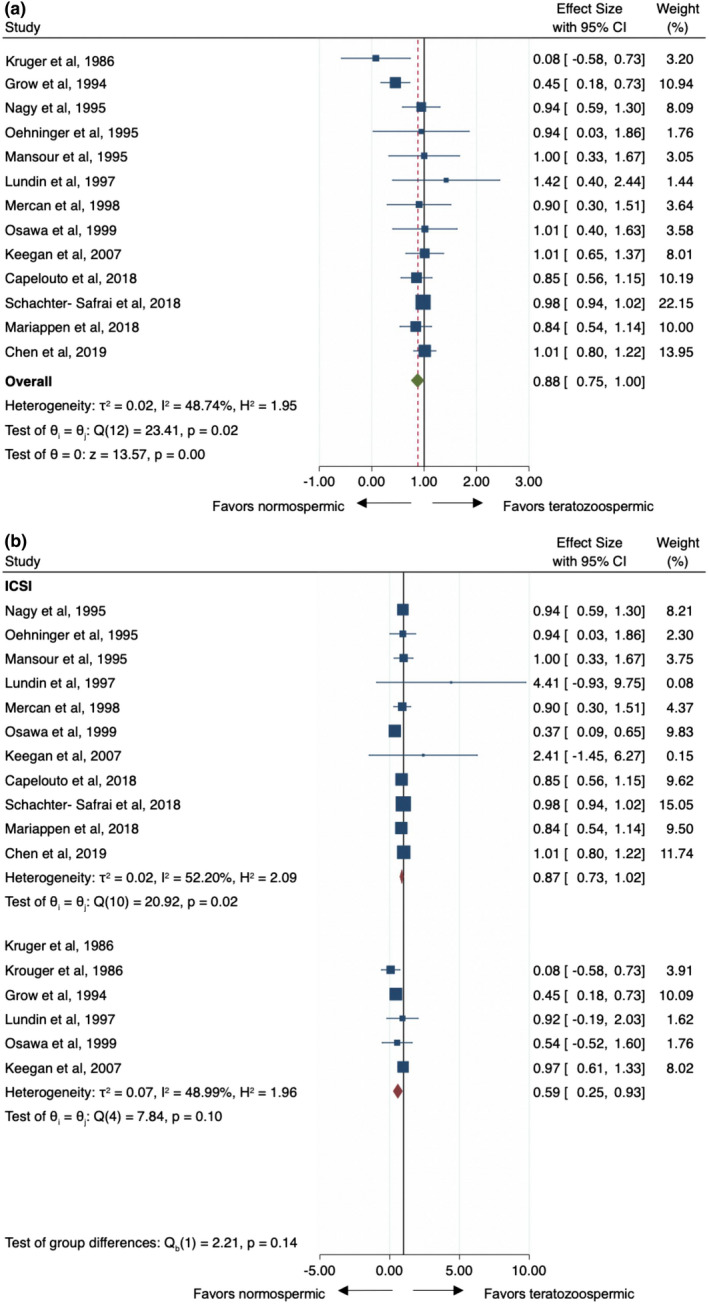
(a) Pooled odds ratio for teratozoospermic men compared to normozoospermic controls for pregnancy rate outcome at assisted reproductive techniques. (b) Subgroup analysis of IVF versus ICSI approach in teratozoospermic men compared to normozoospermic controls for pregnancy rate outcome CI, confidence interval; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection
3.5. Oligospermia and pregnancy rates
Of the 17 studies included, only seven (Capelouto et al., 2018; Kasman et al., 2021; Mariappen et al., 2018; Mercan et al., 1998; Nagy et al., 1995; Oehninger et al., 1995; Schachter‐Safrai et al., 2019) reported data regarding the association of sperm concentration and ART outcomes. All the studies (Capelouto et al., 2018; Kasman et al., 2021; Mariappen et al., 2018; Mercan et al., 1998; Nagy et al., 1995; Oehninger et al., 1995; Schachter‐Safrai et al., 2019) reported outcomes from IVF/ICSI. The reference threshold for defining normospermic patients ranged from 10 × 106/ml to 20 × 106/ml across the studies. Odds ratio estimates varied from 0.75 to 1.20 with OR <1 indicating lower probability of pregnancy with oligospermia. The pooled OR estimate was similar under a fixed or random effect model: 0.88 (95% CI: 0.75–1; Figure 2) in absence of study heterogeneity (Q = 6.09, d.f. = 6, p = 0.41; I 2 = 1.46%). No single study was found to significantly affect the heterogeneity statistic when excluded from analysis. Furthermore, the funnel plot suggests that there was no small‐study effect (Egger test, p=0.63), which supports the absence of publication bias. The “Trim and Fill” method suggested that two “missing” studies would be necessary to adjust asymmetry from the funnel plot Figure S2b. On meta‐regression analysis, there were no significant trends between patient age, number of cycles per study as well as year of publication (Fig. S4). There was a modest negative association between normal concentration threshold and clinical pregnant rate (Coeff: −0.04, SE: 0.02, p = 0.039; Fig. S4) suggesting that lower sperm concentrations were associated with lower pregnancy rates.
FIGURE 2.
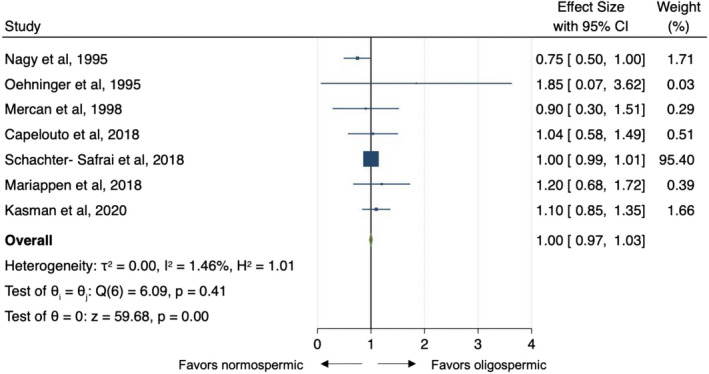
Pooled odds ratio for oligospermic men compared to normospermic controls for pregnancy rate outcome at assisted reproductive techniques. CI, confidence interval
3.6. Asthenospermia and pregnancy rates
Of the 17 studies included, nine studies reported data regarding the effect of impaired motility on ART outcomes. Eight studies (Capelouto et al., 2018; Kasman et al., 2021; Mariappen et al., 2018; Mercan et al., 1998; Nagy et al., 1995; Oehninger et al., 1995; Palermo et al., 1995; Schachter‐Safrai et al., 2019) reported outcomes from IVF/ICSI technique while only one experience (Ben‐Chetrit et al., 1995) from IVF alone. The reference threshold for defining normal proportion of motile spermatozoa ranged from 20% to 40% across the studies analysed. Odds ratio estimates varied from 0.61 to 1.22 with OR <1 indicating lower probability of pregnancy rate with asthenospermia. The pooled OR estimate was the same both under a fixed‐ or random‐effect model: 0.88 (95%CI: 0.73–1.03; Figure 3) acknowledging the existence of substantial study heterogeneity (Q = 19.1, d.f. = 8, p = 0.01; I 2 = 58%). No single study was found to significantly affect the heterogeneity statistic when excluded from analysis. Furthermore, the funnel plot suggests that there was no small‐study effect (Egger test, p = 0.72), which supports the absence of publication bias. The “Trim and Fill” method suggested that there were no “missing” studies necessary for inclusion to remove potential asymmetry from the funnel plot Figure S2c. Similar to the meta‐regression analysis of concentration, higher motility thresholds were associated with effect sizes closer to the null effect point (Coeff: −0.04, SE: 0.02, p = 0.039; Fig. S5) suggesting that more severe asthenospermia may be associated with lower pregnancy rates.
FIGURE 3.
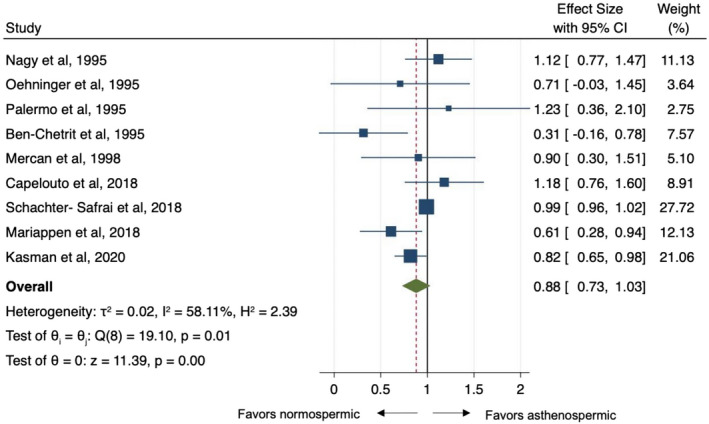
Pooled odds ratio for asthenospermic men compared to normospermic controls for pregnancy rate outcome at assisted reproductive techniques. CI, confidence interval
4. DISCUSSION
Overall, the current report failed to identify an association between semen parameters (i.e. sperm motility, concentration and morphology) and pregnancy rates with ART. While early studies suggested a possible relationship between sperm morphology and clinical pregnancy rate, later studies in the meta‐analysis did not find any relationship. Importantly, some secondary analyses suggested that lower sperm concentration and motility levels are associated with lower pregnancy rates. However, the current metanalysis suggests that current ART techniques are able to overcome impairments in semen quality.
To date, more than 8 million children have been born with the assistance of IVF, and over 2.5 million cycles are being performed annually, resulting in over 500,000 deliveries, with continued studies examining factors associated with outcomes (Fauser, 2019). Oocyte quality is a well‐known predictor of IVF success with a recent meta‐analysis highlighting the association between intra‐ and/or extra‐cytoplasmatic oocyte morphologies and IVF outcomes (Setti et al., 2011). On the other hand, the influence of the male gamete on IVF success is less certain. Previously, a meta‐analysis by Hotaling et al. (2011) reported that isolated teratozoospermia was not correlated with clinical pregnancy (OR 1.18, 95% CI, 0.83–1.67). However, other semen parameters have not been rigorously examined.
A major strength of this present study is the comprehensive assessment of the different semen parameters over a long time period of observation and evolution of the practice of ART (i.e. 1986–2020). While early studies suggested that lower sperm morphology led to impaired pregnancy rates, current studies displayed to association between sperm morphology and pregnancy rate. The current report was limited to conventional semen parameters (i.e. sperm concentration, motility and morphology). However, Ribas‐Maynou et al. (2021) recently demonstrated an inverse relationship between sperm DNA fragmentation, implantation and clinical pregnancy rate. In their meta‐analysis, the authors reported a 32% and 28% lower implantation and clinical pregnancy rate for cycles with abnormal sperm DNA fragmentation. Moreover, the association was stronger for IVF compared to ICSI suggesting ICSI may be able to overcome sperm damage.
It is important to highlight that the current analysis identified a temporal effect on the outcomes from ART and semen quality. There was a trend over the years where the more recent experiences did not identify an association between semen quality and PR which may reflect continued quality control and improvements in ART. In addition, granular data on semen parameters were not available in the majority of the reports. However, an analysis comparing bounds of semen parameter reference ranges suggested lower quality may be associated with lower pregnancy rates for both concentration and motility. As a secondary analysis, the results should be interpreted with caution. Nevertheless, it is possible that men with the most severely impaired semen quality may display compromised ART outcomes.
Our study has limitations that warrant discussion. First, despite the fact that the experiences included cover a long time period and different geographical regions of the world, the overall number of studies analysed is relatively small. Second, it was not possible to explore the isolated effect of each semen abnormality as most studies did not report abnormalities in isolation. Next, we were limited by specific thresholds of semen quality that were reported. It is conceivable that lower parameters (e.g. <1 M sperm/mL, <5% motility, 0% morphology) or a combination of impaired parameters (e.g. Oligo–Astheno–Teratospermic [OAT] syndrome) may be associated with ART outcomes. Finally, the outcome analysed was clinical pregnancy rate rather than the ultimate goal of a couple utilizing ART which is a live birth.
5. CONCLUSION
The current analysis found no association between semen quality (as measured by concentration, motility or morphology) and clinical pregnancy rates utilizing ART. While future investigations are necessary to explore the association between semen parameters and other ART outcomes (e.g. fertilization, implantation, birth and perinatal health), our study suggests that ART is able to overcome modest impairments in semen quality.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI‐CARE Agreement.
CONFLICT OF INTEREST
Authors have no conflict of interest to disclose.
RELEVANT DISCLOSURES
Authors have nothing to disclose.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1
Table S2
Del Giudice, F. , Belladelli, F. , Chen, T. , Glover, F. , Mulloy, E. A. , Kasman, A. M. , Sciarra, A. , Salciccia, S. , Canale, V. , Maggi, M. , Ferro, M. , Busetto, G. M. , De Berardinis, E. , Salonia, A. , & Eisenberg, M. L. (2022). The association of impaired semen quality and pregnancy rates in assisted reproduction technology cycles: Systematic review and meta‐analysis. Andrologia, 54, e14409. 10.1111/and.14409
Francesco Del Giudice and Federico Belladelli have equally contributed to this study.
PROSPERO Registration Number: ID‐240844
[Correction added on May 18, 2022, after first online publication: CRUI funding statement has been added.]
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- Akanji Tijani, H. , & Bhattacharya, S. (2010). The role of intrauterine insemination in male infertility. Human Fertility (Cambridge, England), 13(4), 226–232. 10.3109/14647273.2010.533811 [DOI] [PubMed] [Google Scholar]
- Ben‐Chetrit, A. , Senoz, S. , Greenblatt, E. M. , & Casper, R. F. (1995). In vitro fertilization outcome in the presence of severe male factor infertility. Fertility and Sterility, 63(5), 1032–1037. 10.1016/s0015-0282(16)57543-8 [DOI] [PubMed] [Google Scholar]
- Boeri, L. , Belladelli, F. , Capogrosso, P. , Cazzaniga, W. , Candela, L. , Pozzi, E. , Valsecchi, L. , Papaleo, E. , Viganò, P. , Abbate, C. , Pederzoli, F. , Alfano, M. , Montorsi, F. , & Salonia, A. (2021) Normal sperm parameters per se do not reliably account for fertility: A case‐control study in the real‐life setting. Andrologia, 53(1), 10.1111/and.13861 [DOI] [PubMed] [Google Scholar]
- Capelouto, S. M. , Nagy, Z. P. , Shapiro, D. B. , Archer, S. R. , Ellis, D. P. , Smith, A. K. , Spencer, J. B. , & Hipp, H. S. (2018). Impact of male partner characteristics and semen parameters on in vitro fertilization and obstetric outcomes in a frozen oocyte donor model. Fertility and Sterility, 110(5), 859–869. 10.1016/j.fertnstert.2018.06.003 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Li, D. , Ni, X. , Zhu, L. , Zhang, N. , Fang, J. , Jiang, W. , & Wang, J. (2020) Effects of the normal sperm morphology rate on the clinical and neonatal outcomes of conventional IVF cycles. Andrologia, 52(5), 10.1111/and.13568 [DOI] [PubMed] [Google Scholar]
- Del Giudice, F. , Busetto, G. M. , De Berardinis, E. , Sperduti, I. , Ferro, M. , Maggi, M. , Gross, M. S. , Sciarra, A. , & Eisenberg, M. L. (2020). A systematic review and meta‐analysis of clinical trials implementing aromatase inhibitors to treat male infertility. Asian Journal of Andrology, 22(4), 360–367. 10.4103/aja.aja_101_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice, F. , Kasman, A. M. , Ferro, M. , Sciarra, A. , De Berardinis, E. , Belladelli, F. , Salonia, A. , & Eisenberg, M. L. (2020). Clinical correlation among male infertility and overall male health: A systematic review of the literature. Investigative and Clinical Urology, 61(4), 355–371. 10.4111/icu.2020.61.4.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Duval, S. , & Tweedie, R. (2000). Trim and fill: A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics, 56(2), 455–463. 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Farquhar, C. , & Marjoribanks, J. (2018). Assisted reproductive technology: an overview of Cochrane Reviews. The Cochrane Database of Systematic Reviews, 8(8), CD010537. 10.1002/14651858.CD010537.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser, B. C. (2019). Towards the global coverage of a unified registry of IVF outcomes. Reproductive Biomedicine Online, 38(2), 133–137. 10.1016/j.rbmo.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Grow, D. R. , Oehninger, S. , Seltman, H. J. , Toner, J. P. , Swanson, R. J. , Kruger, T. F. , & Muasher, S. J. (1994). Sperm morphology as diagnosed by strict criteria: probing the impact of teratozoospermia on fertilization rate and pregnancy outcome in a large in vitro fertilization population. Fertility and Sterility, 62(3), 559–567. 10.1016/s0015-0282(16)56946-5 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring Inconsistency in meta‐analyses. BMJ (Clinical Research ed.), 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth, B. , Harris, A. , & Mortimer, D. (2007). The cost effectiveness of intracyctoplasmic sperm injection (ICSI). Journal of Assisted Reproduction and Genetics, 24(12), 571–577. 10.1007/s10815-007-9175-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotaling, J. M. , Smith, J. F. , Rosen, M. , Muller, C. H. , & Walsh, T. J. (2011). The relationship between isolated teratozoospermia and clinical pregnancy after in vitro fertilization with or without intracytoplasmic sperm injection: A systematic review and meta‐analysis. Fertility and Sterility, 95(3), 1141–1145. 10.1016/j.fertnstert.2010.09.029 [DOI] [PubMed] [Google Scholar]
- Kasman, A. M. , Del Giudice, F. , & Eisenberg, M. L. (2020). New insights to guide patient care: the bidirectional relationship between male infertility and male health. Fertility and Sterility, 113(3), 469–477. 10.1016/j.fertnstert.2020.01.002 [DOI] [PubMed] [Google Scholar]
- Kasman, A. M. , Li, S. , Zhao, Q. , Behr, B. , & Eisenberg, M. L. (2021). Relationship between male age, semen parameters and assisted reproductive technology outcomes. Andrology, 9(1), 245–252. 10.1111/andr.12908 [DOI] [PubMed] [Google Scholar]
- Keegan, B. R. , Barton, S. , Sanchez, X. , Berkeley, A. S. , Krey, L. C. , & Grifo, J. (2007). Isolated teratozoospermia does not affect in vitro fertilization outcome and is not an indication for intracytoplasmic sperm injection. Fertility and Sterility, 88(6), 1583–1588. 10.1016/j.fertnstert.2007.01.057 [DOI] [PubMed] [Google Scholar]
- Kruger, T. F. , Menkveld, R. , Stander, F. S. , Lombard, C. J. , Van der Merwe, J. P. , van Zyl, J. A. , & Smith, K. (1986). Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertility and Sterility, 46(6), 1118–1123. 10.1016/s0015-0282(16)49891-2 [DOI] [PubMed] [Google Scholar]
- Lopes, S. , Jurisicova, A. , & Casper, R. F. (1998). Gamete‐specific DNA fragmentation in unfertilized human oocytes after intracytoplasmic sperm injection. Human Reproduction (Oxford, England), 13(3), 703–708. 10.1093/humrep/13.3.703 [DOI] [PubMed] [Google Scholar]
- Lundin, K. , Söderlund, B. , & Hamberger, L. (1997). The relationship between sperm morphology and rates of fertilization, pregnancy and spontaneous abortion in an in‐vitro fertilization/intracytoplasmic sperm injection programme. Human Reproduction (Oxford, England), 12(12), 2676–2681. 10.1093/humrep/12.12.2676 [DOI] [PubMed] [Google Scholar]
- Mansour, R. T. , Aboulghar, M. A. , Serour, G. I. , Amin, Y. M. , & Ramzi, A. M. (1995). The effect of sperm parameters on the outcome of intracytoplasmic sperm injection. Fertility and Sterility, 64(5), 982–986. 10.1016/s0015-0282(16)57914-x [DOI] [PubMed] [Google Scholar]
- Mantel, N. , & Haenszel, W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute, 22(4), 719–748. [PubMed] [Google Scholar]
- Mariappen, U. , Keane, K. N. , Hinchliffe, P. M. , Dhaliwal, S. S. , & Yovich, J. L. (2018). Neither male age nor semen parameters influence clinical pregnancy or live birth outcomes from IVF. Reproductive Biology, 18(4), 324–329. 10.1016/j.repbio.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Mercan, R. , Oehninger, S. , Muasher, S. J. , Toner, J. P. , Mayer, J. Jr , & Lanzendorf, S. E. (1998). Impact of fertilization history and semen parameters on ICSI outcome. Journal of Assisted Reproduction and Genetics, 15(1), 39–45. 10.1023/a:1022578322024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, Z. P. , Liu, J. , Joris, H. , Verheyen, G. , Tournaye, H. , Camus, M. , Derde, M. C. , Devroey, P. , & Van Steirteghem, A. C. (1995). The result of intracytoplasmic sperm injection is not related to any of the three basic sperm parameters. Human Reproduction (Oxford, England), 10(5), 1123–1129. 10.1093/oxfordjournals.humrep.a136104 [DOI] [PubMed] [Google Scholar]
- National Institute of Health and Department of Health and Human Services , “Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies” (2015) Available from: https://www.nhlbi.nih.gov/health‐pro/guidelines/in‐develop/cardiovascular‐riskreduction/tools/cohort
- Oehninger, S. , Veeck, L. , Lanzendorf, S. , Maloney, M. , Toner, J. , & Muasher, S. (1995). Intracytoplasmic sperm injection: Achievement of high pregnancy rates in couples with severe male factor infertility is dependent primarily upon female and not male factors. Fertility and Sterility, 64(5), 977–981. 10.1016/s0015-0282(16)57913-8 [DOI] [PubMed] [Google Scholar]
- Ola, B. , Afnan, M. , Sharif, K. , Papaioannou, S. , Hammadieh, N. , & L.R. Barratt, C . (2001). Should ICSI be the treatment of choice for all cases of in‐vitro conception? Considerations of fertilization and embryo development, cost effectiveness and safety. Human Reproduction, 16(12), 2485–2490. 10.1093/humrep/16.12.2485 [DOI] [PubMed] [Google Scholar]
- Osawa, Y. , Sueoka, K. , Iwata, S. , Shinohara, M. , Kobayashi, N. , Kuji, N. , & Yoshimura, Y. (1999). Assessment of the dominant abnormal form is useful for predicting the outcome of intracytoplasmic sperm injection in the case of severe teratozoospermia. Journal of Assisted Reproduction and Genetics, 16(8), 436–442. 10.1023/a:1020573609836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo, G. D. , Cohen, J. , Alikani, M. , Adler, A. , & Rosenwaks, Z. (1995). Intracytoplasmic sperm injection: a novel treatment for all forms of male factor infertility. Fertility and Sterility, 63(6), 1231–1240. 10.1016/s0015-0282(16)57603-1 [DOI] [PubMed] [Google Scholar]
- Palermo, G. , Joris, H. , Devroey, P. , & Van Steirteghem, A. C. (1992). Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet (London, England), 340(8810), 17–18. 10.1016/0140-6736(92)92425-f [DOI] [PubMed] [Google Scholar]
- Ribas‐Maynou, J. , Yeste, M. , Becerra‐Tomás, N. , Aston, K. I. , James, E. R. , & Salas‐Huetos, A. (2021). Clinical implications of sperm DNA damage in IVF and ICSI: updated systematic review and meta‐analysis. Biological Reviews of the Cambridge Philosophical Society, 96(4), 1284–1300. 10.1111/brv.12700 [DOI] [PubMed] [Google Scholar]
- Schachter‐Safrai, N. , Karavani, G. , Reuveni‐Salzman, A. , Gil, M. , & Ben‐Meir, A. (2019). Which semen analysis correlates with favorable Intracytoplasmic morphologically selected sperm injection (IMSI) outcomes? European Journal of Obstetrics, Gynecology, and Reproductive Biology, 234, 85–88. 10.1016/j.ejogrb.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Setti, A. S. , Figueira, R. C. , Braga, D. P. , Colturato, S. S. , Iaconelli, A. Jr , & Borges, E. Jr (2011). Relationship between oocyte abnormal morphology and intracytoplasmic sperm injection outcomes: a meta‐analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 159(2), 364–370. 10.1016/j.ejogrb.2011.07.031 [DOI] [PubMed] [Google Scholar]
- Sousa, M. , & Tesarik, J. (1994). Ultrastructural analysis of fertilization failure after intracytoplasmic sperm injection. Human Reproduction (Oxford, England), 9(12), 2374–2380. 10.1093/oxfordjournals.humrep.a138455 [DOI] [PubMed] [Google Scholar]
- Stroup, D. F. , Berlin, J. A. , Morton, S. C. , Olkin, I. , Williamson, G. D. , Rennie, D. , Moher, D. , Becker, B. J. , Sipe, T. A. , & Thacker, S. B. (2000). Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA, 283(15), 2008–2012. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- Tesarik, J. , Rienzi, L. , Ubaldi, F. , Mendoza, C. , & Greco, E. (2002). Use of a modified intracytoplasmic sperm injection technique to overcome sperm‐borne and oocyte‐borne oocyte activation failures. Fertility and Sterility, 78(3), 619–624. 10.1016/s0015-0282(02)03291-0 [DOI] [PubMed] [Google Scholar]
- Thoma, M. E. , McLain, A. C. , Louis, J. F. , King, R. B. , Trumble, A. C. , Sundaram, R. , & Buck Louis, G. M. (2013). Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertility and Sterility, 99(5), 1324–1331.e1. 10.1016/j.fertnstert.2012.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Steeg, J. W. , Steures, P. , Eijkemans, M. J. C. , F. Habbema, J. D. , Hompes, P. G. A. , Kremer, J. A. M. , van der Leeuw‐Harmsen, L. , Bossuyt, P. M. M. , Repping, S. , Silber, S. J. , Mol, B. W. J. , & van der Veen, F. (2011). Role of semen analysis in subfertile couples. Fertility and Sterility, 95(3), 1013–1019. 10.1016/j.fertnstert.2010.02.024 [DOI] [PubMed] [Google Scholar]
- van Weert, J. M. , Repping, S. , Van Voorhis, B. J. , van der Veen, F. , Bossuyt, P. M. , & Mol, B. W. (2004). Performance of the postwash total motile sperm count as a predictor of pregnancy at the time of intrauterine insemination: A meta‐analysis. Fertility and Sterility, 82(3), 612–620. 10.1016/j.fertnstert.2004.01.042 [DOI] [PubMed] [Google Scholar]
- Verheyen, G. , Tournaye, H. , Staessen, C. , De Vos, A. , Vandervorst, M. , & Van Steirteghem, A. (1999). Controlled comparison of conventional in‐vitro fertilization and intracytoplasmic sperm injection in patients with asthenozoospermia. Human Reproduction (Oxford, England), 14(9), 2313–2319. 10.1093/humrep/14.9.2313 [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO Laboratory Manual for the Examination and Procession of Human Semen. (2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1
Table S2
Data Availability Statement
Research data are not shared.


