Abstract
Background
There has been limited evaluation of health‐related quality of life (HRQOL) in rectal cancer patients receiving neoadjuvant chemoradiotherapy. HRQOL outcomes in the National Surgical Adjuvant Breast and Bowel Project R‐04 trial are examined in this article.
Methods
Between 2004 and 2010, R‐04 patients were invited to enroll in the HRQOL substudy, with questionnaires administered before randomization, after completion of chemoradiotherapy, and 1‐year after surgery. HRQOL measures included: Functional Assessment of Cancer Therapy for colorectal cancer (FACT‐C); Short Form‐36v.2 Vitality scale; a treatment‐specific symptom scale; and the FACT neurotoxicity scale. A 5‐year postsurgery assessment was added to the protocol in 2012. Mixed‐effects models examined neoadjuvant therapy treatment effects in the 1‐year sample and models that explored associations of host factors and treatment impact on 5‐year HRQOL.
Results
A total of 1373 patients completed baseline HRQOL and at least one additional assessment. The average age was 58 years (range, 23–85 years), male (68%), and 59% Stage II. There were no statistically significant differences in HRQOL outcomes by treatment arm, but HRQOL worsened from baseline to postneoadjuvant chemoradiotherapy, with statistically significant effect sizes changes ranging from 0.6 (Vitality) to 0.9 (FACT‐C Trial Outcome Index). Neurotoxicity was greater in the oxaliplatin‐treated groups. Obese/overweight patients had statistically significantly worse FACT‐C Trial Outcome Index scores than did underweight/normal weight groups. At 5 years, younger patients and those with normal baseline weight had statistically significantly better physical function scores and older patients had better mental health outcomes.
Conclusions
HRQOL did not differ across the four R‐04 treatment arms; however, host factors explained significant variation in posttreatment HRQOL. ClinicalTrials.gov: NCT00058474 (https://ClinicalTrials.gov/ct2/show/NCT00058474).
Lay summary
This article reports on the health‐related quality of life (HRQOL) outcomes of patients treated with four different chemotherapy regimens combined with radiation in rectal cancer patients before definitive surgical treatment.
There were no significant differences in HRQOL by treatment regimen, but all patients experienced decreased vitality (energy) and physical functioning. By 1 year after treatment, most patients had returned to pretreatment vitality and physical functioning, with the exception of increased neurotoxicity.
In a subsample of patients assessed at 5 years after surgery, physical function was better in those who at pretreatment were younger, normal weight, and had better performance status. Mental function was better in those who at pretreatment were older and had better performance status.
Keywords: clinical trial, health‐related quality of life, long‐term survivors, neoadjuvant chemoradiotherapy, neurotoxicity, rectal cancer
Short abstract
.
INTRODUCTION
Systemic treatment approaches for surgically curable rectal cancer have been studied for the past 3 decades. The National Surgical Adjuvant Breast and Bowel Project (NSABP) explored combinations of treatments to enhance survival in trials focused on adjuvant chemotherapy and radiotherapy. 1 , 2 The most recent rectal cancer trial, NSABP R‐04, randomized 1608 patients with clinical Stage II or III disease, between 2004 and 2010, initially comparing infusional 5‐fluorouracil (5‐FU) to oral capecitabine (CAPE) concomitantly with radiotherapy preoperatively, hypothesizing that oral therapy might be more convenient and have equal efficacy. 3 The primary end point for the trial was locoregional tumor control. While this trial was recruiting, oxaliplatin (OXA) was found to be a promising colon cancer adjuvant treatment. In 2005, NSABP R‐04 was amended to further randomize patients to receive 5‐FU or CAPE, with or without OXA, resulting in a 2 × 2 factorial design, creating four separate treatment groups instead of two. When OXA was added to the trial protocol, doses of 5‐FU and CAPE were reduced to decrease toxicity of the combined regimen and were administered for only 5 days per week instead of the 7 days per week used initially. 3 There were only 293 patients randomized to the two‐arm study before the 2005 amendment.
The R‐04 trial analysis found no statistically significant differences between 5‐FU and CAPE in locoregional tumor control, disease‐free survival, and overall survival; or for OXA versus no OXA for these three end points. 3 However, patients receiving OXA had statistically significant greater overall and Grade 3 and 4 diarrhea. Study details and outcomes have been reported previously. 3 , 4
The R‐04 trial also included a health‐related quality of life (HRQOL) substudy assessing the neoadjuvant treatment regimens (before and after adjuvant therapy assessments). One‐year posttreatment surgical procedure impacts (sphincter‐sparing surgery [SSS] vs. abdominal‐perineal resection [APR]) were assessed with treatment‐targeted questions for bowel, bladder, and sexual function as secondary HRQOL outcomes. 5 HRQOL scores measured at 1 year showed no difference for patients treated with SSS compared with those who received APR; however, there were statistically significant differences in colorectal cancer–specific symptoms according to surgery type. Patients who underwent APR reported worse body image than patients who underwent SSS and had greater problems with micturition. In contrast, patients who underwent SSS reported worse gastrointestinal symptoms and weight loss. Prompted by these interesting findings, in 2012, the protocol was amended to add a 5‐year HRQOL assessment to capture long‐term effects of rectal cancer surgical treatments as well as additional assessments that focused on long‐term survivorship.
In this article, we focus on the short‐ and long‐term impacts of neoadjuvant chemoradiotherapy (NAC) on HRQOL, and host factors (i.e., demographic, medical, performance status) associated with subsequent HRQOL outcomes. The short‐term evaluation of HRQOL related to NAC was a secondary objective of the original R‐04 protocol. Because there were no clinical treatment outcome differences across the 4 arms of the trial (as reported in 2015), we delayed analyses for the HRQOL substudy until after the 5‐year assessments were completed in May 2016. In 2018, funding was received as part of the National Cancer Institute Moonshot Program to focus on new methods for evaluation of treatment toxicity, and analysis of the R‐04 trial data was proposed to investigate the potential effects of host factors on treatment tolerability and HRQOL outcomes. Thus, the results described in this article go beyond the initially proposed R‐04 protocol analyses and allow a first evaluation of long‐term HRQOL at 5 years in this sample of patients.
METHODS AND MATERIALS
R‐04 enrolled and randomized 1608 patients from 2004 to 2010. 3 All patients who spoke English, French, or Spanish were invited to participate in the HRQOL substudy. The HRQOL questionnaire was administered before randomization, after completion of NAC (before definitive surgery), and at 1‐year postsurgery. If NAC was delayed, the first follow‐up questionnaire was completed after radiotherapy to capture the patient's assessment at the end of therapy rather than at a fixed time point.
The 1‐ and 5‐year questionnaires were self‐administered at regularly scheduled office visits before the clinical visit whenever possible. If the patient was not accessible in person, staff were encouraged to mail the questionnaire or collect responses by telephone. Patients who discontinued protocol therapy for reasons other than recurrence or a second primary were expected to continue the HRQOL assessments on schedule. If a patient declined to complete a questionnaire or it was not completed for any reason, the clinical site submitted a Missing Data Form to the statistical coordinating center, which described the reason for missing data, such as staff failure to administer, patient refusal, or the patient being too ill.
The analytic objectives of this article are to: (1) compare HRQOL outcomes for the four preoperative chemoradiotherapy rectal cancer treatments; (2) identify pretreatment patient characteristics (host factors) associated with treatment toxicity at 1 and 5 years after surgery; and (3) examine changes in HRQOL from 1‐year postrandomization to the 5‐year follow‐up visit.
The majority of the HRQOL measures were administered previously in the NSABP C‐06 colon cancer adjuvant therapy trial. 6 In this article, we report results for the following.
The Functional Assessment of Cancer Therapy Questionnaire for colorectal cancer (FACT‐C) 7 (included at all time points)
The FACT Neurotoxicity Scale (FACT‐NTX), modified for use in NSABP C‐07 8 (included at all time points)
The Short Form‐36 (SF‐36) version 2 Vitality Scale 9 (included at all time points)
The NSABP Symptom Checklist (SCL‐17) with an 11‐item 5‐FU–specific symptom scale, 6 plus 6 additional items relevant for the added drugs/administration issues (i.e., continuous infusion) (administered first year only)
The SF‐36v.2 health survey (included in the 5‐year assessment only) 9
We examined the FACT‐C Trial Outcome Index (FACT‐C TOI), which includes 22 to 24 items dependent on whether patient has an ostomy; the SCL‐17; the FACT‐NTX13; the FACT‐NTX4; and the four‐item SF‐36 Energy/Fatigue Scale (SF‐36 Vitality). The FACT‐C TOI is a sum of scores from the physical well‐being subscale, functional well‐being subscale, and colorectal cancer subscale of the FACT‐C, with a range of 0 to 84, and a higher score representing better HRQOL. The SCL‐17 score is the average of 17 items scored on a 0 to 100 range, with a higher score representing greater symptom bother. The FACT‐NTX13 has a 0 to 52 range and the FACT‐NTX4 a 0 to 16 range, with higher scores representing greater toxicity. The SF‐36 Vitality Scale is scored on a T‐score metric (mean = 50, SD = 10, in US general population), with a higher score indicating more energy.
We also examined the SF‐36 Physical and Mental Health Component Summary Scores (PCS and MCS), which are weighted combinations of eight multi‐item scales assessing physical function (10 items), role limitations because of physical health problems (four items), bodily pain (two items), general health perceptions (five items), vitality (four items), social functioning (two items), role limitations because of emotional problems (three items), and emotional well‐being (five items). The PCS scoring algorithm has positive weights for the physical functioning, role limitations because of physical health problems, bodily pain, general health perceptions, and vitality scales, and negative weights for the social functioning, role limitations because of emotional problems, and emotional well‐being scales. The MCS scoring algorithm includes positive weights for the emotional well‐being, role limitations because of emotional problems, social functioning, and vitality scales, and negative weights for the physical functioning, role limitations because of physical health problems, bodily pain, and general health perceptions scales. 10
Statistical design and analysis
Primary hypotheses were the following: (1) patients receiving CAPE would have less severe symptoms and better HRQOL than those receiving infusional 5‐FU and (2) patients receiving OXA would experience greater neurotoxicity than those not receiving it at end of treatment and at 1 year. A secondary hypothesis, for the long‐term follow‐up sample, was that poorer 1‐year FACT‐C‐TOI scores and neurotoxicity symptoms would predict worse SF‐36 MCS and PCS 5 years after surgery.
Before examining multivariable models of change in HRQOL from baseline to postradiotherapy and to 1 year after surgery, we examined baseline bivariate associations among sex, age, clinical stage, body mass index (BMI), intended type of rectal surgery (SSS or not), Karnofsky Performance Status (KPS), and race/ethnicity. We then estimated least square means by treatment group (5‐FU, 5‐FU + OXA, CAPE, and CAPE+OXA) from mixed effects models (autoregressive covariance structure, and residual [restricted] maximum likelihood estimation), controlling for: (1) sex (female); (2) age (continuous); (3) clinical stage; (4) BMI (<18.5, 18.5–<25.0, 25.0–<30.0, ≥30.0); (5) plans for SSS or not; (6) KPS (90–100, 70–80, 50–60); and (7) race/ethnicity (Hispanic, non‐Hispanic Black, non‐Hispanic White, non‐Hispanic other/unknown). We also examined interactions of sex (1) with the other variables (2–7).
Additionally, we explored changes on FACT‐C TOI and Vitality scores between baseline and 1 year and compared these with 5 years in the long‐term follow‐up sample. We also examined associations of baseline host factors (e.g., demographics, medical variables, KPS), with subsequent outcomes. Differences between patients in the analytic sample and those excluded because of missing data were evaluated using between‐group t tests for continuous variables and χ2 for categorical variables. Because of the exploratory nature of most comparisons, statistical significance was set at p < .05 without correction for multiple comparisons.
We estimated 3 separate models to obtain adjusted SF‐36 PCS and MCS at the 5‐year assessment. The first model included baseline demographic and medical characteristics (age, race/ethnicity, BMI, KPS); a chemotherapy treatment arm was added for the second model; and 1‐year assessments of FACT‐NTX4 and FACT‐C TOI were added to the third model.
RESULTS
Analytic sample and patient characteristics
Fig 1 shows the disposition of patients available in R‐04 for the primary HRQOL study examining baseline, postneoadjuvant, and 1‐year postsurgery HRQOL data. From 1608 patients randomized, 1373 completed a baseline questionnaire and at least one of the two follow‐up assessments. The amendment adding OXA after trial launch modified the doses of 5‐FU and capecitabine, resulting in more 5‐FU and CAPE‐only patients than OXA patients. The final line of the CONSORT diagram shows the subset of the analytic sample randomized to the four‐arm study with consistent fluoropyrimidine doses. We compared the SCL‐17 scores after treatment for the two and four arms (before and after OXA amendment) for 5‐FU and CAPE and found no statistically significant differences (data not shown); as a result, we combined the 5‐FU and CAPE groups for all further analyses (all patients evaluable for study, n = 1373).
FIGURE 1.
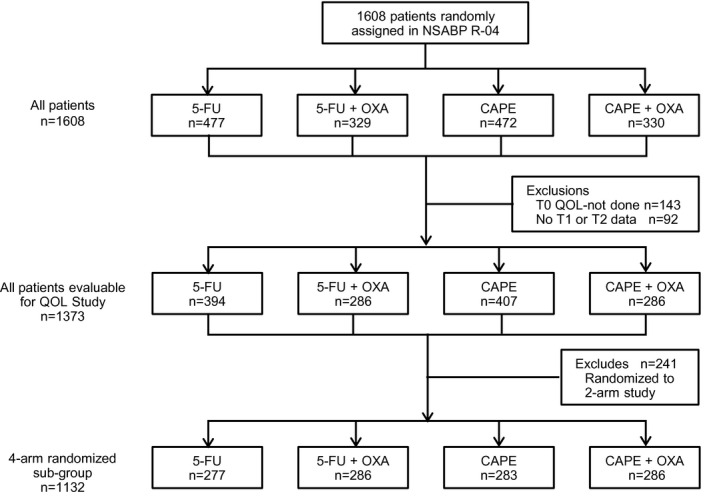
CONSORT Diagram: NSABP Protocol R‐04. 5‐FU indicates 5‐fluorouracil; CAPE, capecitabine; OXA, oxaliplatin; QOL, quality of Life; T0, baseline; T1, postradiotherapy; T2, 1‐year postsurgery; T5, year 5.
Table 1 compares the 1373 analytic sample patients with the 235 who were excluded. Average age of the former was 58 (range, 23–85). Most were male (68%), overweight or obese (73% BMI ≥25), had Stage II cancer (59%), and KPS between 90 and 100 (85%). Those in the analytic sample were significantly more likely to be overweight or obese (p < .0001) and have a better KPS score (p = .02) than those excluded. Because only one patient had a KPS score of 50 to 60, those with KPS 50 to 80 scores are merged here and in subsequent tables.
TABLE 1.
Baseline Characteristics of Patients in and not in Analytic Sample
| Covariate | Not in sample (n = 235) | In sample (n = 1373) | Test |
|---|---|---|---|
| Age, mean (SD) [range] | 57.5 (11.7) [22.3–86.2] | 57.6 (11.3) [23.5–85.4] | t(1606) = 0.18, p = .860 |
| Sex, n (%) | |||
| Male | 149 (63.4) | 939 (68.4) | |
| Female | 86 (36.6) | 434 (31.6) | χ2(1) = 2.3, p = .131 |
| Race/ethnicity, n (%) | |||
| Hispanic | 12 (5.1) | 73 (5.3) | |
| Non‐Hispanic Black | 12 (5.1) | 67 (4.9) | |
| Non‐Hispanic White | 190 (80.9) | 1165 (84.9) | |
| Non‐Hispanic Other/unknown | 21 (8.9) | 68 (4.9) | χ2(3) = 6.2, p = .104 |
| Body mass index, n (%) | |||
| <18.5 underweight | 8 (3.4) | 17 (1.2) | |
| 18.5–<25.0 normal | 94 (40.0) | 358 (26.1) | |
| 25.0–<30.0 overweight | 65 (27.7) | 499 (36.3) | |
| ≥30.0 obese | 68 (28.9) | 499 (36.3) | χ 2(3) = 27.3, p < .001 |
| Clinical stage, n (%) | |||
| II | 138 (58.7) | 815 (59.4) | |
| III | 97 (41.3) | 557 (40.6) | χ 2(1) = 0.04, p = .848 |
| Karnofsky Performance Status (KPS)a, n (%) | |||
| Fully active (90–100) | 184 (79.3) | 1162 (84.6) | |
| Restricted/ambulatory (50–80) | 51 (21.7) | 211 (15.4) | χ 2(1) = 5.9, p = .015 |
| Intent to save sphincter, n (%) | |||
| No | 65 (27.7) | 357 (26.0) | |
| Yes | 170 (72.3) | 1016 (74.0) | χ 2(1) = 0.29, p = .593 |
| Treatment, n (%) | |||
| 5‐FU | 83 (35.3) | 394 (28.7) | |
| 5‐FU + OXA | 43 (18.3) | 286 (20.8) | |
| CAPE | 65 (27.7) | 407 (29.6) | |
| CAPE+OXA | 44 (18.7) | 286 (20.8) | χ 2(3) = 4.3, p = .231 |
Note: Covariates reported as n (%).
Abbreviations: 5‐FU, 5‐fluorouracil; CAPE, capecitabine; OXA, oxaliplatin.
(KPS 90–100) fully active, able to carry on all predisease performance without restriction; (KPS 70–80) restricted in physically strenuous activity but ambulatory; and (KPS 50–60) ambulatory and capable of all self‐care but unable to perform any work activities.
We also examined reasons for missing data, finding that ~70% was due to staff oversight. In a minority of instances, missing data were due to refusal or poor health. Thus, we considered missing data to be missing at random.
Bivariate associations and multivariable models
Baseline HRQOL means by characteristics at randomization are provided in Table 2, and statistically significant (p < .05) associations are summarized. Age was significantly correlated with fewer symptoms, more vitality, and better cancer‐specific HRQOL, but all correlations were small. Females reported less vitality than males. Hispanics and non‐Hispanic Blacks reported more symptoms (SCL‐17) than non‐Hispanic Whites or other/unknown. Underweight (BMI <18.5) patients had significantly worse scores on all scales except for those with normal weight (BMI 18.5–<25.0) on the FACT‐C TOI, and of those who were obese (BMI ≥30.0) on the FACT‐NTX13. Stage III patients had worse FACT‐C TOI and more symptoms (SCL‐17) than those with Stage II disease. Lower KPS was associated with worse scores on all measures.
TABLE 2.
Unadjusted Health‐Related Quality of Life Scale Means by Patient Characteristics at Baseline, n = 1373
| Characteristic | FACT‐C TOI | SF‐36 Vitality | FACT‐NTX13 | FACT‐NTX4 |
NSABP SCL‐17 |
|---|---|---|---|---|---|
| Age | r = 0.17, p < .001 | r = 0.09, p = .001 | r = 0.02, p = .503 | r = −0.04, p = .230 | r = −0.14, p < .001 |
| Sex | |||||
| Male (n = 939) | 65.3a | 54.0a | 2.9a | 0.63a | 7.6a |
| Female (n = 434) | 65.1a | 51.6b | 2.8a | 0.71a | 8.0a |
| Test | t(1365) = 0.26, p = .796 | t(1367) = 3.98, p < .001 | t(598) = 0.54, p = .589 | t(572) = −0.67, p = .505 | t(758) = −0.91, p = .364 |
| Race/ethnicity | |||||
| Hispanic (n = 85) | 59.3bc | 53.1a | 4.2ab | 1.3a | 11.2a |
| Non‐Hispanic Black (n = 79) | 57.4c | 50.7a | 4.5a | 1.5a | 10.8a |
| Non‐Hispanic White (n = 1355) | 66.3a | 53.4a | 2.6b | 0.5b | 7.3b |
|
Non‐Hispanic Other/unknown (n = 89) |
61.7b | 52.9a | 4.4a | 1.2a | 8.8b |
| Test | F(3,1363) = 18.35, p < .001 | F(3,1365) = 1.39, p = .246 | F(3,1085) = 6.00, p = .001 | F(3,1086) = 9.90, p ≤ .001 | F(3,1364) = 11.22, p ≤ .001 |
| Body mass index | |||||
| <18.5 underweight (n = 17) | 59.7b | 45.8b | 5.1a | 1.7a | 12.0a |
| 18.5–<25.0 normal (n = 358) | 62.7ab | 52.2a | 2.5b | 0.6b | 8.4b |
| 25.0–<30.0 overweight (n = 499) | 66.7a | 54.4a | 2.5b | 0.5b | 7.1b |
| ≥30.0 obese (n = 499) | 65.9a | 53.1a | 3.5ab | 0.9b | 7.7b |
| Test | F(3,1363) = 8.56, p < .001 | F(3,1365) = 5.97, p < .001 | F(3,1085) = 4.52, p = .004 | F(3,1086) = 4.54, p = .004 | F(3,1364) = 4.04, p = .007 |
| Clinical stage | |||||
| II (n = 815) | 66.1a | 53.5a | 2.9a | 0.6a | 7.2b |
| III (n = 557) | 64.0b | 52.8a | 3.0a | 0.7a | 8.5a |
| Test | t(1364) = 2.92, p = .004 | t(1366) = 1.34, p = .179 | t(1086) = −0.36, p = .721 | t(798) = −0.82, p = .411 | t(1076) = −3.26, p = .001 |
| Karnofsky Performance Status (KPS)a | |||||
| Fully active (90–100; n = 1162) | 66.7a | 54.2a | 2.6b | 0.6b | 7.1b |
| Restricted/ambulatory (50–80; n = 211) | 57.4b | 47.8b | 4.6a | 1.1a | 10.9a |
| Test | t(261) = 8.64, p < .001 | t(272) = 7.64, p < .001 | t(200) = −4.00, p < .001 | t(200) = −2.90, p = .004 | t(247) = −5.39, p < .001 |
| Intent to save sphincter | |||||
| No (n = 357) | 63.5b | 52.7a | 3.1a | 0.7a | 8.2a |
| Yes (n = 1016) | 65.9a | 53.4a | 2.8a | 0.7a | 7.6a |
| Test | t(1365) = −3.07, p = .002 |
t(1367) = −1.15, p = .249 |
t(1087) = 0.70, p = .482 | t(1088) = 0.17, p = .862 | t(579) = 1.27, p = .205 |
| Treatment | |||||
| 5‐FU (n = 394) | 65.8a | 53.8ab | 3.0ab | 0.7a | 7.9a |
| 5‐FU + OXA (n = 286) | 64.4a | 53.2ab | 2.8ab | 0.7a | 7.7a |
| CAPE (n = 407) | 65.0a | 52.1b | 3.3a | 0.7a | 8.0a |
| CAPE+OXA (n = 286) | 65.8a | 54.1a | 2.4b | 0.5a | 7.0a |
| Test | F(3,1363) = 0.84, p = .473 | F(3,1365) = 2.61, p = .050 | F(3,1085) = 1.82, p = .141 | F(3,1086) = 1.05, p = .371 | F(3,1364) = 1.23, p = .298 |
Note: Student t test and analysis of variance with overall F and Duncan multiple range test used. FACT‐C‐TOI (0 − 84: higher score = better health‐related quality of life), SF‐36 Vitality T‐score (higher score = more vitality), FACT‐NTX13 (0–52: higher score = greater toxicity), FACT‐NTX4 sensory (0–16: higher score = greater toxicity), NSABP SCL‐17 (0–100: higher score = greater symptom bother).
Abbreviations: 5‐FU, 5‐fluorouracil; CAPE, capecitabine; NSABP SCL‐17, National Surgical Adjuvant Breast and Bowel Project Symptom Checklist; NTX, neurotoxicity; OXA, oxaliplatin; SF‐36, Short‐Form 36‐item Survey; TOI, Trial Outcome Index.
(KPS 90–100) Fully active, able to carry on all predisease performance without restriction; (KPS 70–80) restricted in physically strenuous activity but ambulatory; (KPS 50–60) ambulatory and capable of all self‐care but unable to perform any work activities.
Means sharing the same superscript letter (a, b, c) for a variable within a column do not differ significantly using Hochberg multiple comparison adjustment, p < .05.
Comparison of HRQOL outcomes by time and treatment assignment
In models examining baseline, after chemoradiotherapy, and 1 year after surgery time points (Table 3), HRQOL worsened after chemoradiotherapy, and there were no significant differences in the change scores for the four treatment arms for the FACT‐C TOI or the SF‐36 Vitality scale. There was slightly less symptom toxicity (SCL‐17) after NAC in the 5‐FU–only arm. The magnitude of worsening (effect size) in HRQOL after NAC compared with baseline ranged from 0.6 (Vitality for CAPE) to 0.9 (FACT‐C TOI and SF‐36 Vitality for 5‐FU + OXA). Neurotoxicity (FACT‐NTX13 and FACT NTX4) continued to worsen 1 year after surgery, but other aspects of HRQOL (SCL‐17, Vitality, FACT‐C TOI) improved from after chemoradiotherapy to 1 year after surgery.
TABLE 3.
Least Square Means by Treatment Group on Health‐Related Quality of Life and Symptoms at Baseline (BL), Post‐Radiotherapy (RT), and 1‐Year Postsurgery (PS)
| Outcome | Baselinea (n = 1373) | Post‐RTa (n = 1314) | 1 y PSa (n = 1040) | Change scores over timeb | ||
|---|---|---|---|---|---|---|
| Post‐RT: BL | 1 y PS: BL | 1 y PS: post‐RT | ||||
| FACT‐C TOI (range, 0–84; higher score = better QOL) | ||||||
| 5‐FU | 59.4a (12.7) | 50.8a (15.0) |
57.8a (12.8) |
–8.7d [0.7] | −1.7 [0.1] | 7.0d [0.6] |
| 5‐FU + OXA | 58.1a (12.1) | 47.0b (15.1) |
56.5a (13.1) |
−11.1d [0.9] | −1.6 [0.1] | 9.6d [0.8] |
| CAPE | 58.7a (13.3) | 49.2ac (14.8) |
56.8a (13.3) |
−9.5d [0.7] | −1.9d [0.1] | 7.5d [0.6] |
| CAPE+OXA | 59.5a (13.3) | 47.3bc (14.3) |
58.3a (12.4) |
−12.2d [0.9] | −1.1 [0.0] | 11.1a [0.9] |
| SF‐36 vitality T‐score (range, 0–100; higher score = more vitality) | ||||||
| 5‐FU | 51.1a (10.6) | 44.9a (10.7) |
49.5a (10.4) |
−6.2d [0.6] | −1.6d [0.2] | 4.6d [0.4] |
| 5‐FU + OXA | 50.8a (9.9) | 42.2b (10.4) |
49.0a (9.5) |
−8.6d [0.9] | −1.8d [0.2] | 6.8d [0.7] |
| CAPE | 49.5a (10.6) | 43.4b (10.9) |
48.2a (10.0) |
−6.1d [0.6] | −1.2 [0.1] | 4.8d [0.5] |
| CAPE+OXA | 51.4a (10.7) | 43.1b (10.4) |
48.9a (10.1) |
−8.3d[0.8] | −2.5d [0.2] | 5.8d [0.6] |
| FACT‐NTX13 (range, 0–52; higher score = greater toxicity) | ||||||
| 5‐FU |
4.7a (4.6) |
5.6c (5.1) |
12.2b (10.4) |
0.9 [0.2] | 7.5d [1.7] | 6.6d [1.4] |
| 5‐FU + OXA |
4.6a (4.4) |
7.6ab (6.3) |
11.1bc (9.3) |
3.0d [0.7] | 6.5d [1.5] | 3.5d [0.8] |
| CAPE |
5.2a (5.8) |
6.7bc (7.0) |
13.9a (11.5) |
1.6d [0.3] | 8.7d [1.5] | 7.1d [1.2] |
| CAPE+OXA |
4.1a (4.0) |
8.1a (6.3) |
10.0c (9.1) |
3.9d [1.0] | 5.9d [1.5] | 2.0d [0.5] |
| FACT‐NTX4 (range, 0–16; higher score = greater toxicity) | ||||||
| 5‐FU | 1.3a (1.9) | 1.5b (2.1) | 5.5c (5.1) | 0.2 [0.1] | 4.2a [2.2] | 4.0d [2.1] |
| 5‐FU + OXA | 1.2a (1.6) | 2.1a (2.3) | 4.7b (4.5) | 0.9d [0.6] | 3.5a [2.2] | 2.6d [1.6] |
| CAPE | 1.4a (2.0) | 2.1ab (2.8) | 6.2a (5.4) | 0.7d [0.4] | 4.9a [2.4] | 4.2d [2.0] |
| CAPE+OXA | 1.1a (1.4) | 2.5a (2.8) | 4.1b (4.3) | 1.4d [1.0] | 3.0a [2.2] | 1.6a [1.2] |
| NSABP SCL‐17 (range, 0–100; higher score, greater symptom bother) | ||||||
| 5‐FU | 10.8a (7.7) | 14.9b (8.9) | 12.9b (8.5) | 4.1d [0.5] | 2.1d [0.3] | −2.0d [0.2] |
| 5‐FU + OXA | 10.5a (6.5) | 16.3a (9.4) | 13.3ab (9.2) | 5.8d [0.9] | 2.8d [0.4] | −3.0d [0.5] |
| CAPE | 10.9a (7.7) | 16.1a (9.5) | 14.5a (10.1) | 5.2d [0.7] | 3.6d [0.5] | −1.6d [0.2] |
| CAPE+OXA | 9.8a (6.9) | 16.5a (9.6) | 12.5b (8.6) | 6.7d [1.0] | 2.7d [0.4] | −4.0d [0.6] |
Note: Results are least‐square means (SD) by treatment group from mixed effect models (baseline, postradiotherapy [RT], and 1‐year postsurgery [PS]) and their change scores over time with effect sizes (ES = unadjusted change score/BL [SD]). Models include treatment, time point, sex, continuous age, race/ethnicity, clinical stage, body mass index, plans for sphincter‐sparing surgery, performance status.
Abbreviations: 5‐FU, 5‐fluorouracil; CAPE, capecitabine; NSABP SCL‐17, National Surgical Adjuvant Breast and Bowel Project Symptom Checklist; NTX, neurotoxicity; OXA, oxaliplatin; SF‐36, Short‐Form 36‐item Survey; TOI, Trial Outcome Index.
Means sharing the same letter (a,b,c) within the first three columns (time point) for the same outcome are not significantly different from each other, Hochberg multiple comparison adjustment, p < .05.
b
Letter d assigned to change scores with p < .05
Table S1 shows the independent variables and their associations with change in outcomes over time. Statistically significant (p < .05) associations are summarized here. Hispanics had more severe symptoms (FACT‐NTX13, SCL‐17) and worse FACT‐C TOI scores than did non‐Hispanic Whites. Obese (BMI ≥30.0) and overweight (BMI 25.0–<30.0) patients had worse FACT‐C TOI scores than did underweight (BMI <18.5) and normal‐weight (BMI 18.5–<25.0) patients. Underweight (BMI <18.5) patients had worse SCL‐17 and lower vitality than all other BMI groups. Better baseline KPS was associated with less severe symptoms (SCL‐17, FACT‐NTX13, FACT‐NTX4) and better SF‐36 Vitality and FACT‐C TOI scores.
Long‐term survivorship outcomes at 5 years
The amendment adding the 5‐year HRQOL component occurred in March 2012. Therefore, all patients who were randomized between 2007 and 2010 who reached the 5‐year time point thereafter were eligible for the 5‐year assessment if they had completed the baseline HRQOL assessment and had not had a recurrence or been lost to follow‐up. Figure S1 delineates those who participated in the 5‐year HRQOL outcome assessment. There were two 5‐year analytic samples: (1) those who completed the baseline and 5‐year surveys (n = 344) and (2) those who completed the baseline, 1‐year, and 5‐year surveys (n = 308). Patients who completed the 5‐year survey were similar in characteristics to the baseline full analytic sample (Table S2), with the exception of having slightly more females, more non‐Hispanic Whites, and obese individuals.
We first examined the change in FACT‐C TOI and SF‐36 Vitality Score over the 5‐year period (Table S3). The SF‐36 Vitality and FACT‐C TOI consistently deteriorated 1 year after surgery in all treatment groups and did not significantly improve at 5 years. The only independent variables significantly associated with change over time for these outcomes were better SF‐36 Vitality Scores for those in the normal‐weight group (p = .02) and better FACT‐C‐TOI (p = .04) and better SF‐36 Vitality scores (p = .007) for those in the highest KPS group (data not shown).
Table 4 shows estimated SF‐36 PCS and MCS by host factors, treatment group, and FACT‐NTX4 and FACT‐C TOI 1 year after chemoradiotherapy from the three separate multivariable models. Younger patients had better PCS scores across all models, and race/ethnicity was not predictive of 5‐year PCS scores in any model; however, patients with normal baseline BMI had significantly higher PCS scores in all three models, with obese patients having the lowest scores. Patients with better baseline KPS scores had significantly better PCS scores at 5 years in Models 1 and 2; however, when the FACT‐C TOI and NTX at 1 year were added in Model 3, baseline KPS was no longer significant. Interestingly, in Model 2, chemotherapy treatment arm predicted differences in PCS (p = .03), with patients who received CAPE having the lowest score at the 5‐year assessment. In Model 3, the FACT‐C TOI at 1 year was significantly associated with the 5‐year PCS (p < .0001), and the adjusted R 2 for this model (0.33) was higher than for Models 1 and 2.
TABLE 4.
Adjusted SF‐36 v2 Summary Scores at Year 5 in 3 Separate Models
| SF‐36 v2 physical component summary (PCS) | SF‐36 v2 mental component summary (MCS) | |||||
|---|---|---|---|---|---|---|
| n = 344 | n = 308 | n = 344 | n = 344 | n = 308 | ||
| Variable |
Model 1 F = 2.77, p = .008, R 2 = 0.0558, adj R 2 = .0356, n = 336 |
Model 2 F = 2.92, p = .002, R 2 = 0.0824, adj R 2 = 0.0542, n = 336 |
Model 3 F = 12.88, p < .001, R 2 = 0.3574, adj R 2 = 0.3296, n = 291 |
Model 1 F = 2.52, p = .016, R 2 = 0.0510, adj R 2 = 0.0307, n = 336 |
Model 2 F = 1.97, p = .036, R 2 = 0.0572, adj R 2 = 0.0282, n = 336 |
Model 3 F = 10.13, p < .001, R 2 = 0.3043, adj R 2 = 0.2743, n = 291 |
| Age (estimate) | beta = −0.13; p = .014 | beta = −0.15; p = .006 | beta = −0.24; p = <.001 | beta = 0.17; p = .003 | beta = 0.17; p = .004 | beta = 0.06; p = .261 |
| Race/ethnicity | ||||||
| Hispanic | 44.1a | 44.1a | 44.4a | 48.8a | 48.8a | 49.8a |
| Non‐Hispanic Black | 45.4a | 45.4a | 45.6a | 46.2a | 46.3a | 46.8a |
| Non‐Hispanic White | 46.3a | 46.4a | 46.5a | 49.2a | 49.1a | 49.0a |
|
Non‐Hispanic Other/unknown |
44.0a | 43.5a | 42.2a | 51.3a | 51.1a | 49.7a |
| Test |
F = 0.36, p = .784 |
F = 0.48, p = .699 | F = 0.96, p = .410 | F = 0.45, p = .714 | F = 0.40, p = .754 | F = 0.24, p = .870 |
| Body mass index | ||||||
| 18.5 to <25.0 normal | 47.1a | 47.1a | 46.5a | 49.3a | 49.3a | 49.1a |
| 25.0 to <30.0 overweight | 45.0ab | 44.9ab | 44.7ab | 48.7a | 48.7a | 49.0a |
| ≥30.0 obese |
42.8 b |
42.5c | 42.8b | 48.6a | 48.5a | 48.4a |
| Test |
F = 4.66, p = .010 |
F = 5.53, p = .004 | F = 4.24, p = .015 | F = 0.11, p = .893 | F = 0.14, p = .873 | F = 0.17, p = .843 |
| Karnofsky Performance Status (KPS)a | ||||||
| Fully active (90–100) | 46.5a | 46.4a | 45.9a | 51.3a | 51.4a | 51.2a |
| Restricted/ambulatory (50–80) | 43.4b | 43.3b | 43.4a | 46.4b | 46.3b | 46.4b |
| Test | p = .045 | p = .047 | p = .089 | p = .004 | p = .003 | p = .004 |
| Treatment | ||||||
| 5‐FU | ‐ | 45.7a | 45.4a | ‐ | 48.3a | 48.1a |
| 5‐FU + OXA | ‐ | 44.7ab | 44.5ab | ‐ | 49.2a | 48.7a |
| CAPE | ‐ | 42.3b | 42.5b | ‐ | 47.8a | 48.4a |
| CAPE+OXA | ‐ | 46.7a | 46.4a | ‐ | 50.0a | 50.0a |
| Test | F = 3.15, p = .025 | F = 2.82, p = .039 | F = 0.71, p = .544 | F = 0.61, p = .609 | ||
| FACT NTX‐4 at year 1 (estimate) | beta = −0.08; p = .505 | beta = 0.07; p = .567 | ||||
| FACT‐C TOI at year 1 (estimate) | beta = 0.43; p ≤ .001 | beta = .47; p ≤ .001 | ||||
Notes. Model 1 examines host factors at baseline, Model 2 adds randomized treatment received, and Model 3 adds FACT NTX4 and FACT‐TOI from 1 year after surgery.
Abbreviations: 5‐FU, 5‐fluorouracil; CAPE, capecitabine; OXA, oxaliplatin; SF‐36, Short‐Form 36‐item Survey.
(KPS 90–100) Fully active, able to carry on all pre‐disease performance without restriction; (KPS 70–80) restricted in physically strenuous activity but ambulatory; (KPS 50–60) ambulatory and capable of all self‐care but unable to perform any work activities.
Means sharing the same letter (a, b, c) for a variable within a column do not differ significantly using Hochberg multiple comparison adjustment, p < .05.
Older patients had significantly better MCS scores in Models 1 and 2 but were no different from younger patients in Model 3. Race/ethnicity was not associated with MCS at 5 years in any model, nor was baseline BMI; however, higher baseline KPS was associated with significantly better MCS scores in all three models. Treatment regimen was not associated with MCS; however, 1‐year FACT‐C TOI scores were strongly associated with 5‐year MCS (p < .0001). In these analyses, the third model explained the most variance in 5‐year MCS score with an adjusted R 2 of 0.27.
DISCUSSION
Although the overall incidence of colon and rectal cancer has been declining because of screening, in recent years there has been an alarming increase in individuals <50 years. 11 Among men and women younger than 50 years, tumors are most commonly diagnosed in the rectum (41% and 36%, respectively). 12 Neoadjuvant chemoradiotherapy is widely used for treatment of rectal cancer, but there has been limited evaluation of the HRQOL of patients who survive for many years after treatment ends. In addition to examining the acute treatment effects of NAC on HRQOL, this article also provides important information about the longer‐term toxicities of this treatment at 1 and 5 years after surgical treatment, along with patient‐related host factors (demographic and medical characteristics) that may influence these outcomes. Using both cancer‐specific and generic HRQOL measures, we examined the contribution of host factors in predicting treatment tolerability and long‐term outcomes.
This HRQOL study was conducted in parallel with one of the largest contemporary trials examining NAC. With this large sample size, we were able to evaluate whether patients reported differences in tolerability between two different fluoropyrimidine therapies, with or without OXA therapy. The R‐04 trial did not find any clinical benefit from the addition of OXA; however, the results of the HRQOL substudy provide detailed information on the treatment experience for patients who may still be receiving either infusional 5‐FU or CAPE with radiation today. Interestingly, we found relatively small differences in HRQOL between the OXA and non–OXA‐containing regimens. However, in a parallel evaluation of R‐04 clinician‐rated toxicity data, our research group detected statistically significant differences in treatment tolerability across the treatments, including greater toxicity in females. 13 At 1 year, the FACT‐C‐TOI and SF‐36 Vitality scores for all treatment regimens were similar. There was no statistically significant difference in neurotoxicity across the four treatment groups in terms of change scores from baseline to 1‐year postsurgery; however, interpretation of these findings is complicated by the lack of detailed data collection on the postsurgical adjuvant chemotherapy that may have contained OXA.
We found that at 1‐year after surgery, HRQOL measured with the FACT‐C‐TOI and the SF‐36 Vitality Scale had returned to near baseline levels, but some elevation in the SCL‐17 treatment‐related symptoms remained, and increased neurotoxicity compared with baseline for all treatment groups (Table 3). Overall, this is good news for rectal cancer patients, even though some treatment‐related symptoms may persist. In other adjuvant therapy trials in breast cancer, we have seen a similar pattern where the functional domains of HRQOL return to pretreatment levels, even though symptoms remain persistently elevated for several years beyond the end of treatment. 14
Predictive modeling suggested that some baseline medical and demographic factors are associated with more favorable 5‐year postsurgery SF‐36 PCS and MCS scores. For example, younger age, normal weight, and higher KPS for PCS, and older age and higher KPS for MCS, were predictive of better long‐term HRQOL. When chemotherapy treatment regimen was added to these models, there was only minimal change in the adjusted R 2 and some evidence that CAPE was associated with slightly worse PCS at 5 years. However, Model 3, which included the FACT‐C‐TOI and neurotoxicity symptoms at 1 year, contributed substantially to predicting 5‐year PCS and MCS. These results suggest it may be possible to identify individuals at risk for poorer HRQOL among longer term survivors. Furthermore, the finding that patients treated with CAPE had significantly worse PCS scores at 5 years compared with 5‐FU needs further evaluation/replication, because infusional 5‐FU might be preferred on this basis.
There are several limitations to the current study. Although this 5‐year prospective HRQOL assessment of rectal cancer survivors is one of the largest available, the late addition of the 5‐year assessment to the HRQOL substudy led to a reduced sample compared to the 1‐year original patient population. Furthermore, it was limited to those who were disease‐free and surviving. As has occurred in other studies, amending clinical trials to add a long‐term survivorship assessment often leads to a reduced and selected sample who respond, based on both patient accessibility and investigator motivation. 15 , 16 However, the survivor sample serves as a well‐characterized prospective cohort, even though it may not reflect the original trial participants, as was seen in our comparison of the 5‐year participants to the baseline HRQOL participants. Not reported here are other important findings related to the surgical treatment itself, which was not randomized (colostomy vs. SSS), but could also be contributing to the long‐term HRQOL assessment. Future evaluation of data from R‐04 examining the long‐term effects of surgery on functioning should contribute to a richer understanding of the HRQOL of these 5‐year survivors.
AUTHOR CONTRIBUTIONS
Patricia A. Ganz: Conceptualization, formal analysis, funding acquisition, methodology, visualization, writing – original draft, and writing – review/editing. Ron D. Hays: Formal analysis, methodology, writing – original draft, and writing – review/editing. Karen L. Spritzer: Formal analysis, methodology, writing – original draft, and writing – review/editing. André Rogatko: Funding acquisition and writing – review/editing. Clifford Y. Ko: Conceptualization and writing – review/editing. Linda H. Colangelo: Data curation, writing – original draft, and writing – review/editing. Amit Arora: Data curation, investigation, and writing – review/editing. Judith O. Hopkins: Data curation, formal analysis, validation, and writing – review/editing. Terry L. Evans: Data curation and writing – review/editing. Greg Yothers: Conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision, writing – original draft, and writing – review/editing.
Funding information
U10CA180868, U10CA180822, UG1CA189867; Roche; Sanofi; 1U01CA232859 as part of the NCI Cancer Moonshot.
CONFLICTS OF INTEREST
The authors made no disclosures.
Supporting information
Supporting information S1: Table S1
ACKNOWLEDGMENTS
U10CA180868, U10CA180822, UG1CA189867; Roche; Sanofi; 1U01CA232859 as part of the NCI Cancer Moonshot.
REFERENCES
- 1. Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R‐02. J Natl Cancer Inst. 2000;92:388–396. [DOI] [PubMed] [Google Scholar]
- 2. Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease‐free survival in patients with carcinoma of the rectum: NSABP R‐03. J Clin Oncol. 2009;27:5124–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allegra CJ, Yothers G, O'Connell MJ, et al. Neoadjuvant 5‐FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical Trial. J Natl Cancer Inst. 2015;107:djv:248. Erratum: J Natl Cancer Inst. 2016;108. Erratum: J Natl Cancer Inst. 2018;110:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R‐04. J Clin Oncol. 2014;32:1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell MM, Ganz PA, Lopa S, et al. Comparative effectiveness of sphincter‐sparing surgery versus abdominoperineal resection in rectal cancer: patient‐reported outcomes in National Surgical Adjuvant Breast and Bowel Project randomized trial R‐04. Ann Surg. 2015;261:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kopec JA, Yothers G, Ganz PA, et al. Quality of life in operable colon cancer patients receiving oral compared with intravenous chemotherapy: results from National Surgical Adjuvant Breast and Bowel Project Trial C‐06. J Clin Oncol. 2007;25:424–430. Erratum: J Clin Oncol. 2007;25:5540–5541. [DOI] [PubMed] [Google Scholar]
- 7. Ward WL, Hahn EA, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy‐Colorectal (FACT‐C) quality of life instrument. Qual Life Res. 1999;8:181–195. [DOI] [PubMed] [Google Scholar]
- 8. Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C‐07. J Clin Oncol. 2007;25:2205–2211. [DOI] [PubMed] [Google Scholar]
- 9. Ware JE Jr, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 10. Laucis NC, Hays RD, Bhattacharyya T. Scoring the SF‐36 in orthopaedics: a brief guide. J Bone Joint Surg Am. 2015;97:1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109:djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. [DOI] [PubMed] [Google Scholar]
- 13. Gresham G, Diniz MA, Razaee ZS, et al. Evaluating treatment tolerability in cancer clinical trials using the Toxicity Index. J Natl Cancer Inst. 2020;112:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ganz PA, Land SR, Geyer CE Jr, et al. Menstrual history and quality‐of‐life outcomes in women with node‐positive breast cancer treated with adjuvant therapy on the NSABP B‐30 trial. J Clin Oncol. 2011;29:1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ganz PA, Hussey MA, Moinpour CM, et al. Late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated on Southwest Oncology Group protocol s8897. J Clin Oncol. 2008;26:1223–1230. [DOI] [PubMed] [Google Scholar]
- 16. Ganz PA, Romond EH, Cecchini RS, et al. Long‐term follow‐up of cardiac function and quality of life for patients in NSABP Protocol B‐31/NRG Oncology: a randomized trial comparing the safety and efficacy of doxorubicin and cyclophosphamide (AC) followed by paclitaxel with AC followed by paclitaxel and trastuzumab in patients with node‐positive breast cancer with tumors overexpressing human epidermal growth factor receptor 2. J Clin Oncol. 2017;35:3942–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information S1: Table S1


