Summary
Objective
Rapid weight gain (RWG) in infancy is strongly associated with subsequent obesity risk, but little is known about the factors driving RWG. This study explored the child and maternal factors associated with infant RWG.
Methods
Data from seven Australian and New Zealand cohorts were used (n = 4542). Infant RWG was defined as a change in weight z‐score ≥0.67 from birth to age 1 year. Univariable and multivariable logistic regression assessed the association between child and maternal factors and infant RWG in each cohort. Meta‐analysis was conducted to obtain pooled effect sizes.
Results
Multivariable analyses revealed boys were more likely to experience RWG (OR 1.42 95% CI 1.22, 1.66) than girls. Higher birth weight in kg (OR 0.09, 95% CI 0.04, 0.20) and gestational age in weeks (OR 0.69, 95% CI 0.48, 0.98) were associated with lower RWG risk. Children who were breastfed for ≥6 months showed lower RWG risk (OR 0.45, 95% CI 0.38, 0.53). Children of native‐born versus overseas‐born women appeared to have higher RWG risk (OR 1.37, 95% CI 0.99, 1.90). Maternal smoking during pregnancy increased RWG risk (OR 1.60, 95% CI 1.28, 2.01), whereas children who started solids ≥6 months (OR 0.77, 95% CI 0.63, 0.93) and children with siblings (OR 0.68, 95% CI 0.57, 0.81) showed lower RWG risk in univariable analysis, but these associations were attenuated in multivariable analysis. No association was found for maternal age, education, marital status and pre‐pregnancy BMI.
Conclusion
Maternal country of birth, smoking status, child sex, birth weight, gestational age, infant feeding and parity were potential determinants of infant RWG.
Keywords: birth weight, determinants, infant, infant feeding, pooled analysis, rapid weight gain
Abbreviations
- BIS
Barwon Infant Study
- BMI
body mass index
- HB
Healthy Beginnings
- InFANT
Infant Feeding Activity and Nutrition Trial
- RWG
rapid weight gain
- WHO
World Health Organization
1. INTRODUCTION
Rapid weight gain (RWG) in infancy has been associated with an array of adverse health outcomes. 1 , 2 Systematic reviews have consistently shown a positive association between infant RWG and subsequent higher obesity risk with two‐ to threefold elevation in risk. 3 , 4 , 5 Emerging evidence has also linked infant RWG with other major risk factors of chronic diseases such as glucose intolerance, hypertension and dyslipidaemia. 6
Infant RWG can serve as a useful intermediate/surrogate marker for early detection of later obesity and associated adverse health outcomes. Prevention and early identification of RWG during infancy provide an important opportunity for optimizing lifelong health. RWG from birth to 1 year of age is more strongly linked to risk of subsequent obesity than RWG from birth to 2 years of age, 5 highlighting the 1st year of life as a critical period for primary prevention.
Despite extensive research highlighting the detrimental effect of infant RWG on later health outcomes, examination of the determinants of RWG during infancy is scarce. 7 The existing studies are limited to reporting factors associated with RWG in early childhood from birth to 2 years of age and focus on studying one or two factors of interest (e.g. infant feeding or maternal smoking). 7 However, as infant RWG is likely influenced by a broad range of factors, understanding how each predicts infant RWG can inform risk prediction and targeted prevention strategies.
Therefore, this study aimed to examine the association between a number of maternal and child factors in the prenatal and postnatal period and RWG during infancy across seven Australian and New Zealand cohorts. By using a uniform analysis approach, results for each factor were summarized and synthesized.
2. METHODS
2.1. Study design and cohorts
The present study included seven Australian and New Zealand cohorts: BIS (Barwon Infant Study), 8 HB (Healthy Beginnings), 9 , 10 , 11 INFANT (Infant Feeding Activity and Nutrition Trial), 12 , 13 INFANT Extend, 14 LIMIT, 15 , 16 NOURISH 17 , 18 and POI. 19 , 20 The cohort characteristics are shown in Table 1. BIS is a population‐based birth cohort, and all other cohorts are randomized controlled trials (RCTs) that aimed to prevent obesity in early childhood. Of the six RCTs, five included women in their first (HB, INFANT, INFANT EXTEND and NOURISH) or any (POI) pregnancies with no restrictions on maternal body mass index (BMI). LIMIT recruited pregnant women with BMI ≥25 kg/m2. Baseline recruitment was undertaken either antenatally or postnatally from 2007 to 2013. All seven cohorts assessed anthropometrics at birth and at follow‐up around 1 year of age (ranged from ages 7.0 to 13.6 months) and collected various maternal and child factors throughout this time. Ethical approval for each study was obtained from relevant institutional ethics committees and informed written consent from participants was obtained.
TABLE 1.
Study characteristics of the seven cohorts: analysis of determinants of rapid weight gain during infancy
| BIS | HB | INFANT | INFANT EXTEND | LIMIT | NOURISH | POI | |
|---|---|---|---|---|---|---|---|
| Study design | Population‐based birth cohort | RCT | Cluster RCT | Cluster RCT | RCT | RCT | RCT |
| Year at baseline | 2010–2013 | 2007 | 2008 | 2011–2012 | 2008–2011 | 2008–2009 | 2009–2010 |
| Participants | Pregnant women and their infants | First time mothers and their infants | First time mothers and their infants | First time mothers and their infants | Pregnant women with BMI≥25 and their infants | First time mothers and their infants | Pregnant women and their infants |
| Location | Geelong, Australia | Southwest Sydney, Australia | Melbourne, Australia | Melbourne, Australia | Adelaide, Australia | Brisbane and Adelaide, Australia |
Dunedin, New Zealand |
| Baseline recruitment | 15‐week gestation | 24–34‐week gestation | Infants aged 3–4 months | Infants aged 3–4 months | 10–20‐week gestation | Infants aged 4–7months | 28–30‐week gestation |
| Intervention | n/a | Infant feeding, family nutrition, physical activity | Infant feeding, physical activity, sedentary behaviours | Infant feeding, physical activity, sedentary behaviours | Lifestyle advice on diet and exercise for pregnant women | Infant feeding | Infant feeding, physical activity, sleep |
| Age at the follow‐up (Mean ± SD, months) | 13.0 ± 0.8 | 12.2 ± 0.8 | 9.2 ± 1.1 | 9.6 ± 2.2 | 7.0 ± 1.9 | 13.6 ± 1.3 | 12.2 ± 0.3 |
| Participants at baseline (n) | 1074 | 667 | 542 | 514 | 2126 | 698 | 802 |
| Participants lost to follow‐up (n) | 200 | 190 | 27 | 136 | 379 | 132 | 126 |
| Participants with missing covariates (n) | 158 | 33 | 32 | 118 | 281 | 45 | 24 |
| Participants included in the final analysis (n) | 716 | 444 | 483 | 260 | 1466 | 521 | 652 |
Abbreviations: BIS, Barwon Infant Study; HB, healthy beginnings; INFANT Extend, Infant Feeding Activity and Nutrition Trial Extend; INFANT, Infant Feeding Activity and Nutrition Trial; POI, prevention of obesity in infancy trial.
2.2. Rapid weight gain during infancy
Across seven cohorts, child length and weight at birth were transcribed from child health records. Child length and weight around 1 year of age were measured using standard protocols by trained staff. WHO growth standards 21 were used to calculate age‐ and sex‐specific weight‐for‐age z‐scores at birth and the follow‐up, and the difference between the time points was calculated. Rapid weight gain was defined as a change in weight for age z‐score ≥0.67, which is clinically equivalent to crossing one centile line in a growth chart. 5 , 22
2.3. Maternal and child factors
A range of maternal and child factors were collected across the cohorts. The following maternal factors were assessed: country of birth (native born vs. overseas born), education level (tertiary education vs. non‐tertiary education), marital status (married vs. not married), smoking status (smoker vs. non‐smoker) and pre‐ or early pregnancy BMI (kg/m2). Child factors included sex (boys vs. girls), birth weight (kg), any breastfeeding duration (<6 vs. ≥6 months), timing of solid introduction (before <6 vs. at or after ≥6 months), parity (≥1 sibling vs. no sibling) and gestational age (weeks). Intervention allocation was also assessed as a determinant in six RCTs. Additional analyses were conducted to evaluate categorical forms of maternal pre‐pregnancy BMI (healthy weight <25 kg/m2 vs. overweight 25–30 kg/m2 vs. obesity≥30 kg/m2), child birth weight (low/normal <4 kg vs. high ≥4 kg) and gestational age (pre‐term<37 weeks vs. full term≥37 weeks) as determinants of infant RWG. Due to the small number of low birth weight children (1.2%–6.9%), they were combined with normal weight children for analyses. Variables collected in ≥2 cohorts were examined as determinants for infant RWG. All determinants were coded consistently across the seven cohorts to enable comparison and meta‐analysis to obtain pooled effects.
2.4. Statistical analysis
All analyses were conducted in Stata 16 with statistical significance set at p < 0.05 (two sided). A descriptive analysis of cohort characteristics was conducted. Analyses were performed to compare the cohort characteristics of those retained and excluded from the current analysis. We conducted separate analyses in each cohort using a consistent analysis approach. As some factors (e.g. child birth weight) may underlie the causal pathways between other factors (e.g. maternal smoking, parity) and RWG, both univariable and multivariable logistic regression analyses were conducted. Univariable logistic regression with infant RWG as the outcome and each maternal and child variable and intervention allocation as the exposure were initially conducted. All maternal and child variables were then included simultaneously in the same multivariable logistic regression model to assess the respective association with infant RWG. Pearson correlations were performed to determine multicollinearity amongst all maternal and child variables. For variables that showed high correlation, the variable that exhibited the stronger association with infant RWG was included in the final multivariable model along with other factors. Child birth weight and gestational age showed a correlation of r > 0.5 across all cohorts, and child birth weight showed a stronger association with infant RWG. No correlations were found for other maternal and child factors. Thus, the final multivariable model included all child and maternal variables except for gestational age. Variables included in the final multivariable model for each cohort are presented in Table S1. The adjusted odds ratio for the association between gestational age and infant RWG was investigated in a separate multivariable model excluding child birth weight. The odds ratios (95% confidence interval) of each variable from univariable and multivariable models were included in a subsequent meta‐analysis. I 2 statistics were used to evaluate heterogeneity across the cohorts. For variables that showed the presence of heterogeneity across studies (I 2 value >50%, p < 0.1), a random‐effects meta‐analysis was conducted to calculate the pooled effect size. Fixed effect meta‐analysis was used to obtain pooled effect size for variables exhibiting no heterogeneity across studies.
2.5. Additional analyses
For variables that showed discrepant results between univariable and multivariable models, meditation analyses were conducted to identify potential mediating effects using the ‘PARAMED’ command. Indirect (mediating) effects from the multivariable model were estimated in each study and meta‐analyses were performed to obtain pooled indirect effects.
Considering the variability in length of follow‐up across studies, additional analyses excluding three cohorts (INFANT, INFANT Extend and LIMIT) that involved follow‐up before age 12 months were also conducted. This additional analysis also tests whether excluding children of women with overweight and obesity from the LIMIT trial influence the pooled effects.
To test the impact of missing covariates on the analyses, multiple imputations by chained equations with 10 data sets were conducted to impute missing child and maternal covariates specifying predictive mean matching to predict continuous variables and logistic regression to predict categorical variables in each cohort. The ‘mi estimate’ command was used to combine estimates from 10 imputed data sets.
3. RESULTS
3.1. Cohort characteristics
The seven cohorts included a total of 6423 women and their children at the study baseline. Excluding those lost to follow‐up and children with missing data on RWG (n = 1190) and covariates (n = 691), resulted in a final sample of 4542 children (70.7%) being included in the analysis (Table 1). Cohort characteristics between those retained and excluded from the analysis in each cohort were similar (data not shown).
Maternal and child characteristics of the seven cohorts are shown in Table 2. Mean maternal age ranged from 26.6 to 32.6 years. Four studies reported maternal country of birth with most women (66.9%–90.6%) being native born. Of the six cohorts with information on maternal education, five included a higher proportion of women with tertiary education (55.1%–64.2%). The exception was HB, which included a greater proportion of non‐tertiary educated women (73.0%). Most women were married across the five cohorts (75.6%–98.1%). The percentage of maternal smoking during pregnancy was 6.1%–17.6% across five cohorts. Apart from LIMIT that consisted of women with BMI ≥25 kg/m2, the mean maternal BMI was comparable across six other cohorts (24.4–25.9 kg/m2). For child factors, all seven cohorts had an equal proportion of boys and girls, and mean birth weights of 3.3–3.6 kg. Three studies reported parity with 41.9%–48.3% of children had no siblings. For any breastfeeding duration, four cohorts (BIS, INFANT, INFANT EXTEND and NOURISH) had more children (63.8%–77.7%) with breastfeeding duration ≥6 months. HB had more children with breastfeeding duration <6 months (60.6%). LIMIT and POI had an even proportion of children in the two breastfeeding duration groups. Five out of six cohorts had more children who started solids before 6 months (59.5%–98.2%). The proportion of preterm babies spans from 0.5% to 11.0% in five cohorts. Percentage RWG from birth to around 1 year of age ranged from 21.6%(POI) to 41.7%(HB).
TABLE 2.
Maternal and child characteristics of seven cohorts: analysis of determinants of rapid weight gain during infancy
| BIS (n = 716) | HB (n = 444) | INFANT (N = 483) | INFANT EXTEND (n = 260) | LIMIT (n = 1466) | NOURISH (n = 521) | POI (n = 652) | |
|---|---|---|---|---|---|---|---|
| Intervention allocation | |||||||
| Control | n/a | 49.5% | 49.3% | 49.2% | 49.8% | 52.0% | 51.2% |
| Intervention | 50.5% | 50.7% | 50.8% | 50.2% | 48.0% | 48.8% | |
| Maternal factors | |||||||
| Age | 31.8 ± 4.3 | 26.6 ± 5.3 | 32.3 ± 4.1 | 32.6 ± 4.1 | 30.0 ± 5.3 | 31.7 ± 4.9 | 32.0 ± 4.9 |
| Country of birth | |||||||
| Native born | 90.6% | 66.9% | 78.9% | 78.5% | n/a | n/a | n/a |
| Overseas born | 9.4% | 33.1% | 21.1% | 21.5% | |||
| Education | |||||||
| Non‐tertiary | 44.3% | 73.0% | 44.9% | 35.8% | n/a | 35.9% | 36.3% |
| Tertiary | 55.7% | 27.0% | 55.1% | 64.2% | 64.1% | 63.7% | |
| Marital status | |||||||
| Married | 75.6% | 90.3% | 98.1% | 96.9% | n/a | n/a | 96.2% |
| Not married | 24.4% | 9.7% | 1.9% | 3.1% | 3.8% | ||
| Smoking during pregnancy | |||||||
| Yes | 12.4% | 17.6% | n/a | n/a | 9.1% | 6.1% | 7.6% |
| No | 87.6% | 82.4% | 90.9% | 93.9% | 92.4% | ||
| Maternal pre‐pregnancy BMI | 25.3 ± 5.3 | 25.1 ± 5.7 | 24.4 ± 5.1 | 24.6 ± 5.1 | 32.4 ± 5.9 | 25.9 ± 5.1 | 25.1 ± 4.9 |
| Normal weight | 58.1% | 59.9% | 64.6% | 63.1% | 51.8% | 58.4% | |
| Overweight | 25.1% | 25.0% | 22.8% | 24.6% | 43.0% | 30.7% | 28.4% |
| Obesity | 16.8% | 15.1% | 12.6% | 12.3% | 57.0% | 17.5% | 13.2% |
| Child factors | |||||||
| Child sex | |||||||
| Boys | 47.6% | 51.8% | 52.4% | 53.1% | 49.3% | 49.5% | 51.4% |
| Girls | 52.4% | 48.2% | 47.6% | 46.9% | 50.7% | 50.5% | 48.6% |
| Birth weight (kg) | 3.5 ± 0.5 | 3.4 ± 0.6 | 3.4 ± 0.6 | 3.3 ± 0.6 | 3.5 ± 0.6 | 3.5 ± 0.4 | 3.6 ± 0.5 |
| <2.5 kg | 2.4% | 5.9% | 6.8% | 6.9% | 3.7% | 0% | 1.2% |
| 2.5–4 kg | 0.2% | 83.8% | 80.8% | 83.1% | 79.1% | 88.3% | 82.5% |
| ≥4 kg | 17.4% | 10.4% | 12.4% | 10.0% | 17.2% | 11.7% | 16.3% |
| Parity | |||||||
| No sibling | 43.6% | n/a | n/a | n/a | 41.9% | n/a |
48.3% 51.7% |
| ≥1 sibling | 56.4% | 58.1% | |||||
| Breastfeeding duration | |||||||
| <6 months | 36.2% | 60.6% | 35.4% | 22.3% | 47.7% | 33.8% | 47.9% |
| ≥6 months | 63.8% | 39.4% | 64.6% | 77.7% | 52.3% | 66.2% | 52.1% |
| Solid introduction | |||||||
| <6 months | 98.2% | 50.2% | 71.2% | 92.7% | n/a | 59.5% | 72.0% |
| ≥6 months | 1.8% | 49.8% | 28.8% | 7.3% | 40.5% | 28.0% | |
| Gestational age (weeks) | 39.5 ± 1.4 | 38.9 ± 2.3 | 38.9 ± 2.0 | 39.3 ± 1.7 | 40.0 ± 1.2 | ||
| Premature (<37 weeks) | 3.5% | n/a | 11.0% | 7.8% | 5.9% | n/a | 0.5% |
| Full term (≥37 weeks) | 96.5% | 89.0% | 92.3% | 94.1% | 99.5% | ||
| Rapid infant weight gain | |||||||
| Yes | 26.1% | 41.7% | 29.0% | 30.4% | 23.6% | 29.6% | 21.6% |
| No | 73.9% | 58.3% | 71.0% | 69.6% | 76.4% | 70.4% | 78.4% |
Note: Values are presented as Mean ± SD or percentage (%).
Abbreviations: BIS, Barwon Infant Study; HB, healthy beginnings; INFANT Extend, Infant Feeding Activity and Nutrition Trial Extend; INFANT, Infant Feeding Activity and Nutrition Trial; POI, prevention of obesity in infancy trial.
Pooled estimates of univariable and multivariable analyses between maternal/child factors and infant RWG from birth to around 1 year of age are presented in Table 3. Children of native‐born women showed a 30% greater tendency to experience RWG than those of overseas‐born women (p = 0.05). Maternal smoking was associated with a 60% higher RWG risk (p < 0.001). Furthermore, boys appeared to show a 13% higher risk of experiencing RWG than girls (p = 0.07). In contrast, higher child birth weight, any breastfeeding duration≥6 months, solid introduction ≥6 months, gestational age and children with siblings were associated with lower RWG risk (OR ranged from 0.11 to 0.77, p < 0.05). The cohort‐specific univariable associations of these factors and infant RWG are shown in Figures 1 and 2. The significant associations for maternal smoking, parity and timing of solid introduction attenuated in the multivariable model (Table 3). The attenuation of the associations was largely attributable to adjustment for child birth weight. Associations of child sex, birth weight, any breastfeeding duration and gestational age with infant RWG were significant in the multivariable model (p < 0.05). Cohort‐specific results from the multivariable model are presented in Figures S1 and S2. No evidence of an association was found for maternal age, education level, marital status, BMI or intervention allocation in all models (Table 3, Figures S3 and S4).
TABLE 3.
Pooled estimates of the association between maternal and child factors and rapid weight gain during infancy from seven cohorts (Barwon Infant Study, Healthy Beginnings, INFANT, INFANT Extend, LIMIT, NOURISH, POI) a
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p‐value | Odds Ratio | 95% CI | p‐value | |
| Intervention allocation (Intervention vs. control) | 0.99 | 0.73, 1.22 | 0.66 | 0.93 | 0.70, 1.22 | 0.58 |
| Maternal factors | ||||||
| Age (years) | 1.00 | 0.99, 1.01 | 0.78 | 1.00 | 0.99, 1.02 | 0.68 |
| Country of birth (Native born vs. overseas born) | 1.30 | 1.00, 1.68 | 0.05 | 1.37 | 0.99, 1.90 | 0.06 |
| Education (non‐tertiary vs. tertiary) | 1.07 | 0.91, 1.26 | 0.41 | 0.89 | 0.72, 1.10 | 0.27 |
| Marital status (Married vs. not married) | 0.93 | 0.69, 1.25 | 0.62 | 1.16 | 0.80, 1.69 | 0.43 |
| Smoking status (Yes vs. no) | 1.60 | 1.28, 2.01 | <0.001 | 1.13 | 0.86, 1.48 | 0.37 |
| Maternal pre‐pregnancy BMI (kg/m2) | 1.00 | 0.99, 1.01 | 0.88 | 1.01 | 0.99, 1.04 | 0.34 |
| Child factors | ||||||
| Child sex (Boys vs. girls) | 1.13 | 0.99, 1.29 | 0.07 | 1.42 | 1.22, 1.66 | <0.001 |
| Birth weight(kg) | 0.11 | 0.05, 0.21 | <0.001 | 0.09 | 0.04, 0.20 | <0.001 |
| Breastfeeding duration (≥6 mo vs. <6 mo) | 0.48 | 0.42, 0.55 | <0.001 | 0.45 | 0.38, 0.53 | <0.001 |
| Solid introduction (≥6 mo vs. <6 mo) | 0.77 | 0.63, 0.93 | 0.001 | 0.88 | 0.71, 1.08 | 0.22 |
| Parity (≥1 sibling vs. no sibling) | 0.68 | 0.57, 0.81 | <0.001 | 0.88 | 0.70, 1.11 | 0.28 |
| Gestational age (weeks) | 0.69 | 0.49, 0.96 | 0.03 | 0.69 | 0.48, 0.98 | 0.04 |
Note: Multivariable model included all maternal and child factors in the same model, birth weight and gestational age was analyzed in separate models.
Univariable model included each factor as the exposure and infant RWG as the outcome.
FIGURE 1.
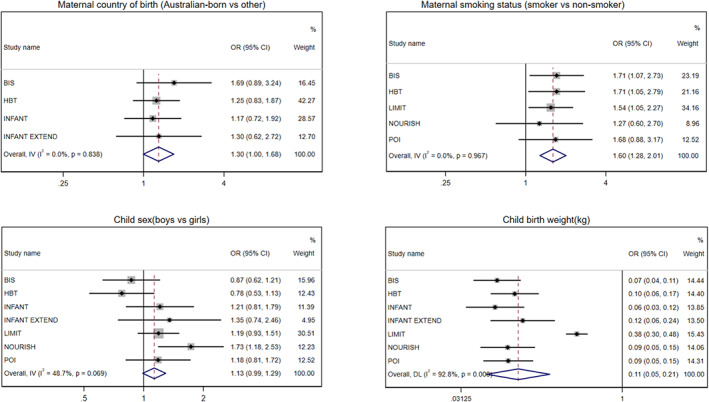
Cohort‐specific and pooled odds ratio (95% confidence interval) of association between maternal and child factors (maternal country of birth, smoking status, child sex and birth weight) and risk of rapid weight gain during infancy. Estimates were obtained from the univariable model without adjustment for covariate. The overall pooled odds ratio was obtained from either fixed or random‐effects meta‐analysis
FIGURE 2.
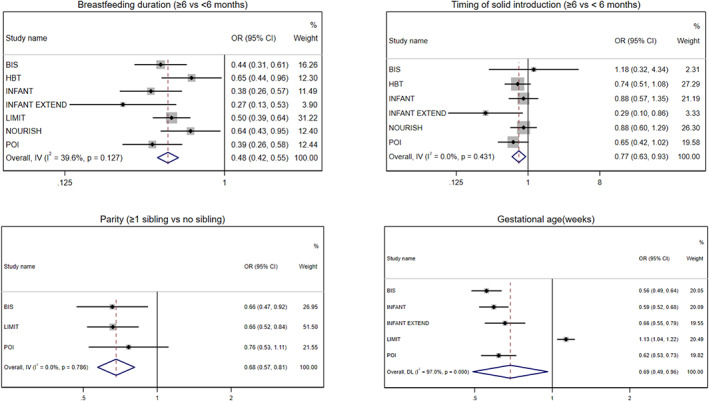
Cohort‐specific and pooled odds ratio (95% confidence interval) of association between child factors (breastfeeding duration, timing of solid introduction, parity and gestational age) and risk of rapid weight gain during infancy. Estimates were obtained from the univariable model without adjustment for covariate. The overall pooled odds ratio was obtained from either fixed or random‐effects meta‐analysis
3.2. Additional analysis
Mediation analyses were undertaken to test the potential mediating effects of child birth weight on the associations between maternal smoking, parity, timing of solid introduction and RWG. Cohort‐specific and pooled indirect effects are shown in Figure S5. Child birth weight mediated the association between maternal smoking and RWG with a pooled indirect effect of 1.46 95% CI 1.21, 1.76. Significant mediating effects of child birth weight on the association between parity (≥1 sibling) and RWG were also revealed (0.71, 95% CI 0.55, 0.93). Child birth weight was found to mediate the association between timing of solid introduction and RWG in two cohorts (INFANT and INFANT EXTEND), but the overall pooled indirect effect was not statistically significant (0.87, 95% CI 0.69, 1.10).
The associations between the categorical form of maternal BMI, birth weight and gestational age with infant RWG are shown in Table S2. Birth weight ≥4 kg (OR 0.17, 95% CI 0.12, 0.23) and full term ≥37 weeks (OR 0.20, 95% CI 0.04, 1.05) were associated with lower odds of infant RWG. RWG risk did not differ by maternal BMI categories. Additional analyses excluding three cohorts showed similar results (Tables S3 and S4).
A total of 5223 (81.5%) participants were included in the analyses with the imputation of missing child and maternal covariates. A comparison of results from multivariable analyses without and with multiple imputations is shown in Table S5 and revealed similar results with comparable effect sizes for all variables except for the maternal country of birth where the associations observed were somewhat attenuated (OR 1.19 95% CI 0.88, 2.37).
4. DISCUSSION
Using data from seven cohorts comprising over 4000 participants, this study assessed the maternal and child determinants of RWG in the 1st year of life. Evidence of significant associations was found for maternal smoking status, child sex, birth weight, gestational age, parity, any breastfeeding duration and timing of solid introduction with infant RWG. The significant association of maternal smoking status, timing of solid introduction and parity with infant RWG attenuated after adjusting for child birth weight. Maternal country of birth also appeared to be a potential determinant of infant RWG. In contrast, maternal age, education level, marital status and pre‐pregnancy BMI were not associated with infant RWG.
Our study findings corroborate results from the limited evidence base on determinants of infant RWG. Congruent with our findings, studies in German 23and Swedish children 24 revealed that maternal smoking during pregnancy increased the risk for RWG from birth to 24 months and from birth to 3–4 or 6 months, and the evidence of association attenuated after adjustment for other factors including birth weight. Our results suggest that the increased RWG risk in children of smoking women was mediated by child birth weight. It has been suggested that in utero exposure to carbon monoxide and nicotine reduces foetal oxygen supply, resulting in intrauterine growth restriction and low birth weight, which in turn promotes RWG during infancy. 25 , 26 Similar to our findings on the maternal country of birth, previous studies reported ethnic differences in RWG from birth to 6 months 27 and growth patterns during infancy. 28 , 29 Ethnic differences in infant growth are likely influenced by sociocultural factors and ethnic variations in infant feeding practices. 30
In agreement with findings from studies of German, 27 Swedish 24 and Canadian children, 31 our study found that neither maternal education nor pre‐pregnancy BMI was predictive of infant RWG. Maternal education and BMI might not directly influence RWG but may be more likely to affect factors associated with RWG. For instance, as a proxy for socioeconomic position, maternal education is likely to influence maternal lifestyle habits (e.g. smoking) and infant feeding, which may, in turn, influence infant RWG. Indeed, Reeske et al. 27 found a higher RWG prevalence in a low versus high maternal education group, but the difference disappeared after adjusting for maternal smoking and infant feeding. Furthermore, our study included cohorts that had a higher proportion of tertiary‐educated women, which may also contribute to a lack of association for maternal education. Higher maternal BMI as a risk factor for obesity in children is widely recognized and may be attributable to genetics and metabolic pathways as well as maternal influences on child lifestyle habits. 32 The lack of association between maternal BMI and infant RWG revealed in our study suggests that infant RWG is unlikely to be an underlying factor contributing to the link between maternal BMI and childhood obesity. However, given the paucity of literature on maternal BMI and infant RWG, further investigation is warranted.
Our finding that boys showed a higher RWG risk than girls is consistent with the existing studies. In a Swedish cohort (n = 1780), girls were 53% and 29% less likely to experience RWG from birth to 3–4 and 6 months, respectively, than boys. 24 Similarly, a German study (n = 370) also found that boys tend to show a greater risk of RWG from birth to 24 months than girls. 23 Sex differences in infant growth patterns have been widely documented. A large Dutch study observed sex differences in both foetal and infant growth with boys exhibiting higher body weight than girls from birth to 12 months. 33 The differences in male and female placental function and biomarkers may explain the sex‐specific growth patterns during infancy. 34 For example, male and female placentae have different protein and gene expressions. In addition, female foetuses seem to have a higher level of certain placental biomarkers than males during the first trimester of pregnancies, resulting in both sex‐specific foetal and infant growth. 34
Associations of similar strengths between higher birth weight and gestational age with lower RWG risk have been shown in other studies. 24 , 27 Rapid growth is commonly observed in low birth weight or preterm infants, 4 thought to be partly due to a recovery from undernutrition in utero. Similar mechanisms apply to gestational age. It is worth noting that WHO growth standards describe normal child growth under optimal environmental conditions and do not account for gestational age. The use of WHO growth standards may inappropriately characterize growth for preterm infants. However, the influence on the current findings is expected to be small as the proportion of preterm infants in our sample is low.
Several studies explored parity as a risk factor for infant RWG. Consistent with our findings, two studies found that children with siblings had a lower risk of RWG than first‐born children. 23 , 35 It is postulated that women with first pregnancy are more likely to have children with low birth weight than women with multiple pregnancies because uterine efficiency for delivering oxygen and nutrients to the foetus tends to increase with more pregnancies. 36 Another explanation is that women in the first pregnancy are more likely to experience breastfeeding problems (e.g. early unintended breastfeeding cessation and formula supplementation), consequently increasing RWG risk. 37 , 38 Indeed, the current study found that the association between parity and RWG risk was mediated by child birth weight.
The beneficial effects of breastfeeding in lowering RWG risk during infancy are well‐documented 24 , 27 , 39 , 40 and are supported by several possible hypotheses. Breastfeeding women may be more likely to practice responsive feeding (i.e. identifying and responding appropriately to infants' hunger and satiety cues), 41 which prevents overfeeding. Specific nutritional components of breast milk (e.g. leptin and ghrelin) may support appetite control and regulation and in turn favourable energy balance and growth patterns. 42 Additionally, breast milk has a lower protein content than infant formula. It has been proposed that excess protein intake may convert to glucose via gluconeogenesis, which could be subsequently stored as fat and contribute to excess body weight and fat gain. 43
The existing literature on the timing of solid introduction focused on its relationship with obesity risk. One study found no evidence of an association between timing of solid introduction and change in weight z‐score from birth to 12 months. 44 A link between early introduction of solids before 4 months and greater obesity risk has been revealed in some studies, 45 likely attributable to a higher intake of energy and protein from the early introduction of solids. 45 Our finding that introduction of solids after 6 months predicted lower infant RWG risk supports these findings. Other factors may confound the association as the significant associations disappeared after controlling for other child and maternal factors.
The current study has several strengths and limitations that warrant discussion. This is the first study that comprehensively assessed the associations of a broad range of maternal and child factors with infant RWG in several cohorts of Australian and New Zealand children. Moreover, mediation analyses were conducted to explore potential meditational pathways. The large sample size and overall synthesis of results across seven cohorts encompassing diverse population groups enhance both the robustness and generalizability of the study findings. It is important to note that the average attrition rate across seven studies is about 30%. However, the comparison of cohort characteristics between those retained and excluded from the current analysis was similar. Analyses with multiple imputations of missing covariates revealed similar results. Although we explored a broad range of factors associated with RWG during infancy, other factors such as maternal alcohol consumption 35 and gestational weight gain, 24 , 27 , 31 which have been previously shown to be risk factors of infant RWG, were not assessed in our study.
This study contributes to the limited body of evidence on the determinants of infant RWG and provides valuable insights on at‐risk groups and preventive strategies for early health promotion programs and interventions. Public health policy and practice could focus on prenatal intervention strategies to help women (particularly those in their first pregnancy) to deliver full‐term children with healthy birth weight such as supporting women to abstain from smoking during pregnancy. Moreover, promote extended breastfeeding and discourage the early introduction of solids during the postnatal period.
5. CONCLUSION
Several maternal and child factors including maternal country of birth, smoking during pregnancy, child sex, birth weight, gestational age, parity and early infant feeding were identified as determinants of RWG in the 1st year of life. The findings will inform the identification of at‐risk groups and the design of targeted strategies for early health promotion and chronic disease prevention programs.
AUTHOR CONTRIBUTIONS
MZ and KJC conceived the study. MZ conducted the statistical analysis, interpreted the results and drafted the manuscript. KJC, KDH, PV, JD, LMW, LB, RT, RB, SM, PDS and MLKT collected data and contributed to results interpretation. All authors critically reviewed and approved the final manuscript.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
Supporting information
Data S1
ACKNOWLEDGEMENTS
We acknowledge the contribution of all parents and children who participated in the seven Australian and New Zealand studies. Open access publishing facilitated by Deakin University, as part of the Wiley ‐ Deakin University agreement via the Council of Australian University Librarians.
Zheng M, Hesketh KD, Vuillermin P, et al. Determinants of rapid infant weight gain: A pooled analysis of seven cohorts. Pediatric Obesity. 2022;17(10):e12928. doi: 10.1111/ijpo.12928
Funding informationMZ is supported by the Australian National Health Medical Research Council Early Career Research Fellowship (GNT 1124283). KDH is supported by an Australian Research Council Future Fellowship (FT130100637).
REFERENCES
- 1. Singhal A. Long‐term adverse effects of early growth acceleration or catch‐up growth. Ann Nutr Metab. 2017;70(3):236‐240. [DOI] [PubMed] [Google Scholar]
- 2. Haschke F, Binder C, Huber‐Dangl M, Haiden N. Early‐life nutrition, growth trajectories, and long‐term outcome. Nestle Nutr Inst Workshop Ser. 2019;90:107‐120. [DOI] [PubMed] [Google Scholar]
- 3. Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life–a systematic review. Obes Rev. 2005;6(2):143‐154. [DOI] [PubMed] [Google Scholar]
- 4. Ong KK, Kennedy K, Castaneda‐Gutierrez E, et al. Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatrica (Oslo, Norway: 1992). 2015;104(10):974‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng M, Lamb KE, Grimes C, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta‐analysis of evidence. Obes Rev. 2018;19(3):321‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musa MG, Kagura J, Pisa PT, Norris SA. Relationship between early growth and CVD risk factors in adolescents. J Dev Orig Health Dis. 2016;7(2):132‐143. [DOI] [PubMed] [Google Scholar]
- 7. Chrestani MA, Santos IS, Horta BL, Dumith SC, de Oliveira Dode MA. Associated factors for accelerated growth in childhood: a systematic review. Matern Child Health J. 2013;17(3):512‐519. [DOI] [PubMed] [Google Scholar]
- 8. Vuillermin P, Saffery R, Allen KJ, et al. Cohort profile: the Barwon infant study. Int J Epidemiol. 2015;44(4):1148‐1160. [DOI] [PubMed] [Google Scholar]
- 9. Wen LM, Baur LA, Rissel C, Wardle K, Alperstein G, Simpson JM. Early intervention of multiple home visits to prevent childhood obesity in a disadvantaged population: a home‐based randomised controlled trial (healthy beginnings trial). BMC Public Health. 2007;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wen LM, Baur LA, Simpson JM, Rissel C, Flood VM. Effectiveness of an early intervention on infant feeding practices and "tummy time": a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):701‐707. [DOI] [PubMed] [Google Scholar]
- 11. Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children's BMI at age 2: randomised controlled trial. BMJ. 2012;344:e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell K, Hesketh K, Crawford D, Salmon J, Ball K, McCallum Z. The infant feeding activity and nutrition trial (INFANT) an early intervention to prevent childhood obesity: cluster‐randomised controlled trial. BMC Public Health. 2008;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell KJ, Lioret S, McNaughton SA, et al. A parent‐focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics. 2013;131(4):652‐660. [DOI] [PubMed] [Google Scholar]
- 14. Campbell KJ, Hesketh KD, McNaughton SA, et al. The extended infant feeding, activity and nutrition trial (InFANT Extend) program: a cluster‐randomized controlled trial of an early intervention to prevent childhood obesity. BMC Public Health. 2016;16:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodd JM. Dietary and lifestyle advice for pregnant women who are overweight or obese: the LIMIT randomized trial. Ann Nutr Metab. 2014;64(3–4):197‐202. [DOI] [PubMed] [Google Scholar]
- 16. Dodd JM, Turnbull D, McPhee AJ, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ. 2014;348:g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daniels LA, Magarey A, Battistutta D, et al. The NOURISH randomised control trial: positive feeding practices and food preferences in early childhood ‐ a primary prevention program for childhood obesity. BMC Public Health. 2009;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daniels LA, Mallan KM, Battistutta D, Nicholson JM, Perry R, Magarey A. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. Int J Obes (Lond). 2012;36(10):1292‐1298. [DOI] [PubMed] [Google Scholar]
- 19. Taylor BJ, Heath AL, Galland BC, et al. Prevention of overweight in infancy (POI.Nz) study: a randomised controlled trial of sleep, food and activity interventions for preventing overweight from birth. BMC Public Health. 2011;11:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor RW, Heath AL, Galland BC, et al. Three‐year follow‐up of a randomised controlled trial to reduce excessive weight gain in the first two years of life: protocol for the POI follow‐up study. BMC Public Health. 2016;16(1):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Length/Height‐for‐Age, Weight‐for‐Age, Weight‐for‐Length, Weight‐for‐Height and Body Mass Index‐for‐Age: Methods and Development. World Health Organization; 2006. [Google Scholar]
- 22. Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch‐up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karaolis‐Danckert N, Buyken AE, Kulig M, et al. How pre‐ and postnatal risk factors modify the effect of rapid weight gain in infancy and early childhood on subsequent fat mass development: results from the multicenter allergy study 90. Am J Clin Nutr. 2008;87(5):1356‐1364. [DOI] [PubMed] [Google Scholar]
- 24. Lindholm A, Bergman S, Alm B, et al. Nutrition‐ and feeding practice‐related risk factors for rapid weight gain during the first year of life: a population‐based birth cohort study. BMC Pediatr. 2020;20(1):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Secker‐Walker RH, Vacek PM, Flynn BS, Mead PB. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstet Gynecol. 1997;89(5, Part 1):648‐653. [DOI] [PubMed] [Google Scholar]
- 26. McGrath‐Morrow SA, Gorzkowski J, Groner JA, et al. The effects of nicotine on development. Pediatrics. 2020;145(3):e20191346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reeske A, Spallek J, Bammann K, et al. Migrant background and weight gain in early infancy: results from the German study sample of the IDEFICS study. PLoS One. 2013;8(4):e60648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bulk‐Bunschoten AM, Pasker‐de Jong PC, van Wouwe JP, de Groot CJ. Ethnic variation in infant‐feeding practices in The Netherlands and weight gain at 4 months. J Hum Lact. 2008;24(1):42‐49. [DOI] [PubMed] [Google Scholar]
- 29. Bolton KA, Kremer P, Laws R, Campbell KJ, Zheng M. Longitudinal analysis of growth trajectories in young children of Chinese‐born immigrant mothers compared with Australian‐born mothers living in Victoria, Australia. BMJ Open. 2021;11(2):e041148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffiths LJ, Tate AR, Dezateux C. Millennium cohort study child health G. do early infant feeding practices vary by maternal ethnic group? Public Health Nutr. 2007;10(9):957‐964. [DOI] [PubMed] [Google Scholar]
- 31. Subhan FB, Colman I, McCargar L, Bell RC. Higher pre‐pregnancy BMI and excessive gestational weight gain are risk factors for rapid weight gain in infants. Matern Child Health J. 2017;21(6):1396‐1407. [DOI] [PubMed] [Google Scholar]
- 32. Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre‐pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta‐analysis. PLoS One. 2013;8(4):e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Broere‐Brown ZA, Baan E, Schalekamp‐Timmermans S, Verburg BO, Jaddoe VW, Steegers EA. Sex‐specific differences in fetal and infant growth patterns: a prospective population‐based cohort study. Biol Sex Differ. 2016;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alur P. Sex differences in nutrition, growth, and metabolism in preterm infants. Front Pediatr. 2019;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oyama M, Nakamura K, Tsuchiya Y, Yamamoto M. Unhealthy maternal lifestyle leads to rapid infant weight gain: prevention of future chronic diseases. Tohoku J Exp Med. 2009;217(1):67‐72. [DOI] [PubMed] [Google Scholar]
- 36. Hinkle SN, Albert PS, Mendola P, et al. The association between parity and birthweight in a longitudinal consecutive pregnancy cohort. Paediatr Perinat Epidemiol. 2014;28(2):106‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Demirci JR, Bogen DL. An ecological momentary assessment of Primiparous Women's breastfeeding behavior and problems from birth to 8 weeks. J Hum Lact. 2017;33(2):285‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hackman NM, Schaefer EW, Beiler JS, Rose CM, Paul IM. Breastfeeding outcome comparison by parity. Breastfeed Med. 2015;10(3):156‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shinn LM, Tangney CC, Busche C, Sharp CM, Mullen MC. Demographic correlates of infant feeding practices and growth performance in the first year of life. Int J Pediatr. 2018;2018:6569204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iguacel I, Álvarez L, Cabero MJ, et al. Rapid infancy weight gain during the complementary feeding period in a cohort of Spanish infants. Child Adolescent Obes. 2019;2(1):63‐78. [Google Scholar]
- 41. Jansen E, Mallan KM, Byrne R, Daniels LA, Nicholson JM. Breastfeeding duration and authoritative feeding practices in first‐time mothers. J Hum Lact. 2016;32(3):498‐506. [DOI] [PubMed] [Google Scholar]
- 42. Lind MV, Larnkjaer A, Molgaard C, Michaelsen KF. Breastfeeding, breast milk composition, and growth outcomes. Nestle Nutr Inst Workshop Ser. 2018;89:63‐77. [DOI] [PubMed] [Google Scholar]
- 43. Stokes A, Campbell KJ, Yu HJ, et al. Protein intake from birth to 2 years and obesity outcomes in later childhood and adolescence: a systematic review of prospective cohort studies. Adv Nutr. 2021;12:1863‐1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klag EA, McNamara K, Geraghty SR, Keim SA. Associations between breast Milk feeding, introduction of solid foods, and weight gain in the first 12 months of life. Clin Pediatr (Phila). 2015;54(11):1059‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pearce J, Taylor MA, Langley‐Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37(10):1295‐1306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


