Abstract
BACKGROUND
Severe COVID-19 infection in pregnancy has been associated with an increase in adverse perinatal outcomes, although studies differ regarding which outcomes are affected. Increased characterization of obstetrical and neonatal outcomes is needed, including details on indications for preterm delivery and additional neonatal adverse outcomes.
OBJECTIVE
This study aimed to determine whether there is a higher rate of adverse perinatal outcomes with severe-to-critical COVID-19 infection compared with nonsevere COVID-19 diagnosed during pregnancy.
STUDY DESIGN
This was a retrospective observational cohort study that compared rates of adverse perinatal outcomes between patients with severe-to-critical and those with nonsevere (asymptomatic, mild, or moderate) COVID-19 infection. Patients had singleton pregnancies and a positive laboratory polymerase chain reaction result for COVID-19. Primary outcomes included hypertensive disorders of pregnancy, cesarean delivery, fetal growth restriction, preterm birth, and neonatal intensive care unit admission. Additional neonatal outcomes analyzed included need for cardiopulmonary resuscitation, low birthweight (<2500 g), 1- or 5-minute Apgar score <7, need for supplemental oxygen, need for intubation, intraventricular hemorrhage, sepsis, respiratory distress syndrome, bronchopulmonary dysplasia, blood transfusion, necrotizing enterocolitis, hypoxic-ischemic encephalopathy, birth trauma, or neonatal death. Appropriate bivariate analyses were used to compare groups. Logistic regression was used to examine primary outcomes while adjusting for confounders.
RESULTS
A total of 441 participants were identified and confirmed via detailed chart review to be pregnant with a singleton pregnancy while diagnosed with COVID-19. Of these, 44 (10%) met National Institutes of Health criteria for severe-to-critical COVID-19 infection. The median gestational age at the time of maternal COVID-19 diagnosis was 36.4 weeks (interquartile range, 29.6–38.6). Severe-to-critical COVID-19 infection had a higher risk of a composite adverse neonatal outcome (36.4% vs 21.4%; P=.03). There was a high incidence of hypertensive disorders of pregnancy overall (20.6%), but this outcome was not higher in the severe-to-critical vs nonsevere group. There were no maternal deaths. There was a low incidence of neonatal COVID-19 test positivity among those tested (1.8%). When adjusting for presence of heart disease and gestational age at COVID-19 diagnosis, severe-to-critical COVID-19 was strongly associated with fetal growth restriction (adjusted odds ratio, 2.73; confidence interval, 1.03–7.25) and neonatal intensive care unit admission (adjusted odds ratio, 3.50; confidence interval, 1.56–7.87). Preterm delivery was more common but was no longer significant after adjustment (adjusted odds ratio, 2.23; confidence interval, 0.99–5.05).
CONCLUSION
Severe-to-critical COVID-19 infection during pregnancy is associated with higher rates of adverse neonatal outcomes and strongly associated with neonatal intensive care unit admission and fetal growth restriction compared with nonsevere disease. There is a high rate of hypertensive disorders of pregnancy overall in all those affected by COVID-19, regardless of severity. Pregnant persons should be counseled on these risks to encourage vaccination, and those with infection during pregnancy should be monitored for fetal growth disorders.
Key words: coronavirus, COVID-19, fever, morbidity, mortality, pandemic, pregnancy, preterm birth, respiratory distress syndrome, SARS-CoV, SARS-CoV-2, sepsis, virus
AJOG Global Reports at a Glance.
Why was this study conducted?
This was a retrospective cohort study conducted to investigate the impact of severe COVID-19 infection on obstetrical and neonatal outcomes.
Key findings
Our study demonstrated increased rates of adverse neonatal outcomes including neonatal intensive care unit admission and increased risk of fetal growth restriction in severe COVID-19 infection compared with nonsevere COVID-19. There was a high rate of hypertensive disorders of pregnancy among all pregnancies affected by COVID-19.
What does this add to what is known?
Our study included detailed characterization of obstetrical and neonatal outcomes in pregnancies with severe COVID-19 disease across a >1-year time span, confirmed findings of increased rates of adverse neonatal outcomes, added details of indications for preterm birth, and found a strong association with fetal growth restriction.
Introduction
The impact of SARS-CoV-2 (COVID-19) during pregnancy has been an ongoing area of investigation with varying results regarding the characterization of maternal outcomes, disease severity, and perinatal and obstetrical outcomes. After several conflicting early reports on rates of maternal adverse outcomes,1, 2, 3, 4, 5, 6, 7 the largest body of data from the Centers for Disease Control and Prevention ultimately confirmed substantially higher rates of hospitalization, intensive care unit (ICU) admission, and mechanical ventilation among pregnant women with COVID-19 compared with nonpregnant persons.5 This was a large and informative data set but was subject to reporting bias, and pregnancy status was only available for some women. In addition, obstetrical and neonatal outcomes were not available. Studies examining the effect of COVID-19 on obstetrical and neonatal outcomes have also been conflicting.8, 9, 10, 11, 12 The most robust study by Metz et al9 examined 1219 pregnant persons positive for COVID-19 and found an increased risk of cesarean delivery, hypertensive disorders of pregnancy, and preterm birth in those with severe-to-critical COVID-19 compared with asymptomatic patients. They did not find an increase in adverse outcomes in the mild-to-moderate group compared with asymptomatic patients.
We sought to elucidate maternal and neonatal outcomes in further detail and confirm these more recent findings of increased adverse perinatal outcomes in those with severe-to-critical COVID-19. We investigated these outcomes through a retrospective observational cohort study examining all pregnant persons with confirmed COVID-19 infection within a large health system. We hypothesized that we would find higher rates of adverse perinatal and neonatal outcomes among severe-to-critical than among nonsevere COVID-19 infections during pregnancy.
Materials and Methods
This was a retrospective observational cohort study examining pregnant persons with polymerase chain reaction (PCR) laboratory-confirmed COVID-19 infection diagnosed between March 1, 2020 and May 5, 2021 across a large hospital system. This hospital system includes a major academic tertiary-care center and 12 regional hospitals. Patients were screened for eligibility if they had a confirmed pregnancy diagnosis and simultaneous diagnosis of COVID-19 using the services of Health Data Compass, an enterprise health data warehouse that integrates patient clinical data from the electronic health record. We identified any patient with a prenatal or delivery encounter using International Classification of Diseases (ICD)-9, ICD-10, Current Procedural Terminology, and positive COVID-19 laboratory codes. Detailed electronic medical record review and data abstraction were performed to ensure that patients met the inclusion criteria of a diagnosis of COVID-19 during a clinically confirmed pregnancy. All study sites had a universal screening process for testing patients on admission to the labor and delivery unit. Additional COVID-19 testing was obtained for inpatients and outpatients presenting with symptoms. Patients were excluded if they did not have a laboratory-confirmed PCR diagnosis of COVID-19, or if they had a multifetal gestation or a known major fetal anomaly. Study data were collected and managed using REDCap (Vanderbilt University, Nashville, TN) electronic data capture tools hosted at the University of Colorado.13 The study was approved by the institutional review board.
Data abstraction included demographics, medical and obstetrical history, COVID-19 treatment, laboratory findings, imaging studies, and birth outcomes. Neonatal outcomes were reviewed for the duration of the neonate's delivery admission. Data accuracy was ensured by detailed chart review and/or reviewed for accuracy by the primary author who is a trained obstetrician. The accuracy of a PCR-confirmed laboratory diagnosis of COVID-19 was verified, including if external laboratory results were available for review. The gestational age at the time of COVID-19 diagnosis was determined using the clinically established estimated due date.
Classification of COVID-19 severity was based on the established National Institutes of Health (NIH) guidelines.14 According to these guidelines, severe COVID-19 disease is defined as peripheral oxygen saturation (SpO2) <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates on imaging >50% of lung fields. Critical disease is defined as respiratory failure, septic shock, and/or multiorgan dysfunction. The “nonsevere COVID-19” cohort included those meeting NIH criteria for asymptomatic disease, mild disease (defined as any signs or symptoms of COVID-19 excluding shortness of breath/dyspnea, or abnormal chest imaging), or moderate illness (defined as evidence of lower respiratory disease during clinical assessment or imaging and an SpO2 ≥94% on room air at sea level). The NIH criteria specify SpO2 criteria at sea level, whereas our study cohort was at approximately 5000 ft of elevation; thus, we translated an SpO2 level of <94% on room air at sea level to any need for oxygen supplementation, which is typically set at a threshold of an SpO2 <92% for pregnant persons at altitude.
Rates of adverse perinatal and neonatal outcomes were compared between those meeting NIH criteria for severe or critical COVID-19 and those with nonsevere disease (asymptomatic, mild, or moderate). Primary perinatal outcomes included cesarean delivery, preterm delivery (<37 weeks), and the placentally mediated disorders of fetal growth restriction and hypertensive disorders of pregnancy. Fetal growth restriction was defined as either an estimated fetal weight or abdominal circumference measuring <10th centile. Hypertensive disorders of pregnancy included gestational hypertension, chronic hypertension with superimposed preeclampsia, preeclampsia with or without severe features, HELLP syndrome, and eclampsia and were classified according to the criteria by the American College of Obstetricians and Gynecologists.15 Secondary perinatal outcomes included rates of pregnancy loss (defined as spontaneous miscarriage at any gestational age or intrauterine fetal demise), blood transfusion, postpartum hemorrhage (defined as >1000 mL of estimated blood loss), venous thromboembolism (VTE), maternal ICU admission, endometritis, and clinically diagnosed chorioamnionitis.
Neonatal outcomes included NICU admission, NICU length of stay, and a composite adverse neonatal outcome defined as the incidence of at least one of the following: NICU admission, incidence of birthweight <2500 g, 1- or 5-minute Apgar score <7, use of supplemental oxygen, intubation, cardiopulmonary resuscitation, incidence of sepsis, respiratory distress syndrome, bronchopulmonary dysplasia, blood transfusion, necrotizing enterocolitis, hypoxic-ischemic encephalopathy, birth trauma, or neonatal death. The incidence of positive neonatal COVID-19 testing was also examined. NICU admission and neonatal testing were not universal for all maternal cases of COVID-19 but provided at the clinical discretion of the neonatal care team. Similarly, placental pathology was not universally sent but performed when clinicians considered it appropriate on the basis of neonatal outcomes.
Descriptive statistics including tests of normality were computed to assess the study variables. Appropriate bivariate analyses were used to compare baseline demographic data and medical history and secondary outcomes including Student t or Mann–Whitney U tests for continuous variables and chi-square or Fisher exact tests for categorical outcomes. Logistic regression was used to identify predictors of primary perinatal outcomes associated with placental disorders (preterm delivery, fetal growth restriction, and hypertensive disorders of pregnancy), and NICU admission, adjusting for any baseline variables found to be associated with severe COVID-19 (P≤.05).
Results
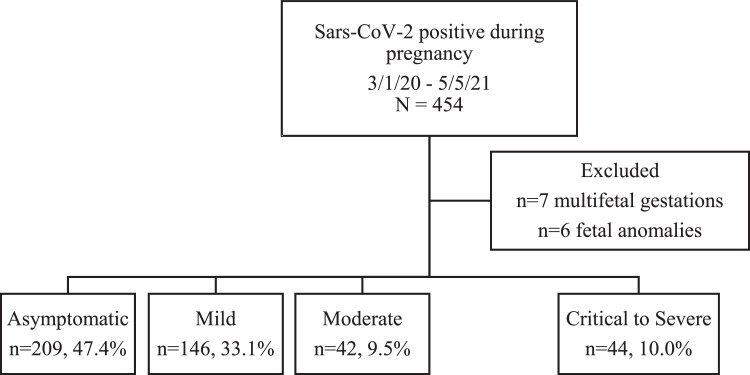
A total of 441 participants were identified and confirmed to have a singleton pregnancy while diagnosed with COVID-19 via laboratory-confirmed PCR. Among these, 397 met criteria for asymptomatic, mild, or moderate infection, and 44 met criteria for critical-to-severe disease (Figure 1). The rates of characteristics defining disease severity for symptomatic patients and rates of specific symptoms are presented in Table 1. Specific symptoms that were more common in the severe-to-critical group included dyspnea, cough, fever, and muscle aches (Table 1).
Figure 1.
COVID-19 severity in the study cohort
Flow diagram demonstrating identified cases meeting inclusion criteria and breakdown of COVID-19 severity classifications.
Hamidi. Severe COVID-19 and perinatal outcomes. Am J Obstet Gynecol Glob Rep 2022.
Table 1.
Disease severity classification
| Mild/moderate COVID-19 (n=188) |
Critical/severe COVID-19 (n=44) |
||||
|---|---|---|---|---|---|
| N | % | n | % | P value | |
| Respiratory failure (ECMO, CPAP, BiPap, ventilation) | 0 | 0.0 | 8 | 18.2 | |
| Nasal cannulaa | 1 | 0.3 | 27 | 61.4 | |
| HFNC | 0 | 0.0 | 1 | 2.3 | |
| BiPap or CPAP | 0 | 0.0 | 0 | 0.0 | |
| Mechanical ventilation | 0 | 0.0 | 8 | 18.2 | |
| ECMO | 0 | 0.0 | 1 | 2.4 | |
| Septic shock | 1 | 0.5 | 0 | 0.0 | |
| Death | 0 | 0 | 0 | 0.0 | |
| Abnormal CXR or chest CT | 10 | 5.3 | 38 | 86.4 | |
| Self-reported dyspnea | 35 | 18.6 | 33 | 75.0 | |
| Other symptoms: | |||||
| Cough | 101 | 53.7 | 33 | 75.0 | .01 |
| Fever | 57 | 30.3 | 25 | 56.8 | <.01 |
| Chest pain or tightness | 13 | 6.9 | 6 | 13.6 | .14 |
| Headache | 45 | 23.9 | 9 | 20.5 | .62 |
| Nausea | 25 | 13.3 | 8 | 18.2 | .40 |
| Vomiting | 14 | 7.5 | 7 | 15.9 | .08 |
| Diarrhea | 10 | 5.3 | 3 | 6.8 | .70 |
| Loss of smell | 45 | 23.9 | 6 | 13.6 | .14 |
| Loss of taste | 38 | 20.2 | 5 | 11.4 | .17 |
| Joint pain | 0 | 0.0 | 0 | 0.0 | — |
| Muscle aches | 41 | 21.8 | 17 | 38.6 | .02 |
| Nasal congestion | 55 | 29.3 | 2 | 4.6 | <.01 |
| Runny nose | 18 | 9.6 | 1 | 2.3 | .14 |
| Sore throat | 36 | 19.2 | 3 | 6.8 | .05 |
| Fatigue | 28 | 14.9 | 11 | 25.0 | .11 |
| Conjunctivitis | 1 | 0.5 | 0 | 0 | 1.0 |
BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; CT, computed tomography; CXR, chest x-ray; ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannula.
aOne patient required nasal cannula and remained in the mild group category because the nighttime oxygen requirement was suspected to be secondary to obstructive sleep apnea and the patient had otherwise only mild symptoms.
Hamidi. Severe COVID-19 and perinatal outcomes. Am J Obstet Gynecol Glob Rep 2022.
The location of diagnosis varied, with most cases diagnosed in the labor and delivery unit (47.9%). The second most common location was an outpatient clinic (40.1%), with the remainder being recognized in the emergency department, a different inpatient setting (such as ICU or medicine floor), or an urgent care setting (9.3%, 2.5%, and 0.2%, respectively). The median gestational age at time of COVID-19 diagnosis across the entire cohort was 36.4 weeks (interquartile range, 29.6–38.6), with 52.4% of participants being diagnosed with COVID-19 preterm (<37 weeks). There was a low incidence of COVID-19 diagnosis in the first trimester (<12 weeks), with a total of 9 participants (2.0%).
Baseline demographics and clinical characteristics were overall similar between those with severe-to-critical and those with nonsevere disease (Table 2). Notably, there were no differences in age, body mass index, history of preterm birth, cesarean delivery, or history of hypertensive disorders. Those with severe-to-critical infection had a higher incidence of underlying heart disease (4.6% vs 0.3%; P<.01) and a lower median gestational age at diagnosis (31.5 vs 37 weeks; P<.01).
Table 2.
Baseline demographic and clinical characteristics
| Characteristic | Asymptomatic, mild, or moderate infection (n=397; 90.0%) Median (IQR) or n (%) | Critical-severe (n=44; 10.0%) Median (IQR) or n (%) | P value |
|---|---|---|---|
| Age (y) | 27.5 (22–32) | 28 (25–33.5) | .15 |
| Gravidity | |||
| 1 | 133 (33.5%) | 14 (31.8%) | .68 |
| 2–4 | 212 (53.4%) | 26 (59.1%) | |
| ≥5 | 52 (13.1%) | 4 (9.1%) | |
| Parity | |||
|
159 (40.1%) | 14 (31.8%) | .29 |
|
238 (59.9%) | 30 (68.2%) | |
| Previous cesarean deliverya | 67 (17.1%) | 4 (9.1%) | .17 |
| Gestational age at diagnosis | 37 (30.1–39) | 31.5 (27.5–34.8) | <.01 |
| Body mass index (kg/m2) | 31.5 (27.1–36) | 32.4 (28–37.1) | .71 |
| Race-ethnicity | |||
|
142 (36.1%) | 10 (23.3%) | .17 |
|
33 (8.4%) | 2 (4.7%) | |
|
38 (9.7%) | 7 (16.3%) | |
|
180 (45.8%) | 24 (55.8%) | |
| History of preterm birth | 28 (7.1%) | 6 (13.6%) | .12 |
| History of any hypertensive disorder of pregnancy | 32 (8.1%) | 3 (6.8%) | 1.00 |
| Tobacco use during pregnancy | 23 (5.8%) | 2 (4.6%) | 1.00 |
| Illicit substance use during pregnancy | 29 (7.3%) | 2 (4.6%) | .76 |
| History of stroke | 0 | 0 | — |
| History of thrombosis | 1 (0.3%) | 1 (2.3%) | .19 |
| HIV infection | 1 (0.3%) | 1 (2.3%) | .19 |
| Asthma | 41 (10.3%) | 8 (18.2%) | .12 |
| Diabetes mellitus | .19 | ||
|
8 (2.0%) | 3 (6.8%) | |
|
33 (8.3%) | 2 (4.6%) | |
| Chronic hypertension | 19 (4.8%) | 4 (9.1%) | .28 |
| Seizure disorder | 9 (2.3%) | 1 (2.3%) | 1.00 |
| Immunosuppressionb | 2 (0.5%) | 0 (0.0%) | 1.00 |
| Any psychiatric or mood disorder | 93 (23.4%) | 14 (31.8%) | .22 |
| Chronic kidney disease | 1 (0.3%) | 0 (0%) | 1.00 |
| Rheumatologic disease | 3 (0.8%) | 1 (2.3%) | .34 |
| Thyroid disease | 19 (4.8%) | 0 (0.0%) | .24 |
| Heart diseasec | 1 (0.25%) | 2 (4.6%) | .03 |
| Chronic liver disease | 1 (0.3%) | 0 (0.0%) | 1.00 |
Data are reported as either median (interquartile range) or number (percentage).
IQR, interquartile range.
aExcluding patients with pregnancy loss in COVID-19–affected pregnancy
bHIV, chronic steroid use, or other immunosuppressants
cHeart disease included 1 patient with arrhythmia and 2 patients with rheumatic heart disease.
Hamidi. Severe COVID-19 and perinatal outcomes. Am J Obstet Gynecol Glob Rep 2022.
Table 3 demonstrates primary and secondary perinatal outcomes. Those with severe-to-critical COVID-19 infection demonstrated higher rates of preterm birth (25% vs 10.2%; P<.01) and fetal growth restriction (13.6% vs 5.3%; P=.03). There was a high but similar rate of hypertensive disorders of pregnancy in both groups (22.7% vs 20.4%; P=.72). The overall cesarean rate and primary cesarean rate were also similar between the groups. Secondary outcomes were significant for earlier gestational age at delivery (38.4 vs 39.1 weeks; P=.04), higher incidence of delivery for nonreassuring fetal status (15.9% vs 5.6%; P<.01), higher maternal ICU admission (31.8 vs 0%; P<.001), and higher rates of VTE (4.6 vs 0.3%; P=.03) in the severe-to-critical group (Table 3).
Table 3.
Perinatal outcomes
| Characteristic | Asymptomatic, mild, or moderate infection (n=397; 90.0%) Median (IQR) or n (%) | Critical-severe (n=44; 10.0%) Median (IQR) or n (%) | P value |
|---|---|---|---|
| Delivery mode: | |||
| Cesarean delivery | 107 (27.2%) | 12 (27.3%) | .99 |
| Vaginal delivery (spontaneous or operative) | 286 (72.8%) Operative: n=4 | 32 (72.7%) Operative: n=2 | |
| Primary cesarean delivery | 60 (56.1%) | 9 (75.0%) | .24 |
| Gestational age at delivery (wk) | 39.1 (37.5–39.4) | 38.4 (36.8–39.4) | .04 |
| Indication for delivery | |||
| Spontaneous | 194 (49.6%) | 14 (31.8%) | <.01 |
| Worsening maternal status | 1 (0.26%) | 1 (2.3%) | |
| Nonreassuring fetal heart tones | 22 (5.6%) | 7 (15.9%) | |
| Other obstetrical indication | 174 (44.5%) | 22 (50%) | |
| Preterm birth (<37 wk) | 40 (10.2%) | 11 (25%) | <.01 |
| Indicated | 27.5% (11/40) | 81.8% (9/11) | |
| Gestational age at preterm delivery | |||
| <28 wk | 3 (7.5%) | 1 (9.1%) | 1.00 |
| 28–36.6 wk | 37 (92.5%) | 10 (91.0%) | |
| Fetal growth restriction | 21 (5.3%) | 6 (13.6%) | .03 |
| Pregnancy outcome | |||
| Live birth | 393 (99.0%) | 44 (100%) | .50 |
| Pregnancy lossa | 4 (1.0%) | 0 (0.0%) | |
| Preterm premature rupture of membranes | 19 (4.8%) | 1 (2.3%) | .71 |
| Endometritis | 8 (2.0%) | 1 (2.3%) | 1.00 |
| Chorioamnionitis | 14 (3.5%) | 0 (0%) | .38 |
| Hypertensive disorder of pregnancy | |||
| Any hypertensive disorder of pregnancy | 81 (20.4%) | 10 (22.7%) | .72 |
| Gestational hypertension | 29 (7.3%) | 5 (11.4%) | .90 |
| Preeclampsia without severe features | 31 (7.8%) | 3 (6.8%) | |
| Preeclampsia with severe features | 20 (5.0%) | 2 (4.6%) | |
| Eclampsia | 1 (0.3%) | 0 (0.0%) | |
| HELLP syndrome | 0 (0.0%) | 0 (0.0%) | |
| Uterine rupture | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Blood transfusion | 2 (0.5%) | 0 (0.0%) | 1.00 |
| Postpartum hemorrhage | 29 (7.4%) | 1 (2.3%) | .34 |
| Maternal intensive care unit admission | 0 (0.0%) | 14 (31.8%) | <.01 |
| Venous thromboembolism | 1 (0.3%) | 2 (4.6%) | .03 |
Data are reported as either median (interquartile range) or number (percentage).
IQR, interquartile range.
aPregnancy loss defined as spontaneous miscarriage at any gestational age or intrauterine fetal demise.
Hamidi. Severe COVID-19 and perinatal outcomes. Am J Obstet Gynecol Glob Rep 2022.
Table 4 demonstrates primary and secondary neonatal outcomes. The primary neonatal outcome of NICU admission was significantly higher in the severe-to-critical group (27.3% vs 7.9%; P<.01). A composite of neonatal adverse outcomes was also significantly higher in the severe-to-critical group (36.4% vs 21.4%; P=.03). Individual secondary neonatal outcomes that were higher in the severe-to-critical group included rates of low birthweight <2500 g (20.9 vs 10.0%; P=.03), 1-minute Apgar <7 (20.5 vs 9.5%; P=.03), use of supplemental oxygen (27.3 vs 11.2%; P<.01), intubation (6.8 vs 1.5%; P=.02), and respiratory distress syndrome (15.9 vs 4.6%; P<.01) (Table 4).
Table 4.
Neonatal outcomes
| Outcome | Asymptomatic, mild, or moderate infection (n=397; 90.0%) Median (IQR) or n (%) | Critical-severe (n=44; 10.0%) Median (IQR) or n (%) | P value |
|---|---|---|---|
| Composite adverse neonatal outcomea | 85 (21.4%) | 16 (36.4%) | .03 |
| Neonatal ICU admission | 31 (7.9%) | 12 (27.3%) | <.01 |
| Neonatal ICU length of stay (d) | 14 (6–27) | 21.5 (7–40) | .39 |
| Birthweight (g) | 3186.5 (2840–3480) | 3125 (2715–3410) | .24 |
| Birthweight <2500 g | 39 (10.0%) | 9 (20.9%) | .03 |
| 1-min Apgar score <7 | 37 (9.5%) | 9 (20.5%) | .03 |
| 5-min Apgar score <7 | 9 (2.3%) | 3 (6.8%) | .08 |
| Use of supplemental oxygen | 44 (11.2%) | 12 (27.3%) | <.01 |
| Required intubation | 6 (1.5%) | 3 (6.8%) | .02 |
| Required CPR | 2 (0.5%) | 0 (0%) | 1.00 |
| Intraventricular hemorrhage | 0 | 0 | — |
| Sepsis | 1 (0.3%) | 0 (0%) | 1.00 |
| Respiratory distress syndrome | 18 (4.6%) | 7 (15.9%) | <.01 |
| Bronchopulmonary dysplasia | 2 (0.5%) | 1 (2.3%) | .27 |
| Blood transfusion | 1 (0.3%) | 1 (2.3%) | .19 |
| Necrotizing enterocolitis | 0 | 0 | — |
| Hypoxic-ischemic encephalopathy | 1 (0.3%) | 0 (0%) | 1.00 |
| Birth trauma | 1 (0.3%) | 0 (0%) | 1.00 |
| Neonatal death | 0 (0%) | 0 (0%) | — |
| Neonatal+COVID-19 PCRb | 2 (0.5%) | 0 (0%) | 1.00 |
Data are reported as either median (interquartile range) or number (percentage).
CPR, cardiopulmonary resuscitation; ICU, intensive care unit; IQR, interquartile range; PCR, polymerase chain reaction.
aComposite adverse neonatal outcome includes at least 1 of the following: neonatal ICU admission, need for CPR, low birthweight (<2500 g), 1- or 5-minute Apgar score <7, need for supplemental oxygen, need for intubation, intraventricular hemorrhage, sepsis, respiratory distress syndrome, bronchopulmonary dysplasia, blood transfusion, necrotizing enterocolitis, hypoxic-ischemic encephalopathy, birth trauma, or neonatal death
bCalculated out of n=111 neonates tested for COVID-19.
Hamidi. Severe COVID-19 and perinatal outcomes. Am J Obstet Gynecol Glob Rep 2022.
There were no maternal or neonatal deaths in our cohort. There was an overall low incidence of neonatal COVID-19 test positivity among those tested (2/111; 1.8%). No differences in rates of pregnancy loss were noted, with a total of 4 pregnancy losses that were all in the nonsevere COVID-19 group (Table 3). These losses included a 15-week, 20-week, and 21-week spontaneous delivery, and one 26-week intrauterine fetal demise. Two losses had evidence of acute chorioamnionitis on placental pathologic examination, and 2 of the spontaneous deliveries presented with a clinical picture consistent with cervical insufficiency.
Table 5 presents the final logistic regression models. Severe-to-critical COVID-19 was predictive of fetal growth restriction (adjusted odds ratio [aOR], 2.73; 95% confidence interval [CI], 1.03–7.25) and NICU admission (aOR, 3.50; 95% CI, 1.56–7.87) when adjusting for gestational age at diagnosis and history of heart disease. Preterm delivery was no longer significant in the severe-to-critical group (aOR, 2.23; 95% CI, 0.99–5.05). Of note, 81.8% of preterm deliveries in the severe-to-critical group were indicated (vs spontaneous), whereas only 27.5% of preterm deliveries were indicated in the nonsevere group. Of the 9 indicated preterm deliveries in the severe group, most occurred during the time of COVID-19 hospitalization (88.9%), and most were secondary to nonreassuring fetal assessment (77.8%). Among the indicated deliveries in the nonsevere group, most were secondary to a hypertensive diagnosis of pregnancy (63.6%). There was a high incidence of hypertensive disorders of pregnancy in the cohort overall (20.6%), but severe-to-critical disease was not associated with this outcome (aOR, 1.30; 95% CI, 0.61–2.76). In addition, severe-to-critical disease was not associated with cesarean delivery (aOR, 1.18; 95% CI, 0.52–2.71) nor primary cesarean delivery (aOR, 0.09; 95% CI, 0.44–1.87).
Table 5.
Adjusted odds ratios of adverse perinatal and neonatal outcomes in severe-to-critical vs nonsevere COVID-19
| Outcomes | Adjusted odds ratios (95% CI) |
|---|---|
| All preterm delivery <37 wk | 2.23 (0.99–5.05) |
| Spontaneous preterm delivery | 0.46 (0.09–2.20) |
| Fetal growth restriction | 2.73 (1.03–7.25)a |
| NICU admission | 3.50 (1.56–7.87)a |
| Risk of cesarean delivery | 0.90 (0.44–1.87) |
| Risk of primary cesarean delivery | 1.18 (0.51–2.71) |
| Risk of hypertensive disorders of pregnancy | 1.30 (0.61–2.76) |
Adjusted for gestational age at the time of COVID-19 diagnosis and preexisting heart disease.
CI, confidence interval; NICU, neonatal intensive care unit.
aSignificant P values defined as P<.05.
Hamidi. Severe COVID-19 and perinatal outcomes. Am J Obstet Gynecol Glob Rep 2022.
Comment
Principal findings
Our study found higher rates of adverse perinatal outcomes among pregnancies affected by severe-to-critical COVID-19 than among those with nonsevere disease. These outcomes included higher rates of fetal growth restriction, NICU admission, and a composite adverse neonatal outcome. Preterm birth was more common in the severe-to-critical group, and this difference neared significance when adjusting for confounders. We did not find higher rates of cesarean delivery or hypertensive disorders in the severe-to-critical group, but there was an overall high rate of hypertensive disorders in the entire cohort (20.6%). Our findings expand the body of evidence supporting the association of COVID-19 with worsened pregnancy outcomes and add additional details on fetal and neonatal outcomes.
Results in the context of what is known
Preterm birth was more common in the severe-to-critical group (25% vs 10.2%; P≤.01). Although this was no longer significant after adjusting for gestational age at diagnosis and heart disease, we suspect that many of the additional adverse neonatal outcomes were driven by the high rate of preterm birth in the severe group. It is also noteworthy that preterm birth was largely driven by indicated deliveries in the severe-to-critical group (81.8%), similarly to what was found by Metz et al.9 Our study adds additional detail on delivery indication, noting that 77.8% were because of nonreassuring fetal status. There was also a high rate of indicated preterm births in the nonsevere group (27.5%). This may be partially explained by the higher incidence of comorbid diagnoses such as hypertensive disorders of pregnancy in all pregnancies affected by COVID-19 regardless of severity, which was indeed the most common indication for preterm delivery in the nonsevere group (63.6%). Although higher rates of adverse neonatal outcomes and low birthweight are expected in the setting of high rates of preterm delivery and NICU admission, these are clinically significant comorbidities.
Our finding that fetal growth restriction was more common in the severe-to-critical COVID-19 group is notable. Aside from isolated case reports,16 previous studies evaluating fetal growth restriction have not shown a consistent correlation with COVID-19 infection; however, these previous studies did not account for severity of infection.17, 18, 19 Although we are cautious about interpreting this finding because of lack of obtained data regarding Doppler studies or other indicators of severe fetal growth restriction, growth restriction is known to be more common in inflammatory states in pregnancy,20 and higher levels of inflammation are a hallmark of severe COVID-19. Because of the retrospective nature of this research, it was difficult to ascertain the timing of COVID-19 infection in relation to the onset of fetal growth restriction. However, we attempted to obtain a general idea of the temporal relationship of COVID-19 diagnosis among the 28 participants with a diagnosis of fetal growth restriction. We did not find a significant difference in the rate of fetal growth restriction diagnosed at any time before a COVID-19 diagnosis. This increased the likelihood of a true difference between the groups, but a causative relationship could not be fully established.
We were surprised not to find a higher rate of hypertensive disorders of pregnancy in the severe group because of the known effect of inflammation in pregnancy on the rates of these disorders.20 Although the lack of increase in rates of hypertensive disorders of pregnancy in the severe-to-critical COVID-19 group differs from the findings of Metz et al,9 it may be explained by the high rate of hypertensive disorders of pregnancies in both severe and nonsevere COVID-19 groups overall (20.6%), which is higher than the background rate of approximately 8.6% in the United States and the rate of 11.1% reported within our health system between 2019 and 2020.21 Hypertensive disorders are emerging as more common with the diagnosis of COVID-19 in pregnancy in general, thus we may have been underpowered to find a difference between the groups.10
We found a higher rate of VTE in the severe COVID-19 group, although the incidence was low overall (3 in the entire cohort). This outcome is difficult to interpret in the context of small numbers and variation in local VTE prophylaxis strategies that changed over time and may have differed among the local and regional hospitals. In addition, although our rate of neonatal test positivity (2/111; 1.8%) is similar to previously published rates,9 it should be interpreted with caution because of lack of uniformity for neonatal testing approaches across sites.
Clinical implications
Our finding of increased adverse neonatal outcomes is particularly useful in counseling patients on the continued risks of COVID-19 infection in pregnancy, especially now that preventative intervention with vaccines is available. Although many of the risks are associated with immediately acute concerns in severe-to-critically ill patients (such as increased indicated preterm birth for nonreassuring fetal status), we additionally confirmed a high rate of hypertensive diagnosis in both groups.9,10 Lastly, our study found an increase in the rate of fetal growth restriction and supports the practice of increased fetal growth surveillance, especially for those who suffer severe-to-critical COVID-19 infection.
Research implications
Continued research into timing of infection and adverse perinatal outcomes would be beneficial. There were limited numbers of participants in the first trimester in our study, and the effect of COVID-19 during this pregnancy time frame remains understudied. Lastly, because of the rarity of stillbirth and pregnancy loss, we were unable to further examine their relationship with COVID-19.
Strengths and limitations
Our study's greatest limitation is its retrospective nature, which limits the ability to draw conclusive relationships between COVID-19 severity and outcomes. Our results were likely skewed toward later gestational ages at time of diagnosis because universal screening protocols for planned admission to labor and delivery are more likely to occur at later gestational ages, which also explains the gestational age difference between our 2 groups, although we did adjust for this in our logistic regressions. There was also potential for confounding between the outcomes of hypertensive disorders of pregnancy and fetal growth restriction that frequently co-occur, although it is biologically plausible that both were caused by the inflammatory nature of COVID-19.20,22 Our study was limited in its ability to separate these 2 outcomes. Overall, there was a low rate of adverse outcomes in our cohort because of a large proportion of asymptomatic positive individuals. Lastly, there were changes in standard-of-care treatments (increased use of steroids and remdesivir) and vaccine availability across the time span of our study, which may have affected outcomes. Because of the timeline of our study within the pandemic, we were unable to assess vaccine status or use of monoclonal antibodies, which were rarely used in this time frame at our system.
There are several strengths of our study. Although this analysis was limited to a single region, it did include a major academic tertiary-care center and outlying regional hospitals, which adds to the generalizability of the results beyond the limitations of studying a single referral site. Our detailed analysis reviews a large number of pertinent clinical outcomes and spans over a year of the pandemic. Our data across this time span reflect that adverse perinatal outcomes remained elevated with COVID-19 even as knowledge of its optimal treatment increased. We were able to perform a detailed chart review and examine a variety of neonatal outcomes in significantly more detail. In addition, different variants of concern have existed during this expanded time frame, thus our review enables a comprehensive updated overview of the effects of COVID-19 on pregnancy.
Conclusions
Severe-to-critical COVID-19 disease is associated with increased risk of fetal growth restriction, NICU admission, and a composite of adverse neonatal outcomes. Indicated preterm birth owing to fetal distress was common. Our study adds additional strength to the existing literature on adverse perinatal outcomes in pregnancy with further detail on neonatal risks, which can aid in counseling patients on the risks of COVID-19 while vaccine hesitancy in this population remains high.
Acknowledgments
This publication was supported by the Health Data Compass Data Warehouse project (healthdatacompass.org), REDCap electronic data capture tools hosted at the University of Colorado, and by National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (Colorado Clinical and Translational Science Awards [CTSA] Program Grant Number UL1 TR002535). Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
The authors report no conflict of interest.
The authors report no funding for this study.
Patient consent was not required because no personal information or details were included.
This publication was supported by the Health Data Compass Data Warehouse project (healthdatacompass.org), REDCap electronic data capture tools hosted at the University of Colorado, and by National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (Colorado Clinical and Translational Science Awards [CTSA] Program Grant Number UL1 TR002535). Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Cite this article as: Hamidi OP, Lijewski V, Sheeder J, et al. Adverse perinatal outcomes in pregnancies affected by severe COVID-19 infection. Am J Obstet Gynecol Glob Rep 2022;XX:x.ex–x.ex.
References
- 1.Badr D, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764–768. doi: 10.1016/j.ajog.2020.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2021;224 doi: 10.1016/j.ajog.2020.11.022. 510.e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lokken EM, Huebner EM, Taylor GG, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225 doi: 10.1016/j.ajog.2020.12.1221. 77.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Portilla RJ, Sotiriadis A, Chatzakis C, et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx) Ultrasound Obstet Gynecol. 2021;57:224–231. doi: 10.1002/uog.23575. [DOI] [PubMed] [Google Scholar]
- 5.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qeadan F, Mensah NA, Tingey B, Stanford JB. The risk of clinical complications and death among pregnant women with COVID-19 in the Cerner COVID-19 cohort: a retrospective analysis. BMC Pregnancy Childbirth. 2021;21:305. doi: 10.1186/s12884-021-03772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pineles BL, Goodman KE, Pineles L, et al. In-hospital mortality in a cohort of hospitalized pregnant and nonpregnant patients with COVID-19. Ann Intern Med. 2021;174:1186–1188. doi: 10.7326/M21-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metz TD, Clifton RG, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conde-agudelo A, Romero R. SARS-COV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68–89. doi: 10.1016/j.ajog.2021.07.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirjani R, Hosseini R, Soori T, et al. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med. 2020;27:1–7. doi: 10.1093/jtm/taaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health . 2022. Coronavirus disease 2019 (COVID-19) treatment guidelines.https://covid19treatmentguidelines.nih.gov/ Available at: Accessed November 13th, 2020. [PubMed] [Google Scholar]
- 15.Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:e237–e260. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 16.Moltner S, Vrijer B De, Banner H. Placental infarction and intrauterine growth restriction following SARS-CoV-2 infection. Arch Gynecol Obstet. 2021;304:1621–1622. doi: 10.1007/s00404-021-06176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullins E, Hudak ML, Banerjee J, et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. 2021;57:573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eltemamy E, Salama S, Salem SM, et al. Assessment of fetal growth and anomalies in the era of COVID-19 pandemic: an Egyptian pilot study. Middle East Fertil Soc J. 2021;26:32. doi: 10.1186/s43043-021-00075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzo G, Mappa I, Maqina P, et al. Effect of SARS-CoV 2 infection during the second half of pregnancy on fetal growth and hemodynamics: a prospective study. Acta Obstet Gynecol Scand. 2021;100:1034–1039. doi: 10.1111/aogs.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalagiri RR, Carder T, Choudhury S, et al. Inflammation in complicated pregnancy and its outcome. Am J Perinatol. 2016;33:1337–1356. doi: 10.1055/s-0036-1582397. [DOI] [PubMed] [Google Scholar]
- 21.Butwick AJ, Druzin ML, Shaw GM, Guo N. Evaluation of US state-level variation in hypertensive disorders of pregnancy. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein JA, Gallagher K, Beck C, Kumar R, Gernand AD. Maternal-fetal inflammation in the placenta and the developmental origins of health and disease. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.531543. [DOI] [PMC free article] [PubMed] [Google Scholar]