Abstract
Doxycilicine is the second‐line treatment of choice for infectious syphilis when treatment with penicillin G is not feasible. To date, difficulties in the penicillin supply chain make it necessary to evaluate and resort to antibiotic therapies which are currently considered a second‐line choice. Moreover, systematic studies comparing the two treatments in affected patients are still few, and many do not consider late and indeterminate latent infections. The objective of this study was to assess the differences in the serological response of the treatment of syphilis infections with benzathine penicillin compared with doxycycline. We built an in‐house database with all patients diagnosed with syphilis infection from January 2010 to January 2020 in the STD Centre of the S.Orsola‐Malpighi Polyclinic of the University of Bologna, located in the North‐east of Italy. We recorded all the principal independent (demographic, social status, reinfection rare, HIV infections, comorbidities, sexual behaviors, and initial TPHA values) and dependent variables (RPR values). We then extrapolated all patients treated with doxycycline (100 mg of doxycycline twice daily for 14 days for infections diagnosed within the first year and a 28 days course for infections older than 1 year or undetermined) and matched in 1:1 ratio numbers with a homogeneous group of patients treated with penicillin G (2.4 million units in a single dose intramuscularly for infections diagnosed within the first year and a cycle consisting in of 2.4 million units administered in a single dose per week for 3 weeks for infections older than 1 year or undetermined) We then analyzed the serological trends and outcomes in the primary, secondary and early latent groups versus late latent and undetermined infections. We retrieved 41 patients for each group with homogeneous initial characteristics. At the end of the 24‐month observation period, a slight difference in a valid RPR reduction rate emerged, with a greater success rate emerged in patients receiving penicillin than those with doxycycline (26 vs. 22, p 0.615). Indeed, patients with latent or indeterminate syphilis treated with doxycycline appear to have a higher rate of serofast than those treated with penicillin. Linear regression analysis showed no strong correlation between the analyzed independent variables and the observed outcomes. Doxycycline had a slightly lower, though not statistically different, success rate when compared with penicillin in treating primary syphilis, but appeared to have a reduced success rate in attaining resolution in late and undetermined syphilis infection.
Keywords: doxycycline, HIV, MSM, penicillin, syphilis, therapy
1. INTRODUCTION
Syphilis is a sexually transmitted disease experiencing for many years an increased incidence in Western populations. 1 This rise may be attributed to a change in sexual behaviors and the reduced diffusion of non‐barrier contraceptives instead of other methods, or none at all. 2 , 3
Prompt diagnosis is essential to prevent the signs and symptoms of disease progression, which may lead to irreversible morbidities.
The pivotal treatment of the disease is antibiotic therapy, of which penicillin represents the first‐line. It has been the treatment of choice for over 50 years, but in the past, it has proved challenging to assess its efficacy in vitro (Treponema pallidum is hard to cultivate) 4 and in vivo (low number of quality studies 5 , 6 ). In addition, allergies to penicillins 7 and difficulties in supplies 8 make its use sometimes complex. Still, it remains the gold standard choice. 9
Long‐acting penicillin G benzathine is the molecule in use. The therapy regimen consists of one dose of 2.4 million units in a single dose intramuscularly for primary, secondary, and early latent syphilis and one quantity of 2.4 million units in a single amount intramuscularly every week for 3 weeks for late latent and undetermined forms. Each dose requires two intramuscular injections, one in each hip or buttock muscle at the same time. 10
The tetracyclines class, especially doxycycline, represents the most used second‐line antibiotic. 11 The regimen in use and widely accepted by CDC and European guidelines consists of doxycycline 100 mg twice daily for 14 days for primary, secondary and early latent syphilis, and twice daily for 28 days for late latent and undetermined forms. 10
Tetracyclines were among the first antibiotics studied as an alternative to penicillin in the treatment of syphilis. Their high tissue diffusivity, broad‐spectrum bactericidal capacity, and seroconversion results in infected patients have made them the most widely used second choice antibiotic. 12
However, there have been limited number of comparative studies on using these second‐line agents for the treatment against the various forms of syphilis. 13 In addition, most studies compare the two treatments in early but not late forms, 6 and also some published works showed discordant results in the ability of tetracyclines versus penicillin to induce seroconversion between one stage and another. 13
Furthermore, we are unable to study in depth the efficacy of other antibiotics in treating syphilis. Penicillin remains the first choice, but the whole world has continually faced difficulties in obtaining penicillin and its derivatives in recent years. 8 , 14 , 15 These issues may be attributed to the low profitability of its production and limited use, supplanted by more common and versatile antibiotics. This meant that the remaining producers are mainly concentrated in China, with consequent fluctuations in price and supply chain to the rest of the world.
We conducted a retrospective cohort study to compare the serological response rates of patients with syphilis treated with penicillin versus those treated with doxycycline in our STD center to evaluate their effectiveness.
2. MATERIALS AND METHODS
We classified the syphilis stages as follows: a diagnosis of early syphilis (E.S.) (primary, secondary, and early latent syphilis) when the T. pallidum infection occurred within the previous year; latent syphilis (L.S.) included late syphilis, with defined manifestations occurring more than 1 year and even decades after initial infection; and undetermined syphilis, which refers to T. pallidum infection with reactive syphilis serologic findings but without clinical manifestations of the disease. 16
We considered cases diagnosed with E.S. or L.S. from January 2010 to January 2020 in our clinic, all of which were tested with serological tests performed by our central laboratory, and built an in‐house database. All patients underwent serial re‐evaluations, with repeat serologies at 1, 3, 6, 9, 12, 18, and 24 months after the first diagnosis.
Patients' age, sex, sexual behaviors, infection, syphilis stage, number of reinfections, TPHA were registered and used as independent variables.
In view of the retrospective and anonymous nature of the study, data access was requested from our control and data management office.
Patients with newly positive TPHA but negative RPR in the first two visits (2 months) were excluded. The emergence of new clinical signs and symptoms after treatment or a four‐fold increase of RPR during the follow‐up period was considered reinfection and excluded.
Inclusion criteria for Group 1 were patients who had assumed doxycycline 100 mg twice daily for 14–28 days according to the stage (14 days for E.S., 28 days for the other forms).
Patients who had performed other antibiotic therapies in the previous and following month were excluded.
Group 2 was extrapolated, with propensity score matching methods, in a 1:1 ratio. We selected patients from our internal database based on the values of the independent variable of Group 1. We selected only patients who had assumed penicillin G benzathine at one dose of 2.4 million units divided into two shots intramuscularly one to three times according to the stage (one dose for E.S., two injections per week for 3 weeks for the other forms). Different penicillin regimens were excluded.
Patients who had performed other antibiotic therapies in the previous and following month were excluded.
The outcome of interest was serological treatment success, defined as negativization of the rapid plasma reagin (RPR) or a decrease in the baseline RPR titer within the 24 months of at least 4‐fold. 17 A failure was defined as the inability to negativize or decrease as expected. 18 Serofast status was also considered a therapy failure, defined as persistently low RPR titers after treatment. 19 The outcomes were stratified on the state of the syphilis stage.
Data processing and statistical analysis were performed using IBM Spss 26.
Registered independent variables (age, sex, HIV infections, syphilis stage, educational qualification, number of reinfections, and TPHA level) were used to extrapolate Group 2 with the propensity score method. The precision for each quantitative parameter was set at 0.2 of tolerance for each variable.
The homogeneity of the two groups was then re‐evaluated:
Quantitative data were expressed by mean ± SD, and an independent sample t‐test was used to compare the two groups.
Qualitative data were described using the χ 2 test or Fisher's exact probability method, and p < 0.05 was considered statistically significant (two‐tailed test).
Outcomes, expressed as RPR levels, were compared as serological difference responses between the two groups and their trend was expressed using Kaplan–Meyer curves.
RPR trends were shown after being stratified between E.S. versus L.S.
Linear regression using the independent variables was then used to assess any values possibly related to the outcome of the seroconversion.
3. RESULTS
We retrieved a total of 1681 patients from our database who had been affected by syphilis.
For Group 1, we retrieved 41 patients who responded to our inclusion criteria. Patients were then stratified between those who underwent 2 week courses of tetracyclines, totaling 24 and corresponding E.S., and those who were treated with 4 week courses, numbering 17.
Group 2 included the same numbers of patients, who were also divided into two sub‐groups of the same number. Patients of Group 2 were selected based on homogeneous age, sex, scholarity and work job, syphilis stage, HIV infection rate, TPHA serological values and syphilis reinfection rate.
All the characteristics of the two main groups were re‐evaluated after matching and were found not statistically significant for each parameter listed in Table 1.
TABLE 1.
Demographic, specifical, and serological characteristics of the two confronted groups
| Group 1 (penicillin) | Group 2 (tetracyclines) | ||
|---|---|---|---|
| Age | 39.78 years Std dev 12,378 | 40.46 years Std dev 11,095 | 0.793 t Student (bilateral sign) |
| Sex | F = 5 M = 36 | F = 4 M = 37 | 0.500 Fisher test (bilateral sign) |
| Scholarity and work job |
7 = high 10 = Low 2 = Unemployed 11 = medium 8 = n.d. 1 = retired 2 = Students |
3 = high 10 = Low 1 = Unemployed 16 = medium 7 = n.d. 2 = retired 2 = Students |
798 Fisher test (bilateral sign) |
| Stage |
Late latent = 17 Early latent = 8 Primary = 7 Secondary = 9 |
Late latent = 17 Early latent = 8 Primary = 7 Secondary = 9 |
1.000 Fisher test (bilateral sign) |
| HIV |
Unaffected = 23 Affected = 18 |
Unaffected = 24 Affected = 17 |
0.500 Fisher test (bilateral sign) |
| TPHA | >640:1 | >640:1 | 0.875 Fisher test (bilateral sign) |
| Reinfection |
No = 26 Yes = 15 |
No = 31 Yes = 10 |
0.337 Fisher test (bilateral sign) |
Note: All accounted variables are homogeneous.
We traced 14 patients for each group who were unable to complete the 24 months of follow‐up, 3 of whom were also unable to complete the 12 months of follow‐up.
The main groups analysis showed mostly no‐differences.
At 12 months, the penicillin group had a slightly higher proportion of patients healed than patients treated with tetracyclines, although not statistically significant (26 vs. 22, p 0.615) (Table 2). At the end of the 24‐month observation period, a slight difference in seronegativization rates emerged, thus not significative, with a greater success rate in patients receiving penicillin than those with doxycycline (37 vs. 27, p 0.14) (Table 2).
TABLE 2.
Difference in seroconversion of the RPR values between the two treatments and differences in the two subgroups of syphilis
| Penicillin versus doxycycline | 12 months | Statistical significance | 24 months | Statistical significance |
|---|---|---|---|---|
| Seroconversion global |
Neg = 26 Pos = 10 (penicillin) versus Neg = 22 Pos = 11 (doxycicline) |
0.615 Fisher test (bilateral sign) |
Neg = 37 Pos = 0 (penicillin) versus Neg = 27 Pos = 4 (doxycicline) |
0.14 Fisher test (bilateral sign) |
|
Seronegativization Primary, secondary and early latent |
1.00 Fisher test (bilateral) | 0.416 Fisher test (bilateral) | ||
|
Seronegativization Late latent and undetermined |
0.212 Fisher test (bilateral) | 0.034 Fisher test (bilateral) |
An insight into this statistical difference revealed, after subgroup analysis, that patients affected by E.S. showed non‐significant differences between the two main groups and a comparable picture of the cure rate (Table 2; Figure 1).
FIGURE 1.
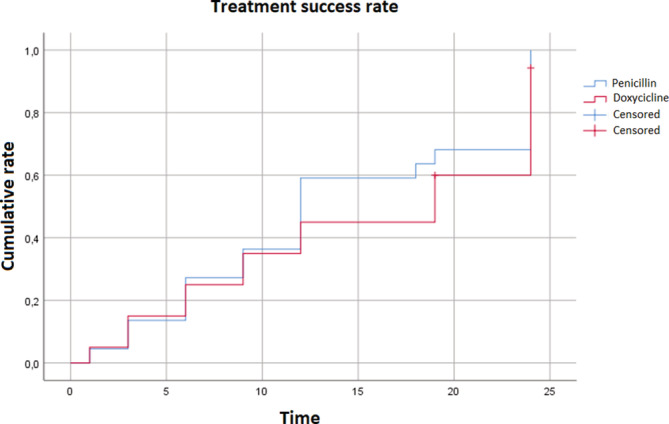
Kaplan–Meyer curves of treatment success based on the RPR after penicillin versus doxyclicines in the 1st subgroup of syphilis infections (primary, secondary and early latent syphilis). Curves are generally overlapping
Meanwhile, the L.S. group treated with doxycycline versus penicillin in Groups 1 and 2 showed significant changes in achieving a valid RPR reduction at the end of the 24 months of observation (p 0.034) (Table 2; Figure 2).
FIGURE 2.
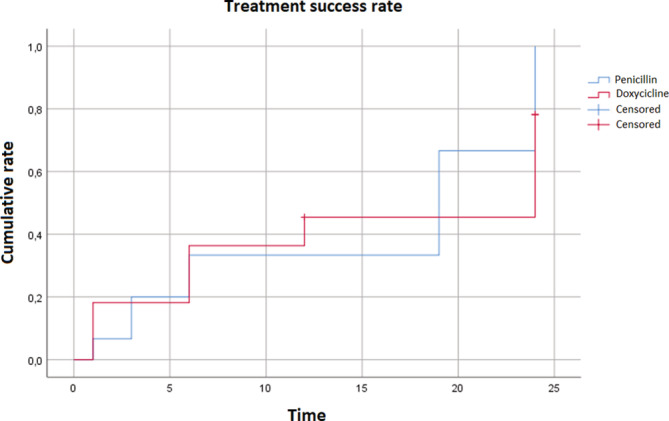
Kaplan–Meyer curves of the RPR trend of penicillin versus doxyclicines in the 2nd subgroup of syphilis infections (late latent and undetermined). The >0.2 deviation rate at 24 months
However, at 12 months, no differences in the speed of achieving seronegativization were found between the main groups and between the E.S. and L.S. subgroups (Table 2; Figures 1 and 2).
The percentage of patients who did not complete the 24‐month follow‐up accounts for 17.1%, balanced between the two groups. The linear regression of evaluated variables did not show any significant statistical difference regarding sex, age, sexual behaviors, HIV infections, number of syphilis reinfections, and TPHA values (Table 3).
TABLE 3.
Linear regression of the independent variables analyzed for the RPR reduction at 24 months
| Linear regression | ||||
|---|---|---|---|---|
| Sign. | Exp (B) | 95% CI per Exp (B) | ||
| Inferior | Superior | |||
| Age | 0.179 | 0.887 | 0.745 | 1.056 |
| Sex | 1.000 | |||
| Male | 1.000 | 2.696 | 0.000 | – |
| Female | 1.000 | 437,108,575.793 | 0.000 | – |
| Sexual behaviors | 0.732 | |||
| MSM | 0.324 | 5.904 | 0.174 | 200.697 |
| MSF | 0.999 | 0.000 | 0.000 | – |
| FSF | 0.762 | 1.673 | 0.060 | 46.907 |
| HIV | 0.181 | 0.068 | 0.001 | 3.492 |
| Reinfections | 0.090 | 19.014 | 0.631 | 573.239 |
| TPHA | 0.996 | |||
Note: No significant differences emerged, although a slight trend in HIV infection and reinfections is observable.
4. DISCUSSION
Our analysis shows similar rates in the seronegativization progress in both groups within the first 12 months.
On the other hand, a substantial difference emerged at the 24th month of follow‐up between the group treated with doxycycline and penicillin. But the different outcomes in patients treated with L.S. may explain the healing rate differences between the two principal groups.
Our data slightly deviates from what is reported in the literature concerning L.S.: patients experience lower RPR seronegativization rates of L.S. when treated with doxycycline. Data shows similar results among the two groups concerning E.S. response to treatment.
Moreover, our univariate model regression showed no confirmed differences but a trend for patients who have had reinfection of syphilis and are infected with HIV to fail RPR seronegativization and maintain a serofast state.
Finally, the work highlighted a tendency of patients with multiple reinfections to have a slight difficulty in achieving an RPR response to therapy with doxycycline, although not statistically confirmable compared to penicillin.
The strength of our study is the use of a statistical method of attenuation of the bias between the two groups (propensity score) and the use of a single‐center database built on medical records constituted by a small number of highly experienced doctors in venereal disease management. In addition, propensity score matching helps to overcome the numerous confounding factors (levels of study/work, number of reinfections, HIV infection and sexual behavior) that could arise in a retrospective study. Moreover, our study included a close follow‐up of the affected patients and considered a subgroup affected by L.S. The latter aspect has been less detailed in other prospective and retrospective studies, and their inclusion in our research brings a complete view of the progress of the two treatments in their respective syphilis forms.
Our study nevertheless presents some weaknesses. The small number of patients could have led to not having the necessary power to establish whether the tendency of certain variables, such as HIV infection or the type of sexual relationships, could have influenced the outcomes significantly. Moreover, other variables in the analytical collection, such as the RPR values and other infectious diseases or comorbidities, were not included due to the limitations of the clinical records used. Finally, the study design, which was retrospective, necessarily presents a certain degree of selection bias.
Our work performed a direct and systematic comparison between syphilis patients and their subgroups treated with the two main antibiotics. This allows us to further our knowledge on the treatment of this disease, reinforcing the message that the response to the two antibiotics is similar for patients with E.S.
At the same time, it highlights how the achievement of a valid RPR reduction may be reduced in patients with L.S., and shows that patients with multiple reinfections could have a slightly increased chance to develop a serofast‐state/the lack of seronegativization when treated with doxycycline. However, we need to interpet this result as several other cofactors may be implied and not analyzed due to the study design and limited group number.
We hope that confidence in the use of doxycycline may increase in treating syphilitic infections, often necessary due to the growing difficulty in finding the first‐choice treatment encountered by smaller centers. At the same time, it highlights how the achievement of a valid RPR reduction may be lower in patients with L.S., and shows that patients with multiple reinfections may have more chance to develop a serofast‐state/the lack of seronegativization when treated with doxycycline.
AUTHOR CONTRIBUTIONS
Study conception and design: Valeria Gaspari, Bianca Maria Piraccini, Corrado Zengarini; data collection: Corrado Zengarini, Miriam Carpanese, Alice Conni; analysis and interpretation of results: Corrado Zengarini, Giulio Vara; draft manuscript preparation: Corrado Zengarini. All authors reviewed the results and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Bologna within the CRUI‐CARE Agreement.
Zengarini C, Carpanese MA, Vara G, Conni A, Piraccini BM, Gaspari V. Analysis of serological treatment response to doxycycline versus benzathine penicillin in syphilis infections, a retrospective single‐center study. Dermatologic Therapy. 2022;35(8):e15586. doi: 10.1111/dth.15586
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Spiteri G, Unemo M, Mårdh O, Amato‐Gauci AJ. The resurgence of syphilis in high‐income countries in the 2000s: a focus on Europe. Epidemiol Infect. 2019;13(147):e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barreiro P. Sexually transmitted infections on the rise in PrEP users. AIDS Rev. 2018;20(1):71. [PubMed] [Google Scholar]
- 3. Ramchandani MS, Golden MR. Confronting rising STIs in the era of PrEP and treatment as prevention. Curr HIV/AIDS Rep. 2019;16(3):244‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edmondson DG, Norris SJ. In vitro cultivation of the syphilis spirochete Treponema pallidum . Curr Protoc. 2021;1(2):e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai T, Qu R, Liu J, Zhou P, Wang Q. Efficacy of doxycycline in the treatment of syphilis. Antimicrob Agents Chemother. 2016;61(1):e01092‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghanem KG, Erbelding EJ, Cheng WW, Rompalo AM. Doxycycline compared with benzathine penicillin for the treatment of early syphilis. Clin Infect Dis. 2006;42(6):e45‐e49. [DOI] [PubMed] [Google Scholar]
- 7. Garcia JFB, Aun MV, Motta AA, Castells M, Kalil J, Giavina‐Bianchi P. Algorithm to guide re‐exposure to penicillin in allergic pregnant women with syphilis: efficacy and safety. World Allergy Organ J. 2021;14(6):100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Araujo RS, ASSD S, Braga JU. Who was affected by the shortage of penicillin for syphilis in Rio de Janeiro, 2013‐2017? Rev Saude Publica. 2020;54:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pastuszczak M, Wojas‐Pelc A. Current standards for diagnosis and treatment of syphilis: selection of some practical issues, based on the European (IUSTI) and U.S. (CDC) guidelines. Postepy Dermatol Alergol. 2013;30(4):203‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janier M, Hegyi V, Dupin N, et al. 2014 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2014;28(12):1581‐1593. [DOI] [PubMed] [Google Scholar]
- 11. Peyriere H, Makinson A, Marchandin H, Reynes J. Doxycycline in the management of sexually transmitted infections. J Antimicrob Chemother. 2018;73(3):553‐563. [DOI] [PubMed] [Google Scholar]
- 12. Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother. 2006;58(2):256‐265. [DOI] [PubMed] [Google Scholar]
- 13. Tsai J‐C, Lin Y‐H, Lu P‐L, et al. Comparison of serological response to doxycycline versus benzathine penicillin G in the treatment of early syphilis in HIV‐infected patients: a multi‐center observational study. PLOS One. 2014;9(10):e109813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nurse‐Findlay S, Taylor MM, Savage M, et al. Shortages of benzathine penicillin for prevention of mother‐to‐child transmission of syphilis: an evaluation from multi‐country surveys and stakeholder interviews. PLoS Med. 2017;14(12):e1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harbarth S, Gundlapalli AV, Stockdale W, Samore MH. Shortage of penicillin G: impact on antibiotic prescribing at a US tertiary care centre. Int J Antimicrob Agents. 2003;21(5):484‐487. [DOI] [PubMed] [Google Scholar]
- 16. French P. Syphilis. BMJ. 2007;334(7585):143‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. French P, Gomberg M, Janier M, Schmidt B, van Voorst VP, Young H. IUSTI: 2008 European guidelines on the management of syphilis. Int J STD AIDS. 2009;20(5):300‐309. [DOI] [PubMed] [Google Scholar]
- 18. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis a systematic review. JAMA. 2014;312(18):1905‐1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seña AC, Zhang X‐H, Li T, et al. A systematic review of syphilis serological treatment outcomes in HIV‐infected and HIV‐uninfected persons: rethinking the significance of serological non‐responsiveness and the serofast state after therapy. BMC Infect Dis. 2015;15(1):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


