Abstract
Several small molecules inhibitors exist for targeting Aurora kinase proteins in somatic cells. In this point of view, we evaluate the specificity of these inhibitors in mouse oocytes, and we demonstrate that MLN 8237 and AZD 1152 are specific for AURKA and AURKC, respectively, only when used at low concentrations.
In Brief Statement
The Aurora protein kinases have critical functions in controlling oocyte meiotic maturation. In this paper, we describe an assay for examining their activation state in oocytes and establish best working doses of three commonly used inhibitors.
The Aurora kinases (AURKs) are serine/threonine protein kinases that are important for cellular division, both in mitosis and meiosis (Nguyen & Schindler, 2017). In mammals, this family consists of three members: Aurora kinase A (AURKA) and Aurora kinase B (AURKB), which are expressed in all cells of the body, and Aurora kinase C (AURKC) which is expressed in germ cells and in some cancer cells (Quartuccio & Schindler, 2015). Because of their critical roles in spindle formation and chromosome segregation during cell division, they are widely studied as potential cancer therapeutic targets. Therefore, several small molecule inhibitors exist that inhibit these kinases (de Groot et al, 2015).
One challenge in targeting specific AURKs with inhibitors is that they share significant sequence and structure similarities (Quartuccio & Schindler, 2015). But, despite sharing homologous domains, each AURK in mouse oocytes appears to have distinct functions, while also having compensatory mechanisms that are observed in knock-out mouse models (Balboula & Schindler, 2014; Blengini et al, 2021; Nguyen et al, 2018; Schindler et al, 2012). For example, a dominant-negative allele of Aurkc shows that AURKC is uniquely required for kinetochore-microtubule surveillance (Balboula & Schindler, 2014), but in oocytes that lack AURKC, AURKA takes over this function and supports meiosis I (Nguyen et al, 2018). To bypass genetic-based compensations, inhibitors are useful to determine Aurora kinase functions. But, although there are several available commercial inhibitors, there is a lack of consensus on which inhibitor concentration is required to inhibit a specific AURK without affecting the activities of other two. The most widely used inhibitors for targeting the AURKs in oocytes are MLN 8237 (MLN) for AURKA, and ZM 447439 (ZM) and AZD 1152 (AZD) for AURKB/C (de Groot et al, 2015; Nguyen et al, 2018; Nikalayevich et al, 2022). The specificity of these inhibitors for each AURK protein depends on their intracellular concentration and the cell type. At high concentrations, they have off-target effects, inhibiting the other family members to varying degrees (de Groot et al, 2015).
To evaluate the specificity of each of these inhibitors in mouse oocytes, we isolated oocytes from wild-type CF-1 females and matured them in vitro to Metaphase I in a range of concentrations for each inhibitor that is used in the oocyte literature. We note that we do not use the standard mineral oil overlay because the drugs are lipid soluble and may partition into the oil. Instead, we use center well organ culture dishes that allow for a larger volume of culture media and have an outer ring for water that prevents evaporation. After resolution of Metaphase I oocyte protein lysates by SDS-PAGE, we performed immunoblotting to detect the activated forms of AURKA and AURKC using a phospho-specific antibody that recognizes the phosphorylated forms of AURKA (pThr288), AURKB (pThr223), and AURKC (pThr198) (pA/B/C). Finally, we quantified the levels of pAURKA and pAURKC; pAURKB was not detectable in our lysates because it is the least abundant of the three isoforms and requires many more oocytes for detection (Nguyen et al, 2018). In oocytes matured in culture media containing MLN, pAURKA was absent with 0.5 μM treatment, the lowest concentration tested. Importantly, pAURKC was significantly reduced by 60% with 5 μM and absent with 10 μM treatments (Fig. 1A,B,C). These data indicate that MLN can specifically inhibit AURKA in mouse oocytes but only when using a range between 0.5–2 μM, and not higher. When we evaluated AURKC inhibitors, we observed similar concentration-dependent off-targeting effects. pAURKC was absent at 0.1 μM of AZD treatment, the lowest concentration used (Fig.1 D,F). However, when we treated oocytes with higher doses of AZD, the data indicate significant inhibition of AURKA at 0.5 μM (40% reduction) and further inhibition as concentrations increased, reaching 80% of inhibition at 10 μM (Fig.1 D,E). These data indicate that AZD is a specific inhibitor of AURKC in mouse oocytes but only when applying concentrations lower than 0.5 μM. Finally, when we evaluated ZM for specificity, the results were concerning. This concern arises because of the prevalence of its use in oocyte-focused experiments. At the lowest concentration where pAURKC is absent (2 μM) (Fig. 1 G, H, I), 50% of AURKA was inhibited. These data indicate the ZM is not a specific inhibitor of AURKC in mouse oocytes because even at the lowest concentration that inhibits AURKC completely, AURKA is partially inhibited.
Figure 1.
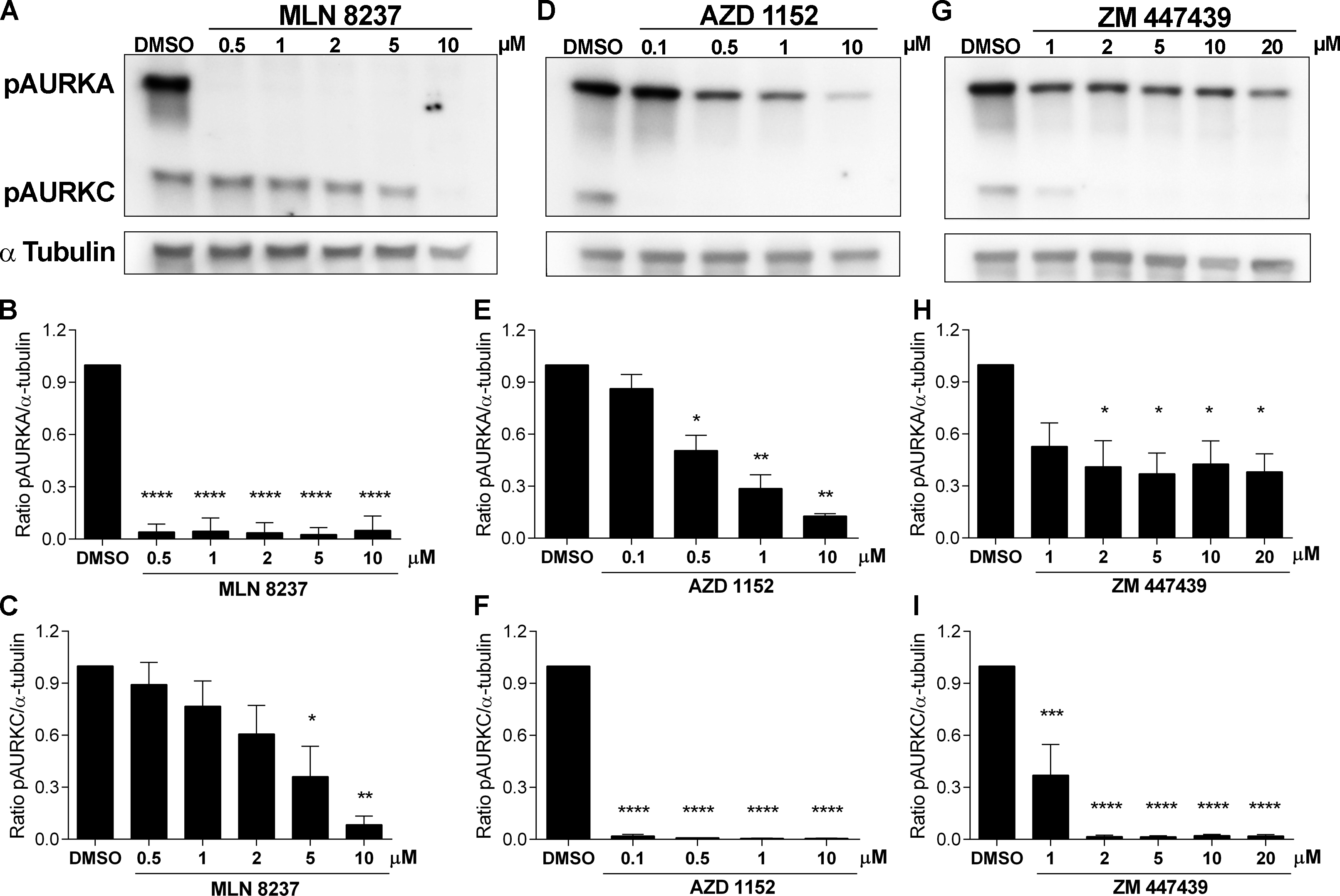
Evaluation of Aurora kinase inhibitor specificity in mouse oocytes. A, D, G are Representative Western blot images of lysates from Metaphase I mouse oocytes matured in vitro in the presence of indicated concentrations of MLN 8237 (Selleckchem, #S1133)(A); AZD 1152 (Selleckchem, #S1147) (D); and ZM 447439 (Tocris Bioscience, #2458) (G). 100 oocytes were loaded per lane. Proteins were separated by electrophoresis in 10% SDS polyacrylamide gels. After blotting, the membranes were incubated overnight at 4°C with primary antibody to detect pAURKA/B/C (1/500; Cell Signaling Technology, #2914) or for 1h to detect α-tubulin (1:1000; Cell Signaling Technology #11H10). Next, the membranes were incubated with anti-rabbit HRP secondary antibody (1:1000; Kindle Bioscience #R1006) for 1h at room temperature. The signals were detected using ECL western blotting detection reagents (Kindle Biosciences, KwikQuant Western Blot Detection Kit, R1002) following the manufacturer’s protocol. Images were analyzed using Image J software (NIH) (Schneider et al, 2012) and were normalized to α-tubulin and set to 1 in DMSO treatment. B, E, H, Quantification of pAURKA from (A), (D) and (G) respectively. C, F, I Quantification of pAURKC from (A), (D) and (G) respectively. Statistical analyses were performed using ANOVA-one way, **** p<0.0001, *** p<0.001, ** p<0.01, * p<0.05. Graphs show the mean ± SEM from 3 independent experiments for MLN and ZM and 2 independent experiments for AZD.
We demonstrate that MLN and AZD can specifically inhibit AURKA and AURKC, respectively, whereas ZM is a pan-AURK inhibitor even at low concentrations in mouse oocytes. MLN and AZD also affected the activity levels of both AURKs in a dose-dependent manner. Because of the number of mice it would require to evaluate pAURKB levels in oocytes, we are unable to conclude the effect of MLN and AZD treatment on AURKB activity. However, because of the compensatory nature of AURKA and AURKC in oocytes, we view off-target effects of these two AURKs the most critical to evaluate. Notably, several of the concentrations that we found to be non-specific were used in other studies where potential off-target effects were not extensively evaluated. It is therefore imperative that the interpretations of results from such studies be taken with caution. Although our results show the potential of MLN and AZD to be specific and discourages the use of ZM, the effective concentrations should be determined for each experimental setting when inhibiting these kinases. Although we have not yet tested other experimental settings, differences in the range of specific concentrations could arise based on experimental methods (i.e. culture media and conditions, chronic inhibition vs. acute inhibition) or the genetic background of the mice. For this reason, we propose and strongly encourage evaluating the activity levels of AURKs using this Western blotting assay when conducting experiments with these and other AURK inhibitors.
Funding Statement
This work was supported by an NIH grant R35 GM136340 to KS.
Footnotes
Declaration of Interest Statement
The authors declare no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported. Karen Schindler is an Associate Editor of Reproduction. Karen Schindler was not involved in the review or editorial process for this paper, on which she is listed as an author
References
- Balboula AZ & Schindler K (2014) Selective disruption of aurora C kinase reveals distinct functions from aurora B kinase during meiosis in mouse oocytes. PLoS Genet, 10(2), e1004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blengini CS, Ibrahimian P, Vaskovicova M, Drutovic D, Solc P & Schindler K (2021) Aurora kinase A is essential for meiosis in mouse oocytes. PLOS Genetics, 17(4), e1009327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot CO, Hsia JE, Anzola JV, Motamedi A, Yoon M, Wong YL, Jenkins D, Lee HJ, Martinez MB, Davis RL, Gahman TC, Desai A & Shiau AK (2015) A Cell Biologist’s Field Guide to Aurora Kinase Inhibitors. Front Oncol, 5, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AL, Drutovic D, Vazquez BN, El Yakoubi W, Gentilello AS, Malumbres M, Solc P & Schindler K (2018) Genetic Interactions between the Aurora Kinases Reveal New Requirements for AURKB and AURKC during Oocyte Meiosis. Current Biology, 28(21), 3458–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AL & Schindler K (2017) Specialize and Divide (Twice): Functions of Three Aurora Kinase Homologs in Mammalian Oocyte Meiotic Maturation. Trends Genet, 33(5), 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikalayevich E, El Jailani S, Dupré A, Cladière D, Gryaznova Y, Fosse C, Buffin E, Touati SA & Wassmann K (2022) Aurora B/C-dependent phosphorylation promotes Rec8 cleavage in mammalian oocytes. Curr Biol, 32(10), 2281–2290.e4. [DOI] [PubMed] [Google Scholar]
- Quartuccio SM & Schindler K (2015) Functions of Aurora kinase C in meiosis and cancer. Front Cell Dev Biol, 3, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler K, Davydenko O, Fram B, Lampson MA & Schultz RM (2012) Maternally recruited Aurora C kinase is more stable than Aurora B to support mouse oocyte maturation and early development. Proc Natl Acad Sci U S A, 109(33), E2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS & Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature methods, 9(7), 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]


