Abstract
Alpha‐band oscillations (8–14 Hz) are essential for attention and perception processes by facilitating the selection of relevant information. Directing visuospatial endogenous (voluntary) attention to a given location consistently results in a power suppression of alpha activity over occipito‐parietal areas contralateral to the attended visual field. In contrast, the neural oscillatory dynamics underlying the involuntary capture of attention, or exogenous attention, are currently under debate. By exploiting the inherent capacity of emotionally salient visual stimuli to capture attention, we aimed to investigate whether exogenous attention is characterized by either a reduction or an increase in alpha‐band activity. Electroencephalographic activity was recorded while participants completed a Posner visuospatial cueing task, in which a lateralized image with either positive, negative, or neutral emotional content competed with a target stimulus presented in the opposite hemifield. Compared with trials with no distractors, alpha power was reduced over occipital regions contralateral to distracting images. This reduction of alpha activity turned out to be functionally relevant, as it correlated with impaired behavioral performance on the ongoing task and was enhanced for distractors with negative valence. Taken together, our results demonstrate that visuospatial exogenous attention is characterized by a suppression of alpha‐band activity contralateral to distractor location, similar to the oscillatory underpinnings of endogenous attention. Further, these results highlight the key role of exogenous attention as an adaptive mechanism for the efficient detection of biologically salient stimuli.
Keywords: alpha oscillations, EEG, emotion, exogenous attention, time‐frequency analyses
Short abstract
Our findings reveal that alpha‐band oscillations do not only index the locus of top‐down visuospatial attention, as it is well known, but also the degree of exogenous attentional capture. Thus, the suppression of alpha‐band power might be taken to indicate the amount of attentional resources diverted to distractor stimuli and, importantly, to describe the advantage of emotional over neutral stimuli.
1. INTRODUCTION
The human brain is constantly exposed to a multitude of stimuli. Hence, in order to discriminate relevant from unnecessary information, an attention system becomes essential. Stimulus selection is accomplished according to internal goals or automatically triggered by certain stimuli, namely endogenous and exogenous attention, respectively (Chica et al., 2011; Corbetta et al., 2008). On the one hand, given that our processing resources are limited, endogenous attention is responsible for ignoring distractors in favor of the ongoing task (Lavie, 2005). On the other hand, if distractor stimuli reach a given saliency threshold, exogenous attention may be captured and cognitive resources diverted to them (Koster et al., 2004).
Alpha‐band oscillations (8–14 Hz) are considered to be crucial for attention and perception, by facilitating the selection of relevant information and inhibiting irrelevant distractors (Foxe & Snyder, 2011; Jensen et al., 2014; Jensen & Mazaheri, 2010; Klimesch, 2012). Consistent evidence supports these theories, showing that modulations of alpha activity correlate with the direction of visuospatial attention. In particular, alpha‐band power has been found to decrease over occipito‐parietal areas contralateral to the attended visual field and/or to increase ipsilaterally (Capilla et al., 2014; Händel et al., 2011; Siegel et al., 2008; Thut et al., 2006; Worden et al., 2000; Wöstmann et al., 2019). In addition, a number of recent studies employing multivariate approaches have shown that selective topography of alpha oscillations precisely tracks the temporal dynamics of visuospatial orienting (Foster et al., 2017; Samaha et al., 2016). Importantly, the modulation of alpha power has an impact on behavioral performance, by enhancing the perception of the target (Capilla et al., 2014; Kelly et al., 2009; Thut et al., 2006) and disrupting the processing of distractors (Payne et al., 2013; Zumer et al., 2014). Furthermore, it has been convincingly demonstrated that alpha‐band fluctuations do not only predict the locus of visuospatial attention but causally drive attention and visual perception (Bagherzadeh et al., 2020; Romei et al., 2010; Thut et al., 2011).
It is worth noting that majority of research has focused on endogenous attention, whilst the oscillatory neural dynamics of exogenous attention have been largely neglected. First evidences of modulations of alpha‐band oscillations under exogenous attention requirements are quite recent and arise from cross‐modal experiments, wherein peripheral non‐predictive (i.e., 50% valid) auditory cues are used to involuntarily orient visuospatial attention (Feng et al., 2017; Keefe & Störmer, 2021; Störmer et al., 2016; Weise et al., 2020). Overall, these studies demonstrate that exogenous cues trigger an oscillatory mechanism within the alpha frequency band and enhance subsequent visual processing, resembling the positive influence of endogenous attention on perception.
However, although all of these studies agree on the relevance of the alpha rhythm, there are substantial discrepancies between them in terms of the pattern of power modulation found. In particular, two studies have shown that alpha power decreases over occipito‐parietal regions, especially in the hemisphere contralateral to the cued side (Feng et al., 2017; Störmer et al., 2016), whereas another found that alpha‐band power increases, rather than decreases, in the hemisphere ipsilateral to the auditory cue (Weise et al., 2020). A fourth study only reported lateralization effects (contra—ipsilateral difference), so the net effect of each hemisphere cannot be estimated (Keefe & Störmer, 2021). This discrepancy in the pattern of alpha power modulation is crucial for the interpretation of the mechanisms underlying visuospatial exogenous attention. Whilst a contralateral decrease in alpha‐band power would imply the facilitation of stimulus processing at attended locations, an ipsilateral alpha increase would indicate the inhibition of the unattended visual field (Foxe & Snyder, 2011; Jensen et al., 2014; Jensen & Mazaheri, 2010; Klimesch, 2012). Weise et al. (2020) suggest that the discrepant results of their study could be due to the fact that they included a distraction task in addition to the spatial cueing task.
The present study sought to shed light on these conflicting findings, by using a type of stimulus that inherently induces distraction: emotional images. It has been pointed out that emotion‐laden distractors grab exogenous attention to a greater extent than neutral stimuli (Carretié, 2014). This is not surprising from an evolutionary perspective, given the importance of detecting biologically salient stimuli such as danger, food, or mating partners, which are, by definition, emotional. We thus harnessed the engaging property of emotional images to investigate the oscillatory mechanisms underlying visuospatial exogenous attention.
We recorded electroencephalographic (EEG) activity while participants performed a Posner cueing task. A visual predictive cue indicated with a validity of 100% the location of an upcoming target, thus ensuring that endogenous attention was oriented to it. An emotional or non‐emotional distractor image appeared simultaneously with the target in the uncued visual field. If exogenous attention were effectively captured by emotion‐laden images, we would expect a suppression of alpha‐band power over posterior regions contralateral to the distractor location. Alternatively, if task‐irrelevant distractors were to be inhibited, we would expect an increase in contralateral alpha power. Our results support the former hypothesis, showing that visuospatial exogenous attention is characterized by a suppression of alpha power that is more prominent over contralateral occipital sites and for distractors with negative valence. This suggests that the desynchronization (reduction) of alpha‐band activity indexes the degree of attentional capture and the resources diverted to distractor stimuli, with the consequent negative impact on behavioral performance.
2. METHOD
2.1. Participants
Thirty‐six volunteers took part in this study (19 females, 29 right‐handed, 21.6 ± 3.5 years old, mean ± SD), although one was excluded from EEG analysis as explained below. They all reported normal or corrected‐to‐normal visual acuity. Before participating in the experiment, they provided informed written consent. The study was approved by the Ethics Committee of the Universidad Autónoma de Madrid and conducted in compliance with the declaration of Helsinki.
2.2. Stimuli and procedure
Participants completed the experimental task while seated 1 m away from the screen inside an electrically shielded, sound‐attenuated room. The task was programmed using Psychtoolbox (v3.0; Brainard, 1997) in Matlab (The MathWorks) and presented through a RGB projector on a back‐projection screen.
The task was divided into four experimental blocks of 105 trials each. The structure of a trial is depicted in Figure 1. A central fixation cross was presented on a gray background, subtending 2.3 × 2.3° of visual angle. After a variable interval ranging from 0.6 to 1 s, an arrow (0.6 × 0.6°) was appended to the fixation cross for 0.1 s, pointing to either the left or the right lower quadrant. This cue indicated with 100% probability the subsequent location of the target stimulus. Participants were instructed to pay covert attention (i.e., without moving their eyes) to the cued location.
FIGURE 1.
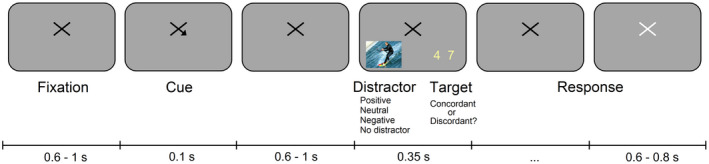
Experimental task. A spatial cue indicated with a probability of 100% the location of an upcoming target stimulus (i.e., a pair a numbers). Participants performed a digit‐parity task while an emotional distractor appeared in the uncued hemifield. No distractors were presented in the control block
After a variable delay of 0.6 to 1 s, a composite target stimulus composed of a pair of yellow numbers was displayed on the screen for 0.35 s. The size of the digits was 1.7 × 2.9° (width × height), and they were presented in either the left or the right lower quadrant at 10.8 × 9.4° from fixation. Participants performed a digit‐parity task during which they were required to report if the pair of numbers was concordant (i.e., both digits odd or both even) or discordant (i.e., one odd and one even), by pressing a key as accurately and fast as possible. They were given unlimited time to respond. After providing the response, the fixation cross turned white for 0.6–0.8 s, indicating the beginning of the next trial. Participants were encouraged to use the time between trials to blink if necessary.
In three out of the four experimental blocks, a distractor stimulus appeared in the uncued location at the same time as the target stimulus. In the remaining block no distractor stimuli were presented, thus acting as a control. The order of presentation of the blocks was randomized across participants. Distracting images were obtained from the EmoMadrid emotional pictures database (Carretié et al., 2019), whose normative data range from −2 to +2 in both emotional arousal and valence dimensions. We selected three groups of 35 images with positive, neutral, and negative valence. Positive and negative images were matched in absolute valence (positive: |1.29| ± 0.148; negative: |−1.28| ± 0.157; t 68 = 0.340, p = .735), whereas the valence of the neutral images did not significantly differ from zero (−0.001 ± 0.130; t 34 = −0.069, p = .945). In a similar way, arousal was matched for emotion‐laden images (positive: 1.27 ± 0.221; negative: 1.24 ± 0.190; t 68 = 0.586, p = .559) and near zero for neutral images (−0.001 ± 0.154; t 34 = −0.052, p = .959).
The size of the distracting images was 14.6 × 11.0° and they were positioned at a visual angle of 10.8 × 9.4°. On each trial, one image was pseudo‐randomly chosen, with the condition that every image appeared three times throughout the whole experiment, whilst images with the same valence did not appear on more than two consecutive trials.
2.3. Self‐reported emotional valence and arousal
At the end of the recording session, participants completed a bidimensional scaling test for each of the 105 images, assessing both their valence and arousal. This last test aimed to confirm that a‐priori categorization of stimuli as positive, negative, and neutral was valid in our sample. The effect of Distractor (positive, negative, neutral) on valence and arousal was statistically tested by means of a one‐way repeated measures analysis of variance (ANOVA). We also conducted a series of t‐tests to statistically check whether positive and negative images differed in absolute valence and arousal, and whether neutral images differed from zero in emotional valence and arousal. Significance threshold was adjusted by Bonferroni correction for multiple comparisons. All statistical analyses were performed using IBM SPSS Statistics 26.
2.4. Behavioral analysis
Behavioral analyses were carried out to verify that distractor stimuli captured exogenous attention. If this were the case, we would expect lower accuracy and longer response times in the digit‐parity task. Trials contaminated with eye movements or blinks were not analyzed, as these could have affected the deployment of covert attention to target stimuli. Trials with extreme response times (either < Q1 – 2.5 × IQR or > Q3 + 2.5 × IQR; Q: quartile, IQR: interquartile range) were also excluded from the analysis.
Target stimuli presented on the right and left hemifields were analyzed separately to account for potential lateralization effects. We statistically tested the effect of Distractor (positive, neutral, negative, and non‐distractor) × Hemifield (right and left) by means of a 4 × 2 ANOVA. In the case of non‐sphericity, the multivariate approximation (Wilks’ lambda) was taken into account. Effect sizes were estimated using the partial eta‐square (η 2 p ) method. Post‐hoc pairwise t‐test analyses were conducted to detect specific differences among conditions, adjusting p‐values by means of the Bonferroni correction when multiple comparisons were performed. In this case we used the unbiased Cohen’s d (also referred to as Hedge’s g) to measure the effect size (Cumming, 2012).
2.5. Recording of the EEG signal
The EEG signal was acquired using an electrode cap (custom‐made by ElectroCap International) with tin electrodes. Fifty‐nine electrodes were placed at the scalp surface following the 10–10 international system. All electrodes were referenced to the nose‐tip and grounded with an electrode located on the forehead. Additionally, horizontal and vertical electro‐oculographic (EOG) activity was recorded to monitor blinks and eye movements. Recordings were continuously digitized at a sampling rate of 420 Hz and on‐line bandpass filtered between 0.3 and 1000 Hz.
2.6. Analysis of the EEG signal
EEG data analysis was carried out using the FieldTrip toolbox (version 20180405; Oostenveld et al., 2011) and in‐house Matlab code. Overall, we aimed to test whether emotionally salient distractors modulate alpha oscillations, similarly to the modulation of alpha‐band power induced by endogenous attention. To this end, we first pre‐processed and removed artifacts from the EEG signal. We then performed a time‐frequency (TF) analysis to identify frequency bands and electrodes of interest. Once these were established, we computed and statistically compared the temporal‐spectral evolution (TSE) curves in response to different distractors and in the absence of distraction. These analysis steps are described in more detail in the following sections.
2.6.1. Pre‐processing
The EEG signal was segmented into 6‐s‐long trials (3.5 s pre‐stimulus) time‐locked to both cue and target/distractor onset. Long epochs were used to avoid edge artifacts in subsequent spectral analyses. Each trial was assigned to one of the four experimental conditions: positive, neutral, negative, or no distractor.
We then carried out a three‐step artifact rejection procedure. First, trials contaminated with eye movements were eliminated, as these may indicate a failure to maintain covert attention. Second, any remaining artifacts were removed by means of independent component analysis (ICA) on a 20‐dimensional space obtained by principal component analysis (PCA). Finally, noisy channels were interpolated based on data recorded on adjacent electrodes.
One participant was not included in the EEG data analysis due to an excessive number of ocular artifacts (>70% of trials contaminated). The average number of artifact‐free trials per condition in the remaining sample was 80.9 ± 14.3 and did not significantly differ across conditions (F 3,102 = 0.514, p = .597).
2.6.2. Time–frequency (TF) analysis
TF analysis aimed to identify the frequency range and electrodes with power modulations. Spectral power was computed for each trial using a Hanning‐tapered sliding window Fourier transform from 0 to 20 Hz in 0.5‐Hz steps and from −0.5 to 1 s in 50‐ms steps. The width of the Hanning window was set to 0.8 s to maximize spectral resolution. Power was normalized by computing the relative percentage change from baseline. In order to determine frequency bands and channels of interest, we computed the average oscillatory activity contralateral to both target and distractor presentation for all conditions together.
2.6.3. Temporal–spectral evolution (TSE) curves
TF analysis revealed power modulations centered around 11 Hz and maximal over O1/O2 electrodes. Based on this, we computed the TSE of alpha‐band oscillations time‐locked to cue and target/distractor onset for these electrodes. To do so, we bandpass filtered single‐trial EEG activity between 9 and 13 Hz and subsequently calculated the absolute value of the Hilbert transform. TSE curves were baseline corrected using a 0.5 s baseline segment.
Contra and ipsilateral TSE curves time‐locked to distractor appearance were statistically compared with trials on which no distractor was present. Each distractor condition (positive, negative, neutral) was tested separately by means of a non‐parametric, permutation‐based approach. In each permutation, trials of the distractor and non‐distractor conditions were randomly shuffled and subjected to a paired t test. This process was repeated 1000 times. To correct for multiple comparisons, the maximum t‐value across time was stored in each repetition. The resultant distribution of the t‐statistic was used to derive corrected p‐values. T‐values above the 95th percentile were considered to be statistically significant.
2.7. Correlation of alpha‐band power with behavioral performance
We then tested whether modulation of alpha‐band activity was correlated with behavioral performance. For this purpose, we calculated the variation in accuracy and response time in the presence of distractors compared with the non‐distractor condition (i.e., distractor—non‐distractor). Thus, an improvement in performance under distraction would be indexed by positive values for accuracy and by negative values for response times. We then computed the correlation coefficient between both accuracy and response time indices and alpha‐band power.
2.8. Correlation between alpha‐band power and self‐reported arousal and valence
Because the largest attentional effects were found for negative distractors, and these tended to be rated as most arousing in the self‐report assessment, we conducted an additional analysis to test whether alpha‐band suppression might be related to emotional arousal. Correlation analysis was conducted on a single‐trial basis. For each participant, we computed the correlation coefficient between alpha‐band power and the self‐reported arousal of the image presented on each single‐trial. Individual correlation values were then subjected to a one‐sample t test compared to zero, to test whether alpha‐band modulations and stimulus arousal were either positively or negatively correlated among individuals. Additionally, we carried out the same procedure to assess whether alpha power and emotional valence were correlated at the single‐trial level.
Finally, we also computed Bayes Factors (BF10) on the correlation coefficients between alpha‐band power and the self‐reported arousal. The aim of this Bayesian analysis was to assess the strength of evidence in favor of the null hypothesis ‐H0‐ over the alternative hypothesis ‐H1‐ (Jeffreys, 1939; see also Dienes, 2014). The BF10 was calculated employing JASP 0.16.0.0 (JASP Team, 2021).
3. RESULTS
3.1. Self‐reported emotional valence and arousal
We first checked whether the participants’ assessment of distractor stimuli actually matched the a‐priori categorization of images as either positive, negative, or neutral. Self‐reported emotional valence was 1.22 ± 0.336 for positive, −1.10 ± 0.364 for negative, and 0.067 ± 0.195 for neutral images. These values correspond to the emotional categories established a priori and significantly discriminate between them (F 2,34 = 310, p < .001, η 2 p = .948; t 35 > 17.7, p < .001, d unb >2.89). Furthermore, self‐reported arousal was 1.02 ± 0.557 for positive, 1.20 ± 0.318 for negative, and −0.039 ± 0.154 for neutral images. As expected, the main effect of arousal was also significant (F 2,34 = 203, p < .001, η 2 p = 0.923), being higher for both positive and negative stimuli compared with neutral ones (t 35 > 10.7, p < .001, d unb >1.75). Therefore, we can be confident that the distractors employed in this study were appropriately assigned to the corresponding emotional category.
We then conducted a more fine‐grained analysis to test whether positive and negative images differed in absolute valence and arousal, and whether valence and arousal was different from zero for neutral images. Because we conducted 4 comparisons, the Bonferroni corrected significance threshold was set to 0.012 (0.05/4). We verified that positive and negative images did not differ in absolute valence (t 35 = 1.66, p = .105, d unb = 0.271). Negative images showed a trend to be assessed as more arousing than positive ones (t 35 = 2.27, p = .030, d unb = 0.370), although this comparison did not reach the corrected threshold for statistical significance. We also verified that arousal did not differ from zero for neutral images (t 35 = 1.52, p = .139, d unb = 0.247), though emotional valence was indeed significantly higher than zero (t 35 = 2.75, p = .009, d unb = 0.448).
3.2. Behavioral results
The ANOVA conducted on the percentage of correct responses in the digit‐parity task showed neither a significant main effect of Hemifield (F 1,35 = 0.407, p = .528, η 2 p = 0.011) nor a significant interaction between Hemifield and Distractor (F 3,105 = 0.117, p = .937, η 2 p = 0.003). In contrast, the main effect of Distractor did result significant (F 3,33 = 3.54, p = .025, η 2 p = 0.243). Subsequent comparisons aimed to identify pairwise differences among distractor types. As we performed 6 comparisons, the corrected threshold for significance was 0.008 (0.05/6). Accuracy on the digit‐parity task showed a trend to be lower when negative distractors were present compared with both positive (t 35 = 2.35, p = .025, d unb = 0.383) and non‐distractor (t 35 = 2.24, p = .032, d unb = 0.364) conditions. Similarly, percentage of correct responses also tended to be lower under the presence of neutral in comparison with positive images (t 35 = 2.13, p = .040, d unb = 0.347), although none of these comparisons reached statistical significance when correcting for multiple comparisons (Figure 2).
FIGURE 2.
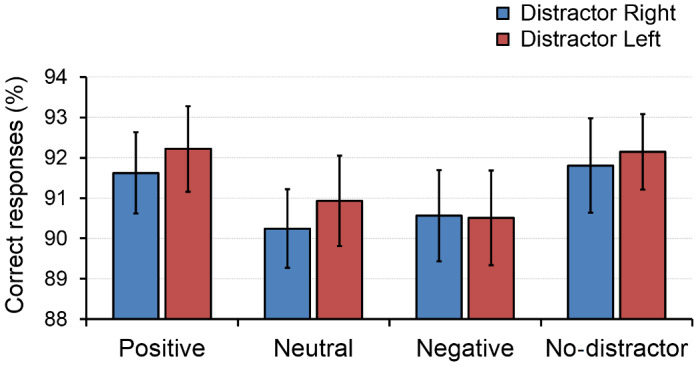
Behavioral results. Percentage of correct responses in the digit‐parity task under the presence of positive, neutral, and negative images, as well as in the absence of distraction. Blue bars indicate distractors presented on the right; red bars indicate left. Error bars represent the standard error of the mean
The ANOVA performed on response times revealed no significant effects (all p > 0.246) and small effect sizes (η 2 p < 0.116). Although participants were instructed to respond as accurately and fast as possible, the lack of time constraints may have prioritized accuracy over speed, thus explaining this null result.
3.3. Time–frequency (TF) results
As can be seen in Figure 3, TF analysis revealed a modulation of power in the alpha frequency range in response to both cue and target/distractor presentation. As expected, following cue onset, alpha‐band power decreased over contralateral posterior sites, reflecting the deployment of endogenous attention to the cued location. Alpha‐band activity was also suppressed in response to the simultaneous presentation of target and distractor, though more bilaterally. Alpha power reduction occurred mainly within the frequency range of 9 to 13 Hz, peaked between 0.3 and 0.7 s after target/distractor onset, and was maximal over occipital electrodes (i.e., O1/O2).
FIGURE 3.
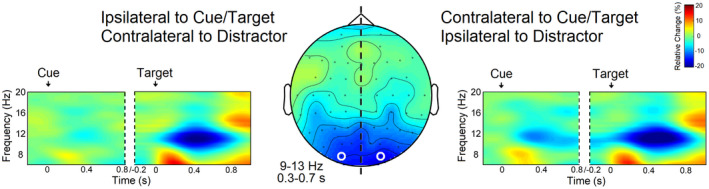
TF results: Alpha‐band power suppression time‐locked to cue and target/distractor. In response to the cue, alpha power decreased over contralateral posterior sites, whereas in response to target/distractor, alpha‐band exhibited a bilateral power reduction. The topography shows the modulation of alpha activity between 9 and 13 Hz and from 0.3 to 0.7 s after target/distractor onset. Occipital electrodes with maximal power suppression are highlighted in white
3.4. Temporal–spectral evolution (TSE) of alpha‐band oscillations
Once we had identified the specific frequency band (9–13 Hz) and electrodes (O1/O2) exhibiting maximal alpha‐band modulation, we calculated the TSE of alpha oscillations relative to cue and target/distractor presentation. As expected, the allocation of endogenous attention to the cued location was characterized by a reduction in alpha‐band amplitude, which was more pronounced contralateral to the focus of attention and peaked at 331 ms (see Figure 4a).
FIGURE 4.
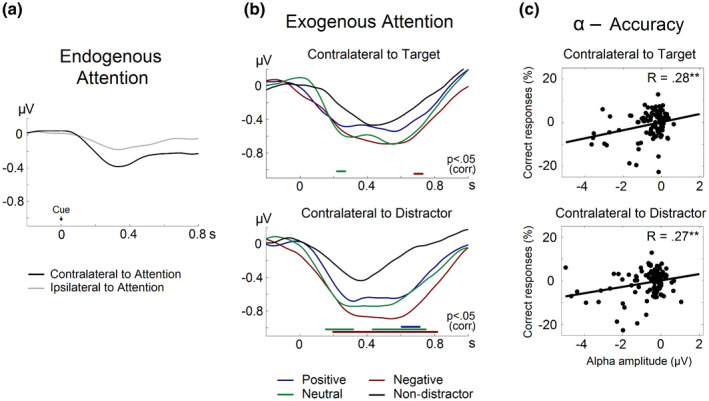
Alpha‐band TSE and correlation with accuracy. (a) TSE of alpha‐band amplitude time‐locked to cue onset. The black line represents alpha‐TSE in the hemisphere contralateral to the focus of endogenous attention; the gray line represents the ipsilateral hemisphere. (b) TSE of alpha‐band amplitude time‐locked to target/distractor onset. Alpha‐TSE contralateral to target and distractor location are shown separately. The figure shows a more pronounced alpha‐band desynchronization contralateral to the presentation of distractor images (positive, neutral, and negative) compared with the absence of distraction. Significant differences between distractor and non‐distractor conditions are indicated by colored horizontal lines (p < .05 corrected for multiple comparisons). (c) Correlation between alpha‐band amplitude and accuracy in the digit‐parity task in the hemisphere contralateral to target and distractor. **p < .01
Exogenous attention captured by distractors followed similar temporal dynamics, with a reduction of alpha amplitude reaching a minimum at 329 ms. Statistical analysis revealed significant differences in alpha‐TSE depending on whether or not distractors were present. As shown in Figure 4b, distractor effects were mainly evident in the hemisphere contralateral to distractor location. In this hemisphere, alpha amplitude exhibited a higher reduction in the presence of negative images in comparison with the control condition between 198 and 817 ms, for neutral images in two intervals, 155–317 and 433–748 ms, and for positive images between 605 and 712 ms. In the hemisphere ipsilateral to distractor presentation two marginal time intervals were found to be significant for negative (679–729 ms) and neutral images (220–270 ms) compared with the absence of distraction (all p < .05 corrected).
3.5. Correlation of alpha‐band power with behavioral performance
Correlation analysis was conducted to check for the existence of a relationship between alpha‐band power and performance in the digit‐parity task. In particular, we sought to test if alpha‐band modulation was related to the level of attention/distraction devoted to the ongoing task. Analysis was carried out on the time window exhibiting effects in the former TSE analysis (i.e., 0.2 to 0.8 s after target/distractor onset).
Our results showed that response times were not significantly correlated with alpha‐band power in either the contralateral (R 103 = −0.13, p = .203) or the ipsilateral hemisphere (R 103 = −0.15, p = .116). As for the behavioral results, this null effect might have been driven by the absence of time limits for responding, thus favoring precision over speed.
In contrast, we did find a significant and positive correlation between accuracy and alpha‐band activity. Thus, the lower the alpha rhythm amplitude, the worse the accuracy (see Figure 4c). Although one would expect this effect to be lateralized, this was not the case, as both alpha‐band amplitude in the contralateral (R 103 = 0.27, p = .006) as well as in the ipsilateral hemisphere (R 103 = 0.28, p = .004) were significantly correlated with accuracy, indicating that the distraction level indexed by alpha‐band amplitude results in impaired behavioral performance.
3.6. Correlation between alpha‐band power and self‐reported arousal and valence
Finally, we conducted a correlation analysis to rule out the possibility that the observed effects could be explained by the arousal of distracting images and not only by their emotional valence. The results of this analysis did not support this explanation, since the correlation between arousal and alpha‐band activity was not statistically significant for either the contralateral (t 34 = 0.516, p = .609, d unb = 0.085) or the ipsilateral (t 34 = 1.46, p = .153, d unb = 0.242) hemisphere.
On the contrary, single‐trial correlation analysis of self‐reported valence and alpha power revealed that lower valence values (i.e., negative distractors) were associated with greater alpha‐band desynchronization. This effect was statistically significant for the hemisphere contralateral to distractor location (t 34 = 2.81, p = .008, d unb = 0.464), but not for the ipsilateral (t 34 = 1.72, p = .094, d unb = 0.284), thus suggesting that the more negative the valence, the greater the capture of attention triggered by emotional distractors.
Additionally, we computed BF10 in order to confirm the abovementioned lack of correlation between alpha‐band power and arousal. H1 for the dimension of arousal was modeled using a Cauchy distribution centered on zero and with an informed prior defined by the standardized unbiased effect size (Cohen’s d) calculated from the t test on valence, expecting both effect sizes to be similar. The cited effect size was small to medium (d unb = 0.464), in line with the findings of other studies analyzing the influence of emotion on exogenous attention through neural measures (such as ERP or fMRI; see Carretié, 2014 for a review). Employing this prior (scale factor = √0.464), the obtained BF10 provided substantial evidence in favor of the H0 for the contralateral hemisphere and anecdotal evidence for the ipsilateral hemisphere (BF10 = 0.212 and BF10 = 0.492, respectively; for more information on the interpretation of BF see e.g. Dienes, 2014). These findings sum additional support against the possibility that the observed effects could be explained by the arousal of distracting images, rather than by their valence.
4. DISCUSSION
In the current study, we investigated the involvement of alpha oscillations in the attentional capture triggered by emotional stimuli. To this end, we recorded EEG activity in participants performing a visuospatial cueing task, in which a target stimulus appeared in the cued location while an image with either positive, negative, or neutral emotional content was simultaneously presented in the opposite hemifield. Our results show that distractor stimuli, particularly those charged with negative emotion, induce a contralateral reduction in alpha power that is correlated with impaired behavioral accuracy.
As expected, we observed a contralateral decrease of occipital alpha power following cue onset, thus replicating the common finding that lateralized alpha oscillations index the deployment of visuospatial endogenous attention (Capilla et al., 2014; Gould et al., 2011; Thut et al., 2006; van Diepen et al., 2016). Lateralization of pre‐stimulus alpha has also been found to be reinforced by increasing the predictive validity of the cue (Dombrowe & Hilgetag, 2014; Gould et al., 2011; van Ede et al., 2012). Hence, by setting the cue validity to 100%, we sought to maximize the expectation of forthcoming target/distractor stimuli and corresponding neural oscillatory effects.
In response to the simultaneous presentation of both target and distractor, the pattern of post‐stimulus alpha suppression became bilateral over occipital electrodes. Alpha‐band activity was reduced contralateral to the location of the target, which is commonly reported when visual stimuli are perceived (Babiloni et al., 2006; Bareither et al., 2014; Harris et al., 2020; Vanni et al., 1997) and has recently been attributed to the attentive processing of target information (Bacigalupo & Luck, 2019; van Diepen et al., 2016). In addition, we found an alpha power suppression contralateral to distractor location, an effect that was particularly marked for images with negative emotional content.
Several authors argue that increases in alpha‐band amplitude ipsilateral to the attended visual field are indicative of reduced processing of distractors (Foxe & Snyder, 2011; Jensen & Mazaheri, 2010; Klimesch, 2012; Payne et al., 2013; van Diepen et al., 2016; Zumer et al., 2014). Consequently, higher levels of alpha‐band power are thought to reflect a state of inhibited neural processing (Foxe & Snyder, 2011; Jensen & Mazaheri, 2010). However, alpha power enhancement also appears in the absence of distractors (Rihs et al., 2007, 2009; van Gerven & Jensen, 2009) and its role in active distractor inhibition has recently been questioned (Fodor et al., 2020; Foster & Awh, 2019; Schroeder et al., 2018). Indeed, Foster and Awh (2019) speculated whether the alpha power increase contralateral to distractors could simply reflect a by‐product of the selection of relevant information rather than suppression of distractors. In an attempt to solve this controversy, it has recently been suggested that target selection and distractor suppression may be two independent processes, both of which are characterized by modulations of alpha oscillations in different regions of the cortex (Wöstmann et al., 2019).
In the present study, the lack of alpha power increase in response to distractor stimuli suggests that these are not being suppressed. Instead, we observed a reduction of post‐stimulus alpha‐band activity contralateral to distractor presentation. This is clearly reminiscent of the endogenous attention mechanism characterized by an alpha‐band desynchronization contralateral to the attended location (Capilla et al., 2014; Rihs et al., 2009; Siegel et al., 2008; Thut et al., 2006; van Diepen et al., 2016) and suggests that attentional resources were allocated to distractor location. In agreement with our results, recent research has also found that exogenous attention driven by non‐predictive auditory cues is indexed by an alpha power reduction contralateral to the cued location (Feng et al., 2017; Störmer et al., 2016; but see Weise et al., 2020). Our findings thus support the notion that the involuntary orientation of attention to distractor stimuli is characterized by a desynchronization of alpha oscillations, which would facilitate distractor processing.
Moreover, alpha‐band activity has been proposed to index the strength of competition between target and distractor stimuli for attentional resources (van Diepen et al., 2016). Emotional stimuli are biologically salient by definition and are known to capture exogenous attention to a greater extent than neutral distractors (for a review see Carretié, 2014). Therefore, they can be considered as natural competitors against target stimuli for attentional resources. Surprisingly, however, we found no studies employing emotion‐laden stimuli to investigate oscillatory correlates of exogenous attention. It has only recently pointed out that the need for proactive and reactive control of emotional distractors is implemented through the modulation of alpha‐band oscillations (Murphy et al., 2020), which might also implicitly reflect the degree of attentional capture.
Interestingly, our results could also be interpreted in the light of predictive coding. Under this view, the brain is regarded as a constructive organ that is actively generating expectations or predictions about the environment and testing them against sensory evidence (Friston, 2005, 2019). Predictions are continuously updated based on prediction errors, i.e., the difference between prior expectations and reality. From this perspective, attention would not be seen as a selection mechanism, but as an emergent property of prediction. Thus, instead of a dichotomous construct (“attending” vs. “ignoring”), attention could be defined as the process of optimizing the precision of predictions and prediction errors (Friston, 2009). Unreliable information will result in sensory attenuation, prioritizing top‐down expectations over sensory evidence. In contrast, prediction errors that convey accurate and newsworthy information will lead to sensory amplification (Friston, 2019). According to this view, our results suggest that emotionally salient stimuli are amplified as bottom‐up sensory processing is emphasized over top‐down expectations. Forward propagation of prediction errors and backward predictions have often been associated with higher (i.e., gamma) and lower (i.e. alpha and beta) frequency bands, respectively (Bastos et al., 2015; Michalareas et al., 2016). However, it has recently been suggested that alpha‐band traveling waves could reflect the computations behind predictive coding in both forward and backward directions (Alamia & VanRullen, 2019). These findings are consistent with our results showing a common mechanism based on the modulation of alpha‐band power for both endogenous top‐down attention and exogenous bottom‐up attentional capture.
Here, we found that alpha desynchronization was maximal when exogenous attention was drawn to negative distractors. This could be taken to reflect a “negativity bias” in attention, in which negative distractors elicit higher indices of attentional capture than positive and neutral stimuli, as previously reported (Horstmann et al., 2006; Huang et al., 2011; Sussman et al., 2013) and supported by several neural indices (for a review see Carretié et al., 2009). Likewise, event‐related potential studies have also revealed that exogenous attention is initially grabbed by images with negative and threatening content (Carretié et al., 2004; Soares et al., 2017). The consequences of ignoring negative stimuli that could signal potential dangers are usually more critical for survival than neutral or even appetitive stimuli, so this is clearly advantageous in evolutionary terms.
Noteworthy, post‐stimulus alpha amplitude in response to distractors began to differ from distractor‐absent trials as early as 155 ms. This early effect in the modulation of alpha‐band oscillations has been to some extent observed in response to exogenously attended stimuli (Feng et al., 2017; Keefe & Störmer, 2021; Störmer et al., 2016). It has been pointed out that exogenous attention to distractors is importantly sustained by the magnocellular visual pathway during the initial stages of visual processing (Carretié et al., 2012, 2017). The magnocellular system is a rapid neural route that processes rudimentary information (Derrington & Lennie, 1984) and has been reported to directly access the prefrontal cortex (Bar et al., 2006), the amygdala (Adolphs, 2004), and the insula (Rodman & Consuelos, 1994), all of them pivotal structures responsible for evaluating stimuli and organizing a response. This could account for the fast alpha‐band mechanism underlying exogenous attention observed in the present study. Interestingly, when distractors are biologically salient, the preference for magnocellular processing remains at subsequent stages (Carretié et al., 2017). In fact, it has been proposed that magnocellular involvement in emotional stimulus processing might play a key role in the abovementioned negativity bias, since it would facilitate the detection of urgent visual events (Carretié et al., 2007). Therefore, we could speculate that the enhanced alpha desynchronization for negative distractors found here may be underpinned by the magnocellular visual pathway.
It is important to mention that mere exposure to emotion‐laden stimuli has also been related to posterior alpha‐band desynchronization (Cui et al., 2013; Knyazev et al., 2008; Mennella et al., 2017; Popov et al., 2012, 2013; Simons et al., 2003). In light of our findings, this effect could be explained by an automatic attentional mechanism triggered by emotionally salient stimuli, which would be reflected in the modulation of alpha oscillations. Nevertheless, it could also be argued that alpha‐band suppression does not reflect an attentional bias to emotional information but its inherent processing. Additional research is needed in order to further disentangle perceptual from attentional effects.
Emotional arousal has also been demonstrated to critically modulate alpha oscillations (Cui et al., 2013; De Cesarei & Codispoti, 2011; Schubring & Schupp, 2019; Simons et al., 2003). Indeed, differences in attention‐related alpha‐band power disappear when emotional stimuli are matched in terms of arousal (Simons et al., 2003). With this in mind, we selected positive and negative images to be equated on arousal values, whilst the neutral stimuli were required to have a score close to zero in this dimension. By analyzing the assessment of emotional valence and arousal provided by our participants, we verified that the neutral stimuli were judged to be low arousal images. Nonetheless, and contrary to our expectations, participants assessed the negative images to be slightly more arousing than the positive ones. Hence, in order to rule out the possibility that the obtained results were rather due to an arousal effect, we analyzed the correlation between alpha power and arousal score on a single‐trial basis. Critically, we found that alpha‐band oscillations were not sensitive to arousal, which was further confirmed by Bayesian analysis, thus allowing us to discard this alternative explanation. The fact that individual participants rated negative images as more arousing than normative scores has also been observed previously (Weinberg & Hajcak, 2010) and could reflect their individual negativity bias. This explanation is even more plausible given the age range of our sample (19 to 28 years), since younger adults tend to be more vulnerable to distraction by negative stimuli and display a stronger negativity bias (Thomas & Hasher, 2006). Therefore, it should be taken into account that generalization of the present results may be limited due to specific characteristics of our sample.
Contrary to the lack of an arousal effect, we did find a significant correlation between alpha‐band amplitude and self‐reported ratings of emotional valence at the single‐trial level. Thus, the more negative the valence, the lower the alpha‐band power. In addition, lower levels of alpha activity were correlated with worse behavioral performance on the ongoing task, which in turn tended to be mostly affected by negative stimuli. Previous research has reported a higher behavioral cost when distractors strongly compete for attentional resources (Fodor et al., 2020; Schroeder et al., 2018; van Diepen et al., 2016). Critically, negative‐laden stimuli have been found to impair performance compared with positive (Horstmann et al., 2006; Sussman et al., 2013), as well as with neutral distractors (Horstmann et al., 2006; Huang et al., 2011; Sussman et al., 2013). Taken together, our results support the view that alpha power suppression indexes the degree of attentional capture and subsequent distraction from the ongoing task, which is particularly pronounced in the case of negative distractors.
Interestingly, trials with positive distractors showed a tendency for improved accuracy. It has been shown that positive emotions can broaden the scopes of both attention and cognition, improving goal‐directed actions (Fredrickson & Branigan, 2005), an effect that could be mediated by the capacity of positive affect to broaden the field of view in attentional tasks (Schmitz et al., 2009). Further research is needed to elucidate if emotionally positive images may play a protective role in the competition for attentional resources, thus preserving task performance.
In conclusion, we have demonstrated that exogenous attention to emotion‐laden visual stimuli is characterized by a pronounced decrease of alpha‐band power contralateral to distraction. This effect evolves rapidly and displays a negativity bias, reflecting that negative stimuli may be automatically prioritized in the competition for attentional resources.
AUTHOR CONTRIBUTIONS
Lydia Arana: Formal analysis; investigation; visualization; writing – original draft; writing – review and editing. María Melcón: Formal analysis; investigation; resources; writing – review and editing. Dominique Kessel: Funding acquisition; investigation; resources; writing – review and editing. Sandra Hoyos: Investigation; writing – review and editing. Jacobo Albert: Resources; writing – review and editing. Luis Carretié: Conceptualization; funding acquisition; supervision; writing – review and editing. Almudena Capilla: Conceptualization; formal analysis; funding acquisition; supervision; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
This work was supported by FEDER/Ministerio de Ciencia, Innovación y Universidades – Agencia Estatal de Investigación, Spain (grants PGC2018‐100682‐B‐I00 and PGC2018‐093570‐B‐I00) and by the Comunidad de Madrid (SAPIENTIA‐CM H2019/HUM‐570) in collaboration with the Universidad Autónoma de Madrid, Spain (grants 2017‐T2/SOC‐5569 and SI1‐PJI‐2019‐00011).
Arana, L. , Melcón, M. , Kessel, D. , Hoyos, S. , Albert, J. , Carretié, L. & Capilla, A. (2022). Suppression of alpha‐band power underlies exogenous attention to emotional distractors. Psychophysiology, 59, e14051. 10.1111/psyp.14051
REFERENCES
- Adolphs, R. (2004). Emotional vision. Nature Neuroscience, 7(11), 1167–1168. 10.1038/nn1104-1167 [DOI] [PubMed] [Google Scholar]
- Alamia, A. , & VanRullen, R. (2019). Alpha oscillations and traveling waves: Signatures of predictive coding? PLoS Biology, 17(10), e3000487. 10.1371/journal.pbio.3000487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni, C. , Vecchio, F. , Bultrini, A. , Romani, G. L. , & Rossini, P. M. (2006). Pre‐ and poststimulus alpha rhythms are related to conscious visual perception: A high‐resolution EEG study. Cerebral Cortex, 16(12), 1690–1700. 10.1093/cercor/bhj104 [DOI] [PubMed] [Google Scholar]
- Bacigalupo, F. , & Luck, S. J. (2019). Lateralized suppression of alpha‐band EEG activity as a mechanism of target processing. Journal of Neuroscience, 39(5), 900–917. 10.1523/JNEUROSCI.0183-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherzadeh, Y. , Baldauf, D. , Pantazis, D. , & Desimone, R. (2020). Alpha synchrony and the neurofeedback control of spatial attention. Neuron, 105(3), 577–587.e5. 10.1016/j.neuron.2019.11.001 [DOI] [PubMed] [Google Scholar]
- Bar, M. , Kassam, K. S. , Ghuman, A. S. , Boshyan, J. , Schmid, A. M. , Schmidt, A. M. , Dale, A. M. , Hämäläinen, M. S. , Marinkovic, K. , Schacter, D. L. , Rosen, B. R. , & Halgren, E. (2006). Top‐down facilitation of visual recognition. Proceedings of the National Academy of Sciences of the United States of America, 103(2), 449–454. 10.1073/pnas.0507062103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareither, I. , Chaumon, M. , Bernasconi, F. , Villringer, A. , & Busch, N. A. (2014). Invisible visual stimuli elicit increases in alpha‐band power. Journal of Neurophysiology, 112(5), 1082–1090. 10.1152/jn.00550.2013 [DOI] [PubMed] [Google Scholar]
- Bastos, A. M. , Vezoli, J. , Bosman, C. A. , Schoffelen, J. M. , Oostenveld, R. , Dowdall, J. R. , De Weerd, P. , Kennedy, H. , & Fries, P. (2015). Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron, 85(2), 390–401. 10.1016/j.neuron.2014.12.018 [DOI] [PubMed] [Google Scholar]
- Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Capilla, A. , Schoffelen, J. M. , Paterson, G. , Thut, G. , & Gross, J. (2014). Dissociated α‐band modulations in the dorsal and ventral visual pathways in visuospatial attention and perception. Cerebral Cortex, 24(2), 550–561. 10.1093/cercor/bhs343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié, L. , Tapia, M. , López‐Martín, S. , & Albert, J. (2019). EmoMadrid: An emotional pictures database for affect research. Motivation and Emotion, 43(6), 929–939. 10.1007/s11031-019-09780-y [DOI] [Google Scholar]
- Carretié, L. (2014). Exogenous (automatic) attention to emotional stimuli: A review. Cognitive, Affective, & Behavioral Neuroscience, 14(4), 1228–1258. 10.3758/s13415-014-0270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié, L. , Albert, J. , López‐Martín, S. , & Tapia, M. (2009). Negative brain: An integrative review on the neural processes activated by unpleasant stimuli. International Journal of Psychophysiology, 71(1), 57–63. 10.1016/j.ijpsycho.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Carretié, L. , Hinojosa, J. A. , López‐Martín, S. , & Tapia, M. (2007). An electrophysiological study on the interaction between emotional content and spatial frequency of visual stimuli. Neuropsychologia, 45(6), 1187–1195. 10.1016/j.neuropsychologia.2006.10.013 [DOI] [PubMed] [Google Scholar]
- Carretié, L. , Hinojosa, J. A. , Martín‐Loeches, M. , Mercado, F. , & Tapia, M. (2004). Automatic attention to emotional stimuli: Neural correlates. Human Brain Mapping, 22(4), 290–299. 10.1002/hbm.20037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié, L. , Kessel, D. , García‐Rubio, M. J. , Giménez‐Fernández, T. , Hoyos, S. , & Hernández‐Lorca, M. (2017). Magnocellular bias in exogenous attention to biologically salient stimuli as revealed by manipulating their luminosity and color. Journal of Cognitive Neuroscience, 29(10), 1699–1711. 10.1162/jocn_a_01148 [DOI] [PubMed] [Google Scholar]
- Carretié, L. , Ríos, M. , Periáñez, J. A. , Kessel, D. , & Álvarez‐Linera, J. (2012). The role of low and high spatial frequencies in exogenous attention to biologically salient stimuli. PLoS One, 7(5), e37082. 10.1371/journal.pone.0037082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica, A. B. , Lasaponara, S. , Chanes, L. , Valero‐Cabré, A. , Doricchi, F. , Lupiáñez, J. , & Bartolomeo, P. (2011). Spatial attention and conscious perception: The role of endogenous and exogenous orienting. Attention, Perception, and Psychophysics, 73(4), 1065–1081. 10.3758/s13414-010-0082-6 [DOI] [PubMed] [Google Scholar]
- Corbetta, M. , Patel, G. , & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Versace, F. , Engelmann, J. M. , Minnix, J. A. , Robinson, J. D. , Lam, C. Y. , … Cinciripini, P. M. (2013). Alpha oscillations in response to affective and cigarette‐related stimuli in smokers. Nicotine and Tobacco Research, 15(5), 917–924. 10.1093/ntr/nts209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming, G. (2012). Understanding the new statistics: Effect sizes, confidence intervals, and meta‐analysis. Routledge. [Google Scholar]
- De Cesarei, A. , & Codispoti, M. (2011). Affective modulation of the LPP and α‐ERD during picture viewing. Psychophysiology, 48(10), 1397–1404. 10.1111/j.1469-8986.2011.01204.x [DOI] [PubMed] [Google Scholar]
- Derrington, A. M. , & Lennie, P. (1984). Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. The Journal of Physiology, 357(1), 219–240. 10.1113/jphysiol.1984.sp015498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes, Z. (2014). Using Bayes to get the most out of non‐significant results. Frontiers in Psychology, 5, 781. 10.3389/fpsyg.2014.00781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowe, I. , & Hilgetag, C. C. (2014). Occipitoparietal alpha‐band responses to the graded allocation of top‐down spatial attention. Journal of Neurophysiology, 112(6), 1307–1316. 10.1152/jn.00654.2013 [DOI] [PubMed] [Google Scholar]
- Feng, W. , Störmer, V. S. , Martinez, A. , McDonald, J. J. , & Hillyard, S. A. (2017). Involuntary orienting of attention to a sound desynchronizes the occipital alpha rhythm and improves visual perception. NeuroImage, 150, 318–328. 10.1016/j.neuroimage.2017.02.033 [DOI] [PubMed] [Google Scholar]
- Fodor, Z. , Marosi, C. , Tombor, L. , & Csukly, G. (2020). Salient distractors open the door of perception: Alpha desynchronization marks sensory gating in a working memory task. Scientific Reports, 10(1), 1–11. 10.1038/s41598-020-76190-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, J. J. , & Awh, E. (2019). The role of alpha oscillations in spatial attention: Limited evidence for a suppression account. Current Opinion in Psychology, 29, 34–40. 10.1016/j.copsyc.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, J. J. , Sutterer, D. W. , Serences, J. T. , Vogel, E. K. , & Awh, E. (2017). Alpha‐band oscillations enable spatially and temporally resolved tracking of covert spatial attention. Psychological Science, 28(7), 929–941. 10.1177/0956797617699167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe, J. J. , & Snyder, A. C. (2011). The role of alpha‐band brain oscillations as a sensory suppression mechanism during selective attention. Frontiers in Psychology, 2, 154. 10.3389/fpsyg.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson, B. L. , & Branigan, C. (2005). Positive emotions broaden the scope of attention and thought‐action repertoires. Cognition & Emotion, 19(3), 313–332. 10.1080/02699930441000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society B, 360(1456), 815–836. 10.1098/rstb.2005.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. (2009). The free‐energy principle: A rough guide to the brain? Trends in Cognitive Sciences, 13(7), 293–301. 10.1016/j.tics.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Friston, K. J. (2019). Waves of prediction. PLOS Biology, 17(10), e3000426. 10.1371/journal.pbio.3000426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, I. C. , Rushworth, M. F. , & Nobre, A. C. (2011). Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. Journal of Neurophysiology, 105(3), 1318–1326. 10.1152/jn.00653.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Händel, B. F. , Haarmeier, T. , & Jensen, O. (2011). Alpha oscillations correlate with the successful inhibition of unattended stimuli. Journal of Cognitive Neuroscience, 23(9), 2494–2502. 10.1162/jocn.2010.21557 [DOI] [PubMed] [Google Scholar]
- Harris, A. M. , Dux, P. E. , & Mattingley, J. B. (2020). Awareness is related to reduced post‐stimulus alpha power: A no‐report inattentional blindness study. European Journal of Neuroscience, 52(11), 4411–4422. 10.1111/ejn.13947 [DOI] [PubMed] [Google Scholar]
- Horstmann, G. , Borgstedt, K. , & Heumann, M. (2006). Flanker effects with faces may depend on perceptual as well as emotional differences. Emotion, 6(1), 28–39. 10.1037/1528-3542.6.1.28 [DOI] [PubMed] [Google Scholar]
- Huang, S. L. , Chang, Y. C. , & Chen, Y. J. (2011). Task‐irrelevant angry faces capture attention in visual search while modulated by resources. Emotion, 11(3), 544–552. 10.1037/a0022763 [DOI] [PubMed] [Google Scholar]
- JASP Team . (2021). JASP (Version 0.16) [Computer software].
- Jeffreys, H. (1939). Theory of probability (1st ed.). Clarendon Press. [Google Scholar]
- Jensen, O. , Gips, B. , Bergmann, T. O. , & Bonnefond, M. (2014). Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends in Neurosciences, 37(7), 357–369. 10.1016/j.tins.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Jensen, O. , & Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience, 4, 186. 10.3389/fnhum.2010.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, J. M. , & Störmer, V. S. (2021). Lateralized alpha activity and slow potential shifts over visual cortex track the time course of both endogenous and exogenous orienting of attention. NeuroImage, 225, 117495. 10.1016/j.neuroimage.2020.117495 [DOI] [PubMed] [Google Scholar]
- Kelly, S. P. , Gomez‐Ramirez, M. , & Foxe, J. J. (2009). The strength of anticipatory spatial biasing predicts target discrimination at attended locations: A high‐density EEG study. European Journal of Neuroscience, 30(11), 2224–2234. 10.1111/j.1460-9568.2009.06980.x [DOI] [PubMed] [Google Scholar]
- Klimesch, W. (2012). Alpha‐band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–617. 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev, G. G. , Bocharov, A. V. , Levin, E. A. , Savostyanov, A. N. , & Slobodskoj‐Plusnin, J. Y. (2008). Anxiety and oscillatory responses to emotional facial expressions. Brain Research, 1227, 174–188. 10.1016/j.brainres.2008.06.108 [DOI] [PubMed] [Google Scholar]
- Koster, E. H. W. , Crombez, G. , Van Damme, S. , Verschuere, B. , & De Houwer, J. (2004). Does imminent threat capture and hold attention? Emotion, 4(3), 312–317. 10.1037/1528-3542.4.3.312 [DOI] [PubMed] [Google Scholar]
- Lavie, N. (2005). Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences, 9(2), 75–82. 10.1016/j.tics.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Mennella, R. , Sarlo, M. , Messerotti Benvenuti, S. , Buodo, G. , Mento, G. , & Palomba, D. (2017). The two faces of avoidance: Time‐frequency correlates of motivational disposition in blood phobia. Psychophysiology, 54(11), 1606–1620. 10.1111/psyp.12904 [DOI] [PubMed] [Google Scholar]
- Michalareas, G. , Vezoli, J. , Van Pelt, S. , Schoffelen, J. M. , Kennedy, H. , & Fries, P. (2016). Alpha‐beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron, 89(2), 384–397. 10.1016/j.neuron.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, J. , Devue, C. , Corballis, P. M. , & Grimshaw, G. M. (2020). Proactive control of emotional distraction: Evidence from EEG alpha suppression. Frontiers in Human Neuroscience, 14, 318. 10.3389/fnhum.2020.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, L. , Guillory, S. , & Sekuler, R. (2013). Attention‐modulated alpha‐band oscillations protect against intrusion of irrelevant information. Journal of Cognitive Neuroscience, 25(9), 1463–1476. 10.1162/jocn_a_00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov, T. , Miller, G. A. , Rockstroh, B. , & Weisz, N. (2013). Modulation of α power and functional connectivity during facial affect recognition. Journal of Neuroscience, 33(14), 6018–6026. 10.1523/JNEUROSCI.2763-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov, T. , Steffen, A. , Weisz, N. , Miller, G. A. , & Rockstroh, B. (2012). Cross‐frequency dynamics of neuromagnetic oscillatory activity: Two mechanisms of emotion regulation. Psychophysiology, 49(12), 1545–1557. 10.1111/j.1469-8986.2012.01484.x [DOI] [PubMed] [Google Scholar]
- Rihs, T. A. , Michel, C. M. , & Thut, G. (2007). Mechanisms of selective inhibition in visual spatial attention are indexed by α‐band EEG synchronization. European Journal of Neuroscience, 25(2), 603–610. 10.1111/j.1460-9568.2007.05278.x [DOI] [PubMed] [Google Scholar]
- Rihs, T. A. , Michel, C. M. , & Thut, G. (2009). A bias for posterior α‐band power suppression versus enhancement during shifting versus maintenance of spatial attention. NeuroImage, 44(1), 190–199. 10.1016/j.neuroimage.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Rodman, H. R. , & Consuelos, M. J. (1994). Cortical projections to anterior inferior temporal cortex in infant macaque monkeys. Visual Neuroscience, 11(1), 119–133. 10.1017/S0952523800011160 [DOI] [PubMed] [Google Scholar]
- Romei, V. , Gross, J. , & Thut, G. (2010). On the role of prestimulus alpha rhythms over occipito‐parietal areas in visual input regulation: Correlation or causation? Journal of Neuroscience, 30(25), 8692–8697. 10.1523/JNEUROSCI.0160-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha, J. , Sprague, T. C. , & Postle, B. R. (2016). Decoding and reconstructing the focus of spatial attention from the topography of alpha‐band oscillations. Journal of Cognitive Neuroscience, 28(8), 1090–1097. 10.1162/jocn_a_00955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, T. W. , De Rosa, E. , & Anderson, A. K. (2009). Opposing influences of affective state valence on visual cortical encoding. Journal of Neuroscience, 29(22), 7199–7207. 10.1523/JNEUROSCI.5387-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, S. C. Y. , Ball, F. , & Busch, N. A. (2018). The role of alpha oscillations in distractor inhibition during memory retention. European Journal of Neuroscience, 48(7), 2516–2526. 10.1111/ejn.13852 [DOI] [PubMed] [Google Scholar]
- Schubring, D. , & Schupp, H. T. (2019). Affective picture processing: Alpha‐ and lower beta‐band desynchronization reflects emotional arousal. Psychophysiology, 56(8), e13386. 10.1111/psyp.13386 [DOI] [PubMed] [Google Scholar]
- Siegel, M. , Donner, T. H. , Oostenveld, R. , Fries, P. , & Engel, A. K. (2008). Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron, 60(4), 709–719. 10.1016/j.neuron.2008.09.010 [DOI] [PubMed] [Google Scholar]
- Simons, R. F. , Detenber, B. H. , Cuthbert, B. N. , Schwartz, D. D. , & Reiss, J. E. (2003). Attention to television: Alpha power and its relationship to image motion and emotional content. Media Psychology, 5(3), 283–301. 10.1207/S1532785XMEP0503_03 [DOI] [Google Scholar]
- Soares, S. C. , Kessel, D. , Hernández‐Lorca, M. , García‐Rubio, M. J. , Rodrigues, P. , Gomes, N. , & Carretié, L. (2017). Exogenous attention to fear: Differential behavioral and neural responses to snakes and spiders. Neuropsychologia, 99, 139–147. 10.1016/j.neuropsychologia.2017.03.007 [DOI] [PubMed] [Google Scholar]
- Störmer, V. S. , Feng, W. , Martinez, A. , McDonald, J. J. , & Hillyard, S. A. (2016). Salient, irrelevant sounds reflexively induce alpha rhythm desynchronization in parallel with slow potential shifts in visual cortex. Journal of Cognitive Neuroscience, 28(3), 433–445. 10.1162/jocn_a_00915 [DOI] [PubMed] [Google Scholar]
- Sussman, T. J. , Heller, W. , Miller, G. A. , & Mohanty, A. (2013). Emotional distractors can enhance attention. Psychological Science, 24(11), 2322–2328. 10.1177/0956797613492774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, R. C. , & Hasher, L. (2006). The influence of emotional valence on age differences in early processing and memory. Psychology and Aging, 21(4), 821–825. 10.1037/0882-7974.21.4.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut, G. , Nietzel, A. , Brandt, S. A. , & Pascual‐Leone, A. (2006). α‐Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. Journal of Neuroscience, 26(37), 9494–9502. 10.1523/JNEUROSCI.0875-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut, G. , Veniero, D. , Romei, V. , Miniussi, C. , Schyns, P. , & Gross, J. (2011). Rhythmic TMS causes local entrainment of natural oscillatory signatures. Current Biology, 21(14), 1176–1185. 10.1016/j.cub.2011.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diepen, R. M. , Miller, L. M. , Mazaheri, A. , & Geng, J. J. (2016). The role of alpha activity in spatial and feature‐ based attention. ENeuro, 3(5), ENEURO.0204‐16.2016. 10.1523/ENEURO.0204-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede, F. , de Lange, F. P. , & Maris, E. (2012). Attentional cues affect accuracy and reaction time via different cognitive and neural processes. Journal of Neuroscience, 32(30), 10408–10412. 10.1523/JNEUROSCI.1337-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerven, M. , & Jensen, O. (2009). Attention modulations of posterior alpha as a control signal for two‐dimensional brain‐computer interfaces. Journal of Neuroscience Methods, 179(1), 78–84. 10.1016/j.jneumeth.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Vanni, S. , Revonsuo, A. , & Hari, R. (1997). Modulation of the parieto‐occipital alpha rhythm during object detection. Journal of Neuroscience, 17(18), 7141–7147. 10.1523/jneurosci.17-18-07141.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, A. , & Hajcak, G. (2010). Beyond good and evil: The time‐course of neural activity elicited by specific picture content. Emotion, 10(6), 767–782. 10.1037/a0020242 [DOI] [PubMed] [Google Scholar]
- Weise, A. , Hartmann, T. , Parmentier, F. , Ruhnau, P. , & Weisz, N. (2020). Increases in parieto‐occipital alpha‐band power reflect involuntary spatial attention due to a task‐distracting deviant sound. BioRxiv. 10.1101/2020.06.29.161992 [DOI] [Google Scholar]
- Worden, M. S. , Foxe, J. J. , Wang, N. , & Simpson, G. V. (2000). Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha‐band electroencephalography increases over occipital cortex. The Journal of Neuroscience, 20(6), RC63 (1‐6). 10.1523/jneurosci.20-06-j0002.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöstmann, M. , Alavash, M. , & Obleser, J. (2019). Alpha oscillations in the human brain implement distractor suppression independent of target selection. The Journal of Neuroscience, 39(49), 9797–9805. 10.1523/JNEUROSCI.1954-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumer, J. M. , Scheeringa, R. , Schoffelen, J. M. , Norris, D. G. , & Jensen, O. (2014). Occipital alpha activity during stimulus processing gates the information flow to object‐selective cortex. PLoS Biology, 12(10), e1001965. 10.1371/journal.pbio.1001965 [DOI] [PMC free article] [PubMed] [Google Scholar]


