Abstract
Zika virus (ZIKV) is an arbovirus belonging to the flavivirus genus and is transmitted in Aedes mosquito vectors. Since its discovery in humans in 1952 in Uganda, ZIKV has been responsible for many outbreaks in South America, Africa, and Asia. Patients infected with ZIKV are usually asymptomatic; mild symptoms include fever, joint and muscle pain, and fatigue. However, severe infections may have neurological implications, such as Guillain‐Barré syndrome and fetal microcephaly. To date, there are no existing approved therapeutic drugs or vaccines against ZIKV infections; treatments mainly target the symptoms of infection. Preventive measures against mosquito breeding are the main strategy for limiting the spread of the virus. Antiviral drug research for the treatment of ZIKV infection has been rapidly developing, with many drug candidates emerging from drug repurposing studies, and compound screening. In particular, several studies have demonstrated the potential of natural products as antivirals for ZIKV infection. Hence, this paper will review recent advances in natural products in ZIKV antiviral drug discovery.
Keywords: antiviral, drug discovery, infectious disease, natural product, Zika virus
1. INTRODUCTION
Zika virus (ZIKV) is an arbovirus belonging to the genus Flavivirus and is mainly transmitted in Aedes mosquito vectors, namely Aedes aegypti and Aedes albopictus. 1 Since its discovery in humans in 1952 in Uganda, ZIKV has been responsible for many outbreaks in South America, 2 , 3 Africa, 4 and Asia, 5 with the first recorded outbreak being in the Federated States of Micronesia in 2007. The World Health Organisation (WHO) reported that as of July 2019, 87 countries have been identified to have indigenous mosquito‐borne transmission of ZIKV. 6 One of the largest outbreaks occurred in French Polynesia in 2013, where over half the population was estimated to be infected with ZIKV. 7 , 8 A retrospective study of this outbreak revealed that 66% of the population was infected with ZIKV, and eight microcephaly cases were diagnosed in neonates. 9 In light of the rapid transmission of ZIKV and its association with neurological disorders, WHO declared ZIKV infection to be a Public Health Emergency of International Concern on 1 February 2016. 10
Although there has been a decrease in the number of reported cases each year in the recent few years, this does not indicate the elimination of ZIKV transmission. In addition to logistical issues in routine surveillance and testing of suspected cases, patients infected with ZIKV are usually asymptomatic; mild symptoms include fever, joint and muscle pain, and fatigue. 11 Such symptoms are often misdiagnosed as other viral infections as they are nonspecific, hence the difficulty in reporting and tracking ZIKV transmission. 12 Furthermore, evidence for ZIKV transmission by sexual intercourse has also been uncovered, indicating that it is not just tropical countries that are at risk of ZIKV outbreaks. 13
Severe ZIKV infections are associated with neurological disorders, such as Guillain‐Barré syndrome in adults. 14 , 15 ZIKV patients who are pregnant also are at increased risk of giving birth to children with congenital Zika syndrome, a group of birth defects, including fetal microcephaly brain calcification, and abnormal brain development. 16 , 17 These complications are not observed in other flaviviral infections, which makes early diagnosis and treatment of ZIKV infections of paramount importance. 18
To date, there are no existing approved therapeutic drugs or vaccines against ZIKV infections; treatments primarily target the symptoms of infection. 19 , 20 These include sufficient rest and taking acetaminophen. Preventive measures against mosquito breeding are the main strategy for limiting the spread of the virus. Antiviral drug research for the treatment of ZIKV infection has been rapidly developing, with many drug candidates emerging from drug repurposing studies, and compound screening. 19 , 21 In particular, several studies have demonstrated the potential of natural products as antivirals for ZIKV infection. 22 Natural products are a key source of compounds for drug discovery, as they tend to have favourable physicochemical properties and lower toxicity. 23 , 24 , 25 Natural products such as flavonoids are abundant in food and plants, and have been demonstrated to have antitumour and anti‐inflammatory properties. 26 , 27 They can also be derived from living organisms which produce secondary metabolites that are nonessential for key processes but enhance survival of the organisms. 28 , 29 Hence, the diversity of compounds in natural products is a library of interest to explore for their antiviral properties against ZIKV. This paper will thus review recent advances in natural products in ZIKV antiviral drug discovery. The origins of the natural products, their chemical classes, and future perspectives will be discussed.
2. GENERAL VIROLOGY
The ZIKV genome consists of a single‐stranded positive‐sense RNA of about 10.7 kb in length. The 5′‐untranslated region (UTR) and 3′‐UTR is about 100 and 420 nucleotides, respectively. Translation of the open reading frame results in a large polyprotein consisting 3423 amino acids. This large polyprotein is processed by host and viral proteases to give rise to three structural and seven nonstructural proteins (Figure 1). The structural proteins C, prM and E, have key roles in viral entry and release of new virions from the host cell. The C protein is responsible for forming the nucleocapsid core surrounding the viral genome; the E protein facilitates virion binding to the receptor on the host cell membrane. The prM protein, once processed, aids in ensuring the spatial structure of the E protein. Similar to other flaviviruses, the nonstructural proteins are part of the viral replication complex in the host cell cytoplasm. 30 Notably, the NS5 protein functions as the viral RNA‐dependent RNA polymerase (RdRp), which reads the positive‐sense RNA genome to give the negative‐sense RNA strand which is then used for translation in producing the large polyprotein precursor. 31 In addition, the NS3 protein contains serine protease and RNA helicase domains, which works to cleave the large polyprotein to give active proteins. The other nonstructural proteins are also involved in viral assembly and replication in the host cell, ensuring that the replication complex is anchored in the ER membrane for effective viral replication. 32
Figure 1.
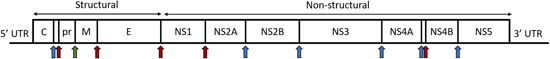
Diagram of the ZIKV genome. Regions coding for each structural and nonstructural protein are demarcated. The genome is translated as a large polyprotein, which is then cleaved by viral NS2B‐NS3 protease complex and host cell proteases to give three structural proteins and seven nonstructural proteins. Arrows in red indicate sites of cleavage by viral NS2B‐NS3 protease; arrows in blue indicate sites of cleavage by host cell proteases; the arrow in green indicates the site of cleavage by host cell furin, resulting in a mature glycoprotein M. [Color figure can be viewed at wileyonlinelibrary.com]
The ZIKV replication cycle is illustrated in Figure 2. When an Aedes mosquito vector carrying ZIKV feeds on a mammalian host, ZIKV virions are released into the bloodstream of the host; binding to receptors on the host cell surface is facilitated by the E protein. One example is the cellular receptor Axl which binds to phosphatidylserine on the surface of the ZIKV. 33 Through receptor‐mediated endocytosis, clathrin‐coated vesicles encapsulate the virus and bud off from the host cell membrane. 34 The low pH environment in the endosome results in the fusion of the viral capsid and the endosome, releasing the nucleocapsid into the cell cytoplasm. 35 The positive‐sense RNA is then translated on the membrane of the rough endoplasmic reticulum and an immature virion is assembled, with the help of the nonstructural proteins. 30 This immature virion moves into the Golgi apparatus, where further post‐translational modifications such as carbohydrate addition and proteolytic cleavage occur on the prM protein. The virion then moves towards the host cell membrane, where it acquires the membrane envelope and is released via exocytosis as a mature virion.
Figure 2.
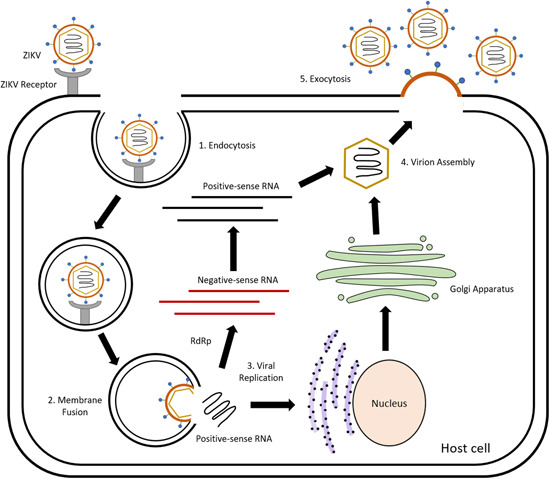
Replication cycle of ZIKV. (1) The E protein on the surface of the ZIKV virus particle recognises and binds to receptors on the cell surface. The virus particles then enter the cell via clathrin‐mediated endocytosis. (2) Fusion of the endosomal membrane and the viral envelope results in the positive‐sense viral RNA being released into the cell cytoplasm, where viral replication occurs. (3) Viral replication follows the secretory pathway in the host cell, facilitated by the viral nonstructural proteins. Viral RNA is translated in the rough endoplasmic reticulum and viral proteins are processed in the Golgi apparatus, where further post‐translational modifications such as carbohydrate addition and proteolytic cleavage occur. Viral NS5 protein which is the RNA‐dependent RNA polymerase (RdRp) synthesises negative‐sense RNA, which is in turn used to produce more copies of the viral positive‐sense RNA. (4) The viral positive‐sense RNA is packaged into the nucleocapsid core (consisting viral proteins synthesised using host cell machinery). (5) The virion then moves towards the host cell membrane, where it acquires the membrane envelope and is released via exocytosis as a mature virion. [Color figure can be viewed at wileyonlinelibrary.com]
The following sections will discuss compounds according to the stage of the ZIKV replication cycle targeted (Table 1). Where possible, similar regions in molecular structures (potential pharmacophores) within each group of compounds will be highlighted. The compounds described here are potential starting points for further hit‐to‐lead or structure‐activity relationship (SAR) studies to improve on the efficacy and physicochemical properties of these potential lead compounds.
Table 1.
Natural products shown to have anti‐ZIKV effects
| S/N | Compound name/Chemical structure/Source | Proposed mode of inhibition | IC50/EC50 | ZIKV strain(s) tested | In vitro cell lines/in vivo animal models studied |
|---|---|---|---|---|---|
| Viral entry inhibitors | |||||
| Inhibitors with direct virucidal effects | |||||
| 1 | Labyrinthopeptin A1 | Binds to viral membrane lipid phosphatidylethanolamine and disrupts viral membrane envelope | A1: 2.0 µM, 1.6 µM | 976, H/PF/2013 | In vitro: Huh‐7 1 |
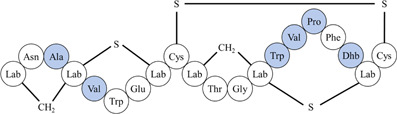
|
A2: 9.6 µM, 3.3 µM | In vivo: injected via i.v and i.p into 4‐week‐old male CD‐1 mice at 10 mg/kg1 | |||
| Labyrinthopeptin A2 | |||||
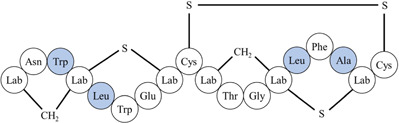
| |||||
| *Differences in amino acid residues are highlighted in blue. Lab: labionin; Dhb: didehydrobutyrine. | |||||
| Source: Actinomadura namibiensis DSM 6313 | |||||
| 2 | Delphinidin | Virucidal effect on virus particles | – | PA259459, MR766 | In vitro: Vero 3 |
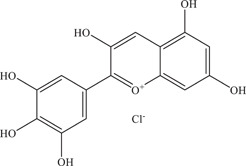
| |||||
| Source: Various fruits 2 | |||||
| 3 | Epigallocatechin gallate (EGCG)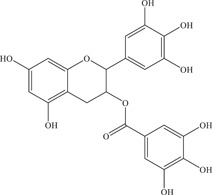
|
Inhibits NS2B‐NS3 viral protease, NS3 helicase, and has virucidal effect on virus particles | NS2B‐NS3pro: 0.73 ± 0.22 µM; 87 ± 1.2 µM | PA259459, MR766, Brazilian strain, Z16006 | In vitro: Vero, 3 , 4 Vero E6, 5 purified ZIKV NS3 helicase, 6 NS2B‐NS3pro 4 , 7 |
| NS3 helicase: 295.7 nM | In silico molecular docking 8 | ||||
| Source: Green tea | 21.4 µM; 0.02 ± 0.003 µM | ||||
| 4 | Baicalein | Inhibits a post‐entry step and has virucidal effect on virus particles | 0.004 µM | PRVABC59 | In vitro: Vero 9 |
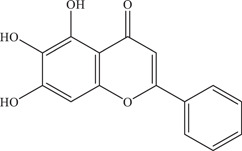
|
In silico molecular docking 9 | ||||
| Source: Scutellaria baicalensis & Scutellaria lateriflora | |||||
| 5 | Baicalin | Inhibits viral entry and has virucidal effect on virus particles | 14 µM | PRVABC59 | In vitro: Vero 9 |
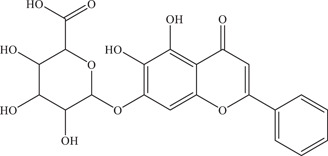
|
In silico molecular docking 9 | ||||
| Source: Scutellaria baicalensis & Scutellaria lateriflora | |||||
| 6 | Gossypol | Virucidal effect on virus particles | 0.21–4.31 µM | PAN2016, R116265, PAN2015, FLR, R103451, | In vitro: Vero, purified E protein 11 |
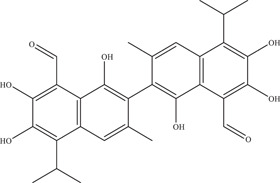
| |||||
| Source: Plants of the Gossypium genus 10 | E protein: 7.12 µM | PRVABC59, PLCal_ZV, IbH 30656, | |||
| MEX 2–81, MR766 | |||||
| 7 | Ginkgolic acid | Inhibits early stages of viral replication, possibly due to its virucidal activity | – | 259249 | In vitro: Vero 12 |
| |||||
| Source: Ginkgo biloba | |||||
| 8 | Emodin | Inhibits viral entry and has virucidal effect on virus particles | 3.2 µM | Brazilian strain | In vitro: Vero, 14 Vero E6 15 |
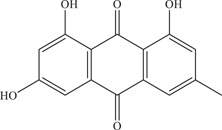
| |||||
| Source: Various plant species 13 | |||||
| 9 | Resveratrol | Inhibits a post‐entry step and has virucidal effect on virus particles | 93% (1 log) reduction in foci | P6740, MR766, PE243 | In vitro: Vero, 14 Huh‐7, 17 HTR‐8/SVneo, 18 Vero E6 18 |
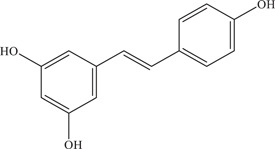
| |||||
| Source: Various plant species 16 | |||||
| 10 | Berberine | Virucidal effect on virus particles | 39.06 µM | Brazilian strain | In vitro: Vero E6 15 |
| |||||
| Source: Berberis vulgaris 19 | |||||
| 11 | Cephalotaxine | Inhibits post‐entry step of viral replication, possibly interfering with viral RNA translation. Virucidal effect also observed. | – | PRVABC59 | In vitro: Vero, 20 A549 20 |
| |||||
| Source: Cephalotaxus drupacea | |||||
| Inhibitors targeting the viral entry process | |||||
| 12 | Nanchangmycin | Blocks clathrin‐mediated endocytosis, inhibiting viral entry | 0.1 µM | MR766, MEX 2‐81, FSS13025 | In vitro: U2OS, 21 human brain microvascular endothelial cells, Vero, JEG‐3 |
| |||||
| Source: Streptomyces nanchangensis | |||||
| 13 | Quercetin | Inhibits NS2B‐NS3 viral protease and viral entry, possibly by targeting the virus particle itself or host cell surface proteins | NS2B‐NS3pro: 1.17 ± 0.22 µM; 2.4 ± 0.2 µM | MR766, Z16006 | In vitro: Vero, 4 , 14 , 23 purified ZIKV NS2B‐NS3pro 4 , 24 |
| |||||
| Source: Various plant species 22 | 2.3 ± 0.50 µM; 19.5 ± 4.8 µg/ml | In silico molecular docking 24 | |||
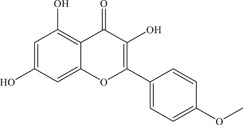
| 14 | Kaempferide | Inhibits viral entry and NS2B‐NS3 viral protease | NS2B‐NS3pro: 7.18 ± 2.16 µM | Z16006 | In vitro: Vero, 4 purified ZIKV NS2B‐NS3pro 4 |
| |||||
| Source: Kaempferia galanga | 5.83 ± 1.92 µM | ||||
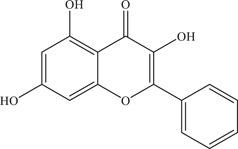
| 15 | Galangin | Inhibits viral entry and NS2B‐NS3 viral protease | NS2B‐NS3pro: 25.68 ± 9.17 µM | Z16006 | In vitro: Vero, 4 purified ZIKV NS2B‐NS3pro 4 |
| |||||
| Source: Alpinia officinarum Hance 25 | 14.36 ± 9.5 µM | ||||
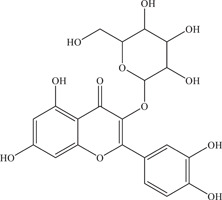
| 16 | Isoquercetin | Inhibits viral entry | 1.2–1.3 µmol/L; 9.7–15.5 µM | PF‐25013‐18, MR766 | In vitro: A549, 26 Huh‐7, 26 SH‐SY5Y, 26 Vero 27 |
|
PLCal_ZV, PRVABC59 | In vivo: injected via i.p. into | |||
| Source: Various plant species 22 | 6–8‐week‐old male or female Ifnar1 −/− mice at 50 mg/kg 27 | ||||
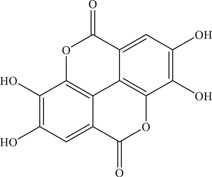
| 17 | Ellagic acid | Reduces cell susceptibility to the virus by binding to host cell surface | 30.86 µM | MR766, H/PF/2013 | In vitro: Vero 28 |
| |||||
| Source: Punica granatum | |||||
| 18 | Curcumin | Inhibits viral entry or binding and NS2B‐NS3 viral protease | NS2B‐NS3pro: 3.5 ± 0.2 µM | PAN2016, R116265, PAN2015, FLR, R103451, PRVABC59, PLCal_ZV, IbH 30656, MEX 2–81, MR766, HD78788 | In vitro: Vero E6, 11 HeLa, 29 purified ZIKV E protein, 11 NS2B‐NS3pro 24 |
| |||||
| Source: Curcuma longa (Turmeric) | 1.90 µM; 5.62–16.57 µM | In silico molecular docking 24 | |||
| 19 | Digitonin | Inhibits viral entry | 3.19–6.52 µM | PAN2016, R116265, PAN2015, FLR, R103451, PRVABC59, PLCal_ZV, IbH 30656, MEX 2–81, MR766 | In vitro: Vero E6 11 |
| |||||
| Source: Digitalis purpurea | |||||
| 20 | Conessine | Inhibits virus‐cell attachment or a postentry step | 7.18–11.60 µM | PAN2016, | In vitro: Vero E6 11 |
|
R116265, PAN2015, FLR, R103451, | ||||
| Source: Holarrhena antidysenterica 30 | PRVABC59, PLCal_ZV, IbH 30656, mosquitostrain MEX 2–81, rhesus macaque strain MR766 | ||||
| Viral replication inhibitors | |||||
| 21 | Cavinafungin | Inhibits host endoplasmic reticulum signal peptidase, preventing viral replication | 150 nM | FSS13025 | In vitro: A549 31 |
| |||||
| Source: Colispora cavincola | |||||
| 22 | Sinefungin | Competitively binds to viral NS5 methyltransferase instead of S‐adenosylmethionine (SAM), prevents the methylation of the cap structure of viral RNA | 1.18 ± 0.05 µM | H/PF/2013, MR766 | In vitro: purified ZIKV NS5 methyltransferase 32 , 33 |
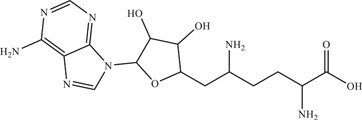
| |||||
| Source: Streptomyces griseoleus | |||||
| 23 | FV13 | Multiple targets, likely to target a postgenome replication step | 1.65 ± 0.86 µM | SV0010/15 | In vitro: LLC/MK2 35 |
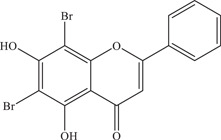
| |||||
| Source: Chrysin‐derivative 34 | |||||
| 24 | FV14 | – | 1.39 ± 0.11 µM | SV0010/15 | In vitro: LLC/MK2 35 |
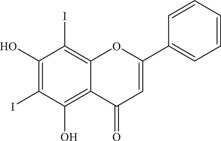
| |||||
| Source: Chrysin‐derivative 34 | |||||
| 25 | Pinocembrin | Inhibits viral RNA and envelope protein synthesis | 17.4 µM | PRVABC59 | In vitro: JEG‐3, 36 Huh‐7 36 |
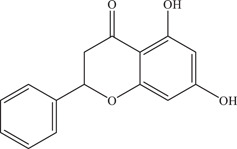
| |||||
| Source: Various plant species 37 | |||||
| 26 | Sophoraflavenone G (SFG) | Inhibits NS5 RdRp activity | 22.61 µM | FSS13025 | |
| |||||
| Source: Sophora flavescens | |||||
| 27 | Cephaeline | Inhibits NS5 RdRp activity and disrupts host cell lysosomal function | NS5 RdRp: 976 nM | MR766, PRVABC59, FSS13025, Brazilian strain | In vitro: HEK293, 39 SNB‐19, Vero E6, purified ZIKV NS5 RdRp |
| |||||
| Source: Carapichea ipecacuanha | 7.60 nM | In silico molecular docking 39 | |||
| 28 | Emetine | Inhibits NS5 RdRp activity and disrupts host cell lysosomal function | NS5 RdRp: 121 nM | MR766, PRVABC59, FSS13025, Brazilian strain | In vitro: HEK293, 39 SNB‐19, Vero E6, purified ZIKV NS5 RdRp |
| |||||
| Source: Carapichea ipecacuanha | 52.9 nM | In vivo: injected via i.p. into 3‐month‐old SJL male mice at 1 mg/kg/day, injected via i.p. into 6–7‐week‐old Ifnar1 −/− mice at 1 mg/kg 39 | |||
| In silico molecular docking 39 | |||||
| 29 | Lycorine | Inhibits NS5 RdRp activity | 0.22–0. 39 µM | KU963796 | In vitro: Vero, 40 A549, Huh‐7, purified ZIKV NS5 RdRp |
|
In vivo: intragastric administration in 4–5‐week‐old AG6 mice at 1–10 mg/kg 40 | ||||
| Source: Various Amaryllidaceae species 41 | In silico molecular docking 40 | ||||
| 30 | Silvestrol | Inhibits host cell factor eIF4A | – | 976, H/PF/2013 | In vitro: A549, 42 primary human hepatocytes |
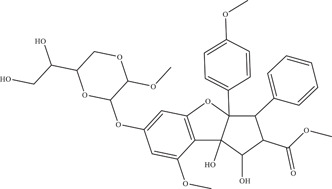
| |||||
| Source: Aglaia foveolata | |||||
| Inhibitors targeting the viral protease | |||||
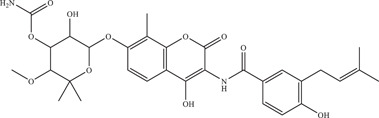
| 31 | Novobiocin |
Competitively binds to NS2B‐NS3 viral protease |
42.63 µM | PRVABC59 |
In vitro: Vero, 43 Huh‐7, purified ZIKV NS2B‐NS3pro |
|
In vivo: injected subcutaneously into dexamethasone‐immunosuppressed BALB/c mice at 100 mg/kg 43 | ||||
| Source: Streptomyces niveus | In silico molecular docking 43 | ||||
| 32 | Apigenin | Noncompetitive inhibitor of NS2B‐NS3 viral protease | 56.3 ± 0.9 µM | – | In vitro: purified ZIKV NS2B‐NS3pro 24 |
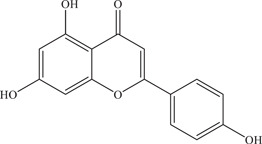
| |||||
| Source: Apiaceae family plants 44 | In silico molecular docking 24 | ||||
| 33 | Astragalin | Inhibits NS2B‐NS3 viral protease | 112 ± 5.5 µM | – | In vitro: purified ZIKV NS2B‐NS3pro 7 |
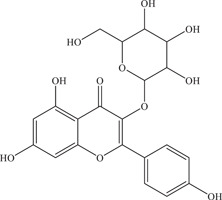
| |||||
| Source: Various plant species 45 | |||||
| 34 | Epicatechin gallate (ECG) | Inhibits NS2B‐NS3 viral protease | 89 ± 1.6 µM | – | In vitro: purified ZIKV NS2B‐NS3pro 7 |
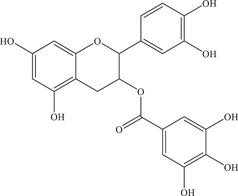
| |||||
| Source: Green tea | |||||
| 35 | Gallocatechin gallate (GCG) | Inhibits NS2B‐NS3 viral protease | 99 ± 1.8 µM | – | In vitro: purified ZIKV NS2B‐NS3pro 7 |
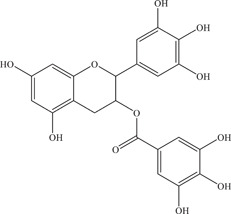
| |||||
| Source: Green tea | |||||
| 36 | Isorhamnetin | Inhibits NS2B‐NS3 viral protease | 15.5 ± 0.7 µM | – | In vitro: purified ZIKV NS2B‐NS3pro 24 |
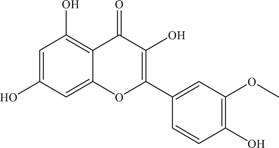
| |||||
| Source: Various plant species 46 | In silico molecular docking 24 | ||||
| 37 | Luteolin | Noncompetitive (allosteric) inhibitor of NS2B‐NS3 viral protease | 2.7 ± 0.3 µM; 53 ± 1.3 µM | – | In vitro: purified ZIKV NS2B‐NS3pro 7 , 24 |
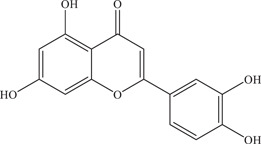
| |||||
| Source: Various plant species 47 | In silico molecular docking 24 | ||||
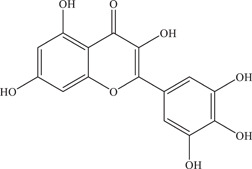
| 38 | Myricetin | Inhibits NS2B‐NS3 viral protease via mixed inhibition | NS2B‐NS3 protease: 1.10–22 µM | Z16006 | In vitro: Vero, 4 purified ZIKV NS2B‐NS3pro 4 , 7 , 24 |
| |||||
| Source: Various plant species 22 | 0.58 ± 0.17 µM | In silico molecular docking 4 , 24 | |||
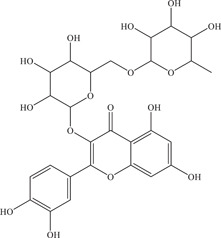
| 39 | Rutin | Inhibits NS2B‐NS3 viral protease | 104 ± 2.9 µM | – | In vitro: purified ZIKV NS2B‐NS3pro 7 |
| |||||
| Source: Various plant species 48 | |||||
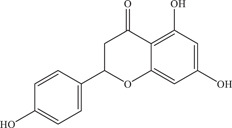
| 40 | Naringenin | Inhibits a postentry step, possible noncompetitive inhibitor of NS2B‐NS3 viral protease | 58.79 µM | BR 2015/15261, BR 2015/15098, BR 2016/16288, PE243 | In vitro: A549 49 , 50 |
|
In silico molecular docking 50 | ||||
| Source: Various citrus fruits 51 | |||||
| Inhibitors with unknown targets | |||||
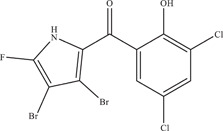
| 41 | Marinopyrrole derivative (Compound 1) | – | 5.95 ± 0.72 µM | NR‐50210 | In vitro: Vero 52 |
| |||||
| Source: Actinomycete strain CNQ‐418 derivative | |||||
| 42 | Kitasamycin | – | 41.7 ± 10.1 µM | PE243 | In vitro: Huh‐7 53 |
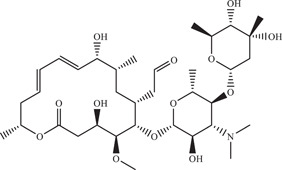
| |||||
| Source: Streptomyces narbonensis | |||||
| 43 | Hippeastrine hydrobromide (HH) | – | 1.95 µM | MR766 | In vitro: human pluripotent stem cell (hPSC)‐derived human cortical neural progenitor cells (hNPCs), human fetal‐like forebrain organoid model 54 |
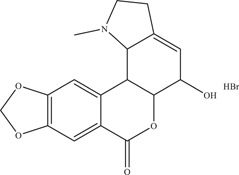
| |||||
| Source: Lycoris radiata | In vivo: injected subcutaneously in 6–8‐week‐old in severe combined immunodeficiency beige (SCID‐beige) mice at 100 mg/kg/day 54 | ||||
| 44 | 18‐oxoferruginol | – | 2.60 ± 0.07 µM | IMT17 | In vitro: Vero 55 |
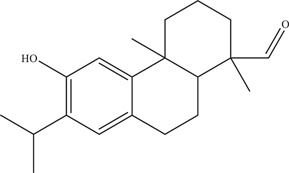
| |||||
| Source: Sequoia sempervirens 56 | |||||
3. VIRAL ENTRY INHIBITORS
Viral entry is a common stage of the viral replication cycle to target, as these inhibitors can be used as prophylactic treatments. 36 In addition, as membrane permeability into the host cell is not a requirement, compounds inhibiting viral entry tend to be less toxic. The process of viral entry itself is multifaceted, with different viral‐host interactions involving host cell surface receptors and the ZIKV E protein. 37 Hence, it is unsurprising that a large number of ZIKV natural product antivirals target this stage of the viral replication cycle.
3.1. Inhibitors with direct virucidal effects
Viral entry inhibitors can be divided into two subclasses, namely those that exhibit direct virucidal effects on the viral envelope itself and those that target host cell receptors or viral‐host interactions.
Labyrinthopeptins are a type of lantibiotic, which is a peptide antibiotic consisting of unusual amino acids. Labyrinthopeptins A1 and A2 (LabyA1 and LabyA2) are peptides produced in the bacteria actinomycete Actinomadura namibiensis DSM 6313. Using high‐content imaging and immunofluorescence, Prochnow et al. 38 showed the broad‐spectrum antiviral activity of LabyA1 and LabyA2 against not just flaviviruses but other viruses such as herpes simplex virus without high toxicity. LabyA1 inhibited ZIKV‐976 with an IC50 value of 2.0 µM; LabyA2 inhibited ZIKV‐976 with an IC50 value of 9.6 µM. Mechanistic studies revealed that LabyA1 and LabyA2 bind directly to virus particles to exhibit their antiviral effect. In particular, the labyrinthopeptins bind to phosphatidylethanolamines present as membrane lipids on the surface of the virus particles. This disrupts the viral envelope, leading to virolysis. Both LabyA1 and LabyA2 were also shown to work synergistically and display good preliminary pharmacokinetic properties when administered in male CD1 mice.
Cirne‐Santos et al. 39 investigated the anti‐ZIKV activity of crude extracts from the Brazilian brown seaweed Dictyota menstrualis. Brown seaweeds of this genus are rich in cyclic diterpenes and have been investigated for their antiviral effects on human immunodeficiency virus. 40 , 41 It was found that fraction FAc‐2 exhibited virucidal effects on ZIKV particles, inhibiting >90% of viral activity at a 20 µg/ml concentration.
In addition to compounds and fractions of microbial and marine origin, plants are a rich source of antivirals. Epigallocatechin gallate (EGCG) is a flavanol found in a large quantity in green tea and has known antiviral effects against many other viruses. 42 Carneiro 22 and colleagues showed the direct virucidal effect of EGCG on ZIKV particles with an EC50 value of 21.4 µM, inhibiting viral entry. Building upon the current findings, another study identified the site of EGCG binding on the E protein of ZIKV, and showed the key amino acid residues of the E protein involved in interacting with the compound via induced‐fit docking and molecular dynamics simulations. 43 Through a similar method, the interaction of EGCG with NS3 helicase was investigated, which showed possible binding of EGCG to both the RNA site and NTPase site of NS3 helicase. 44 In vitro NTPase activity inhibition by EGCG was shown to be 295.7 nM, confirming the results obtained in silico. EGCG was also identified in another screen of six structurally related polyphenols conducted by Vázquez‐Calvo et al., 45 where the virucidal effects of both delphinidin and EGCG on ZIKV was observed. A concentration of 10 µM was able to decrease viral plaque formation by approximately 10‐fold. Further analysis via quantitative reverse transcription‐polymerase chain reaction revealed both delphinidin and EGCG to result in a reduction of viral RNA in the infected culture supernatant.
Flavones baicalein and baicalin from Chinese herbs Scutellaria baicalensis and Scutellaria lateriflora were investigated for their anti‐ZIKV effect in Vero‐infected cells. 46 Baicalein had the lowest EC50 value of 0.004 µM when it was introduced post‐entry; baicalin had the lowest EC50 value of 14 µM when it was introduced at the point of entry. Both compounds also showed direct virucidal effect on ZIKV particles. In silico studies revealed the two compounds had the strongest binding affinity to viral NS5. In addition, both baicalein and baicalin have long half‐lives of 15.01 and 9.65 h respectively, and have favourable pharmacokinetic properties, making them ideal candidates for lead optimisation. 47
Gossypol was identified in a natural product library screen of Vero‐infected cells by Gao et al., 48 exhibiting the highest inhibitory activity among the hits identified against various ZIKV strains (IC50 range: 0.21–4.31 µM). Time‐of‐addition experiments revealed its potent virucidal effect on virus particles; enzyme‐linked immunoassay and surface plasmon resonance experiments confirmed the high binding affinity of gossypol for the ZIKV E protein (EC50: 7.12 µM; KD: 2.19 µM).
Ginkgolic acid is a phenolic acid extractedfrom the seed coats or leaves of Ginkgo biloba. Plaque‐forming assays showed ginkgolic acid to be able to block ZIKV replication in Vero cells in a dose‐dependent manner. 51 Time‐of‐addition experiments showed that ginkgolic acid affects the early stages of viral replication, possibly due to its virucidal activity.
Emodin, an anthraquinone derivative that can be found in various Chinese herbs, was shown by to reduce ZIKV viral titre in Vero cells (IC50: 3.2 µM). 49 Emodin reduced viral entry by 42.31% and produced a virucidal effect on virus particles. It was postulated that the virucidal effect is due to the high affinity of emodin for the phospholipid bilayer of the viral envelope. This is evidenced by a change in the hydrodynamic radii of ZIKV virus particles before and after incubation with emodin when measured via dynamic light scattering, possibly indicating a disruption of the phospholipid bilayer of virus particles.
Resveratrol is produced by several plant species, and is usually extracted from red grapes and red wine. 50 Resveratrol has been widely researched on for its antioxidant, anti‐inflammatory, and anticancer properties. The anti‐ZIKV activity of resveratrol and its mechanism of action were explored. 52 Results showed treatment of Huh7‐infected cells with 80 µM resveratrol reduced the number of foci formed by 93% (1 log) and is likely to have direct virucidal activity and post‐entry inhibition of the ZIKV replication cycle.
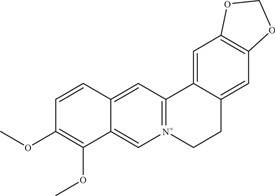
Berberine, an isoquinoline alkaloid isolated from Berberis vulgaris, was assessed by Batista and lab for its anti‐ZIKV activity on Vero cells. 49 Berberine reduced ZIKV viral titre with an IC50 value of 39.06 µM and was identified to do so via a direct virucidal effect.
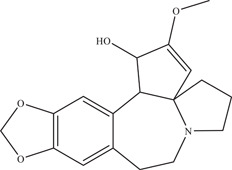
Cephalotaxine is an alkaloid isolated from the plant Cephalotaxus drupacea, shown by Lai, Ho, and Lu 53 to inhibit ZIKV infection in A549 cells in a dose‐dependent manner. Further analysis revealed that viral RNA was reduced in Vero cells when cephalotaxine was added post‐infection and throughout infection, indicating that cephalotaxine exhibits inhibition after virus entry. It was hypothesised that cephalotaxine works by interfering with viral RNA translation. In addition, a virucidal effect on ZIKV particles was observed.
Many of the compounds mentioned above do not just exhibit virucidal effects, but are bifunctional in targeting two different stages of the ZIKV replication cycle, such as EGCG which targets both viral entry and NS3 helicase. This bifunctional aspect could be leveraged on in drug development as for the same dose, dual effects are achieved.
3.2. Inhibitors targeting the viral entry process
These inhibitors prevent the successful internalisation of the virus by disrupting viral‐host cell interactions or by blocking host cell factors that mediate the endocytosis of the viral capsid. 54
The same study carried out by Cirne‐Santos and colleagues on crude extracts from D. menstrualis. 39 In addition to fraction FAc‐2 exhibiting virucidal effects on ZIKV particles, fraction F‐6 from the crude extract was found to reduce viral activity via inhibition of viral adsorption into host cells.
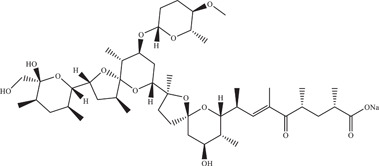
Nanchangmycin is an antibiotic produced by Streptomyces nanchangensis and shown to inhibit Gram‐positive bacteria. Rausch 56 and co‐workers carried out a high‐throughput image‐based screen on a library of bioactive compounds against ZIKV and identified nanchangmycin to be a potent inhibitor with an IC50 of 0.1 µM in U2OS cells. Nanchangmycin also exhibited inhibitory effects on other viruses with the same internalisation pathway as ZIKV such as chikungunya and sindbis viruses. However, nanchangmycin had no effect on parainfluenza 5 virus which does not share the same internalisation pathway as ZIKV. This suggests that nanchangmycin likely acts by blocking clathrin‐mediated endocytosis.
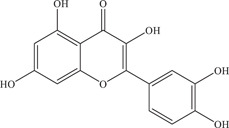
Zou 55 and colleagues further added on to the research by investigating the inhibitory effects of several flavonoids on ZIKV‐infected Vero cells in vitro. 56 Plaque reduction assays revealed the flavonols quercetin (IC50: 2.30 ± 0.50 µM), myricetin (IC50: 0.58 ± 0.17 µM), galangin (IC50: 14.36 ± 9.5 µM), and kaempferide (IC50: 5.83 ± 1.92 µM) to be potent inhibitors of ZIKV infection, with high CC50 values indicating low cytotoxicity. The flavanol EGCG was the most potent among the five compounds, with an IC50 of 0.02 ± 0.003 µM and CC50 of more than 500 µM. Time‐of‐addition assays showed that these flavonoids mediate the highest inhibition when co‐incubated with the virus, inhibiting viral entry. In addition, a biochemical enzymatic assay with ZIKV NS2B‐NS3 protease was performed, confirming that these five compounds are also able to inhibit ZIKV NS2B‐NS3 protease activity, with EC50 values ranging from 0.73 to 25.68 µM. Based on the data obtained, Zou 55 and colleagues postulated that the IC50 values decreased when the number of hydroxyl groups increased on the molecule. Furthermore, the galloyl group on EGCG seems to be responsible for the formation of crucial hydrogen bonds that give rise to the low IC50 value of EGCG. This study has thus showed the dual function of flavonoid inhibitors in inhibiting both ZIKV viral entry and ZIKV NS2B‐NS3 protease activity.
Isoquercetin, a glycoside derivative of quercetin which is a flavonol found in various plant species, was also found to have anti‐ZIKV activity in several studies, being able to reduce viral replication in Vero‐infected cells (IC50: 1.2–1.3 µmol/L). 57 Preliminary studies in immunocompromised mice (Ifnar1 −/− ) vulnerable to ZIKV infection showed the protective efficacy of isoquercetin in increasing survival and reducing weight loss. Another study reported the IC50 of isoquercetin in multiple cell lines (A549, Huh‐7, SH‐SY5Y) to be between 9.7 and 15.5 µM, and showed that isoquercetin targets the initial stages of ZIKV infection, particularly that of virus internalisation. 58
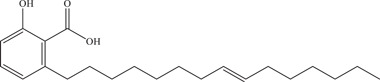
Another study on ellagic acid, a polyphenol identified and isolated from the P. granatum plant (commonly known as pomegranate), demonstrated its possible mechanism of action by binding to host cell surface and reducing cell susceptibility to the virus. 59
Curcumin is a component of turmeric, and has been shown to have antibacterial, antifungal and antiviral effects. 60 Mounce 61 and co‐workers showed the anti‐ZIKV effect of curcumin on HeLa cells to be through inhibition of virus binding to the cell surface (IC50: 1.90 µM), as transfection of ZIKV bypassed curcumin inhibition. Derivatives of curcumin such as bisdemethoxycurcumin and demethoxycurcumin showed similar inhibitory activity as unmodified curcumin.
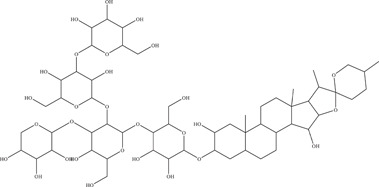
Digitonin is a steroidal saponin in the class of terpenoids, which are characterised by the five‐carbon isoprene structure. Digitonin was a positive hit in a natural product library screen by Gao 48 and colleagues, with IC50 values against various ZIKV strains ranging from 3.19 to 6.52 µM. Time‐of‐addition experiments showed digitonin to inhibit viral entry.
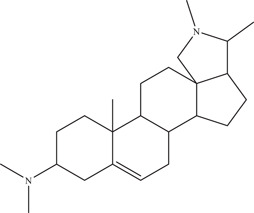
Conessine, a steroidal alkaloid, was another hit identified in the natural product library screen by Gao 48 and colleagues, with IC50 values ranging from 7.18 to 11.60 µM against a variety of ZIKV strains. From time‐of‐addition experiments, it is likely that conessine targets the virus‐cell attachment or a post‐entry step of the ZIKV replication cycle.
Several studies have attempted to identify the mechanism of inhibition of these natural products. A flavonoid binding pocket was identified on the Dengue virus (DENV) E protein surface, where it was shown that flavonoids such as EGCG, baicalein and baicalin docked in the same region between domain I and domain II of different subunits of E protein. 62 This same flavonoid binding pocket might be present in ZIKV, resulting in many flavonoids being inhibitors of viral entry. The viral E protein is a glycoprotein that mediates virion binding to host cell receptors, inhibition of binding results in inhibition of viral attachment and entry into cells. In addition, the trihydroxyphenyl group on aromatic ring R2 seen in delphinidin and EGCG seem to confer the anti‐ZIKV activity, as compounds like catechin and epicatechin lacking the trihydroxyphenyl group do not inhibit ZIKV infection. 45 Another possible explanation is that the mildly acidic nature of the trihydroxyphenyl impairs the acidification of the endosome, inhibiting the release of the viral genome into the host cell cytoplasm. It has also been suggested that the type of sugar bound to the aglycone group of the flavonoid may affect the anti‐ZIKV activity of the flavonoid, as seen in isoquercetin versus quercetin. 58 Taken together, the findings point toward possible pharmacophores essential for the inhibition of ZIKV viral entry of these natural products.
4. VIRAL REPLICATION INHIBITORS
Viral replication inhibitors are those that target the postentry stages of the replication cycle, inhibiting ZIKV viral proteins released from the viral capsid or host cell factors involved in viral replication and assembly. Host cell factors such as Golgi‐specific Brefeldin A‐resistant guanine nucleotide exchange factor 1 (GBF1) involved in Golgi‐to‐ER recycling and fatty acid synthase have been shown to be crucial in viral replication. 30 , 63
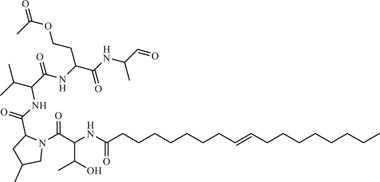
Estoppey and colleagues explored the anti‐flaviviral effect of cavinafungin, a lipopeptide from the fungus Colispora cavincola with IC50 of 150 nM against ZIKV in A549 cells. 64 Through CRISPR/Cas9 chemogenomic profiling, host cell endoplasmic reticulum (ER) signal peptidase was found as the target of cavinafungin. Cavinafungin inhibits the ER signal peptidase, which in turn results in parts of the viral polyprotein precursor not being cleaved to give individual viral proteins (Figure 1). As partially cleaved fragments of the polyprotein are unstable, viral replication is inhibited and viral titre is reduced.
Sinefungin is a structural analogue of S‐adenosylmethionine (SAM) produced by Streptomyces griseoleus. SAM is a natural substrate of many methyltransferases, making sinefungin a pan‐methyltransferase competitive inhibitor. The ZIKV nonstructural protein NS5 contains a methyltransferase domain which methylates the viral mRNA cap. Coutard and lab demonstrated that sinefungin was able to inhibit ZIKV 2′‐O‐methyltransferase activity with an IC50 of 1.18 ± 0.05 µM via radioactive methyltransferase assay. 65 Subsequently, Hercik and colleagues presented the crystal structure of the ZIKV NS5 methyltransferase domain with sinefungin bound and made suggestions for possible fragment‐based drug design that might improve the specificity and binding affinity of sinefungin. 66 Tao and lab followed up with the synthesis of sinefungin derivatives and reported the IC50 of certain derivatives in BHK cells to be as low as 6.33 ± 1.93 µM, as compared to sinefungin (IC50 > 50 µM). 67
Suroengrit and colleagues conducted a screen of eight selected flavonoid derivatives (including flavones, flavanones and chalcones) against DENV serotype 2 (DENV2) using plaque titration of the culture supernatants of LLC‐MK2 cells. 68 From the screen, FV13 and FV14, two halogenated chrysins, showed strong inhibition of DENV2. Upon further testing with ZIKV, both compounds showed potent anti‐ZIKV activity with IC50 values of 1.65 ± 0.86 µM and 1.39 ± 0.11 µM, respectively. Further investigations into the mechanism of action of FV13 showed that FV13 acts at the early post‐attachment stage, where multiple targets are possible. FV13 and FV14 have more favourable pharmacokinetic profiles than chrysin, with high efficacy and low cytotoxicity, making them ideal inhibitors to further investigate.
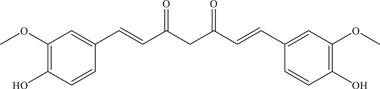
Pinocembrin was identified in an immunofluorescence‐based high throughput screen of a library of 483 flavonoid derivatives, inhibiting ZIKV infection in JEG‐3 cells with an IC50 of 17.4 µM. 69 It was reported to decrease both positive‐ and negative‐sense ZIKV RNA levels; Western blot analysis also showed a decrease in ZIKV envelope protein levels. Hence, it is likely that pinocembrin works by inhibiting post‐entry stages in the ZIKV replication cycle.
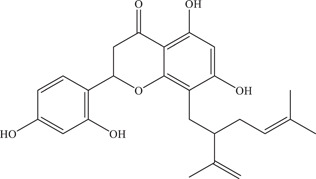
Sophoraflavenone G (SFG) was identified from an extract of Sophora flavescens, a plant used in Chinese medicine, to be able to inhibit ZIKV and DENV replication. 70 An in vitro RdRp activity assay revealed that SFG inhibits ZIKV RdRp with an IC50 of 22.61 µM. SFG is a suggested starting hit compound from which lead optimization can be carried out on, as significant levels of cytotoxicity were seen at higher doses of SFG.
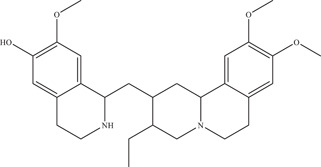
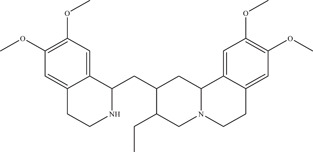
Emetine is a drug currently used in the treatment of amebiasis, a parasitic infection of the intestines by Entamoeba histolytica. 71 Emetine can be extracted from the plant Carapichea ipecacuanha, and was identified in a drug‐repurposing screen on HEK293 cells (IC50: 52.9 nM). 72 Further time‐of‐addition and ZIKV NS5 polymerase experiments revealed NS5 RdRp to be the target of emetine (IC50: 121 nM). In vivo treatment of emetine on two distinct mouse models (SJL and Ifnar1 −/ −) showed an approximate 10‐fold decrease in circulating ZIKV. In addition, emetine was also found to disrupt lysosome function. The same study also looked at cephaeline, the structural desmethyl analogue of emetine, which also demonstrated anti‐ZIKV properties (IC50: 7.60 nM), targeting ZIKV NS5 RdRp (IC50: 976 nM) and the autophagy‐dependent virus infection pathway. 72
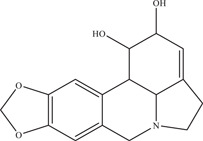
Lycorine, a benzyl phenethylamine alkaloid, was tested both in vitro on a variety of cell types (EC50: 0.22–0.39 µM) and in vivo by Chen et al. 73 Cellular thermal shift assay carried out on ZIKV NS5 showed that lycorine directly binds to ZIKV NS5, inhibiting its RdRp activity and reducing viral RNA replication. Treatment of ZIKV‐infected AG6 mice with 10 mg/kg lycorine improved survival rate by 83% and reduced viral RNA in the brain and liver.
Silvestrol is a flavagline isolated from the plant Aglaia foveolate known to inhibit the host cell factor DEAD‐box RNA helicase eukaryotic initiation factor‐4A (eIF4A), an essential component in viral RNA translation in Ebola virus and coronaviruses. 74 Using immunofluorescence microscopy, Elgner 75 and co‐workers showed the dose‐dependent reduction in A549‐infected cells upon treatment with 5–50 nM of silvestrol. Inhibition of viral RNA translation by silvestrol was shown to be through inhibition of cellular eIF4A.
4.1. Inhibitors targeting the viral protease
A common target for the development of inhibitors is the ZIKV NS2B‐NS3 protease, which is key in the production of mature viral proteins for further viral replication. 76 , 77 The nonstructural protein NS3 is a serine protease, with a catalytic site consisting of the triad of serine‐135, histidine‐51 and aspartate‐75. 78 The protease activity of NS3 requires the interaction with the cofactor domain of nonstructural protein NS2B. The NS2B‐NS3 viral protease is an attractive target for ZIKV drug discovery as the active site of the protease is highly conserved among other flaviviruses as well, showing promise for the possibility of developing a broad‐spectrum flavivirus inhibitor. 79 Target‐based studies beginning with ZIKV NS2B‐NS3 protease as a viral target explored compounds that bind to and inhibit protease activity. Often, in vitro biochemical enzymatic assays are performed to characterise the method of inhibition of these compounds.
The antibiotic novobiocin is an aminocoumarin produced by Streptomyces niveus, which acts by inhibiting bacterial DNA gyrase and hence preventing bacterial cell division. In an in silico structure‐based screening of 8227 compounds for ZIKV NS2B‐NS3 protease inhibitors, novobiocin was identified and validated for its anti‐ZIKV activity, with an IC50 of 42.63 µM in Vero cells. 80 Further in vitro studies are consistent with the hypothesis of novobiocin acting on the ZIKV NS2B‐NS3 protease. In addition, subcutaneous administration of novobiocin at 100 mg/kg every 12 h from 1 to 13 dayspost‐infection (dpi) to dexamethasone‐immunosuppressed mice led to a higher survival rate and lesser weight loss compared to untreated mice. Mean viral loads in the blood and organ tissues were reduced by more than two‐log fold in novobiocin‐treated mice as compared to untreated mice just 5 dpi.
With ZIKV NS2B‐NS3 protease as a target, Roy et al. 81 carried out an in vitro biochemical enzymatic assay of nine natural products. From the enzymatic assay, six compounds were shown to inhibit NS2B‐NS3 protease significantly in a non‐competitive manner. These six compounds are mainly flavonoids, namely the flavones luteolin (IC50: 2.7 ± 0.3 µM) and apigenin (IC50: 56.3 ± 0.9 µM), flavonols myricetin (IC50: 1.3 ± 0.1 µM), quercetin (IC50: 2.4 ± 0.2 µM) and isorhamnetin (IC50: 15.5 ± 0.7 µM), and the natural phenol curcumin (IC50: 3.5 ± 0.2 µM). Through molecular docking, the explanations for the different IC50 values were elucidated, such as myricetin being the strongest inhibitor as it forms six hydrogen bonds with four different amino acid residues on ZIKV NS3 protease. With the knowledge of key residue interactions and how binding is affected, inhibitors can be better designed with higher affinity for NS2B‐NS3 protease.
Also utilising NS2B‐NS3 protease as a target, Lim 82 and co‐workers performed an in vitro biochemical enzymatic assay with a fluorogenic peptide, determining the inhibition kinetics for 22 polyphenols. Among the 22 polyphenols tested at 100 µM, seven compounds reduced NS2B‐NS3 protease activity by more than 40%. These seven compounds were further validated to obtain IC50 values. The compounds are: the flavone luteolin (IC50: 53 ± 1.3 µM), the flavonols myricetin (IC50: 22 ± 0.2 µM), astragalin (IC50: 112 ± 5.5 µM), and rutin (IC50: 104 ± 2.9 µM), the flavanols EGCG (IC50: 87 ± 1.2 µM), epicatechin gallate (IC50: 89 ± 1.6 µM), and gallocatechin gallate (IC50: 99 ± 1.8 µM). Comparing the structures of the compounds and the inhibition values, one of the observations made was that the presence of the hydroxyl group on flavonols is important in the inhibitory activity of flavonols against ZIKV NS2B‐NS3 protease.
Naringenin, a flavanone found in several citrus fruits, was shown to inhibit ZIKV replication in A549 cells in a dose‐dependent manner with an IC50 of 58.79 µM. 83 , 84 Time‐of‐addition assays revealed the stage naringenin inhibits to be between virus replication and assembly of the ZIKV replication cycle. Molecular docking showed that naringenin can act as a non‐competitive inhibitor of the NS2B‐NS3 viral protease, which could be a possible target of naringenin. The low bioavailability of naringenin prompted the study of chemical derivatives of naringenin by Mendes and colleagues, where ether derivatives demonstrated anti‐ZIKV effects on A549‐infected cells (IC50 values range from 6.76 to 69.33 µM). 85 However, these ether derivatives were shown to have higher toxicity than that of naringenin.
The suggested SAR for flavonoids against ZIKV NS2B‐NS3 protease has been researched on in multiple studies, with results being in agreement with each other. Continuing on from the SAR established for viral entry, Zou 55 and colleagues extended the pharmacophore region of flavonoids beyond the trihydroxyphenyl group to include the ester functional group (galloyl group). In addition, Lim 82 and co‐workers established a similar SAR as Zou 55 where the presence of hydroxyl groups at C3′, C4′, and C5′ of the B‐ring is key in the inhibition of NS2B‐NS3 protease. As opposed to the findings from Gaudry et al., 58 glycosylation at C7 of the A‐ring decreases NS2B‐NS3 protease inhibition, though this glycosylation may be important in inhibiting viral entry instead. The binding pocket of these flavonoids on the ZIKV NS2B‐NS3 protease complex is likely to be on an allosteric site, based on the enzyme kinetics performed by Roy and colleagues. 81
5. INHIBITORS WITH UNKNOWN TARGETS
Inhibitors with unknown targets were identified in phenotypic screening assays, which are a common way of identifying potential antivirals. A large number of compounds can be tested, increasing the probability of identifying a hit inhibiting ZIKV infection with acceptable IC50 and CC50 values. Improvements in technology have enabled the establishment of high throughput screening assays that are becoming increasingly cost‐efficient and improving in accuracy.
A marinopyrrole derivative was identified from a screen of a library of natural product derivatives carried out by Bernatchez and co‐workers. 86 This compound was obtained after structural simplification and optimization studies on marinopyrroles, which are produced by Actinomycete strain CNQ‐418. Of the 24 compounds screened, one was able to reduce cytopathic effect (CPE) in Vero cells with an EC50 of 5.95 ± 0.72 µM.
Kitasamycin was identified in a high‐content immunofluorescence screening of U.S. Food and Drug Administration (FDA)‐approved drugs. 87 Kitasamycin is a macrolide antibiotic produced by Streptomyces narbonensis, and has been shown to inhibit Gram‐positive bacteria and mycoplasma by binding to bacterial ribosomal RNA, hence inhibiting bacterial protein synthesis. Though the mechanism of the anti‐ZIKV effect of kitasamycin is still unknown, the measured EC50 is 41.7 ± 10.1 µM in Huh7 cells, indicating the potential of kitasamycin as a promising compound with anti‐ZIKV activity.
Hippeastrine hydrobromide (HH), first isolated from Lycoris radiata, is another steroidal alkaloid identified in a high‐content chemical screen using human pluripotent stem cell‐derived cortical neural progenitor cells (IC50: 1.95 µM). 88 In a human fetal‐like forebrain organoid model, treatment with 25 µM of HH for 3 days was effective in reducing the percentage of cells infected with ZIKV. This is especially important as ZIKV infection is often associated with neurological disorders. 18 This finding was further shown in severe combined immunodeficiency beige (SCID‐beige) mice, where immunohistochemical analysis revealed that HH was able to reduce infection in various regions of the brain. However, the mechanism of action of HH remains to be investigated.
18‐oxoferruginol is an abietane diterpenoid whose anti‐ZIKV activity was evaluated in vitro. 89 Among the abietane analogues tested, 18‐oxoferruginol presented the best selectivity index of 13.51 with a CC50 value of 35.09 ± 5.20 µM and an EC50 value of 2.60 ± 0.07 µM.
Instead of isolated compounds, several studies also investigated the anti‐ZIKV effect of plant extracts as a starting point for the identification of novel anti‐ZIKV agents. 59 , 90 , 91 , 92 , 93 , 94 , 95 Most of these extracts are polyphenol‐rich, such as extracts from Polygonum cuspidatum, 93 Psiloxylon mauritianum, 92 Schinus terebenthifolius Raddi, 91 Punica granatum, 59 Doratoxylon apetalum, 90 Aphloia theiformis, 94 and silymarin, an extract from the plant Silybum marianum L. Gaertn. 95 From these extracts, natural products with novel chemical scaffolds can be identified. For instance, Kuo et al. showed the anti‐flaviviral activity of the methanolic extract from Polygonum cuspidatum, in which major constituents include quercetin, resveratrol, and emodin. 93 Flow cytometry‐based infection analysis on Vero‐infected cells showed the significant inhibition of ZIKV entry by this P. cuspidatum extract.
6. CONCLUSION AND FUTURE PERSPECTIVES
Ever since the first outbreak of ZIKV in Micronesia in 2007, much research has been carried out in identifying anti‐ZIKV compounds, through drug‐repurposing screens or looking to the relatively untapped source of natural compounds. Natural products of microbial, plant and marine origins have great structural diversity, as evidenced by the many chemical classes. This allows for the discovery of novel chemical scaffolds which can serve as parent compounds for further SAR investigations to further improve on the efficacy and safety profile of the compound through synthetic processes. This method of identifying hit compounds saves time as these molecules have evolutionary preoptimized biological targets, increasing the prospect of identifying a positive hit.
As shown by the 44 isolated compounds and six plant extracts discussed in the earlier sections, current knowledge of the anti‐ZIKV natural products show that these compounds target different stages in the ZIKV replication cycle, ranging from host cell factors such as eIF4A to ZIKV viral proteins such as the NS2B‐NS3 protease. 55 , 75 , 81 , 82 This, therefore, raises the possibility of combinatorial therapy in the treatment of ZIKV infections to increase the efficacy of treatment. This has been explored by Gao et al. 48 in the combinatorial effects of gossypol with other natural products such as curcumin. Results show a two‐ to threefold enhancement in the inhibition of ZIKV infection in Vero cells, with little effect on cytotoxicity. High synergism was observed when both D. menstrualis acetylated crude extract fraction FAc‐2 and ribavirin, a synthetic nucleoside with known antiviral activity against other RNA and DNA viruses, were added together. 96 ZIKV replication was inhibited by around 90%, which was much more than the addition of the compounds effects of these compounds individually. Many of these natural products isolated are flavonoids or polyphenols, whose antiviral activity is known to be caused by hydrogen bond interactions of the phenolic hydroxyl groups with viral or host cell factors. 85 Strategies to improve the inhibitory activity of these natural products include improving lipophilicity for greater interaction with biological membranes and better permeability of the compound into the host cell. However, these structural modifications should also take into account the current established SAR of these flavonoids, such as the number and positions of hydroxyl groups, the hybridization of carbons, and the glycosylation of the compound.
Nonetheless, there are still certain areas of research that provide great potential in the discovery of ZIKV natural product antivirals, such as marine sources of natural products. The marine environment has also been another important source of natural products, providing several compounds that have been approved by FDA as antibiotics and anticancer drugs. 97 , 98 Sources of these natural products include marine microorganisms, algae, sponges, jellyfish and rocky corals. 99 Marine natural products as ZIKV antivirals are still a relatively unexplored area, which is surprising as organisms in the marine environment such as marine actinomycetes and cyanobacteria have provided many compounds that have progressed into clinical trials and even been approved for clinical use. 100 This could be due to perceived challenges presented by natural product drug discovery, such as the extra resources and time required to isolate and identify compounds responsible for the antiviral effect. For compounds with moderate to high complexity, chemical synthesis might not be a viable option. However, with the development of better technology, it has become much easier. It was reported that with the relevant skills and resources, the structures of individual compounds in extracts can be solved within 2 weeks. 101
In many studies the in vitro antiviral assays were only tested in a particular cell line or only using a particular ZIKV strain as shown in Table 1. Thus, the results of the compound may be cell line‐ or strain‐specific instead, which renders the compound ineffective in treating ZIKV infection where ZIKV infects a variety of cell types. 54 , 56 This is particularly important as there have been significant differences observed in the pathogenesis of African ZIKV strains as compared to Asian strains. For example, Aubry et al. 102 reported that African ZIKV strains are more transmissible by mosquitoes, and are associated with fetal loss rather than birth defects. Differences in growth ability were also reported, where the African ZIKV strains showed greater ability to infect human placental trophoblasts. 103 Hence, it would be interesting to explore the differences in drug effect across these different ZIKV strains, particularly comparing between the African and Asian lineages.
Additionally, more work in characterising the activity of the compound in several representative cell types is required, before taking the compound forward to in vivo studies. Several of these compounds have only been tested in cell‐free systems against particular ZIKV proteins such as the NS2B‐NS3 protease, which does not rule out the possibility of secondary drug targets, such as other viral or host factors. At the same time, preliminary information on the pharmacokinetic properties of the drug is not considered. The advantage, however, of using purified viral proteins is in the ability to determine the mode of inhibition of the drug, where further characterisation of specific molecular interactions of the pharmacophores and amino acid residues can be elucidated. As the end goal is still towards testing in living organisms, further validation in cell culture is essential. Majority of the studies perform cell‐based assays by infecting cell lines with ZIKV and quantifying reduction in viral titre via plaque assays. A common choice of cell line is Vero, a kidney epithelial cell line obtained from the African green monkey. Although Vero is derived from normal kidney cells and not immortal cells, its origin is non‐human. Hence, evaluating the drug effect in other immortalised human cell lines or in primary human cell lines is necessary. Although human A549 was shown to be as effective as Vero E6 in its susceptibility to ZIKV infection, more physiologically relevant cell lines can be used, such as the human brain microvascular endothelial cells used by Rausch et al. 56 , 104 Such cell lines will be helpful in replicating the natural host environment of ZIKV.
In any drug discovery programme, in vivo testing in animal models is essential in evaluating the pharmacokinetic properties of the compound—understanding its preclinical safety and efficacy profile and establish a therapeutic dose. Of the 44 natural compounds highlighted, only six of these compounds have been tested in mouse models. 38 , 57 , 72 , 73 , 80 , 88 Though the particular mouse models used differ, the mice are all immunocompromised, either with double knockout of the Ifnar1 gene or dexamethasone‐immunosuppressed. This is because it is known that mice with genetic knockouts in the type I interferon signalling pathway are more susceptible to flavivirus infection. 105 However, ethical concerns arising from the use of animal models present a high barrier to entry. Furthermore, inter‐species biological differences may skew the results obtained.
To circumvent and improve on existing limitations of animal models, three‐dimensional (3D) cell cultures and organoid models have been developed. In the study by Zhou and colleagues, a human pluripotent stem cell‐derived forebrain organoid model was utilised to study the effect of hippeastrine hydrobromide on ZIKV infection in the brain. 88 This organoid model is used in the study of brain disorders such as microcephaly, which is one of the possible effects of severe ZIKV infection. 106 Especially for complex organs like the brain, two‐dimensional monolayer or free‐floating neurosphere cell cultures might not be able to replicate the same diversity and composition of the various cell types that constitute the organ. Being able to mimic the in vivo physiology with lesser ethical concerns make organoid cultures an ideal model system for antiviral development. Nonetheless, culturing organoids is still a relatively new technique, requiring extensive training and equipment. Specialised equipment to visualise readouts is required, especially if the study is focused on particular segments of the 3D culture. Issues with regards to accessing the target host cell receptors and visualising CPE have also been raised. 107 Finally, studying organoids in isolation neglects interactions arising from the immune and circulatory system being connected to organ, which may affect the results of ZIKV infection.
Several of these natural products may still require optimisation of their pharmacokinetic properties. For instance, Batista and colleagues have recognised the barriers in bringing the natural products berberine and emodin forward in pharmaceutical development, such as low aqueous solubility and bioavailability. 49 For this reason, the development of semisynthetic or synthetic structures which were conceptually derived from a natural product have been on the rise, where a natural product compound is used as a start point for the introduction of synthetic modifications. 108 This was explored in a few of the studies with chemically modified flavones 68 or ethers and esters lipophilic naringenin derivatives. 85
In areas where ZIKV infection is endemic, coinfections with other arboviruses such as DENV and the West Nile virus (WNV) are common. 109 This is an additional concern as DENV‐specific antibodies have been reported to result in a higher susceptibility to ZIKV infection by the Brazilian isolate through the antibody‐dependent enhancement mechanism. 110 Thus, it is worthwhile to consider looking at natural products that can act as antivirals against a broad‐spectrum of arboviruses instead of just ZIKV in isolation, as carried out by numerous studies. 38 , 45 , 56 , 64 , 68 , 90
To conclude, viral infections are seasonal in that there are cyclical patterns in infectiousness due to changes in pathogen survival rates and host susceptibility. 111 Although there seems to be a decrease in the number of reported cases of ZIKV infection, as long as the vectors for ZIKV infection continue to exist, the search for antivirals against ZIKV remains an urgent need. Moreover, ZIKV infection could also occur vertically from mother to fetus. Hence, research on ZIKV antivirals should continue to prevent future outbreaks. Natural products have lower costs of production, are environmentally friendly, and tend to have more favourable physicochemical properties such as lower cytotoxicity compared to synthetic compounds. This is especially important as ZIKV infections tend to be endemic in developing countries, where cost is an important factor in considering the available therapeutic options. Furthermore, as ZIKV infection has been associated with fetal microcephaly, the patient profile of anti‐ZIKV drugs includes pregnant women, indicating the need for a more favourable safety profile. Hence, natural products are a viable source of antivirals against ZIKV infection, which continues to be a persistent epidemic worldwide.
ACKNOWLEDGEMENTS
This research received no external funding, and the authors declare no conflict of interest. This study is supported by MOE Tier 2 2017 (MOE2017‐T2‐1‐078; MOE2017‐T2‐2‐014) and NRF‐CRP21‐2018‐0004.
Biographies
Yuhui Deborah Fong graduated with a B.Sc. in Life Sciences (2020) and is currently doing her PhD at National University of Singapore (NUS) with Associate Professor Justin Jang Hann Chu. She is currently exploring the screening of compounds to identify antivirals against human enteroviruses.
Associate Professor Justin Jang Hann Chu is currently the Assistant Dean for Academic Affairs and a faculty member in the Department of Microbiology and Immunology, Yong Loo Lin School of Medicine, National University of Singapore https://medicine.nus.edu.sg/mbio/about-us/our-people/academic-staff/justin-chu.html. He is holding a Joint Senior Principal Investigator in the Institute of Molecular and Cell Biology (IMCB), A*STAR. A/P Chu is also the Director of the largest research based high containment Biosafety Level 3 Facility in Singapore which is located in the Yong Loo Lin School of Medicine, NUS. A/P Chu is actively engaged in the study of the molecular biology of human enteroviruses that cause HFMD as well as mosquito‐borne viruses including Dengue, Zika, Japanese Encephalitis and Chikungunya. The outcomes of these studies are helping to pave the roadmap towards the development of a number of antiviral strategies. Eight patents and numerous scientific awards have been received from his current research. A/P Chu has published over 100 international peer‐reviewed scientific publications, six book chapters and over 150 conference papers. A number of these scientific papers are published in prestigious journals including Science, Science Translational Medicine, Nature Communications, PNAS, PLoS Pathogens. A/P Chu is currently serving as the associate editor and reviewer for a number of peer‐reviewed journals in the areas of medical virology and anti‐viral strategies. He is the current President of the Asia‐Pacific Society of Medical Virology and the President of the Singapore Society for Microbiology and Biotechnology.
Fong YD, Chu JJH. Natural products as Zika antivirals. Med Res Rev. 2022;42:1739‐1780. 10.1002/med.21891
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
REFERENCES
- 1. Westaway EG, Brinton MA, Gaidamovich SY, et al. Flaviviridae. Intervirology. 1985;24:183‐192. [DOI] [PubMed] [Google Scholar]
- 2. Campos GS, Bandeira AC, Sardi SI. Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885‐1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas‐region of the Americas, May 2015‐January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:55‐58. [DOI] [PubMed] [Google Scholar]
- 4. Grard G, Caron M, Mombo IM, et al. Zika Virus in Gabon (Central Africa)—2007: A New Threat from Aedes albopictus . PLoS Negl Trop Dis. 2014;8:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olson JG, Ksiazek TG, Suhandiman G, Triwibowo V. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75:389‐393. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization . WHO. 2019;2019 . [Google Scholar]
- 7. Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain‐Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19:1‐3. [DOI] [PubMed] [Google Scholar]
- 8. Cao‐Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20:1085‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013‐15: a retrospective study. Lancet. 2016;387:2125‐2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization , WHO.
- 11. Musso D, Nilles EJ, Cao‐Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20:O595‐O596. [DOI] [PubMed] [Google Scholar]
- 12. Duffy MR, Chen T‐H, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536‐2543. [DOI] [PubMed] [Google Scholar]
- 13. Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao‐Lormeau V‐M. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuler‐Faccini L, Ribeiro EM, Feitosa IML, et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:59‐62. [DOI] [PubMed] [Google Scholar]
- 15. Dirlikov E, Major CG, Mayshack M, et al. Guillain‐Barré Syndrome during ongoing Zika virus transmission—Puerto Rico, January 1‐July 31, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:910‐914. [DOI] [PubMed] [Google Scholar]
- 16. Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet. 2016;388:898‐904. [DOI] [PubMed] [Google Scholar]
- 17. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects‐‐reviewing the evidence for causality. N Engl J Med. 2016;374:1981‐1987. [DOI] [PubMed] [Google Scholar]
- 18. Chen T, He X, Zhang P, et al. Research advancements in the neurological presentation of flaviviruses. Rev Med Virol. 2019;29:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alam A, Imam N, farooqui A, Ali S, Malik MZ, Ishrat R. Recent trends in ZikV research: a step away from cure. Biomed Pharmacother. 2017;91:1152‐1159. [DOI] [PubMed] [Google Scholar]
- 20. Wahid B, Ali A, Rafique S, Idrees M. Current status of therapeutic and vaccine approaches against Zika virus. Eur J Intern Med. 2017;44:12‐18. [DOI] [PubMed] [Google Scholar]
- 21. Munjal A, Khandia R, Dhama K, et al. Advances in developing therapies to combat Zika virus: current knowledge and future perspectives. Front Microbiol. 2017;8:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carneiro BM, Batista MN, Braga ACS, Nogueira ML, Rahal P. The green tea molecule EGCG inhibits Zika virus entry. Virology. 2016;496:215‐218. [DOI] [PubMed] [Google Scholar]
- 23. Wright GD. Unlocking the potential of natural products in drug discovery. Microb Biotechnol. 2019;12:55‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Motika SE, Hergenrother PJ. Re‐engineering natural products to engage new biological targets. Nat Prod Rep. 2020;37:1395‐1403. 10.1039/d0np00059k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen B. A new golden age of natural products drug discovery. Cell. 2015;163:1297‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang HK. The therapeutic potential of flavonoids. Expert Opin Investig Drugs. 2000;9:2103‐2119. [DOI] [PubMed] [Google Scholar]
- 27. Cragg GM, Pezzuto JM. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract. 2016;25:41‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdel‐Razek AS, El‐Naggar ME, Allam A, Morsy OM, Othman SI. Microbial natural products in drug discovery. Processes. 2020;8:1‐19. [Google Scholar]
- 29. Yi M, Lin S, Zhang B, Jin H, Ding L. Antiviral potential of natural products from marine microbes. Eur J Med Chem. 2020;207:112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sager G, Gabaglio S, Sztul E, Belov GA. Role of host cell secretory machinery in Zika Virus Life Cycle. Viruses. 2018;10:2013‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez AK, Muñoz AL, Segura NA, Rangel HR, Bello F. Molecular characteristics and replication mechanism of dengue, zika and chikungunya arboviruses, and their treatments with natural extracts from plants: an updated review. EXCLI J. 2019;18:988‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hossein F. An overview of the current medical literature on Zika virus. Biophys Rev. 2020;12:1133‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meertens L, Labeau A, Dejarnac O, et al. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep. 2017;18:324‐333. [DOI] [PubMed] [Google Scholar]
- 34. Kaufmann B, Rossmann MG. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes Infect. 2011;13:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chambers TJ, Hahn CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649‐88. [DOI] [PubMed] [Google Scholar]
- 36. Agrelli A, de Moura RR, Crovella S, Brandão LAC. ZIKA virus entry mechanisms in human cells. Infect Genet Evol. 2019;69:22‐29. [DOI] [PubMed] [Google Scholar]
- 37. Zhou Y, Simmons G. Development of novel entry inhibitors targeting emerging viruses. Expert Rev Anti Infect Ther. 2012;10:1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prochnow H, Rox K, Birudukota NVS, et al. Labyrinthopeptins exert broad‐spectrum antiviral activity through lipid‐binding‐mediated virolysis. J Virol. 2020;94:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cirne‐Santos CC, Barros CDS, Gomes MWL, et al. Nat Prod Commun. 2019;14:1‐7. [Google Scholar]
- 40. Pereira HS, Leão‐Ferreira LR, Moussatché N, et al. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV‐1). Antiviral Res. 2004;64:69‐76. [DOI] [PubMed] [Google Scholar]
- 41. De Souza Pereira H, Leão‐Ferreira LR, Moussatché N, et al. Effects of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis on HIV‐1 reverse transcriptase. Planta Med. 2005;71:1019‐1024. [DOI] [PubMed] [Google Scholar]
- 42. Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti‐infective properties of epigallocatechin‐3‐gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168:1059‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharma N, Murali A, Singh SK, Giri R. Epigallocatechin gallate, an active green tea compound inhibits the Zika virus entry into host cells via binding the envelope protein. Int J Biol Macromol. 2017;104:1046‐1054. [DOI] [PubMed] [Google Scholar]
- 44. Kumar D, Sharma N, Aarthy M, Singh SK, Giri R. Mechanistic insights into Zika Virus NS3 helicase inhibition by epigallocatechin‐3‐Gallate. ACS Omega. 2020;5:11217‐11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vázquez‐Calvo A, de Oya NJ, Martín‐Acebes MA, Garcia‐Moruno E, Saiz JC. Antiviral properties of the natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front Microbiol. 2017;8:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oo A, Teoh BT, Sam SS, Bakar SA, Zandi K. Baicalein and baicalin as Zika virus inhibitors. Arch Virol. 2019;164:585‐593. [DOI] [PubMed] [Google Scholar]
- 47. Li M, Shi A, Pang H, et al. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J Ethnopharmacol. 2014;156:210‐215. [DOI] [PubMed] [Google Scholar]
- 48. Gao Y, Tai W, Wang N, et al. Viruses. 2019;11:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Batista M. N., Braga A. C. S., Fernandes Campos G. R., et al. Natural products isolated from oriental medicinal herbs inactivate Zika Virus. Viruses. 2019;11:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu C‐F, Yang J‐Y, Wang F, Wang X‐X. Resveratrol: botanical origin, pharmacological activity and applications: Resveratrol: botanical origin, pharmacological activity and applications. Chin J Nat Med. 2013;11:1‐15. [Google Scholar]
- 51. Campos D, Navarro S, Llamas‐Gonzalez YY, Sugasti M, Gonzalez‐Santamaria J. Broad antiviral activity of ginkgolic acid against Chikungunya, Mayaro, Una, and Zika Viruses. Viruses. 2020;12:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mohd A, Zainal N, Tan KK, AbuBakar S. Resveratrol affects Zika virus replication in vitro. Sci Rep. 2019;9:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lai ZZ, Ho YJ, Lu JW. Cephalotaxine inhibits Zika infection by impeding viral replication and stability. Biochem Biophys Res Commun. 2020;522:1052‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee JK, Shin OS. Int J Mol Sci. 2019;20:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zou M, Liu H, Li J, et al. Structure‐activity relationship of flavonoid bifunctional inhibitors against Zika virus infection. Biochem Pharmacol. 2020;177:113962. [DOI] [PubMed] [Google Scholar]
- 56. Rausch K, Hacket B, Weinbren N, et al. Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against Zika Virus. Cell Rep. 2017;18:804‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong G, He S, Siragam V, et al. Antiviral activity of quercetin‐3‐β‐O‐D‐glucoside against Zika virus infection. Virol. Sin. 2017;32:545‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gaudry A, Bos S, Viranaicken W, et al. The flavonoid isoquercitrin precludes initiation of Zika virus infection in human cells. Int J Mol Sci. 2018;19:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Acquadro S, Civra A, Cagliero C, et al. Punica granatum leaf ethanolic extract and ellagic acid as inhibitors of Zika virus infection. Planta Med. 2020;86:1363‐1374. 10.1055/a-1232-5705 [DOI] [PubMed] [Google Scholar]
- 60. Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int. 2014;2014:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res. 2017;142:148‐157. [DOI] [PubMed] [Google Scholar]
- 62. Ismail NA, Jusoh SA. Molecular docking and molecular dynamics simulation studies to predict flavonoid binding on the surface of DENV2 E protein. Interdiscip Sci: Comput Life Sci. 2016;9:499‐511. [DOI] [PubMed] [Google Scholar]
- 63. Martín‐Acebes MA, Vázquez‐Calvo Á, Saiz JC. Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses. Prog Lipid Res. 2016;64:123‐137. [DOI] [PubMed] [Google Scholar]
- 64. Estoppey D, Lee CM, Janoschke M, et al. The natural product cavinafungin selectively interferes with Zika and Dengue virus replication by inhibition of the host signal peptidase. Cell Rep. 2017;19:451‐460. [DOI] [PubMed] [Google Scholar]
- 65. Coutard B, Barral K, Lichière J, et al. Zika virus methyltransferase: structure and functions for drug design perspectives. J Virol. 2017;91:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hercik K, Brynda J, Nencka R, Boura E. Structural basis of Zika virus methyltransferase inhibition by sinefungin. Arch Virol. 2017;162:2091‐2096. [DOI] [PubMed] [Google Scholar]
- 67. Tao Z, Cao R, Yan Y, et al. Design, synthesis and in vitro anti‐Zika virus evaluation of novel Sinefungin derivatives. Eur J Med Chem. 2018;157:994‐1004. [DOI] [PubMed] [Google Scholar]
- 68. Suroengrit A, Yuttithamnon W, Srivarangkul P, et al. Halogenated Chrysins Inhibit Dengue and Zika virus infectivity. Sci Rep. 2017;7:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Le Lee J, Loe MWC, Lee RCH, Chu JJH. Antiviral activity of pinocembrin against Zika virus replication. Antiviral Res. 2019;167:13‐24. [DOI] [PubMed] [Google Scholar]
- 70. Sze A, Olagnier D, Hadj SB, et al. Sophoraflavenone G restricts Dengue and Zika virus infection via RNA polymerase interference. Viruses. 2017;9:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scholar E. in xPharm: The Comprehensive Pharmacology Reference . Elsevier Inc; 2009:1‐4. [Google Scholar]
- 72. Yang S, Xu M, Lee EM, et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov. 2018;4:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen H, Lao Z, Xu J, et al. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology. 2020;546:88‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chu J, Galicia‐Vázquez G, Cencic R, et al. CRISPR‐mediated drug‐target validation reveals selective pharmacological inhibition of the RNA helicase, eIF4A. Cell Rep. 2016;15:2340‐2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Elgner F, Sabino C, Basic M, Ploen D, Grünweller A, Hildt E. Inhibition of Zika virus replication by silvestrol. Viruses. 2018;10:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. H S, R H. Recent advances in targeting viral proteases for the discovery of novel antivirals. Curr Top Med Chem. 2010;10:323‐345. [DOI] [PubMed] [Google Scholar]
- 77. Hsu J, Wang H‐C, Chen G‐W, Shih S‐R. Antiviral drug discovery targeting to viral proteases. Curr Pharm Des. 2006;12:1301‐1314. [DOI] [PubMed] [Google Scholar]
- 78. Voss S, Nitsche C. Inhibitors of the Zika virus protease NS2B‐NS3. Bioorg Med Chem Lett. 2020;30:126965. [DOI] [PubMed] [Google Scholar]
- 79. Nitsche C. Proteases from dengue, West Nile and Zika viruses as drug targets. Biophys Rev. 2019;11:157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yuan S, Chan JFW, Den‐Haan H, et al. Structure‐based discovery of clinically approved drugs as Zika virus NS2B‐NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antiviral Res. 2017;145:33‐43. [DOI] [PubMed] [Google Scholar]
- 81. Roy A, Lim L, Srivastava S, Lu Y, Song J. PLoS One. 2017;12:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lim H, Nguyen TTH, Kim NM, Park JS, Jang TS, Kim D. Inhibitory effect of flavonoids against NS2B‐NS3 protease of ZIKA virus and their structure activity relationship. Biotechnol Lett. 2017;39:415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cataneo AHD, Kuczera D, Koishi AC, et al. The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Sci Rep. 2019;9:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Salehi B, Fokou PVT, Sharifi‐Rad M, et al. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de LA, Mendes O, Ponciano CS, et al. The anti‐Zika virus and anti‐tumoral activity of the citrus flavanone lipophilic naringenin‐based compounds. Chem Biol Interact. 2020;331:1‐12. [DOI] [PubMed] [Google Scholar]
- 86. Bernatchez JA, Yang Z, Coste M, et al. Development and validation of a phenotypic high‐content imaging assay for assessing the antiviral activity of small‐molecule inhibitors targeting Zika Virus. Antimicrob Agents Chemother. 2018;62:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pascoalino BS, Courtemanche G, Cordeiro MT, Gil LHVG, Freitas‐Junior LH. Zika antiviral chemotherapy: identification of drugs and promising starting points for drug discovery from an FDA‐approved library. F1000Research. 2016;5:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhou T, Tan L, Cederquist GY, et al. High‐content screening in hPSC‐neural progenitors identifies drug candidates that inhibit Zika virus infection in fetal‐like organoids and adult brain. Cell Stem Cell. 2017;21:274‐283.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sousa FTG, Nunes C, Sabino C, González‐cardenete MA. Anti‐Zika virus activity of several abietane‐type ferruginol analogues. J. Sao Paulo Inst. Trop. Med. 2020;62:2‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Haddad JG, Koishi AC, Gaudry A, et al. Doratoxylon apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells. Int J Mol Sci. 2019;20:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oliveira MBS, Valentim IB, Rocha TS, et al. Schinus terebenthifolius Raddi extracts: from sunscreen activity toward protection of the placenta to Zika virus infection, new uses for a well‐known medicinal plant. Ind. Crop. Prod. 2020;152:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Clain E, Haddad JG, Koishi AC, et al. The polyphenol‐rich extract from psiloxylon mauritianum, an endemic medicinal plant from reunion island, inhibits the early stages of Dengue and Zika virus infection. Int J Mol Sci. 2019;20:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kuo YT, Liu CH, Li JW, et al. Identification of the phytobioactive Polygonum cuspidatum as an antiviral source for restricting dengue virus entry. Sci Rep. 2020;10:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Clain E, Sinigaglia L, Koishi AC, et al. Extract from Aphloia theiformis, an edible indigenous plant from Reunion Island, impairs Zika virus attachment to the host cell surface. Sci Rep. 2018;8:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. da Silva TF, Ferraz AC, Almeida LT, et al. Antiviral effect of silymarin against Zika virus in vitro. Acta Trop. 2020;211:105613. [DOI] [PubMed] [Google Scholar]
- 96. Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Broad‐spectrum antiviral activity of Virazole: 1‐beta‐D‐ribofuranosyl‐1,2,4‐triazole‐3‐carboxamide. Science. 1972;177:705‐706. [DOI] [PubMed] [Google Scholar]
- 97. Li R. Marinopyrroles: unique drug discoveries based on marine natural products. Med Res Rev. 2016;36:169‐189. [DOI] [PubMed] [Google Scholar]
- 98. Pereira F. Have marine natural product drug discovery efforts been productive and how can we improve their efficiency? Expert Opin. Drug Discov. 2019;14:717‐722. [DOI] [PubMed] [Google Scholar]
- 99. Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep. 2019;36:122‐173. [DOI] [PubMed] [Google Scholar]
- 100. Abdelmohsen UR, Balasubramanian S, Oelschlaeger TA, et al. Potential of marine natural products against drug‐resistant fungal, viral, and parasitic infections. Lancet Infect Dis. 2017;17:e30‐e41. [DOI] [PubMed] [Google Scholar]
- 101. Singh SB, Barrett JF. Empirical antibacterial drug discovery‐‐foundation in natural products. Biochem Pharmacol. 2006;71:1006‐1015. [DOI] [PubMed] [Google Scholar]
- 102. Aubry F, Jacobs S, Darmuzey M, et al. Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nat Commun. 2021;12:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sheridan MA, Balaraman V, Schust DJ, Ezashi T, Michael Roberts R, Franz AWE. PLoS One. 2018;13:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vicenti I, Boccuto A, Giannini A, Dragoni F, Saladini F, Zazzi M. Comparative analysis of different cell systems for Zika virus (ZIKV) propagation and evaluation of anti‐ZIKV compounds in vitro. Virus Res. 2018;244:64‐70. [DOI] [PubMed] [Google Scholar]
- 105. Dowall SD, Graham VA, Rayner E, et al. PLoS Negl Trop Dis 10.1371/JOURNAL.PNTD.0004658 [DOI] [PMC free article] [PubMed]
- 106. Bershteyn M, Kriegstein AR. Cerebral organoids in a dish: progress and prospects. Cell. 2013;155:19‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sridhar A, Simmini S, Ribeiro CMS, et al. A perspective on organoids for virology research. Viruses. 2020;12:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Patridge E, Gareiss P, Kinch MS, Hoyer D. An analysis of FDA‐approved drugs: natural products and their derivatives. Drug Discov Today. 2016;21:204‐207. [DOI] [PubMed] [Google Scholar]
- 109. Mercado‐Reyes M, Acosta‐Reyes J, Navarro‐Lechuga E, et al. Dengue, chikungunya and zika virus coinfection: results of the national surveillance during the zika epidemic in Colombia. Epidemiol Infect. 2019;147:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Castanha PMS, Nascimento EJM, Braga C, et al. Dengue Virus‐Specific Antibodies Enhance Brazilian Zika Virus Infection. J Infect Dis. 2017;215:781‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin Microbiol Infect. 2012;18:946‐954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


