Abstract
Background
Chronic obstructive pulmonary disease (COPD) is diagnosed and its severity graded by traditional spirometric parameters (forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) and FEV1, respectively) but these parameters are considered insensitive for identifying early pathology. Measures of small airway function, including forced expiratory flow between 25% and 75% of vital capacity (FEF25-75), may be more valuable in the earliest phases of COPD. This study aimed to determine the prevalence of low FEF25-75 in ever-smokers with and without airflow limitation (AL) and to determine whether FEF25-75 relates to AL severity.
Method
A retrospective analysis of lung function data of 1458 ever-smokers suspected clinically of having COPD. Low FEF25-75 was defined by z-score<−0.8345 and AL was defined by FEV1/FVC z-scores<−1.645. The severity of AL was evaluated using FEV1 z-scores. Participants were placed into three groups: normal FEF25-75/ no AL (normal FEF25-75/AL−); low FEF25-75/ no AL (low FEF25-75/AL−) and low FEF25-75/ AL (low FEF25-75/AL+).
Results
Low FEF25-75 was present in 99.9% of patients with AL, and 50% of those without AL. Patients in the low FEF25-75/AL− group had lower spirometric measures (including FEV1 FEF25-75/FVC and FEV3/FVC) than those in the normal FEF25-75/AL− group. FEF25-75 decreased with AL severity. A logistic regression model demonstrated that in the absence of AL, the presence of low FEF25-75 was associated with lower FEV1 and FEV1/FVC even when smoking history was accounted for.
Conclusions
Low FEF25-75 is a physiological trait in patients with conventional spirometric AL and likely reflects early evidence of impairment in the small airways when spirometry is within the ‘normal range’. FEF25-75 likely identifies a group of patients with early evidence of pathological lung damage who warrant careful monitoring and reinforced early intervention to abrogate further lung injury.
Keywords: COPD epidemiology, Lung Physiology, Respiratory Measurement
WHAT IS ALREADY KNOWN ON THIS TOPIC
Studies have demonstrated that small airway dysfunction is prevalent in chronic obstructive pulmonary disease (COPD) and can be seen in some susceptible individuals without airflow limitation (AL), but studies using forced expiratory flow between 25% and 75% of vital capacity (FEF25-75) as measure of small airways function have generally included only a small number of patients with or at risk of developing COPD.
WHAT THIS STUDY ADDS
Low FEF25-75 is present in essentially in all of patients with AL and detected in 50% of those without AL, which was associated with lower lung function indices than those with normal spirometry. This highlights that low FEF25-75 is a physiological feature in patients with AL and likely signifies earlier lung injury in the small airways before classical AL of COPD is present.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Low FEF25-75 without AL might detect a group of patients at risk of developing COPD, where evidence-based preventative strategies could be emphasised and implemented, thereby avoiding progressive lung damage.
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease most commonly caused by significant exposure to noxious particles and, pathophysiologically, includes small airway disease and parenchymal destruction.1–4 COPD is diagnosed based on subjective (respiratory symptoms, history of exposure to risk factors) and objective (physiologically by spirometry) assessments.5 The Global Initiative for Obstructive Lung Disease (GOLD), defines airflow limitation (AL) using a fixed forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio and severity defined by FEV1 % predicted.5 Other bodies recommend using the lower limit of normal (LLN) based on z-scores for the ratio to define AL and stratify the severity of the disease as this is thought to be less biased at the extremes of age.6 7
COPD is a slowly progressive disease in most individuals8 and FEV1/FVC and FEV1 lack the diagnostic sensitivity to identify early lung pathology.9 10 As only a proportion of smokers develop COPD,11 identifying individuals with early lung damage who are most at risk of developing overt COPD would enable a focused effort to prevent pathological progression.
The role of small airways in COPD has been explored in several studies.3 12–14 Small airways loss precedes the development of emphysema and AL in pathological studies investigated by microcomputed tomographic radiology.2 3 12 Further, in a longitudinal study of alpha-1 antitrypsin deficiency (AATD) patients using forced expiratory flow between 25% and 75% of vital capacity (FEF25-75) as a measure of small airway,15 a reduced FEF25-75 without AL was associated with worse health status and a faster subsequent decline in FEV1 and appeared to precede AL defined by spirometry.15 This, and other studies, suggest that measures of small airways function (SAF; especially FEF25-75) may be more sensitive to early damage than traditional spirometric measures.16–20
We hypothesised that low FEF25-75, would be ubiquitous in patients with AL, as this has been demonstrated to precede the development of AL.21 22 Furthermore, we hypothesised that patients with low FEF25-75 but without AL would have physiological indicators of the risk of developing AL, even after the correction for potential confounders such as smoking history.
The study had five main aims:
To investigate the prevalence of low FEF25-75 in cigarette smokers with and without AL.
To assess whether low FEF25-75 without AL was associated with lower lung function measurements within the normal range, which might reflect an increased risk for developing AL.
To assess the relationships between FEF25-75 and other spirometric measures.
To assess the relationships between FEF25-75 and AL severity in established COPD.
To determine whether the presence of low FEF25-75 without AL was associated with lower lung function measurements, even after correction for potential confounders.
Methods
Study design and setting
This was a retrospective, cross-sectional study of anonymised data from patients known to have or suspected of having COPD who underwent routine pulmonary function test at University Hospitals Birmingham National Health Service Foundation Trust, UK. The study included data obtained between 1 January 2016 and 30 April 2021 and all patients who had lung function during this period were screened for inclusion.
Eligibility criteria
All participants attending for lung function within the study period with the following included:
Symptoms suggestive of COPD (breathlessness and/or a persistent cough).
Age 30 years or older.
>10 pack-years history of cigarette smoking.
Either a confirmed diagnosis or suspected of having COPD by a senior physician.
All traditional spirometric measures including FEF25-75 were reported.
Participants were excluded if they had COPD related to AATD, a history/diagnosis of other chronic lung diseases or significant structural changes in the lung (such as bronchiectasis) defined radiologically. Patients with emphysema identified radiologically; however, were not excluded.
Study measures
Patients’ demographic data were collected. Smoking history included smoking status at the time of testing (ex-smoker or current smoker), pack-years history and years since quitting smoking. The smoking exposure was categorised into light (<20 pack-year), moderate (20–40 pack-years) and heavy (>40 pack-year).23 Regular medication use was documented.
FEV1, FVC, FEV1/FVC, FEF25-75, FEV in the first 3 s (FEV3), and FEV3/FVC were assessed. Corrected FEF25-75 for lung volume (FEF25-75/FVC) was also assessed.17 Lung function assessments used the Ultima PF Pulmonary Lung Function System (Medical Graphics UK, Tewkesbury, UK) and were performed in accordance with national guidelines.24 In this study, predicted values for routine spirometric measures were derived from the European Community for Steel and Coal.25 The z-score for the routine spirometric measures were calculated using the Global Lung Function Initiative 2012 formula.7
The z-scores for FEF25-75 and FEV1/FVC were used to define abnormality. A cut-off z-score for normality for FEF25-75 was chosen of −0.8435 as this has shown to predict COPD development.21 The LLN (ie, z-score −1.645) was used for FEV1/FVC to define AL, as recommended in the American Thoracic Society/European Respiratory Society guidelines.6 7 Using these thresholds, participants were grouped into three groups: normal FEF25-75/ no AL (normal FEF25-75/AL−); low FEF25-75/ no AL (low FEF25-75/AL−); and low FEF25-75/ AL (FEF25-75/AL+). AL severity was defined using FEV1 z-score,26 to classify five severity groups.
FEF25-75 z-score was compared with z-scores of other physiological measures where available.
Statistical analysis
Statistical analysis was performed using IBM SPSS software (V.26). Data were not normally distributed, hence Kruskal-Wallis H tests were used throughout with the median and IQR reported. Where Kruskal-Wallis H tests were significant, a Mann-Whitney U test was conducted. For variables used in group definitions (FEF25-75 and FEV1/FVC), no statistical analysis was conducted, except where the definition did not cause the variable to differ. Here, Mann-Whitney U tests was performed to determine the differences. Categorical variables were assessed using χ2 or Fisher’s exact test, with the count and percentage reported. The relationship of FEF25-75 z-score with z-score of other physiological measures and whether smoking behaviours have impact on the relationships were assessed using weight least-square regression. Coefficient of determination (r2) was reported throughout. Curvilinear regression was used to determine the relationship between FEF25-75 % predicted or FEF25-75/FVC with % predicted or ratio of other physiological measures, with r2 reported throughout.
Logistic regression was performed to identify factors associated with the presence of low FEF25-75. χ2 and Mann-Whitney U test were used to identify relevant univariable risk factors and significant variables were included in the univariate logistic regression and ORs with 95% CIs reported. Significant variables in univariate analyses were included in the subsequent multivariate analysis. Variables, which were associated with multicollinearity (defined by variable inflation factor (VIF) >10) with other variables, were not included in the multivariate logistic regression. A p<0.05 was considered statistically significant throughout. For group comparisons, p values were adjusted using the Benjamini-Hochberg method27 with adjusted p value significance level set at p<0.05. No power calculations were conducted for this pragmatic study.
Patient and public involvement
Patients and/or the public did not take part in the development, conduct, reporting or dissemination of this study.
Results
Participant’s selection
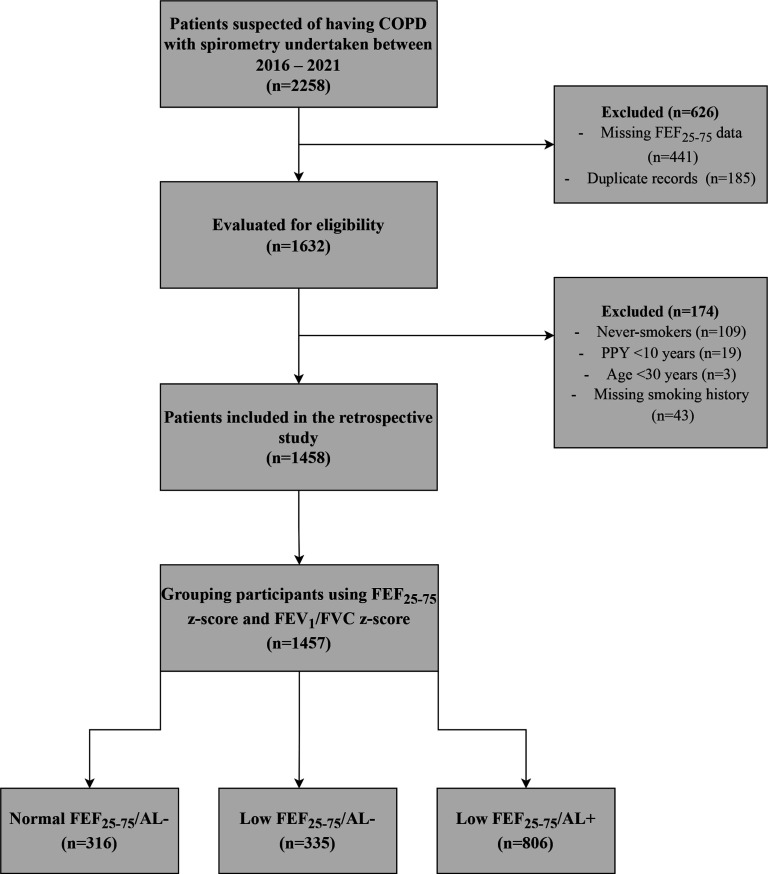
On initial screening, the dataset included 2258 records. After assessing for eligibility, 1458 ever-smokers were included (see figure 1 for a flow chart including reasons for exclusion). These participants were placed into the three groups based on the predefined criteria: normal FEF25-75/AL− (n=316); low FEF25-75/AL− (n=335) and low FEF25-75/AL+ (n=806). One participant did not meet any of the grouping criteria and was therefore excluded from the final analysis.
Figure 1.
Flow chart of the retrospective study. This figure shows the selection of patients according to eligibility criteria. One participant did not meet any of the group definition and was therefore not included in the grouping analysis. AL, airflow limitation; COPD, chronic obstructive pulmonary disease; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PPY, pack per year.
Prevalence of low FEF25-75
All but one participant with AL had low FEF25-75 (806/807; 99.9%). Of those without AL, 51.4% (335/650) had low FEF25-75.
Demographics and clinical characteristics
Baseline demographics for the eligible participants and groups are shown in table 1. The average age was higher in low FEF25-75/AL+ group (median 65 years; IQR 58–73) vs both normal FEF25-75/AL− group (median 63 years (IQR 54.75–72); p=0.012) and low FEF25-75/AL− group (median 63 years (IQR 54.75–72); p=0.025). There were no differences in sex across groups. Body mass index (BMI) was lower (p<0.001) in low FEF25-75/AL+ group than both normal FEF25-75/AL− group (median BMI 25.67; IQR 21.88–29.82 vs 30.20; IQR 25.34–34.71) and low FEF25-75/AL− group (median BMI 28.94; IQR 25.33–34.071).
Table 1.
Baseline demographics of the included participants
| Variable | Total n=1458 |
Normal FEF25-75/AL− n=316 |
Low FEF25-75/AL− n=335 |
Low FEF25-75/AL+ n=806 |
| Age (years) | 64 (56.75–72) | 63 (54.75–72) | 63 (54.75–72) | 65 (58–73)*† |
| Sex (male: female) n | 744: 714 | 168: 148 | 150: 184 | 425: 382 |
| Race (n, %) | ||||
| Caucasian | 1382 (94.8) | 286 (90.5)†‡ | 321 (96.1) | 774 (94.8) |
| Black | 22 (1.5) | 9 (2.8)† | 1 (0.3) | 12 (1.5) |
| Asian | 49 (3.4) | 19 (6.0)‡ | 11 (3.3) | 19 (2.3) |
| Others | 5 (0.3) | 2 (0.6) | 1 (0.3) | 2 (0.2) |
| Smoking status (n, %) | ||||
| Current smokers | 842 (57.8) | 163 (51.6)‡ | 197 (59) | 482 (59.7) |
| Ex-smokers | 616 (42.2) | 153 (48.4)‡ | 137 (41) | 325 (40.3) |
| Smoking exposure (n, %) | ||||
| Light | 216 (14.8) | 73 (23.1)†‡ | 43 (12.8) | 100 (12.4) |
| Moderate | 568 (39) | 138 (43.7) | 133 (39.7) | 297 (36.8) |
| Heavy | 673 (46.2) | 105 (33.2)†‡ | 159 (47.5) | 409 (50.7) |
| Pack-year | 40 (25–55) | 31 (20–45)†‡ | 40 (26–55) | 41 (28–59) |
| Years quit§ | 10 (3–20) | 11 (4–24.50)‡ | 10 (4–20) | 8 (3–15) |
| BMI (kg/m2) | 27.32 (23.09–31.95) | 30.20 (25.34–34.71) | 28.94 (25.33–34.07) | 25.67 (21.88–29.82)*† |
Data are presented as median and IQR, unless otherwise stated.
In the group comparisons, the significance level for adjusted p value was set at 0.05.
*Significantly different from low FEF25-75/AL−.
†Significantly different from low FEF25-75/AL+.
‡Significantly different from normal FEF25-75/AL−.
§Only assessed in ex-smokers.
AL, airflow limitation; BMI, body mass index; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Participants in normal FEF25-75/AL− group had generally smoked less (less heavy smokers and a lower pack-year history) compared with low FEF25-75/AL− group and low FEF25-75/AL+ group, with no differences between the latter 2.
Expectedly, patients in low FEF25-75/AL+ group used more COPD-associated medications than those in normal FEF25-75/AL− group or low FEF25-75/AL− group, including short-acting beta-2 agonists (SABA), inhaled corticosteroids (ICS)/long-acting beta-2 agonists (LABA) and long-acting muscarinic antagonists (LAMA) (p<0.001 for all). Interestingly, participants in low FEF25-75/AL− group used more COPD medications (including SABA and ICS/LABA) than normal FEF25-75/AL− group (p<0.001 for all). Details of the medications used across groups are provided in online supplemental table E1.
bmjresp-2022-001385supp001.pdf (1.3MB, pdf)
Physiological assessment of lung function
Table 2 shows the baseline spirometric measures for the three groups. All spirometric measures were lower in low FEF25-75/AL− group than normal FEF25-75/AL− group (p<0.001).
Table 2.
Baseline spirometric measures of the included participants
| Variable | Total n=1458 |
Normal FEF25-75/AL− n=316 |
Low FEF25-75/AL− n=335 |
Low FEF25-75/AL+ n=806 |
| FEV1 | ||||
| z-score | −2.09 (−3.16 to −1.11) | −0.44 (−1.00 to 0.20) | −1.67 (−2.26 to −1.18)* | −2.97 (−3.70 to −2.12)*† |
| % predicted | 67.05 (47.65 to 84.12) | 93.68 (85.60 to 103.92) | 74.05 (64.50 to 82.43)* | 50.91 (37.25 to 66.36)*† |
| FVC | ||||
| z-score | −0.50 (−1.15 to 0.20) | −0.50 (−1.15 to 0.20)†‡ | −1.34 (−2.02 to −0.63) | −1.19 (−2.02 to −0.35) |
| % Predicted | 84.43 (71.96 to 96.75) | 93.16 (83.37 to 103.37)†‡ | 80.44 (70.60 to 91.37) | 82.05 (69.22 to 95.38) |
| FEV1/FVC ratio§ | ||||
| z-score | −1.93 (−3.34 to −0.63) | 0.09 (−0.30 to 0.46) | −1.00 (−1.33 to −0.60)* | −3.18 (−4.11 to −2.34) |
| % | 63 (48 to 74) | 79 (76 to 83) | 71 (68 to 75)* | 50 (39 to 59) |
| FEF25-75§ | ||||
| z-score | −1.96 (−2.80 to −1.01) | −0.16 (−0.49 to 0.25) | −1.37 (−1.67 to −1.11) | −2.72 (−3.2 to −2.18)† |
| % Predicted | 40.56 (21.08 to 67.58) | 95.25 (83 to 110.51) | 56.02 (47.76 to 63.70) | 22.74 (14.80 to 33.76)† |
| FEF25-75/FVC ratio | 48.28 (27.25 to 79.86) | 104.80 (90.10 to 122.99) | 68.02 (59.16 to 79.86)* | 28.73 (19.90 to 40.47)*† |
| FEV3/FVC ratio | 85.44 (74.22 to 92.26) | 94.65 (92.10 to 96.83) | 90.75 (87.73 to 93.46)* | 75.13 (64.70 to 82.67)*† |
Data are presented as median and IQR. In the groups’ comparisons, the significance level for adjusted p value was set at 0.05.
*Significantly different from normal FEF25-75/AL−.
†Significantly different from low FEF25-75/AL−.
‡Significantly different from low FEF25-75/AL+.
§Statistical test was only undertaken for differences between groups where a definition did cause the variable to differ.
AL, airflow limitation; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expiratory volume in 1 s; FEV3, FEV in 3 s; FVC, forced vital capacity.
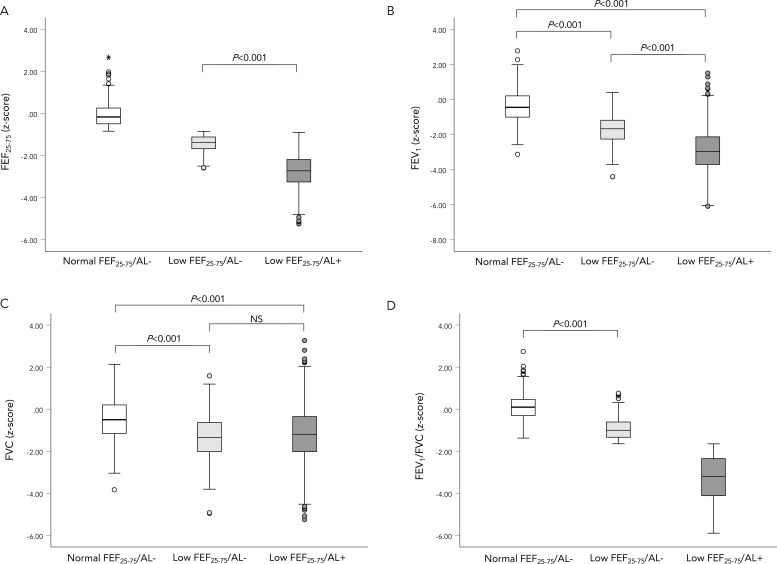
Participants in low FEF25-75/AL+ group had lower lung function (p<0.001 for all comparisons) than both low FEF25-75/AL− group and normal FEF25-75/AL− group. FVC z-score and FVC % predicted did not differ between low FEF25-75/AL+ group and low FEF25-75/AL− group. The distribution of FEF25-75 z-score, FEV1 z-score, FEV1/FVC z-score and FVC z-score across groups are shown graphically in figure 2. The distribution of FEF25-75% predicted, FEF25-75/FVC, FEV1 % predicted, FVC % predicted, FEV1/FVC ratio and FEV3/FVC ratio across groups are shown in online supplemental figure E1.
Figure 2.
Distribution of spirometric measures across study groups. A box plot demonstrating the distribution of z-scores of spirometric measures across groups. The plot shows median, IQR, minimum and maximum. (A) The distribution of FEF25-75 z-score across groups. (B) The distribution of FEV1/FVC z-score across groups. (C) The distribution of FEV1 z-score across groups. (D) The distribution of FVC z-score across groups. For figures (A, D), statistical test was only done for differences between groups where a definition did cause the variable to differ, and the reported p values are for the Mann-Whitney U test. For figures (B, C), the presented p values are for Mann-Whitney U test, and the Kruskal Wallis tests p values for both figures were<0.001. AL, airflow limitation; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; NS, not significant.
The relationship of FEF25-75 with AL severity
Participants with AL were grouped according to AL severity. Table 3 summarises baseline demographics and measures of small airways of these participants. In this cohort, patients with very severe disease were younger than those with lesser severity (p<0.001 for all comparisons). There were no differences between subgroups for sex or ethnicity, although BMI was lower in patients with very severe disease compared with moderately severe patients (median BMI 23.43 (IQR 19.62–28.73) vs 26.99 (IQR 22.85–30.36), p=0.01). Of note, smoking status and pack-year history did not differ across severity groups but those with the most severe disease had stopped smoking later than the other groups.
Table 3.
Baseline demographics and FEF25-75 across AL severity
| Variable | Mild n=177 |
Moderate n=111 |
Moderately severe n=120 |
Severe n=263 |
Very severe n=135 |
| Age (years) | 65 (57–75) | 67 (60–75) | 67 (58.50–74) | 69 (61–73) | 59 (53–64)*†‡§ |
| Sex (male: female) n | 92: 86 | 50: 61 | 72: 48 | 138: 125 | 73: 62 |
| Smoking status (n, %) | |||||
| Current smokers | 113 (63.5) | 59 (53.2) | 72 (60) | 159 (60.5) | 79 (58.5) |
| Ex-smokers | 65 (36.5) | 52 (46.8) | 48 (40) | 104 (39.5) | 56 (41.5) |
| Pack-year | 40 (26.75–55) | 41 (25–53) | 43 (29–60) | 44 (30–62) | 38 (23–63) |
| Years quit | 12 (3–21.50) | 9 (3–16) | 9 (2.25–19.50) | 7 (3–14) | 5 (2–10)* |
| BMI (kg/m2) | 25.78 (22.96–28.68) | 26.17 (21.29–30.42) | 26.99 (22.85–30.36) | 25.52 (21.92–30.66) | 23.43 (19.62–28.73)‡ |
| FEF25-75 | |||||
| z-score | −1.94 (−2.18 to −1.69) | −2.28 (−2.57 to −2.07)* | −2.56 (−2.82 to −2.32)*† | −3.01 (−3.26 to −2.78)*†‡ | −3.77 (−4.11 to −3.52)*†‡§ |
| % Predicted | 40.50 (33.74 to 48.48) | 32.50 (26.49 to 38.56)* | 25.76 (21.40 to 29.61)*† | 17.60 (13.95 to 21.62)*†‡ | 10.32 (8.76 to 13.67)*†‡§ |
| FEF25-75/FVC | 41.93 (30.95 to 48.58) | 38.11 (29.23 to 47.09) | 31.61 (24.04 to 40.27)*† | 23.28 (18.08 to 31.43)*†‡ | 15.68 (13.26 to 22.33)*†‡§ |
Data are presented as median and IQR unless otherwise stated. Severity of AL are stratified using FEV1 z-score.
In the groups’ comparisons, the significance level for adjusted p value was set at 0.05.
*Significantly different from mild.
†Significantly different from moderate.
‡Significantly different from moderately severe.
§Significantly different from severe.
AL, airflow limitation; BMI, body mass index; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV3, forced expiratory volume in 3 s; FVC, forced vital capacity.
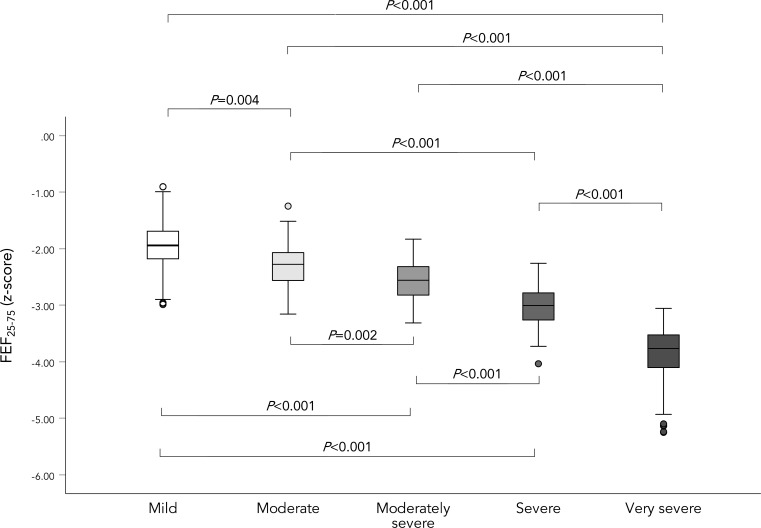
FEF25-75 z-score worsened in a stepwise manner as the severity of AL increased (p<0.001; see figure 3). Of note, even in mild AL, FEF25-75 % predicted was substantially impaired (median 40.50% (IQR 33.74–48.48) and 41.93% (IQR 30.95–48.58) for FEF25-75 /FVC; see online supplemental figure E2).
Figure 3.
Distribution of FEF25-75 z-score across AL severity. A box plot demonstrating the distribution of FEF25-75 z-score across AL severity. The plot shows median, IQR, minimum and maximum. AL severity was assessed using FEV1 z-score. The presented p values are for Mann-Whitney U test, and the Kruskal Wallis tests p value was <0.001 for all. AL, airflow limitation; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity.
The relationship of FEF25-75 with other lung function parameters
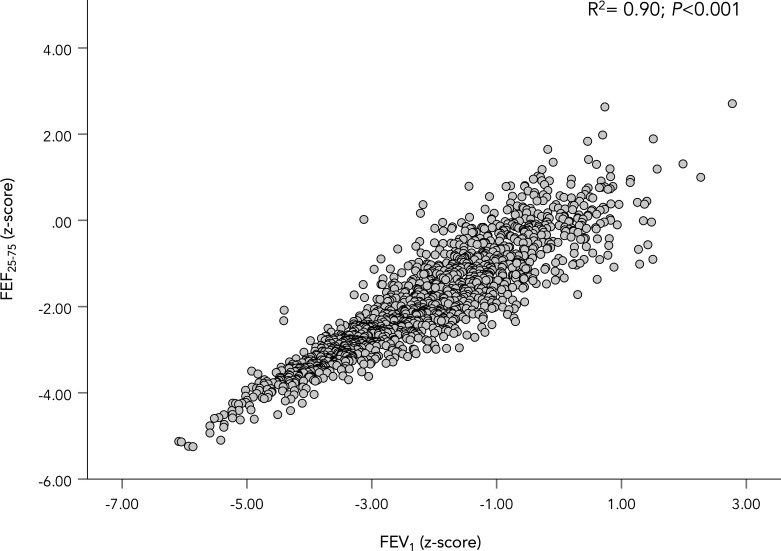
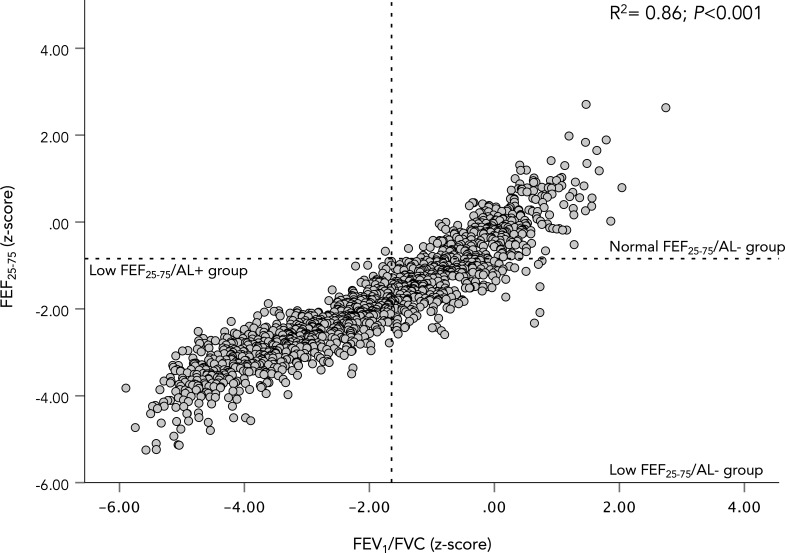
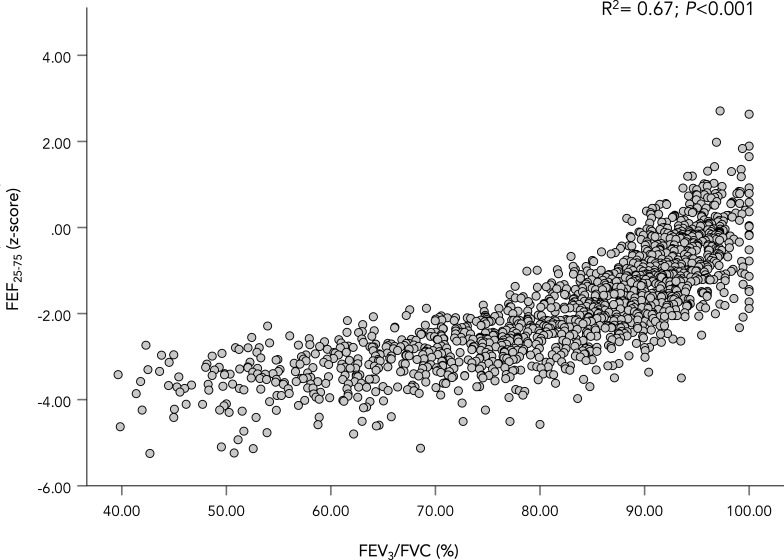
Including all participants (n=1458), FEF25-75 z-score demonstrated a strong relationship to FEV1 (r2=0.90, p<0.001; see figure 4) and FEV1/FVC z-score (r2=0.86, p<0.001; see figure 5), but a weaker relationship to FVC z-score (r2=0.17, p<0.001). FEF25-75 z-score also demonstrated strong relationship to FEV3/FVC % (r2=0.69, p<0.001; see figure 6).
Figure 4.
FEV1 z-score plotted against FEF25-75 z-score. A scatter plot showing the relationship between FEF25-75 z-score and FEV1 z-score. The coefficient of determination (r2) for the WLS regression is shown in the figure along with its p value. FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expired volume in 1 s; WLS, weight-least square.
Figure 5.
FEV1/FVC z-score plotted against FEF25-75 z-score. A scatter plot showing the relationship between FEF25-75 z-score and FEV1/FVC z-score. The plot is divided according to groups definition. The coefficient of determination (r2) for the WLS regression is shown in the figure along with its p value. AL, airflow limitation; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expired volume in 1 s; FVC, forced vital capacity; WLS, weight-least square.
Figure 6.
FEV3/FVC plotted against FEF25-75 z-score. A scatter plot showing the relationship between FEF25-75 z-score and FEV3/FVC. The coefficient of determination (r2) for the WLS regression is shown in the figure along with its p value. FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expired volume in 1 s; FVC, forced vital capacity; WLS, weight-least square.
In the multiple regression analyses (n=1458; accounting for pack-year history), FEF25-75 z-score showed strong relationship to FEV1 z-score (r2=0.90, p<0.001) and FEV1/FVC (r2=0.86, p<0.001), and weak relationship to FVC z-score (r2=0.18, p<0.001). Pack-years was not a statistically significant predictor in any of the regression models.
In the multiple regression analysis for ex-smokers (n=616; accounting for years since quitting smoking), FEF25-75 z-score showed a strong relationship to FEV1 z-score (r2=0.89, p<0.001) and FEV1/FVC z-score (r2=0.88, p<0.001), and a weak relationship to FVC z-score (r2=0.22, p<0.001). Years since quitting was a significant predictor in all models (p<0.001 for all models, except in the model for FEV1 z-score p=0.017).
FEF25-75 % predicted and FEF25-75/FVC ratio also showed strong relationship to other spirometric measures, presented in online supplemental table E3. The relationships of FEF25-75 % predicted and FEF25-75/FVC with FEV1/FVC and FEV1 % predicted are graphically shown in online supplemental figures E3 and E4.
The association of the presence of low FEF25-75 with low lung function measurements
A regression model was built to assess whether the presence of low FEF25-75 without AL was associated with lower lung function measurements (see table 4). In the univariate analysis, pack-years, sex, FEV1 z-score, FVC z-score and FEV1/FVC z-score were significant factors related to the presence of low FEF25-75. All significant variables were included in the multivariate analysis except FVC z-score because of multicollinearity with other spirometric measures (VIF=30.94). The multivariate analysis demonstrated that females had a 33.22 times higher OR of having low FEF25-75 compared with males (95% CI, 8.19 to 134.72). The multivariate analysis also showed that the presence of low FEF25-75 was associated with a lower FEV1 z-score and FEV1/FVC z-score even when in the normal range. Of the significant factors in univariate analysis, pack-years was no longer significant in the multivariate analysis.
Table 4.
Logistic regression of the association of the presence of low FEF25-75 with low lung function measurement in participants without AL
| Variable | Univariate | Multivariate* | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.004 | 0.991 to 1.018 | 0.55 | |||
| Pack-years | 1.009 | 1.003 to 1.015 | 0.002 | 0.988 | 0.971 to 1.005 | 0.168 |
| Smoking status† | ||||||
| Current smokers | 1.340 | 0.983 to 1.827 | 0.064 | |||
| Sex‡ | ||||||
| Female | 1.383 | 1.016 to 1.883 | 0.039 | 33.225 | 8.194 to 134.723 | <0.001 |
| FEV1 z-score | 0.136 | 0.100 to 0.185 | <0.001 | 0.001 | 0.00008 to 0.005 | <0.001 |
| FEV1/FVC z-score | 0.043 | 0.027 to 0.068 | <0.001 | 0.00001 | 0.000001 to 0.0003 | <0.001 |
| FVC z-score | 0.449 | 0.377 to 0.536 | <0.001 | |||
This tables demonstrate the logistic regression of the association of the presence of low FEF25-75 with low lung function measurements in participants without AL (n=651 (those normal FEF25-75 n=316 vs those with low FEF25-75 n=335)).
Low FEF25-75 was defined by z-score<−0.8435.
Statistically significant p values are written in bold.
*The multivariate regression model showed a Nagelkerke R2=0.942 and Hosmer-Lemeshow p value=0.999.
†The reference category was ex-smokers.
‡The reference category was male.
AL, airflow limitation; BMI, body mass index; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Discussion
This cross-sectional study of commonly measure of SAF (FEF25-75) in smokers suspected of having COPD highlights four important points.
First, low FEF25-75 (considered indicative of impairment in the small airways) is a constant feature of those who have developed AL, with and without correction for FVC.
Second, there was a significant reduction in FEF25-75 even in mild AL, suggesting a substantial disruption of SAF prior to crossing the AL diagnostic criteria. Indeed, once AL is established, there is a strong association between FEF25-75 z-score across AL severity.
Third, evidence of low FEF25-75 is common (51.4%) in symptomatic ever-smokers even without AL and is associated with lower lung function parameters (even while in the normal range) compared with those with normal FEF25-75 and normal FEV1/FVC. This suggests that even when routine spirometry appears ‘normal’, those with low FEF25-75 may have physiological evidence suggesting decline compared with health. This group of patients likely have early lung injury reflecting small airway impairment. Our data support the notion that such patients may form a cohort that would benefit from close monitoring, to ascertain progression potentially leading to COPD and support to mitigate such an outcome.
Fourth, the relationship between FEF25-75 and FEV1 and FEV1/FVC is maintained even following adjustment for smoking history, indicating it is independent of cigarette load. Further, the logistic regression demonstrated that the presence of low FEF25-75 was associated with lower FEV1 and FEV1/FVC, after correcting for smoking status. This suggests there are a group of smokers who are pathophysiologically different, consistent with a ‘susceptible’ cohort. Further study is needed to understand the mechanisms underpinning this potential susceptibility.
In the regression model, sex was related to low FEF25-75 in the absence of AL, with females 33 times more likely to have low FEF25-75, although with a wide 95% CI. In the AATD study by Stockley et al there was also a higher proportion of females with low FEF25-75 than males compared with those with normal spirometry and AL.15 This study and the AATD study highlight that females have a greater likelihood of a low FEF25-75 in the absence of AL. Given that females with COPD have greater small airway impairments than males28 and females are at higher risk of developing COPD than males with similar smoking histories,29 our finding and those of Stockley et al indicate that low FEF25-75 (which is likely suggestive of impairment in the small airways) is likely to be greater in females before developing overt AL. Studies have reported that females have small tracheal cross-sectional area compared with males.30 31 This may be similar throughout the bronchial tree explaining why females are most likely to have low FEF25-75 without AL than males. However, confirming this will require more comprehensive studies.
In the current study, age was higher in the low FEF25-75/AL+ group than the normal FEF25-75/AL− group and low FEF25-75/AL− group, but was reduced in those with very severe AL compared with all other severities of AL. In a complex disease such as COPD, decline rates are variable. Age (as a surrogate of time) might account for some of the differences in baseline lung function between the low FEF25-75/AL− group and low FEF25-75/AL+ group. However, age was not a significant factor accounting for the presence of low FEF25-75 in multivariate regression modelling. The contribution of ageing on the presence of low FEF25-75 can only be confirmed by longitudinal follow-up, which would also enhance our understanding of the relationship between small and large airways function in COPD and might support new monitoring and treatment strategies.
Smoking exposure was similar between low FEF25-75/AL− group and low FEF25-75/AL+ group and did not differ across increasing AL severity (as grouped by FEV1 z-score) nor was associated with low FEF25-75 in multivariate analysis. These results suggest that smoking exposure alone cannot explain the physiological differences between groups. Tsushima et al reported similar findings, demonstrating that smokers with COPD had similar pack-year history compared with those designated at-risk of COPD,16 although Mirsadraee et al suggested this reflected a lower smoke exposure.17 This latter study used GOLD criteria and % predicted to define groups while our study used the z-scores to define abnormality in FEV1/FVC and FEF25-75. The physiological criteria used may account for some differences in study findings.
The FEV3/FVC has been used to detect mild lung injury in the absence of AL.32 Morris et al reported that, compared with those with normal FEV3/FVC, patients with a lower ratio had lower FEV1, higher residual volume (RV)/total lung capacity (TLC), higher RV, higher TLC and lower transfer factor for carbon monoxide (TLco), potentially highlighting the presence of early physiological impairment including air trapping and impaired gas exchange.32 Our study demonstrated that FEV3/FVC was lower (although within normal range) in the low FEF25-75/AL− group than in normal FEF25-75/AL− group and was strongly associated with the FEF25-75 z-score, providing further support that the FEF25-75 z-score is likely detecting early lung pathology in this group. FEF25-75/FVC has also been used in the early detection of COPD17 and again this measure was also lower in low FEF25-75/AL− group, further supporting the FEF25-75 z-score.
This study found that the use of ICS/LABA was as high in the low FEF25-75/AL− group as in the low FEF25-75/AL+ group, despite no AL in former group. This contradicts the recommendation by NICE guidelines that the use of ICS/LABA should be for those with spirometrically confirmed AL.33 Therefore, the absence of AL in low FEF25-75/AL− group raises concern regarding the reason for prescribing such high levels of ICS/LABA. ICS/LABA combinations contains high dose of ICS characterised by high potency, and adverse effects, including community-acquired pneumonia, glucose dysregulation and adrenal suppression.34 There are two possible reasons why patients in low FEF25-75/AL− group are prescribed ICS/LABA. First, the current study used the LLN to define AL, whereas the fixed 70% cut-off is still widely used in clinical practice. This could explain that some patients were given ICS/LABA following the confirmation of AL using the fixed ratio cut-off. Second, given the lack of evidence on how to treat patients with symptoms of COPD despite no AL, the patients might have experienced worse respiratory symptoms, requiring physicians to escalate therapy, by the addition of ICS. Whether using COPD medications (and especially ICS) to treat patients without AL is of benefit in the patients described here, requires appropriate randomised control trials. An RCT by Han et al is ongoing, which evaluates using LABA/LAMA in patients with COPD symptoms but no AL to determine whether such medication is effective in such patients35 and the same should be done with ICS.
Several studies have assessed FEF25-75 in COPD. FEF25-75 % predicted was lower (though not necessarily abnormal) in patients at risk of developing COPD.16 Correction of FEF25-75 for FVC also identifies early pathological changes prior to COPD development17 and expiratory flow rates (including FEF25-75) detected abnormality in those with normal FEV1/FVC.36 Our findings, together with other studies strengthen FEF25-75 (expressed as either % predicted or z-score) as a valuable marker of impairment in the small airways before classically defined AL is present.15–17 21 36
Concerns about the use of FEF25-75 in clinical management have been raised, for example, in a large cross-sectional study using FEF25-75 z-score.37 That study concluded that FEF25-75 did not provide additional information to current spirometric measures used in clinical practice, which contrasts with the close relationship demonstrated in our study. However, the study by Quanjer et al37 included a large and mixed population of participants including a variety of lung diseases. The lack of utility of a test in a general population does not negate its use in a selected one, a concept supported in the study of a highly selected population (AATD), where low FEF25-75 % predicted in the absence of AL was associated with a reduced health status and a subsequent faster decline in lung function.15 In addition, that study suggested that low FEF25-75 preceded the development of macroscopic emphysema, a classic component of the PiZZ genetic variant.
A 10-year longitudinal study demonstrated that non-AATD patients with low FEF25-75 z-score had a higher incidence rate of developing COPD than those with normal FEF25-75 z-score (41.8% vs 7.4%, p<0.001).21 The authors used the same normality cut-off for as used in the current study.21 Considering that small airways dysfunction seems to precede AL15 and the fact that loss of >70% of small airways has to occur before COPD becomes detectable by FEV1/FVC,12 patients with FEF25-75 z-score<−0.8435 described by Kwon et al possibly had impairment in their small airways that would have worsened over time due to the continual exposure to risk factors, leading to the development of AL.21
Our study provides evidence to support the use of FEF25-75 (expressed as z-score) as an assessment tool in patients potentially at risk of developing COPD. We suggest that patients with FEF25-75 <-0.8453 should be considered a phenotypic group that likely reflects early impairment in the small airways. This group of patients should be monitored and early preventive measures (most importantly, smoking cessation) should be objectively supported and encouraged especially when there is progression. In this group, the reduction of environmental-related exposure (ie, pollution, work related exposure and biomass fuel exposure) may also be beneficial in stabilising progression to COPD. Moreover, pharmacological treatments such as extra-fine particles inhalers may be of particular use in this group, as they achieve higher deposition in the small airways.38–40 However, this concept clearly requires further research to determine whether such treatments are of value for this group. Other measures of small airways have also demonstrated value in the early detection of COPD.18 20 41 In this study, we chose FEF25-75 because of its availability already in routine physiological assessment.
Our study has limitations. It was a cross-sectional study but the value of FEF25-75 as a monitoring tool has also been demonstrated longitudinally15 21 22 and our study provided a larger sample confirming the prevalence of low FEF25-75 in smokers with and without AL. FEF25-75 is a highly variable spirometric measure but we used FEF25-75 z-score to optimise the interpretive accuracy. This was also a retrospective study, meaning that available data were limited to routine lung function tests, although this is more representative of the real-world approach to such strategies. Studies have shown that RV/TLC is also a potential marker for SAF.42–45 However, the data analysed in this study was limited to spirometric measures and did not include lung volumes such as RV and TLC. Therefore, further studies should evaluate whether low FEF25-75 is associated with low RV/TLC. We pragmatically used<−0.8453 z-score cut-off to define low FEF25-75, which is different from the LLN for other lung function parameters. The ERS/ATS guidelines highlight that no satisfactory outcome-based thresholds for lung function have been defined and that further research is needed to establish a comprehensive disease-specific clinical approach to interpretation.46 The chosen cut-off for our study has also been used by others and shown to significantly predict COPD development,21 indicating it likely reflects early impairment in the small airways.
In conclusion, low FEF25-75 z-score is a physiological feature present in patients with AL and also in symptomatic patients in the absence of AL. These findings highlight the potential importance of FEF25-75 as marker of small airways impairment, and importantly, in the detection of early pathological features of COPD. FEF25-75 is part of routine lung function assessment, and therefore, closely monitoring patients with low FEF25-75 and considering early interventions may be central to improving health and prognosis.
Footnotes
Contributors: NYA was the study's guarantor, responsible for conducting the study, had access to the data, and controlled the decision to publish the study. NYA and ES designed and planned the study. NYA and MA analysed the data. NYA wrote the initial manuscript. ES, RAS and JS reviewed the data and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding: This study was conducted as part of PhD studentship for NYA. NYA is supported by King Saud bin Abdulaziz University for health sciences through the Saudi Arabian Cultural Bureau in the UK.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The lung function data used and evaluated during this study can be made available from the corresponding author, NYA, on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The data study was approved by the Health Research Authority (HRA – project number 274729) and the South Birmingham Research Ethics Committee (Reference number 20/WM/0024).
References
- 1.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–53. 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968;278:1355–60. 10.1056/NEJM196806202782501 [DOI] [PubMed] [Google Scholar]
- 3.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567–75. 10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koo H-K, Vasilescu DM, Booth S, et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med 2018;6:591–602. 10.1016/S2213-2600(18)30196-6 [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease . The global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report, 2022. Available: https://goldcopd.org/2022-gold-reports/ [Accessed 21 Feb 2022].
- 6.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948. 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 7.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011;365:1184–92. 10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 9.Herpel LB, Kanner RE, Lee SM, et al. Variability of spirometry in chronic obstructive pulmonary disease: results from two clinical trials. Am J Respir Crit Care Med 2006;173:1106–13. 10.1164/rccm.200506-975OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennock BE, Rogers RM, McCaffree DR. Changes in measured spirometric indices. What is significant? Chest 1981;80:97–9. 10.1378/chest.80.1.97 [DOI] [PubMed] [Google Scholar]
- 11.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645–8. 10.1136/bmj.1.6077.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest 2013;143:1436–43. 10.1378/chest.12-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosken CH, Wiggs BR, Paré PD, et al. Small airway dimensions in smokers with obstruction to airflow. Am Rev Respir Dis 1990;142:563–70. 10.1164/ajrccm/142.3.563 [DOI] [PubMed] [Google Scholar]
- 14.Gennimata S-A, Palamidas A, Karakontaki F, et al. Pathophysiology of evolution of small airways disease to overt COPD. COPD 2010;7:269–75. 10.3109/15412555.2010.497515 [DOI] [PubMed] [Google Scholar]
- 15.Stockley JA, Ismail AM, Hughes SM, et al. Maximal mid-expiratory flow detects early lung disease in α(1)-antitrypsin deficiency. Eur Respir J 2017;49:1602055. 10.1183/13993003.02055-2016 [DOI] [PubMed] [Google Scholar]
- 16.Tsushima K, Sone S, Yoshikawa S, et al. Clinical differences in the global initiative for chronic obstructive lung disease stage 0. Respir Med 2006;100:1360–7. 10.1016/j.rmed.2005.11.021 [DOI] [PubMed] [Google Scholar]
- 17.Mirsadraee M, Boskabady MH, Attaran D. Diagnosis of chronic obstructive pulmonary disease earlier than current global initiative for obstructive lung disease guidelines using a feasible spirometry parameter (maximal-mid expiratory flow/forced vital capacity). Chron Respir Dis 2013;10:191–6. 10.1177/1479972313507461 [DOI] [PubMed] [Google Scholar]
- 18.Boeck L, Gensmer A, Nyilas S, et al. Single-breath washout tests to assess small airway disease in COPD. Chest 2016;150:1091–100. 10.1016/j.chest.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 19.Verbanck S. Physiological measurement of the small airways. Respiration 2012;84:177–88. 10.1159/000341742 [DOI] [PubMed] [Google Scholar]
- 20.Oxhoj H, Bake B, Wilhelmsen L. Ability of spirometry, flow-volume curves and the nitrogen closing volume test to detect smokers. A population study. Scand J Respir Dis 1977;58:80–96. [PubMed] [Google Scholar]
- 21.Kwon DS, Choi YJ, Kim TH, et al. FEF(25-75%) values in patients with normal lung function can predict the development of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2020;15:2913–21. 10.2147/COPD.S261732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazzan E, Semenzato U, Turato G, et al. Symptomatic smokers without COPD have physiological changes heralding the development of COPD. ERJ Open Res 2022;8. 10.1183/23120541.00202-2022. [Epub ahead of print: 27 06 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y-H, Shin M-H, Kweon S-S, et al. Cumulative smoking exposure, duration of smoking cessation, and peripheral arterial disease in middle-aged and older Korean men. BMC Public Health 2011;11:94. 10.1186/1471-2458-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sylvester KP, Clayton N, Cliff I, et al. ARTP statement on pulmonary function testing 2020. BMJ Open Respir Res 2020;7:e000575. 10.1136/bmjresp-2020-000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party standardization of lung function tests, European community for steel and coal. official statement of the European respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed] [Google Scholar]
- 26.Quanjer PH, Pretto JJ, Brazzale DJ, et al. Grading the severity of airways obstruction: new wine in new bottles. Eur Respir J 2014;43:505–12. 10.1183/09031936.00086313 [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 28.Tam A, Churg A, Wright JL, et al. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016;193:825–34. 10.1164/rccm.201503-0487OC [DOI] [PubMed] [Google Scholar]
- 29.Amaral AFS, Strachan DP, Burney PGJ, et al. Female smokers are at greater risk of airflow obstruction than male smokers. UK Biobank. Am J Respir Crit Care Med 2017;195:1226–35. 10.1164/rccm.201608-1545OC [DOI] [PubMed] [Google Scholar]
- 30.Martin TR, Castile RG, Fredberg JJ, et al. Airway size is related to sex but not lung size in normal adults. J Appl Physiol 1987;63:2042–7. 10.1152/jappl.1987.63.5.2042 [DOI] [PubMed] [Google Scholar]
- 31.Sheel AW, Guenette JA, Yuan R, et al. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol 2009;107:1622–8. 10.1152/japplphysiol.00562.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris ZQ, Coz A, Starosta D. An isolated reduction of the FEV3/FVC ratio is an indicator of mild lung injury. Chest 2013;144:1117–23. 10.1378/chest.12-2816 [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Care Excellence . NICE guidelines - Chronic obstructive pulmonary disease in over 16s: diagnosis and management, 2019. Available: https://www.nice.org.uk/guidance/ng115 [Accessed 21 Feb 2022]. [PubMed]
- 34.Ejiofor S, Turner AM. Pharmacotherapies for COPD. Clin Med Insights Circ Respir Pulm Med 2013;7:CCRPM.S7211–34. 10.4137/CCRPM.S7211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han MK, Ye W, Kim D-Y, et al. Design of the redefining therapy in early COPD study. Chronic Obstr Pulm Dis 2020;7:382–9. 10.15326/jcopdf.7.4.2020.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelb AF, Yamamoto A, Verbeken EK, et al. Normal routine spirometry can mask COPD/Emphysema in symptomatic smokers. Chronic Obstr Pulm Dis 2021;8:124–34. 10.15326/jcopdf.2020.0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quanjer PH, Weiner DJ, Pretto JJ, et al. Measurement of FEF25-75% and FEF75% does not contribute to clinical decision making. Eur Respir J 2014;43:1051. 10.1183/09031936.00128113 [DOI] [PubMed] [Google Scholar]
- 38.Pirina P, Foschino Barbaro MP, Paleari D, et al. Small airway inflammation and extrafine inhaled corticosteroids plus long-acting beta2-agonists formulations in chronic obstructive pulmonary disease. Respir Med 2018;143:74–81. 10.1016/j.rmed.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 39.van den Berge M, De Backer J, Van Holsbeke C, et al. Functional respiratory imaging assessment of budesonide/glycopyrrolate/formoterol fumarate and glycopyrrolate/formoterol fumarate metered dose inhalers in patients with COPD: the value of inhaled corticosteroids. Respir Res 2021;22:191. 10.1186/s12931-021-01772-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usmani OS, Scichilone N, Mignot B, et al. Airway deposition of Extrafine inhaled triple therapy in patients with COPD: a model approach based on functional respiratory imaging computer simulations. Int J Chron Obstruct Pulmon Dis 2020;15:2433–40. 10.2147/COPD.S269001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piorunek T, Kostrzewska M, Stelmach-Mardas M, et al. Small airway obstruction in chronic obstructive pulmonary disease: potential parameters for early detection. Adv Exp Med Biol 2017;980:75–82. 10.1007/5584_2016_208 [DOI] [PubMed] [Google Scholar]
- 42.Mahut B, Caumont-Prim A, Plantier L, et al. Relationships between respiratory and airway resistances and activity-related dyspnea in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2012;7:165–71. 10.2147/COPD.S29745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crisafulli E, Pisi R, Aiello M, et al. Prevalence of small-airway dysfunction among COPD patients with different gold stages and its role in the impact of disease. Respiration 2017;93:32–41. 10.1159/000452479 [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Li X-Y, Yuan L-R, et al. Evaluation of small airway function and its application in patients with chronic obstructive pulmonary disease (review). Exp Ther Med 2021;22:1386. 10.3892/etm.2021.10822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li K, Gao Y, Pan Z, et al. Influence of emphysema and air trapping heterogeneity on pulmonary function in patients with COPD. Int J Chron Obstruct Pulmon Dis 2019;14:2863–72. 10.2147/COPD.S221684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanojevic S, Kaminsky DA, Miller M. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2021;2101499. 10.1183/13993003.01499-2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001385supp001.pdf (1.3MB, pdf)
Data Availability Statement
Data are available on reasonable request. The lung function data used and evaluated during this study can be made available from the corresponding author, NYA, on reasonable request.