Abstract
Ketamine, an anesthetic available since 1970, and esketamine, its newer S‐enantiomer, provide a novel approach for the treatment of depression and other psychiatric disorders. At subanesthetic doses, the two drugs, along with their older congener, phencyclidine (PCP), induce a transient, altered mental state by blocking the N‐methyl‐D‐aspartate (NMDA) receptor for glutamate, the primary excitatory neurotransmitter in the mammalian central nervous system. This multidisciplinary review examines the pharmacology/direct effects on consciousness, effectiveness in depression and acute suicidality, and safety of these fast‐acting NMDA antagonists. To capture the essence of 60 years of peer‐reviewed literature, we used a semi‐structured approach to the subtopics, each of which required a different search strategy. We review the evidence for the three primary reported benefits of the two clinical drugs when used for depression: success in difficult‐to‐treat patients, rapid onset of action within a day, and immediate effects on suicidality. Key safety issues include the evidence—and lack thereof—for the effects of repeatedly inducing this altered mental state, and whether an adequate safety margin exists to rule out the neurotoxic effects seen in animal studies. This review includes evidence from multiple sources that raise substantial questions about both safety and effectiveness of ketamine and esketamine for psychiatric disorders.
Keywords: drug approval, esketamine, evidence‐based toxicology, ketamine hydrochloride, phencyclidine, phencyclidine‐related disorders, United States Food and Drug Administration
1. INTRODUCTION
Three chemically psychoactive drugs are formally known as non‐competitive, non‐selective antagonists of the N‐methyl‐D‐aspartate (NMDA) neuroreceptors. The most widely used is ketamine, which has been licensed in the United States since 1970 for anesthesia 1 but also became an illicit party drug in many countries, and more recently was repurposed as an off‐label treatment for depression and other psychiatric conditions. The newest of this group is esketamine, licensed in the United States 2 and Europe 3 in 2019 for treatment‐resistant depression. The oldest and most potent congener is phencyclidine (PCP), which was abandoned as an anesthetic but widely used as an illegal street drug known to induce hallucinations and bizarre and violent behavior. 4 All three drugs share a chemically related structure, have similar direct effects on consciousness, and have a primary mechanism of action as fast‐acting antagonists of the NMDA receptor. Comparative details are shown in Table 1.
TABLE 1.
Fast‐acting, non‐selective, non‐competitive NMDA receptor antagonists
| Phencyclidine (PCP) | Ketamine (Ketalar) | Esketamine (Spravato) | |
|---|---|---|---|
| Primary use | Illegal street drug | Anesthetic | Treatment‐resistant depression |
| Other uses | Anesthetic (withdrawn) | Psychiatric disorders | Acute suicidality |
| Illegal street drug | |||
| Route of administration | Snorted/inhaled | IV/IM/snorted/inhaled | Nasal spray device |
| Abuse & dependence a | High potential | Moderate‐low potential | Moderate‐low potential |
Abbreviations: IV, intravenous; IM, intramuscular.
US Drug Enforcement Administration Controlled Substance Schedule II and III.
The latest interest in ketamine and esketamine was spawned by the proposition that subanesthetic doses of these agents might be useful in depression and acute suicidality. One group of investigators in 2019 hailed the repurposed ketamine as “A paradigm shift for depression research and treatment,” 5 citing a rapid onset of action compared with standard antidepressants and success in difficult‐to‐treat patients. However, other commentaries raised questions about both safety and efficacy.
We conducted a narrative, multidisciplinary review to assess the risks and benefits and to identify answered and unanswered questions about clinical use of these fast‐acting NMDA antagonists. This broad subject in which scientific knowledge evolved over many decades was divided into the following subtopics: Pharmacology, Effectiveness in Depression/Suicidality, and Safety. Our search strategy is shown in Box 1.
BOX 1. Search strategy and selection criteria.
We searched PubMed in April 2021 and identified 21,298 studies indexed to ketamine, 5546 studies of phencyclidine, and 309 studies of esketamine. In addition to the peer‐reviewed literature, we also searched Drugs@FDA for review documents of these types: summary, medical, statistical, and pharmacology/toxicology reviews.
Because of the different history and clinical uses, the selected studies necessarily differed among the three study drugs. Because phencyclidine has no current medical use, studies focused primarily on abuse and recreational use. With a 60‐year history, ketamine studies included toxicology, pharmacology, use in anesthesia, abuse, and off‐label use at subanesthetic doses for psychiatric disorders. Esketamine, recently and specifically developed by a major pharmaceutical company for the narrow purpose of a novel therapy for treatment‐resistant depression had a portfolio of recent peer‐reviewed studies for a newly‐approved drug, along with extensive public disclosures from the United States Food and Drug Administration review and approval process.
2. PHARMACOLOGY
The two most widely distributed neurotransmitters in the human and mammalian central nervous system (CNS) are glutamate, with excitatory effects on neurons, and gamma‐aminobutyric acid (GABA), which inhibits neuronal depolarization and signaling. 6 The glutamate excitatory neurotransmitter, in turn, primarily binds to three different receptor types: NMDA, Alpha Amino 3 Hydroxy 5 Methyl 4 Isoxazolepropionate (AMPA), and kainate. Blocking the NMDA receptor produces two effects in animals and humans: anesthesia and loss of consciousness at higher doses, and an altered state of consciousness that occurs at subanesthetic doses or as concentrations declined as the drug was metabolized and eliminated. 7
The earliest NMDA receptor antagonist to be tested in humans was PCP, which induced effects similar to psychosis as explored in published clinical studies in 1959. 8 , 9 The PCP congener ketamine was specifically designed for a shorter duration of anesthetic effect and fewer adverse effects on mood, behavior, perception, cognition, and memory. 10 Esketamine, in turn, is the S‐enantiomer of ketamine and is currently licensed as a nasal spray (NS) for treatment‐resistant depression. 2 Esketamine has 2 to 4 times the affinity for the NMDA receptor as the other optical isomer, R‐ketamine. 11 Ketamine is primarily administered by intravenous infusion (IV) or via the intramuscular (IM) route. 1 Chemical structures of the fast‐acting NMDA antagonists are shown in Figure 1.
FIGURE 1.
Chemical structure of phencyclidine, ketamine, and esketamine.
2.1. Effects on consciousness
At IV dose of 1–4.5 mg/kg, ketamine produces rapid‐onset surgical anesthesia at an infusion rate of 0.5 mg/kg/min. 1 At subanesthetic doses of 0.5 mg/kg administered over 40 min, the primary direct effect of ketamine is to induce an altered mental state of consciousness. Similar effects on consciousness occur with esketamine with 56–84 mg administered through 28‐mg nasal spray devices. With IV ketamine or inhaled nasal administration of esketamine, the onset of altered consciousness is rapid, with symptoms peaking at 40 min and resolving in most but not all patients by 1.5 h. The altered mental state roughly mirrors the pharmacokinetics of nasal spray and IV administration, with maximum concentration (Cmax) at 40 min, and resolution typically occurring in the first phase of rapid elimination of 2–4 h, but with a terminal half‐life of 7–12 h. 12 However, in some individuals the altered mental state persists after the drug is no longer present, a topic examined below in the discussion of safety.
The altered mental state induced by these three NMDA antagonists varies by individual, drug potency, and dose. It involves widely varied changes in perception (hallucinations and visions), mood (euphoria and suicidality), behavior (sedation and violence), and cognition (reduced but not enhanced).
Although the altered mental state induced by these NMDA antagonists has probably not varied over the 60 years of medical use, the terminology and measurement scales used to describe the same event have evolved. In reports of ketamine for anesthesia, these effects were called “emergence reactions,” 1 presumably because they occurred as plasma concentrations declined to subanesthetic doses as the drug infusion was discontinued. Next, the NMDA antagonists were investigated as a possible mechanism for the psychosis seen in schizophrenia. These investigators used the Brief Psychiatric Rating Scale (BPRS), a common instrument in that disorder, and reported that ketamine caused substantial increases in the BPRS in both normal and schizophrenic patients. 13 , 14 , 15 Finally, as esketamine was being evaluated for use in depression, the same altered mental state was described as “dissociation” and another measurement scale was deployed, the Clinician Administered Dissociative States Scale (CADSS). 2 The CADSS was developed to evaluate Post Traumatic Stress Disorder (PTSD). 16
A retrospective study published in 2020 17 sought to capture without fixed scales the altered state of consciousness induced by single infusions of ketamine for depression. The retrospective study pooled the results for clinician‐administered questionnaires used in five sub‐studies conducted over the preceding 13 years. The 10 most frequent patient‐reported side effects among 44 symptoms reported by 5% or more are shown in Table 2. The complete symptom list included “feeling weird, strange, or bizarre” (78%), difficulty speaking (49%), and euphoria (27%). Direct effects of PCP, an even more potent NMDA antagonist than ketamine, were less systematically measured since it was primarily an illicit street drug in the late 1960s and 1970s. However, a 2018 review described the acute effects of PCP as psychosis, hallucinations, delusions, and thought disorders. 4 Reports and diagnostic guidance for treating PCP intoxication 18 also include an alert for belligerent, assaultive, and other violent behavior.
TABLE 2.
Most frequent patient‐reported effects of ketamine infusion for depression 17
| Percent | Rank | |
|---|---|---|
| Feeling weird, strange, or bizarre | 78 | 1 |
| Spacey | 74 | 2 |
| Woozy/loopy | 72 | 3 |
| Dissociation | 62 | 4 |
| Visual distortions | 57 | 5 |
| Floating | 55 | 6 |
| Numbness | 53 | 7 |
| Difficulty speaking | 49 | 8 |
| Delayed verbal response | 40 | 9 |
| Confusion | 38 | 10 |
3. EFFECTIVENESS IN DEPRESSION
The clinical testing of ketamine and esketamine for depression differed in multiple dimensions. Ketamine was explored as a novel depression treatment beginning more than two decades ago. Studies of ketamine for depression were conducted primarily by academic psychiatric practices that mounted trials of modest size. Esketamine was developed 15 years later by a major pharmaceutical company specifically seeking the United States Food and Drug Administration (FDA) approval for treatment‐resistant depression with a full‐scale preclinical and clinical testing regimen similar to that required for a new molecular entity.
3.1. Ketamine effectiveness testing
The earliest widely cited ketamine trial in patients with depression was conducted in 2000 by psychiatrists based at the Yale University School of Medicine Department of Psychiatry without pharmaceutical industry financial support. 19 Seven patients meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM_IV) criteria for major depression completed a randomized crossover design. Patients were administered ketamine 0.5 mg/kg IV over 40‐min period, or a sham placebo infusion at 1‐week intervals. The authors reported that at 72 h after infusion the 25‐item Hamilton Depression Rating Scale (HAMD) declined by 14 standard deviation (SD) (±10) points (42%) after a single infusion of ketamine compared with 0 SD (±12) points with sham treatment. Depression scores returned to baseline within 2 weeks of the active treatment infusion.
This seminal report attracted substantial attention in the psychiatric research community, with more than 40 peer‐reviewed reports published over the next 15 years that varied from a small open‐label case series to a randomized, active drug‐controlled clinical trial. Since ketamine was an inexpensive, readily available anesthetic drug, it lent itself to projects conducted by research‐oriented psychiatrists without requiring the tens of millions of dollars and global organization needed to finance a full‐scale drug development program.
The clinical trials establishing the effectiveness of ketamine for depression, treatment‐resistant depression, and bipolar depression were assessed in four systematic reviews published in 2014 and 2015. 20 , 21 , 22 , 23 Each systematic review examined 3 to 13 clinical trials. The underlying clinical trials, design, characteristics, and effects on depression are shown in Table 3. The clinical trial evidence cited in the reviews consistently demonstrated two characteristics of ketamine as a treatment for depression: (1) a substantial effect on depression scores, (2) a rapid onset of action that was immediately evident at 24 h. This was—in contrast to standard Selective Serotonin Reuptake Inhibitor (SSRI) antidepressants which normally take several weeks to establish a statistically significant reduction in depression rating scale scores. In all four systematic reviews the effect size as measured by the standardized mean difference (SMD) or Hedge's g was “large” according to statistical guidelines. 24 The included trials had other consistent features. The patient population had major depression or bipolar depression; the trials featured a single infusion of ketamine 0.5 mg/kg infused over 40 min or a close equivalent. The effects on depression were measured at various time intervals after infusion, ranging from 4 h to 14 days. However, all the included trials in the four systematic reviews shown in Table 3 included assessments at 24 h.
TABLE 3.
Systematic reviews of ketamine clinical trials for depression: effects at 24 h
| Trials selected (K) | Trials, single infusion | Total Patients (N) | SMD [95% CI] a | N on active drug (range) | Depression rating scales | |
|---|---|---|---|---|---|---|
| Caddy et al Cochrane Database Syst Rev 2015 20 b | 3 | 3 | 54 | −1.42 [−2.26,‐0.57] | 4–9 | HAMD = 1; MADRS = 1; BDI = 1 |
| Coyle et al Hum Psychopharmacol Clin Exp 21 2015 | 13 | 11 | 291 | −1.24 [−1.56,‐0.93] c | NR | MADRS or HAMD |
| Fond et al Psychopharmacology 23 2014 | 9 | 9 | 192 | −0.99 [−1.23,‐0.75] | 9–47 | HAMD = 5; MADRS = 4 |
| Lee et al Gen Hosp Psych 22 2015 | 5 | 5 | 150 | −1.01 [−1.34,‐0.69] | 15–47 | HAMD = 1; MADRS = 4; BDI = 1 |
Abbreviations: BDI, Beck Depression Inventory; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery Asberg Depression Rating Scale, NR, not reported; SMD = standardized mean difference.
SMD effect size: Small = 0.2; Medium = 0.5; Large = 0.8.
Subset of ketamine v placebo w/depression rating scale score.
Hedge's g, 95% CI.
The clinical trials included in the systematic reviews also shared limitations in assessing the safety and effectiveness of ketamine as a treatment for depression. The study sizes were small, ranging from 4 to 47 patients receiving the active drug. The trials with one exception featured a single infusion of ketamine, and none measured the effect later than 14 days, leaving duration of benefit uncertain but openly questioned in the report texts. With one exception discussed below, none of the trials featured a design with blinded active‐drug controls. This was especially relevant given a drug that within 40 min of administration induced an altered state of consciousness that would be immediately evident to the patient and probably the investigator. The altered state of consciousness would compromise blinding in the four clinical trials with a crossover design.
An alternative approach to systematic review is to examine the largest, placebo‐controlled, rater‐blinded, fully randomized trial in this group. In 2013, a team of 12 investigators at the Baylor College of Medicine and the Icahn School of Medicine conducted a single‐infusion trial of ketamine at two medical centers among patients diagnosed with DSM_VI major depressive disorder and an inadequate response to at least three previous trials of antidepressant medication. 21 The patients were randomized in a 2–1 ratio to receive 0.5 mg/kg of ketamine (N = 47) or 0.045 mg/kg of midazolam (N = 25), both infused over 40 min. The primary end point was the difference in the reduction of depression at 24 h as measured by the Montgomery Asberg Depression Rating Scale (MADRS), a 0–60 rating scale. At 24 h the ketamine group's MADRS scores had declined from a mean 32.6 to 14.77 (55%). Scores for the midazolam controls had declined from a mean of 31.1 to 22.72 (26%). The study reported a 7.9 point least squares (LS) mean difference [95% confidence interval (CI) 3.2, 12.71] in MADRS scores, and an effect size of Cohen's d of 0.81, a borderline large effect. Among the ketamine depression studies identified in the four systematic reviews, this study was the largest (47 patients on active drug) and the only study with two or more sites. It featured enhanced blinding through use of a benzodiazepine active control with a rapid‐onset altered state of consciousness. The patient population was limited to treatment‐resistant depression, suggesting a possible additional benefit over the standard of care. One of the meta‐analyses scored this trial with a medium risk of bias and “Yes” for conflict of interest. 21 This occurred presumably because four of the twelve co‐authors reported they were consulting for pharmaceutical companies; one study center institution and two coauthors declared they held use‐patents on ketamine should the FDA approve it for depression.
3.2. Esketamine effectiveness testing
The portfolio of pre‐approval clinical trial testing of the esketamine nasal preparation was designed through communication between the sponsor, Janssen Pharmaceuticals, and the FDA. Design and duration of phase 3 pivotal trials, doses to be tested, definition of the treatment‐resistant depression indication, primary and secondary end points as well as other issues were negotiated in nine meetings between Janssen and the FDA between 2012 and 2016. 27 This review of effectiveness will focus on the phase 3 esketamine trials that were agreed to during these negotiations and that were relied on by the FDA as evidence supporting the approval of esketamine.
3.2.1. Esketamine phase 3 trial design
The study population was treatment‐resistant depression, specifically defined as patients with major depression who had failed prior trials of any two other antidepressant medications. The esketamine doses were 56 and 84 mg per session administered in 28‐mg nasal spray devices. The primary end point was change in the severity of depression measured by MADRS. The duration of drug effect—an issue unresolved in most of the ketamine studies—was addressed through intensity of treatment (twice weekly infusions) and a primary end point with effect on depression measured at 28 days. The esketamine phase 3 trials contained a novel requirement that had the effect of introducing an active control to the “placebo” control group. At the beginning of the trials, both the active drug and comparison group (all of which failed two previous antidepressant trials) were switched to a third antidepressant of a different class. This additional requirement was notable for two reasons. The FDA required it because of ethical concerns about randomizing patients with sustained major depression to an inactive placebo. Also, it had the effect of enhancing the blinding of the studies because the early onset side effects of a different class of antidepressant would also serve as active controls.
3.2.2. Esketamine phase 3 trial results in treatment‐resistant depression
The design and results of the esketamine phase 3 trials are shown in Table 4 and Figure 2. The tolerability of the treatment was established through dropout rates that were lower than the 20–40% seen in trials of many standard antidepressants. 27 In TRANSFORM 1–3 trials, 28 , 29 , 30 study reports showed that 91%, 86.8%, and 88.4% completed the full 4 weeks of treatment, respectively. In all three trials, a substantial reduction in LS mean MADRS scores from baseline was recorded: 50%, 57.8%, and 28%, respectively.
TABLE 4.
Esketamine phase 3 clinical trials in treatment‐resistant depression
| Study | Patient Population | Treatment Groups a | Baseline MADRS score (0–60) | Change from Baseline @ 4 weeks | LS Mean fr Placebo (95% CI) | p Value v Placebo |
|---|---|---|---|---|---|---|
| Fedgchin et al. Int J Neuropsychopharmacol 2019 (Study 3001,TRANSFORM‐1) 28 | N = 346, age 18–64 | Esk 56 mg twice weekly + AD | 37.4 | −19.0 | −4.1 (−7.7 to −0.5) | p < 0.05 |
| Esk 84 mg twice weekly + AD | 37.8 | −18.8 | −3.2 (−6.9 to +0.5) | NS p = 0.088 | ||
| Placebo twice weekly + AD | 37.5 | −14.8 | — | — | ||
| Popova et al. Am J Psychiatry 2019 (Study 3002, TRANSFORM‐2) 29 | N = 223, age 18–64 | Esk 56 or 84 mg twice weekly + AD | 37.0 | −21.4 | −4.0 (−7.31 to −0.64) | p < 0.05 |
| Placebo twice weekly + AD | 37.3 | −17.0 | — | — | ||
| Ochs‐Ross et al. Am J Geriatr Psychiatry 2020 (Study 3005, TRANSFORM‐3) 30 | N = 137, age > 64 | Esk 28, 56, or 84 mg twice weekly + AD | 35.5 | −10.0 | −3.6 (−7.20 to +0.07) | NS p = 0.059 |
| Placebo twice weekly + AD | 34.8 | −6.3 | — | — |
Abbreviations: AD, anti‐depressant; LS Mean, least squares mean; MADRS, Montgomery Asberg Depression Rating Scale; NS, not statistically significant.
Both treatment and placebo group were switched to a new antidepressant.
FIGURE 2.
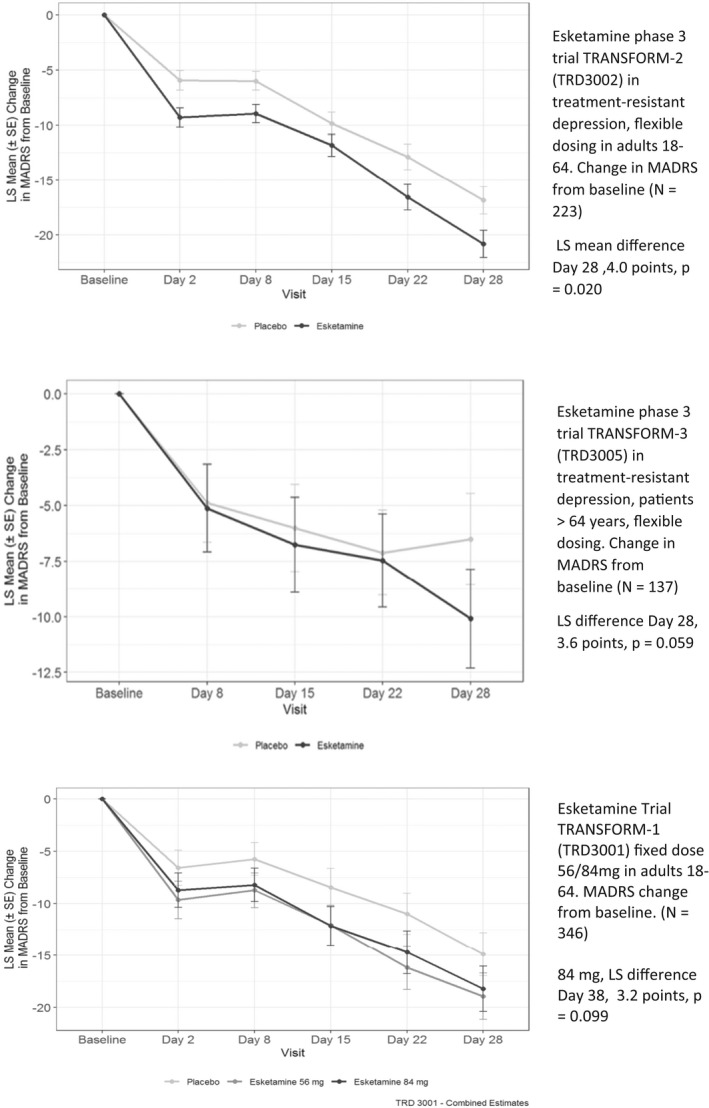
Successful and unsuccessful phase 3 clinical trials of esketamine for treatment‐resistant depression. Note: Both treatment and placebo patients switched to a new antidepressant at baseline. Abbreviations: MADRS, Montgomery‐Asberg Depression Rating Scale; LS Means, least squares means; SE, standard error. Flexible Dosing = 56‐84 mg. Source: Adapted from Kim J, Chen Q27
However, results for the primary efficacy end point were smaller and sometimes not statistically significantly different from the active‐controlled comparison groups that were only switched to a new class of antidepressant. The largest treatment effect was seen in the flexible dose TRANSFORM‐2 trial. At day 28, the MADRS score in the esketamine‐treated patients was reduced by a LS mean of 21.4 (SD 12.3) points, compared with 17.0 (13.9) points in the placebo group. Thus, the treatment achieved a LS mean difference of 4 points (95% CI – 7.31 to – 0.64). In TRANSFORM‐3, the trial in patients age 65 years and older, the MADRS scores were not statistically significantly different between treatment and controls at any time point. The results of the two fixed dose groups (esketamine 56 mg and 86 mg twice weekly) in TRANSFORM‐1 were difficult to interpret and were judged by the FDA as not providing the legally required “substantial evidence” of efficacy. In the lower dose, 56‐mg group, the LS mean MADRS score reduction was 4.1 points larger than the placebo at day 28, a result that was statistically significant. In the higher dose group, 84 mg, the LS mean difference was 3.2 points and was not statistically significant. The results were also the reverse of the expected dose–response relationship and published report declared the study failed to meet its primary end point. 28
3.2.3. Esketamine effectiveness within 24 h
The FDA had granted esketamine breakthrough drug status on the basis of a phase 2 trial that demonstrated treatment benefit at 24 h, which the FDA judged a major potential advance over standard antidepressants that typically took 4 weeks to achieve a positive effect on depression. 27 To further establish the rapid onset, the FDA required a secondary end point of a 50% reduction in MADRS scores achieved at day 2 and maintained through day 28 with one lapse allowed. In none of the three phase, 3 trials was this end point achieved. Also, as shown in Figure 2, the active placebo group that was switched to a new antidepressant also showed parallel trends in reduction in MADRS over time.
3.2.4. Esketamine effects on suicidality
Three clinical trials to establish the benefits of esketamine were conducted in hospitalized patients with major depressive disorder and active suicidal ideation with intent. Design and results of these trials are shown in Table 5. Unlike the phase 3 trials above, eligibility requirements did not require failure of two prior antidepressant therapies. All three trials were funded and conducted by Janssen Pharmaceuticals. Active suicidal ideation with intent was defined as patients in the psychiatric inpatient unit or emergency department who responded affirmatively to questions asking whether they were thinking about killing themselves and also had an intent to act on those thoughts. All enrolled patients were given comprehensive care while hospitalized for 5 or more days.
TABLE 5.
Esketamine trials in patients with active suicidal ideation with intent
| Study | Patient Population | Treatment Groups | Difference in Suicidality (CGI‐SS‐r) @ 24 h a | Baseline MADRS score (0–60) | Change from baseline @ 24 h | LS Mean fr placebo (SE) | p Value v Placebo |
|---|---|---|---|---|---|---|---|
| Canuso et al. Am J Psychiatry 2018 (PERSEVERE) 31 | N = 68, age 18–64 Acutely suicidal b | Esk 84 mg twice weekly + AD | NS | 38.5 | −19.2 | ‐7.2 (2.85) | p = < 0.05 |
| Placebo twice weekly + Ad c | — | 38.8 | −12.0 | — | — | ||
| Fu et al. J Clin Psychiatry 2020 (ASPIRE‐1) 33 | N = 226, age 18–64 Acutely suicidal | Esk 84 mg twice weekly + AD | NS | 41.3 | −16.4 | −3.8 (1.39) | p < 0.05 |
| Placebo twice weekly + AD | — | 41.0 | −12.8 | — | — | ||
| Ionescu et al. Neuropsychopharmacol 2021 (ASPIRE‐2) 32 | N = 230, age 18–64 Acutely suicidal | Esk 84 mg twice weekly + AD | NS | 39.5 | −15.7 | −3.9 (1.39) | p < 0.05 |
| Placebo twice weekly + AD | — | 39.9 | −12.4 | — | — |
Abbreviations: AD, anti‐depressant; CGI‐SS‐r, Clinical Global Impression‐Severity of Suicidalty‐revised; LS Mean, least squares mean; MADRS, Montgomery Asberg Depression Rating Scale; NS, Not statistically significant; SE, standard error.
Difference from placebo by Analysis of Covariance.
Acutely suicidal is suicidal ideation with intent assessed in emergency department or hospital inpatient unit.
Standard‐of‐care treatment (initial psychiatric hospitalization and newly initiated or optimized oral antidepressant therapy).
Changes in suicidality were assessed with varied but multiple measurement scales in the three trials, but all included assessments with Clinical Global Impression‐Severity of Suicidality‐revised (CGI‐SS‐r). The trials also evaluated depression using the MADRS, similar to the phase 3 efficacy trials, over a similar period of 28 days. Unlike the phase 3 trials with flexible dosing, all patients were administered the higher esketamine 84 mg dose twice weekly, but the dose was reduced if tolerability was judged an issue. The comparison group was administered saline and a bittering agent in identical nasal spray devices.
The scientific objective of a rapid reduction in suicidality with esketamine treatment compared to usual care was not achieved in any of the three clinical trials. In the published results summarized in Table 5, the CGI‐SS‐r was reduced in all patient groups, but the reduction was not statistically significantly different between the esketamine and untreated controls at any time point. The lack of drug effect on suicidality was apparently not sensitive to method of measurement. No statistically significant difference was seen in the Beck Scale for Suicidal Ideation, 31 in the Clinician Reported Frequency of Suicidal Thinking, or the Patient‐Reported Frequency of Suicidal Thinking. 32 , 33
The effect on depression symptoms measured by the MADRS in the active suicidal ideation trials was similar to the phase 3 trials for initial approval. The baseline MADRS severity scores were higher and the score reductions were larger in the active suicidal ideation trials, but differences with usual care controls were similar to the phase 3 clinical trials (Tables 4 and 5). At 28 days statistically significant LS mean difference in MADRS between treatment and usual care controls was 3.8 points and 3.9 points in ASPIRE‐1 and ASPIRE‐2, respectively. 32 , 33
3.2.5. Effectiveness discussion
The promising results seen in the small, single‐infusion, single‐center trials of racemic ketamine were generally not replicated in the larger, multi‐site trials of esketamine nasal spray. The esketamine trials were also subject to FDA site inspections, data integrity checks, and other forms of independent scrutiny. Only one phase 3 esketamine trial in treatment‐resistant depression was judged to have unequivocally demonstrated a statistically significant benefit at 28 days. 29 In that trial the treatment difference from placebo was 4 points on the MADRS 0–60 rating scale. The two trials that did not reach statistical significance had favorable trends of less than 4 points.
The benefit of rapid onset that earned esketamine an FDA designation of breakthrough status was also not replicated in the phase 3 trials in treatment‐resistant depression. The trials' failure on this end point appeared to result from two factors: (1) A 50% reduction in MADRS scores at 24 h was an ambitious drug effect measure; (2) As Figure 2 illustrates, the placebo group switched to a new antidepressant that also had rapid score declines at 24 h. These data and Figure 2 suggest that most of the benefit in treating depression came not from the pharmacological effect of ketamine and esketamine on depression, but mostly from the major change in the modality of treatment to a special office visit, an IV infusion or nasal device; blood pressure monitoring, and supervision for 2 h.
The trials to document the expected benefits of a rapid reduction in suicidality were an unambiguous failure in all three trials using multiple measurement scales at different time points. Two trials did demonstrate a statistically significant effect on depression measured by MADRS at 28 days, but the difference of less than 4 points varied little from standard antidepressants in severely ill patients. 34
The FDA was criticized in multiple peer‐reviewed commentaries for approving esketamine even though two out of three of the phase 3 trials did not document a benefit. 35 , 36 , 37 Other criticisms included a benefit size that was not clinically meaningful, including older patients in the indication even though they were excluded from the sole successful trial, and relaxing the definition of “treatment‐resistant depression” from three to two prior failed antidepressants. The FDA defended its decision to approve esketamine in a journal editorial, noting that 40% of standard antidepressant trials also failed to establish a statistically significant benefit. 38
One year after the FDA approval of esketamine in 2019, the agency granted Janssen Pharmaceuticals an expanded indication for esketamine for patients with acute suicidal ideation or behavior. The assessment report, which was not made public, was apparently based on the three failed clinical trials of suicidality reviewed above. Although an FDA‐approved indication normally means “substantial evidence” of benefit, the indication contained the unusual qualifier, “The effectiveness of SPRAVATO in preventing suicide or reducing suicidal ideation or behavior has not been demonstrated.” 2
4. SAFETY
4.1. Neurotoxicology of 3 NMDA Antagonists
In infant, juvenile, and adolescent mice, rats, dogs, and monkeys, the administration of the fast‐acting NMDA antagonists induced development of abnormal structures inside neurons, and then as exposure, dose, and duration increased, with apoptotic cell death. 25 , 26 Selected neurotoxicity studies are shown in Table 6. The earliest biomarker of neurotoxicity was vacuolization—first seen in 1989 in electronic micrography of adult rat brain cells treated with a single dose of PCP, ketamine, and other NMDA antagonists. Vacuoles in animals are non‐functional sacs filled with cytoplasm but not mitochondria, usually attached to the cell membrane. After short exposures, this early‐onset form of neurotoxicity appeared to resolve in adults. But an expanding number of toxicology studies reached the conclusion that administration of NMDA antagonists was the most neurotoxic in developing brains where synaptogenesis and synaptic refinement occurred, eliminating unused connections. 26 These findings resulted in safety concerns since ketamine was already widely used as an anesthetic in children. The 2020 ketamine label contains a warning that “administration of anesthetic and sedation drugs that block NMDA receptors…increase neuronal apoptosis in the developing brain and result in long‐term cognitive deficits when used for longer than 3 h. The clinical significance of these findings is not clear.”. 1
TABLE 6.
Selected neurotoxicity animal studies of fast‐acting NMDA antagonists
| Study | Study drugs | Animals | Duration | Key findings | Limitations |
|---|---|---|---|---|---|
| Olney et al Science 1989 25 | PCP, Ketamine, MK‐801 | Adult rats | Single infusions; repeat tests for 4 days | Formation of multiple vacuoles in singulate and retrospenial cortical neurons | Effects diminished 12 h later |
| Sun et al Addiction Biology 2014 39 | Ketamine | 24 juvenile cynomolgus monkeys | 1 mg/kg IV daily for 1, 6 months | Apoptotic nuronal cell death at 6 months in pre‐frontal cortex; behavioral changes | Effects not seen at 1 month |
| Yeung Tox et al Tox Ltrs 2010 40 | Ketamine | 6 cynomogus /Crab‐eating Macaques +18 mice | 30 mg/kg daily IV for 1, 3, or 6 months | Hyperphosphorylated tau in prefrontal and enorhinal cortical sections at 1, 3, 6 months | Effects not seen at 1 month |
| Janssen TOX10415 42 | Esketamine | 10 adult female rats | Single dose 0.9–9 mg/kg IN | Negative for neuronal vacuoles at 4 h | Uncertain for necrosis at 7 days |
| Janssen TOX11374 42 | Ketamine | 16 adult rats | Single dose 0,4,15, 60 mg/kg IV | Minimal neuronal vacuolization at layer 1 of retrospenial cortex at 60 mg/kg. | NOEL 1.6 fold AUC for vacuolization |
Abbreviations: AUC, area under the curve; IN, intranasal; IV, intravenous; NOEL, no effects level; PCP, phencyclidine.
Other studies suggested neurotoxic effects at older ages, at subanesthetic doses, and with repeated exposure typical of illicit or recreational use. Repeat daily administration of ketamine over 6 months to adolescent cynomolgus monkeys resulted in decreased locomotion and neuronal cell death through apoptosis in the prefrontal cortex. 39 Another ketamine study in two species of adolescent monkeys that evaluated daily ketamine administration for 1, 3, and 6 months detected in brain section imaging hyperphosphorylated tau, a biomarker for Alzheimer's disease. 40 These changes at 3 and 6 or more months were not seen in shorter duration exposures. A study of human chronic ketamine non‐medical users compared with controls showed reduced cortical thickness and poorer cognitive performance. 41
Toxicology studies of esketamine were also conducted to support the FDA approval in 2019. The three neurotoxicity studies the FDA accepted, however, were limited to a single dose in adult rats. One study detected vacuolization but not apoptotic cell death. 42
4.2. Managing an altered mental state
The most prevalent adverse effect of subanesthetic doses or illicit use of NMDA antagonists is the rapid induction of an altered mental state, as described previously. The primary precaution for managing this risk is the administration of the drugs under medical supervision and then monitoring following administration.
To manage the rapid‐onset altered mental state of esketamine, the FDA created one of the most comprehensive set of precautions, called Risk Evaluation and Management Strategies (REMS), that it had required for any outpatient drug. 43 The program included 24 different outpatient provider requirements, including provider training, certification, monitoring, and adverse drug event reporting on every patient, and sponsor audits to ensure compliance at certified administration centers. However, none of these mandatory precautions applied to the US network of centers offering off‐label ketamine infusions. 44 There was also evidence that ketamine was also being compounded by some pharmacies for use in a nasal device at home. 45
Despite short‐term monitoring provided in many forms of NMDA antagonist administration, there is evidence that the altered consciousness or sedative effects persist beyond 1.5–2 h in some individuals. Edward Domino, a lead developer of ketamine, remained puzzled 30 years later why the altered mental state could recur or continue even “if the drug is no longer present in the brain.” 7 The FDA's Division of Pharmacovigilance (DPV) examined this issue for ketamine in 2014 and concluded: “DPV identified cases for which the duration of psychiatric reactions following ketamine exposure ranged from 3 h to several weeks, and time to onset ranged from immediate to several weeks.” 46 The FDA division recommended a “special note” for the ketamine labeling warning of this safety risk, but this recommendation was not implemented. A study of ketamine administered to nine healthy volunteers included follow‐up clinical interviews at 8 and 24 h. 15 It reported that four out of nine patients reported a recurrence of their altered mental state at a “delayed time after ketamine.” The FDA medical review of esketamine noted that in the single phase 1 study featuring a longer period of monitoring of sedation effects, six patients (25%) reported adverse events of somnolence with an average duration of 6.5 h (range 1.6–20 h). 27
Despite these multiple studies with longer periods of monitoring, the esketamine short‐term phase 3 trials included only psychiatric symptom evaluations at 40 min to 1.5 h.
4.3. Risks of repeat dosing
A central but unanswered safety question about NMDA antagonists for clinical use is the cumulative effects of repeatedly inducing an altered state of consciousness. Safety concerns were also based on the animal toxicology studies of drug‐induced abnormal neuron structures reviewed above.
Little evidence is available for the effects of repeated exposure to ketamine in clinical use for the treatment of depression and other disorders. A narrative review of the side effects reported in 60 ketamine depression studies declared it could not evaluate long‐term effects “because insufficient data were available regarding the side effects of repeat‐dosing and possible cumulative and long‐term risks.”. 47
The effects of repeated exposure to intranasal esketamine were also an issue for the human clinical testing for FDA approval. It was relevant because the approved treatment regimen provided for 34 to 56 administrations to patients treated for 1 year's time. 2 In addition, as noted above, the animal studies accepted in the FDA toxicology review provided only a single administration. The phase 3 efficacy trials in humans provided limited insight into the long‐term safety of esketamine because they were short‐term studies–28 days. To assess risks of longer term exposure, the FDA accepted as pivotal an open‐label, long‐term, 7‐phase clinical trial that enrolled 802 patients who were transferred into the study after successfully completing one of the esketamine phase 3 trials, or enrolled directly. The sponsor stopped the study when 100 patients completed 12 months of esketamine therapy, which the FDA accepted as an acceptable level of exposure for a drug approved for chronic or long‐term therapy. 38 , 48 The long‐term exposure was assessed among 580 patients who had responded to treatment with at least a 50% reduction in MADRS. By three measures, cognitive function was either unchanged or improved from baseline, except in the subgroup of patients age 65 or older, who had a decline in two of the three measures.
4.4. Tolerance, overdose, and addiction
Illicit use of PCP and ketamine to induce euphoria or hallucinogenic states has waxed and waned over decades, and in different global regions. PCP became a prominent drug of abuse in the United States in the 1970s, with safety concerns fueled by media accounts of bizarre behaviors including cannibalism, murder, and users gouging out their eyes. 4 In addition to short‐term intoxication, there were reports of psychosis lasting 4 to 6 weeks. 10 The similar but less potent ketamine became a popular party drug in the United Kingdom in the decade from 2000 to 2010, with respondents in surveys of dance clubs showing 16% reporting current use of ketamine a mean of 4 times per month. 49 A retrospective study of recreational ketamine users reported cognitive decline among frequent users, but not infrequent users. 50 In the United States, PCP is still classified as a Schedule II controlled substance (high potential for abuse) even though other dangerous drugs with no approved medical use are listed in the more restrictive Schedule I. Ketamine and esketamine are Schedule III, indicating moderate‐to‐low abuse potential. 1 , 2
In the clinical trials of esketamine, tolerance to its direct effects on consciousness was observed. In the successful phase 3 efficacy trial, the altered mental state, measured by the CADSS scale at 40 min, decreased by 50% from day 1 to day 25. 29 Studies in rats, monkeys, and frequent human users of illicit ketamine confirmed that tolerance develops with repeated exposure. 50
A safety review of illicit ketamine noted that another acute risk was accidents while experiencing altered consciousness. 50 A survey of 90 ketamine recreational users reported that 13% had been involved in an accident. 51 In Hong Kong, 9% of drivers in fatal car crashes tested positive for ketamine in a 2005 survey. 51
4.5. Safety discussion
Considering the scientific studies reviewed in this assessment, we find that neither ketamine nor esketamine has been shown to be safe for extended clinical use to treat depression. At subanesthetic doses, ketamine is a well‐documented drug of addiction and abuse. Images from animal studies demonstrate damage to neurons with even brief exposure. With extended use, the abnormal vacuoles created may exceed the self‐repair capacity of the neurons and trigger apoptotic cell death. The altered mental state induced by ketamine and esketamine varies with dose, duration, and patient mental health. However, extensive studies now demonstrate that altered consciousness varies from a mild, transient, but pleasant euphoria to hallucinations, delusions, and other forms of clinically significant psychosis. Although transient in most patients, the altered mental state can be persistent or recur even after the drug has been eliminated from circulation.
The studies of longer‐term use ketamine and esketamine were limited and often flawed. The ketamine depression studies were too short to provide useful information on this issue, with most limited to a single infusion. The long‐term esketamine trial 48 that the FDA relied on demonstrated many defects in study design. It was open label. The main phase was limited to responders. It was terminated after just 100 patients reached the 1‐year mark. Almost any drug intervention can be made to appear safe or beneficial if unblinded and limited to a small group of patients who, from the onset, had already responded well. Limited studies of longer term ketamine and PCP abusers and recreational users show direct adverse effects on cognition but contain little systematic information about dose, purity, or frequency of use.
The expanding use of fast‐acting NMDA receptor antagonists for psychiatric disorders is a significant risk to the public. The appropriate clinical use and safety precautions need to be reassessed from a public health and regulatory perspective.
AUTHOR CONTRIBUTIONS
TJM drafted the manuscript. TJM, DRM, AA, and GCA contributed to the study conception and design. TJM, AA, and DRM led the data collection and review. TJM, DRM, GCA, and AA provided critical review of drafts and approved the final manuscript.
CONFLICT OF INTEREST
GCA is a current member and past Chair of FDA's Peripheral and Central Nervous System Advisory Committee; is a co‐founding Principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a member of OptumRx's National P&T Committee. These arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict‐of‐interest policies.
ETHICS APPROVAL
This review of previously published documents is exempt from review.
CONSENT FOR PUBLICATION
All coauthors approved the final version of this manuscript and agreed to submit it for publication.
Moore TJ, Alami A, Alexander GC, Mattison DR. Safety and effectiveness of NMDA receptor antagonists for depression: A multidisciplinary review. Pharmacotherapy. 2022;42:567‐579. doi: 10.1002/phar.2707
[Correction added on July 8, 2022 after first online publication. One of the affiliations of Donald R. Mattison has been corrected in this version.]
REFERENCES
- 1. Prescribing information for KETALAR (ketamine hydrochloride) Injection for Intravenous or Intramuscular Use, CIII. Package insert. Par Pharmaceutical; 2020. [Google Scholar]
- 2. Prescribing information for SPRAVATO (esketamine) Nasal Spray, CIII. Package insert. Janssen Pharmaceuticals, Inc.; 2020. [Google Scholar]
- 3. European Medicines Agency . Spravato [Internet]. European Medicines Agency Web Site. 2019. Cited September 24, 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/spravato
- 4. Bertron JL, Seto M, Lindsley CW. DARK classics in chemical neuroscience: phencyclidine (PCP). ACS Chem Nerosci. 2018;9:2459‐2474. [DOI] [PubMed] [Google Scholar]
- 5. Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: a paradigm shift for depression research and treatment. Neuron. 2019;101:774‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A‐S, White LE. Neuroscience. 5th ed. Sinauer Associates is an imprint of Oxford University Press; 2011. [Google Scholar]
- 7. Domino EF, Warner DS. Taming the ketamine Tiger. Anesthesiology. 2010;113:678‐684. [DOI] [PubMed] [Google Scholar]
- 8. Bodi T, Share I, Levy H, Moyer JH. Clinical trial of phencyclidine (sernyl) in patients with psychoneurosis. Antibiotic Med Clin Ther. 1959;6:79‐84. [PubMed] [Google Scholar]
- 9. Rosenbaum G, Cohen BD, Luby ED, Gottlieb JS, Yelen D. Comparison of sernyl with other drugs: simulation of schizophrenic performance with sernyl, LSD‐25, and amobarbital (amytal) sodium; I. attention, motor function, and proprioception. AMA Arch Gen Psychiatry. 1959;1:651‐656. [DOI] [PubMed] [Google Scholar]
- 10. Lodge D, Mercier MS. Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacol. 2015;172:4254‐4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh JB, Fedgchin M, Daly EJ, et al. A double‐blind, randomized, placebo‐controlled, dose‐frequency study of intravenous ketamine in patients with treatment‐resistant depression. Am J Psychiatry. 2016;173:816‐826. [DOI] [PubMed] [Google Scholar]
- 12. Elayan IM. Secondary Pharmacology/Toxicology Review: Drug: SPRAVATO (Esketamine) [Internet]. Food and Drug Administration, Center for Drug Evaluation and Research; 2019. [Google Scholar]
- 13. Overall JE, Gorham DR. The brief psychiatric rating scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97‐99. [PubMed] [Google Scholar]
- 14. Malhotra AK, Pinals DA, Weingartner H, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301‐307. [DOI] [PubMed] [Google Scholar]
- 15. Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455‐467. [DOI] [PubMed] [Google Scholar]
- 16. Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the clinician‐administered dissociative states scale (CADSS). J Trauma Stress. 1998;11:125‐136. [DOI] [PubMed] [Google Scholar]
- 17. Acevedo‐Diaz EE, Cavanaugh GW, Greenstein D, et al. Comprehensive assessment of side effects associated with a single dose of ketamine in treatment‐resistant depression. J Affect Disord. 2020;263:568‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bey T, Patel A. Phencyclidine intoxication and adverse effects: a clinical and pharmacological review of an illicit drug. Cal J Emerg Med. 2007;8:9‐14. [PMC free article] [PubMed] [Google Scholar]
- 19. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351‐354. [DOI] [PubMed] [Google Scholar]
- 20. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database of Systematic Reviews [Internet]. 2015. Cited May 17, 2021. https://www.readcube.com/articles/10.1002%2F14651858.CD011612.pub2 [DOI] [PubMed]
- 21. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta‐analysis. Hum Psychopharmacol. 2015;30:152‐163. [DOI] [PubMed] [Google Scholar]
- 22. Lee EE, Della Selva MP, Liu A, Himelhoch S. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta‐analysis. Gen Hosp Psychiatry. 2015;37:178‐184. [DOI] [PubMed] [Google Scholar]
- 23. Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta‐analysis. Psychopharmacology (Berl). 2014;231:3663‐3676. [DOI] [PubMed] [Google Scholar]
- 24. Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P T. 2008;33:700‐711. [PMC free article] [PubMed] [Google Scholar]
- 25. Olney JW, Labruyere J, Price MT. Pathological changes induced in Cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360‐1362. [DOI] [PubMed] [Google Scholar]
- 26. Kaindl AM, Ikonomidou C. Glutamate antagonists are neurotoxins for the developing brain. Neurotox Res. 2007;11:203‐218. [DOI] [PubMed] [Google Scholar]
- 27. Kim J, Chen Q. Clinical Review: NDA 211243 Esketamine (Spravato) [Internet]. US Food and Drug Administration, Center for Drug Evaluation and Research; 2019:287. [Google Scholar]
- 28. Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed‐dose Esketamine nasal spray combined with a new Oral antidepressant in treatment‐resistant depression: results of a randomized, double‐blind, active‐controlled study (TRANSFORM‐1). Int J Neuropsychopharmacol. 2019;22:616‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed Esketamine nasal spray combined with a newly initiated Oral antidepressant in treatment‐resistant depression: a randomized double‐blind active‐controlled study. Am J Psychiatry. 2019;176:428‐438. [DOI] [PubMed] [Google Scholar]
- 30. Ochs‐Ross R, Daly EJ, Zhang Y, et al. Efficacy and safety of Esketamine nasal spray plus an Oral antidepressant in elderly patients with treatment‐resistant depression—TRANSFORM‐3. Am J Geriatr Psychiatry. 2020;28:121‐141. [DOI] [PubMed] [Google Scholar]
- 31. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal Esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double‐blind, randomized placebo‐controlled study. Am J Psychiatry. 2018;175:620‐630. [DOI] [PubMed] [Google Scholar]
- 32. Ionescu DF, Fu D‐J, Qiu X, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double‐blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2021;24:22‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fu D‐J, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double‐blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81:19m13191. [DOI] [PubMed] [Google Scholar]
- 34. Kirsch I, Deacon BJ, Huedo‐Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta‐analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turner EH. Esketamine for treatment‐resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry. 2019;6:977‐979. [DOI] [PubMed] [Google Scholar]
- 36. Gastaldon C, Papola D, Ostuzzi G, Barbui C. Esketamine for treatment resistant depression: a trick of smoke and mirrors? Epidemiol Psychiatr Sci. 2019;29:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cosgrove L, Naudet F, Högberg G, Shaughnessy AF, Cristea IA. Reconceptualising treatment‐resistant depression as difficult‐to‐treat depression. Lancet Psychiatry. 2021;8:11‐13. [DOI] [PubMed] [Google Scholar]
- 38. Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment‐resistant depression — first FDA‐approved antidepressant in a new class. N Engl J Med. 2019;381:1‐4. [DOI] [PubMed] [Google Scholar]
- 39. Sun L, Li Q, Li Q, et al. Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys. Addict Biol. 2014;19:185‐194. [DOI] [PubMed] [Google Scholar]
- 40. Yeung LY, Wai MSM, Fan M, et al. Hyperphosphorylated tau in the brains of mice and monkeys with long‐term administration of ketamine. Toxicol Lett. 2010;193:189‐193. [DOI] [PubMed] [Google Scholar]
- 41. Zhong J, Wu H, Wu F, et al. Cortical thickness changes in chronic ketamine users. Front Psych. 2021;12:645471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mathew SV. Pharmacology/Toxicology NDA Review and Evaluation: NDA 211243 SPRAVATO (Esketamine) [Internet]. US Food and Drug Administration, Center for Drug Evaluation and Research; 2019:70 https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000PharmR.pdf [Google Scholar]
- 43. Marc . Risk Evaluation and Mitigation Strategy (REMS) Document: SPRAVATO (Esketamine Hydrochloride) REMS Program [Internet]. US Food and Drug Administration, Center for Drug Evaluation and Research; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000REMS.pdf [Google Scholar]
- 44. Ketamine Directory ‐ Find verified ketamine treatment providers [Internet]. Ketamine Directory. 2022. Cited May 7, 2022. https://www.ketaminedirectory.com
- 45. FDA alerts health care professionals of potential risks associated with compounded ketamine nasal spray [internet]. Food and Drug Administration web site. FDA. 2022. Cited May 7, 2022. https://www.fda.gov/drugs/human‐drug‐compounding/fda‐alerts‐health‐care‐professionals‐potential‐risks‐associated‐compounded‐ketamine‐nasal‐spray
- 46. Mundkur M, Cotter S, Wong J. Pharmacovigilance and Drug Utilization Review: Ketalar (Ketamine Hydrochloride). Food and Drug Administration, Center for Drug Evaluation and Research; 2018. [Google Scholar]
- 47. Short B, Fong J, Galvez V, Shelker W, Loo CK. Side‐effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78. [DOI] [PubMed] [Google Scholar]
- 48. Wajs E, Aluisio L, Holder R, et al. Esketamine nasal spray plus Oral antidepressant in patients with treatment‐resistant depression: assessment of long‐term safety in a phase 3, open‐label study (SUSTAIN‐2). J Clin Psychiatry. 2020;81. doi: 10.4088/JCP.19m12891 [DOI] [PubMed] [Google Scholar]
- 49. McCambridge J, Winstock A, Hunt N, Mitcheson L. 5‐year trends in use of hallucinogens and other adjunct drugs among UKdance drug users. Eur Addict Res. 2007;13:57‐64. [DOI] [PubMed] [Google Scholar]
- 50. Morgan CJA, Curran HV, Independent Scientific Committee on Drugs . Ketamine use: a review. Addiction. 2012;107:27‐38. [DOI] [PubMed] [Google Scholar]
- 51. Muetzelfeldt L, Kamboj SK, Rees H, Taylor J, Morgan CJA, Curran HV. Journey through the K‐hole: phenomenological aspects of ketamine use. Drug Alcohol Depend. 2008;95:219‐229. [DOI] [PubMed] [Google Scholar]


