Abstract
Several studies have reported differences in the morphological characteristics of motoneurons and the contractile properties of motor units of male and female rats. However, differences in spinal motoneuron activity between the sexes are not well understood. This study investigates the electrophysiological properties of spinal α‐motoneurons in male and female Wistar rats under pentobarbital anaesthesia. Fast and slow types of tibial motoneurons were recorded intracellularly in 15 male and 15 female rats, and the measured parameters were compared statistically using two‐way ANOVA and Tukey post hoc tests. The membrane properties, action potential parameters and firing characteristics were not different between sexes, though significant differences were observed in the properties of fast and slow motoneuron types within both sex groups. We conclude that the sex‐related differences observed in motor performance between male and female rats are largely due to differences in muscle mass, the proportion of muscle fibre types and the related motor unit contractile properties, while the mechanisms of motor control dependent on the electrophysiological activity of motoneurons are similar between the sexes. These findings are significant, as they indicate that results of experiments investigating electrophysiological properties can be reliably compared between sexes.
Keywords: membrane properties, motoneuron, rat, rhythmic firing, sex differences
The study indicates that the intrinsic properties, excitability and firing characteristics of spinal motoneurons innervating hind limb muscles do not differ between male and female rats, and therefore, results of experiments investigating electrophysiological properties can be reliably compared between sexes. We conclude that the observed sex differences in motor performance are primarily due to peripheral differences in muscle organisation and motor unit contractile properties.
List of abbreviations
- AHP
afterhyperpolarisation
- AHP–HDT
afterhyperpolarisation half‐decay time
- amp
amplitude
- AP
action potential
- APhalf‐width
action potential duration measured at the level of half‐amplitude
- DT
doublet threshold
- ESF
early‐state firing
- f–I
frequency current relationship
- freq
frequency
- i.p.
intraperitoneally
- i.v.
intravenously
- ISI
initial interspike interval
- max
maximum
- min
minimum
- MN
motoneuron
- Rheo
rheobase
- RIN
input resistance
- RINSag ratio
ratio between peak and plateau input resistance
- RMP
resting membrane potential
- SSF
steady‐state firing
- vs.
versus
- VT
voltage threshold
1. INTRODUCTION
Skeletal muscles are strikingly different in male and female animals, particularly in their basic morphometric characteristics, the number of motor units and their contractile properties. Systematic studies on the sexual dimorphism of the rat medial gastrocnemius muscle have indicated that male and female rats differ considerably in the size and strength of the muscle as a whole and the morphometric parameters of the muscle fibres (Mierzejewska‐Krzyżowska et al., 2011). Sexual dimorphism is also evident as differences in the number, innervation ratio, proportion and contractile properties of particular types of motor units (Celichowski & Drzymała, 2006; Celichowski & Drzymała‐Celichowska, 2007). Differences in the morphology of cell bodies of motoneurons (MNs) innervating the medial gastrocnemius muscle (Mierzejewska‐Krzyżowska et al., 2014, 2019) suggest that the electrophysiological properties of MNs (including the input resistance and other parameters determining cell excitability and the threshold and frequencies of rhythmic firing) may also differ between male and female rats. If this is the case, these differences would affect central mechanisms of motor control of the same muscle in both sexes. The electrophysiological properties of the MNs innervating rat muscles have been investigated by numerous research studies performed on either male (Bączyk et al., 2013; Carp et al., 2008; Krutki et al., 2015) or female rats (Bakels & Kernell, 1993; Beaumont & Gardiner, 2003; Cormery et al., 2005; Li et al., 2007; MacDonell et al., 2015). Data from male and female rats are less frequently combined in a single study (Cholanian et al., 2017; Nishimura et al., 2018). Surprisingly, the studies cited above show numerous divergent or partially overlapping ranges of values for some electrophysiological parameters. It is therefore difficult to determine whether sexual dimorphism is responsible for differences in the membrane and firing properties of spinal MNs, as the results of these independent research projects could be affected by different experimental conditions, the strain (Wistar, Sprague–Dawley, Fischer) and age of rats used (Kalmar et al., 2009), and the intensity of their daily motor activities (Bączyk et al., 2013; Beaumont & Gardiner, 2003; Beaumont et al., 2004; Button et al., 2008; Cormery et al., 2005; Krutki et al., 2015).
This study is the first to compare the electrophysiological properties of MNs of male and female rats of the same age (6 months old), under consistent experimental conditions in one series of experiments. The presence of differences between the sexes in the basic properties of MNs would require us to reconsider the results of some previous reports and would emphasise the importance of using only one sex of rat in spinal MN experiments. We used intracellular recording techniques to investigate membrane and firing properties of MNs innervating muscles of the hind limbs via the tibial nerve. Fast and slow types of tibial MNs were evaluated separately, as they differ considerably in their electrophysiological properties, and play different roles in the control of contractions of fast and slow motor units (Gardiner & Kernell, 1990).
2. MATERIALS AND METHODS
2.1. Animals
Experiments were performed on 30 six‐month‐old Wistar rats: 15 females (mean body weight 281 ± 46 g) and 15 males (mean body weight 464 ± 56 g). Animals were housed in standard cages (two per cage), with unlimited access to food and water in a room with a reverse light–dark cycle (12 h/12 h). Humidity and temperature were maintained at 55% ± 10% and 22 ± 2°C, respectively. All procedures were designed to minimise the suffering of animals; our methods were approved by the Local Ethics Committee and performed in accordance with the Polish Law on the Protection of Animals and European Union guidelines.
2.2. Surgical preparation
Rats were anaesthetised deeply with an intraperitoneal injection of sodium pentobarbital (initial dose 60 mg kg−1 i.p., supplemented with additional doses of 10 mg kg−1 h−1 i.v.). Appropriate depth of anaesthesia was indicated by a lack of pinna and withdrawal reflexes during preparation and by continuous observation of heart rate (300–360 beats per minute) using electrocardiography during the recording session. At the end of the experiments, animals were euthanised using an overdose of pentobarbital sodium (180 mg kg−1 i.p.).
Initial surgical procedures included catheterisation of the jugular vein for drug administration and a tracheotomy for insertion of a tracheal tube. The rats were artificially ventilated (SAR‐830, CWE), and the level of CO2 in the expired air was measured (Capstar 100, CWE) and maintained in a range of 2%–4% by adjusting air volume and ventilation rate. Pancuronium bromide (Pancuronium, Jelfa) was administered intravenously at regular intervals (initial dose 0.4 mg kg−1 i.v., supplemented every 30 min with doses of 0.2 mg kg−1 i.v.) to induce and maintain paralysis of muscles during the recording session. Respiratory movements were minimised by creating a pneumothorax on the side of recording.
The tibial nerve (providing innervation to the triceps surae, plantaris, tibialis posterior, flexor digitorum longus and flexor hallucis longus muscles) was dissected and mounted on a bipolar silver wire electrode for stimulation. Laminectomy was performed over the L4–L5 spinal segments to expose the surface of the spinal cord. The dura mater was then removed, and small holes were made in the pia for the insertion of glass micropipettes.
Animals were fixed using clamps in a metal frame, and the exposed regions of the lumbar spinal cord and dissected nerves were covered with paraffin oil in small pools formed by skin flaps. The core body and oil temperature were continuously monitored and kept within physiological limits (37 ± 1°C) using a thermostatically controlled heating system (Fine Science Tools).
2.3. Stimulation and recording
The tibial nerve was stimulated with electrical pulses (3 Hz, duration 0.1 ms, amplitude up to 0.5 V) generated by a square‐pulse stimulator (GRASS Instruments; model S88). Glass micropipettes (tips broken to 1.5–2.0 μm in external diameter, with impedances of 10–20 MΩ, filled with a 2 M solution of potassium citrate) were introduced into the spinal cord with a motor‐driven manipulator in 2 μm steps. Recordings from single MNs were obtained using an intracellular amplifier system (AxoClamp model 2B stimulator, Axon Instruments) in bridge mode or discontinuous current‐clamp mode (current switch rate mode 5.5–8 kHz), with electrode capacitance maximally compensated. MNs innervating hind limb muscles were identified on the basis of antidromic action potentials evoked by stimulation of the nerve (Figure 1a). The antidromic character of a spike was confirmed by its constant and short latency and ‘all‐or‐nothing’ appearance. Following antidromic identification, intracellular depolarising currents were injected into the MN to induce an orthodromic action potential (AP). For each MN, an average of 20 orthodromic spikes were used to calculate basic AP properties, including AP amplitude (AP amp), AP duration measured at the level of half‐amplitude (AP half‐width), afterhyperpolarisation amplitude (AHP amp) and AHP half‐decay time (AHP–HDT) (Figure 1b). Only stable recordings with resting membrane potentials (RMPs) of at least −50 mV and AP amplitudes over 50 mV with a positive overshoot were included in the dataset.
FIGURE 1.
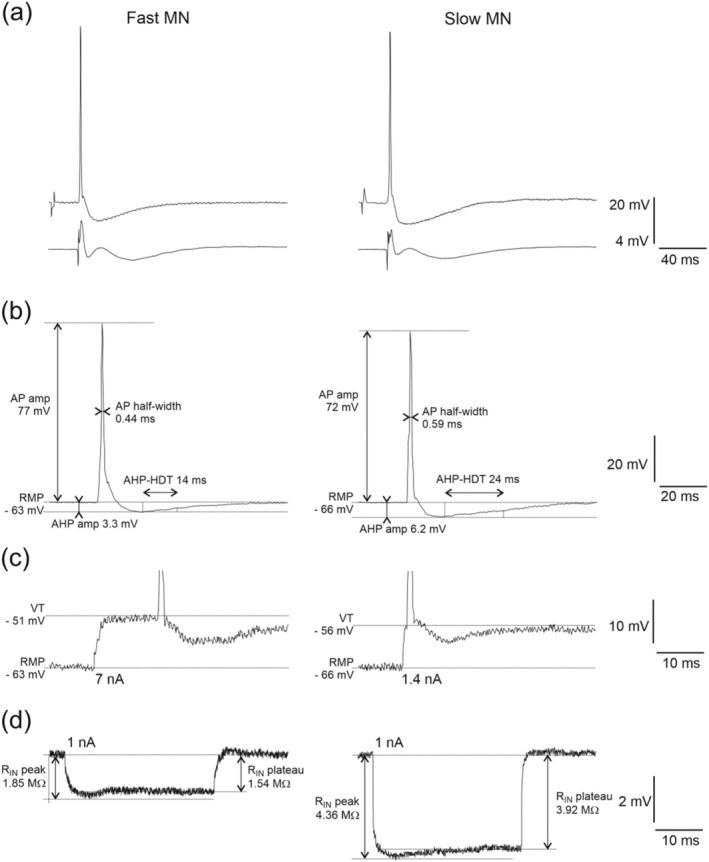
Examples of recordings from a fast MN (left column) and slow MN (right column). (a) Antidromic action potentials evoked by stimulation of the tibial nerve recorded intracellularly (upper traces), and incoming volleys recorded from the surface of the spinal cord at the dorsal root entry zone (lower traces). (b) Orthodromic action potentials evoked by intracellular stimulation with indicated measured parameter (AHPamp, afterhyperpolarisation amplitude; AHP–HDT, afterhyperpolarisation half‐decay time; AP half‐width, action potential half‐width; APamp, action potential amplitude). (c) Expanded voltage traces of the rheobase spikes evoked with the intracellular current injection of 7 nA (for fast MN) or 1.4 nA (for slow MN). Horizontal lines indicate the resting membrane potential (RMP) and spike voltage threshold (VT) for each example. (d) Responses to the intracellular injection of 1 nA hyperpolarising current pulse for calculation of peak input resistance (RIN peak) and plateau input resistance (RIN plateau)
The peak and plateau input resistance (RIN) of each MN was calculated from the average deflection in membrane potential evoked by 40 short pulses (100 ms) of 1 nA hyperpolarising current (Figure 1d). Rheobase (Rheo) was determined as the minimum amplitude of current required to elicit a single spike within 50 ms of a 500 ms square‐wave pulse (Figure 1c). The membrane voltage at which an action potential was elicited (voltage threshold, VT) was also determined from these recordings as the point at which the first derivative of the voltage reached 10 mV ms−1 (Sekerli et al., 2004).
Injections of square‐wave pulses of depolarising current lasting 500 ms were applied at increasing amplitudes in steps of 0.1–2 nA to evoke rhythmic discharges of MNs until blocking occurred before the end of the 500 ms period (Figure 2a), and the minimum and maximum currents for rhythmic firing were determined. The linear relationship between the discharge frequency and injected current (f–I) was assessed for each MN based on the equation ‘y = ax + b’, where ‘a’ determines the slope of the relationship (Figure 2b). The f–I slope was determined for the initial interspike interval (ISI), by calculating the instantaneous frequency and current of the first ISI; the early‐state firing (ESF), by calculation of the mean of the first three intervals; and the steady‐state firing (SSF), by calculation of the mean of the last three intervals. The f–I relation for the ISI was used to determine whether a secondary range was present in a motoneuron (Figure 2b, left panel) by visual inspection. If distinct primary and secondary ranges occurred, the f–I slope was calculated for the primary range. The doublet threshold (DT) was also determined from these recordings as the current intensity necessary to evoke the initial doublet of spikes (with the first interspike interval <5 ms). Moreover, the ratio of the minimum current for rhythmic firing to rheobase was calculated for each MN. During the experimental session, all recordings were amplified (Axon Instruments; AxoClamp model 2B) and were passed through an analog‐to‐digital 16‐bit converter (National Instruments, USB‐6341) at a sampling rate of 10 kHz. The recordings were then stored offline for further analysis.
FIGURE 2.
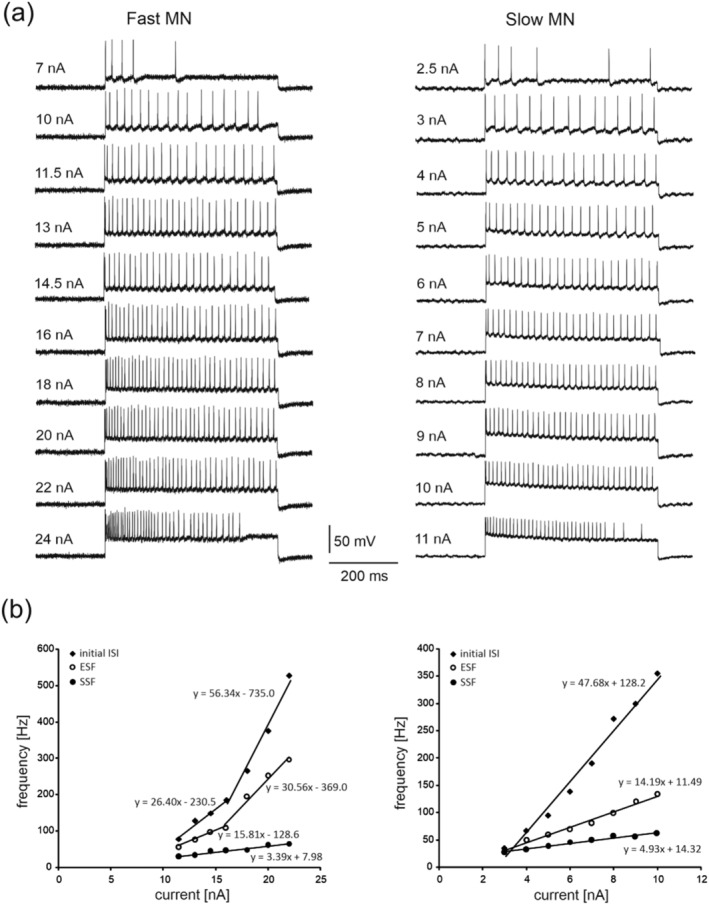
Rhythmic firing of the same fast MN (left column) and slow MN (right column) as in Figure 1. (a) Examples of MN firing in response to a 500 ms intracellular depolarising current of increasing intensity. Note that the MNs begin rhythmic firing during the entire 500 ms at a current intensity of 11.5 nA (fast MN) and 3 nA (slow MN) and continue steady‐state firing up to 22 nA (fast MN) and 10 nA (slow MN); (b) the f–I curves plotted for the two MNs, based on all rhythmic firing recordings. The frequencies of initial interspike interval (ISI), early‐state firing (ESF), and steady‐state firing (SSF) are presented for each MN. Note the two distinct linear ranges of firing determined for the ISI and ESF for the fast MN, and the single linear range of firing for the slow MN. The regression lines were determined according to the equations provided for each plot
2.4. Statistical analysis
All data were presented as mean ± SD. All data groups were tested for normal distribution (Shapiro–Wilk test) and equal variance (F test). The data were analysed using a two‐way analysis of variance (ANOVA) with sex (female vs. male) and MN type (fast vs. slow) as fixed factors. Tukey's HSD post hoc tests were performed to compare the pairs of means. The significance level was set at p < 0.05. The Pearson correlation coefficient was calculated for the relationship between Rheo and input conductance and between RMP and VT, and the linear regression was then derived. An equal‐slope test was performed to compare the slopes of regression lines derived for these correlations.
3. RESULTS
3.1. Motoneuron sampling
A total of 135 MNs were analysed: 70 in the female group and 65 in the male group. Typically, four to eight MNs were successfully recorded in one experiment. All cells were antidromically identified as tibial MNs. All recorded cells were identified as fast or slow MNs on the basis of AHP–HDT, which was ˂20 ms for fast and ˃20 ms for slow MNs (Beaumont & Gardiner, 2002). The proportion of slow MNs was similar in both groups (21/70 cells [30%] in the female group and 19/65 cells [29%] in the male group).
3.2. Membrane and action potential properties
As shown in Table 1, no significant differences in membrane properties and AP characteristics were observed when F and S MNs were compared in male and female rats. In contrast, significant differences between fast and slow MNs were identified in the AHP–HDT, which were shorter for fast MNs (F 1,131 = 929.41, p ˂ 0.0001, post hoc tests p ˂ 0.0001 for females, and p ˂ 0.0001 for males), and in the AHP amplitude, which were lower for fast MNs (F 1,131 = 36.84, p ˂ 0.0001, post hoc tests p ˂ 0.0001 for females, p = 0.0002 for males). Moreover, values of input resistance were higher for slow MNs (the peak RIN: F 1,131 = 35.59, p ˂ 0.0001, post hoc tests p = 0.0002 for females, p = 0.0016 for males; the plateau RIN: F 1,131 = 36.84, p ˂ 0.0001, post hoc tests p = 0.0002 for females, p = 0.0017 for males). Additionally, a shorter AP half‐width was noted for fast MNs in males (F 1,131 = 12.36, p = 0.0006, post hoc tests p = 0.0087).
TABLE 1.
Basic membrane and action potential properties of male and female MNs
| Females (n = 70) | Males (n = 65) | |||
|---|---|---|---|---|
| Fast (n = 49) | Slow (n = 21) | Fast (n = 46) | Slow (n = 19) | |
| RMP (mV) | −64.54 ± 6.92 | −61.75 ± 4.66 | −67.45 ± 5.63 | −63.10 ± 6.21 |
| AP amp (mV) | 74.29 ± 10.16 | 69.71 ± 9.71 | 76.04 ± 10.05 | 69.48 ± 8.43 |
| AP half‐width (ms) | 0.52 ± 0.07 | 0.56 ± 0.08 | 0.50 ± 0.07 | 0.57 ± 0.08** |
| AHP amp (mV) | 3.29 ± 0.99 | 4.74 ± 1.56** | 3.26 ± 1.23 | 4.83 ± 1.62** |
| AHP–HDT (ms) | 12.36 ± 1.74 | 23.42 ± 2.09** | 12.02 ± 1.61 | 23.46 ± 2.17** |
| RIN peak (MΩ) | 2.76 ± 1.02 | 4.18 ± 1.68** | 2.56 ± 0.88 | 3.89 ± 0.93** |
| RIN plateau (MΩ) | 2.30 ± 0.88 | 3.58 ± 1.44** | 2.15 ± 0.81 | 3.32 ± 0.83** |
| RINSag ratio | 1.19 ± 0.07 | 1.17 ± 0.07 | 1.20 ± 1.44 | 1.17 ± 0.06 |
Note: Values averaged across all recorded neurons in each group are presented as the mean ± SD. No significant differences were found between male and female rats.
Abbreviations: AHPamp, afterhyperpolarisation amplitude; AHP–HDT: afterhyperpolarisation half‐decay time; APamp, action potential amplitude; APhalf‐width, action potential duration measured at the level of half‐amplitude; n, number of MNs; RIN, input resistance; RINSag ratio, ratio between peak and plateau input resistance; RMP, resting membrane potential.
Significant differences compared to fast MNs at p < 0.05.
Significant differences compared to fast MNs at p < 0.01 (two‐way ANOVA with sex and MN type as fixed factors and Tukey's HSD post hoc tests).
3.3. Threshold and rhythmic firing properties
Threshold and firing properties of MNs were not found to differ between male and female rats (Table 2). However, the rheobase values were higher for fast MNs in both female and male groups, compared to slow MNs (F 1,131 = 82.57, p ˂ 0.0001, post hoc tests p ˂ 0.0001 for females, p ˂ 0.0001 for males), and this was also the case for the doublet threshold (F 1,131 = 63.59, p ˂ 0.0001, post hoc tests p ˂ 0.0001 for females, p ˂ 0.0001 for males). A significant correlation was found between rheobase and input conductance in MNs (see Figure 3). The Pearson correlation coefficients in female and male groups were 0.56 (p ˂ 0.05) and 0.65 (p ˂ 0.05) for fast MNs and 0.64 (p ˂ 0.05) and 0.62 (p ˂ 0.05) for slow MNs, respectively. The slopes of the regression lines did not differ between female and male MNs (p = 0.73 and p = 0.32, for fast and slow MNs, respectively, an equal‐slope test). As the ability of MNs to produce discharges can be affected by changes in membrane potential, the relationship between RMP and VT was analysed separately for fast and slow MNs. Figure 4 presents the significant correlations between these two parameters in both female and male rats. The Pearson correlation coefficients in females were 0.51 (p ˂ 0.05) and 0.72 (p ˂ 0.05) for fast and slow MNs, respectively. In males, these values were 0.52 (p ˂ 0.05) and 0.71 (p ˂ 0.05). The slopes of the regression lines did not differ between female and male MNs (p = 0.78 and p = 0.84, for fast and slow MNs, respectively, an equal‐slope test), suggesting that variations in RMP influence MN firing abilities equally in both sexes.
TABLE 2.
Threshold and firing properties of female and male MNs
| Females (n = 70) | Males (n = 65) | |||
|---|---|---|---|---|
| Fast (n = 49) | Slow (n = 21) | Fast (n = 46) | Slow (n = 19) | |
| Rheo (nA) | 9.37 ± 4.85 | 2.32 ± 1.52** | 9.85 ± 4.75 | 2.77 ± 1.25 ** |
| VT (mV) | −45.11 ± 0.16 | −49.26 ± 6.34 | −44.50 ± 7.33 | −48.10 ± 5.88 |
| Min current (nA) | 14.50 ± 7.15 | 3.71 ± 2.20** | 13.74 ± 6.32 | 4.13 ± 1.61** |
| Min current/Rheo | 1.45 ± 0.16 | 1.57 ± 0.29 | 1.44 ± 0.24 | 1.54 ± 0.25 |
| Max current (nA) | 25.69 ± 9.45 | 10.35 ± 4.40** | 28.34 ± 6.99 | 12.49 ± 3.99** |
| Min initial ISI freq (Hz) | 83.44 ± 63.88 | 49.37 ± 26.99 | 89.48 ± 68.59 | 37.53 ± 31.28* |
| Max initial ISI freq (Hz) | 504.57 ± 109.01 | 429.53 ± 124.72 | 527.37 ± 133.68 | 405.16 ± 126.45 |
| Initial ISI f–I slope (Hz·nA−1) | 47.02 ± 17.45 | 54.86 ± 20.57 | 40.10 ± 16.04 | 52.00 ± 19.71 |
| DT (nA) | 19.17 ± 8.82 | 7.97 ± 3.80** | 19.05 ± 6.71 | 8.89 ± 3.47** |
| Min ESF freq (Hz) | 50.55 ± 15.46 | 38.24 ± 16.89* | 49.65 ± 16.6 | 29.69 ± 11.29** |
| Max ESF freq (Hz) | 224.85 ± 110.57 | 162.45 ± 75.64 | 247.34 ± 96.00 | 164.14 ± 78.24* |
| Min SSF freq (Hz) | 30.32 ± 7.74 | 22.76 ± 6.84** | 29.63 ± 7.76 | 22.18 ± 8.32** |
| Max SSF freq (Hz) | 76.33 ± 23.60 | 61.46 ± 18.19 | 82.17 ± 26.64 | 61.32 ± 20.06* |
| SSF f–I slope (Hz·nA−1) | 4.08 ± 1.55 | 5.72 ± 2.67** | 3.59 ± 1.16 | 4.79 ± 1.69* |
Note: Values averaged across all recorded neurons in each group are presented as the mean ± SD. No significant differences were found between male and female rats.
Abbreviations: DT, doublet threshold; initial ISI f–I slope: the slope of the initial interspike interval frequency–current relationship; max current, maximum current for rhythmic firing; max ESF freq, maximum early‐state firing frequency; max initial ISI freq, maximum initial interspike interval frequency; max SSF freq, maximum steady‐state firing frequency; min current, minimum current for rhythmic firing; min current/Rheo, ratio of the minimum SSF current to rheobase; min ESF freq, minimum early‐state firing frequency; min initial ISI freq, minimum initial interspike interval frequency; min SSF freq, minimum steady‐state firing frequency; n, number of MNs; Rheo, rheobase current; SSF f–I slope, the slope of the steady‐state firing frequency–current relationship; VT, voltage threshold for spike generation.
Significant differences compared to fast MNs at p < 0.05.
Significant differences compared to fast MNs at p < 0.01 (two‐way ANOVA with sex and MN type as fixed factors and Tukey's HSD post hoc tests).
FIGURE 3.
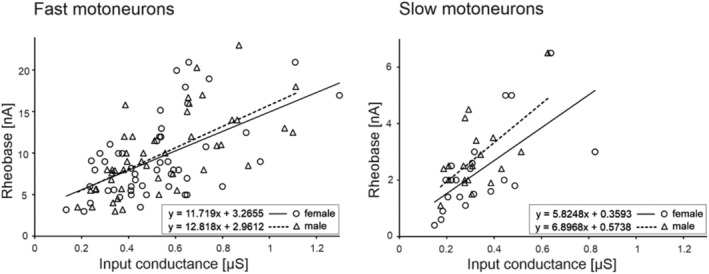
The relationships between rheobase and plateau input conductance (the reciprocal of the plateau RIN) were plotted for fast MNs (left) and slow MNs (right). The regression lines were determined separately for female and male MNs, according to the equations provided in each plot. Note the higher slopes of regression lines for fast MNs compared to slow MNs, but similar slopes for female and male MNs within fast and slow groups (p = 0.73 and p = 0.32, respectively, an equal‐slope test)
FIGURE 4.
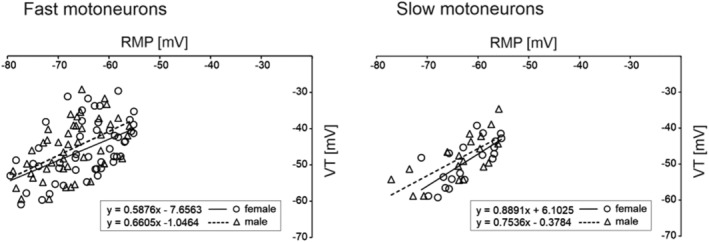
Correlations between resting membrane potential (RMP) and voltage threshold (VT) for fast MNs (left) and slow MNs (right). The regression lines were determined separately for female and male MNs, according to the equations provided in each plot. For each pair of correlations, the slopes of the regression lines are similar for female and male MNs (p = 0.78 and p = 0.84, respectively, an equal‐slope test)
Figure 2a provides examples of the steady‐state firing of two female MNs (fast and slow) at progressively increasing amplitudes of depolarising current. The firing frequency/current (f–I) curves plotted for the three properties investigated for these two representative MNs are presented in Figure 2b as the initial interspike interval (ISI), the early‐state firing (ESF) and the steady‐state firing (SSF), based on all rhythmic firing recordings (Figure 1a). MN rhythmic firing properties that are crucial for muscle activation and force production are listed in Table 2. The MN firing and threshold properties did not differ significantly between females and males within fast and slow MN groups, though numerous differences in the firing properties of these MN types were evident within both female and male groups. The minimum currents required to produce rhythmic firing were higher for fast MNs (F 1,131 = 74.46, p ˂ 0.0001, post hoc tests p ˂ 0.0001 for females and p ˂ 0.0001 for males), as were the maximum currents for rhythmic firing (F 1,131 = 107.25, p ˂ 0.0001, post hoc tests p ˂ 0.0001 for females and p ˂ 0.0001 for males). Moreover, the minimum SSF frequencies were higher for fast MNs in both sex groups (F 1,131 = 21.62, p ˂ 0.0001, post hoc tests p = 0.0131 for females and p = 0.0035 for males), while the maximum SSF frequencies were significantly higher for fast MNs in the male group only (F 1,131 = 12.64, p = 0.0005, post hoc tests p = 0.0115). The mean slopes of the f–I relationships were significantly higher for slow MNs (F 1,131 = 25.44, p ˂ 0.0001, post hoc tests p ˂ 0.0001 for females and p = 0.0169 for males). The minimum ESF frequencies were higher for fast MNs in both sex groups (F 1,131 = 25.44, p ˂ 0.0001, post hoc tests p = 0.0220 for females and p ˂ 0.0001 for males), while the maximum ESF frequencies were significantly higher for fast MNs only in the male group (F 1,131 = 13.15, p = 0.0004, post hoc tests p = 0.0103). Finally, the minimum ISI frequency was significantly higher for fast MNs in the male group only (F 1,131 = 12.49, p = 0.0005, post hoc tests p = 0.0081).
4. DISCUSSION
This study found no significant difference in the basic electrophysiological properties of male and female rat MNs. These results were unexpected, given the numerous studies that indicate considerable sexual dimorphism in muscle mass, morphology, motor innervation, fibre biochemical composition and motor unit contractility (Celichowski & Drzymała‐Celichowska, 2007; Drzymała‐Celichowska & Krutki, 2015; Eason et al., 2000; English et al., 1999; English & Widmer, 2003; Mierzejewska‐Krzyżowska et al., 2011), which appear to be essential for the obvious differences in motor performance observed between male and female animals (Laudato et al., 2021; Mortreux et al., 2021) and humans (Hicks et al., 2001; Roberts et al., 2020). Differences between sexes in the electrophysiological properties of MNs would have a significant impact on the recruitment of motor units and the rate of force development during contractions.
Data from the literature suggests that, during muscle activity, the MN firing rate corresponds to the steep part of the force–frequency curve of the motor unit response (Hennig & Lømo, 1987; Kernell, 1979). It has been shown that all motor unit types of the medial gastrocnemius are stronger in male rats and have longer twitch contraction and relaxation times. The steep parts of the force–frequency relationships of motor units are also shifted towards the lower frequencies in male rats, compared to female rats (Celichowski & Drzymała, 2006). It might, therefore, be expected that the frequencies of rhythmic discharges of MNs, which strongly influence the force–frequency relationships of motor units, should also differ between male and female rats; however, this was not confirmed by our data.
Small but significant differences in the average soma diameter have been reported between the medial gastrocnemius MNs of male and female rats (the diameter in males is higher by just 5.5% on average, Mierzejewska‐Krzyżowska et al., 2014). Cell anatomical features (such as soma size and dendritic arborization extent) can influence electrophysiological properties, and smaller MNs typically have higher input resistance (Manuel & Zytnicki, 2011), lower rheobase (Krutki et al., 2017; Zengel et al., 1985) and higher excitability (Henneman, 1957) than larger MNs. Moreover, the firing frequencies and intracellular current required to induce rhythmic firing appear to be significantly lower in small MNs compared to large MNs (Cormery et al., 2005; Kernell, 1979; Krutki et al., 2017). The smaller size of female MNs was, therefore, expected to influence the threshold properties of MNs. Surprisingly, the passive and threshold MN properties were not found to differ between male and female rats.
It should be noted that the rheobase and input conductance was similarly linearly correlated for both male and female MNs (see Figure 3), suggesting that different sizes of individual MNs across the sample are responsible for the diversity of parameters within each group, rather than alterations in membrane conductance.
The membrane and firing properties of MNs in both male and female rats varied widely. For example, across the entire MN population, the plateau input resistance varied between 0.77 and 6.76 MΩ, the rheobase between 0.4 and 23 nA and the voltage threshold between −29.09 and −69.79 mV. A similar range of values was observed for the minimum current necessary to evoke rhythmic firing (between 0.5 and 30 nA), and the slopes of f–I relationships (between 1.6 and 11.9 Hz nA−1 for SSF, between 5.6 and 33.1 Hz nA−1 for ESF and between 13.4 and 93 Hz nA−1 for the initial ISI). These results suggest that it is necessary to distinguish between subpopulations of fast and slow types of MNs, consistent with previous observations that their size, intrinsic properties and excitability vary in conjunction with the type of muscle fibre they are innervating (Beaumont & Gardiner, 2002; Gardiner, 1993). Indeed, the results of this study indicate that, for a majority of the membrane and firing properties measured, there are substantial differences between slow and fast MNs when these subpopulations are analysed separately in either male or female rats (see Tables 1 and 2). This suggests that a similar functional organisation of the MN pools exists in the spinal cords of male and female rats.
Our study was performed in the anaesthetised preparation, which makes it possible to measure basic MN properties under highly reduced synaptic influences. On the other hand, MNs in the awake state of an animal are under neuromodulatory control from inhibitory and excitatory postsynaptic potentials, from descending or peripheral input as well as by presynaptic factors. Thus, one cannot rule out that sex differences in MN activity might be present in awake rats.
The results of this comparative study have practical applications. If putative sex differences had been revealed in relation to certain physiological properties, this would provide an important argument for explaining the difficulty (or even impossibility) of comparing respective data from research performed using sexually diverse experimental groups. The consequences of such differences would be important for understanding the functional diversity of motor control systems between sexes. The lack of difference in electrophysiological properties of spinal MNs observed in this study between male and female rats is surprising, particularly given the obvious differences in body weight (adult male rats are twice as heavy as their female counterparts of the same age), the mass of the central nervous system (which is approximately 20% heavier in males) and the resulting differences in several aspects of motor performance. However, our results also indicate that at least part of the central mechanisms controlling the motor output of individual MN types does not differ between the sexes. This suggests that the differences frequently noted by independent studies between the measured properties of MNs in male or female rats are likely to be due to other causes, such as rat breed, age, motor activity and a variety of other conditions and interventions that contribute to the development of MN properties.
5. CONCLUSIONS
In summary, this study demonstrates that, despite previously reported differences in motor output and MN morphology, the electrophysiological properties of spinal MNs innervating hind limbs do not differ significantly between male and female rats. The mean values of membrane and rhythmic firing properties are similar in both sexes, though values measured for individual MNs vary widely in each population. We therefore suggest that the intrinsic properties, excitability and mechanisms controlling the firing activity of MNs are not related to sex, and that the obvious differences in motor performance between males and females are primarily due to peripheral differences in muscle mass, proportion of muscle fibre types and contractile properties.
CONFLICT OF INTEREST
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHORS CONTRIBUTIONS
Experiments were performed at the Department of Neurobiology, Poznań University of Physical Education. JC and PK conceived and designed the study. MB, HDC and PK performed experiments, collected and analysed data; all authors interpreted the results, and HDC and PK drafted the manuscript. All authors revised and approved the final version of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15745.
ACKNOWLEDGEMENTS
The study was supported by the Polish National Science Centre, grant no. 2018/31/B/NZ7/01028.
Drzymała‐Celichowska, H. , Celichowski, J. , Bączyk, M. , & Krutki, P. (2022). The electrophysiological properties of hindlimb motoneurons do not differ between male and female rats. European Journal of Neuroscience, 56(3), 4176–4186. 10.1111/ejn.15745
Edited by: Francisco Alvarez
Funding information Polish National Science Centre, Grant/Award Number: 2018/31/B/NZ7/01028
DATA AVAILABILITY STATEMENT
All supporting data and materials can be accessed at the corresponding author host institution at the Department of Neurobiology, Poznań University of Physical Education, Poland.
REFERENCES
- Bączyk, M. , Hałuszka, A. , Mrówczyński, W. , Celichowski, J. , & Krutki, P. (2013). The influence of a 5‐wk whole body vibration on electrophysiological properties of rat hindlimb spinal motoneurons. Journal of Neurophysiology, 109, 2705–2711. 10.1152/jn.00108.2013 [DOI] [PubMed] [Google Scholar]
- Bakels, R. , & Kernell, D. (1993). Matching between motoneurone and muscle unit properties in rat medial gastrocnemius. Journal of Physiology, 463, 307–324. 10.1113/jphysiol.1993.sp019596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont, E. , & Gardiner, P. (2002). Effects of daily spontaneous running on the electrophysiological properties of hindlimb motoneurones in rats. Journal of Physiology, 540, 129–138. 10.1113/jphysiol.2001.013084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont, E. , & Gardiner, P. F. (2003). Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle & Nerve, 27, 228–236. 10.1002/mus.10308 [DOI] [PubMed] [Google Scholar]
- Beaumont, E. , Houlé, J. D. , Peterson, C. A. , & Gardiner, P. F. (2004). Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle & Nerve, 29, 234–242. 10.1002/mus.10539 [DOI] [PubMed] [Google Scholar]
- Button, D. C. , Kalmar, J. M. , Gardiner, K. , Marqueste, T. , Zhong, H. , Roy, R. R. , Edgerton, V. R. , & Gardiner, P. F. (2008). Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? Journal of Physiology, 586, 529–544. 10.1113/jphysiol.2007.141499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp, J. S. , Tennissen, A. M. , Mongeluzi, D. L. , Dudek, C. J. , Chen, X. Y. , & Wolpaw, J. R. (2008). An in vitro protocol for recording from spinal motoneurons of adult rats. Journal of Neurophysiology, 100, 474–481. 10.1152/jn.90422.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celichowski, J. , & Drzymała, H. (2006). Differences between properties of male and female motor units in the rat medial gastrocnemius muscle. Journal of Physiology and Pharmacology, 57, 83–93. [PubMed] [Google Scholar]
- Celichowski, J. , & Drzymała‐Celichowska, H. (2007). The number of motor units in the medial gastrocnemius muscle of male and female rats. Journal of Physiology and Pharmacology, 58, 821–828. [PubMed] [Google Scholar]
- Cholanian, M. , Wealing, J. , Levine, R. B. , & Fregosi, R. F. (2017). Developmental nicotine exposure alters potassium currents in hypoglossal motoneurons of neonatal rat. Journal of Neurophysiology, 117, 1544–1552. 10.1152/jn.00774.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormery, B. , Beaumont, E. , Csukly, K. , & Gardiner, P. (2005). Hindlimb unweighting for 2 weeks alters physiological properties of rat hindlimb motoneurones. Journal of Physiology, 568, 841–850. 10.1113/jphysiol.2005.091835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzymała‐Celichowska, H. , & Krutki, P. (2015). Slow motor units in female rat soleus are slower and weaker than their male counterparts. Journal of Muscle Research and Cell Motility, 36, 287–295. 10.1007/s10974-015-9408-2 [DOI] [PubMed] [Google Scholar]
- Eason, J. M. , Schwartz, G. , Shirley, K. A. , & English, A. W. (2000). Investigation of sexual dimorphism in the rabbit masseter muscle showing different effects of androgen deprivation in adult and young adult animals. Archives of Oral Biology, 45, 683–690. 10.1016/s0003-9969(00)00030-3 [DOI] [PubMed] [Google Scholar]
- English, A. W. , Eason, J. , Schwartz, G. , & Shirley, A. (1999). Sexual dimorphism in the rabbit masseter muscle: Myosin heavy chain composition of neuromuscular compartments. Cells, Tissues, Organs, 164, 179–191. 10.1159/000016658 [DOI] [PubMed] [Google Scholar]
- English, A. W. , & Widmer, C. G. (2003). Sex differences in rabbit masseter muscle function. Cells, Tissues, Organs, 174, 87–96. 10.1159/000070577 [DOI] [PubMed] [Google Scholar]
- Gardiner, P. F. (1993). Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. Journal of Neurophysiology, 69, 1160–1170. 10.1152/jn.1993.69.4.1160 [DOI] [PubMed] [Google Scholar]
- Gardiner, P. F. , & Kernell, D. (1990). The "fastness" of rat motoneurones: Time‐course of afterhyperpolarization in relation to axonal conduction velocity and muscle unit contractile speed. Pflügers Archiv, 415(6), 762–766. 10.1007/BF02584018 [DOI] [PubMed] [Google Scholar]
- Henneman, E. (1957). Relation between size of neurons and their susceptibility to discharge. Science, 126(3287), 1345–1347. 10.1126/science.126.3287.1345 [DOI] [PubMed] [Google Scholar]
- Hennig, R. , & Lømo, T. (1987). Gradation of force output in normal fast and slow muscles of the rat. Acta Physiologica Scandinavica, 130, 133–142. 10.1111/j.1748-1716.1987.tb08119.x [DOI] [PubMed] [Google Scholar]
- Hicks, A. L. , Kent‐Braun, J. , & Ditor, D. S. (2001). Sex differences in human skeletal muscle fatigue. Exercise and Sport Science Reviews, 29, 109–112. 10.1097/00003677-200107000-00004 [DOI] [PubMed] [Google Scholar]
- Kalmar, J. M. , Button, D. C. , Gardiner, K. , Cahill, F. , & Gardiner, P. F. (2009). Caloric restriction does not offset age‐associated changes in the biophysical properties of motoneurons. Journal of Neurophysiology, 101, 548–557. 10.1152/jn.90617.2008 [DOI] [PubMed] [Google Scholar]
- Kernell, D. (1979). Rhythmic properties of motoneurones innervating muscle fibres of different speed in m. gastrocnemius medialis of the cat. Brain Research, 160, 159–162. 10.1016/0006-8993(79)90612-7 [DOI] [PubMed] [Google Scholar]
- Krutki, P. , Hałuszka, A. , Mrówczyński, W. , Gardiner, P. F. , & Celichowski, J. (2015). Adaptations of motoneuron properties to chronic compensatory muscle overload. Journal of Neurophysiology, 113, 2769–2777. 10.1152/jn.00968.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutki, P. , Mrówczyński, W. , Bączyk, M. , Łochyński, D. , & Celichowski, J. (2017). Adaptations of motoneuron properties after weight‐lifting training in rats. Journal of Applied Physiology, 123, 664–673. 10.1152/japplphysiol.00121.2017 [DOI] [PubMed] [Google Scholar]
- Laudato, J. A. , Tice, A. L. , Cal, J. A. , Gordo, B. S. , & Steiner, J. L. (2021). Effects of alcohol on skeletal muscle contractile performance in male and female mice. PLoS ONE, 16(8), e0255946. 10.1371/journal.pone.0255946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Murray, K. , Harvey, P. J. , Ballou, E. W. , & Bennett, D. J. (2007). Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. Journal of Neurophysiology, 97, 1236–1246. 10.1152/jn.00995.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonell, C. W. , Power, K. P. , Chopek, J. W. , Gardiner, K. R. , & Gardiner, P. F. (2015). Extensor motoneurone properties are altered immediately before and during fictive locomotion in the adult decerebrate rat. Journal of Physiology, 593, 2327–2342. 10.1113/JP270239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel, M. , & Zytnicki, D. (2011). Alpha, beta and gamma motoneurons: Functional diversity in the motor system's final pathway. Journal of Integrative Neuroscience, 10, 243–276. 10.1142/S0219635211002786 [DOI] [PubMed] [Google Scholar]
- Mierzejewska‐Krzyżowska, B. , Bukowska, D. , & Celichowski, J. (2019). The differences in the structure of the motor nucleus of the medial gastrocnemius muscle in male and female rats. Annals of Anatomy, 221, 93–100. 10.1016/j.aanat.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Mierzejewska‐Krzyżowska, B. , Bukowska, D. , Taborowska, M. , & Celichowski, J. (2014). Sex differences in the number and size of motoneurons innervating rat medial gastrocnemius muscle. Anatomy Histology Embryology, 43, 182–189. 10.1111/ahe.12060 [DOI] [PubMed] [Google Scholar]
- Mierzejewska‐Krzyżowska, B. , Drzymała‐Celichowska, H. , & Celichowski, J. (2011). Gender differences in the morphometric properties of muscle fibers and the innervation ratio of motor units in the rat medial gastrocnemius muscle. Anatomy Histology Embryology, 40, 249–255. 10.1111/j.1439-0264.2011.01066.x [DOI] [PubMed] [Google Scholar]
- Mortreux, M. , Rosa‐Caldwell, M. E. , Stiehl, I. D. , Sung, D. M. , Thomas, N. T. , Fry, C. S. , & Rutkove, S. B. (2021). Hindlimb suspension in Wistar rats: Sex‐based differences in muscle response. Physiological Reports, 9, e15042. 10.14814/phy2.15042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, K. , Ohta, M. , Saito, M. , Morita‐Isogai, Y. , Sato, H. , Kuramoto, E. , Yin, D. X. , Maeda, Y. , Kaneko, T. , Yamashiro, T. , Takada, K. , Oh, S. B. , Toyoda, H. , & Kang, Y. (2018). Electrophysiological and morphological properties of α and γ motoneurons in the rat trigeminal motor nucleus. Frontiers in Cellular Neuroscience, 24, 12–19. 10.3389/fncel.2018.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, B. M. , Nuckols, G. , & Krieger, J. W. (2020). Sex differences in resistance training: A systematic review and meta‐analysis. Journal of Strength Conditioning Research, 34, 1448–1460. 10.1519/JSC.0000000000003521 [DOI] [PubMed] [Google Scholar]
- Sekerli, M. , Del Negro, C. A. , Lee, R. H. , & Butera, R. J. (2004). Estimating action potential thresholds from neuronal time‐series: New metrics and evaluation of methodologies. IEEE Transactions Biomedical Engineering, 51, 1665–1672. 10.1109/TBME.2004.827531 [DOI] [PubMed] [Google Scholar]
- Zengel, J. E. , Reid, S. A. , Sypert, G. W. , & Munson, J. B. (1985). Membrane electrical properties and prediction of motor‐unit type of cat medial gastrocnemius motoneurons in the cat. Journal of Neurophysiology, 53, 1323–1344. 10.1152/jn.1985.53.5.1323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data and materials can be accessed at the corresponding author host institution at the Department of Neurobiology, Poznań University of Physical Education, Poland.


