Abstract
Background
Advanced age is a risk factor for unfavorable outcome in community‐acquired bacterial meningitis, but clinical characteristics and outcome in meningitis patients of 80 years or older have not been defined.
Methods
We compared clinical characteristics and outcome of community‐acquired bacterial meningitis patients aged 80 years or older and adults under 80 years old within a prospective nationwide cohort study.
Results
Out of 2140 episodes identified between March 2006 and July 2018, 149 occurred in patients aged 80 years or older (7%). Common predisposing factors other than age were diabetes mellitus (25 of 148 [17%]), otitis or sinusitis (30 of 136 [22%]), and pneumonia (23 of 141 [16%]). The triad of fever, neck stiffness and altered consciousness was present in 60 of 139 (43%). The most common causative pathogen was Streptococcus pneumoniae (99 of 149 [66%]). Atypical causative pathogens, such as Listeria monocytogenes, Staphylococcus aureus, and Escherichia coli, occurred more often compared to younger patients (49 of 149 [33%] vs 362 of 1991 [18%]; p < 0.001). Patients of 80 years and older had high case fatality rate (75 of 149 [50%]), but 45 of 149 (30%) had a favorable outcome. Characteristics associated with an unfavorable outcome were absence of otitis or sinusitis, presence of aphasia, mono‐ or hemiparesis, a lower score on the Glasgow Coma Scale, a higher heart rate, a higher blood C‐reactive protein concentration and CSF leukocytes <100 per mm3.
Conclusions
Bacterial meningitis in patients of 80 years of older is associated with high rates of unfavorable outcome and death. Atypical causative pathogens such as L. monocytogenes, S. aureus, and E. coli occur commonly and should be considered when starting empirical antimicrobial therapy in this age group.
Keywords: clinical characteristics, community‐acquired bacterial meningitis, pathogens, prospective cohort study, very old patients
Key points
Patients of 80 years and older constitute 7% of all adult bacterial meningitis cases.
Bacterial meningitis is more often caused by atypical pathogens, including Listeria monocytogenes, Escherichia coli and Staphylococcus aureus, in patients aged 80 years or older as compared to younger patients.
The case fatality rate in patients above 80 years of age is high (50%), but 30% of the patients had a favorable outcome.
Why does this paper matter?
Hospital care of patients aged 80 years and older with bacterial meningitis is different compared to younger patients. As atypical pathogens occur more often, these should be considered when choosing empirical antibiotic therapy. Furthermore, this paper helps recognizing the disease and its complications in this patient category.
INTRODUCTION
Bacterial meningitis is associated with high case fatality rates. 1 Age is an important risk factor for the development of bacterial meningitis with neonates and older adults at the highest risk. 1 One of the most robust predictors of an unfavorable outcome in this disease is advance age. 2 Previous studies showed that Streptococcus pneumoniae is the most common causative pathogen of meningitis in older patients, which by itself has been associated with unfavorable disease outcome in several studies. 3 , 4 So far, no studies have focused on the very advanced in age, those 80 years or older, 3 , 4 , 5 , 6 while this group is proportionally increasing within the population. The very advanced of age may have different living circumstances, for example living in nursing homes, which may resemble characteristics and outcome of nosocomial meningitis rather than community‐acquired meningitis with specific causative pathogens and outcome characteristics. 7 , 8 In this study, we studied clinical characteristics and outcome of patients aged 80 years or older with community‐acquired bacterial meningitis from a prospective nationwide cohort study in the Netherlands.
METHODS
The MeninGene study is a nationwide prospective cohort study on community‐acquired bacterial meningitis in the Netherlands. Patients aged 16 years and older were included between March 2006 and July 2018. They were identified following a daily update by the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM), which receives approximately 85% of the CSF isolates of patients with bacterial meningitis. 9 , 10 The daily update included the names of hospitals and attending physicians. Subsequently, treating physicians were informed about the study by telephone. Patients or legal representatives received written information about the study and were asked to give written informed consent. Treating physicians could also contact the researchers 24/7 to include a patient in the study. Data were prospectively collected through online case record forms (CRFs), including clinical characteristics, laboratory results, microbiological results, clinical course, treatment, and outcome.
We included all patients with a proven bacterial meningitis, defined as bacterial pathogen in the cerebrospinal fluid (CSF), or the combination of a positive PCR, blood culture or CSF antigen test combined with at least one individual predictors of bacterial meningitis as defined by Spanos et al., consisting of a cerebrospinal fluid glucose concentration <340 mg/L (1.9 mmol/L), CSF:blood glucose ratio <0.23, protein concentration >2200 mg/L, white blood cell count >2000 per microliter or >1180 polymorphonuclear leukocytes per microliter. 11 We excluded all patients who developed bacterial meningitis in the hospital or within 1 week after discharge, following head trauma or a neurosurgical procedure within 1 month of presentation, or those who had a neurosurgical device.
In the current study, we evaluated patients aged 80 years and older and compared them with patients aged 16–79 years. Patients with a medical history including diabetes mellitus, asplenia, alcoholism, infection with the human immunodeficiency virus and patients using immunosuppressive drugs were considered immunocompromised. Neurologic examination was performed at admission and at discharge. Outcome was scored on the Glasgow Outcome Scale (GOS), ranging from 1 (death) to 5 (mild or no disability) and then dichotomized in favorable (GOS 5) and unfavorable outcome (GOS 1–4). All causes of death were classified by two physicians into systemic (septic shock, cardiorespiratory failure, multi‐organ failure, myocardial infarction and other systemic causes) or neurologic (brain herniation, cerebrovascular complications, intractable seizures, withdrawal of care due to poor neurologic prognosis, or other neurological complications) as published previously. 3 , 12 , 13 The Cohen's kappa for inter‐rater agreement of cause of death was 0.68.
Group differences were tested with the Fisher's test for categorical variables and the Mann–Whitney U test for continuous variables. All analyses were conducted in R (version 4.0.3). p‐Values <0.05 were considered statistically significant. To assess the association of clinical characteristics with outcome, we performed a univariable logistic regression analysis providing odds ratios (ORs) and 95% confidence intervals (95% CIs). Potential predictors were selected based on previous research and pathophysiological interest. 2 , 14 Linearity of the association of continuous variables and outcome was assessed by visual inspection. The variable was grouped when there was no linear relationship.
RESULTS
A total of 2140 episodes of community‐acquired bacterial meningitis were included, of which 149 episodes occurred in 149 patients aged 80 or older (7%; Figure 1). Over time, the proportion of patients aged 80 years or older remained stable: 49 of 739 (7%) from 2006 to 2010, 52 of 654 (8%) from 2010 to 2014 and 48 of 747 (6%) from 2014 to July 2018 (p = 0.50). One hundred of 149 patients were female (67%). An immunocompromised state was found in 36 patients (24%), which was most often due to diabetes mellitus (25 of 148 patients [17%]; Table 1). Distant infection foci were identified in 51 of 145 patients (35%) and consisted of pneumonia in 23 of 141 (16%), otitis or sinusitis in 30 of 136 (22%), and endocarditis in 4 of 135 patients (3%).
FIGURE 1.
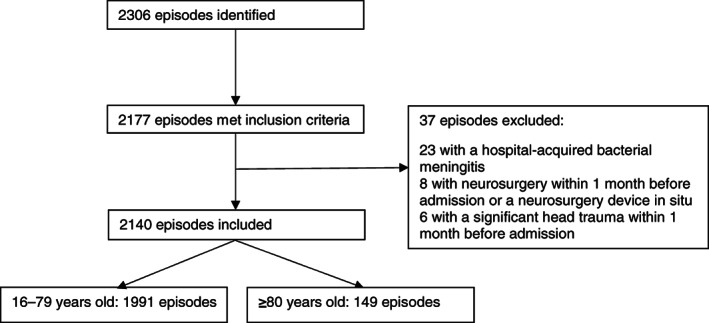
Flowchart of patient inclusion. Out of 2306 patients prospectively included in the MeninGene study, 2177 episodes met the inclusion criteria for the present study, of which 37 had 1 or more exclusion criteria. One hundred and forty‐nine of 2140 episodes were in patients aged 80 years or older
TABLE 1.
Clinical characteristics and outcome
| Characteristic | 16–79 years old | ≥ 80 years old | P‐value |
|---|---|---|---|
| Age a | 60 (46–68) | 84 (81–86) | |
| Female sex | 962/1991 (48) | 100/149 (67) | <0.001 |
| Medical history | |||
| Immunocompromised | 533/1986 (27) | 36/149 (24) | 0.50 |
| Diabetes mellitus | 248/1958 (13) | 25/148 (17) | 0.16 |
| Immunosuppressant use | 165/1963 (8) | 16/147 (11) | 0.29 |
| Alcoholism | 119/1980 (6) | 0/149 (0) | <0.001 |
| Cancer | 242/1984 (12) | 30/149 (20) | 0.007 |
| Extrameningeal infection | 856/1961 (44) | 51/145 (35) | 0.056 |
| Pneumonia | 168/1892 (9) | 23/141 (16) | 0.006 |
| Endocarditis | 28/1889 (1) | 4/135 (3) | 0.16 |
| Otitis or sinusitis | 698/1899 (37) | 30/136 (22) | <0.001 |
| Symptoms on admission | |||
| Headache | 1412/1725 (82) | 66/98 (67) | 0.001 |
| Neck stiffness | 1337/1891 (74) | 97/136 (71) | 0.62 |
| Rash | 166/1772 (9) | 2/130 (2) | 0.001 |
| Heart rate (beats/min) b | 99 (83–112) | 100 (89–114) | 0.048 |
| Systolic blood pressure (mmHg) c | 142 (125–162) | 150 (135–170) | <0.001 |
| Diastolic blood pressure (mmHg) d | 80 (69–90) | 80 (70–93) | 0.069 |
| Body temperature e | 38.9 (37.8–39.6) | 38.9 (37.6–39.6) | 0.71 |
| ≥38°C | 1408/1924 (73) | 103/143 (72) | 0.77 |
| Triad of symptoms f | 743/1853 (40) | 60/139 (43) | 0.48 |
| Score on Glasgow Coma Scale g | 11 (9–14) | 10 (8–13) | 0.005 |
| GCS <14 (altered mental status) | 1380/1960 (70) | 116/148 (78) | 0.039 |
| GCS <8 (coma) | 395/1960 (20) | 40/148 (27) | 0.057 |
| Seizures | 146/1883 (8) | 9/133 (7) | 0.89 |
| Cranial nerve palsy | 141/1701 (8) | 9/124 (7) | 0.87 |
| Aphasia‐mono‐ or hemiparesis | 379/1411 (21) | 34/118 (29) | 0.066 |
| Blood chemical tests | |||
| Erythrocyte sedimentation rate (mm/h) h | 40 (21–69) | 50 (34–77) | 0.012 |
| Thrombocytes (×109/L) i | 198 (148–254) | 193 (143–253) | 0.50 |
| Indices of CSF inflammation | |||
| Leukocytes (cells/μl) j | 2483 (588–6884) | 1593 (440–3986) | 0.001 |
| <100 | 189 (10) | 21 (15) | 0.087 |
| 100–999 | 419 (22) | 35 (24) | 0.53 |
| >999 | 1274 (68) | 87 (61) | 0.097 |
| Protein (g/L) k | 3.8 (2.2–6.0) | 4.8 (2.9–8.0) | 0.001 |
| CSF:blood glucose ratio l | 0.06 (0.01–0.28) | 0.04 (0.01–0.21) | 0.47 |
| Causative pathogens | |||
| Identified by blood culture | 1300/1707 (76) | 109/128 (85) | 0.02 |
| Identified by CSF culture | 18,879/1991 (94) | 136/149 (91) | 0.14 |
| S. pneumoniae | 1398/1991 (70) | 99/149 (66) | 0.35 |
| N. meningitidis | 231/1991 (12) | 1/149 (1) | <0.001 |
| L. monocytogenes | 105/1991 (5) | 19/149 (13) | <0.001 |
| S. aureus | 28/1991 (1) | 6/149 (4) | 0.027 |
| E. coli | 13/1991 (1) | 4/149 (3) | 0.026 |
| Other organisms | 216/1991 (11) | 20/149 (13) | 0.34 |
Note: Data presented as n/N (%) or median (IQR). Group differences were tested with a Fisher's exact test for categorical variables and a Mann–Whitney U test for continuous variables.
Abbreviations: CSF, cerebrospinal fluid; ICU, Intensive Care Unit.
Age was known in 1991 patients <80 years and 149 patients ≥80 years.
Heart rate was known in 1888 <80 years and 144 ≥80 years.
Systolic blood pressure was known in 1903 <80 years and 146 ≥80 years.
Diastolic blood pressure was known in 1906 <80 years and 146 ≥80 years.
Body temperature was known in 1924 <80 years and 143 ≥80 years.
The triad of symptoms consisted of fever‐neck stiffness and change in mental status.
GCS was known in 1960 <80 years and 148 ≥80 years.
ESR was known in 814 <80 years and 63 ≥80 years.
Number of blood thrombocytes was known in 1872 <80 years and 136 ≥80 years.
Number of CSF leukocytes was known in 1884 <80 years and 144 ≥80 years.
CSF protein was known in 1875 <80 years and 143 ≥80 years.
CSF:blood glucose ratio was known in 1765 <80 years and 137 ≥80 years.
In 67 of 135 patients (50%), symptoms were present for more than 24 h and 15 of 139 patients (11%) had received antibiotic pretreatment. Common presenting features were altered mental status (defined as a score on the Glasgow Coma Scale below 14) in 116 of 148 patients (78%), fever in 103 of 143 (72%), neck stiffness in 97 of 136 (71%) and headache in 66 of 98 (67%). The classical triad of fever, altered mental status and neck stiffness was reported in 60 of 139 patients (43%). Presenting features were comparable to patients younger than 80 years old (Table 1), although headache was less common in patients aged 80 years or older (66 of 98 [67%] vs 1412 of 1725 [82%], p = 0.001). Cranial imaging on admission was performed in 130 of 147 patients aged 80 years or older (88%), showing recent abnormalities in 39 patients (30%): most commonly sinus opacification (15 of 130 patients [12%]), mastoid opacification (13 of 130 patients [10%]) and hypodensities consistent with recent cerebral infarction (11 of 130 patients [8%]).
CSF examination revealed a median white blood cell (WBC) count of 1593 per mm 3 (IQR 440–3986), which was lower than in patients younger than 80 years (2483 cells per mm3 [IQR 588–6884], p = 0.001). CSF cultures revealed the causative pathogen in 136 of 149 patients (91%), blood cultures in 11 additional patients (7%) and CSF PCR/culture negative in 2 patients (1%). The most common causative pathogens were S. pneumoniae in 99 patients (66%), Listeria monocytogenes in 19 patients (13%), and Staphylococcus aureus in 6 (4%), of which none were methicillin‐resistant S. aureus (MRSA). Other pathogens were Neisseria meningitidis (n = 1 [1%]), Haemophilus influenzae (n = 4 [3%]), Escherichia coli (n = 4 [3%]), Streptococcus agalactiae (n = 5 [3%]), Streptococcus dysgalactiae subspecies equisimilis in (n = 3 [2%]), Streptococcus bovis (n = 2 [1%]), Streptococcus salivarius (n = 2 [1%]), and Streptococcus thermophilus, non‐specified viridans group streptococci, Enterococcus faecalis and Pseudomonas aeruginosa all in 1 patient (1%). Atypical pathogens (not S. pneumoniae or N. meningitidis) were thus responsible for 49 of 149 cases (33%), which was more than in patients below 80 years of age (362 of 1991 [18%]; p < 0.001). Immunocompromising conditions were found in 29% of these 49 patients. Listeria monocytogenes, S. aureus, and E. coli were more common in patients of 80 years or older than younger patients (p = 0.004 for listerial meningitis; p = 0.03 for S. aureus; and p = 0.03 for E. coli).
The started empirical antibiotic treatment at the moment of suspicion of bacterial meningitis consisted of a third‐generation cephalosporin and amoxicillin in 91 of 139 patients (65%). Twenty‐one patients (15%) received monotherapy with a third‐generation cephalosporin, 17 (12%) received monotherapy with amoxicillin or penicillin and 10 received another antibiotic regimen (7%). Adjunctive dexamethasone treatment according to guidelines, 4 times per day 10 mg for 4 days and started with the first dose of antibiotics, was started in 111 of 144 patients (77%). Third‐generation cephalosporin monotherapy was started in 2 of 19 patients with a L. monocytogenes meningitis (11%). For 6 patients with a S. aureus meningitis, initial antibiotic therapy consisted of a third‐generation cephalosporin or amoxicillin in 3 patients (50%) and flucloxacillin in 3 patients with known endocarditis or known positive blood cultures (50%).
Complications occurred in a large proportion of the patients over 80 years old (91 of 145 [63%]; Table 2). Systemic complications occurred in 74 of 145 patients (51%): pneumonia in 34 of 124 patients (27%), respiratory failure in 56 of 140 patients (40%), and circulatory shock in 24 of 136 patients (18%). Neurological complications were reported in 47 of 141 patients (33%): seizures in 32 of 139 patients (23%), focal neurologic deficits in 20 of 126 patients (16%), and cerebrovascular accidents in 12 of 126 patients (10%). Patients aged 80 years or older were more likely to develop systemic complications (74 of 145 [51%] vs 633 of 1940 [33%], p < 0.001) and seizures (32 of 137 [23%] vs 245 of 1845 [13%], p = 0.001) than younger patients.
TABLE 2.
Clinical course and outcome
| Characteristic | 16–79 years old | ≥80 years old | P‐value |
|---|---|---|---|
| Clinical course | |||
| Admitted to the ICU | 1025/1696 (52) | 52/147 (35) | <0.001 |
| Systemic complications | 633/1940 (33) | 74/145 (51) | <0.001 |
| Respiratory failure | 469/1899 (25) | 56/140 (40) | <0.001 |
| Pneumonia | 277/1844 (15) | 34/124 (27) | 0.001 |
| Circulatory shock | 186/1818 (10) | 24/136 (18) | 0.009 |
| Neurological complications | 638/1930 (33) | 47/141 (33) | 0.93 |
| Seizures | 251/1879 (13) | 32/139 (23) | 0.003 |
| Cerebrovascular accidents | 202/1838 (11) | 12/126 (10) | 0.76 |
| Focal neurological deficits | 426/1818 (23) | 20/126 (16) | 0.062 |
| Score on Glasgow Outcome Scale | |||
| 1 (death) | 286/1968 (15) | 75/149 (50) | <0.001 |
| 2 (vegetative state) | 6/1968 (0.3) | 0/149 (0) | >0.99 |
| 3 (severe disability) | 94/1968 (5) | 6/149 (4) | 0.84 |
| 4 (moderate disability) | 316/1968 (16) | 23/149 (15) | 0.82 |
| 5 (mild or no disability) | 1266/1968 (64) | 45/149 (30) | <0.001 |
| Discharge location | |||
| Home | 1269/1583 (80) | 25/74 (34) | <0.001 |
| Rehabilitation center | 231/1583 (15) | 31/74 (42) | <0.001 |
| Nursing home | 83/1583 (5) | 18/74 (24) | <0.001 |
| Focal neurologic deficits at discharge a | 279/1411 (20) | 12/62 (19) | >0.99 |
Note: Data presented as n/N (%) or median (IQR). Group differences were tested with a Fisher's exact test.
Abbreviation: ICU, intensive care unit.
Focal neurologic deficits included mono‐, hemiparesis, aphasia and cranial nerve palsies.
The case fatality rate of patients aged 80 years or older was 50% (75 of 149 patients), compared to 286 of 1968 patients (15%, p < 0.001) in younger patients. Forty‐five patients (30%) aged 80 years or older had a favorable outcome in spite of their advanced age. Death was attributed to systemic complications in 50 of 72 patients (69%, for three patients the cause of death was unknown). Most commonly, causes of death were cardiorespiratory failure in 26 patients (36%), septic shock in 12 (17%), withdrawal of care due to poor neurological prognosis in 14 (19%) and cerebral infarction or hemorrhage in 7 (10%; Figure 2). Patient aged 80 years or older were more likely to die due to systemic complications than younger patients (50 of 72 [69%] vs 114 of 268 [43%], p < 0.001). Out of the 74 patients aged 80 years or older who survived, discharge destination was home in 32%, a rehabilitation center in 42% and a nursing home in 24%. In a univariable analysis in patients aged 80 years and older (Table 3), variables present on admission that were associated with an unfavorable outcome were absence of otitis or sinusitis, presence of aphasia, mono‐ or hemiparesis, a lower score on the Glasgow Coma Scale, higher heart rate, high blood C‐reactive protein concentration and CSF leukocytes <100 per mm3.
FIGURE 2.
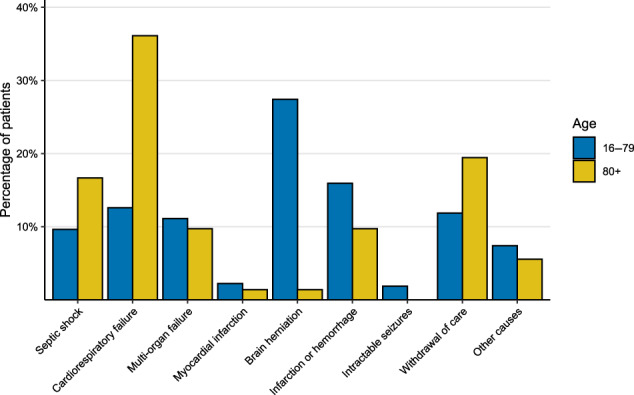
Distribution of the cause of death within age groups. Figure displaying the percentage of patients within the age groups of 80 years or older and 16–79 years old (y‐axis), dying due to specific causes of death (x‐axis). Patients aged 80 years or older most commonly died due to cardiorespiratory failure while younger patients most commonly died due to brain herniation
TABLE 3.
Factors associated with an unfavorable outcome
| Characteristic | Favorable outcome (N = 45) | Unfavorable outcome (N = 104) | Univariable odds ratio for unfavorable outcome (95% CI) |
|---|---|---|---|
| Age a | 83 (81–86) | 84 (82–86) | 1.07 (0.97–1.20) |
| Otitis or sinusitis | 15/43 (35) | 15/93 (16) | 0.36 (0.15–0.83) |
| Pneumonia | 9/44 (20) | 14/97 (14) | 0.66 (0.26–1.71) |
| Immunocompromised state | 12/45 (27) | 24/104 (23) | 0.83 (0.37–1.88) |
| Cancer | 5/45 (11) | 25/104 (24) | 2.53 (0.97–7.94) |
| Seizures | 1/43 (2) | 8/90 (9) | 4.10 (0.72–77.3) |
| Fever (≥38°C) | 34/44 (77) | 69/99 (70) | 0.68 (0.29–1.51) |
| Aphasia, mono‐ or hemiparesis | 7/40 (18) | 27/78 (35) | 2.50 (1.02–6.81) |
| Glasgow coma scale score b | 12 (10–14) | 10 (8–12) | 0.78 (0.67–0.88) |
| Heart rate (beats/min) c | 94 (85–105) | 100 (92–118) | 1.32 (1.09–1.65) |
| Systolic blood pressure (mmHg) d | 157 (142–175) | 150 (132–168) | 0.89 (0.78–1.02) |
| C‐reactive protein (mg/L) e | 94 (46–222) | 222 (123–373) | 1.05 (1.02–1.08) |
| CSF leukocytes (per mm3) | 1831 (902–4751) | 1446 (263–3895) | |
| <100 | 3/44 (7) | 18/99 (18) | 0.27 (0.06–0.88) |
| 100–999 | 9/44 (20) | 26/99 (26) | 0.56 (0.22–1.33) |
| 1000–10,000 | 30/44 (68) | 49/99 (49) | Reference |
| >10,000 | 2/44 (5) | 6/99 (6) | 0.54 (0.08–2.54) |
| CSF protein (g/L) f | 3.88 (2.22–6.24) | 5.16 (3.05–8.45) | 1.06 (0.98–1.15) |
| CSF:blood glucose ratio | 0.13 (0.02–0.27) | 0.03 (0.01–0.16) | |
| <0.25 | 30/42 (71) | 81/95 (85) | 2.48 (0.98–6.25) |
| 0.25–0.50 | 11/42 (26) | 12/95 (13) | Reference |
| >0.50 | 1/42 (2) | 2/95 (2) | 1.83 (0.15–42.77) |
| Positive blood culture | 29/37 (78) | 80/91 (88) | 2.01 (0.71–5.46) |
| Pneumococcal meningitis | 28/45 (62) | 71/104 (68) | 1.31 (0.62–2.70) |
Note: Data presented as n/N (%) or median (interquartile range).
Age known for all episodes.
Odds ratio for an increase of one point, known for 45 episodes with favorable and 103 episodes with unfavorable outcome.
Odds ratio for an increase of 10 beats/min, known for 45 episodes with favorable and 99 episodes with unfavorable outcome.
Odds ratio for an increase of 10 mmHg, known for 45 episodes with favorable and 101 episodes with unfavorable outcome.
Odds ratio for an increase of 10 mg/L, known for 44 episodes with favorable and 97 episodes with unfavorable outcome.
CSF protein known for 42 episodes with favorable and 101 episodes with unfavorable outcome.
DISCUSSION
Our study shows that community‐acquired bacterial meningitis in patients very advanced in age is a severe disease with a case fatality rate (50%), although 30% has a favorable outcome. Although patients aged 80 years or older do not present differently compared to younger patients, atypical causative pathogens including L. monocytogenes, S. aureus, and E. coli occur more frequently and should be taken in account when starting empirical antibiotic therapy.
Patients very advanced in age presented with similar clinical features than younger patients. This is in contrast with previous studies. 3 , 5 , 6 A prospective study including 696 Dutch patients with bacterial meningitis showed that 257 patients of 60 years and older were less likely to present with neck stiffness, fever and headache, while focal cerebral deficits were more common. 3 A prospective study including 675 Spanish patients reported lower proportion of patients with neck stiffness and headache, and more hemiparesis and seizures in 185 patients aged 65 years or older as compared to younger patients. 5 A retrospective study including 205 Spanish patients aged 65 years or older reported lower rates of neck stiffness (62%) as compared to younger patients (84%). 6 This might be explained by the fact that we evaluated the very advance in age, defined as 80 years or older, whereas previous studies had the age cut‐off at 60–65 years. Our finding that patients very advance of age do not present with atypical clinical features, should not imply that making the diagnosis in these patients is easy. The differential diagnosis of a decreased level of consciousness and fever in this population has a broad differential diagnosis, ranging from a delirium to bacterial meningitis. If meningitis cannot be excluded with a high level of certainty, one should keep a low threshold to perform lumbar puncture because cerebrospinal fluid examination is essential to establish the diagnosis of meningitis.
We found that in the majority of patients aged 80 years and older cranial imaging is performed in the initial diagnostic work‐up (88%), although this rarely led to the identification of relevant intracranial abnormalities. Using clinical criteria as described in several guidelines ‐ consisting of new onset seizures, focal neurologic deficits, severe immunodeficiency and a severely altered mental status defined as a score on the Glasgow Coma Scale below 10—the number of scans can be reduced. 8 , 15
Streptococcus pneumoniae was the most common causative pathogen occurring in about two‐thirds of patients. However, atypical pathogens, such as L. monocytogenes (13%), S. aureus (4%) and E. coli (3%) occurred in relatively high proportions among patients very advantaged in age. Our findings are in line with previous studies reporting that S. pneumoniae is the most common pathogen in older patients, and listerial meningitis is relatively common. 3 , 16 Another study has shown that atypical or (multi)resistant pathogens are common in patients older than 64, especially when they reside in long‐term care facilities. 17 The high proportion of atypical pathogens could also be explained by age‐related changes in immune function, making older patients more susceptible for invasive bacterial infections, 18 or by relative high rates of skin infections in this population. 19
Our data show that empirical antimicrobial therapy for older patients should include antibiotics active against L. monocytogenes, S. aureus, and E. coli. This underlines the importance of empirical treatment with amoxicillin, in combination a third generation cephalosporin—ceftriaxone or cefotaxime—, as recommended by the guidelines of the European Society for Clinical Microbiology and Infectious Diseases (ESCMID). 15 We identified two patients with meningitis a causative pathogen not covered by this recommended treatment: one patient with P. aeruginosa and one with E. faecalis meningitis, who both died. Administering ceftazidime as the third‐generation cephalosporin is recommended for meningitis after neurosurgery or trauma and could be considered for patients very advanced of age, as it covers P. aeruginosa. In countries with high rates of pneumococcal resistance for ceftriaxone, like the United States, addition of vancomycin, which covers E. faecalis but not P. aeruginosa, is recommended.
We found that despite the advanced age of this population, 30% of patients have a favorable outcome. This implies that in bacterial meningitis patients over 80 years old, the initial treatment should aim for a full recovery. Treatment restrictions should not be based on age alone, but take comorbidity, premorbid functioning and severity of disease on presentation into account. Characteristics associated with a favorable outcome were those associated with reduced severity of systemic inflammation such as lower heartrate and blood C‐reactive protein concentration, and the presence of otitis or sinusitis. Furthermore, the absence of characteristics associated with neurological damage such as a low level of consciousness and focal neurological deficits was predictive of a favorable outcome.
The mortality rate in patients of 80 years or older was high (50%), compared to previous studies on older patients with bacterial meningitis which used a cut‐off of 60‐years and older. 3 , 5 , 6 Besides by age itself, the high mortality could be explained by several factors such as a higher risk of meningitis due to pathogens that have been associated severe disease, including S. pneumoniae, L. monocytogenes, S. aureus and E. coli. 2 , 14 , 20 , 21 Older patients have more comorbidities such as cancer and pneumonia, which also have been associated with poor outcome in this disease. 22 , 23 Our study also shows that patients aged 80 or older were less likely to be admitted to the ICU indicating that physicians may limit invasive treatments in this population because of comorbidity, previous dependence on care or according to the patient's or family's wishes. All these factors may contribute to the high mortality rate in this age group.
There are several limitations to this study. First, we only included patients who underwent lumbar puncture. Older patients may not receive a lumbar puncture due to an atypical or uncharacteristic clinical presentation or a poor overall prognosis. Also, patients with a severe septic shock or coagulation disorders, for whom the lumbar puncture was postponed initially, and died before CSF examination was performed, were not evaluated in this study. Furthermore, patients with space‐occupying lesions on CT who could not undergo a lumbar puncture were not included either. All these factors may lead to a selection bias and underestimation of the mortality rate. Second, only a small proportion of our included patients had negative CSF cultures (9%) because the NRLBM only identifies patients with a positive CSF culture. Patients pretreated with antibiotics, which increases the probability of negative CSF cultures, may therefore be underrepresented in our study. Third, we did not evaluate living circumstances prior to hospital admission. Therefore, we cannot draw conclusions on the influence of living circumstances on pathogen distribution and clinical course. Nonetheless, this large nationwide study allowed us to describe a representative group of very old patients with community‐acquired bacterial meningitis.
In conclusion, bacterial meningitis in patients of 80 years of older is associated with a high mortality rate, although 30% have a good recovery despite their old age. Atypical causative pathogens such as L. monocytogenes, S. aureus, and E. coli occur commonly and should be considered when starting empirical antimicrobial therapy in this age group. Primary preventive strategies, such as the 23‐valent polysaccharide pneumococcal vaccination (PPV‐23) which is given to adults aged 60 years or older since 2020, or secondary prevention with the 13‐valent pneumococcal conjugate (PCV‐13) and PPV‐23, 15 may decrease the impact of this disease in the population very advantaged of age. Maximized herd protection may also help older adults, who have a poor immunological response, to be fully immunized. 8
CONFLICT OF INTEREST
Nina M. van Sorge receives consultancy fees from Pfizer, MSD, and GSK (fees paid to Amsterdam UMC); In addition, Nina M. van Sorge has a patent WO 2013/020090 A3 (inventors: N.M. van Sorge/V. Nizet) outside the submitted work with royalties paid to University of California San Diego. Other authors: no conflicts of interest.
AUTHOR CONTRIBUTIONS
Thijs M. van Soest: substantial contribution to acquisition of data, analysis and interpretation of data, drafting the article, final approval. Nora Chekrouni: substantial contribution to acquisition of data, revising the article, final approval. Nina M. van Sorge: substantial contribution to acquisition of data, revising the article, final approval. Matthijs C. Brouwer: substantial contribution to conception and design, revising the article, final approval. Diederik van de Beek: substantial contribution to conception and design, revising the article, final approval.
SPONSOR'S ROLE
The sponsor had no role in design, methods, subject recruitment, data collections, analysis and preparation of the paper.
van Soest TM, Chekrouni N, van Sorge NM, Brouwer MC, van de Beek D. Community‐acquired bacterial meningitis in patients of 80 years and older. J Am Geriatr Soc. 2022;70(7):2060‐2069. doi: 10.1111/jgs.17766
Funding information This work was supported by the Netherlands Organization for Health Research and Development (ZonMw; NWO‐Vidi Grant [917.17.308] to Matthijs C. Brouwer; NWO‐Vici‐Grant [grant number 918.19.627 to Diederik van de Beek]); the Academic Medical Center (AMC Fellowship to Diederik van de Beek); and the European Research Council (ERC Consolidator grant to M.C.B., ERC Starting grant to Diederik van de Beek). The Netherlands Reference Laboratory for Bacterial Meningitis is supported by the National Institute of Public health and the Environmental Protection, Bilthoven.
Previous presentation of data: Data of this study were presented (online) at the 31st ECCMID, the European Congress of Clinical Microbiology and Infectious Diseases, on July 12th, 2021.
REFERENCES
- 1. van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community‐acquired bacterial meningitis. Nat Rev Dis Primers. 2016;2:16074. [DOI] [PubMed] [Google Scholar]
- 2. van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849‐1859. [DOI] [PubMed] [Google Scholar]
- 3. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. Community‐acquired bacterial meningitis in older people. J Am Geriatr Soc. 2006;54:1500‐1507. [DOI] [PubMed] [Google Scholar]
- 4. Erdem H, Kilic S, Coskun O, et al. Community‐acquired acute bacterial meningitis in the elderly in Turkey. Clin Microbiol Infect. 2010;16:1223‐1229. [DOI] [PubMed] [Google Scholar]
- 5. Cabellos C, Verdaguer R, Olmo M, et al. Community‐acquired bacterial meningitis in elderly patients: experience over 30 years. Medicine (Baltimore). 2009;88:115‐119. [DOI] [PubMed] [Google Scholar]
- 6. Domingo P, Pomar V, de Benito N, Coll P. The spectrum of acute bacterial meningitis in elderly patients. BMC Infect Dis. 2013;13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146‐154. [DOI] [PubMed] [Google Scholar]
- 8. van de Beek D, Brouwer MC, Koedel U, Wall EC. Community‐acquired bacterial meningitis. Lancet. 2021;398:1171‐1183. [DOI] [PubMed] [Google Scholar]
- 9. Spanjaard L, Bol P, Ekker W, Zanen HC. The incidence of bacterial meningitis in the Netherlands—a comparison of three registration systems, 1977–1982. J Infect. 1985;11:259‐268. [DOI] [PubMed] [Google Scholar]
- 10. Bijlsma MW, Bekker V, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. Epidemiology of invasive meningococcal disease in the Netherlands, 1960‐2012: an analysis of national surveillance data. Lancet Infect Dis. 2014;14:805‐812. [DOI] [PubMed] [Google Scholar]
- 11. Spanos A, Harrell FE Jr, Durack DT. Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. JAMA. 1989;262:2700‐2707. [PubMed] [Google Scholar]
- 12. van de Beek D, de Gans J. Dexamethasone and pneumococcal meningitis. Ann Intern Med. 2004;141:327. [DOI] [PubMed] [Google Scholar]
- 13. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol. 2006;5:123‐129. [DOI] [PubMed] [Google Scholar]
- 14. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community‐acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis. 2016;16:339‐347. [DOI] [PubMed] [Google Scholar]
- 15. van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22(Suppl 3):S37‐S62. [DOI] [PubMed] [Google Scholar]
- 16. Koopmans MM, Bijlsma MW, Brouwer MC, van de Beek D, van der Ende A. Listeria monocytogenes meningitis in the Netherlands, 1985–2014: a nationwide surveillance study. J Infect. 2017;75:12‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marwick C, Santiago VH, McCowan C, Broomhall J, Davey P. Community acquired infections in older patients admitted to hospital from care homes versus the community: cohort study of microbiology and outcomes. BMC Geriatr. 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu W, Wong G, Hwang YY, Larbi A. The untwining of immunosenescence and aging. Semin Immunopathol. 2020;42:559‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheinfeld N. Infections in the elderly. Dermatol Online J. 2005;11:8. [PubMed] [Google Scholar]
- 20. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bichon A, Aubry C, Dubourg G, et al. Escherichia coli spontaneous community‐acquired meningitis in adults: a case report and literature review. Int J Infect Dis. 2018;67:70‐74. [DOI] [PubMed] [Google Scholar]
- 22. Pomar V, Benito N, Lopez‐Contreras J, Coll P, Gurgui M, Domingo P. Characteristics and outcome of spontaneous bacterial meningitis in patients with cancer compared to patients without cancer. Medicine (Baltimore). 2017;96:e6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Figueiredo AHA, Brouwer MC, Bijlsma MW, van der Ende A, van de Beek D. Community‐acquired pneumonia in patients with bacterial meningitis: a prospective nationwide cohort study. Clin Microbiol Infect. 2020;26:513 e7‐513 e11. [DOI] [PubMed] [Google Scholar]


