Abstract
Efficient cell‐to‐cell communication is essential for tissue development, homeostasis, and the maintenance of cellular functions after injury. Tunneling nanotubes (TNTs) have emerged as a new important method of cell‐to‐cell communication. TNTs are primarily established between stressed and unstressed cells and can transport a variety of cellular components. Mitochondria are important trafficked entities through TNTs. Transcellular mitochondria transfer permits the incorporation of healthy mitochondria into the endogenous network of recipient cells, changing the bioenergetic profile and other functional properties of the recipient and may allow the recipient cells to recuperate from apoptotic processes and return to a normal operating state. Mesenchymal cells (MSCs) can form TNTs and transfer mitochondria and other constituents to target cells. This occurs under both physiological and pathological conditions, leading to changes in cellular energy metabolism and functions. This review summarizes the newly described capacity of melatonin to improve mitochondrial fusion/fission dynamics and promote TNT formation. This new evidence suggests that melatonin's protective effects could be attributed to its ability to prevent mitochondrial damage in injured cells, reduce senescence, and promote anastasis, a natural cell recovery phenomenon that rescues cells from the brink of death. The modulation of these new routes of intercellular communication by melatonin could play a key role in increasing the therapeutic potential of MSCs.
Keywords: intercellular communication, ischemic injury, mesenchymal stem cells, mitochondria, tunneling nanotubes
1. INTRODUCTION
Efficient cell‐to‐cell communication is essential for tissue development, homeostasis, and the maintenance of cellular functions after injury. There are a variety of means by which cells communicate; these include exosomes, secreted microRNAs, or gap junctions. 1 , 2 , 3 Tunneling nanotubes (TNTs), initially observed by Roustom and coworkers in 2004 in cultured rat pheochromocytoma PC12 cells 4 and later found in other cell lines, including primary rat neurons, astrocytes, and immune cells, 5 , 6 have emerged as a new important method of cell‐to‐cell communication. TNTs are thin‐extended membrane protrusions that connect cells over long distances allowing the exchange of various‐sized cargoes, such as small molecules (e.g., calcium ions), macromolecules (nucleic acids, proteins, etc.), and organelles (vesicles, lysosomes, mitochondria, autophagosomes, etc.) between cells. 7 When TNTs connect multiple cells, this leads to functional cellular networks. 8
Interest in TNTs has significantly increased despite the difficulties in studying them in in vivo experiments, mainly due to the preservation of their delicate structure, the possible confusion with other cellular protrusions, and their functional heterogeneity. Homotypic and heterotypic TNTs, however, play a vital role in disease progression and therapy. TNTs have been linked to developmental processes and signaling between different cell types, including immune cells, mesenchymal stem/stromal cells (MSCs), astrocytes, and neurons, and have been implicated in pathological conditions such as cancer progression and chemotherapy resistance, neurodegeneration 9 and neuroprotection, 10 as well as anastasis, a natural cell recovery phenomenon that rescues cells from the brink of death. 11 TNTs are therefore identified as novel bridges of intercellular communication in physiological and pathological life processes (Figure 1). Here, we review the processes involved in TNTs cellular communication highlighting their role in MSCs cell survival after stress and their possible modulation by melatonin.
Figure 1.
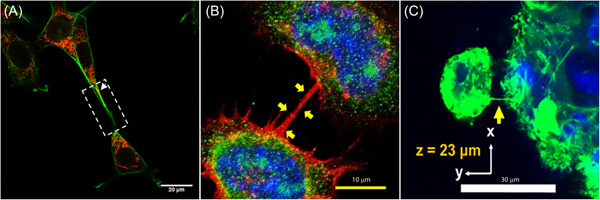
Tunneling nanotubes (TNTs) in different cell types. (A) Confocal image of a TNT (white arrow) between HT22 cells exposed to oxygen‐glucose deprivation and then treated with melatonin. The overlay shows the co‐localization (yellow) of phalloidin (green) and MitoTracker Deep Red (red) indicating the presence of mitochondria within TNT. 100 (B) Confocal image of TNT (yellow arrows) between CAD cells stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (blue), phalloidin (red), and Ca2+/calmodulin‐dependent protein kinase II (βCaMKII, green). The overlay shows the βCaMKII‐positive puncta inside a TNT (yellow). Adapted with permission from Vargas et al. 25 C, Confocal image of TNT (yellow arrow) between mesenchymal stem cells (MSCs) and an MSC spheroid stained with Hoechst 33342 (blue) and phalloidin (green). Adapted with permission from Zhang et al. (Copyright [2018] American Chemical Society). 124
2. TUNNELING NANOTUBES STRUCTURE AND FORMATION
TNTs are nano‐scaled membranous tubes of about 50–200 nm diameter and up to several cell diameters long, freely hovering in the medium. TNTs are different entities from filopodia. Indeed, for example, in cell cultures, TNTs are free‐floating while filopodia, which are unable to mediate vesicular transport, are connected to the cell plate. Moreover, the regulatory mechanisms responsible for filopodia and TNTs formation work in direct opposition; when a cell encounters a TNT, it retracts its existing filopodia. 12
To date, two different mechanisms have been proposed to form TNTs. The first commonly referred to as the “cell dislodgement mechanism,” describes two cells closely opposed to each other, fusing transiently and retaining a thin membrane thread while they move apart. 13 The second, known as the actin‐driven protrusion mechanism, proposes the formation of a filipodium‐like protrusion from one cell to another, followed by membrane fusion of the tip upon physical contact. In both mechanisms, actin plays a key role since drugs inhibiting actin polymerization block TNT formation. 14
Onfelt and coworkers 13 described two classes of TNTs between human monocyte‐derived macrophages distinguished by their cytoskeletal composition and functional properties. Thin TNTs (diameter < 0.7 nm) contain only F‐actin, whereas thicker TNTs (diameter > 0.7 nm) contain both F‐actin and microtubules (microtubule‐containing TNTs; MT‐TNTs). Thick TNTs appear linked to bidirectional transfers of cellular components, including organelles, whereas thin TNTs containing only F‐actin exhibit unidirectional transfers. 15 Thus, TNTs seem heterogeneous in their membrane structure and cell type‐specific. Many do not contain microtubules since tubulin blockage does not affect their formation in all cell types.
Stressed and unstressed cells establish different types of TNTs. Wang and Gerdes, for example, showed that UV‐treated PC12 cells assembled a type of TNT characterized by continuous microtubule formations (MT‐TNTs). 16 These TNTs were different from those formed between untreated cells. They also showed that MT‐TNTs could transfer mitochondria and participate in the rescue effects. 16 TNT connections were also observed among neurons and astrocytes. However, only cells containing injured mitochondria at an early apoptotic stage formed MT‐TNTs, 16 suggestings that connections are dependent on the vitality status of the cells.
In HBEC‐3 cells, it has been shown that cell density affects the ability to form TNTs. In this model, blockage of tubulin did not influence TNTs formation. 17 The authors identified two types of TNTs named TNT1 and TNT2. TNT1 formed a network within groups of cells or linked isolated cells and were probably formed through a directed filopodia‐like protrusion, while TNT2 only connected closely adjacent cells. It should be noted that the bulk of the information on TNTs formation between cells originated from experimental work in cell culture since studying TNTs in vivo is significantly more difficult. However, TNT‐like structures exist during the embryonic development of chicks 18 and fish. 19 TNT‐like structures connecting bone marrow‐derived MHC class II cells have been reported in the corneal stroma of GFP chimeric mice 20 and between osteoclasts in the bone destruction sites of arthritic rats. 21 Recently, Chen and colleagues provided in vivo evidence of intercellular transportation of proteins via TNTs in brain cells. They reported TNT development between apical dendrites and nearby astrocytic somas/processes in the prefrontal cortex of adult mice, which allowed intercellular communication, including prion transport. 22
3. HOW AND WHERE DO NANOTUBES FORM?
The formation of TNTs is mainly a feature of cells under stress. Hydrogen peroxide or serum deprivation, for example, induces oxidative stress and increases TNTs formation in cultured astrocytes and neurons. 6 In astrocytes, TNTs are primarily established between stressed and unstressed cells, and their formation seems to be mainly controlled by the transcription factor p53. In stressed neurons and astrocytes, p53 stimulation induces both caspase‐3 upregulation and TNT formation. It has been proposed that oxidative insults cause p53 activation that, in turn, upregulates Epidermal Growth Factor Receptor (EGFR) expression, activates the Akt/PI3K/mTOR pathway, and induces M‐Sec overexpression. 6 M‐Sec, first described as tumor necrosis factor‐alpha‐induced protein 2, 23 in cooperation with the RalA small GTPase and the exocyst complex, trigger F‐actin polymerization that induces the initiation of membrane protrusions for TNT formation from the cell membrane. 23 , 24 Another pathway involved in TNTs formation and TNT‐mediated transfer of cargoes between neurons may be the Wnt/Ca2+ pathway, an intracellular cascade involved in actin cytoskeleton remodeling. In this pathway, the Ca2+/calmodulin‐dependent protein kinase II (CaMKII) is identified as a key molecule in modulating the TNT‐mediated transfer of cargoes. 25 The calcium‐binding protein S100A4 and its receptor RAGE are other critical elements for the formation of stable protrusions and for guiding TNT growth between astrocytes and neurons. Caspase‐3 cleaves intracellular S100A4, decreasing its concentration. This creates a chemical gradient from a low concentration in initiating cells toward a high concentration in target cells, which is followed by the cell finding the target. Interestingly, whereas for TNT formation between astrocytes, S100A4 seems to be sufficient for cell targeting, neuronal activation is also required for the TNT formation between astrocytes and neurons, suggesting that healthy target cells are needed to successfully build nanotubes. 26 TNTs between stressed and unstressed cells were also observed by Kretschmer and coworkers in prostate cancer cells (PCa) as well as from stressed PCa and osteoblasts; 27 they also occur in T cells triggered by apoptosis by FasL. 28 , 29
4. INTERCELLULAR EXCHANGE OF CELLULAR COMPONENTS
TNTs transport a variety of cellular components, including organelles, proteins, calcium ions, viruses, and bacteria. Mitochondria are important trafficked entities through TNTs. Transcellular mitochondria transfer enables their incorporation into the endogenous network of recipient cells, changing the bioenergetic profile and other functional properties of the recipient. 30
Miro1 (mitochondrial Rho‐GTPase 1, synonym: RhoT1), is a calcium‐sensitive adaptor protein that connects the mitochondrion and the KIF5 motor protein through additional proteins, like Miro2, TRAK1, TRAK2, and Myo19, seems to be an important player in mitochondrial transfer. Miro1 overexpression causes a more efficient mitochondrial transfer from mesenchymal stem cells (MSCs) to damaged neural cells improving survival and recovery. 31 , 32 Conversely, knocking down Miro1 inhibits TNTs generation and MSCs rescue efficacy. 33 Miro1 also enhances the transfer of mitochondria from MSCs to neuron‐like PC12 cells experiencing ischemic damage, 32 improving recovery and cell proliferation.
Lysosomes have been identified as another important cargo within the TNTs structures. Lysosomes are membrane‐bound organelles whose primary function is to digest macromolecules and degrade cellular waste. The mechanism of lysosome transfer can be both diffusive and active, the latter involving ATP and motor proteins like dyneins and kinesins, which regulate the movement of lysosomes along microtubules. 34 Lysosomes were initially observed in TNTs connecting macrophage cells 13 and then reported in other cells connected through TNTs. 34 The study of the intercellular transport of lysosomes revealed that TNTs can also facilitate the transport of viruses and proteins, with the latter being linked to various neurodegenerative diseases. 35 , 36 Calcium ion flux has also been documented to be relevant to TNT physiology. Calcium ion flux is mediated through Cx42 gap junctions (GJs) and by IP3 which stimulates calcium ion release from the endoplasmic reticulum. 37
Other cargoes transported by TNTs include microRNA (miRNA), lipids, proteins, and likely many other cell products. miRNA transfer has been investigated mainly in cancer cells and has been identified as a mechanism for tumor cells to spread novel resistance gene mutations. 38 Recently, the presence of lipid droplets within TNTs has been demonstrated in endothelial cells by Astanina and coworkers; 39 they reported that under angiogenic conditions the number of lipid droplets and TNTs formation increased significantly.
5. TUNNELING NANOTUBES AND MESENCHYMAL STEM CELLS
MSCs are stromal cells, nonhematopoietic in nature, which can differentiate into multiple cell types. MSCs can be isolated from dental tissue, bone marrow, adipose tissue, umbilical cord, placenta, and other sources. MSCs possess the capacity to form TNTs and gap junctions, and transfer mitochondria and other constituents to target cells. This occurs under both physiological and pathological conditions, leading to changes in cell energy metabolism and functions. Spees and coworkers described the mitochondrial donation from MSCs initially in 2006. 40 The authors demonstrated that cocultivation of human bone marrow MSCs (hMSCs) and A549p cells, which were incapable of aerobic respiration and growth because of defective mtDNA, fostered the cells to acquire functional mitochondria provided by the donor hMSCs. Accordingly, Liu and coworkers demonstrated that MSCs repaired postischemic endothelial cells with dysfunctional mitochondria by transferring functional mitochondria from healthy MSCs via TNTs in vitro. 41 In a murine model of lipopolysaccharide (LPS)‐induced acute lung injury, mitochondrial transfer from MSCs to pulmonary alveolar epithelial cells occurred after establishing gap junctions with the injured lung epithelial cells; this resulted in the regeneration of the damaged alveoli. 42
The therapeutic efficacy of MSCs via TNTs‐mediated transfer of mitochondria has also been shown in preclinical studies related to ischemia, oxidative stress, mitochondrial damage, inflammation, and respiratory illness; likewise, they form gap‐junction, secrete immunomodulatory factors, growth factors, and release exosomes/microvesicles. 43 Because of these effects, MSCs have been frequently evaluated for their regenerative applications. MSCs have been shown to influence immunity, 44 increase epithelial cell proliferation, 45 and support recovery of ischemic tissues. 46 MSCs can protect cells from injury and promote tissue repair, 47 and when injected into the myocardium after infarction can reduce the incidence of scar formation. 48
It remains unclear, however, how MSCs mediate their rescue efficacy. Four potential but nonexclusive mechanisms have been proposed: (i) direct cell‐to‐cell signaling; (ii) paracrine signaling with soluble secreted factors such as hormones and proteins; (iii) homing of released exosomes or microvesicles that contain immunoregulatory molecules and other molecules including RNA; and (iv) mitochondrial trafficking via TNTs or microvesicles. 49 It is believed that, as a strategy that can restore the bioenergetic needs of damaged cells, mitochondrial transfer is by itself an effective reparative means to rescue different types of damaged cells. Below is discussed the potential role of mitochondrial transfer through MSCs after an ischemic insult and how it can aid in repairing and regenerating injured and damaged cells.
6. TUNNELING NANOTUBES, MESENCHYMAL STEM CELLS, AND ISCHEMIA
Research on TNTs and ischemia has mainly been related to nanotube building by MSCs when applied as a therapeutic tool for stroke in the brain and after myocardial infarction. During an ischemic insult, the lack of blood flow causes irreversible damage to neurons and the loss of numerous neuronal networks. Cells directly affected by the lack of glucose and oxygen in the ischemic core die by necrosis. In the surrounding penumbra, neurons, astrocytes, microglia, oligodendrocytes, pericytes, and endothelial cells respond in different ways to the damaging events caused by excitotoxicity, oxidative stress, and inflammation. The fate of neurons is multifactorial, and survival relies on communication among all the different players. Neurons are connected to each other forming an extensive communication network through synaptic transmission; failures at the synaptic level cause neuronal disintegration and transsynaptic degeneration ending in severe neuronal dysfunction. 50 Glial cells, neurons, and blood vessels are strictly interconnected to maintain cerebral blood flow. 51 Moreover, the integrity and functionality of the blood–brain barrier are critical features for the preservation and recovery of neural tissues. 52 The work of this “neurovascular unit” 51 seems to involve several factors, including TNTs. 53 Babenko and coworkers observed the formation of TNTs in cocultures of MSCs with either rat astrocytes or neuron‐like PC12 pheochromocytoma cells exposed to oxygen‐glucose deprivation. Their study demonstrated that mitochondria transported through TNTs restored cell proliferation and respiration affected by the ischemic insult. 32 A recent in vivo study demonstrated that the infusion of bone marrow MSCs to ischemic rats caused an effective TNTs‐mediated mitochondrial transfer to promote recovery from ischemic stroke. 54
Published reports documented similar results in cardiac stroke. Thus, TNTs–mediated mitochondrial transfer from bone marrow MSCs to H9c2 cells rescued cardiomyocytes from ischemia/reperfusion damage. 55 Similarly, a study by Figeac et al. demonstrated that mouse cardiomyocytes triggered human adipose MSCs to release several soluble factors related to cardiac protection through TNTs formation. 56 A protective role of TNTs was also observed between human bone marrow MSCs and human umbilical cord vein endothelial cells subjected to oxygen and nutrient deprivation. 41
7. MELATONIN: A POSSIBLE NEW ROLE IN MSCS COMMUNICATION?
Melatonin, produced by the pineal gland and secreted in blood and cerebrospinal fluid, has numerous biological functions. Other cells also synthesize melatonin, but they release it into the extracellular space but not in the blood. 57 , 58 , 59 Many melatonin's actions are mediated by membrane receptors, MT1 and MT2; these are members of the G‐protein coupled receptor superfamily. Melatonin may also interact with other intracellular molecules, including the so‐called MT3 receptor, the nuclear receptor ROR/RZR, and calmodulin. 60 , 61 Some functions of melatonin, such as its direct radical scavenging actions, are receptor independent.
There is a large amount of evidence confirming that melatonin protects tissues from ischemia/reperfusion injury; 62 , 63 , 64 , 65 the protective actions involve several mechanisms including a reduction of oxidative damage, 63 , 66 , 67 inhibition of the mitochondrial permeability transition pore (MPTP), and activation of uncoupling proteins (UCPs). 68 Melatonin also regulates the inflammatory reaction by modulating pro‐ and anti‐inflammatory cytokines 69 , 70 and preventing the expression of cyclooxygenase (COX) and inducible nitric oxide synthase (iNOS). 71
Robust data have recently documented the role of melatonin in mitochondrial function. 72 , 73 , 74 Melatonin is synthesized in, taken up by, and concentrated in mitochondria, and the presence of the MT1 receptor on the mitochondrial membranes isolated from brain lysates has been reported. 75 Mitochondria are highly dynamic and energy‐producing organelles that act as a signal transduction hub to regulate physiological activities such as intracellular calcium homeostasis, apoptosis, and autophagy. 76 , 77 Mitochondria are also the primary source of reactive oxygen species (ROS) as a by‐product of oxidative phosphorylation; moreover, ROS accumulation and oxidative stress are elevated during mitochondrial dysfunction. 78 , 79 Oxidative stress alters the mitochondrial electron transport chain activity and the cardiolipin structure; these changes facilitate the opening of the mitochondrial permeability transition pore, promoting apoptosis, autophagy, and eventually cell death. 80 , 81 Melatonin is a powerful antioxidant and decreases ROS levels by directly scavenging free radicals and stimulating the activities of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPX), 74 , 82 , 83 and by regulating the activity of the complex I and III, which reduce ROS generation. 84
Mitochondria form a dynamic network that continuously changes size, shape, and location. Mitochondrial dynamics is a critical part of cell quality control and is managed through several mechanisms, including biogenesis, fission, and fusion. 85 , 86 , 87 Mitochondrial fission is characterized by the division of a mitochondrion into two daughter mitochondria; this process is regulated by dynamin‐related protein 1 (DRP1), mitochondrial fission protein 1 (FIS1), and mitochondrial fission factor (Mff). 88 In contrast, mitochondrial fusion is the union of two mitochondria forming a large new mitochondrion. This is generally regulated by mitofusin‐1 (MFN1), mitofusin‐2 (MFN2), and optic atrophy 1 (OPA1), three GTPase‐dependent proteins in the dynamin superfamily. 86 , 89 , 90 The balance between fission and fusion is crucial to ensure a steady energy supply and cellular homeostasis. Mitochondrial fission occurs during cardiolipin oxidation, promoting the mitochondrial apoptosis pathway, and is always accompanied by mitophagy, an autophagic response specifically targeting damaged mitochondria. 91 , 92 Mitochondrial fusion, on the other hand, permits the content exchange between two different mitochondria, which maintain biochemical and genetic uniformity via the elimination of ROS and mutated DNA, and allows for the repolarization of membranes. 93
Mitochondrial dynamics are critical for MSCs to acquire the optimal morphology, enabling cells to quickly and adaptively respond to environmental stresses, affecting their self‐renewal, differentiation, and fate. 94 , 95 , 96 Melatonin has important effects on mitochondrial dynamics and dysfunctions. Melatonin can improve mitochondrial biogenesis in ischemia/reperfusion‐induced myocardial injury, affect fission/fusion dynamics in the diabetic retina, 97 regulate mitochondrial dynamics and alleviate neuronal damage in prion diseases, 98 and improve mitochondrial dynamics and functions in an experimental model of obesity and associated diabetes 99 (Figure 2).
Figure 2.
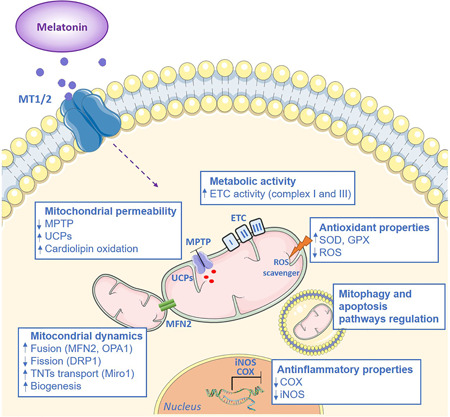
The protective role of melatonin on mitochondrial functions. Melatonin exerts several protective actions on mitochondria that involve its antioxidant properties, the inhibition of mitochondrial permeability, and the enhancement of metabolic activity. In addition, melatonin regulates mitochondrial dynamics and promotes tunneling nanotube formation. COX, cyclooxygenase; ETC, electron transport chain; DRP1, dynamin‐related protein 1; GPX, glutathione peroxidase; iNOS, inducible nitric oxide synthase; MT1/2, melatonin receptors 1 or 2; I, II, III, mitochondrial complexes; MPTP, mitochondrial permeability transition pore; MFN2, mitofusin‐2; OPA1, optic atrophy 1; Miro1, mitochondrial Rho‐GTPase 1; ROS, reactive oxygen species; SOD, superoxide dismutase; UCPs, uncoupling proteins
Using cultured hippocampal neurons (HT22 cells) subjected to oxygen/glucose deprivation (OGD), we recently reported that the addition of melatonin to the medium in which the cells were grown reduced ROS formation and restored the mitochondrial fusion/fission dynamics affected by OGD, thereby preventing mitochondrial dysfunction. Notably, while the stress induced by OGD by itself increased TNTs formation in HT22 cells, the presence of melatonin in the medium resulted in an increased number of TNT connections between cells. In addition, in the presence of melatonin, we found a higher number of mitochondria containing TNTs compared to the OGD and the control condition. 100 The improved mitochondrial biogenesis and dynamics and the increased donation of mitochondria may support mitochondrial respiration in suffering cells and may foster cell recovery via anastasis. 11 Yip and colleagues published almost simultaneously similar results. 101 These authors using both in vitro and in vivo experimental approaches showed that in the injured area TNTs transfer mitochondria from healthy to apoptotic cells allowing them to recover. In the in vivo MCAO rat model, purified healthy mitochondria pretreated with melatonin were injected 60 min after reperfusion into the infarcted site. The injection reduced mitochondrial DNA damage, oxidative stress levels, apoptotic neurons, and the infarct volume, and enhanced the number of intact and functional mitochondria in the damaged neurons. Using mitochondrial trackers, the authors showed that the injected healthy mitochondria were transferred via TNTs. Our findings 100 and those of Yip and colleagues 101 underpin the importance of melatonin in helping to maintain the function of damaged mitochondria and potentially supporting cell recovery. The molecular mechanisms by which melatonin enhances TNT formation and functionality remain unknown and deserve intensive study. A modulatory effect of melatonin on the cytoskeleton has been reported in different types of cells 102 , 103 , 104 and cytoskeletal rearrangements seem to protect the neuro‐cytoskeleton from damage caused by free radicals, which, in turn, contributes to cell survival.
Melatonin is also involved in exosome biogenesis and release. 105 Exosomes are small microvesicles of endosomal origin harboring biomolecules with the ability to alter the function of target cells often distant from the original host cell. 106 A recent publication has shown that exosomes of bone marrow MSCs pre‐conditioned with melatonin had benefits in MSCs via paracrine effects and improved their therapeutic efficacy on I/R induced acute renal failure. 107 In line with these observations, Pournaghi et al. demonstrated that melatonin alters exosomal size and production in bovine granulosa cells in a dose‐dependent manner. 108 Liu and co‐workers examined a potential correlation between melatonin receptors and exocytosis. The authors demonstrated the involvement of the MT2 receptor and the GSK‐3β/CRMP2‐axis in the exocytosis rat hippocampal neurons suggesting that this pathway may be relevant for the release of extracellular vesicles. 109 Several authors reported that melatonin treatment alters the unsaturated/saturated fatty acid ratio by increasing the flexibility of the cell membrane by increasing the release of exosomes and their delivery to the target cells. 108
MSCs transplantation has emerged as promising means for restoring tissue and organ functions. There are many types of MSCs, for example, cord blood mesenchymal stem cells (UCBMSCs), bone marrow mesenchymal stem cells (BMSCs), adipose‐derived mesenchymal stem cells (ADMSCs), dental pulp MSCs, and limbal MSCs. 61 Recent work has shown that melatonin increases MSC survival and induces a synergistic effect that alleviates inflammation, apoptosis, and oxidative stress. 110 This evidence is in line with the data that identified the melatonin receptors MT1 and MT2 on BMSC. 61 , 111 The injection of MSCs is a potentially effective treatment strategy in the developing injured brain, given their potent neuroregenerative properties and favorable immunological profile. 112 The possibility of obtaining large quantities of MSCs from birth‐associated tissues, especially from the placenta and the umbilical cord, makes them excellent potential candidates for use in neonatal care. Transplantation of MSCs promotes functional neurologic recovery in various models of neonatal cerebral damage by promoting neurogenesis, oligodendrogenesis, and axonal remodeling and rewiring. 113 , 114 Donega et al., for example, found that after neonatal hypoxic‐ischemic brain injury, intranasal MSCs transplantation improves behavioral outcomes and fosters neuronal and oligodendrocyte regeneration in mice. 115 It is important to consider that following the injection of MSCs many of them die or fail to proliferate due to reduced nutrition or growth factors. Recently, melatonin was found to improve the homing ability and decrease the apoptotic rate of MSC after transplantation. 110 Indeed, in the ischemic brain, melatonin pretreatment increased the survival of MSCs in vitro by reducing apoptosis after transplantation. 116 Melatonin also protects MSCs from senescence‐associated mitochondrial dysfunction. A recent study by Lee and coworkers showed that treatment with melatonin rescued replicative senescent MSCs and improved functional recovery in a murine hindlimb ischemia model by increasing blood flow perfusion and neovascularization, an effect that was associated with enhanced mitophagy and improved mitochondrial function. 117 Mitochondrial dysfunction and excessive ROS production are primary causes of senescence in MSCs; 118 , 119 between senescent cells there is a high prevalence of intercellular bridges recognized as membrane‐bound TNTs containing both actin and tubulin, which allow the transfer of large cargo including mitochondria between senescence cells. 120
It is obviously crucial to clarify the effects of melatonin on MSCs transplantation and how they improve stem cell survival. Possible mechanisms include modulation of the inflammatory microenvironment, secretion of survival‐promoting neurotrophic factors, stimulation of the plasticity and neural activity in damaged host tissue, and renewing synaptic transmission. 121 These combined actions of melatonin may rescue from senescence and anastasis and enhance in vivo stem cells' capacity to survive and interact with different cell types in the microenvironment, 122 displaying the ability to connect to target cells through TNTs development (Figure 3).
Figure 3.
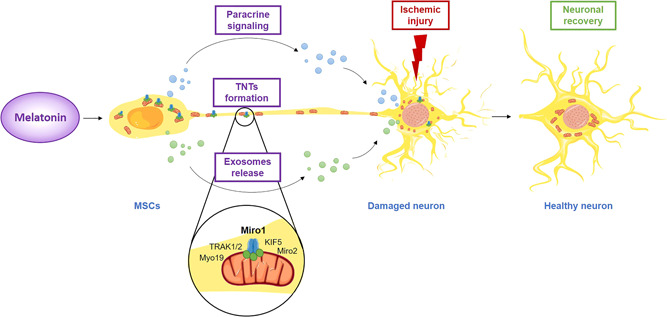
Melatonin as a potential modulator in mesenchymal stem cell communication and neuronal recovery. Melatonin has a variety of effects on MSCs, which include paracrine signaling through modulation of the inflammatory microenvironment or secretion of survival‐promoting neurotrophic factors, exosomes release, and TNTs formation. TNTs transport a variety of cellular components, including organelles, proteins, calcium ions, viruses, and bacteria. This representation highlights the new role of melatonin as a promising agent able to promote mitochondrial transfer via TNTs in MSCs leading to neuronal recovery. KIF5, kinesin‐related protein 5; Miro1, mitochondrial Rho‐GTPase 1; Miro2, mitochondrial Rho‐GTPase 2; MSCs, mesenchymal stem cells; Myo19, myosin XIX; TNTs, tunneling nanotubes; TRAK1/2, trafficking kinesin‐binding protein 1 and 2
8. CONCLUSIONS
Research progress has led to the identification of several actions of melatonin that would be beneficial for its use in tissue regeneration. MSCs represent the new frontier of regenerative medicine. The regenerative capacity of MSCs involves several processes: (i) direct cell‐to‐cell signaling; (ii) paracrine signaling with soluble secreted factors such as hormones and proteins; (iii) homing of released exosomes or microvesicles that contain immunoregulatory agents and other molecules including RNA; and (iv) mitochondrial trafficking via TNTs or microvesicles. The transfer of mitochondria via TNTs has been identified as a new mechanism for mitochondrial movement. This transfer of critical cellular components allows the recipient cells to regain viability and develop normal cellular functions. In this scenario, particular attention should be directed at the newly‐described capacity of melatonin to promote both the TNT formation and the release of microvesicles. The modulation of these new routes of intercellular communication by melatonin could play a key role in increasing the therapeutic potential of MSCs in a large number of diseases, including neurological and immune disorders, cerebral and cardiac ischemia, diabetes, and bone and cartilage diseases. As summarized in this review, the findings suggest that melatonin's protective effects are attributed not only to its capacity to prevent mitochondrial damage in injured cells but also to promote anastasis, a natural cell recovery phenomenon that rescues cells from the brink of death. 123 Thus, we speculate that an important function of melatonin is to stimulate the transfer of highly‐functional mitochondria through TNTs from healthy to apoptotic cells with faltering mitochondria, allowing the recipient cells to recuperate from apoptotic processes and return to a normal functional state.
AUTHOR CONTRIBUTIONS
Francesca Luchetti, Silvia Carloni, Maria G. Nasoni, and Walter Balduini contributed to the collection, interpretation, and writing of the manuscript. Russel J. Reiter critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by a grant from the University of Urbino Carlo Bo to W. Balduini (DR‐473_2018). Open access funding provided by Universita degli Studi di Urbino Carlo Bo within the CRUI‐CARE Agreement.
Luchetti F, Carloni S, Nasoni MG, Reiter RJ, Balduini W. Tunneling nanotubes and mesenchymal stem cells: new insights into the role of melatonin in neuronal recovery. J Pineal Res. 2022;73:e12800. 10.1111/jpi.12800
Contributor Information
Francesca Luchetti, Email: francesca.luchetti@uniurb.it.
Walter Balduini, Email: walter.balduini@uniurb.it.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125‐132. [DOI] [PubMed] [Google Scholar]
- 2. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landschaft D. Gaps and barriers: gap junctions as a channel of communication between the soma and the germline. Semin Cell Dev Biol. 2020;97:167‐171. [DOI] [PubMed] [Google Scholar]
- 4. Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303(5660):1007‐1010. [DOI] [PubMed] [Google Scholar]
- 5. McCoy‐Simandle K, Hanna SJ, Cox D. Exosomes and nanotubes: control of immune cell communication. Int J Biochem Cell Biol. 2016;71:44‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Cui J, Sun X, Zhang Y. Tunneling‐nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 2011;18(4):732‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abounit S, Zurzolo C. Wiring through tunneling nanotubes: from electrical signals to organelle transfer. J Cell Sci. 2012;125(Pt 5):1089‐1098. [DOI] [PubMed] [Google Scholar]
- 8. Ariazi J, Benowitz A, De Biasi V, et al. Tunneling nanotubes and gap junctions‐their role in long‐range intercellular communication during development, health, and disease conditions. Front Mol Neurosci. 2017;10:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tiwari V, Koganti R, Russell G, Sharma A, Shukla D. Role of tunneling nanotubes in viral infection, neurodegenerative disease, and cancer. Front Immunol. 2021;12:680891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nair S, Rocha‐Ferreira E, Fleiss B, et al. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: Role of mitochondria, inflammation, and reactive oxygen species. J Neurochem. 2021;158(1):59‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reiter RJ, Sharma R, Rosales‐Corral S. Melatonin, tunneling nanotubes and anastasis: cheating cell death. Melatonin Research. 2021;4(4):566‐580. [Google Scholar]
- 12. Delage E, Cervantes DC, Pénard E, et al. Differential identity of filopodia and tunneling nanotubes revealed by the opposite functions of actin regulatory complexes. Sci Rep. 2016;6:39632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Onfelt B, Nedvetzki S, Benninger RK, et al. Structurally distinct membrane nanotubes between human macrophages support long‐distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177(12):8476‐8483. [DOI] [PubMed] [Google Scholar]
- 14. Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, Gerdes HH. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 2009;583(9):1481‐1488. [DOI] [PubMed] [Google Scholar]
- 15. Gerdes HH, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20(4):470‐475. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22(7):1181‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dubois F, Bénard M, Jean‐Jacques B, et al. Investigating tunneling nanotubes in cancer cells: guidelines for structural and functional studies through cell imaging. BioMed Res Int. 2020;2020:2701345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teddy JM, Kulesa PM. In vivo evidence for short‐ and long‐range cell communication in cranial neural crest cells. Development. 2004;131(24):6141‐6151. [DOI] [PubMed] [Google Scholar]
- 19. Caneparo L, Pantazis P, Dempsey W, Fraser SE. Intercellular bridges in vertebrate gastrulation. PLoS One. 2011;6(5):e20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180(9):5779‐5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang JQ, Takahashi A, Gu JY, et al. In vitro and in vivo detection of tunneling nanotubes in normal and pathological osteoclastogenesis involving osteoclast fusion. Laboratory Investigation. 2021;101(12):1571‐1584. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Cao J. Astrocyte‐to‐neuron transportation of enhanced green fluorescent protein in cerebral cortex requires F‐actin dependent tunneling nanotubes. Sci Rep. 2021;11(1):16798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hase K, Kimura S, Takatsu H, et al. M‐Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nature Cell Biol. 2009;11(12):1427‐1432. [DOI] [PubMed] [Google Scholar]
- 24. Ohno H, Hase K, Kimura S. M‐Sec: Emerging secrets of tunneling nanotube formation. Commun Integr Biol. 2010;3(3):231‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vargas JY, Loria F, Wu YJ, et al. The Wnt/Ca(2+) pathway is involved in interneuronal communication mediated by tunneling nanotubes. EMBO J. 2019;38(23):e101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun X, Wang Y, Zhang J, et al. Tunneling‐nanotube direction determination in neurons and astrocytes. Cell Death Dis. 2012;3:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kretschmer A, Zhang F, Somasekharan SP, et al. Stress‐induced tunneling nanotubes support treatment adaptation in prostate cancer. Sci Rep. 2019;9(1):7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arkwright PD, Luchetti F, Tour J, et al. Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes. Cell Res. 2010;20(1):72‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luchetti F, Canonico B, Arcangeletti M, et al. Fas signalling promotes intercellular communication in T cells. PLoS One. 2012;7(4):e35766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shanmughapriya S, Langford D, Natarajaseenivasan K. Inter and Intracellular mitochondrial trafficking in health and disease. Ageing Res Rev. 2020;62:101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmad T, Mukherjee S, Pattnaik B, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Babenko V, Silachev D, Popkov V, et al. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules. 2018;23(3):687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahmad T, Mukherjee S, Pattnaik BR, et al. Miro 1 knockdown in stem cells inhibits mitochondrial donation mediated rescue of bronchial epithelial injury. Biophys J. 2013;104(2):659a. [Google Scholar]
- 34. Murray LMA, Krasnodembskaya AD. Concise review: intercellular communication via organelle transfer in the biology and therapeutic applications of stem cells. Stem Cells. 2019;37(1):14‐25. [DOI] [PubMed] [Google Scholar]
- 35. Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV‐infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254(2):142‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Victoria GS, Zurzolo C. The spread of prion‐like proteins by lysosomes and tunneling nanotubes: Implications for neurodegenerative diseases. J Cell Biol. 2017;216(9):2633‐2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lock JT, Parker I, Smith IF. Communication of Ca(2+) signals via tunneling membrane nanotubes is mediated by transmission of inositol trisphosphate through gap junctions. Cell Calcium. 2016;60(4):266‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desir S, Dickson EL, Vogel RI, et al. Tunneling nanotube formation is stimulated by hypoxia in ovarian cancer cells. Oncotarget. 2016;7(28):43150‐43161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Astanina K, Koch M, Jungst C, Zumbusch A, Kiemer AK. Lipid droplets as a novel cargo of tunnelling nanotubes in endothelial cells. Sci Rep. 2015;5:11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103(5):1283‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu K, Ji K, Guo L, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia‐reperfusion model via tunneling nanotube like structure‐mediated mitochondrial transfer. Microvasc Res. 2014;92:10‐18. [DOI] [PubMed] [Google Scholar]
- 42. Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone‐marrow‐derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Med. 2012;18(5):759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qu R, Li Y, Gao Q, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27(4):355‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815‐1822. [DOI] [PubMed] [Google Scholar]
- 45. Donnelly JM, Engevik A, Feng R, et al. Mesenchymal stem cells induce epithelial proliferation within the inflamed stomach. Am J Physiol: Gastrointest Liver Physiol. 2014;306(12):G1075‐G1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yong KW, Choi JR, Mohammadi M, Mitha AP, Sanati‐Nezhad A, Sen A. Mesenchymal stem cell therapy for ischemic tissues. Stem Cells Int. 2018;2018:8179075‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11(5):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deleglise B, Lassus B, Soubeyre V, et al. Dysregulated neurotransmission induces trans‐synaptic degeneration in reconstructed neuronal networks. Sci Rep. 2018;8(1):11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Terasaki Y, Liu Y, Hayakawa K, et al. Mechanisms of neurovascular dysfunction in acute ischemic brain. Curr Med Chem. 2014;21(18):2035‐2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agnati LF, Fuxe K. Extracellular‐vesicle type of volume transmission and tunnelling‐nanotube type of wiring transmission add a new dimension to brain neuro‐glial networks. Philos Trans R Soc London Ser B. 2014;369(1652). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu K, Guo L, Zhou Z, Pan M, Yan C. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res. 2019;123:74‐80. [DOI] [PubMed] [Google Scholar]
- 55. Han H, Hu J, Yan Q, et al. Bone marrow‐derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol Med Rep. 2016;13(2):1517‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Figeac F, Lesault PF, Le Coz O, et al. Nanotubular crosstalk with distressed cardiomyocytes stimulates the paracrine repair function of mesenchymal stem cells. Stem Cells. 2014;32(1):216‐230. [DOI] [PubMed] [Google Scholar]
- 57. Acuña‐Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997‐3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reiter RJ, Ma Q, Sharma R. Melatonin in mitochondria: mitigating clear and present dangers. Physiology. 2020;35(2):86‐95. [DOI] [PubMed] [Google Scholar]
- 59. Venegas C, García JA, Escames G, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52(2):217‐227. [DOI] [PubMed] [Google Scholar]
- 60. Reiter RJ, Tan DX, Fuentes‐Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010;181:127‐151. [DOI] [PubMed] [Google Scholar]
- 61. Slominski RM, Reiter RJ, Schlabritz‐Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351(2):152‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abolhasanpour N, Alihosseini S, Golipourkhalili S, Badalzadeh R, Mahmoudi J, Hosseini L. Effect of melatonin on endoplasmic reticulum‐mitochondrial crosstalk in stroke. Arch Med Res. 2021;52(7):673‐682. [DOI] [PubMed] [Google Scholar]
- 63. Cervantes M, Morali G, Letechipia‐Vallejo G. Melatonin and ischemia‐reperfusion injury of the brain. J Pineal Res. 2008;45(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 64. Cheung RT. The utility of melatonin in reducing cerebral damage resulting from ischemia and reperfusion. J Pineal Res. 2003;34(3):153‐160. [DOI] [PubMed] [Google Scholar]
- 65. Hardeland R. Melatonin and inflammation‐story of a double‐edged blade. J Pineal Res. 2018;65(4):e12525. [DOI] [PubMed] [Google Scholar]
- 66. Galano A, Guzman‐Lopez EG, Reiter RJ. Potentiating the benefits of melatonin through chemical functionalization: possible impact on multifactorial neurodegenerative disorders. Int J Mol Sci. 2021;22(21):11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maharaj DS, Glass BD, Daya S. Melatonin: new places in therapy. Biosci Rep. 2007;27(6):299‐320. [DOI] [PubMed] [Google Scholar]
- 68. Tan DX, Manchester LC, Qin L, Reiter RJ. Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci. 2016;17(12):2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez‐Gallego J. A review of the molecular aspects of melatonin's anti‐inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 70. Yu GM, Kubota H, Okita M, Maeda T. The anti‐inflammatory and antioxidant effects of melatonin on LPS‐stimulated bovine mammary epithelial cells. PLoS One. 2017;12(5):e0178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tocharus J, Chongthammakun S, Govitrapong P. Melatonin inhibits amphetamine‐induced nitric oxide synthase mRNA overexpression in microglial cell lines. Neurosci Lett. 2008;439(2):134‐137. [DOI] [PubMed] [Google Scholar]
- 72. He C, Wang J, Zhang Z, et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte's quality under in vitro conditions. Int J Mol Sci. 2016;17(6):939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61(3):253‐278. [DOI] [PubMed] [Google Scholar]
- 74. Suofu Y, Li W, Jean‐Alphonse FG, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci USA. 2017;114(38):E7997‐E8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang X, Sirianni A, Pei Z, et al. The melatonin MT1 receptor axis modulates mutant Huntingtin‐mediated toxicity. J Neurosci. 2011;31(41):14496‐14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13(12):780‐788. [DOI] [PubMed] [Google Scholar]
- 78. Angelova PR, Abramov AY. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018;592(5):692‐702. [DOI] [PubMed] [Google Scholar]
- 79. Annesley SJ, Fisher PR. Mitochondria in health and disease. Cells. 2019;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ambekar T, Pawar J, Rathod R, et al. Mitochondrial quality control: epigenetic signatures and therapeutic strategies. Neurochem Int. 2021;148:105095. [DOI] [PubMed] [Google Scholar]
- 81. Baechler BL, Bloemberg D, Quadrilatero J. Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy. 2019;15(9):1606‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hardeland R. Melatonin and the electron transport chain. Cell Mol Life Sci. 2017;74(21):3883‐3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rodriguez C, Mayo JC, Sainz RM, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 84. Petrosillo G, Venosa ND, Pistolese M, et al. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia‐ reperfusion: role of cardiolipin. FASEB J. 2006;20(2):269‐276. [DOI] [PubMed] [Google Scholar]
- 85. Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62(3):341‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Xie LL, Shi F, Tan Z, Li Y, Bode AM, Cao Y. Mitochondrial network structure homeostasis and cell death. Cancer Sci. 2018;109(12):3686‐3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu YJ, McIntyre RL, Janssens GE, Houtkooper RH. Mitochondrial fission and fusion: a dynamic role in aging and potential target for age‐related disease. Mech Ageing Dev. 2020;186:111212. [DOI] [PubMed] [Google Scholar]
- 89. Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101(45):15927‐15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tabara LC, Morris JL, Prudent J. The complex dance of organelles during mitochondrial division. Trends Cell Biol. 2021;31(4):241‐253. [DOI] [PubMed] [Google Scholar]
- 91. Ma K, Chen G, Li W, Kepp O, Zhu Y, Chen Q. Mitophagy, mitochondrial homeostasis, and cell fate. Front Cell Dev Biol. 2020;8:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhu H, Toan S, Mui D, Zhou H. Mitochondrial quality surveillance as a therapeutic target in myocardial infarction. Acta physiologica. 2021;231(3):e13590. [DOI] [PubMed] [Google Scholar]
- 93. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li Q, Gao Z, Chen Y, Guan MX. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell. 2017;8(6):439‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lv J, Yi Y, Qi Y, et al. Mitochondrial homeostasis regulates definitive endoderm differentiation of human pluripotent stem cells. Cell Death Discov. 2022;8(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ren L, Chen X, Chen X, Li J, Cheng B, Xia J. Mitochondrial dynamics: fission and fusion in fate determination of mesenchymal stem cells. Front Cell Dev Biol. 2020;8:580070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chang JY, Yu F, Shi L, Ko ML, Ko GY. Melatonin affects mitochondrial fission/fusion dynamics in the diabetic retina. J Diabetes Res. 2019;2019:8463125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang X, Zhao D, Wu W, et al. Melatonin regulates mitochondrial dynamics and alleviates neuron damage in prion diseases. Aging. 2020;12(11):11139‐11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Agil A, Chayah M, Visiedo L, et al. Melatonin improves mitochondrial dynamics and function in the kidney of zucker diabetic fatty rats. J Clin Med. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nasoni MG, Carloni S, Canonico B, et al. Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic‐like injury in hippocampal HT22 cells. J Pineal Res. 2021;71(1):e12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yip HK, Dubey NK, Lin KC, et al. Melatonin rescues cerebral ischemic events through upregulated tunneling nanotube‐mediated mitochondrial transfer and downregulated mitochondrial oxidative stress in rat brain. Biomed Pharmacother. 2021;139:111593. [DOI] [PubMed] [Google Scholar]
- 102. Benitez‐King G. Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res. 2006;40(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 103. Cecon E, Oishi A, Jockers R. Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br J Pharmacol. 2018;175(16):3263‐3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Moreno ACR, Saito RF, Tiago M, et al. Melatonin inhibits human melanoma cells proliferation and invasion via cell cycle arrest and cytoskeleton remodeling. Melatonin Res 2020;3(2):194‐209. [Google Scholar]
- 105. Novais AA, Chuffa LGA, Zuccari D, Reiter RJ. Exosomes and melatonin: where their destinies intersect. Front Immunol. 2021;12:692022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huang‐Doran I, Zhang CY, Vidal‐Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 2017;28(1):3‐18. [DOI] [PubMed] [Google Scholar]
- 107. Zahran R, Ghozy A, Elkholy SS, El‐Taweel F, El‐Magd MA. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia‐reperfusion injury in a rat model. Int J Urol. 2020;27(11):1039‐1049. [DOI] [PubMed] [Google Scholar]
- 108. Pournaghi M, Khodavirdilou R, Saadatlou MAE, et al. Effect of melatonin on exosomal dynamics in bovine cumulus cells. Process Biochem. 2021;106:78‐87. [Google Scholar]
- 109. Liu D, Wei N, Man HY, Lu Y, Zhu LQ, Wang JZ. The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ. 2015;22(4):583‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hu C, Li L. Melatonin plays critical role in mesenchymal stem cell‐based regenerative medicine in vitro and in vivo. Stem Cell Res Ther. 2019;10(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27(2):101‐110. [DOI] [PubMed] [Google Scholar]
- 112. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726‐736. [DOI] [PubMed] [Google Scholar]
- 113. Shen LH, Li Y, Chen J, et al. One‐year follow‐up after bone marrow stromal cell treatment in middle‐aged female rats with stroke. Stroke. 2007;38(7):2150‐2156. [DOI] [PubMed] [Google Scholar]
- 114. Yasuhara T, Hara K, Maki M, et al. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic‐ischemic rats. J Cereb Blood Flow Metab. 2008;28(11):1804‐1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Donega V, Nijboer CH, van Velthoven CT, et al. Assessment of long‐term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res. 2015;78(5):520‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee SJ, Jung YH, Oh SY, Yun SP, Han HJ. Melatonin enhances the human mesenchymal stem cells motility via melatonin receptor 2 coupling with Galphaq in skin wound healing. J Pineal Res. 2014;57(4):393‐407. [DOI] [PubMed] [Google Scholar]
- 117. Lee JH, Yoon YM, Song KH, Noh H, Lee SH. Melatonin suppresses senescence‐derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L‐mitophagy pathway. Aging cell. 2020;19(3):e13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang Y, Liu Y, Chen E, Pan Z. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. 2020;382(3):457‐462. [DOI] [PubMed] [Google Scholar]
- 119. Ma L, Liu Q, Tian M, Tian X, Gao L. Mechanisms of melatonin in anti‐aging and its regulation effects in radiation‐induced premature senescence. Radiat Med Protect. 2021;2(01):33‐37. [Google Scholar]
- 120. Walters HE, Cox LS. Intercellular transfer of mitochondria between senescent cells through cytoskeleton‐supported intercellular bridges requires mTOR and CDC42 signalling. Oxid Med Cell Longevity. 2021;2021:6697861‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben‐Hur T. Neuroprotective effect of transplanted human embryonic stem cell‐derived neural precursors in an animal model of multiple sclerosis. PLoS One. 2008;3(9):e3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pluchino S, Cusimano M, Bacigaluppi M, Martino G. Remodelling the injured CNS through the establishment of atypical ectopic perivascular neural stem cell niches. Arch Ital Biol. 2010;148(2):173‐183. [PubMed] [Google Scholar]
- 123. Tang HL, Tang HM, Mak KH, et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol Biol Cell. 2012;23(12):2240‐2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang J, Whitehead J, Liu Y, Yang Q, Leach JK, Liu GY. Direct observation of tunneling nanotubes within human mesenchymal stem cell spheroids. J Phys Chem B. 2018;122(43):9920‐9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


