Abstract
A short period (1–2 h) of hypothermic oxygenated machine perfusion (HOPE) after static cold storage is safe and reduces ischemia‐reperfusion injury‐related complications after liver transplantation. Machine perfusion time is occasionally prolonged for logistical reasons, but it is unknown if prolonged HOPE is safe and compromises outcomes. We conducted a multicenter, observational cohort study of patients transplanted with a liver preserved by prolonged (≥4 h) HOPE. Postoperative biochemistry, complications, and survival were evaluated. The cohort included 93 recipients from 12 European transplant centers between 2014–2021. The most common reason to prolong HOPE was the lack of an available operating room to start the transplant procedure. Grafts underwent HOPE for a median (range) of 4:42 h (4:00–8:35 h) with a total preservation time of 10:50 h (5:50–20:50 h). Postoperative peak ALT was 675 IU/L (interquartile range 419–1378 IU/L). The incidence of postoperative complications was low, and 1‐year graft and patient survival were 94% and 88%, respectively. To conclude, good outcomes are achieved after transplantation of donor livers preserved with prolonged (median 4:42 h) HOPE, leading to a total preservation time of almost 21 h. These results suggest that simple, end‐ischemic HOPE may be utilized for safe extension of the preservation time to ease transplantation logistics.
Keywords: clinical research/practice, graft survival, ischemia reperfusion injury (IRI), liver allograft function/dysfunction, liver transplantation/hepatology, organ acceptance, organ perfusion and preservation, organ procurement and allocation, solid organ transplantation
Short abstract
Lung allografts procured in controlled donation after circulatory death with use of abdominal normothermic regional perfusion combined with lung retrieval is safe for lung grafts which show equivalent outcomes to graft transplanted after donation after brain death.
Abbreviations
- AKI
acute kidney injury
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BAR
balance of risks
- BMI
body mass index
- CVVH
continuous veno‐venous hemofiltration
- DBD
donation after brain death
- DCD
donation after circulatory death
- DHOPE
dual hypothermic oxygenated machine perfusion
- EAD
early allograft dysfunction
- HAT
hepatic artery thrombosis
- HCC
hepatocellular carcinoma
- HOPE
hypothermic oxygenated machine perfusion
- INR
international normalized ratio
- IQR
interquartile range
- MELD
model for end‐stage liver disease
- mpEAD
machine perfusion early allograft dysfunction
- NAS
nonanastomotic biliary strictures
- NMP
normothermic machine perfusion
- PNF
primary nonfunction
- PRS
post‐reperfusion syndrome
- PVT
portal vein thrombosis
- SCS
static cold storage
- yGT
gamma‐glutamyl transferase
1. INTRODUCTION
The use of machine perfusion to preserve donor livers is one of the most important advances in liver transplantation in the past decade. Hypothermic oxygenated machine perfusion (HOPE) is performed at 4–12°C with an acellular perfusion solution at low perfusion pressures and flow rates. 1 Hypothermic oxygenation of mitochondria induces metabolic programming within 1 h, thereby decreasing mitochondrial succinate accumulation and uploading adenosine triphosphate levels. 2 Reperfusion of livers treated by end‐ischemic HOPE is, therefore, associated with less oxidative injury and mitochondrial damage with subsequently less downstream inflammation. 2 , 3 , 4
The results of two recently published randomized controlled trials comparing end‐ischemic HOPE versus static cold storage (SCS) confirm the beneficial effects of this technique on clinical outcomes. 5 , 6 In the transplantation of donation after circulatory death (DCD) livers, 2 h of HOPE after SCS reduced the incidence of ischemia‐reperfusion‐related complications after transplantation, including a 68% reduction in symptomatic nonanastomotic biliary strictures (NAS), when compared to grafts preserved with SCS alone. 5 In the transplantation of livers from high‐risk donation after brain death (DBD) donors, approximately 2 h of end‐ischemic HOPE reduced the incidence of early allograft dysfunction (EAD) and postoperative complications. 6
Whereas a brief period (usually 1–2 h) of HOPE after SCS improves post‐transplant outcomes, machine perfusion time may occasionally be prolonged because of unforeseen transplant logistics. For example, when the donor liver is reallocated to another recipient in the last minute, or in the event of a difficult recipient hepatectomy. 7 , 8 , 9 Good outcomes after prolonged normothermic machine perfusion (NMP) up to 20 h have been reported previously, 10 but the use of prolonged HOPE is still unexplored. In a preclinical study of porcine and discarded human livers, HOPE could succesfully be prolonged for up to 24 h, followed by normothermic reperfusion.11 However, clinical data are currently lacking and it remains unknown whether postoperative outcomes are compromised when HOPE is prolonged beyond 2 h.
The objective of this multicenter study was, therefore, to evaluate outcomes after transplantation of donor livers preserved by prolonged (≥4 h) HOPE. We hypothesize that good outcomes after prolonged HOPE can be achieved, which are comparable to those previously reported for regular HOPE.
2. METHODS
2.1. Study design
European liver transplant centers with a clinical HOPE program were approached for participation in this multicenter observational cohort study. All cases of prolonged (≥4 h) HOPE‐preserved donor livers and recipients were eligible for inclusion in the study. There were no exclusion criteria. The study was designed as a stage 1 study according to the IDEAL‐D (Idea, Exploration, Assessment, Long‐term study outcomes for Devices) framework. 12 , 13 , 14 IDEAL stage 1 (‘Idea’) studies describe the first use of a procedure or device, either as a planned or unplanned approach with short‐term clinical outcomes as endpoints. The study was approved by the Institutional Review Board of the University Medical Center Groningen (RR 202100366) and adhered to the Declaration of Helsinki and the Declaration of Istanbul. The first and last authors had full access to all data in the study and take responsibility for its integrity and the data analysis. The study complied with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. 15
2.2. Liver procurement, preservation, and transplantation
Donor livers were obtained, preserved, and transported to recipient transplant centers according to standard national practice. The transplantation surgery and postoperative care were performed according to standard local practice. According to national legislation, livers from DCD donors in Italy were retrieved with in situ normothermic regional perfusion. The Liver Assist device (XVIVO), VitaSmart (Medica), or a custom‐made device by the Bergamo group was used for end‐ischemic HOPE of the liver. The devices enable pressure‐controlled, single or dual perfusion using a centrifugal pump or roller pump to provide a continuous flow through the portal vein and, in case of dual perfusion, a pulsatile flow through the hepatic artery. The Bergamo devices was based on a heart‐lung machine combined with an oxygenator with heat‐exchange performance (Quadrox‐i) and a cardiotomy reservoir (VHK). The perfusion systems were filled with an acellular preservation solution. The perfusion pressure was set to 20–30 mmHg in the hepatic artery and 3–9 mmHg in the portal vein. The temperature of the perfusion fluid was maintained between 8–12°C. Oxygenation of the perfusion solution was provided by membrane oxygenators supplying 100% oxygen to the preservation solution to target partial oxygen pressures of at least 70 kPa. Livers included in this study were perfused for at least 4 h.
2.3. Survey
An online questionnaire was sent to the program leader from each participating center. The survey included 10 questions about the centers’ experience with HOPE and the surgeon's view on prolonged HOPE.
2.4. Data collection
Data were collected at each center and anonymously stored in a single electronic database. Donor and recipient characteristics included age, the body mass index (BMI), the model for end‐stage liver disease (MELD) score, the donor risk index (DRI), the Eurotransplant DRI (ET‐DRI), and the balance of risks (BAR) score. The DRI is calculated based on the following donor characteristics: age, race, height, cause of death, DCD, and whether it was a partial or split graft. 16 By adding the latest laboratory gamma‐glutamyl transferase (yGT) of the donor and rescue allocation, the ET‐DRI was developed. 17 The BAR score is calculated based on the following variables: MELD score, retransplantation, whether the recipient was on life support preoperatively, recipient age, cold ischemia time, and donor age. 18 Postoperative outcomes included the occurrence of post‐reperfusion syndrome (PRS) (as defined below), postoperative laboratory values up to day 7 (lactate, aspartate aminotransferase [AST], alanine aminotransferase [ALT], yGT, bilirubin, creatinine, and international normalized ratio [INR]), intensive‐care and total hospital length of stay, primary non‐function (PNF), EAD, portal vein or hepatic artery thrombosis, NAS, acute kidney injury (AKI), complications according to Clavien‐Dindo grading, and 1‐year graft and patient survival.
2.5. Definitions
Post‐reperfusion syndrome was defined as (1) a decrease in mean arterial blood pressure ≥30 mmHg below baseline, lasting for ≥1 min, within 5 min after reperfusion (Aggarwal criteria 19 ), or (2) a fall in mean arterial blood pressure on reperfusion <50 mmHg either sustained ≥30 min and/or requiring ≥0.15 µg/kg/min norepinephrine, >2 U/h vasopressin, or infusion of epinephrine (Watson crtiteria 20 ). Primary nonfunction was defined as nonlife sustaining graft function leading to graft loss or retransplantation within the first week after liver transplantation. Both the definition of EAD according to Olthoff et al. 21 as well as a modified machine perfusion EAD (mpEAD) were used. Since the definition of EAD according to Olthoff et al. was coined prior to the introduction of machine perfusion, it does not take into account the so‐called washout effect of liver transaminases during/after machine perfusion (ILTS guidelines 2021 22 ). Hence, transaminases are likely to be lower in recipients transplanted by a machine‐perfused graft. The mpEAD was defined as the presence of 1 or more of the following on postoperative day 7: bilirubin ≥10 mg/dl, INR ≥1.6, or lactate ≥2 mmol/L in the absence of vascular complications. Vascular thrombosis was defined as a radiologically or surgically proven thrombus of the portal vein or hepatic artery. NAS were defined as any nonanastomotic biliary complication leading to surgical or endoscopic intervention within 12 months after liver transplantation, in the absence of concomitant hepatic artery thrombosis, or anastomotic stenosis. 23 Biliary leakage was defined as fluid with an elevated bilirubin level in the abdominal drain or intra‐abdominal fluid on or after postoperative day three or the need for radiological intervention owing to biliary collections or relaparotomy due to biliary peritonitis. 24
Acute kidney injury was defined as (1) increase serum creatinine by ≥0.3 mg/dl within 48 h after transplantation or, (2) increase in serum creatinine ≥1.5 times baseline, or (3) urine volume <0.5 ml/kg/h for 6 h. 25 Graft survival was defined as the time between liver transplantation and retransplantation or death. Graft survival was censored for patients dying with a functional graft. Patient survival was defined as the time between liver transplantation and all‐cause death.
2.6. Statistical analysis
Continuous variables are expressed as median and interquartile range, unless stated otherwise. Categorical variables are expressed as frequencies and proportions (%). Kaplan–Meier survival curves were used to graphically depict patient and graft survival. In one subanalysis, outcomes after prolonged HOPE were compared between DBD and DCD livers. Another subanalysis compared outcomes after prolonged single HOPE versus dual HOPE. Categorical variables were compared using Chi‐square test and continuous variables with a Mann–Whitney U test. p‐values < .05 were considered statistically significant. Data were analyzed with IBM SPSS Statistics version 24 (IBM Corporation) and Prism 8.
3. RESULTS
3.1. Study population
A total of 93 patients were transplanted with a donor liver after prolonged HOPE. In this cohort, the median donor age was 57 (50–68) years and 46% of livers were from DCD donors (Table 1). In case of DCD liver donation, median functional warm ischemia time was 32 (26–52) minutes. The median DRI and ET‐DRI scores were 2.24 (1.88–2.45) and 1.96 (1.81–2.30), respectively. The median recipient age was 59 (53–65) years and the majority was male (78%). Prior to liver transplantation, the median BAR score was 5 (3–8). The most common indications for liver transplantation were alcoholic cirrhosis (22%), hepatocellular carcinoma (17%), and nonalcoholic steatohepatitis (16%).
TABLE 1.
Baseline characteristics
| Characteristics | Patients (n = 93)n (%) or median(IQR) |
|---|---|
| Donor | |
| Age—year | 57 (50–68) |
| Male sex—no. (%) | 60 (65%) |
| Body mass index—kg/m2 | 25 (23–28) |
| Type of donor—no. (%) | |
| DBD | 50 (54%) |
| DCD | 43 (46%) |
| Donor Risk Index a | 2.24 (1.88–2.45) |
| Eurotransplant Donor Risk Index b | 1.96 (1.81–2.30) |
| Recipient | |
| Age—year | 59 (53–65) |
| Male sex—no. (%) | 73 (78%) |
| Body‐mass index—kg/m2 | 27 (23–29) |
| Laboratory MELD score c | 12 (9–19) |
| Balance of risk score d | 5 (3–8) |
| Indication for transplantation—no. (%) | |
| Alcoholic cirrhosis | 20 (22%) |
| HCC | 16 (17%) |
| NASH | 15 (16%) |
| HCV | 14 (15%) |
| HBV | 6 (6.5%) |
| Cholangiopathy | 7 (7.5%) |
| Retransplantation | 3 (3.2%) |
| AIH | 2 (2.2%) |
| Other | 10 (11%) |
| Child Pugh Score—no. (%) | |
| A | 36 (39%) |
| B | 33 (35%) |
| C | 21 (23%) |
| Missing | 3 (3.2%) |
| Machine perfusion | |
| Type of machine perfusion—no. (%) | |
| HOPE | 38 (41%) |
| DHOPE | 55 (59%) |
| Indication for prolonged HOPE—no. (%) | |
| Operating room logistics | 34 (37%) |
| Difficult recipient hepatectomy | 27 (29%) |
| Uncontrolled DCD in Italy | 18 (19%) |
| Change of recipient | 10 (11%) |
| Split liver on the pump | 4 (4.3%) |
| Portal venous pressure—mmHg | 4 (3–5) |
| Hepatic artery pressure—mmHg | 25 (24–25) |
| Portal venous flow start—ml/min | 230 (140–310) |
| Portal venous flow end—ml/min | 251 (150–486) |
| Hepatic artery flow start—ml/min | 49 (38–82) |
| Hepatic artery flow end—ml/min | 75 (66–115) |
| Temperature—°C | 9 (7–10) |
| Oxygenation—kPa | 97 (81–106) |
Abbreviations: AIH, autoimmune hepatitis; DBD, donation after brain death; DCD, donation after circulatory death; HBV, viral hepatitis B; HCC, hepatocellular carcinoma; HCV, viral hepatitis C; MELD, model for end‐stage liver disease; NASH, nonalcoholic steatohepatitis.
The donor risk index includes seven donor and graft characteristics that are significantly and independently associated with increased failure of deceased donor liver transplants. 16
The Eurotransplant donor risk index was based on the donor risk index by adding the latest laboratory yGT of the donor and rescue allocation. 17
The laboratory MELD score ranges from 6 to 40 with higher scores indicating more advanced disease.
The balance of risk score is a scoring system that was developed to detect unfavorable combinations of donor and recipient factors on the risk of graft failure after liver transplantation. 18
3.2. Survey outcomes and center characteristics
Twelve transplant centers in 6 European countries (the Netherlands, Germany, Belgium, Italy, Switzerland, and France) contributed to the study. The median number of liver transplantations performed per year in the participating centers was 80 (60–130) (Table S1). In 5 centers, both single and dual vessel HOPE were performed, and in the other centers either single HOPE (n = 4) or dual HOPE (n = 3) was performed. In the participating centers, the median proportion of livers preserved by HOPE was 30% (8%–100%) for DCD livers and 23% (16%–29%) for DBD livers. A dedicated on‐call organ perfusionist team was available for machine perfusion in 8 out of 12 centers. Among the surveyed surgeons, the reported duration up to which they would currently feel comfortable perfusing a liver with HOPE averaged 6 h. For prolonged machine perfusion in particular, dual HOPE was preferred over single HOPE by 8 out of 12 centers.
3.3. Preservation time
Median SCS time prior to machine perfusion was 5:31 h (range 1:39–13:43 h). Median duration of prolonged HOPE was 4:42 h (range 4:00–8:35 h). The median total out‐of‐body preservation time was 10:50 h (range 5:50–20:50 h) (Figure 1).
FIGURE 1.
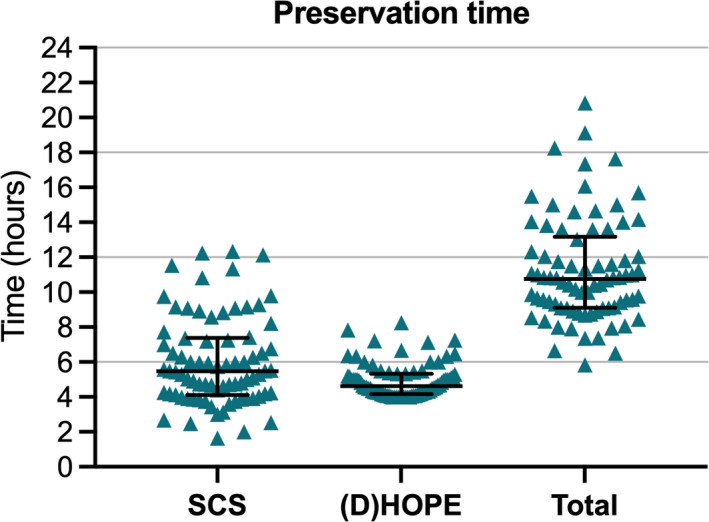
Preservation times in hours. Static cold storage time was defined as the time between in situ cold donor flush and connection to machine perfusion. Total preservation time was defined as the time from in situ cold donor flush and reperfusion in the recipient. Shown here are individual values and the median with interquartile range. HOPE, (dual) hypothermic oxygenated machine perfusion; SCS, static cold storage [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Hypothermic oxygenated machine perfusion
Fifty‐nine percent of livers were preserved with dual HOPE, compared with 41% single HOPE. The most common reason to prolong HOPE was for unforeseen operating room logistics (37%). Median pressure settings for machine perfusion were 4 mmHg (3–5 mmHg) in the portal vein and 25 mmHg (24–25 mmHg) in the hepatic artery. Flow in the portal vein increased from 230 ml/min (140–310 ml/min) after initiation of machine perfusion to 251 ml/min (150–486 ml/min) before disconnection. Flow in the hepatic artery increased from 49 ml/min (38–82 ml/min) to 75 ml/min (66–115 ml/min). The median temperature of the preservation solution was 9°C.
3.5. Postoperative outcomes
The median follow‐up time was 19 months (6–33 months). In the first 7 days after liver transplantation, peak ALT was 675 IU/L (419–1378 IU/L) and peak AST was 1130 IU/L (722–2517 IU/L) (Figure 2). Levels of yGT peaked around postoperative day 7 and were low at 1 and 3 months after liver transplantation. Bilirubin levels were low at 1 and 3 months after liver transplantation. The incidence of PRS was 12% (Table 2). Twenty‐four hours after reperfusion, median lactate concentration was 1.3 mmol/L (1.0–2.3 mmol/L). One graft was lost due to PNF (1%), 33 grafts (36%) met the criteria for EAD according to Olthoff criteria, and 13 grafts (14%) met the criteria according to the mpEAD. One graft developed a thrombus of the portal vein (1%) and 2 grafts (2%) were retransplanted for HAT. For 4 patients (4%), CVVH was required after they developed postoperative AKI. Within 12 months after liver transplantation, 1 patient developed NAS in the transplanted graft. The median duration of stay on the ICU was 4 days (2–7 days) and the total hospital length of stay was 19 days (14–29 days). Actuarial 1‐year graft survival was 93.5%, and patient survival was 88.2% (Figure 3). No serious adverse device events or device malfunctions were reported.
FIGURE 2.
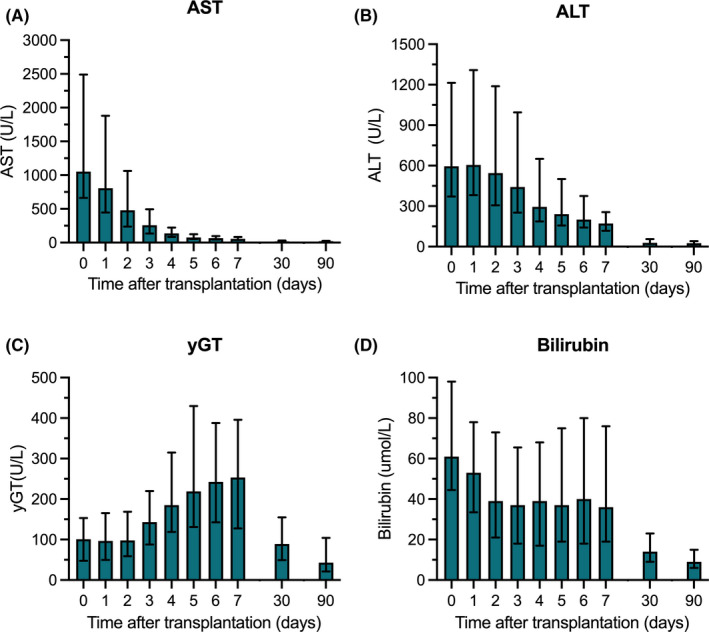
Postoperative biochemistry. Levels of AST (A), ALT (B), yGT (C), and total bilirubin (D) in the first postoperative week and at 1 and 3 months after liver transplantation. Shown here are the median and interquartile range. ALT, alanine aminotransferase; AST, aspartate aminotransferase; yGT, gamma‐glutamyl transferase [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Outcomes after liver transplantation (n = 93 patients)
| Event | n (%) or median (IQR) |
|---|---|
| Post‐reperfusion syndrome a | 11 (12%) |
| Serum lactate—mmol/L | |
| Peak lactate after reperfusion | 4.5 (2.9–6.4) |
| Lactate 24 h after reperfusion | 1.3 (1.0–2.3) |
| Peak transaminases—IU/L | |
| ALT | 675 (419–1378) |
| AST | 1130 (722–2517) |
| Primary non‐function b | 1 (1.1%) |
| Early allograft dysfunction c | 33 (35%) |
| Machine perfusion‐early allograft dysfunction d | 13 (14%) |
| Vascular complications | |
| Portal vein thrombosis e | 1 (1.1%) |
| Hepatic artery thrombosis f | 2 (2.2%) |
| Kidney failure requiring CVVH g | 4 (4.3%) |
| Duration of stay—days | |
| In the intensive care unit | 4 (2–7) |
| In the hospital | 19 (14–29) |
| Biliary complications | |
| Non‐anastomotic biliary strictures h | 1 (1.1%) |
| Anastomotic biliary stricture | 3 (3.2%) |
| Biliary leakage i | 4 (4.3%) |
| Postoperative complications j | |
| Clavien‐Dindo 3B | 12 (13%) |
| Clavien‐Dindo 4A | 14 (15%) |
| Clavien‐Dindo 4B | 5 (5.4%) |
| Clavien‐Dindo 5 | 4 (4.3%) |
| Retransplantation within 1 year | 5 (5.4%) |
| Primary non‐function | 1 (1.1%) |
| Hepatic artery thrombosis | 2 (2.2%) |
| Portal vein thrombosis | 1 (1.1%) |
| Multi‐organ failure with secondary liver failure | 1 (1.1%) |
| Patient death within 1 year | 6 (6.5%) |
| Multi‐organ failure | 1 (1.1%) |
| Small‐cell lung carcinoma | 1 (1.1%) |
| Myocardial infarction | 1 (1.1%) |
| Sepsis | 1 (1.1%) |
| Aspergillosis pneumonia | 1 (1.1%) |
| Duodenal perforation with erosive bleeding | 1 (1.1%) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CVVH, continuous veno‐venous hemofiltration.
Hemodynamic instability after reperfusion defined as post‐reperfusion syndrome with a decrease in mean arterial pressure >30% below baseline, lasting for ≥1 min, within 5 min after reperfusion (Aggarwal criteria 6 ), or as vasoplegia with a fall in mean arterial pressure on reperfusion to <50 mmHg either sustained >30 min and/or requiring >0.15 µg/kg/min norepinephrine, >2 U/h vasopressin, or infusion of epinephrine (significant hypotension resistant to pressors).
Nonlife sustaining graft function leading to graft loss or retransplantation within 7 days after liver transplantation.
Presence of one or more of the following: bilirubin ≥10 mg/dl on postoperation day 7, INR ≥1.6 on postoperative day 7, and ALT or AST >2000 IU/L within the first 7 days (Olthoff criteria).
Presence of 1 or more of the following: bilirubin ≥10 mg/dl on postoperative day 7, INR ≥1.6 on postoperative day 7, lactate ≥2 mmol/L on postoperative day 7 in the absence of vascular complications (mpEAD).
Radiologically or surgically proven thrombosis of the portal vein within 12 month after liver transplantation.
Radiologically or surgically proven thrombosis of the hepatic artery within 12 months after liver transplantation.
Kidney failure defined as (1) increase serum creatinine by ≥0.3 mg/dl within 48 h after transplantation or (2) increase in serum creatinine ≥1.5 times baseline or (3) urine volume <0.5 ml/kg/h for 6 h. Assessed within 30 days after liver transplantation.
Radiological appearance of irregularities and beading dilatation of the intrahepatic bile ducts and/or the presence of cavitations and bile lakes leading to surgical or endoscopic intervention within 12 months after liver transplantation.
Biliary leakage as defined by the International Study Group for Liver Surgery. 24
The complication with the highest grade according to Clavien‐Dindo was scored. Complications were assessed within 30 days after liver transplantation.
FIGURE 3.
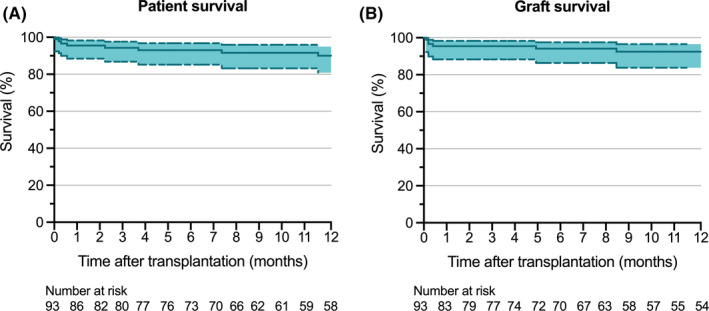
One‐year graft and patient survival after liver transplantation. Kaplan–Meier survival curves are shown for 1‐year patient (A) and graft (B) survival. Dashed lines represent the 95% confidence interval [Color figure can be viewed at wileyonlinelibrary.com]
In a subanalysis, outcomes after prolonged HOPE of 43 DCD versus 50 DBD grafts were compared (Table 3). Inherent to DCD liver transplantation, the incidence of PRS was higher, when compared to transplantation of DBD grafts, albeit not reaching significance (19% vs. 6%, p = .053). There were no major differences in other postoperative outcomes between recipients of DCD or DBD livers, including PNF (0% vs. 2%, p = .35), EAD (40% vs. 32%, p = .45), and NAS (0% vs. 2%, p = .35). One DCD liver recipient underwent retransplantation versus four DBD liver recipients (p = .23). When comparing outcomes after transplantation of livers preserved by prolonged HOPE or DHOPE, no major differences were found (Table S2).
TABLE 3.
Outcomes after liver transplantation in recipients of grafts from DBD versus DCD donors (n = 93 patients)
| Event |
DBD (n = 50)n (%) or median (IQR) |
DCD (n = 43)n (%) or median (IQR) |
p‐value |
|---|---|---|---|
| Post‐reperfusion syndrome a | 3 (6.0%) | 8 (19%) | .053 |
| Serum lactate—mmol/L | |||
| Peak lactate after reperfusion | 4.9 (3.3–7.2) | 3.8 (2.7–5.6) | .117 |
| Lactate 24 h after reperfusion | 1.2 (1.1–2.0) | 1.4 (1.0–2.6) | .948 |
| Peak AST—IU/L | 997 (619–2517) | 1306 (792–2647) | .146 |
| Peak ALT—IU/L | 671 (335–1097) | 706 (450–1907) | .195 |
| Primary nonfunction b | 1 (2.0%) | 0 (0.0%) | .351 |
| Early allograft dysfunction c | 16 (32%) | 17 (40%) | .449 |
| Machine perfusion‐early allograft dysfunction d | 7 (14%) | 6 (14%) | .995 |
| Vascular complications | |||
| Portal vein thrombosis e | 1 (2.0%) | 0 (0.0%) | .351 |
| Hepatic artery thrombosis f | 2 (4.0%) | 0 (0.0%) | .185 |
| Nonanastomotic biliary strictures g | 1 (2.0%) | 0 (0.0%) | .351 |
| Kidney failure treated with CVVH h | 2 (4.0%) | 2 (4.7%) | .300 |
| Median duration of stay—days | |||
| In the intensive care unit | 5 (3–9) | 4 (2–6) | .030 |
| In the hospital | 19 (13–36) | 19 (15–27) | .551 |
| Retransplantation within 1 year | 4 (8.0%) | 1 (2.3%) | .226 |
| Patient death within 1 year | 4 (8.0%) | 2 (4.7%) | .484 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CVVH, continuous veno‐venous hemofiltration; DBD, donation after brain death; DCD, donation after circulatory death; mpEAD, machine perfusion early allograft dysfunction.
Hemodynamic instability after reperfusion defined as post‐reperfusion syndrome with a decrease in mean arterial pressure >30% below baseline, lasting for ≥1 min, within 5 min after reperfusion (Aggarwal criteria 6 ), or as vasoplegia with a fall in mean arterial pressure on reperfusion to <50 mmHg either sustained >30 min and/or requiring >0.15 µg/kg/min norepinephrine, >2 U/h vasopressin, or infusion of epinephrine (significant hypotension resistant to pressors). 7
Nonlife sustaining graft function leading to graft loss or retransplantation within 7 days after liver transplantation.
Presence of 1 or more of the following: bilirubin ≥10 mg/dl on postoperative day 7, INR ≥1.6 on postoperative day 7, and ALT or AST >2000 IU/L within the first 7 days. 21
Presence of 1 or more of the following: bilirubin ≥10 mg/dl on postoperative day 7, INR ≥1.6 on postoperative day 7, lactate ≥2 mmol/L on postoperative day 7 in the absence of vascular complications (mpEAD).
Radiologically or surgically proven thrombosis of the portal vein within 12 month after liver transplantation.
Radiologically or surgically proven thrombosis of the hepatic artery within 12 months after liver transplantation.
Radiological appearance of irregularities and beading dilatation of the intrahepatic bile ducts and/or the presence of cavitations and bile lakes leading to surgical or endoscopic intervention within 12 months after liver transplantation.
Kidney failure defined as (1) increase serum creatinine by ≥0.3 mg/dl within 48 h after transplantation or (2) increase in serum creatinine ≥1.5 times baseline or (3) urine volume <0.5 ml/kg/h for 6 h. Assessed within 30 days after liver transplantation. 25
4. DISCUSSION
An increasing number of transplant centers worldwide have implemented a short (1–2 h) period of HOPE after conventional cold storage to resuscitate donor livers prior to transplantation. Despite limited clinical data, machine perfusion is occasionally prolonged, mostly because of unforeseen logistical issues at the recipient center. This is the first study on the outcomes after transplantation of donor livers preserved by prolonged HOPE in a large multicenter observational cohort. We demonstrate that good outcomes may be achieved after prolonged HOPE, with total preservation times up to almost 21 h.
The present study reveals that the majority of grafts rapidly clear lactate after reperfusion and reach a physiological INR at postoperative day 1. Low peak transaminase levels were observed in the first postoperative week and bilirubin was low at 1 and 3 months after liver transplantation. These patterns in postoperative biochemistry are comparable to previously published studies investigating short‐term HOPE. 5 , 6 , 26 , 27 , 28 While machine perfusion time was more than doubled compared with machine perfusion preservation times in the DHOPE‐DCD and HOPE ECD‐DBD clinical trials, equivalent postoperative outcomes are observed. 5 , 6 In particular, we show equally low rates of vascular and biliary complications and excellent graft and patient survival (Tables S3 and S4). Outcomes after prolonged HOPE are also comparable to the benchmark outcome values in DBD (Table S3) and DCD (Table S4) liver transplantation of non‐machine perfused grafts. 29 , 30 While prolonged cold ischemia is a well‐known risk factor for postoperative complications in DCD liver transplantation, the results of this study suggest HOPE can be used to prolong preservation time without impairing postoperative outcome.
The results of the present study show that the combination of SCS and prolonged HOPE is safe in liver grafts with high risks and achieves comparable outcomes as low risk benchmark cohorts, which may have several clinical implications. In clinical practice, there is a need for an extension of the preservation time due to the lack of intensive care beds or to bridge theater capacity (frequently from early morning hours to mornings or noon), where the here described approach with a median overall preservation time of 11 h appears very beneficial. Additionally, the indication for machine perfusion could be recipient‐orientated rather than based on donor characteristics only. This way, grafts can be preserved by HOPE during a difficult recipient hepatectomy with less time pressure. Answers to the survey in this study include the potential of prolonged HOPE to accept two livers at the same time (so that one graft is preserved longer with HOPE), or to enable transplantation at daytime.
Prolonged preservation has previously been achieved clinically using NMP, or by supercooling in an experimental setting. 10 , 31 , 32 Previous studies showed that NMP enables a prolongation of liver preservation and overnight organ care. 10 , 33 The results of the present study suggest that similar results can be achieved with HOPE. Prolonged preservation by HOPE compared with NMP, can be advantageous since the organ is maintained in a hypometabolic state with minimal production of coagulation factors and waste products, reducing the need to adjust the perfusate composition, minimizing labor and reducing resources. Moreover, in case the perfusion system fails (e.g., failure of the oxygenator), the liver would still be preserved at hypothermia, limiting the risk of warm ischemia‐induced injury and graft loss. By experimental supercooling of discarded human livers to −4°C, the total preservation time was successfully extended to 27 h. 34 The Zürich group reported 1‐week preservation of human livers with their custom‐made normothermic perfusion device. 32 Both techniques, however, are still in a preclinical phase.
A limitation of this study is its retrospective design. Based on this, there are inherent differences in organ procurement and implantation techniques between the centers contributing to this study. Despite such variations in center practice, all transplants performed with prolonged HOPE led to excellent outcomes across different technical variations (HOPE and DHOPE) and intra‐posttransplant management. While this study includes livers preserved by both prolonged HOPE and DHOPE, preclinical studies suggest that both techniques are equally effective. 35 , 36 A formal matched control group was not included in this study, but outcomes were compared to previously published studies instead. The descriptive nature of the study is in line with the IDEAL‐D framework for translational device studies in Stage 1 (“Idea”). Notably, there is currently no consensus on the definition of “prolonged” machine perfusion. The cutoff at 4 h in the present study was based on a doubling of the current standard machine perfusion time of 2 h.
To further investigate the safety and feasibility of prolonged DHOPE, we have initiated a prospective, pseudo‐randomized, clinical trial (IDEAL‐D stage 2) comparing prolonged DHOPE (≥4 h) to regular short‐term (1–2 h) DHOPE (DHOPE‐PRO trial, NTR NL8740; www.trialregister.nl). 37
We conclude that good outcomes can be achieved after transplantation of donor livers preserved with prolonged (median 4:42 h) HOPE in experienced centers, leading to a total preservation time of almost 21 h. These results suggest that simple, end‐ischemic HOPE may be utilized for safe extension of the preservation time to ease transplantation logistics.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Table S1‐S4
Brüggenwirth IMA, Mueller M, Lantinga VA, et al. Prolonged preservation by hypothermic machine perfusion facilitates logistics in liver transplantation: A European observational cohort study. Am J Transplant. 2022;22:1842–1851. doi: 10.1111/ajt.17037
Robert J. Porte and Vincent E. de Meijer share last authorship.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. de Meijer VE, Fujiyoshi M, Porte RJ. Ex situ machine perfusion strategies in liver transplantation. J Hepatol. 2019;70(1):203‐205. doi: 10.1016/j.jhep.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 2. Schlegel A, Muller X, Mueller M, et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine. 2020;60:103014. doi: 10.1016/j.ebiom.2020.103014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brüggenwirth IMA, van Leeuwen OB, Müller M, et al. The importance of adequate oxygenation during hypothermic machine perfusion. JHEP Rep. 2021;3(1):100194. doi: 10.1016/j.jhepr.2020.100194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schlegel A, de Rougemont O, Graf R, Clavien P‐A, Dutkowski P. Protective mechanisms of end‐ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58(2):278‐286. doi: 10.1016/j.jhep.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 5. van Rijn R, Schurink IJ, de Vries Y, et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N Engl J Med. 2021;384(15):1391‐1401. doi: 10.1056/nejmoa2031532 [DOI] [PubMed] [Google Scholar]
- 6. Czigany Z, Pratschke J, Froněk J, et al. Hypothermic oxygenated machine perfusion (HOPE) reduces early allograft injury and improves post‐transplant outcomes in extended criteria donation (ECD) liver transplantation from donation after brain death (DBD): results from a multicenter randomized controlled trial (HOPE ECD‐DBD). Ann Surg. 2021. doi: 10.1097/SLA.0000000000005110 [DOI] [PubMed] [Google Scholar]
- 7. De Carlis R, Lauterio A, Ferla F, Di Sandro S, Sguinzi R, De Carlis L. Hypothermic machine perfusion of liver grafts can safely extend cold ischemia for up to 20 hours in cases of necessity. Transplantation. 2017;101(7):e223‐e224. doi: 10.1097/TP.0000000000001753 [DOI] [PubMed] [Google Scholar]
- 8. Dondossola D, Ravaioli M, Lonati C, et al. The role of ex situ hypothermic oxygenated machine perfusion and cold preservation time in extended criteria donation after circulatory death and donation after brain death. Liver Transpl. 2021;27(8):1130‐1143. doi: 10.1002/lt.26067 [DOI] [PubMed] [Google Scholar]
- 9. Pavicevic S, Uluk D, Reichelt S, et al. Hypothermic oxygenated machine perfusion for extended criteria donor allografts‐preliminary experience with extended organ preservation times in the setting of organ reallocation. Artif Organs. 2021. doi: 10.1111/aor.14103 [DOI] [PubMed] [Google Scholar]
- 10. Cardini B, Oberhuber R, Fodor M, et al. Clinical implementation of prolonged liver preservation and monitoring through normothermic machine perfusion in liver transplantation. Transplantation. 2020;104(9):1917‐1928. doi: 10.1097/TP.0000000000003296 [DOI] [PubMed] [Google Scholar]
- 11. Brüggenwirth IMA, van Leeuwen OB, de Vries Y, et al. Extended hypothermic oxygenated machine perfusion enables ex situ preservation of porcine livers for up to 24 hours. JHEP Rep. 2020;2(2): 100092. doi: 10.1016/j.jhepr.2020.100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105‐1112. doi: 10.1016/S0140-6736(09)61116-8 [DOI] [PubMed] [Google Scholar]
- 13. Hirst A, Philippou Y, Blazeby J, et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg. 2019;269(2):211‐220. doi: 10.1097/SLA.0000000000002794 [DOI] [PubMed] [Google Scholar]
- 14. Bilbro NA, Hirst A, Paez A, et al. The IDEAL reporting guidelines: a delphi consensus statement stage specific recommendations for reporting the evaluation of surgical innovation. Ann Surg. 2021;273(1):82‐85. doi: 10.1097/SLA.0000000000004180 [DOI] [PubMed] [Google Scholar]
- 15. Elm EV, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806‐808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng S, Goodrich NP, Bragg‐Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783‐790. doi: 10.1111/j.1600-6143.2006.01242.x [DOI] [PubMed] [Google Scholar]
- 17. Braat AE, Blok JJ, Putter H, et al. The Eurotransplant donor risk index in liver transplantation: ET‐DRI. Am J Transplant. 2012;12(10):2789‐2796. doi: 10.1111/j.1600-6143.2012.04195.x [DOI] [PubMed] [Google Scholar]
- 18. Dutkowski P, Oberkofler CE, Slankamenac K, et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end‐stage liver disease era. Ann Surg. 2011;254(5):745‐753; discussion 753. doi: 10.1097/SLA.0b013e3182365081 [DOI] [PubMed] [Google Scholar]
- 19. Aggarwal S, Kang Y, Freeman JA, Fortunato FL, Pinsky MR. Postreperfusion syndrome: cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19(4 suppl 3):54‐55. Accessed May 14, 2020. https://ohsu.pure.elsevier.com/en/publications/postreperfusion‐syndrome‐cardiovascular‐collapse‐following‐hepati‐2 [PubMed] [Google Scholar]
- 20. Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia‐important lessons from the first 12 cases. Transplantation. 2017;101(5):1084‐1098. doi: 10.1097/TP.0000000000001661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16(8):943‐949. doi: 10.1002/lt.22091 [DOI] [PubMed] [Google Scholar]
- 22. Martins PN, Rizzari MD, Ghinolfi D, et al. Design, analysis, and pitfalls of clinical trials using ex situ liver machine perfusion: the international liver transplantation society consensus guidelines. Transplantation. 2021;105(4):796‐815. doi: 10.1097/TP.0000000000003573 [DOI] [PubMed] [Google Scholar]
- 23. Jong IEM, Overi D, Carpino G, et al. Persistent biliary hypoxia and lack of regeneration are key mechanisms in the pathogenesis of post‐transplant non‐anastomotic strictures. Hepatology. 2021. doi: 10.1002/hep.32166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brooke‐Smith M, Figueras J, Ullah S, et al. Prospective evaluation of the International Study Group for Liver Surgery definition of bile leak after a liver resection and the role of routine operative drainage: an international multicentre study. HPB. 2015;17(1):46. doi: 10.1111/HPB.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. 2012;120(4):c179‐c184. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 26. Dutkowski P, Polak WG, Muiesan P, et al. First comparison of hypothermic oxygenated PErfusion versus static cold storage of human donation after cardiac death liver transplants: an international‐matched case analysis. Ann Surg. 2015;262(5):764‐771; discussion 770–1. doi: 10.1097/SLA.0000000000001473 [DOI] [PubMed] [Google Scholar]
- 27. van Rijn R, Karimian N, Matton APM, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104(7):907‐917. doi: 10.1002/bjs.10515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Carlis R, Schlegel A, Frassoni S, et al. How to preserve liver grafts from circulatory death with long warm ischemia? A retrospective Italian cohort study with normothermic regional perfusion and hypothermic oxygenated perfusion. Transplantation. 2021. doi: 10.1097/TP.0000000000003595 [DOI] [PubMed] [Google Scholar]
- 29. Muller X, Marcon F, Sapisochin G, et al. Defining benchmarks in liver transplantation: a multicenter outcome analysis determining best achievable results. Ann Surg. 2018;267(3):419‐425. doi: 10.1097/SLA.0000000000002477 [DOI] [PubMed] [Google Scholar]
- 30. Schlegel A, van Reeven M, Croome K, et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J Hepatol. 2021. doi: 10.1016/j.jhep.2021.10.004 [DOI] [PubMed] [Google Scholar]
- 31. de Vries RJ, Tessier SN, Banik PD, et al. Subzero non‐frozen preservation of human livers in the supercooled state. Nat Protoc. 2020;15(6):2024‐2040. doi: 10.1038/s41596-020-0319-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eshmuminov D, Becker D, Bautista Borrego L, et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol. 2020;38(2):189‐198. doi: 10.1038/s41587-019-0374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watson CJE, Randle LV, Kosmoliaptsis V, Gibbs P, Allison M, Butler AJ. 26‐hour storage of a declined liver before successful transplantation using ex vivo normothermic perfusion. Ann Surg. 2017;265(1):e1‐e2. doi: 10.1097/SLA.0000000000001834 [DOI] [PubMed] [Google Scholar]
- 34. de Vries RJ, Tessier SN, Banik PD, et al. Supercooling extends preservation time of human livers. Nat Biotechnol. 2019;37(10):1131‐1136. doi: 10.1038/s41587-019-0223-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Vries Y, Brüggenwirth IMA, Karangwa SA, et al. Dual versus single oxygenated hypothermic machine perfusion of porcine livers: impact on hepatobiliary and endothelial cell injury. Transplant Direct. 2021;7(9):e741. doi: 10.1097/TXD.0000000000001184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schlegel A, Kron P, De Oliveira ML, Clavien P‐A, Dutkowski P. Is single portal vein approach sufficient for hypothermic machine perfusion of DCD liver grafts? J Hepatol. 2016;64(1):239‐241. doi: 10.1016/j.jhep.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 37. Brüggenwirth IMA, Lantinga VA, Rayar M, et al. Prolonged dual hypothermic oxygenated machine preservation (DHOPE‐PRO) in liver transplantation: study protocol for a stage 2, prospective, dual‐arm, safety and feasibility clinical trial. BMJ Open Gastroenterol. 2022;9(1):e000842. doi: 10.1136/BMJGAST-2021-000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S4
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


