ABSTRACT
Seed dormancy varies greatly between species, clades, communities, and regions. We propose that fireprone ecosystems create ideal conditions for the selection of seed dormancy as fire provides a mechanism for dormancy release and postfire conditions are optimal for germination. Thus, fire‐released seed dormancy should vary in type and abundance under different fire regimes. To test these predictions, we compiled data from a wide range of fire‐related germination experiments for species in different ecosystems across the globe. We identified four dormancy syndromes: heat‐released (physical) dormancy, smoke‐released (physiological) dormancy, non‐fire‐released dormancy, and non‐dormancy. In fireprone ecosystems, fire, in the form of heat and/or chemical by‐products (collectively termed ‘smoke’), are the predominant stimuli for dormancy release and subsequent germination, with climate (cold or warm stratification) and light sometimes playing important secondary roles. Fire (heat or smoke)‐released dormancy is best expressed where woody vegetation is dense and fires are intense, i.e. in crown‐fire ecosystems. In such environments, seed dormancy allows shade‐intolerant species to take advantage of vegetation gaps created by fire and synchronize germination with optimal recruitment conditions. In grassy fireprone ecosystems (e.g. savannas), where fires are less intense but more frequent, seed dormancy is less common and dormancy release is often not directly related to fire (non‐fire‐released dormancy). Rates of germination, whether controls or postfire, are twice as fast in savannas than in mediterranean ecosystems. Fire‐released dormancy is rare to absent in arid ecosystems and rainforests. The seeds of many species with fire‐released dormancy also possess elaiosomes that promote ant dispersal. Burial by ants increases insulation of seeds from fires and places them in a suitable location for fire‐released dormancy. The distribution of these dormancy syndromes across seed plants is not random – certain dormancy types are associated with particular lineages (phylogenetic conservatism). Heat‐released dormancy can be traced back to fireprone floras in the ‘fiery’ mid‐Cretaceous, followed by smoke‐released dormancy, with loss of fire‐related dormancy among recent events associated with the advent of open savannas and non‐fireprone habitats. Anthropogenic influences are now modifying dormancy‐release mechanisms, usually decreasing the role of fire as exaptive effects. We conclude that contrasting fire regimes are a key driver of the evolution and maintenance of diverse seed dormancy types in many of the world's natural ecosystems.
Keywords: Cerrado, Cistaceae, crown fire, Fabaceae, fire heat, mediterranean, myrmecochory, Poaceae, Rhamnaceae, Rutaceae, savanna, seasonality, seed dormancy syndrome, smoke, surface fire
I. INTRODUCTION
Seed dormancy is an adaptive trait that prevents seeds from initiating germination under present or expected future conditions that would lead to a low probability of germination success or seedling survival. In such cases, germination is delayed and seeds remain stored in the soil as a persistent seed bank that can last from a few months to many decades, pending the arrival of conditions that are more suitable for seedling recruitment. There is an abundant literature on the taxonomy, ecology, physiology, and biogeography of seed dormancy and the conditions that break it (Fenner & Thompson, 2005; Finch‐Savage & Leubner‐Metzger, 2006; Baskin & Baskin, 2014; Long et al., 2015; Gioria et al., 2020). Dormant seeds are produced by many plant lineages occurring in numerous habitat types and under contrasting climates and disturbance regimes throughout the world.
The mechanisms for overcoming seed dormancy are diverse and provide the basis for the classification of the main dormancy types (Baskin & Baskin, 2014; Baskin & Baskin, 2021): morphological dormancy (MD), physical dormancy (PY), and physiological dormancy (PD) (see glossary in Table 1). Some seeds may have multiple mechanisms where they combine physiological and either morphological or physical dormancy. Independently of how seeds are released from dormancy, commencement of germination requires appropriate environmental levels of moisture, warmth and oxygen. Thus, there may be a period (typically days or months, but sometimes longer) between inherent (primary) dormancy release and commencement of germination, in which seeds are temporally under imposed (secondary) dormancy (see Table 1; Baskin & Baskin, 2014; Thompson et al., 2003; Brits & Manning, 2019). Unless otherwise stated, dormancy here refers to primary dormancy.
Table 1.
Glossary
| Seed dormancy and germination | |
| Dormancy | That seed state when germination will not proceed even though external conditions may be favourable. Where this is controlled internally through retarded embryo maturity or metabolic inactivity it is referred to as primary (innate or inherent) dormancy. Dormancy may also be imposed environmentally through the lack of suitable hydrothermal conditions when it is referred to as imposed (secondary) dormancy. Unless otherwise stated, ‘dormancy’ refers to the primary class in this review. There are three basic types of (primary) dormancy, depending on the mechanism of release: morphological, physical and physiological dormancy. Some seeds may have multiple mechanisms where they combine physiological and either morphological or physical dormancy. |
| Morphological dormancy (MD) | Dormancy is maintained in an underdeveloped embryo that requires a period of post‐dispersal maturation (after‐ripening) before the seed is ready to germinate. Metabolism occurs during dormancy but at a low rate even in the presence of conditions suitable for germination. |
| Physical dormancy (PY) | Dormancy is maintained as the seed coat is impermeable to water and/or oxygen so that metabolism cannot occur. The coat needs to be fractured, or a special pore opened, physically to enable water and/or oxygen uptake to initiate metabolism and thus enable germination to proceed. |
| Physiological dormancy (PD) | Dormancy is controlled by a physiological mechanism(s) that prevents the embryo from growing. It is broken when respiration inhibitors are leached from the seed, or conversely essential cofactors are supplied from the surroundings (e.g. karrikinolide in smoke), or physiological mechanisms are activated by a change in the surroundings (e.g. light to produce phytochrome far‐red) to enable the metabolic initiation of germination. |
| Fire‐released dormancy | A concise term for heat‐released and smoke‐released dormancy syndromes (Table 2). |
| Dormancy syndrome | A correlated suite of traits that is coordinated to maintain seed dormancy during storage, execute seed dormancy release in response to a specified stimulus, and respond quickly to favourable germination conditions when they become available. We define four dormancy syndromes in the main text and Table 2. |
| Heat‐stimulated germination | Heat per se does not stimulate germination but breaks dormancy that allows germination to proceed later, i.e. once suitable hydrothermal conditions are met. Thus, this term is equivalent to the heat‐released dormancy syndrome as described in the text and in Table 2. |
| Smoke‐stimulated germination | In physiologically dormant seeds, specific smoke chemicals break dormancy and allow germination to proceed. These chemicals may be absorbed by dry seeds but, once the wet season begins, they are more likely to be absorbed dissolved in the soil solution during imbibition so that germination proceeds without further delay. Thus, this term is equivalent to the smoke‐released dormancy syndrome as described in the text and in Table 2. Smoke chemicals may also hasten the rate of germination of non‐dormant seeds among some species. |
| Imposed (secondary) dormancy | That state following primary‐dormancy release where metabolic activity continues to be suppressed as external conditions remain unsuitable for germination. In species with heat‐responsive seeds, this state is maintained between the fire event and the first substantial postfire rains but may be minimal among smoke‐responsive seeds if the chemicals are only absorbed once the seeds have imbibed. |
| Postfire regeneration strategies | |
| Obligate resprouters | Plants that are long lived and rely on resprouting to regenerate after fire. These plants do not produce seedlings after fire because they lack a seed bank. Obligate resprouters may reproduce from seeds late in the fire‐free interval, but the terminology for seeders and resprouters refers to postfire conditions. Seed dormancy is rare in these species. Obligate resprouters have a polypyric life cycle (Pausas & Keeley, 2014). |
| Obligate seeders | Plants that are killed by fire and rely on seedlings from a persistent seed bank to regenerate after fire. Seeds that are soil stored have fire‐released dormancy. Thus, they recruit massively after fire and either complete their lifespan before the next fire (and only exist as a soil seed bank thereafter) or are fire killed (monopyric life cycle). |
| Facultative seeders (= facultative resprouters) | Plants that are able to both resprout and recruit from dormant seeds after fire (polypyric life cycle). |
| Fire‐regime types | |
| Crown‐fire regime | Fires in woody‐plant‐dominated ecosystems that burn all vegetation components including crowns of the dominant species (woody‐plant‐fuelled fires). Fires are typically of high intensity (foliage of all species is incinerated) and moderate frequency (>20‐year intervals). Fires in most mediterranean‐type forests, woodlands and shrublands, and closed‐cone pine forests, are of this type. |
| Surface‐fire regime | Fires that spread in the herbaceous (grass‐fuelled fires) or litter (litter‐fuelled fires) layer and rarely reach the crowns of the overstorey (if present). Fires are typically of low intensity but high frequency (<5‐year intervals). Fires in the understorey of some forests and woodlands (understorey fires), and in savannas and grasslands, are of this type. |
Given the diversity of dormancy types, seed dormancy can be viewed as an assemblage of disparate traits that have evolved in unrelated lineages and environments (Silveira et al., 2012; Willis et al., 2014) and can provide benefits under multiple selective frameworks. For instance, dormancy may confer adaptive benefits under unpredictable rainfall regimes (Rees, 1994), under strongly seasonal climates (Venable, 2007; Rubio de Casas et al., 2017; Collette & Ooi, 2021), and in the presence of extended cold periods (Wyse & Dickie, 2018); it may also enable survival of ingestion by vertebrate dispersers [endozoochory (Malo & Suárez, 1996; Baes, de Viana & Sühring, 2002)]. In turn, dormancy release may take advantage of recruitment opportunities provided by vegetation gaps (Vázquez‐Yanes & Orozco‐Segovia, 1993; Figueroa, 2003), especially those created by fire (Keeley, 1991; Keeley & Fotheringham, 2000; Paula & Pausas, 2008). A recent review concluded that, at the global scale, the abundance of seed banks is not related to climate but to clades assembling in “unpredictable environments” (Gioria et al., 2020), although fire, as a well‐known stochastic phenomenon, was not mentioned.
Here, we propose that seed dormancy is most adaptive in ecosystems where seedling recruitment is controlled by large, intermittently formed vegetation gaps created by periodic disturbances. Thus, seed dormancy will be best expressed in highly flammable ecosystems subject to periodic fires that create swathes of bare ground suitable for germination and recruitment (Fig. 1). Such recurring fires (i) create large bare, nutrient‐enriched patches suitable for germination and seedling recruitment; (ii) select for seeds with strong heat tolerance or avoidance properties; and (iii) provide both the signal (cue) and the mechanism (environmental inducer) for breaking dormancy. In addition, we propose that differences in fire regimes (e.g. in frequency and intensity) create different selective environments for seed dormancy and thus help to explain variability in the prevalence and characteristics of seed dormancy in different regions.
Fig. 1.
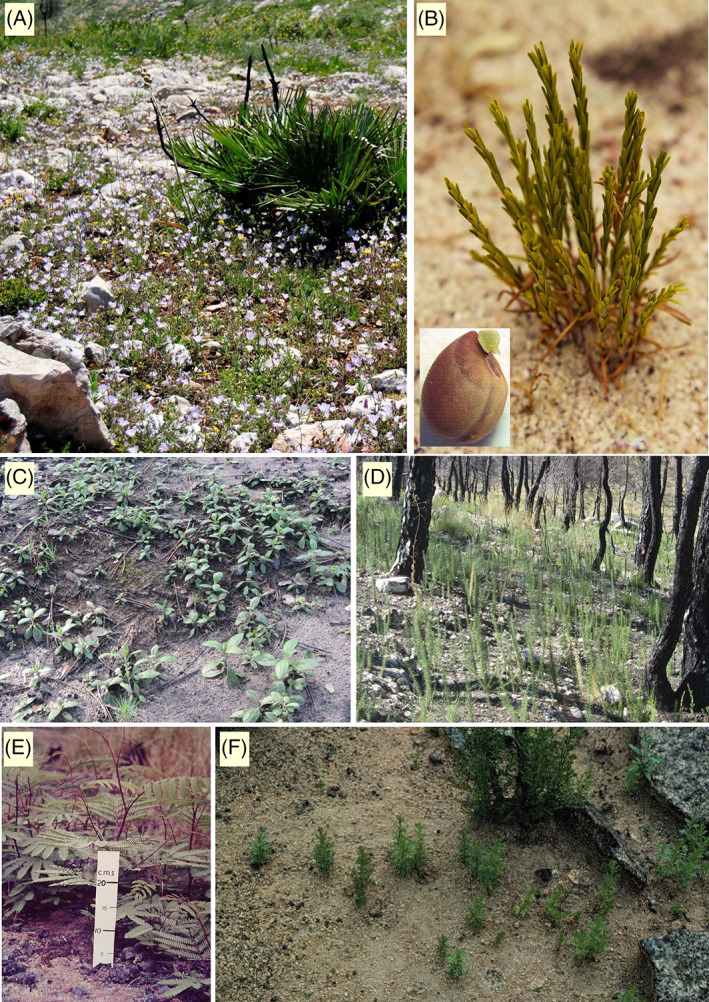
Photographs of seedlings establishing after fire. (A) Malva cretica (Malvaceae) 1 year postfire, plants in flower ~15 cm tall. (B) Clump of seedlings of Stachystemon axillaris (Euphorbiaceae; smoke‐released) arising from an ant nest 8 months after a summer fire; largest seedling 8 cm tall. Interfire recruitment is negligible in this species. Inset is their semi‐hard seed with an elaiosome beside the hilum that attracts ants (mainly Camponotus cf claripes) that use the appendage to carry the seed when transporting it to their nests. Seed 3 mm long. (C) Cistus cf albidus (Cistaceae; hard‐seeded), 3.5 months postfire, ~ 2 cm tall. (D) Ulex parviflorus (Fabaceae; hard‐seeded) 1 year postfire, ~ 25 cm tall. (E) Paraserianthes lophantha (Fabaceae; hard‐seeded) 1 year postfire, showing germination restricted to parts of the soil receiving moderate fire temperatures (foreground too hot, background too cool). (F) Adenostoma fasciculatum (Rhamnaceae), largest 10 cm tall. A is from E Spain (E. Laguna); B from SW Australia (B. Lamont, inset A. Tinker); C and D from E Spain (J. Pausas); E from SW Australia [B. Dell, from Groom & Lamont (2015) with permission] and F from California (J. Keeley).
In a seminal paper, Keeley (1991) provided the first review on this topic, based on the Californian mediterranean flora. During the following 30‐year period, seed ecology in the context of fire has expanded to all corners of the globe; heat and smoke germination experiments have been performed on many species; and phylogenetic analyses have greatly improved our understanding of the evolution of key plant traits. It is now time to review and integrate the lessons learned across ecosystems and lineages. We first review the mechanisms by which seeds are released from dormancy in fireprone ecosystems, and under what conditions these mechanisms have been selected. We have compiled examples from a wide range of germination experiments that show the fitness benefits of seed dormancy (via enhanced germination) in various ecosystems around the world. Then, based on published molecular phylogenies, we trace the origin and evolutionary dynamics of seed dormancy in different lineages. Finally, we review alternative drivers to fire for promoting seed dormancy, noting that cold or warm stratification and light are also known to have alternative or supplementary roles in breaking dormancy.
II. FIRE AS A DORMANCY‐RELEASE MECHANISM
(1). Dormancy syndromes
Three factors determine under what circumstances seed dormancy is adaptive (Donohue et al., 2010): (i) dormancy (specifically as a property of long‐lived seeds) can be an optimal strategy when aboveground environments are unpredictable for recruitment (bet‐hedging); (ii) dormancy (specifically dormancy release) can be a mechanism to detect suitable conditions for recruitment (environmental matching); and (iii) non‐dormancy (rapid germination) can allow escape from pending threats to recruitment (e.g. short growing season, seed predation). All three factors operate in fireprone ecosystems (Table 2). However, postfire conditions are especially optimal for germination and establishment of many species (i.e. environmental matching is favoured) because fires create extensive vegetation gaps that have high resource availability, minimal competition, and low pathogen load. Thus, they offer a unique window of opportunity for recruitment, especially among weakly competitive, shade‐intolerant plants. To benefit from these conditions, germination needs to proceed as soon as conditions are suitable after fire. Thus, seeds are required to both survive the passage of fire and also to detect the fire gap created to start germinating. Seeds possess two ways to sense a fire event and enable germination to occur as soon as postfire conditions are suitable: they use the two properties of fire, heat and/or smoke, to release dormancy and prime the seeds to germinate. On the basis of these fire properties and the associated dormancy‐release types we define four dormancy syndromes (Table 1) that account for the different types of dormancy release and germination and their associated properties in fireprone ecosystems (Table 2; see online Supporting Information, Table S1).
Table 2.
Dormancy syndromes present in fireprone ecosystems, and their main ecological characteristics (for more details see the main text and Table S1)
| Dormancy syndromes | ||||
|---|---|---|---|---|
| Heat‐released dormancy | Smoke‐released dormancy | Non‐fire‐released dormancy | Non‐dormancy | |
| Benefit | Environmental matching | Environmental matching | Bet‐hedging | Quick establishment, granivory avoidance |
| Selection driver | Postfire vegetation gaps | Postfire vegetation gaps | Fire‐independent vegetation gaps | Reliable environmental conditions |
| Response trait | Fire‐stimulated germination | Fire‐stimulated germination | Heat tolerance, seed longevity | – |
| Seed properties | Long‐lived, impermeable, hard | Long‐lived, permeable | Long‐lived | Short‐lived |
| Basic dormancy type | Physical | Physiological | Variable | – |
| Dormancy release mechanism | Fire heat | Chemicals from combustion | Moisture, warmth | – |
| Ancillary promoters | Smoke, scarification | Ash, scarification, heat, light/dark | Seed decay, temperature stratification | – |
| Annual response | No germination (dormant) | No germination (dormant) | Germinates or remains dormant | Germination or seed mortality |
| Dormancy release | Abrupt | Abrupt | Gradual | – |
| Imposed dormancy | Strong | Weak | Variable | Weak |
| Peak in seedling numbers | Postfire | Postfire | Postfire, any time | Unrelated to fire |
| Fire response | High germination | High germination | Low germination | Seed mortality |
| Environments a | Mediterranean, warm temperate | Mediterranean, warm temperate | Savannas, cool temperate (non‐fireprone) | Rainforests, deserts, saline/rocky habitats |
| Fire regime | Moderately frequent crown fires | Moderately frequent crown fires | Rare or frequent surface fires | Rarely burns |
| Heat and smoke responses b | H+S+, H+S* | H+S+, H*S+ | H*S* | H–S*, H*S–, H–S– |
| Prominent examples | Most Fabaceae, Cistaceae, Malvaceae | Many Lamiaceae, Rutaceae, Ericaceae, Poaceae | Some Fabaceae, Poaceae, Polygalaceae | Obligate resprouters, fleshy‐fruited, shade‐tolerant species |
–, does not apply.
Environments where the type is most prominent; species with non‐dormant seeds may occur under any environment and fire regime, including fireprone ecosystems (e.g. obligate resprouters in mediterranean or savanna ecosystems).
See Fig. S11 for details. Dormancy released (+), inhibited (−) or unaffected (*) by heat (H) or smoke (S).
(a). Heat‐released dormancy syndrome
The best‐known mechanism for detecting a vegetation gap created by fire is the presence of a high temperature threshold for breaking physical dormancy that exceeds soil temperatures experienced during summer and is compatible with fire heat. By raising the dormancy‐breaking temperature threshold, seeds remain dormant in the soil seed bank until fire simultaneously creates swathes of bare ground and breaks physical dormancy. Seeds will then germinate as soon as the appropriate hydrothermal conditions arise (e.g. following the first substantial postfire rains). These seeds are ‘hard’, with a thick, impermeable seed coat that (i) prevents the uptake of water (sometimes oxygen; Brits & Manning, 2019) that would otherwise induce germination if temperatures were moderate; (ii) maintains low internal moisture levels that increase longevity and heat tolerance without reducing protein integrity (Considine & Considine, 2016); and (iii) inhibits pathogen invasion and agents of decay. The heat from fire fractures, or dislodges special heat‐sensitive tissues in, the seed coat to create a ‘water gap’ (Nandi, 1998; Gama‐Arachchige et al., 2013; Brits & Manning, 2019). This renders the seed permeable to water and oxygen and enables germination to proceed. Germination occurs later, once the hydrothermal requirements for germination are met. Thus, there may be a prolonged interval (imposed dormancy) between the breaking of dormancy (when fire occurs) and germination (when the levels of moisture and temperature are adequate). We call this suite of traits, that covers primary dormancy maintenance and release, followed by imposed dormancy maintenance and release, then germination, the heat‐released (physical) dormancy syndrome (Tables 2, S1). Note that ‘heat‐stimulated germination’ is often used as an equivalent short‐hand term (see Table 1). Well‐known examples of species with this syndrome include most Cistaceae (an essentially Mediterranean family; Thanos et al., 1992) and most legumes (Fabaceae) (Fig. 2), and it has been identified in another 12 angiosperm families, including Malvaceae, Rhamnaceae and Convolvulaceae (Baskin, Baskin & Li, 2000; Appendix S1, Fig. S1).
Fig. 2.

Percentage of seeds germinating under different treatments among species of Cistaceae and Fabaceae. (A) For 32 Mediterranean Basin Cistaceae species (340 germination tests), treatments are: control (light green), winter temperatures (cold stratification; blue), summer temperatures (warm stratification; red), and heat treatments simulating fire (orange) at different temperatures (70°C to 150°C) and exposure times (1 to 10 min). Note that even at relatively high heat pulses (e.g. 120°C for 10 min), germination is higher than for the control. Data collated from Herranz, Ferrandis & Martínez‐Sánchez (1999), Luna (2020), Luna et al. (2007, 2008); Luna, Chamorro & Pérez (2019), Moreira & Pausas (2012), Moreira et al. (2010), Navarro‐Cano, Rivera & Barberà (2009), Pérez‐García & González‐Benito (2006), Scuderi et al. (2010) and Thanos et al. (1992). (B) For 55 species of Western Australian Fabaceae, treatments are control (cont., green) mechanical scarification (Scar., grey), and application of boiling water for 0.5, 1, 2 or 5 min (data from Bell, Plummer & Taylor, 1993). Note that all heat pulses stimulate germination more than scarification. (C) Fabaceae, comparison of germination between the control and after a heat pulse (100°C for 1 min) for species from the Brazilian Cerrado savanna (Sav, yellow; 46 species; Daibes et al., 2017, 2019) and a SE Australian shrubland (WT = warm temperate, pink; 38 species; Auld & O'Connell, 1991). Note the different response between shrublands (crown fires) and savannas (surface fires; no response). Boxplots represent the median (horizontal thick line), the first and third quartiles (box), and the 1.5 interquartile range (whiskers). Asterisks refer to significant differences in relation to the control treatment (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Heat tolerance may be critical for seed survival; viability may be retained even under treatments of 150°C (Fig. 2A). Perhaps the record is 20% survival of seeds of a South African legume at 215°C for 1 min (Virgilia oroboides; Jeffery, Holmes & Rebelo, 1988). Sunlight is incapable of heating soil to these temperatures but such levels of heat can be received from intense fires (Brits, 1986; Auld & Bradstock, 1996; Merritt et al., 2007). The mechanism of seed heat tolerance seems to be associated with the fact that the tissues are highly desiccated (Tangney et al., 2019). The heat dose needed for breaking dormancy and the magnitude and shape of the heat response may depend on characteristics of the seed coat (thickness and structure of the cuticle, epidermis, palisade and parenchyma, and type of water gap; Nandi, 1998; Hradilová et al., 2019), seed size (smaller seeds may be more heat tolerant and have lower thresholds; Tavşanoğlu & Çatav, 2012), seed age (Downes et al., 2015), and whether it is wet or dry heat (van Klinken, Flack & Pettit, 2006; Erickson, 2015; Liyanage & Ooi, 2017). A few non‐dormant seeds are often present even among species with high heat responses/tolerances (e.g. 10% germination among the controls in category A3 of Fig. S2). Both heat tolerances and temperature thresholds for dormancy release should be subject to selection and can be expected to vary with the intensity of recurrent fires but the topic has received little study (Tangney et al., 2019).
In regions with especially cold winters (uplands, high latitudes) that are also fireprone in the warm part of the year, seeds may require a cold pretreatment in addition to fire to yield maximum levels of dormancy release (Keeley, 1991). This has been confirmed for a few species where fire (heat) and cold stratification treatments have been applied separately and together, with the combined treatment often being most effective (Figs S3, S4).
(b). Smoke‐released dormancy syndrome
Another major mechanism by which species can take advantage of fire‐induced gaps (postfire germination) is through certain chemicals present in smoke, ash, or charred wood (collectively termed ‘smoke’ here, although they may have different effects). These compounds are organic [e.g. karrikin (a pyranobutenolide), glyceronitrile] or inorganic (e.g. nitrogen oxides) by‐products of the pyrolysis of biomass that diffuse in a volatile state or percolate through the soil profile after rain to reach the seeds (Roche, Koch & Dixon, 1997; Stevens et al., 2007; Ghebrehiwot et al., 2013). In physiologically dormant seeds, these cofactors link to specific proteins to catalyse the production of hydrolytic enzymes that enable respiration to commence and thus break dormancy (Keeley & Fotheringham, 1997, 2000; van Staden et al., 2004; Waters et al., 2013; Keeley & Pausas, 2018; Lamont, He & Yan, 2019a). In addition, there is some evidence that karrikins may increase the rate of water uptake via aquaporins in meristematic cells (Jain, Kulkarni & van Staden, 2006; Ghebrehiwot et al., 2008).
Seeds of this syndrome may bypass a period of imposed dormancy because smoke compounds usually reach the seed dissolved in rainwater, and this water often creates suitable germination conditions (seeds with this syndrome are water permeable). Thus, dormancy release and germination are likely to occur contemporaneously in the field unless rain occurs during periods of unsuitable temperatures (as is also the case for heat‐released seeds; MacKenzie et al., 2016). We call this syndrome smoke‐released (physiological) dormancy (Table 2). Note that ‘smoke‐stimulated germination’ can be used as an equivalent short‐hand term (Table 1).
This dormancy syndrome is phylogenetically widespread, with many examples among Lamiaceae, Ericaceae, Rutaceae, Asteraceae, Poaceae, Proteaceae, Euphorbiaceae and Restionaceae (Keeley & Pausas, 2018; Carthey et al., 2018; Fig. 3; Tables S2–S4, Appendix S1). It is unambiguously tied to fire but species rarely have an absolute requirement for smoke. Typically, there is an increase in germination with increasing smoke dosage until an optimum is reached beyond which germination rates gradually fall (Fig. S5A, B). Germination of species with little seed dormancy are either unaffected by smoke or germination gradually decreases as smoke concentration increases (Fig. S5C). There are no records of viability loss in the presence of smoke although it is rarely examined on the assumption that it does not occur.
Fig. 3.

Smoke‐released dormancy. (A) Changes in germination percentage (relative to the control) after treatments with fire‐derived compounds (charred wood or nitrogen‐containing compounds) for 60 species in three families (N = 99 records) widespread in the Mediterranean Basin (collated from Moreira & Pausas, 2018). Values within each box are number of records/number of species. (B) Proportion of species in Poaceae (boxplot and coloured symbols) and Lamiaceae (asterisks) with germination stimulated by smoke in different ecosystems with woody‐fuelled crown‐fire or grass‐fuelled surface‐fire regimes under warm (red) and cool (blue) climates. (C) Percentage of species that show a significant positive response to smoke for eight ecosystem types. The ecosystems are ordered in increasing abundance of smoke‐responsive species, from tropical rainforest and cool temperate shrublands to the greatest dependence among mediterranean ecosystems. The colour of the labels indicates the main fire regime: no fires (black), surface fires (blue), crown fires (red); dry shrublands may also show some low‐intensity crown fires. Boxplots represent the median (horizontal thick line), the first and third quartiles (box), and the 1.5 interquartile range (whiskers). Red points are the raw data. For further details of the data plotted in B see Tables S2 and S3; for C, including full names of the ecosystems, see Table S4.
Smoke is more likely to break dormancy and stimulate germination the higher the initial level of dormancy, and the older the seed is if an after‐ripening period is required for embryos to reach maturity (Merritt et al., 2006; Downes et al., 2015; Newton et al., 2021). Variability in smoke responses is observed at both the individual seed level within batches and among plants, even within a given population, possibly as an inverse function of seed mass (smaller seeds are more smoke‐responsive; Ma, Wu & Ooi, 2018). Assuming such variation to have a genetic basis, this must provide abundant opportunities for adaptation to changing climate and fire regimes but has received little study.
The positive effect of smoke is observed for germination experiments in the dark as well as in light: under dark conditions, germination enhanced by smoke is similar to that enhanced by light; however, maximum germination is usually attained in the presence of both light and smoke (Fig. S6). The latter is consistent with experimental evidence indicating that smoke compounds increase the sensitivity of seeds to light (Nelson et al., 2010). Smoke may also enhance the germination rate of grasses (Hodges et al., 2021), which might give them a temporal advantage during establishment. Seedling growth rates may also be enhanced (Blank & Young, 1998; Moreira et al., 2010; Ghebrehiwot et al., 2012), including root growth (Moreira et al., 2010), which further increases fitness benefits at this critical life stage.
Heat‐released physical dormancy is typically tested by heat treatments under laboratory conditions (Fig. 2). Smoke‐released physiological dormancy is also tested in the laboratory (Fig. 3), but unlike heat, smoke effects can also be tested easily in field plots by spraying with smoke water (Tormo, Moreira & Pausas, 2014). Thus, it is possible to evaluate responses of the whole seed bank to smoke (community‐scale approach). Studies that have tested the effects of smoke on the soil seed bank under crown‐fire ecosystems consistently show an overall increase in seedling density, species richness, and seedling biomass (Fig. S7).
(c). Non‐fire‐released dormancy syndrome
The two fire‐released dormancy syndromes described above are prominent, if not dominant, in many fireprone ecosystems (e.g. Fig. 3C). In some of these ecosystems, there may be species with soil‐stored seed banks that do not respond to either heat or smoke, although the seeds can withstand fire as well as those with fire‐released dormancy. We call this syndrome non‐fire‐released dormancy (Tables 2, S1). Here, dormancy release does not follow abruptly after fire, but is induced by a variety of environmental stimuli that sometimes include an indirect role for fire or any other disturbance that opens the vegetation. For example, the postfire decrease in albedo (due to blackening of the landscape) may enhance cool/warm or wet/dry cycles and thus promote dormancy release among hard seeds (Baskin & Baskin, 1984; Brits & Manning, 2019). Some mediterranean annuals have a strictly photoblastic response and are unaffected by smoke (Merritt et al., 2006). In subtropical savannas, some species require wet, hot‐summer stratification (van Klinken et al., 2006; Zhang et al., 2020).
In other cases, germination starts as soon as the seed coat decays sufficiently, i.e. permeability increases over time (Lonsdale, 1993; Brits & Manning, 2019). The need to satisfy an after‐ripening requirement is another possibility for germination that is out of phase with fire (Downes et al., 2015). Examples occur not just in fireprone ecosystems such as savannas (Daibes et al., 2017, 2019; Ibañez Moro et al., 2021) but also in many non‐fireprone ecosystems (Figueroa, 2003). Among typically hard‐seeded families, a few Fabaceae and Cistaceae may also show non‐fire‐released dormancy in non‐fireprone habitats (Pérez‐García & González‐Benito, 2006; Sautu et al., 2006). While fire‐released dormancy allows the detection of vegetation gaps for matching germination to optimal conditions, non‐fire‐released dormancy takes advantage of the longevity of seeds that ensures the efficacy of this bet‐hedging strategy when suitable (non‐fire) conditions arise (Table 2).
(d). Non‐dormancy syndrome
Finally, many species in fireprone ecosystems do not have dormant seeds and thus lack a soil seed bank (Non‐dormancy syndrome; Tables 2, S1). Their adaptive advantages centre around rapid germination, thus avoiding seed predation, time‐based viability loss, or conditions unsuitable for germination, especially seasonal drought. The germination of some species with little dormancy, even among species in non‐fireprone ecosystems (including cultivars), can be experimentally stimulated by fire properties (heat or smoke). This has caused some researchers to question the role of fire in shaping dormancy; however, relevant explanations may still include a role for fire (see Section V.5).
For Fabaceae in a Brazilian Cerrado savanna, 30% of 48 species examined were non‐dormant (Fig. S8, Table S5). Surprisingly, all but one of these retained some viability after pretreatment at 200°C for 1 min, and 48% were unaffected by less‐intense heat. Such a high level of heat tolerance among non‐dormant seeds is consistent with the inevitability of fire in these savannas. Almost all perennial species rely on resprouting for persistence under such frequent fire regimes. Among some woody species, lack of dormancy is notable among obligate resprouters with their fleshy fruits dispersed over long distances by vertebrates; they do not take advantage of postfire conditions for recruitment, which can occur several to many years after fire (Pausas & Keeley, 2014). Some species with fire‐released dormancy may show no dormancy in populations that inhabit non‐flammable habitats. For example, populations of Erica coccinea (Ericaceae) that occur among non‐fireprone rocky outcrops in South Africa show 86% germination in the presence or absence of smoke (i.e. they are essentially non‐dormant) (Leonard, West & Ojeda, 2018). By contrast, populations in the surrounding flammable shrubland show 16% germination in controls compared with 82% in the presence of smoke.
(2). Spatial and temporal components of germination
Germination experiments provide information on the expression of the various dormancy syndromes by different taxa and ecosystems (Figs 2, 3), but seedling recruitment under field conditions is a more complex process. First, the vertical distribution of seeds is important. Successful emergence in the field will depend on the extent to which the heat pulse (as a function of fire intensity) or smoke reaches the seed and is capable of breaking dormancy and stimulating germination at a given soil depth. Seeds on the soil surface or beneath highly combustible litter may be charred or incinerated whereas seeds located deep in the soil may not receive enough heat or smoke to release dormancy (Fig. 4A, B; Fig. S9). In addition, germinants must be able to reach the surface before their metabolic reserves are exhausted. In fact, most germinable seeds are located just beneath the surface and gradually diminish to negligible levels by a depth of 8 cm (Fig. 4C). The ability of germinants to reach the surface falls linearly to be negligible once this same depth is reached (Fig. 4D, E), and is usually negatively related to seed size (Fontenele et al., 2020; Tangney et al., 2020). The depth to which species are buried is a function of time, seed characteristics (size, surface texture, shape), soil properties (litter thickness, soil texture and density, which also affect heat transfer), and the extent to which seeds are buried by dispersal agents (Saatkamp, Pochlod & Lawrence, 2014; Fig. 4).
Fig. 4.
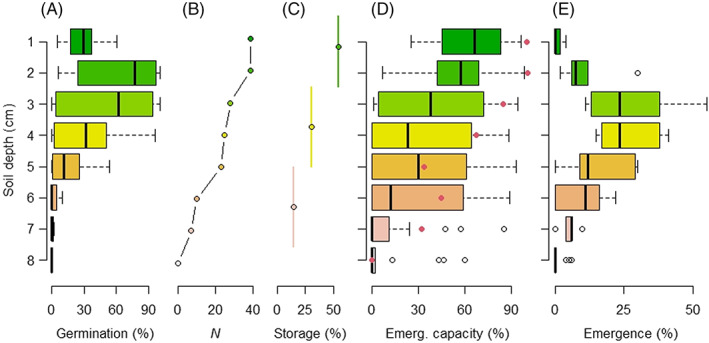
Examples of postfire biotic responses through the soil profile in fireprone Australian ecosystems. (A) Percentage germination of seeds sown at increasing depths based on laboratory experiments using a range of fire‐type surface temperatures for 39 legume species. Boxplots indicate variability among species. From Auld & O'Connell (1991). (B) Number of species (N) out of 39 with germination levels exceeding 5% at that depth for the same experiment as A. (C) Percentage of germinable seeds present in three soil layers from three eucalypt woodland samples (68 species identified; Read et al., 2000). (D) Ability of germinants to reach the surface (emergence capacity, %) at different sown depths for 17 species (boxplot; Tangney et al., 2020) and for Grevillea speciosa (red dots; Auld & Denham, 2005). (E) Field observations of percentage of seedlings emerging relative to total seeds present for Grevillea speciosa and Acacia suaveolens (from Auld & Denham, 2005). As these two species have seeds with elaiosomes the low emergence levels in the top layers compared with D could be due to a combination of high fire intensity (seed mortality, as in A) and deeper burial of seeds by ants (compared with C). Boxplots represent the median (horizontal thick line), the first and third quartiles (box), and the 1.5 interquartile range (whiskers).
Furthermore, there is much patchiness in fire intensity, seed density, and species distribution at the microscale, due to the scattered distribution of vegetation and fuel loads. Local variation in soil properties (e.g. texture) is also expected to control the extent to which heat and soil water can reach the seeds. These vertical × horizontal interactions produce complex spatial patterns of seedling emergence in the field (Fig. S10; Lamont, Witkowski & Enright, 1993; Odion & Davis, 2000). To complicate the picture further, there is inherent variability in seed size and fire‐released dormancy within and among plants (as with any other trait) that also contributes to the spatial variability of recruitment. Once emerged (Fig. 4D, E), the fate of seedlings will depend on additional constraints such as rainfall, nutrient availability, herbivory, and shade.
(3). Ant dispersal of dormant seeds: a fire‐persistence trait
Some seeds with dormancy have an aril attractive to ants, called an elaiosome. In these cases, ants act as dispersal agents without harming the seeds, and bury them in their nests (myrmecochory). Myrmecochory promotes short‐distance dispersal (including uphill, in contrast to passive dispersal), protection from granivores, placement of the seed at a suitable depth for germination and emergence, and access to the extra nutrients associated with ant nests. If these are its primary functions then myrmecochory should be best represented in vegetation types where ants are active, as in all but the coldest parts of the Earth. Berg (1975), followed by others (Hughes & Westoby, 1992; Auld, 1996; Giladi, 2006; Lengyel et al., 2010), suggested that ant dispersal and burial could also serve both to insulate seeds from intense fires and to place them at a depth where the heat pulse/smoke concentration is conducive to breaking dormancy and stimulating germination. If these are its primary functions, then myrmecochory should be expressed most often in fireprone habitats with high ant activity.
We noted that many genera with elaiosomes are especially species‐rich in crown‐fire ecosystems with high ant diversity, such as some Proteaceae [Grevillea (350 species), Leucospermum] and Fabaceae [Acacia (1100 species), Daviesia, Ulex] (Auld, 1996; Lengyel et al., 2010). Further, there is a strong correlation between species occurring in fireprone habitats and possession of elaiosomes, with the reverse in non‐fireprone habitats (e.g. Acacia and Rutaceae in Australia, and worldwide Polygalaceae; Table 3). Thus, most Acacia species with heat‐released dormancy possess elaiosomes, but the few species not subject to fire heat (i.e. those in deserts) lack elaiosomes. Among Rutaceae, most species with smoke‐responsive seeds in highly fireprone sclerophyll vegetation have elaiosomes (e.g. Boronia, Phebalium). The few citrus species in rainforests (non‐fireprone) have non‐dormant seeds with colourful arils that attract bird dispersers (Table 3). Polygalaceae is ant‐dispersed in fireprone ecosystems (mediterranean and warm temperate climates), whereas in other ecosystems (rainforest, cold deserts) its species are vertebrate‐dispersed with fleshy seeds or fruits (Table 3). Most fireprone Rhamnaceae (tribe Pomaderreae) bear elaiosomes and have heat‐released dormancy, with
Table 3.
Association between binary traits (Yes/No) for clades possessing many species but varying in seed dormancy. Association between traits is given as number of species possessing (Y) or not possessing (N) each trait (YY: have both; YN: only trait 1; NY: only trait 2; NN: neither trait present). Acacia and Rutaceae refer to Australian species only. Traits include heat‐ and smoke‐released dormancy (Heat, Smoke; from germination experiments), hard‐seededness, fireproneness of the habitat (including crown and surface fires), presence of an elaiosome, and a thick seed coat (>150 μm). Data are from Data S1, except for Leucadendron (from Newton et al., 2021). Emboldened numbers are the highest in each foursome. P‐values refer to Pearson's Chi‐squared test (of the contingency table of the two traits) with simulated P‐values. In all cases, the residuals are positive for YY or NN (species over‐represented) and negative for YN or NY (species under‐represented)
| Clade | Trait 1 | Trait 2 | YY/YN, NY/NN | P‐value |
|---|---|---|---|---|
| Cistaceae | Heat | Hard seeds | 35/0, 8/5 | 0.0008 |
| Heat | Fireprone | 36/0, 5/13 | <0.0001 | |
| Fireprone | Hard seeds | 49/2, 5/4 | 0.0020 | |
| Acacia a | Heat | Hard seeds | 98/0, 0/11 | <0.0001 |
| Heat | Fireprone | 90/0, 1/11 | <0.0001 | |
| Heat | Elaiosome | 87/4, 4/9 | <0.0001 | |
| Fireprone | Elaiosome | 83/1, 3/8 | <0.0001 | |
| Fireprone | Hard seeds | 91/1, 0/11 | <0.0001 | |
| Lupinus | Heat | Hard seeds | 17/0, 0/5 | <0.0001 |
| Heat | Fireprone | 18/0, 1/5 | <0.0001 | |
| Fireprone | Hard seeds | 18/1, 0/5 | 0.0001 | |
| Leucadendron b | Heat/smoke | Thick seed coat | 10/2*, 5*/22* | 0.0005 |
| Rhamnaceae (dry fruited only) c | Heat | Hard seeds | 47/0, 3/3 | 0.001 |
| Heat | Fireprone | 46/0, 3/2 | 0.010 | |
| Fireprone | Hard seeds | 49/2, 1/1 | – d | |
| Fireprone | Elaiosome | 36/15, 1/1 | – d | |
| Polygalaceae e | Fireprone | Elaiosome | 6/8, 0/14 | 0.010 |
| Rutaceae f | Fireprone | Elaiosome | 113/13, 6/81 | <0.0001 |
| Smoke | Fireprone | 37/0, 14/29 | <0.0001 | |
| Smoke | Elaiosome | 36/1, 10/33 | <0.0001 |
For Acacia, we assumed that all species with heat (100°C)‐stimulated germination are hard seeded as all 45 examined to date have this trait (Burrows, Alden & Robinson, 2018).
All Leucadendron species occur in fireprone ecosystems; *serotinous species have thin seed coats. Seeds with a thick seed coat (>150 μm) are not necessarily impermeable but they absorb water at a much slower rate and show smoke‐ and/or heat‐stimulated germination (Lamont, Gómez Barreiro & Newton, 2021; Newton et al., 2021).
We considered only Rhamnaceae with dry fruits; the same statistics for those with fleshy fruits were not significant (P > 0.10) essentially because they lack all these traits. Data for both dry and fleshy‐fruited species are provided in Data S1.
Insufficient variability to perform statistics.
Chi‐squared test performed at genus level, weighted by the number of species in each genus, and considering the genus Polygala, the largest genus by far (~700 species), as five lineages, one on each continent. On merging the five lineages as a single large genus, the relationship remained significant (P = 0.011).
Ceanothus the notable exception in California, whose fireprone flora possesses few ant‐dispersed species (Rundel et al., 2018). Mimosoid legumes bear an elaiosome in sclerophyll shrublands to forests in Australia [Lengyel et al. (2010) for Acacia] but not in rarely burnt deserts (Burrows et al., 2018), nor in the frequently burnt savannas of Africa (Vachellia; Garner & Witkowski, 1997) and South America (Mimosa; Gunn, 1984), nor in rainforests [Albizia (Diabate et al., 2005); Adenanthera, Castanosperumum (Hopkins & Graham, 1987)]. Only two elaiosome‐bearing species are listed among 52 rainforest species that we compiled (see Section V.5) and neither is endemic.
We conclude that ant dispersal of dormant seeds is prevalent in fireprone ecosystems, and especially crown‐fire ecosystems. Among alternative explanations, crypsis from granivorous vertebrates cannot be dismissed (Christian & Stanton, 2004), but it is hard to explain why this is not equally important in rainforests or arid ecosystems. More comparative studies are needed to determine the generality of our findings, and to explain the relative absence of ant seed dispersal in the highly fireprone Californian flora (Rundel et al., 2018). It would be of interest to know the extent to which abundances of aril‐consuming ants are affected by these contrasting environments. At present, we suggest that ant seed dispersal is an under‐rated fire‐persistence trait and exposure of seeds to milder levels of heat via burial is likely to be its primary function. Experimental research comparing burial depth with optimal conditions for dormancy release of species with or without elaiosomes remains to be undertaken.
(4). Consequences of seed dormancy for stand structure
As a consequence of delaying germination to postfire conditions, recruitment of species with fire‐released dormancy is restricted to these conditions (i.e. to the first wet period after fire), and thus populations of these species tend to form single cohorts of the same postfire age (Pate et al., 1985; Keeley, 1991; Fig. 1). This is especially notable among non‐resprouting species (obligate seeders; Table 1). Postfire facultative species (Table 1) are characterized by stands of mixed ages as recruitment after each fire coexists with postfire resprouting (Lamont & Witkowski, 2021). Mixed‐age stands are even more prominent among obligate resprouters as their recruitment is independent of fire (seed production and germination occur long after the passage of fire) and is related more to environmental fluctuations. Among the obligate seeders with fire‐released dormancy that form single postfire cohorts within each fire cycle (monopyric species; Pausas & Keeley, 2014), we find (i) those that survive during the entire inter‐fire period, store seeds throughout this period, and are killed by each fire; and (ii) those that are short‐lived and complete their life cycles in a few years (before the next fire), and their seeds remain dormant in the soil until the next fire (fire ephemerals; Pate et al., 1985). A special case of the latter are postfire annuals (pyroendemics; Keeley & Pausas, 2018). No other disturbance creates these types of habitat‐defined species, highlighting the role of fire as a selective agent in a plant's reproductive cycle (He, Lamont & Pausas, 2019).
III. SELECTIVE ENVIRONMENTS
Our collation of data for 586 species in the various fireprone environments shows that germination of 42% of the species was stimulated by heat and/or smoke, 49% were unaffected by both (i.e. were both heat and smoke tolerant), and 9% were inhibited by heat and/or smoke (Fig. S11). The overall importance of fire‐released dormancy is highest in crown fire ecosystems (mediterranean ecosystems excluding central Chile, followed by other warm temperate ecosystems), lower in surface fire savanna ecosystems (especially the Cerrado), and negligible in rainforests (Figs 2, 3, 5, S11, Tables S2–S5). In this section, we further generalize the patterns of fire‐related dormancy syndromes by contrasting the world's two major vegetation–climate–fire‐regime complexes: crown‐fire regimes in mediterranean and warm temperate ecosystems and surface fire regimes in savannas and grasslands (see Tables S2 and S6 for the regions included in each subcategory).
Fig. 5.

Raw data (×) and median ± 25% percentiles for total germination (%, x‐axis) and rate of germination (days to 50% of total germination or mean daily rate, y‐axis, log scale) for 89 species (shrubs, forbs, grasses) from either a mediterranean climate (moderate inter‐fire interval, crown‐fire regime; red symbols) or a savanna/grassland climate (frequent fires, surface‐fire regime; black symbols). Open circle = no‐smoke control; filled circle = smoke pretreatment; open square = no‐heat control; filled square = fire‐type heat pretreatment. Collated from 293 tests undertaken in 12 studies (Jeffery et al., 1988; Crosti et al., 2006; Travlos, Economou & Karamanos, 2007; Reyes & Trabaud, 2009; Tavşanoğlu, 2011; Chou, Cox & Wester, 2012; Ribeiro, Pedrosa & Borghetti, 2013; Galindez et al., 2016; Paredes et al., 2018; López‐Mársico et al., 2019; Fernandes et al., 2020; Gorgone‐Barbosa et al., 2020). Inset: the best‐fit curves with 95% confidence intervals (GAM, P < 0.0001; untransformed data).
(1). Woody ecosystems with crown‐fire regimes
Fire spreads readily where vegetation is dry and continuous. Thus, ecosystems with both dense woody vegetation and a seasonal climate (with a dry, highly flammable period) are typically subject to crown fires. This includes forests, woodlands and shrublands that burn at relatively high intensity (the whole shoot mass burns) in mediterranean environments (with summer drought) as well as other warm temperate environments with a drier warm season in summer (e.g. southeastern Australia) (Table S6). In such crown‐fire ecosystems, the dense canopy of woody vegetation prevents successful recruitment of shade‐intolerant plants, and thus seed dormancy allows them to avoid germinating in deep shade. In these environments, the main creators of gaps suitable for seedling recruitment are wildfires (Keeley et al., 2012). Thus, many mediterranean species have seeds that respond to heat and/or smoke that take advantage of vegetation gaps for postfire germination when recruitment conditions are optimal. Thus, crown‐fire ecosystems are the paradigm for fire‐released dormancy syndromes, including dormancy released by heat (e.g. many legumes, Figs 2C, S2), smoke (e.g. many Poaceae, Fig. 3B), or by both. Fire thus promotes germination in 80% of Fabaceae species in mediterranean regions and 100% in other warm temperate regions (Fig. S8B). In these ecosystems, seed dormancy is especially prevalent among high‐light‐requiring, shade‐intolerant plants, and dormancy‐breaking thresholds act as a mechanism for detecting vegetation gaps created by fire.
Consistent with these results, most species in the Restionaceae, a sister family to Poaceae common in South African shrublands (with high‐intensity fires), have smoke‐released dormancy (85%; Table S2; He, Lamont & Manning, 2016). Mediterranean ecosystems show an even spread among the dormancy‐release mechanisms (heat, smoke, or both, each accounting for 20–25%) than other warm temperate ecosystems (Fig. S11), and are the regions with the greatest representation of fire‐released dormancy (Figs 3, S11). Most mediterranean shade‐intolerant species can resist the heat of a fire (even where the heat does not break dormancy; see H*S+ and H*S* in Fig. S11). Central Chile has a mediterranean climate but, historically experiences fewer fires compared with the other mediterranean regions (Keeley et al., 2012); interestingly, fire‐released dormancy is less prominent here with only 27% of species responding to fire (H+S+, H+S*, H*S+; Fig. S11), although heat resistance remains high.
(2). Grassy ecosystems with surface‐fire regimes
(Sub)tropical savannas and grasslands are the world's most extensive fireprone ecosystems, with more frequent but less‐intense fires than crown‐fire ecosystems. Postfire germination in these vegetation types has only been studied in depth recently, and given their broad distribution and variability, our understanding of seed ecology in this biome is more restricted. In savannas and grasslands, light is not a strong limiting factor, and thus pressure to evolve mechanisms for gap detection is less intense than in crown‐fire ecosystems. Yet seed tolerance to heat should still provide a fitness benefit, as fires are frequent and may still signal an improvement in recruitment conditions.
In savannas, seeds tolerate fires but species with fire‐released dormancy (by heat, smoke, or both) average only 23% (Fig. S11; Daibes et al., 2017, 2019; Ibañez Moro et al., 2021). For the hard‐seeded legume family, 42% (of 144 species) studied in six savannas (Fig. S8) showed heat‐released dormancy, while this figure was 86% (of 128 species) in four crown‐fire ecosystems (Fig. S8B). Smoke appears more effective than heat in savannas, especially in South America (Zirondi et al., 2019a; Zirondi, Silveira & Fidelis, 2019b). Smoke also tends to be more important in crown‐fire than surface‐fire ecosystems at the community level (Fig. 3C) and family scale (Poaceae, Lamiaceae; Fig. 3B). The few studies on the role of fire in the germination of the Florida scrub with a subtropical savanna climate also show some species with smoke‐released seed dormancy (King & Menges, 2018).
We have identified nine possible reasons for limited fire‐released dormancy in grassy savanna environments compared with crown‐fire systems: (i) the absence of a continuous woody cover suggests that light may not be a strong limiting factor, such that a fire may not be required to create optimal conditions for germination; (ii) the shorter inter‐fire interval in savannas prevents most non‐resprouting plants (for which a seed bank is essential) from reaching maturity, and thus they are excluded; (iii) the fact that almost all perennials in savanna ecosystems are resprouters (they become fire‐tolerant before reaching maturity) reduces the selective pressure for efficient postfire seedling recruitment; (iv) the fact that most grasses quickly resprout and cover the soil provides a selective pressure for rapid postfire germination; (v) short inter‐fire intervals reduce the likelihood of seeds getting buried deeply; (vi) low fire intensity and warm climates limit the ability to identify temperature thresholds substantially different from seasonal temperatures; (vii) more generally, the high frequency of fire (<5 year intervals, often 1–2 years) results in limited differences in recruitment conditions for the inter‐fire and postfire periods, so that other factors, such as unusually wet years, become more important for recruitment; (viii) the unique availability of wet summer heat as a possible dormancy‐release mechanism that may or may not be associated with a recent fire (van Klinken et al., 2006); and (ix) fire‐stimulated flowering (for which savannas are renowned; Lamont & Downes, 2011; Pausas, 2017) may result in rapid production of non‐dormant seeds that can take advantage of improved conditions for germination immediately postfire.
At present, we cannot dismiss any of these non‐exclusive possibilities. However, it is important to recognize the limited research performed on the seed ecology of these ecosystems, especially regarding possible smoke effects on non‐woody species (Carthey et al., 2018). The information currently available indicates that savannas have more species with non‐dormant seeds and less with fire‐released dormancy than crown‐fire ecosystems, and this is especially clear in the Brazilian Cerrado (Fig. S8A). Nevertheless, most seeds show high heat tolerance (Fig. S8B) that confirms a long evolutionary history associated with fire, and ensures that viability is maintained until conditions are suitable for germination, whether or not this follows a fire.
Another trait yet to receive attention is the relative rate of germination. Savannas have an extremely short fire cycle and the soil is soon re‐covered by resprouting grasses. Thus, there is a strong pressure for seeds to germinate rapidly postfire. Consistent with this expectation, germination rates are, on average, twice as fast for the seeds of savanna plants (lower y‐axis values, Fig. 5), coupled with their lower seed dormancy levels (high % germination; x‐axis, Fig. 5) and reduced responses to fire. In general, heat has a greater positive effect on breaking dormancy than smoke (t‐test: P = 0.005), especially in crown‐fire ecosystems (Fig. 5). In mediterranean ecosystems, higher total germination is correlated with a more rapid germination rate, slow‐germinating seeds tending to be the untreated controls; for species in savannas the rapid germination rate is independent of treatment (total germination). Since the correlation between percentage germination and germination rate is strong for mediterranean ecosystems (Fig. 5, inset), determining total germination could be regarded as a higher priority for these ecosystems than determining germination rate. Germination rate may deserve more attention in grassy ecosystems as this is more likely to be independent of total germination (Hodges et al., 2021).
IV. MACRO‐EVOLUTIONARY PATTERNS OF FIRE‐RELEASED SEED DORMANCY
What is the evolutionary history of fire‐released seed dormancy syndromes among plants? Is it consistent with dormancy as a fire adaptation? We propose that fire‐released dormancy must be ancient within the evolutionary history of flowering plants because (i) the capacity for seed dormancy was present among their earliest ancestors [at least 130 million years ago (Ma); Willis et al., 2014; Friis et al., 2015], (ii) fire is an ancient process on Earth (from 420 Ma; Scott, 2018), (iii) fireprone ecosystems have been common at least since the mid‐Cretaceous (100 Ma) when flowering plants first became prominent (Shi et al., 2022), (iv) many other fire‐adapted traits appeared during the Cretaceous and proliferated during the Paleogene–Neogene (Lamont et al., 2019a), and (v) fire‐released dormancy provides strong fitness benefits to plants (as shown in previous sections).
On the basis of anatomical observations of fossil seeds, there is evidence of physical dormancy in Anacardiaceae from the middle Eocene (43 Ma; Baskin et al., 2000). Extant Anacardiaceae are mostly confined to rainforests and lack dormancy so the significance of this record is unclear: it coincided with the mid‐Eocene Climatic Maximum (Zachos, Dickens & Zeebe, 2008) so physical dormancy could have been associated with abundant fires then and the family could have moved into rainforests later. There are no fossil records for major extant families with physical dormancy; in addition, fossil seeds provide no clues as to the presence of physiological dormancy. The task is further complicated by difficulties in locating fossil charcoal in dry (fireprone) ecosystems [e.g. absence of peatland deposits (Keeley et al., 2012; Lamont et al., 2019a)]. Thus, our understanding of the evolution of seed dormancy relies on ancestral trait reconstructions using time‐based phylogenies, dated from fossil records, rather than on direct fossil evidence of dormancy.
We document three evolutionary pathways for seed dormancy here: (i) origin of fire‐released dormancy from ancestral lineages in non‐fireprone ecosystems (lacking seed dormancy); (ii) dormancy changes from crown‐fire‐adapted lineages to surface‐fire regimes; and (iii) loss of fire‐released dormancy (i.e. evolution of non‐dormancy) by ancestral lineages moving from fireprone ecosystems to non‐fireprone ecosystems (Table S7).
(1). Onset of fire‐released dormancy
There is no single origin of fire‐released dormancy; both heat‐ and smoke‐released dormancy had multiple phylogenetic origins (i.e. convergent evolution; Keeley et al., 2012, Keeley & Pausas, 2018), and are ancient traits (Fig. 6). For example, a large number of Fabaceae species (in both subfamilies Faboideae and Caesalpinioideae) has physical dormancy sensitive to the heat of fire. It is plausible that this trait was ancestral in Fabaceae that evolved 90 Ma (Li et al., 2015; Lamont et al., 2019a; Fig. 6) from a Fabales rainforest parent clade, with soft seeds that lacked dormancy (Lamont et al., 2019a). Cistaceae, and other closely related Malvales such as Bixacaeae, are dominated by species with heat‐released dormancy (Figs 2, 7; Baskin et al., 2000) that in the case of Cistaceae mostly occur in fireprone Mediterranean shrublands. They evolved from a clade of tropical trees (that lack dormancy) in non‐fireprone ecosystems more than 70 Ma (Figs 6, 7), then migrated to surface‐fire ecosystems (in which the trees do not get burnt) where they acquired dormancy, then to crown‐fire ecosystems (as small shrubs) where heat‐released dormancy peaked. Some terminal species now occupy non‐fireprone habitats and lack heat‐released dormancy but not necessarily dormancy (non‐fire‐released dormancy – hard seeds; Fig. 7). Recent research shows that a 99‐million‐year‐old fossil Phylica (Rhamnaceae) from a fireprone ecosystem exhibited floral and leaf traits identical to modern Phylica (Shi et al., 2022). Since the modern taxa also inhabit fireprone ecosystems (fynbos) and have hard and arillate (ant‐dispersed) seeds, it indicates that these traits have been maintained for at least 99 million years (My) in Rhamnaceae. Such compelling fossil evidence demonstrates the importance of the fire regime in driving the direction of evolution in this family.
Fig. 6.
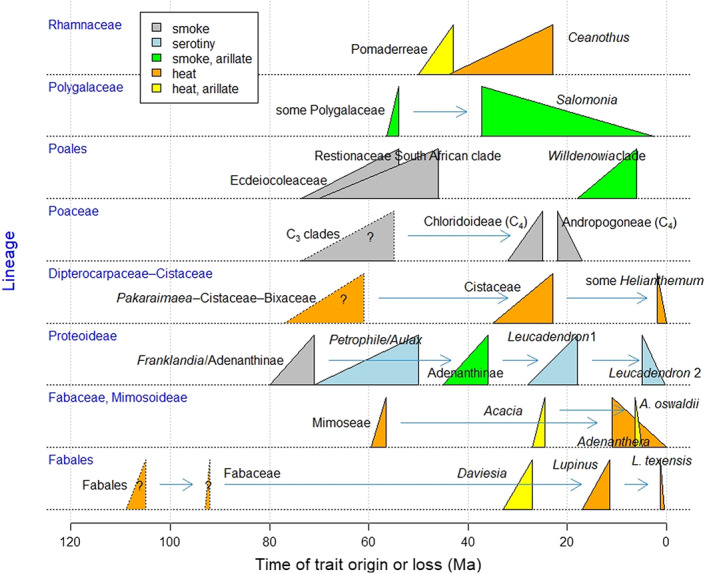
Acquisition or loss of traits related to dormancy release and/or ant dispersal among various lineages or clades over geological time [x axis; million years ago (Ma)] extracted from trait assignments to dated molecular phylogenies. The base of each triangle extends from the stem to crown age of the corresponding lineage. Their colour refers to the trait that is acquired or lost in that period (a question mark indicates uncertain reconstruction of the trait at present). Triangles with a positive hypotenuse (right leaning) indicate trait acquisition, triangles with a negative hypotenuse (left leaning) indicate trait loss. Arrows link ancestral clades with derived clades of the same lineage. A. oswaldii refers to Acacia oswaldii. See Table S7 for details and sources.
Fig. 7.

Dated phylogeny for major clades in the New and Old World Cistaceae together with closely related ancestral clades (from Aparicio et al., 2017; Heckenhauer et al., 2017). Monotoideae and Dipterocarpoideae are subfamilies in the Dipterocarpaceae. Crocanthemum includes Hudsonia; the seed traits of both are poorly known. Pie charts at the tips show the fraction of species that occur in crown‐fire ecosystems (red), surface‐fire ecosystems (orange), those with physical dormancy – hard seeds (green), and those with heat‐released dormancy (blue) (from Data S1). Blank sectors mean that the trait is absent. Letters at the tips refer to growth forms in the clade (T, tree; S, shrub or subshrub; H, herb/annual). Black dots indicate the crown age of diversification of the corresponding clade. The plot below the phylogeny shows the trend (smoothed lines) of the four variables over time (using the midpoint between the stem and crown age of each lineage). Note that recent lineages (mainly shrubs) tend to be in ecosystems with crown fires, with heat‐released physical dormancy, as opposed to the oldest lineages (trees) that lack hard seeds and are in non‐fireprone (rainforest) ecosystems or (more recently) those with surface fires (savannas) (except the monotypic Pakairamaea in South America savannas that is hard‐seeded). Heat rel. = heat released.
The smoke‐released dormancy syndrome shows a similar general pattern of evolution to the heat‐released dormancy syndrome (Fig. 6, Table S7). It may not be as ancient in origin as heat‐released dormancy, but there are no indications of smoke sensitivity arising from heat sensitivity: both appear separately and at multiple times from non‐dormant ancestors. Notable clades with smoke‐responsive ancestors include the proteoid Proteaceae, from 80 Ma (Sauquet et al., 2009; Lamont & He, 2012) and the graminoid Restionaeae, from 70 Ma (Brown & Botha, 2004; Litsios et al., 2014; He et al., 2016; Fig. 6), both in mediterranean South Africa; and the small graminoid Ecdeiocoleaceae, endemic to SW Australia (Poales), separating from its non‐fireprone sisters, Flagellaria–Joinvillea, at a stem age of 74 Ma (Fig. 6; Table S7). Another illuminating case is the Rutaceae (Fig. 8): the oldest lineages occur in non‐flammable rainforest and lack dormancy (Hopkins & Graham, 1987), in contrast to the younger lineages that inhabit sclerophyll vegetation subject to crown fires (forest to shrublands). These separated from Flindersia–Lunasia at 45–30 Ma which remained in rainforests; interestingly, Flindersia later migrated to the savannas of N Australia where its non‐dormancy has been retained. It is in these flammable habitats where the shrubby Boroniaeae and Eriostemon clades acquired smoke sensitivity. Note that the major forest genera Boronia and Zieria have maintained their smoke‐released dormancy even when in savannas.
Fig. 8.

Dated molecular phylogeny of the main genera in Rutaceae, subfamily Amyridoideae, that occur in Oceania (plus Vepris in Africa). For each genus, symbols indicate the current biome (sclerophyll woodlands and shrublands, savanna, rainforest) and associated fire regime (crown, surface, or no fires), seed dormancy, experimental evidence for smoke‐sensitive germination (at least for those species examined), and dispersal mode (vertebrate dispersal refers mainly to birds, but in some cases includes mammals, such as primates dispersing Vepris). Missing symbols indicate missing information. Note that it is unlikely that smoke‐germination experiments have been conducted on rainforest species as it is not fireprone; nevertheless, many of these are non‐dormant. Symbols with two colours indicate species inhabiting two habitats or with uncertainty. Undated nodes are arbitrarily located at the midpoint of the stem. Despite some phylogenetic uncertainties, it is clear that rainforest habitats and vertebrate dispersal are ancestral, while crown‐fire habitats and ant dispersal are derived. Phylogeny from Paetzold et al. (2018) and Bayly et al. (2013); smoke‐released dormancy and ant dispersal from Data S1; other dispersal modes from Lieberman et al. (1979), Bayly et al. (2013), Gould & Gabriel (2015), Appelhans et al. (2018) and Paetzold et al. (2018).
It is interesting that some of the fire‐associated lineages evolved elaiosomes about the same time as they acquired fire‐released dormancy [e.g. Pomaderreae (Rhamnaceae)] while others were much later [e.g. Acacia (Caesalpinioideae)] (Fig. 6). Elaiosomes appeared among two heat‐ and two smoke‐released clades at later dates (41–18 Ma), clearly making this trait advanced within lineages (Fig. 6), possibly as the fire regime became more intense over time, and supporting the primary role of ant dispersal as a fire‐persistence trait (Section II.3). C3 grasses evolved smoke‐released dormancy in the period 73.5–55 Ma (trait assignments have yet to be undertaken to increase precision on these dates; Fig. 6). Triodia in semiarid Australia is a C4 grass that seems to have independently acquired smoke‐released dormancy (Erickson, 2015). The genus arose in a savanna environment 22 Ma (Table S7); as the savanna turned semi‐arid, and thus less fireprone, seedling establishment became more reliant on the increasingly stochastic fire regime with greater reliance on seed dormancy.
(2). Dormancy changes under surface‐fire regimes
The expansion of surface‐fire regimes during the Neogene is well established (Scheiter et al., 2012). We noted above (Section III.2) that levels of fire‐released dormancy are lower under surface‐fire regimes, and there is evidence of some lineages losing fire‐released dormancy when moving to surface‐fire environments. This is illustrated by the C4 grass tribes, Andropogoneae and Paniceae, which lost smoke‐released dormancy on migrating to the summer‐wet grasslands, 22–17 Ma (Table S7). The seeds of Lupinus diffusus, arising in Florida pine savannas just 0.3 Ma, have remained hard, as is typical of the genus, but are not as heat‐tolerant as their forebears (Campbell‐Martínez et al., 2019). Mimosa (Fabaceae) is well represented in the South American Cerrado where it appeared only 4 Ma (Simon et al., 2009). Most mimosas here do not have heat‐released dormancy (Daibes et al., 2019) but this could be because their ancestors migrated directly from the Amazon rainforest (Simon et al., 2009), where heat‐released dormancy is absent, rather than losing this trait. However, this would not explain their high heat tolerances that must then have been acquired on entering the Cerrado. The non‐smoke‐released Salomonia (Polygalaceae) separated from the soil‐stored, ant‐dispersed Polygala on entering the savannas of N Australia and China from 37 Ma (Table S7).
(3). Loss of fire‐released dormancy in non‐fireprone ecosystems
The absence of fire‐released seed dormancy among species in clades where dormancy is otherwise present is a derived state. For instance, among hard‐seeded Cistaceae, the speciose Helianthemum contains examples retaining hard seeds that evolved <2 Ma but do not respond to fire‐type heat. These occur in non‐fireprone habitats, such as gypsum outcrops and salt‐lake margins, or remain fireprone but have become annuals and germinate under appropriate hydrothermal conditions independent of fire (Pérez‐García & González‐Benito, 2006; Copete et al., 2009; Aparicio et al., 2017; Figs 6, 7; Table S7). Within the Fabaceae, genera confined to non‐fireprone habitats may lack dormancy (in rainforest, Castanospermum is recalcitrant and Adenanthera evolved 11 Ma; in desert, Mariosousa evolved 7 Ma, Lupinus texensis germination is suppressed by wet heat; in saline river margins, Lupinus alba subsp. termis evolved 0.5 Ma). Non‐fire‐released dormancy in non‐fireprone ecosystems must rely on other mechanisms for breaking dormancy that deserve further study (Table 2). By contrast, Acacia (Fabaceae), with 1100 species, is renowned for its hard seeds almost completely impermeable to water but whose germination approaches 100% after immersion in boiling water (Bell, 1999; Burrows et al., 2018), and also possesses ant‐attracting elaiosomes. However, at least 11 species are soft‐seeded and lack elaiosomes; they are associated with deserts or open vegetation that rarely burns. Acacia originated 28 Ma from ancestors that were already hard‐seeded but lacked elaiosomes, whereas the A. oswaldii subclade, with vestigial non‐functional or no elaiosomes, arose only 6.4 Ma as central Australia became arid and less fireprone (Miller et al., 2013).
The few cases of ecotypic variation are especially instructive as they not only show evolution in action, but also confirm the functional link between fire regime and fire‐related traits. They indicate that some cost must be involved in maintaining these traits as they are lost readily in the absence of fire. For instance, populations of Erica coccinea (Ericaceae) occurring on rock outcrops (non‐flammable) in South Africa have non‐dormant seeds while their widespread parent populations in highly fireprone shrublands have smoke‐released dormancy (Leonard et al., 2018). Similarly, the heat‐released dormant seeds of Prunella vulgaris (Lamiaceae) in South American savanna grasslands lost their heat requirement on entering the rainforest (Godoy et al., 2011).
V. ALTERNATIVE DRIVERS
The main evolutionary pressures faced by plants can be grouped into (i) environmental factors, (ii) disturbance regimes, and (iii) biotic interactions (ecology in 3D; Pausas & Lamont, 2018). Thus, while the role of fire in seed dormancy is key, it would be naïve to suggest that it is always the only, or even the primary, driver of seed dormancy across the plant kingdom. At the global scale, different evolutionary pressures contribute to shaping variability in the dominance of a given trait (Finch‐Savage & Leubner‐Metzger, 2006; Donohue et al., 2010; Dalling et al., 2020). Here we consider alternative drivers to fire in fireprone ecosystems. In non‐fireprone ecosystems, the role of these alternative drivers may have a different ranking.
(1). Vegetation gaps of anthropogenic origin
Many fireprone landscapes are strongly urbanized, especially in the Mediterranean Basin where human occupancy is both high and has a long history. Under such conditions, areas cleared of vegetation are abundant (urban margins, roads, abandoned farmland). Within such gaps, a fraction of seeds of species with fire‐released dormancy can germinate and recruit successfully (Baeza & Roy, 2008). The higher summer temperatures due to a lack of tree cover or increased nutrient content due to household‐waste dumping and decomposition of excrement can stimulate dormancy‐release mechanisms. Recruitment is never as high as under postfire conditions (Figs 1, 2, 9, S7; Table S8) so that selection will still favour fire‐caused gaps but with an overall change towards lower dormancy‐breaking thresholds in the anthropogenic gaps. Given that fire was common in mediterranean ecosystems long before the humanization of these landscapes, dormancy release and germination in anthropogenic gaps is likely an exaptation developed from previous natural conditions (fire‐generated gaps).
Fig. 9.

Percentage germination of 68 populations or species subjected to simulated fire‐ and summer‐type (warm stratification) temperature conditions (C., Cistus; F., Fumana; U., Ulex; A., Acacia; M., Mimosa) throughout the world. Dotted line gives the 1:1 relationship, i.e. points above the line have higher germination levels after fire heat than after summer heat. In the key, the number before the taxon refers to the number of populations (for a species) or species (for a genus or family) corresponding to the number of points with that symbol; numbers in parentheses indicate the reference from which the data were obtained (see Table S8 for additional details). Note that all points at or below the line are for species in savannas [S]. 1, Moreira & Pausas (2012); 2, Luna (2020); 3, Ooi et al. (2014); 4, Newton et al. (2021); 5, Elliott, Fischer & LeRoy (2011); 6, Haines et al. (2007); 7, Karaguzel et al. (2004); Quinlivan (1968); 8, Tieu et al. (2001); 9, Hall et al. (2017); 10, Mbalo & Witkowski (1997); 11, Zupo, Baeza & Fidelis (2016); 12, Gorgone‐Barbosa et al. (2016); 13, Zhang et al. (2020).
Much depends on the extent of human‐driven modifications relative to the lability of the trait. For instance, cultivation reduces hard‐seededness in legumes, partly because fire is no longer present as a dormancy‐release mechanism and because soft‐seededness is a preferred trait for domestication (Maass, 2006; Huss & Gierlinger, 2021). Acacia mearnsii, native to SE Australia, does not germinate at all unless seeds receive a heat pulse from fire (Riveiro et al., 2020); however, it germinates well without any heat treatment in South African plantations (Kulkarni, Sparg & van Staden, 2007). Smoke‐released dormancy in Calluna vulgaris (N Europe) also varies with the history of anthropogenic fires in its various populations (Vandvik et al., 2014). Hence, recruitment in anthropogenic gaps among species otherwise considered to possess fire‐released dormancy is likely. These cases do not provide evidence against the primary role of fire in trait evolution but show how fire adaptations can change with shifts in the fire regime. Documenting such changing optimal conditions for dormancy release and germination at the local scale can provide basic information for conservation management (Baeza & Roy, 2008).
(2). Hot climates
It has been argued that temperature thresholds for heat‐released seed dormancy could have evolved as a response to high summer temperatures in some fireprone ecosystems (Ooi et al., 2014; Luna, 2020). In these cases, fire heat is regarded as simply an extreme form of summer heat, with dormancy breakage by fire viewed as an exaptation. However, the overwhelming evidence among crown‐fire species of much higher levels of germination, often by an order of magnitude, after fire‐type heat compared with summer‐type heat under experimental conditions (Figs 2, 9) demonstrates that it is a specific fire‐adapted trait.
That summer heat has little role in increasing fitness in crown‐fire forest and shrublands is shown by (i) massive recruitment only occurring following fire (Fig. 1); (ii) in the absence of fire, soil temperatures exceeding 70°C would not be reached on a regular basis; (iii) high summer temperatures only reach a small fraction of the seed bank (the few seeds lying on top of the soil); (iv) germination would occur every year even under hostile conditions for recruitment (such as a thick litter layer or strong competition from parents); and (v) the likelihood of incineration before reaching maturity would be high. By contrast, summer‐type heat in savanna ecosystems may be just as, or more, effective than fire heat. As noted in Section III, this is associated with lower levels of dormancy and alternative methods of breaking dormancy, such as wet summer heat (van Klinken et al., 2006).
Our collation of the available data indicates a strong association between species presence in ecosystems with intense crown fires, possession of hard seeds, and presence of heat‐released dormancy (Acacia, Lupinus, Cistaceae; Table 3). In the case of Cistaceae, warm stratification before fire has no enhancing effect on germination (Luna, 2020), and when applied early after fire, primary dormancy is probably restored in most seeds (Lamont, Burrows & Korczynskyj, 2022). As a consequence of the strong association between heat‐released dormancy and fire, the geographic distribution of species with such traits and those with opposing traits (in each clade) are quite different. For instance, Acacia species with seed dormancy occur mainly in the thickly wooded, highly fireprone regions within 500 km of the Australian coast, whereas those lacking dormancy occur in the warmer/drier inland regions with open shrublands where fires are rare and of low intensity (Fig. 10). In addition, species diversity is much greater in the former, as fire is a strong driver of speciation (He et al., 2019). Most Cistaceae occur in fireprone ecosystems, but those that lack dormancy are associated with microsites that do not burn (e.g. dry habitats with low cover such as gypsum soils; Pérez‐García & González‐Benito, 2006). Phylogenetic analysis shows that fire‐released dormancy is the ancestral condition in Cistaceae whereas non‐dormancy and non‐fire‐released dormancy are derived conditions (Fig. 7).
Fig. 10.

Distribution of Acacia species in Australia (A) with heat‐released dormancy with elaiosomes and (B) without heat‐released dormancy and lacking elaiosomes. Darker colours indicate a higher number of Acacia species. These maps show markedly different distributions, with the former species typically associated with forests and shrublands that burn with high fire intensity (SW and SE), and the latter associated with arid ecosystems and coastal dunes that rarely burn, and savannas that burn frequently at low intensity. This pattern would not be expected if high summer temperature was the driver of heat‐released dormancy. Species trait data from Data S1 (species with contradictory data among sources are omitted); species distribution from GBIF (doi: 10.15468/dl.vzyhj8).
The distribution of Aspalathus in South Africa is strongly associated with the most highly fireprone, mediterranean parts of the Cape (Cowling et al., 2018): its diversity peaks at the highest values of two fire indicators for the region: Normalized Difference Vegetation Index (NDVI; i.e. greatest biomass, and thus highest fire intensity) and rainfall seasonality (fireproneness), and not under the hottest/driest climates. All Leucadendron species occur in the fireprone fynbos of the Cape region and not under the less‐fireprone, hotter/drier karoo to the north‐east or the hotter/wetter forests to the east. Most species are serotinous; however, those that are not serotinous have relatively thick‐coated seeds with germination stimulated by smoke (Newton et al., 2021; Table 3). Rhamnaceae is another family with many species in the world's mediterranean ecosystems (Onstein & Linder, 2016). It includes species with fleshy fruits dispersed by vertebrates, such as Ziziphus and Rhamnus, and species with dry seeds, such as Pomaderris and Ceanothus. Fleshy‐fruited plants do not form a persistent soil seed bank and their germination is not tied to fire. For the dry‐fruited Rhamnaceae, there is a strong tendency for those inhabiting fireprone ecosystems to have heat‐released germination (Table 3). Thus, results for all clades considered here (Table 3) are consistent with the expectation that high‐intensity fires are the principal driver of heat‐released dormancy.
The fact that hot climates are not the prime driver of fire‐released dormancy does not mean that temperature has no role in dormancy release. Summer temperatures are likely to be a driver for increasing dormancy‐breaking heat thresholds at temperatures higher than the historical summer temperatures (Zomer et al., 2022), so they are relevant in an evolutionary sense. In hot arid ecosystems, physical seed dormancy may be adaptive to survive the hot, dry periods but with germination cued instead by sporadic rains following physical deterioration of the seed coat (Daws et al., 2007); here, heat‐released dormancy would be a clear disadvantage.
Species whose germination is promoted by smoke are usually excluded from any discussion on the role of summer heat in breaking dormancy. However, under highly seasonal climates, a bout of warmth (summer–autumn) might herald the onset of cool, moist conditions (winter) suitable for germination (Appendix S2, Table S9). In the absence of fire, such annual dormancy‐breaking occurrences would be maladapative (except for annuals; Baker et al., 2005). But, in association with fire, this dual environmental stimulus might prove to be a ‘fail‐safe’ mechanism for promoting successful recruitment in fire‐generated gaps. Thus, for fire ephemeral shrubs, Baker et al. (2005) only obtained significant germination for seeds soil‐stored over summer and treated later with smoke water. Warm stratification responses of dormant seeds in association with smoke or heat, including additive effects, have also been reported among other species in Australia (Merritt et al., 2007; Hodges et al., 2019).
(3). Cold winters
Many fireprone ecosystems may experience near‐freezing temperatures at some point during the year. Since attempts to germinate at low temperatures are likely to lead to frost damage and death, cold stratification that heralds the end of winter and the onset of warmer growing conditions could be a driver of dormancy release. Most mediterranean species do not show any significant effect of cold stratification [Cistaceae (Luna et al., 2008; Tavşanoğlu, 2011); some Ceanothus (Huffman, 2006)]. Since cold stratification is a metabolic process, it requires seeds to be kept moist; since hard seeds are impermeable it might explain why these two traits are rarely coupled. However, in some regions with cold winters that are also fireprone in the warm part of the year, some species can be expected to benefit from cold stratification to yield maximum levels of dormancy release following fire. Such additive benefits were observed among the 10 species we collated that received both heat and cold stratification treatments (Figs S3, S4).
(4). Plant–animal relations
Biotic interactions are a major evolutionary pressure on plants, with the seeds of many species consumed by insects, birds, or mammals. We argue herein that ant‐attracting elaiosomes are likely to be an adaptation for heat protection of the seed. For vertebrates, physical dormancy may be adaptive for resisting gut passage [endozoochory (Malo & Suárez, 1996; Jaganathan, Yule & Liu, 2016)], which may have contributed to its evolution in some species. But this seems unlikely to have had a key role in fireprone ecosystems, as the great advantages provided by large postfire gaps that enable massive recruitment are unmatched as the prime selective agent. In addition, plants dispersed via endozoochores tend to have fleshy fruits with weakly dormant seeds (especially in non‐fireprone habitats, such as rainforests), whereas most plants with strong dormancy have small dry fruits/seeds that are not dispersed by vertebrates and are characteristic of fireprone habitats (Table 3).
Counter‐intuitively, it may be the case that endozoochory is the derived condition in some clades, having evolved from fireprone ancestors. Thus, bird dispersal associated with large, brightly coloured arils is a recent development among fireprone ancestors in Acacia (A. cyclops, with hard seeds in rarely burnt coastal dunes), Polygalaceae (Polygala arillata, confined to Asian rainforests, is terminal among polygalas) and in Rutaceae (Acronychia/Melicope in Pacific rainforests; Fig. 8).
(5). Fire‐released dormancy in non‐fireprone ecosystems
When we examine traits related to fire‐released seed dormancy across different ecosystems worldwide, rainforests and deserts rank by far the lowest (Fig. 3C). However, there are cases of a positive effect of fire (heat or smoke) on germination in species that are not subject to fire under natural conditions. This has raised questions about the critical role of fire in shaping dormancy. Despite such experiments lacking ecological realism, they do not negate the arguments for a primary role for fire in promoting seed dormancy in other contexts. Fire may break dormancy in some seeds that have evolved dormancy under evolutionary frameworks unrelated to fire. For instance, cold non‐fireprone environments may possess some species with hard seeds whose dormancy can also be experimentally broken by heat [northern Japan (Tsuyuzaki & Miyoshi, 2009); central Asia cold desert (Long et al., 2012)]. This is because the mechanisms for breaking dormancy by cold or heat are much the same (fire mimicking; Lamont & He, 2017a) – any extreme temperatures can cause fracture of seed coat tissues and opening of water gaps (Brits & Manning, 2019). Dormancy under such inhospitable environments is foremost a way of maximizing seed longevity (bet‐hedging; Table 2), and dormancy release can also be induced by seed coats deteriorating over time or from natural scarification (Long et al., 2012).
In addition, some species currently in non‐fireprone ecosystems have descended from ancestors that inhabited fireprone ecosystems (Figs 7, 8; Lamont & He, 2017a), and may simply have retained their fire‐adapted traits (stabilization sensu Lamont et al., 2019a). For instance, we reviewed smoke responses for 52 rainforest species (Fig. 3C, Table S10), and found only one (Jacaranda copaia, Bignoniaceae) in the Amazon Basin that shows smoke‐released dormancy (we dismissed positive records for three hard‐seeded species that were scarified first by the researchers). This species has wide environmental tolerances and is also common in the fireprone monsoon forests of Central America (Scotti‐Saintagne et al., 2013). Since the authors (Ferraz, Arruda & Van Staden, 2013) did not state where their seeds were collected, it is possible that even this positive record may not be accurate. In addition, rainforests and savannas have waxed and waned in extent over the last 10 million years at least, so that this kind of ecological mismatch is not unexpected (Simon et al., 2009; Pausas & Bond, 2020). Of the 52 species in 36 families present in rainforests for which we have data on seed smoke pretreatments, at least 28 families contain species outside these rainforests that possess smoke‐released seed dormancy (the others are hard‐seeded or are unexamined; Table S10). While this does not guarantee that rainforest species have a genetic predisposition to adopt smoke‐released dormancy, it greatly increases the likelihood that they could do so.
Overall, examples of fire‐released dormancy in non‐fireprone ecosystems are rare, with germination occurring in response to other stimuli, or are for species that may not be confined to non‐fireprone habitats, and/or are likely to have evolved relatively recently from fireprone ancestors. Thus we consider that they contribute little to understanding the ecology of fire‐related seed dormancy.
VI. CONCLUSIONS
Seed dormancy is most adaptive in fireprone ecosystems where fire acts as a mechanism for releasing dormancy and heralding the creation of a postfire environment that is optimal for germination and seedling survival.
In fireprone ecosystems, four dormancy syndromes can be recognized: heat‐released physical dormancy, smoke‐released physiological dormancy, non‐fire‐released dormancy, and non‐dormancy. The first two (fire‐released dormancy) are adaptive to match germination with the optimal conditions for establishment; the third is a bet‐hedging strategy; and the fourth is a strategy for rapid germination (e.g. granivore avoidance). The distribution of these different dormancy syndromes across the ‘tree of life’ is not random – certain dormancy types are linked to particular lineages (phylogenetic conservatism), as well as vegetation types, climates, and fire regimes.
Species with fire‐released dormancy tend to be shade‐intolerant and favoured by vegetation gaps, have dry seeds/fruits that are small and dispersed over short distances, and are most prominent among evergreen shrubs (especially non‐resprouters), herbs and grasses in fireprone ecosystems.
Dormancy syndromes vary with the fire regime. Fire‐released dormancy is especially prominent where woody vegetation is dense and fires are intense, i.e. crown‐fire regimes. In such environments, fire‐released dormancy allows shade‐intolerant species to detect vegetation gaps and to match germination with the optimal recruitment conditions created by fire. In grassy fireprone ecosystems (savannas, surface‐fire regimes), where fires are less intense but more frequent, seed heat tolerance may be maintained but dormancy is less common and dormancy release is often unrelated to fire (non‐fire‐released dormancy). Ecosystems where fires are uncommon (rainforest, deserts) may also have species with dormant seeds, but fire‐released dormancy is irrelevant.
In many lineages, there is a close association between species possessing elaiosomes and inhabiting a fireprone ecosystem. Elaiosomes provide a mechanism for seed burial that protects the seed from the full heat of a fire and optimizes the chances of dormancy release. However, more research is needed to understand this trait fully and its relationship with other evolutionary pressures, such as seed predation.
Heat‐ and smoke‐released dormancy syndromes originated many tens of millions of years ago when lineages first became subject to fires, and the subsequent gains and losses of fire‐released dormancy can be traced to changes in the fire regime from more intense, then more frequent but less intense, and finally to fire‐free.
There are numerous lines of evidence that support fire regimes as the key driver of the evolution of seed dormancy and its release. Climate (hot summers, cold winters), plant–animal interactions, and more recently, anthropogenic intervention, have at best played modifying roles in these fireprone ecosystems.
Supporting information
Table S1. Dormancy‐release syndromes present in fireprone ecosystems, and their ecological properties.
Appendix S1. Taxonomic patterns of fire‐released dormancy.
Fig. S1. Summary of our collation of fire responses among seven families with at least some fire‐responsive members (totalling 1155 species worldwide).
Fig. S2. Illustrations of six categories of dormancy‐breaking responses that can be recognized among Fabaceae.
Fig. S3. Summary of interaction between cold stratification and fire‐related treatments for five species (Ceanothus americanus, Ceanothus jepsonii, Discaria pubescens, Lupinus sulphureus, Silphium terebinthenaceum) in individual studies treated with heat and 13 populations of Eschscholzia californica treated with smoke.
Fig. S4. Interaction between cold stratification and fire‐related cues in the germination of ten species with hard seeds in fireprone ecosystems, and 13 populations of Eschscholzia californica.
Table S2. Germination results for smoke applied to Poaceae species in various ecosystems.
Table S3. Effect of smoke on the germination of Lamiaceae species aggregated into two environments: savanna (tropical and subtropical savannas and shortgrass prairie) and shrublands of the Mediterranean Basin.
Table S4. Summary of germination experiments with smoke in different ecosystem types.
Fig. S5. Illustrations of three categories of smoke‐released dormancy/germination responses that can be recognized in various ecosystems worldwide.
Fig. S6. Interactions between smoke and light on seed germination.
Fig. S7. Effect of smoke on soil seed banks in different crown‐fire ecosystems.
Fig. S8. Summary of data in Table S5 for Fabaceae on a % species per environment and fire‐regime basis.
Table S5. Viability and germination characteristics of the Fabaceae in four major biome types throughout the world, and their surface or crown fire regimes, based on the six categories in Fig. S2 plus an additional three categories (C1,2,3) with low viability and germination.
Fig. S9. Soil temperatures during experimental fires.
Fig. S10. Transect through a recently burnt sclerophyll shrubland in California.
Fig. S11. Percentage of species with release from dormancy stimulated, inhibited, or unaffected by heat and/or smoke in four environment categories.
Table S6. Descriptions of biogeographic regions used in Fig. S11.
Table S7. Changes in seed traits through evolutionary time in response to changes in climate and fire regime.
Table S8. List of references used to construct Fig. 9, with additional information.
Appendix S2. Timing of germination (seasonality effects).
Table S9. Summary of seasonal aspects of maintaining and breaking seed dormancy in three major biome types with contrasting fire regimes over 30 months, with one fire in crown‐fire ecosystems and two fires in surface‐fire ecosystems.
Table S10. Smoke‐stimulated germination in rainforest species.
Data S1. Available at https://doi.org/10.6084/m9.figshare.19126823.v4
VII. ACKNOWLEDGEMENTS
J.G.P.s contribution to this study was performed within the framework of projects FocScales (PROMETEO/2021/040; Generalitat Valenciana) and FIROTIC (PGC2018‐096569‐B‐I00; Spanish government); B.B.L.s contribution was privately supported. We thank Dylan Korczynskyj, Tianhua He, Gert Brits, Justin Collette, Mark Ooi and Mike Lawes for comments and assistance, and Alison Cooper for a critical editing of this long article.
REFERENCES
References identified with an asterisk (*) are cited only in the supporting information.
- * Abella, S. R. A. R. , Springer, J. D. S. D. & Covington, W. W. C. W. (2007). Seed banks of an Arizona Pinus ponderosa landscape: responses to environmental gradients and fire cues. Canadian Journal of Forest Research 37, 552–567. [Google Scholar]
- * Abu, Y. , Romo, J. T. , Bai, Y. & Coulman, B. (2016). Priming seeds in aqueous smoke solutions to improve seed germination and biomass production of perennial forage species. Canadian Journal of Plant Science 96, 551–563. [Google Scholar]
- * Adkins, S. W. & Peters, N. C. B. (2001). Smoke derived from burnt vegetation stimulates germination of arable weeds. Seed Science Research 11, 213–222. [Google Scholar]
- * Afolayan, A. J. , Meyer, J. J. M. & Leeuwner, D. V. (1997). Germination in Helichrysum aureonitens (Asteraceae): effects of temperature, light, gibberellic acid, scarification and smoke extract. South African Journal of Botany 63, 22–24. [Google Scholar]
- * Agboola, D. , Ebofin, A. , Aduradola, A. & Ajiboye, A. (2005). The effect of presowing treatments on the germination of seeds of two savannah tree legumes. Indian Forester 131, 701–710. [Google Scholar]
- Aparicio, A. , Martín‐Hernanz, S. , Parejo‐Farnés, C. , Arroyo, J. , Yeşilyurt, E. B. , Zhang, M.‐L. , Rubio, E. & Albaladejo, R. G. (2017). Phylogenetic reconstruction of the genus Helianthemum (Cistaceae) using plastid and nuclear DNA‐sequences: systematic and evolutionary inferences. Taxon 66, 868–885. [Google Scholar]
- * Appanah, S. & Turnbull, J. M. (1998). A Review of Dipterocarps: Taxonomy, Ecology, and Silviculture. Jakarta, Indonesia: CIFOR. [Google Scholar]
- Appelhans, M. S. , Wen, J. , Duretto, M. , Crayn, D. & Wagner, W. L. (2018). Historical biogeography of Melicope (Rutaceae) and its close relatives with a special emphasis on Pacific dispersals. Journal of Systematics and Evolution 56, 576–599. [Google Scholar]
- * Arcamone, J. R. & Jaureguiberry, P. (2018). Germination response of common annual and perennial forbs to heat shock and smoke treatments in the Chaco Serrano, central Argentina. Austral Ecology 43, 567–577. [Google Scholar]
- * Auld, T. D. (1986). Population dynamics of the shrub Acacia suaveolens (Sm.) Willd.: fire and the transition to seedlings. Australian Journal of Ecology 11, 373–385. [Google Scholar]
- Auld, T. D. (1996). Ecology of the Fabaceae in the Sydney region: fire, ants and the soil seedbank. Cunninghamia 4, 531–551. [Google Scholar]
- Auld, T. D. & Bradstock, R. A. (1996). Soil temperatures after the passage of a fire: do they influence the germination of buried seeds? Australian Journal of Ecology 21, 106–109. [Google Scholar]
- Auld, T. D. & Denham, A. J. (2005). A technique to estimate the pre‐fire depth of burial of Grevillea seeds by using seedlings after fire. Australian Journal of Botany 53, 401–405. [Google Scholar]
- * Auld, T. D. , Denham, A. J. & Turner, K. (2007). Dispersal and recruitment dynamics in the fleshy‐fruited Persoonia lanceolata (Proteaceae). Journal of Vegetation Science 18, 903–910. [Google Scholar]
- Auld, T. D. & O'Connell, M. A. (1991). Predicting patterns of post‐fire germination in 35 eastern Australian Fabaceae. Australian Journal of Ecology 16, 53–70. [Google Scholar]
- Baes, P. O. , de Viana, M. L. & Sühring, S. (2002). Germination in Prosopis ferox seeds: effects of mechanical, chemical and biological scarificators. Journal of Arid Environments 50, 185–189. [Google Scholar]
- Baeza, J. & Roy, J. (2008). Germination of an obligate seeder (Ulex parviflorus) and consequences for wildfire management. Forest Ecology and Management 256, 685–693. [Google Scholar]
- Baker, K. S. , Steadman, K. J. , Plummer, J. A. , Merritt, D. J. & Dixon, K. W. (2005). Dormancy release in Australian fire ephemeral seeds during burial increases germination response to smoke water or heat. Seed Science Research 15, 339–348. [Google Scholar]
- * Baldwin, I. T. & Morse, L. (1994). Up in smoke: II. Germination of Nicotiana attenuata in response to smoke‐derived cues and nutrients in burned and unburned soils. Journal of Chemical Ecology 20, 2373–2391. [DOI] [PubMed] [Google Scholar]
- Baskin, C. C. & Baskin, J. M. (2014). Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd Edition . Amsterdam: Elsevier. [Google Scholar]
- * Baskin, C. C. , Baskin, J. M. , Yoshinaga, A. , Cordell, S. , Drake, D. , Gleason, S. & Welton, P. (2004). Seed Germination Ecology of Hawaiian Montane Species: A Continuation of Efforts to Acquire, Organize, and Share Data to Facilitate Propagation and Restoration Efforts. Hawaii Conservation Alliance, Honolulu. https://laukahi.org/wpcontent/uploads/2021/06/Baskin_HCA_update_2004_10.pdf. [Google Scholar]
- Baskin, J. M. & Baskin, C. C. (1984). Environmental conditions required for germination of prickly sida (Sida spinosa). Weed Science 32, 786–791. [Google Scholar]
- Baskin, J. M. & Baskin, C. C. (2021). The great diversity in kinds of seed dormancy: a revision of the Nikolaeva–Baskin classification system for primary seed dormancy. Seed Science Research 31, 249–277. [Google Scholar]
- Baskin, J. M. , Baskin, C. C. & Li, X. (2000). Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology 15, 139–152. [Google Scholar]
- * Bautista‐Rodríguez, E. I. , del Lagunes‐Espinoza, L. C. , Lara‐Viveros, F. M. , Castelán‐Estrada, M. , Conde‐Martínez, V. , Bautista‐Rodríguez, E. I. , del Lagunes‐Espinoza, L. C. , Lara‐Viveros, F. M. , Castelán‐Estrada, M. & Conde‐Martínez, V. (2017). Comparison of pre‐germination treatments in Lupinus spp. and their effects on germination and related solutes. Botanical Sciences 95, 577–590. [Google Scholar]
- Bayly, M. J. , Holmes, G. D. , Forster, P. I. , Cantrill, D. J. & Ladiges, P. Y. (2013). Major clades of Australasian Rutoideae (Rutaceae) based on rbcL and atpB sequences. PLoS One 8, e72493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, D. T. (1999). The process of germination in Australian species. Australian Journal of Botany 47, 475–517. [Google Scholar]
- Bell, D. T. , Plummer, J. A. & Taylor, S. K. (1993). Seed germination ecology in southwestern Western Australia. The Botanical Review 59, 24–73. [Google Scholar]
- Berg, R. Y. (1975). Myrmecochorous plants in Australia and their dispersal by ants. Australian Journal of Botany 23, 475–508. [Google Scholar]
- Blank, R. R. & Young, J. A. (1998). Heated substrate and smoke: influence on seed emergence and plant growth. Rangeland Ecology & Management 51, 577–583. [Google Scholar]
- * Bouchenak‐Khelladi, Y. , Maurin, O. , Hurter, J. & van der Bank, M. (2010). The evolutionary history and biogeography of Mimosoideae (Leguminosae): an emphasis on African acacias. Molecular Phylogenetics and Evolution 57, 495–508. [DOI] [PubMed] [Google Scholar]
- * Bradstock, R. A. & Auld, T. D. (1995). Soil temperature during experimental bushfire in relation to fire intensity: consequences for legume germination and fire management in South‐eastern Australia. Journal of Applied Ecology 32, 76–84. [Google Scholar]
- * Bradstock, R. A. , Auld, T. D. , Ellis, M. E. & Cohn, J. S. (1992). Soil temperatures during bushfires in semi‐arid, mallee shrublands. Australian Journal of Ecology 17, 433–440. [Google Scholar]
- Brits, G. J. (1986). Influence of fluctuating temperatures and H2O2 treatment on germination of Leucospermum cordifolium and Serruina florida (Proteaceae) seeds. South African Journal of Botany 52, 286–290. [Google Scholar]
- Brits, G. J. & Manning, J. C. (2019). Seed structure and physiology in relation to recruitment ecology in Leucospermum (Proteaceae) in fynbos. Australian Journal of Botany 67, 290–308. [Google Scholar]
- Brown, N. A. C. & Botha, P. A. (2004). Smoke seed germination studies and a guide to seed propagation of plants from the major families of the Cape Floristic Region, South Africa. South African Journal of Botany 70, 559–581. [Google Scholar]
- * Brown, N. A. C. , Jamieson, H. & Botha, P. A. (1994). Stimulation of germination in South African species of Restionaceae by plant‐derived smoke. Plant Growth Regulation 15, 93–100. [Google Scholar]
- * Brown, N. A. C. & van Staden, J. (1997). Smoke as a germination cue: a review. Plant Growth Regulation 22, 115–124. [Google Scholar]
- Burrows, G. E. , Alden, R. & Robinson, W. A. (2018). The lens in focus – lens structure in seeds of 51 Australian Acacia species and its implications for imbibition and germination. Australian Journal of Botany 66, 398–413. [Google Scholar]
- Campbell‐Martínez, G. , Thetford, M. , Miller, D. L. & Pérez, H. E. (2019). Seedling emergence of Lupinus diffusus in response to abrasion in an electric seed scarifier. Native Plants Journal 20, 14–24. [Google Scholar]
- Carthey, A. J. R. , Tims, A. , Geedicke, I. & Leishman, M. R. (2018). Broadscale patterns in smoke‐responsive germination from the south‐eastern Australian flora. Journal of Vegetation Science 29, 737–745. [Google Scholar]
- * Çatav, S. S. , Küçükakyüz, K. , Akbaş, K. & Tavşanoğlu, Ç. (2014). Smoke‐enhanced seed germination in Mediterranean Lamiaceae. Seed Science Research 24, 257–264. [Google Scholar]
- * Chamorro, D. & Moreno, J. M. (2019). Effects of water stress and smoke on germination of Mediterranean shrubs with hard or soft coat seeds. Plant Ecology 220, 511–521. [Google Scholar]
- Chou, Y.‐F. , Cox, R. D. & Wester, D. B. (2012). Smoke water and heat shock influence germination of shortgrass prairie species. Rangeland Ecology & Management 65, 260–267. [Google Scholar]
- Christian, C. E. & Stanton, M. L. (2004). Cryptic consequences of a dispersal mutualism: seed burial, elaiosome removal, and seed‐bank dynamics. Ecology 85, 1101–1110. [Google Scholar]
- * Clark, D.L. & Wilson, M.V. (2000). Promoting regeneration of native species In Willamette Valley upland prairies. In p. 20 pp. Technical Report, U.S. Fish and Wildlife Service.
- * Clarke, P. J. , Davison, E. A. & Fulloon, L. (2000). Germination and dormancy of grassy woodland and forest species: effects of smoke, heat, darkness and cold. Australian Journal of Botany 48, 687–699. [Google Scholar]
- * Clarke, S. & French, K. (2005). Germination response to heat and smoke of 22 Poaceae species from grassy woodlands. Australian Journal of Botany 53, 445–454. [Google Scholar]
- * Coates, F. (1996). Ecological and biogeographical correlates of rarity in two narrow endemics in Tasmania: Spyridium microphyllum (F. Muell. ex Reisseck) Druce and Spyridium obcordatum (Hook. f.) W.M. Curtis. Ph.D. Theses, University of Tasmania, Hobart.
- * Cochrane, A. , Brown, K. , Cunneen, S. & Kelly, A. (2001). Variation in seed production and germination in 22 rare and threatened Western Australian Verticordia (Myrtaceae). Journal of the Royal Society of Western Australia 84, 103. [Google Scholar]
- * Collette, J. C. & Ooi, M. K. J. (2017). Germination ecology of the endangered species Asterolasia buxifolia (Rutaceae): smoke response depends on season and light. Australian Journal of Botany 65, 283–291. [Google Scholar]
- Collette, J. C. & Ooi, M. K. J. (2021). Distribution of seed dormancy classes across a fire‐prone continent: effects of rainfall seasonality and temperature. Annals of Botany 127, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine, M. J. & Considine, J. A. (2016). On the language and physiology of dormancy and quiescence in plants. Journal of Experimental Botany 67, 3189–3203. [DOI] [PubMed] [Google Scholar]
- * Cook, L. G. , Hardy, N. B. & Crisp, M. D. (2015). Three explanations for biodiversity hotspots: small range size, geographical overlap and time for species accumulation. An Australian case study. New Phytologist 207, 390–400. [DOI] [PubMed] [Google Scholar]
- Copete, M. A. , Ferrandis, P. , Martínez‐Duro, E. , Herranz, J. M. , Domínguez, F. & Albert, M. J. (2009). Helianthemum polygonoides Peinado, Mart. Parras, Alcaraz and Espuelas. In Poblaciones en peligro: viabilidad demográfica de la flora vascular amenazada de España. Populations in Peril: Demographic Viability of Threatened Spanish Vascular Flora (eds Iriondo J., Albert M. J., Giménez‐Benavides L., Domínguez‐Lozano F. and Escudero A.), pp. 93–96. Madrid: Ministerio de Medio Ambiente, y Medio Rural y Marino. [Google Scholar]
- Cowling, R. M. , Gallien, L. , Richardson, D. M. & Ojeda, F. (2018). What predicts the richness of seeder and resprouter species in fire‐prone Cape fynbos: rainfall reliability or vegetation density? Austral Ecology 43, 614–622. [Google Scholar]
- * Crisp, M. D. , Cayzer, L. , Chandler, G. T. & Cook, L. G. (2017). A monograph of Daviesia (Mirbelieae, Faboideae, Fabaceae). Phytotaxa 300, 1–308. [Google Scholar]
- Crosti, R. , Ladd, P. G. , Dixon, K. W. & Piotto, B. (2006). Post‐fire germination: the effect of smoke on seeds of selected species from the central Mediterranean basin. Forest Ecology and Management 221, 306–312. [Google Scholar]
- * Cuello, N. , López‐Mársico, L. & Rodríguez, C. (2020). Field burn versus fire‐related cues: germination from the soil seed bank of a South American temperate grassland. Seed Science Research 30, 206–2014. [Google Scholar]
- Daibes, L. F. , Pausas, J. G. , Bonani, N. , Nunes, J. , Silveira, F. A. O. & Fidelis, A. (2019). Fire and legume germination in a tropical savanna: ecological and historical factors. Annals of Botany 123, 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daibes, L. F. , Zupo, T. , Silveira, F. A. O. & Fidelis, A. (2017). A field perspective on effects of fire and temperature fluctuation on Cerrado legume seeds. Seed Science Research 27, 74–83. [Google Scholar]
- Dalling, J. W. , Davis, A. S. , Arnold, A. E. , Sarmiento, C. & Zalamea, P.‐C. (2020). Extending plant defense theory to seeds. Annual Review of Ecology, Evolution, and Systematics 51, 123–141. [Google Scholar]
- * Davis, T. D. , George, S. W. , Upadhyaya, A. & Persons, J. (1991). Improvement of seedling emergence of Lupinus texensis Hook. following seed scarification treatments. Journal of Environmental Horticulture 9, 17–21. [Google Scholar]
- Daws, M. I. , Davies, J. , Pritchard, H. W. , Brown, N. A. C. & van Staden, J. (2007). Butenolide from plant‐derived smoke enhances germination and seedling growth of arable weed species. Plant Growth Regulation 51, 73–82. [Google Scholar]
- * Dayamba, S. D. , Sawadogo, L. , Tigabu, M. , Savadogo, P. , Zida, D. , Tiveau, D. & Oden, P. C. (2010). Effects of aqueous smoke solutions and heat on seed germination of herbaceous species of the Sudanian savanna‐woodland in Burkina Faso. Flora 205, 319–325. [Google Scholar]
- * Dayamba, S. D. , Tigabu, M. , Sawadogo, L. & Oden, P. C. (2008). Seed germination of herbaceous and woody species of the Sudanian savanna‐woodland in response to heat shock and smoke. Forest Ecology and Management 256, 462–470. [Google Scholar]
- * Debouck, D. G. , Toro, O. , Paredes, O. M. , Johnson, W. C. & Gepts, P. (1993). Genetic diversity and ecological distribution of Phaseolus vulgaris (Fabaceae) in northwestern South America. Economic Botany 47, 408–423. [Google Scholar]
- Diabate, M. , Munive, A. , Faria, S. M. D. , Ba, A. , Dreyfus, B. & Galiana, A. (2005). Occurrence of nodulation in unexplored leguminous trees native to the West African tropical rainforest and inoculation response of native species useful in reforestation. New Phytologist 166, 231–239. [DOI] [PubMed] [Google Scholar]
- * Dittus, D. A. & Muir, J. P. (2010). Breaking germination dormancy of Texas native perennial herbaceous legumes. Native Plants Journal 11, 5–10. [Google Scholar]
- * Dixon, K. W. , Roche, S. & Pate, J. S. (1995). The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 101, 185–192. [DOI] [PubMed] [Google Scholar]
- Donohue, K. , Rubio de Casas, R. , Burghardt, L. , Kovach, K. & Willis, C. G. (2010). Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics 41, 293–319. [Google Scholar]
- Downes, K. S. , Light, M. E. , Pošta, M. & van Staden, J. (2015). Fire‐related cues and the germination of eight Conostylis (Haemodoraceae) taxa, when freshly collected, after burial and after laboratory storage. Seed Science Research 25, 286–298. [Google Scholar]
- * Eastwood, R. J. , Drummond, C. S. , Schifino‐Wittmann, M. T. & Hughes, C. E. (2008). Diversity and evolutionary history of lupins–insights from new phylogenies. In Lupins for Health and Wealth (eds Palta J. A. and Berger J. B.), pp. 346–354. International Lupin Association, Canterbury. [Google Scholar]
- * Edwards, E. J. , Osborne, C. P. , Strömberg, C. A. E. , Smith, S. A. & C 4 Grasses Consortium (2010). The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591. [DOI] [PubMed] [Google Scholar]
- * Elliott, C. W. , Fischer, D. G. & LeRoy, C. J. (2011). Germination of three native Lupinus species in response to temperature. Northwest Science 85, 403–410. [Google Scholar]
- * Ely, C. (2016). Smoking Grass: Germination responses of six native Poaceae species to smoke water treatments. Master Thesis, Evergreen State College, USA.
- * Enright, N. J. , Goldblum, D. , Ata, P. & Ashton, D. H. (1997). The independent effects of heat, smoke and ash on emergence of seedlings from the soil seed bank of a heathy eucalyptus woodland in Grampians (Gariwerd) National Park, western Victoria. Australian Journal of Ecology 22, 81–88. [Google Scholar]
- * Enright, N. J. & Kintrup, A. (2001). Effects of smoke, heat and charred wood on the germination of dormant soil‐stored seeds from a Eucalyptus baxteri heathy‐woodland in Victoria, SE Australia. Austral Ecology 26, 132–141. [Google Scholar]
- * Enright, N. J. & Lamont, B. B. (1989). Seed banks, fire season, safe sites and seedling recruitment in five co‐occurring Banksia species. Journal of Ecology 77, 1111–1122. [Google Scholar]
- Erickson, T.E. (2015). Seed dormancy and germination traits of 89 arid zone species targeted for mine‐site restoration in the Pilbara region of Western Australia. PhD Thesis, University of Western Australia.
- Fenner, M. & Thompson, K. (2005). The Ecology of Seeds. Cambridge University Press, Cambridge. [Google Scholar]
- Fernandes, A. F. , Oki, Y. , Fernandes, G. W. & Moreira, B. (2020). The effect of fire on seed germination of campo rupestre species in the South American Cerrado. Plant Ecology 222, 45–55. [Google Scholar]
- Ferraz, I. D. K. , Arruda, Y. M. B. C. & Van Staden, J. (2013). Smoke‐water effect on the germination of Amazonian tree species. South African Journal of Botany 87, 122–128. [Google Scholar]
- * Fichino, B. S. , Dombroski, J. R. G. , Pivello, V. R. & Fidelis, A. (2016). Does fire trigger seed germination in the neotropical savannas? Experimental tests with six cerrado species. Biotropica 48, 181–187. [Google Scholar]
- Figueroa, J. A. (2003). Seed germination in temperate rain forest species of southern Chile: chilling and gap‐dependency germination. Plant Ecology 166, 227–240. [Google Scholar]
- * Figueroa, J. A. & Cavieres, L. A. (2012). The effect of heat and smoke on the emergence of exotic and native seedlings in a Mediterranean fire‐free matorral of central Chile. Revista Chilena de Historia Natural 85, 101–111. [Google Scholar]
- * Figueroa, J. A. , Cavieres, L. A. , Gómez‐González, S. , Montenegro, M. M. & Jaksic, F. M. (2009). Do heat and smoke increase emergence of exotic and native plants in the matorral of central Chile? Acta Oecologica 35, 335–340. [Google Scholar]
- Finch‐Savage, W. & Leubner‐Metzger, G. (2006). Seed dormancy and the control of germination. New Phytologist 171, 501–523. [DOI] [PubMed] [Google Scholar]
- Fontenele, H. G. V. , Cruz‐Lima, L. F. S. , Pacheco‐Filho, J. L. & Miranda, H. S. (2020). Burning grasses, poor seeds: post‐fire reproduction of early‐flowering Neotropical savanna grasses produces low‐quality seeds. Plant Ecology 221, 1265–1274. [Google Scholar]
- * Forest, F. , Chase, M. W. , Persson, C. , Crane, P. R. & Hawkins, J. A. (2007). The role of biotic and abiotic factors in evolution of ant dispersal in the milkwort family (Polygalaceae). Evolution 61, 1675–1694. [DOI] [PubMed] [Google Scholar]
- Friis, E. M. , Crane, P. R. , Pedersen, K. R. , Stampanoni, M. & Marone, F. (2015). Exceptional preservation of tiny embryos documents seed dormancy in early angiosperms. Nature 528, 551–554. [DOI] [PubMed] [Google Scholar]
- Galindez, G. , Ceccato, D. V. , Malagrina, G. M. , Pidal, B. , Chilo, G. N. , Bach, H. G. , Fortunato, R. H. & Ortega Baes, F. P. (2016). Physical seed dormancy in native legume species of Argentina. Boletín de la Sociedad Argentina de Botánica 51, 73–78. [Google Scholar]
- Gama‐Arachchige, N. S. , Baskin, J. M. , Geneve, R. L. & Baskin, C. C. (2013). Identification and characterization of ten new water gaps in seeds and fruits with physical dormancy and classification of water‐gap complexes. Annals of Botany 112, 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Gamage, H. K. , Memmott, P. , Firn, J. & Schmidt, S. (2014). Harvesting as an alternative to burning for managing spinifex grasslands in Australia. Advances in Ecology 2014, ID 430431. [Google Scholar]
- * Garduza‐Acosta, B. , Lagunes‐Espinoza, L. C. , Bautista‐Muñoz, C. C. , García‐de‐los‐Santos, G. , Zaldívar‐Cruz, J. M. & Hernández‐Flores, A. (2020). Germination of Crotalaria and Lupinus (Fabaceae) seeds submitted to different pre‐germination treatments and their effect on enzymatic activity during early germination. Brazilian Journal of Biology 80, 23–29. [DOI] [PubMed] [Google Scholar]
- Garner, R. D. & Witkowski, E. T. F. (1997). Variations in seed size and shape in relation to depth of burial in the soil and pre‐ dispersal predation in Acacia nilotica, A. tortilis and Dichrostachys cinerea . South African Journal of Botany 63, 371–377. [Google Scholar]
- * Gashaw, M. & Michelsen, A. (2002). Influence of heat shock on seed germination of plants from regularly burnt savanna woodlands and grasslands in Ethiopia. Plant Ecology 159, 83–93. [Google Scholar]
- Ghebrehiwot, H. M. , Kulkarni, M. G. , Kirkman, K. P. & van Staden, J. (2008). Smoke‐water and a smoke‐isolated butenolide improve germination and seedling vigour of Eragrostis Tef (Zucc.) Trotter under high temperature and low osmotic potential. Journal of Agronomy and Crop Science 194, 270–277. [Google Scholar]
- Ghebrehiwot, H. , Kulkarni, M. , Kirkman, K. & van Staden, J. (2012). Smoke and heat: influence on seedling emergence from the germinable soil seed bank of mesic grassland in South Africa. Plant Growth Regulation 66, 119–127. [Google Scholar]
- Ghebrehiwot, H. M. , Kulkarni, M. G. , Szalai, G. , Soós, V. , Balázs, E. & Van Staden, J. (2013). Karrikinolide residues in grassland soils following fire: implications on germination activity. South African Journal of Botany 88, 419–424. [Google Scholar]
- * Ghebrehiwot, H. M. , Kulkarni, M. G. , Kirkman, K. P. & Van Staden, J. (2009). Smoke solutions and temperature influence the germination and seedling growth of South African mesic grassland species. Rangeland Ecology & Management 62, 572–578. [Google Scholar]
- Giladi, I. (2006). Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112, 481–492. [Google Scholar]
- * Gilmour, C. A. , Crowden, R. K. & Koutoulis, A. (2000). Heat shock, smoke and darkness: partner cues in promoting seed germination in Epacris tasmanica (Epacridaceae). Australian Journal of Botany 48, 603–609. [Google Scholar]
- Gioria, M. , Pyšek, P. , Baskin, C. C. & Carta, A. (2020). Phylogenetic relatedness mediates persistence and density of soil seed banks. Journal of Ecology 108, 2121–2131. [Google Scholar]
- Godoy, O. , Saldaña, A. , Fuentes, N. , Valladares, F. & Gianoli, E. (2011). Forests are not immune to plant invasions: phenotypic plasticity and local adaptation allow Prunella vulgaris to colonize a temperate evergreen rainforest. Biological Invasions 13, 1615–1625. [Google Scholar]
- * Gómez‐González, S. , Paula, S. , Cavieres, L. A. & Pausas, J. G. (2017). Postfire responses of the woody flora of Central Chile: insights from a germination experiment. PLoS One 12, e0180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Gómez‐González, S. , Sierra‐Almeida, A. & Cavieres, L. A. (2008). Does plant‐derived smoke affect seed germination in dominant woody species of the Mediterranean matorral of central Chile? Forest Ecology and Management 255, 1510–1515. [Google Scholar]
- Gorgone‐Barbosa, E. , Daibes, L. F. , Novaes, R. B. , Pivello, V. R. & Fidelis, A. (2020). Fire cues and germination of invasive and native grasses in the Cerrado. Acta Botanica Brasilica 34, 185–191. [Google Scholar]
- Gorgone‐Barbosa, E. , Pivello, V. R. , Baeza, M. J. & Fidelis, A. (2016). Disturbance as a factor in breaking dormancy and enhancing invasiveness of African grasses in a Neotropical Savanna. Acta Botanica Brasilica 30, 131–137. [Google Scholar]
- Gould, L. & Gabriel, D. N. (2015). Wet and dry season diets of the endangered Lemur catta (ring‐tailed lemur) in two mountainous rocky outcrop forest fragments in south‐central Madagascar. African Journal of Ecology 53, 320–330. [Google Scholar]
- Groom, P. K. & Lamont, B. B. (2015). Plant Life of Southwestern Australia. Adaptations for Survival. Berlin, Germany: De Gruyter Open. [Google Scholar]
- Gunn, C. R. (1984). Fruits and Seeds of Genera in the Subfamily Mimosoideae (Fabaceae), p. 194. Springfield, Virginia: Technical Bulletin, US Department of Agriculture. [Google Scholar]
- Haines, L. , Ennis, I. L. , Blanchon, D. J. & Triggs, C. M. (2007). Propagating the pale‐flowered kumarahou (Pomaderris hamiltonii) and kumarahou (Pomaderris kumeraho) from seeds. New Zealand Journal of Botany 45, 91–100. [Google Scholar]
- Hall, S. A. , Newton, R. J. , Holmes, P. M. , Gaertner, M. & Esler, K. J. (2017). Heat and smoke pre‐treatment of seeds to improve restoration of an endangered Mediterranean climate vegetation type. Austral Ecology 42, 354–366. [Google Scholar]
- * He, T. , Lamont, B. B. & Downes, K. S. (2011). Banksia born to burn. New Phytologist 191, 184–196. [DOI] [PubMed] [Google Scholar]
- He, T. , Lamont, B. B. & Manning, J. (2016). A Cretaceous origin for fire adaptations in the Cape flora. Scientific Reports 6, 34880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, T. , Lamont, B. B. & Pausas, J. G. (2019). Fire as a key driver of Earth's biodiversity. Biological Reviews 94, 1983–2010. [DOI] [PubMed] [Google Scholar]
- * He, T. , Pausas, J. G. , Belcher, C. M. , Schwilk, D. W. & Lamont, B. B. (2012). Fire‐adapted traits of Pinus arose in the fiery Cretaceous. New Phytologist 194, 751–759. [DOI] [PubMed] [Google Scholar]
- Heckenhauer, J. , Samuel, R. , Ashton, P. S. , Turner, B. , Barfuss, M. H. J. , Jang, T.‐S. , Temsch, E. M. , Mccann, J. , Salim, K. A. , Attanayake, A. M. A. S. & Chase, M. W. (2017). Phylogenetic analyses of plastid DNA suggest a different interpretation of morphological evolution than those used as the basis for previous classifications of Dipterocarpaceae (Malvales). Botanical Journal of the Linnean Society 185, 1–26. [Google Scholar]
- * Herranz, J. M. , Ferrandis, P. & Martínez‐Sánchez, J. J. (1998). Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecology 136, 95–103. [Google Scholar]
- Herranz, J. M. , Ferrandis, P. & Martínez‐Sánchez, J. J. (1999). Influence of heat on seed germination of nine woody Cistaceae species. International Journal of Wildland Fire 9, 173–182. [Google Scholar]
- Hodges, J. A. , Price, J. N. , Nicotra, A. B. , Neeman, T. & Guja, L. K. (2021). Smoke and heat accelerate and increase germination in fire‐prone temperate grassy ecosystems. Ecosphere 12, e03851. [Google Scholar]
- Hodges, J. A. , Price, J. N. , Nimmo, D. G. & Guja, L. K. (2019). Evidence for direct effects of fire‐cues on germination of some perennial forbs common in grassy ecosystems. Austral Ecology 44, 1271–1284. [Google Scholar]
- Hopkins, M. & Graham, A. W. (1987). The viability of seeds of rainforest species after experimental soil burials under tropical wet lowland forest in north‐eastern Australia. Australian Journal of Ecology 12, 97–108. [Google Scholar]
- Hradilová, I. , Duchoslav, M. , Brus, J. , Pechanec, V. , Hýbl, M. , Kopecký, P. , Smržová, L. , Štefelová, N. , Vaclávek, T. , Bariotakis, M. , Machalová, J. , Hron, K. , Pirintsos, S. & Smýkal, P. (2019). Variation in wild pea (Pisum sativum subsp. elatius) seed dormancy and its relationship to the environment and seed coat traits. PeerJ 7, e6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman, D. W. (2006). Production, losses, and germination of Ceanothus fendleri seeds in an Arizona Ponderosa pine forest. Western North American Naturalist 66, 365–373. [Google Scholar]
- Hughes, L. & Westoby, M. (1992). Fate of seeds adapted for dispersal by ants in Australian sclerophyll vegetation. Ecology 73, 1285–1299. [Google Scholar]
- Huss, J. C. & Gierlinger, N. (2021). Functional packaging of seeds. New Phytologist 230, 2154–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez Moro, A. V. , Bravo, S. J. , Abdala, N. R. , Borghetti, F. , Chaib, A. M. & Galetto, L. (2021). Heat shock effects on germination and seed survival of five woody species from the Chaco region. Flora 275, 151751. [Google Scholar]
- * Jabaily, R. S. , Shepherd, K. A. , Gardner, A. G. , Gustafsson, M. H. G. , Howarth, D. G. & Motley, T. J. (2014). Historical biogeography of the predominantly Australian plant family Goodeniaceae. Journal of Biogeography 41, 2057–2067. [Google Scholar]
- Jaganathan, G. K. , Yule, K. & Liu, B. (2016). On the evolutionary and ecological value of breaking physical dormancy by endozoochory. Perspectives in Plant Ecology, Evolution and Systematics 22, 11–22. [Google Scholar]
- Jain, N. , Kulkarni, M. G. & van Staden, J. (2006). A butenolide, isolated from smoke, can overcome the detrimental effects of extreme temperatures during tomato seed germination. Plant Growth Regulation 49, 263–267. [Google Scholar]
- * Jaureguiberry, P. & Díaz, S. (2015). Post‐burning regeneration of the Chaco seasonally dry forest: germination response of dominant species to experimental heat shock. Oecologia 177, 689–699. [DOI] [PubMed] [Google Scholar]
- * Jefferson, L. V. , Pennacchio, M. , Havens, K. , Forsberg, B. , Sollenberger, D. & Ault, J. (2008). Ex situ germination responses of Midwestern USA prairie species to plant‐derived smoke. The American Midland Naturalist 159, 251–256. [Google Scholar]
- Jeffery, D. J. , Holmes, P. M. & Rebelo, A. G. (1988). Effects of dry heat on seed germination in selected indigenous and alien legume species in South Africa. South African Journal of Botany 54, 28–34. [Google Scholar]
- * Jurado, E. , Márquez‐Linares, M. & Flores, J. (2011). Effect of cold storage, heat, smoke and charcoal on breaking seed dormancy of Arctostaphylos pungens HBK (Ericaceae). Phyton 80, 101–105. [Google Scholar]
- Karaguzel, O. , Cakmakci, S. , Ortacesme, V. & Aydinoglu, B. (2004). Influence of seed coat treatments on germination and early seedling growth of Lupinus varius l. Pakistan Journal of Botany 36, 65–74. [Google Scholar]
- * Kaye, T. N. & Kuykendall, K. (2001). Effects of scarification and cold stratification on seed germination of Lupinus sulphureus ssp. kincaidii . Seed Science & Technology 29, 663–668. [Google Scholar]
- * Kazancı, D. D. & Tavşanoğlu, Ç. (2019). Heat shock‐stimulated germination in Mediterranean Basin plants in relation to growth form, dormancy type and distributional range. Folia Geobotanica 54, 85–98. [Google Scholar]
- Keeley, J. E. (1991). Seed germination and life history syndromes in the California chaparral. The Botanical Review 57, 81–116. [Google Scholar]
- * Keeley, J. E. & Baer‐Keeley, M. (1999). Role of charred wood, heat‐shock, and light in germination of postfire phrygana species from the eastern Mediterranean Basin. Israel Journal of Plant Sciences 47, 11–16. [Google Scholar]
- * Keeley, J. E. & Bond, W. J. (1997). Convergent seed germination in South African fynbos and Californian chaparral. Plant Ecology 133, 153–167. [Google Scholar]
- Keeley, J. E. , Bond, W. J. , Bradstock, R. A. , Pausas, J. G. & Rundel, P. W. (2012). Fire in Mediterranean Ecosystems: Ecology, Evolution and Management. New York, NY: Cambridge University Press. [Google Scholar]
- Keeley, J. E. & Fotheringham, C. J. (1997). Trace gas emissions and smoke‐induced seed germination. Science 276, 1248–1250. [Google Scholar]
- * Keeley, J. E. & Fotheringham, C. J. (1998). Smoke‐induced seed germination in California chaparral. Ecology 79, 2320–2336. [Google Scholar]
- Keeley, J. E. & Fotheringham, C. J. (2000). Role of fire in regeneration from seeds. In Seeds: The Ecology of Regeneration in Plant Communities (ed. Fenner M.), pp. 311–330. CAB International, Wallingford. [Google Scholar]
- * Keeley, J. E. & Keeley, S. C. (1986). Chaparral and fires. Fremontia 14, 6–9. [Google Scholar]
- Keeley, J. E. & Pausas, J. G. (2018). Evolution of ‘smoke’ induced seed germination in pyroendemic plants. South African Journal of Botany 115, 251–255. [Google Scholar]
- * Keith, D. A. (1997). Combined effects of heat shock, smoke and darkness on germination of Epacris stuartii Stapf., an endangered fire‐prone Australian shrub. Oecologia 112, 340–344. [DOI] [PubMed] [Google Scholar]
- * Kenny, B. J. (2000). Influence of multiple fire‐related germination cues on three Sydney Grevillea (Proteaceae) species. Austral Ecology 25, 664–669. [Google Scholar]
- * Kildisheva, O. A. , Dixon, K. W. , Silveira, F. A. O. , Chapman, T. , Sacco, A. D. , Mondoni, A. , Turner, S. R. & Cross, A. T. (2020). Dormancy and germination: making every seed count in restoration. Restoration Ecology 28, S256–S265. [Google Scholar]
- King, R. A. & Menges, E. S. (2018). Effects of heat and smoke on the germination of six Florida scrub species. South African Journal of Botany 115, 223–230. [Google Scholar]
- * Konsam, B. , Phartyal, S. S. , Kumar, M. & Todaria, N. P. (2017). Life after fire for understory plant community in subtropical Chir pine forest of Garhwal Himalaya. Indian Forester 143, 759–766. [Google Scholar]
- Kulkarni, M. G. , Sparg, S. G. & van Staden, J. (2007). Germination and post‐germination response of Acacia seeds to smoke‐water and butenolide, a smoke‐derived compound. Journal of Arid Environments 69, 177–187. [Google Scholar]
- Lamont, B. B. , Burrows, G. E. & Korczynskyj, D. (2022). High summer temperatures do not interact with fire to promote germination among seeds of Cistaceae: a reinterpretation of Luna (2020) with extra data on wet/dry conditions. Plant Ecology 223, 141–149. [Google Scholar]
- Lamont, B. B. & Downes, K. S. (2011). Fire‐stimulated flowering among resprouters and geophytes in Australia and South Africa. Plant Ecology 212, 2111–2125. [Google Scholar]
- * Lamont, B.B. , El‐ahmir, S.M. , Lim, S.L. , Groom, P.K. & He, T. (2017a). Contribution of transition and stabilization processes to speciation is a function of the ancestral trait state and selective environment in Hakea. bioRxiv, 207373.
- Lamont, B.B. , Gómez Barreiro, P. & Newton, R.J. (2021). Seed‐coat thickness explains contrasting germination responses to smoke and heat by Leucadendron species. bioRxiv . doi: 10.1101/2021.12.16.472977 [DOI]
- Lamont, B. B. & He, T. (2012). Fire‐adapted Gondwanan Angiosperm floras evolved in the Cretaceous. BMC Evolutionary Biology 12, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont, B. B. & He, T. (2017a). Fire‐proneness as prerequisite for the evolution of fire‐adapted traits. Trends in Plant Science 22, 278–288. [DOI] [PubMed] [Google Scholar]
- * Lamont, B. B. & He, T. (2017b). When did a Mediterranean‐type climate originate in southwestern Australia? Global and Planetary Change 156, 46–58. [Google Scholar]
- * Lamont, B. B. , He, T. & Downes, K. (2013). Adaptive responses to directional trait selection in the Miocene enabled Cape proteas to colonize the savanna grasslands. Evolutionary Ecology 27, 1099–1115. [Google Scholar]
- * Lamont, B. B. , He, T. & Lim, S. L. (2016). Hakea, the world's most sclerophyllous genus, arose in southwestern Australian heathland and diversified throughout Australia over the past 12 million years. Australian Journal of Botany 64, 77–88. [Google Scholar]
- * Lamont, B. B. , He, T. & Pausas, J. G. (2017b). South African geoxyles evolved in response to fire; frost came later. Evolutionary Ecology 31, 603–617. [Google Scholar]
- * Lamont, B. B. , He, T. & Yan, Z. (2019a). Evolutionary history of fire‐stimulated resprouting, flowering, seed release and germination. Biological Reviews 94, 903–928. [DOI] [PubMed] [Google Scholar]
- * Lamont, B. B. , He, T. & Yan, Z. (2019b). Fire as a pre‐emptive evolutionary trigger among seed plants. Perspectives in Plant Ecology, Evolution and Systematics 36, 13–23. [Google Scholar]
- * Lamont, B. B. & Wiens, D. (2003). Are seed set and speciation rates always low among species that resprout after fire, and why? Evolutionary Ecology 17, 277–292. [Google Scholar]
- Lamont, B. B. & Witkowski, E. T. F. (2021). Plant functional types determine how close postfire seedlings are from their parents in a species‐rich shrubland. Annals of Botany 127, 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont, B. B. , Witkowski, E. T. F. & Enright, N. J. (1993). Post‐fire litter microsites: safe for seeds, unsafe for seedlings. Ecology 74, 501–512. [Google Scholar]
- Lengyel, S. , Gove, A. D. , Latimer, A. M. , Majer, J. D. & Dunn, R. R. (2010). Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: a global survey. Perspectives in Plant Ecology, Evolution and Systematics 12, 43–55. [Google Scholar]
- Leonard, J. , West, A. G. & Ojeda, F. (2018). Differences in germination response to smoke and temperature cues in ‘pyrophyte’ and ‘pyrofuge’ forms of Erica coccinea (Ericaceae). International Journal of Wildland Fire 27, 562–568. [Google Scholar]
- Li, H.‐L. , Wang, W. , Mortimer, P. E. , Li, R.‐Q. , Li, D.‐Z. , Hyde, K. D. , Xu, J.‐C. , Soltis, D. E. & Chen, Z.‐D. (2015). Large‐scale phylogenetic analyses reveal multiple gains of actinorhizal nitrogen‐fixing symbioses in angiosperms associated with climate change. Scientific Reports 5, 14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Liang, Q. , Deng, H. P. , He, P. , Fang, W. , Jiang, H. & Luo, T. (2020). Effects of plant‐derived smoke on seed germination of species common in subtropical China. Applied Ecology and Environmental Research 18, 5175–5185. [Google Scholar]
- Lieberman, D. , Hall, J. B. , Swaine, M. D. & Lieberman, M. (1979). Seed dispersal by baboons in the Shai Hills, Ghana. Ecology 60, 65–75. [Google Scholar]
- * Linder, H. P. & Vlok, J. H. (1991). The morphology, taxonomy and evolution of Rhodocoma (Restionaceae). Plant Systematics and Evolution 175, 139–160. [Google Scholar]
- Litsios, G. , Wüest, R. O. , Kostikova, A. , Forest, F. , Lexer, C. , Linder, H. P. , Pearman, P. B. , Zimmermann, N. E. & Salamin, N. (2014). Effects of a fire response trait on diversification in replicated radiations. Evolution 68, 453–465. [DOI] [PubMed] [Google Scholar]
- * Liyanage, G. S. , Offord, C. A. & Sommerville, K. D. (2020). Techniques for breaking seed dormancy of rainforest species from genus Acronychia . Seed Science and Technology 48, 159–165. [Google Scholar]
- Liyanage, G. S. & Ooi, M. K. J. (2017). Do dormancy‐breaking temperature thresholds change as seeds age in the soil seed bank? Seed Science Research 27, 1–11. [Google Scholar]
- Long, R. L. , Gorecki, M. J. , Renton, M. , Scott, J. K. , Colville, L. , Goggin, D. E. , Commander, L. E. , Westcott, D. A. , Cherry, H. & Finch‐Savage, W. E. (2015). The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews 90, 31–59. [DOI] [PubMed] [Google Scholar]
- * Long, R. L. , Stevens, J. C. , Griffiths, E. M. , Adamek, M. , Powles, S. B. & Merritt, D. J. (2011). Detecting karrikinolide responses in seeds of the Poaceae. Australian Journal of Botany 59, 609–619. [Google Scholar]
- Long, Y. , Tan, D. Y. , Baskin, C. C. & Baskin, J. M. (2012). Seed dormancy and germination characteristics of Astragalus arpilobus (Fabaceae, subfamily Papilionoideae), a central Asian desert annual ephemeral. South African Journal of Botany 83, 68–77. [Google Scholar]
- Lonsdale, W. M. (1993). Losses from the seed bank of Mimosa pigra: soil micro‐organisms vs. temperature fluctuations. Journal of Applied Ecology 30, 654–660. [Google Scholar]
- López‐Mársico, L. , Farías‐Moreira, L. , Lezama, F. , Altesor, A. & Rodríguez, C. (2019). Light intensity triggers different germination responses to fire‐related cues in temperate grassland species. Folia Geobotanica 54, 53–63. [Google Scholar]
- Luna, B. (2020). Fire and summer temperatures work together breaking physical seed dormancy. Scientific Reports 10, 6031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Luna, B. , Chamorro, D. & Pérez, B. (2019). Effect of heat on seed germination and viability in species of Cistaceae. Plant Ecology & Diversity 12, 151–158. [Google Scholar]
- Luna, B. , Moreno, J. M. , Cruz, A. & Fernández‐González, F. (2007). Heat‐shock and seed germination in a group of Mediterranean plant species growing in a burned area: an approach based on plant functional types. Environmental and Experimental Botany 60, 324–333. [Google Scholar]
- Luna, B. , Pérez, B. , Céspedes, B. & Moreno, J. M. (2008). Effect of cold exposure on seed germination of 58 plant species comprising several functional groups from a mid‐mountain Mediterranean area. Écoscience 15, 478–484. [Google Scholar]
- Ma, H. , Wu, H. & Ooi, M. K. J. (2018). Within population variation in germination response to smoke cues: convergent recruitment strategies and different dormancy types. Plant and Soil 427, 281–290. [Google Scholar]
- Maass, B. L. (2006). Changes in seed morphology, dormancy and germination from wild to cultivated hyacinth bean germplasm (Lablab purpureus: Papilionoideae). Genetic Resources and Crop Evolution 53, 1127–1135. [Google Scholar]
- MacKenzie, B. D. E. , Auld, T. D. , Keith, D. A. , Hui, F. K. C. & Ooi, M. K. J. (2016). The effect of seasonal ambient temperatures on fire‐stimulated germination of species with physiological dormancy: a case study using Boronia (Rutaceae). PLoS One 11, e0156142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo, J. E. & Suárez, F. (1996). Cistus ladanifer recruitment: not only fire, but also deer. Acta Oecologica 17, 55–60. [Google Scholar]
- * Marques, A. , Costa, M.‐C.D. , Farrant, J.M. , Hilhorst, H. , Buitink, J. , Ligterink, W. , Leprince, O. , Pelletier, S. , Gabaldon, T. , Schranz, M.E. , Delahaie, J. , Julca, I. , Marcet‐Houben, M. , Nijveen, H. , Derks, M. , et al. (2019). A blueprint of seed desiccation sensitivity in the genome of Castanospermum australe. BioRxiv , 10.1101/665661. [DOI]
- * Martin, R. E. , Miller, R. L. & Cushwa, C. T. (1975). Germination response of legume seeds subjected to moist and dry heat. Ecology 56, 1441–1445. [Google Scholar]
- * Martinat, J. E. & Fuentes, E. (2016). Efecto de las altas temperaturas en la germinación de Fabaceae forrajeras de las Sierras Chicas de Córdoba, Argentina. Iheringia, Série Botânica 71, 5–12. [Google Scholar]
- Mbalo, B. A. & Witkowski, E. T. F. (1997). Tolerance to soil temperatures experienced during and after the passage of fire in seeds of Acacia karroo, A. tortilis and Chromolaena odorata: a laboratory study. South African Journal of Botany 63, 421–425. [Google Scholar]
- * McClain, K. (2016). Seed Germination Requirements for Four Fire‐Recruiter Chaparral Shrubs. Senior Honors Projects 91. John Carroll University. http://collected.jcu.edu/honorspapers/ [Google Scholar]
- Merritt, D. , Kristiansen, M. , Flematti, G. , Turner, S. , Ghisalberti, E. , Trengove, R. & Dixon, K. (2006). Effects of a butenolide present in smoke on light‐mediated germination of Australian Asteraceae. Seed Science Research 16, 29–36. [Google Scholar]
- Merritt, D. J. , Turner, S. R. , Clarke, S. & Dixon, K. W. (2007). Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany 55, 336–344. [Google Scholar]
- Miller, J. T. , Murphy, D. J. , Ho, S. Y. W. , Cantrill, D. J. & Seigler, D. (2013). Comparative dating of Acacia: combining fossils and multiple phylogenies to infer ages of clades with poor fossil records. Australian Journal of Botany 61, 436–445. [Google Scholar]
- * Milne, L. (1988). Palynology of a late Eocene lignitic sequence from the western margin of the Eucla Basin, Western Australia. Memoirs of the Association of Australasian Palaeontologists 1988, 285–310. [Google Scholar]
- * Mojzes, A. & Kalapos, T. (2015). Plant‐derived smoke enhances germination of the invasive common milkweed (Asclepias syriaca L.). Polish Journal of Ecology 63, 280–285. [Google Scholar]
- * Mojzes, A. & Kalapos, T. (2016). Positive germination response of oriental mustard (Sisymbrium orientale L., Brassicaceae) to plant‐derived smoke. Brazilian Journal of Botany 39, 959–963. [Google Scholar]
- * Montalvo, A. M. , Feist‐Alvey, L. J. & Koehler, C. E. (2002). The effect of fire and cold treatments on seed germination of annual and perennial populations of Eschscholzia californica (Papaveraceae) in Southern California. Madroño 49, 207–227. [Google Scholar]
- Moreira, B. & Pausas, J. G. (2012). Tanned or burned: the role of fire in shaping physical seed dormancy. PLoS One 7, e51523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, B. & Pausas, J. G. (2018). Shedding light through the smoke on the germination of Mediterranean Basin flora. South African Journal of Botany 115, 244–250. [Google Scholar]
- Moreira, B. , Tormo, J. , Estrelles, E. & Pausas, J. G. (2010). Disentangling the role of heat and smoke as germination cues in Mediterranean Basin flora. Annals of Botany 105, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Morris, E. C. (2000). Germination response of seven east Australian Grevillea species (Proteaceae) to smoke, heat exposure and scarification. Australian Journal of Botany 48, 179–189. [Google Scholar]
- * Morrone, O. , Aagesen, L. , Scataglini, M. A. , Salariato, D. L. , Denham, S. S. , Chemisquy, M. A. , Sede, S. M. , Giussani, L. M. , Kellogg, E. A. & Zuloaga, F. O. (2012). Phylogeny of the Paniceae (Poaceae: Panicoideae): integrating plastid DNA sequences and morphology into a new classification. Cladistics 28, 333–356. [DOI] [PubMed] [Google Scholar]
- * Murphy, D. J. , Brown, G. K. , Miller, J. T. & Ladiges, P. Y. (2010). Molecular phylogeny of Acacia Mill. (Mimosoideae: Leguminosae): evidence for major clades and informal classification. Taxon 59, 7–19. [Google Scholar]
- * Naghipour, A. A. , Bashari, H. , Khajeddin, S. J. , Tahmasebi, P. & Iravani, M. (2016). Effects of smoke, ash and heat shock on seed germination of seven species from Central Zagros rangelands in the semi‐arid region of Iran. African Journal of Range & Forage Science 33, 67–71. [Google Scholar]
- Nandi, O. I. (1998). Ovule and seed anatomy of Cistaceae and related Malvanae. Plant Systematics and Evolution 209, 239–264. [Google Scholar]
- Navarro‐Cano, J. A. , Rivera, D. & Barberà, G. G. (2009). Induction of seed germination in Cistus heterophyllus (Cistaceae): a rock rose critically endangered in Spain. Research Journal of Botany 4, 10–16. [Google Scholar]
- Nelson, D. C. , Flematti, G. R. , Riseborough, J. A. , Ghisalberti, E. L. , Dixon, K. W. & Smith, S. M. (2010). Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the United States of America 107, 7095–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, R. J. , Mackenzie, B. D. E. , Lamont, B. B. , Gomez‐Barreiro, P. , Cowling, R. M. & He, T. (2021). Fire‐mediated germination syndromes in Leucadendron (Proteaceae) and their functional correlates. Oecologia 196, 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odion, D. C. & Davis, F. W. (2000). Fire, soil heating, and the formation of vegetation patterns in chaparral. Ecological Monographs 70, 149–169. [Google Scholar]
- * O'Dowd, D. J. & Gill, A. M. (1986). Seed dispersal syndromes in Australian Acacia. In Seed Dispersal (ed. Murray D. R.), pp. 87–121. Sydney: Academic Press. [Google Scholar]
- Onstein, R. E. & Linder, H. P. (2016). Beyond climate: convergence in fast evolving sclerophylls in Cape and Australian Rhamnaceae predates the mediterranean climate. Journal of Ecology 104, 665–677. [Google Scholar]
- Ooi, M. K. J. , Denham, A. J. , Santana, V. M. & Auld, T. D. (2014). Temperature thresholds of physically dormant seeds and plant functional response to fire: variation among species and relative impact of climate change. Ecology and Evolution 4, 656–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetzold, C. , Kiehn, M. , Wood, K. R. , Wagner, W. L. & Appelhans, M. S. (2018). The odd one out or a hidden generalist: Hawaiian Melicope (Rutaceae) do not share traits associated with successful Island colonization. Journal of Systematics and Evolution 56, 621–636. [Google Scholar]
- Paredes, M. V. F. , da Cunha, A. L. N. , Musso, C. , Aires, S. S. , Sato, M. N. & Miranda, H. S. (2018). Germination responses of native and invasive Cerrado grasses to simulated fire temperatures. Plant Ecology & Diversity 11, 193–203. [Google Scholar]
- * Pastore, J. F. B. & Abbott, J. R. (2012). Taxonomic notes and new combinations for Asemeia (Polygalaceae). Kew Bulletin 67, 801–813. [Google Scholar]
- * Pastore, J. F. B. , Abbott, J. R. , Neubig, K. M. , Berg, C. V. D. , Mota, M. C. D. A. , Cabral, A. & Whitten, W. M. (2019). Phylogeny and biogeography of Polygala (Polygalaceae). Taxon 68, 673–691. [Google Scholar]
- Pate, J. S. , Casson, N. E. , Rullo, J. & Kuo, J. (1985). Biology of fire ephemerals of the sandplains of the kwongan of south‐western Australia. Functional Plant Biology 12, 641–655. [Google Scholar]
- Paula, S. & Pausas, J. G. (2008). Burning seeds: germinative response to heat treatments in relation to resprouting ability. Journal of Ecology 96, 543–552. [Google Scholar]
- Pausas, J. G. (2017). Homage to L. M. Coutinho: fire adaptations in cerrado plants. International Journal of Wildland Fire 26, 249–251. [Google Scholar]
- Pausas, J. G. & Bond, W. J. (2020). Alternative biome states in terrestrial ecosystems. Trends in Plant Science 25, 250–263. [DOI] [PubMed] [Google Scholar]
- Pausas, J. G. & Keeley, J. E. (2014). Evolutionary ecology of resprouting and seeding in fire‐prone ecosystems. New Phytologist 204, 55–65. [DOI] [PubMed] [Google Scholar]
- Pausas, J. G. & Lamont, B. B. (2018). Ecology and biogeography in 3D: the case of the Australian Proteaceae. Journal of Biogeography 45, 1469–1477. [Google Scholar]
- * Pausas, J.G. & Lamont, B.B. (2022) Data from: fire‐released seed dormancy ‐ a global synthesis. Figshare. 10.6084/m9.figshare.19126823.v4 [accessed 31.03.2022]. [DOI] [PMC free article] [PubMed]
- * Peel, M. C. , Finlayson, B. L. & Mcmahon, T. A. (2007). Updated world map of the Köppen‐Geiger climate classification. Hydrology and Earth System Sciences Discussions 4, 439–473. [Google Scholar]
- * Penman, T. D. , Binns, D. , Allen, R. , Shiels, R. & Plummer, S. (2008). Germination responses of a dry sclerophyll forest soil‐stored seedbank to fire related cues. Cunninghamia 10, 547–555. [Google Scholar]
- * Pérez‐Fernández, M. A. & Rodríguez‐Echeverría, S. (2003). Effect of smoke, charred wood, and nitrogenous compounds on seed germination of ten species from woodland in Central‐Western Spain. Journal of Chemical Ecology 29, 237–251. [DOI] [PubMed] [Google Scholar]
- Pérez‐García, F. & González‐Benito, M. E. (2006). Seed germination of five Helianthemum species: effect of temperature and presowing treatments. Journal of Arid Environments 65, 688–693. [Google Scholar]
- Quinlivan, B. J. (1968). Seed coat impermeability in the common annual legume pasture species of Western Australia. Australian Journal of Experimental Agriculture 8, 695–701. [Google Scholar]
- * Ramos, D. M. , Liaffa, A. B. S. , Diniz, P. , Munhoz, C. B. R. , Ooi, M. K. J. , Borghetti, F. & Valls, J. F. M. (2016). Seed tolerance to heating is better predicted by seed dormancy than by habitat type in Neotropical savanna grasses. International Journal of Wildland Fire 25, 1273–1280. [Google Scholar]
- * Ramos, D. M. , Valls, J. F. M. , Borghetti, F. & Ooi, M. K. J. (2019). Fire cues trigger germination and stimulate seedling growth of grass species from Brazilian savannas. American Journal of Botany 106, 1190–1201. [DOI] [PubMed] [Google Scholar]
- * Randriatafika, F. , Rabenantoandro, J. & Rajoharison, R. A. (2007). Analyses of seed germination of littoral forest native species in southeastern Madagascar. In Biodiversity, Ecology and Conservation of Littoral Forest Ecosystems in Southeastern Madagascar, Tolagnaro (Fort Dauphin) (eds Ganzhorn J. U., Goodman S. M. and Vincelette M.), p. Washington DC: Smithsonian Institution Press, 119. [Google Scholar]
- * Read, T. R. & Bellairs, S. M. (1999). Smoke affects the germination of native grasses of New South Wales. Australian Journal of Botany 47, 563–576. [Google Scholar]
- Read, T. R. , Bellairs, S. M. , Mulligan, D. R. & Lamb, D. (2000). Smoke and heat effects on soil seed bank germination for the re‐establishment of a native forest community in New South Wales. Austral Ecology 25, 48–57. [Google Scholar]
- Rees, M. (1994). Delayed germination of seeds: a look at the effects of adult longevity, the timing of reproduction, and population age/stage structure. The American Naturalist 144, 43–64. [Google Scholar]
- Reyes, O. & Trabaud, L. (2009). Germination behaviour of 14 Mediterranean species in relation to fire factors: smoke and heat. Plant Ecology 202, 113–121. [Google Scholar]
- Ribeiro, L. C. , Pedrosa, M. & Borghetti, F. (2013). Heat shock effects on seed germination of five Brazilian savanna species. Plant Biology 15, 152–157. [DOI] [PubMed] [Google Scholar]
- * Rivas, M. , Reyes, O. & Casal, M. (2006). Influence of heat and smoke treatments on the germination of six leguminous shrubby species. International Journal of Wildland Fire 15, 73–80. [Google Scholar]
- Riveiro, S. F. , Cruz, Ó. , Casal, M. & Reyes, O. (2020). Fire and seed maturity drive the viability, dormancy, and germination of two invasive species: Acacia longifolia (Andrews) Willd. and Acacia mearnsii De Wild. Annals of Forest Science 77, 60. [Google Scholar]
- * Robles‐Díaz, E. , Flores, J. & Yáñez‐Espinosa, L. (2016). Paths of water entry and structures involved in the breaking of seed dormancy of Lupinus . Journal of Plant Physiology 192, 75–80. [DOI] [PubMed] [Google Scholar]
- * Roche, S. , Dixon, K. W. & Pate, J. S. (1998). For everything a season: amoke‐induced seed germination and seedling recruitment in a Western Australian Banksia woodland. Australian Journal of Ecology 23, 111–120. [Google Scholar]
- Roche, S. , Koch, J. M. & Dixon, K. W. (1997). Smoke enhanced seed germination for mine rehabilitation in the southwest of Western Australia. Restoration Ecology 5, 191–203. [Google Scholar]
- * Roeder, M. , Yang, W. & Tomlinson, K. (2019). Influence of smoke, heat and fire on germination of woody species occurring in the dry valleys of southwest China. Journal of Plant Ecology 12, 931–940. [Google Scholar]
- Rubio de Casas, R. , Willis, C. G. , Pearse, W. D. , Baskin, C. C. , Baskin, J. M. & Cavender‐Bares, J. (2017). Global biogeography of seed dormancy is determined by seasonality and seed size: a case study in the legumes. New Phytologist 214, 1527–1536. [DOI] [PubMed] [Google Scholar]
- Rundel, P. W. , Arroyo, M. T. K. , Cowling, R. M. , Keeley, J. E. , Lamont, B. , Pausas, J. G. & Vargas, P. (2018). Fire and plant diversification in mediterranean‐climate regions. Frontiers in Plant Science 9, 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Ruprecht, E. , Fenesi, A. , Fodor, E. I. & Kuhn, T. (2013). Prescribed burning as an alternative management in grasslands of temperate Europe: the impact on seeds. Basic and Applied Ecology 14, 642–650. [Google Scholar]
- Saatkamp, A. , Pochlod, P. & Lawrence, V. D. (2014). The functional role of soil seed bank in natural communities. In Seeds: The Ecology of Regeneration in Plant Communities (ed. Gallagher R. S.), pp. 263–295. Boston, MA: CABI Publishing. [Google Scholar]
- Sauquet, H. , Weston, P. H. , Anderson, C. L. , Barker, N. P. , Cantrill, D. J. , Mast, A. R. & Savolainen, V. (2009). Contrasted patterns of hyperdiversification in Mediterranean hotspots. Proceedings of the National Academy of Sciences of the United States of America 106, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautu, A. , Baskin, J. M. , Baskin, C. C. & Condit, R. (2006). Studies on the seed biology of 100 native species of trees in a seasonal moist tropical forest, Panama, Central America. Forest Ecology and Management 234, 245–263. [Google Scholar]
- Scheiter, S. , Higgins Steven, I. , Osborne Colin, P. , Bradshaw, C. , Lunt, D. , Ripley Brad, S. , Taylor Lyla, L. & Beerling David, J. (2012). Fire and fire‐adapted vegetation promoted C4 expansion in the late Miocene. New Phytologist 195, 653–666. [DOI] [PubMed] [Google Scholar]
- * Schramm, P. & Johnson, R. (1981). Seed conditioning and germination of New Jersey tea (Ceanothus americanus; Rhamnaceae). In Proceedings of the 6th North American Prairie Conference pp. 222–226. Ohio Biological Survey Biological Notes.
- * Schwilk, D. W. & Zavala, N. (2012). Germination response of grassland species to plant‐derived smoke. Journal of Arid Environments 79, 111–115. [Google Scholar]
- Scott, A. C. (2018). Burning Planet: The Story of Fire Through Time. Oxford, England: Oxford Univeristy Press. [Google Scholar]
- * Scott, K. , Setterfield, S. , Douglas, M. & Andersen, A. (2010). Soil seed banks confer resilience to savanna grass‐layer plants during seasonal disturbance. Acta Oecologica 36, 202–210. [Google Scholar]
- Scotti‐Saintagne, C. , Dick, C. W. , Caron, H. , Vendramin, G. G. , Troispoux, V. , Sire, P. , Casalis, M. , Buonamici, A. , Valencia, R. , Lemes, M. R. , Gribel, R. & Scotti, I. (2013). Amazon diversification and cross‐Andean dispersal of the widespread Neotropical tree species Jacaranda copaia (Bignoniaceae). Journal of Biogeography 40, 707–719. [Google Scholar]
- Scuderi, D. , Gregorio, R. D. , Toscano, S. , Cassaniti, C. & Romano, D. (2010). Germination behaviour of four mediterranean Cistus L. species in relation to high temperature. Ecological Questions 12, 171–180. [Google Scholar]
- * Shaddad, M. A. , Radi, A. F. , Abdel‐Rahman, A. M. & Azooz, M. M. (1990). Response of seeds of Lupinus termis and Vicia faba to the interactive effect of salinity and ascorbic acid or pyridoxine. Plant and Soil 122, 177. [Google Scholar]
- Shi, C. , Wang, S. , Cai, H. , Zhang, H. , Long, X. , Tihelka, E. , Song, W. , Feng, Q. , Jiang, R. , Cai, C. , Lombard, N. , Li, X. , Yuan, J. , Zhu, J. , Yang, H. , et al. (2022). Fire‐prone Rhamnaceae with South African affinities in Cretaceous Myanmar amber. Nature Plants 8, 125–135. 10.1038/s41477-021-01091-w. [DOI] [PubMed] [Google Scholar]
- Silveira, F. A. O. , Ribeiro, R. C. , Oliveira, D. M. T. , Fernandes, G. W. & Lemos‐Filho, J. P. (2012). Evolution of physiological dormancy multiple times in Melastomataceae from Neotropical montane vegetation. Seed Science Research 22, 37–44. [Google Scholar]
- Simon, M. F. , Grether, R. , De Queiroz, L. P. , Skema, C. , Pennington, R. T. & Hughes, C. E. (2009). Recent assembly of the Cerrado, a Neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proceedings of the National Academy of Sciences of the United States of America 106, 20359–20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Smith, M. A. , Bell, D. T. & Loneragan, W. A. (1999). Comparative seed germination ecology of Austrostipa compressa and Ehrharta calycina (Poaceae) in a Western Australian Banksia woodland. Australian Journal of Ecology 24, 35–42. [Google Scholar]
- * Smith, N. J. C. , Zahid, D. M. , Ashwath, N. & Midmore, D. J. (2008). Seed ecology and successional status of 27 tropical rainforest cabinet timber species from Queensland. Forest Ecology and Management 256, 1031–1038. [Google Scholar]
- * Souza, M. L. , Silva, D. R. P. , Fantecelle, L. B. , de Lemos Filho, J. P. , Souza, M. L. , Silva, D. R. P. , Fantecelle, L. B. & de Lemos Filho, J. P. (2015). Key factors affecting seed germination of Copaifera langsdorffii, a Neotropical tree. Acta Botanica Brasilica 29, 473–477. [Google Scholar]
- Stevens, J. C. , Merritt, D. J. , Flematti, G. R. , Ghisalberti, E. L. & Dixon, K. W. (2007). Seed germination of agricultural weeds is promoted by the butenolide 3‐methyl‐2H‐furo[2,3‐c]pyran‐2‐one under laboratory and field conditions. Plant and Soil 298, 113–124. [Google Scholar]
- * Stuurwold, J.E. (1972). The effects of moist‐heat treatments and stratification on germination of prairie plant seeds. Honors Theses, Western Michigan University, Michigan.
- * Tang, Y. , Boulter, S. L. & Kitching, R. L. (2003). Heat and smoke effects on the germination of seeds from soil seed banks across forest edges between subtropical rainforest and eucalypt forest at Lamington National Park, south‐eastern Queensland, Australia. Australian Journal of Botany 51, 227–237. [Google Scholar]
- Tangney, R. , Merritt, D. J. , Callow, J. N. , Fontaine, J. B. & Miller, B. P. (2020). Seed traits determine species responses to fire under varying soil heating scenarios. Functional Ecology 34, 1967–1978. [Google Scholar]
- Tangney, R. , Merritt, D. J. , Fontaine, J. B. & Miller, B. P. (2019). Seed moisture content as a primary trait regulating the lethal temperature thresholds of seeds. Journal of Ecology 107, 1093–1105. [Google Scholar]
- Tavşanoğlu, C. (2011). Fire‐related cues (heat shock and smoke) and seed germination in a Cistus creticus population in southwestern Turkey. Ekoloji 20, 99–104. [Google Scholar]
- Tavşanoğlu, Ç. & Çatav, Ş. S. (2012). Seed size explains within‐population variability in post‐fire germination of Cistus salviifolius . Annales Botanici Fennici 49, 331–340. [Google Scholar]
- * Tavşanoğlu, Ç. , Ergan, G. , Çatav, Ş. S. , Zare, G. , Küçükakyüz, K. & Özüdoğru, B. (2017). Multiple fire‐related cues stimulate germination in Chaenorhinum rubrifolium (Plantaginaceae), a rare annual in the Mediterranean Basin. Seed Science Research 27, 26–38. [Google Scholar]
- * Teketay, D. (1996a). Germination ecology of twelve indigenous and eight exotic multipurpose leguminous species from Ethiopia. Forest Ecology and Management 80, 209–223. [Google Scholar]
- * Teketay, D. (1996b). The effect of different pre‐sowing seed treatments, temperature and light on the germination of five Senna species from Ethiopia. New Forests 11, 155–171. [Google Scholar]
- Thanos, C. A. , Georghiou, K. , Kadis, C. & Pantazi, C. (1992). Cistaceae: a plant family with hard seeds. Israel Journal of Botany 41, 251–263. [Google Scholar]
- * Thomas, P. B. , Morris, E. C. & Auld, T. D. (2003). Interactive effects of heat shock and smoke on germination of nine species forming soil seed banks within the Sydney region. Austral Ecology 28, 674–683. [Google Scholar]
- Thompson, K. , Ceriani, R. M. , Bakker, J. P. & Bekker, R. M. (2003). Are seed dormancy and persistence in soil related? Seed Science Research 13, 97–100. [Google Scholar]
- * Tieu, A. , Dixon, K. W. , Meney, K. A. & Sivasithamparam, K. (2001). The interaction of heat and smoke in the release of seed dormancy in seven species from southwestern Western Australia. Annals of Botany 88, 259–265. [Google Scholar]
- * Tonnabel, J. , Mignot, A. , Douzery, E. J. P. , Rebelo, A. G. , Schurr, F. M. , Midgley, J. , Illing, N. , Justy, F. , Orcel, D. & Olivieri, I. (2014). Convergent and correlated evolution of major life history traits in the angiosperm genus Leucadendron (Proteaceae). Evolution 68, 2775–2792. [DOI] [PubMed] [Google Scholar]
- * Toon, A. , Crisp, M. D. , Gamage, H. , Mant, J. , Morris, D. C. , Schmidt, S. & Cook, L. G. (2015). Key innovation or adaptive change? A test of leaf traits using Triodiinae in Australia. Scientific Reports 5, 12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo, J. , Moreira, B. & Pausas, J. G. (2014). Field evidence of smoke‐stimulated seedling emergence and establishment in Mediterranean Basin flora. Journal of Vegetation Science 25, 771–777. [Google Scholar]
- Travlos, I. S. , Economou, G. & Karamanos, A. J. (2007). Seed germination and seedling emergence of Spartium junceum L. in response to heat and other pre‐sowing treatments. Journal of Agronomy 6, 152–156. [Google Scholar]
- Tsuyuzaki, S. & Miyoshi, C. (2009). Effects of smoke, heat, darkness and cold stratification on seed germination of 40 species in a cool temperate zone in northern Japan. Plant Biology 11, 369–378. [DOI] [PubMed] [Google Scholar]
- van Klinken, R. D. , Flack, L. K. & Pettit, W. (2006). Wet‐season dormancy release in seed banks of a tropical leguminous shrub is determined by wet heat. Annals of Botany 98, 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staden, J. , Jäger, A. K. , Light, M. E. & Burger, B. V. (2004). Isolation of the major germination cue from plant‐derived smoke. South African Journal of Botany 70, 654–659. [Google Scholar]
- Vandvik, V. , Töpper, J. P. , Cook, Z. , Daws, M. I. , Heegaard, E. , Måren, I. E. & Velle, L. G. (2014). Management‐driven evolution in a domesticated ecosystem. Biology Letters 10, 20131082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez‐Yanes, C. & Orozco‐Segovia, A. (1993). Patterns of seed longevity and germination in the tropical rainforest. Annual Review of Ecology and Systematics 24, 69–87. [Google Scholar]
- Venable, D. L. (2007). Bet hedging in a guild of desert annuals. Ecology 88, 1086–1090. [DOI] [PubMed] [Google Scholar]
- * Verkerke, W. (1985). Ovules and seeds of the Polygalaceae. Journal of the Arnold Arboretum 66, 353–394. [Google Scholar]
- Waters, M. T. , Scaffidi, A. , Flematti, G. R. & Smith, S. M. (2013). The origins and mechanisms of karrikin signalling. Current Opinion in Plant Biology 16, 667–673. [DOI] [PubMed] [Google Scholar]
- * Wiggers, M. S. , Kirkman, L. K. , Boyd, R. S. & Hiers, J. K. (2013). Fine‐scale variation in surface fire environment and legume germination in the longleaf pine ecosystem. Forest Ecology and Management 310, 54–63. [Google Scholar]
- * Williams, P. R. , Congdom, R. A. , Grice, A. C. & Clarke, P. J. (2003). Fire‐related cues break seed dormancy of six legumes of tropical eucalypt savannas in north‐eastern Australia. Austral Ecology 28, 507–514. [Google Scholar]
- * Williams, P. R. , Congdon, R. A. , Grice, A. C. & Clarke, P. J. (2005). Germinable soil seed banks in a tropical savanna: seasonal dynamics and effects of fire. Austral Ecology 30, 79–90. [Google Scholar]
- Willis, C. G. , Baskin, C. C. , Baskin, J. M. , Auld, J. R. , Venable, D. L. , Cavender‐Bares, J. , Donohue, K. & de Casas, R. R. (2014). The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytologist 203, 300–309. [DOI] [PubMed] [Google Scholar]
- * Wills, T. J. & Read, J. (2002). Effects of heat and smoke on germination of soil‐stored seed in a south‐eastern Australian sand heathland. Australian Journal of Botany 50, 197–206. [Google Scholar]
- Wyse, S. V. & Dickie, J. B. (2018). Ecological correlates of seed dormancy differ among dormancy types: a case study in the legumes. New Phytologist 217, 477–479. [DOI] [PubMed] [Google Scholar]
- * Yao, L. , Naeth, M. A. & Mollard, F. P. O. (2017). Ecological role of pyrolysis by‐products in seed germination of grass species. Ecological Engineering 108, 78–82. [Google Scholar]
- * Yeşilyurt, E. B. , Erik, S. & Tavşanoğlu, Ç. (2017). Inter‐population variability in seed dormancy, seed mass and germination in Helianthemum salicifolium (Cistaceae), a hard‐seeded annual herb. Folia Geobotanica 52, 253–263. [Google Scholar]
- Zachos, J. C. , Dickens, G. R. & Zeebe, R. E. (2008). An early Cenozoic perspective on greenhouse warming and carbon‐cycle dynamics. Nature 451, 279–283. [DOI] [PubMed] [Google Scholar]
- * Zaman, S. , Padmesh, S. & Tawfiq, H. (2009). Effect of pre‐germination treatments on seed germination of Helianthemum lippii (L.) Dum.Cours. Desert Plants 25, 18–21. [Google Scholar]
- Zhang, Y. , Zhang, K. , Ji, Y. & Tao, J. (2020). Physical dormancy and soil seed bank dynamics in seeds of Melilotus albus (Fabaceae). Flora 266, 151600. [Google Scholar]
- * Zhou, J. , da Silva, J. T. & Ma, G. (2014). Effects of smoke water and karrikin on seed germination of 13 species growing in China. Central European Journal of Biology 9, 1108–1116. [Google Scholar]
- Zirondi, H. L. , de Pinho José, H. , Daibes, L. F. & Fidelis, A. (2019a). Heat and smoke affect the germination of flammable resprouters: Vellozia species in the Cerrado. Folia Geobotanica 54, 65–72. [Google Scholar]
- Zirondi, H. L. , Silveira, F. A. O. & Fidelis, A. (2019b). Fire effects on seed germination: heat shock and smoke on permeable vs impermeable seed coats. Flora 253, 98–106. [Google Scholar]
- Zomer, M. , Moreira, B. & Pausas, J.G. (2022) Fire and summer temperatures interact to shape seed dormancy thresholds. Annals of Botany, 10.1093/aob/mcac047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zuloaga‐Aguilar, S. , Briones, O. & Orozco‐Segovia, A. (2011). Seed germination of montane forest species in response to ash, smoke and heat shock in Mexico. Acta Oecologica 37, 256–262. [Google Scholar]
- * Zuloaga‐Aguilar, S. , Briones, O. , Orozco‐Segovia, A. , Zuloaga‐Aguilar, S. , Briones, O. & Orozco‐Segovia, A. (2010). Effect of heat shock on germination of 23 plant species in pine–oak and montane cloud forests in western Mexico. International Journal of Wildland Fire 19, 759–773. [Google Scholar]
- Zupo, T. , Baeza, M. J. & Fidelis, A. (2016). The effect of simulated heat‐shock and daily temperature fluctuations on seed germination of four species from fire‐prone ecosystems. Acta Botanica Brasilica 30, 514–519. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dormancy‐release syndromes present in fireprone ecosystems, and their ecological properties.
Appendix S1. Taxonomic patterns of fire‐released dormancy.
Fig. S1. Summary of our collation of fire responses among seven families with at least some fire‐responsive members (totalling 1155 species worldwide).
Fig. S2. Illustrations of six categories of dormancy‐breaking responses that can be recognized among Fabaceae.
Fig. S3. Summary of interaction between cold stratification and fire‐related treatments for five species (Ceanothus americanus, Ceanothus jepsonii, Discaria pubescens, Lupinus sulphureus, Silphium terebinthenaceum) in individual studies treated with heat and 13 populations of Eschscholzia californica treated with smoke.
Fig. S4. Interaction between cold stratification and fire‐related cues in the germination of ten species with hard seeds in fireprone ecosystems, and 13 populations of Eschscholzia californica.
Table S2. Germination results for smoke applied to Poaceae species in various ecosystems.
Table S3. Effect of smoke on the germination of Lamiaceae species aggregated into two environments: savanna (tropical and subtropical savannas and shortgrass prairie) and shrublands of the Mediterranean Basin.
Table S4. Summary of germination experiments with smoke in different ecosystem types.
Fig. S5. Illustrations of three categories of smoke‐released dormancy/germination responses that can be recognized in various ecosystems worldwide.
Fig. S6. Interactions between smoke and light on seed germination.
Fig. S7. Effect of smoke on soil seed banks in different crown‐fire ecosystems.
Fig. S8. Summary of data in Table S5 for Fabaceae on a % species per environment and fire‐regime basis.
Table S5. Viability and germination characteristics of the Fabaceae in four major biome types throughout the world, and their surface or crown fire regimes, based on the six categories in Fig. S2 plus an additional three categories (C1,2,3) with low viability and germination.
Fig. S9. Soil temperatures during experimental fires.
Fig. S10. Transect through a recently burnt sclerophyll shrubland in California.
Fig. S11. Percentage of species with release from dormancy stimulated, inhibited, or unaffected by heat and/or smoke in four environment categories.
Table S6. Descriptions of biogeographic regions used in Fig. S11.
Table S7. Changes in seed traits through evolutionary time in response to changes in climate and fire regime.
Table S8. List of references used to construct Fig. 9, with additional information.
Appendix S2. Timing of germination (seasonality effects).
Table S9. Summary of seasonal aspects of maintaining and breaking seed dormancy in three major biome types with contrasting fire regimes over 30 months, with one fire in crown‐fire ecosystems and two fires in surface‐fire ecosystems.
Table S10. Smoke‐stimulated germination in rainforest species.
Data S1. Available at https://doi.org/10.6084/m9.figshare.19126823.v4


