Sir,
An 83-year-old gentleman with non-valvular atrial fibrillation, diabetes, and hypertension on 5 mg BD apixaban, 30 mg TDS diltiazem, 40 mg telmisartan, and linagliptin + metformin, presented with a right parietal subdural hematoma following a traumatic fall. He complained of headache, had no focal neurologic deficits, and was managed conservatively with anti-edema measures. However, a burr hole surgery with hematoma evacuation was done on day 7 given the progressively increasing midline shift (7 mm). He developed persistent left focal seizures on the second postoperative day, and did not respond to levetiracetam and lacosamide. Over the next 24 h, he developed left-sided hemiparesis with drowsiness. The magnetic resonance imaging (MRI) of the brain revealed a reduction in the midline shift without any evidence of vascular insult [Figure 1]. Continuous electroencephalogram (EEG) monitoring revealed diffuse slowing with C4-T4 spike-wave activity. He was diagnosed with focal status epilepticus and started on midazolam 0.2 mg/kg body weight. A burst suppression pattern was achieved in 24 h following which it was tapered and stopped and clobazam was added. He regained full sensorium with no focal deficit over the next 3–4 days. He was discharged on day 7, and levetiracetam was switched to brivaracetam at discharge (levetiracetam: brivaracetam 10:1 switching ratio applied) considering its better adverse effect profile (treatment at discharge: 100 mg BD brivaracetam, 200 mg BD lacosamide, and 10 mg BD clobazam).
Figure 1.
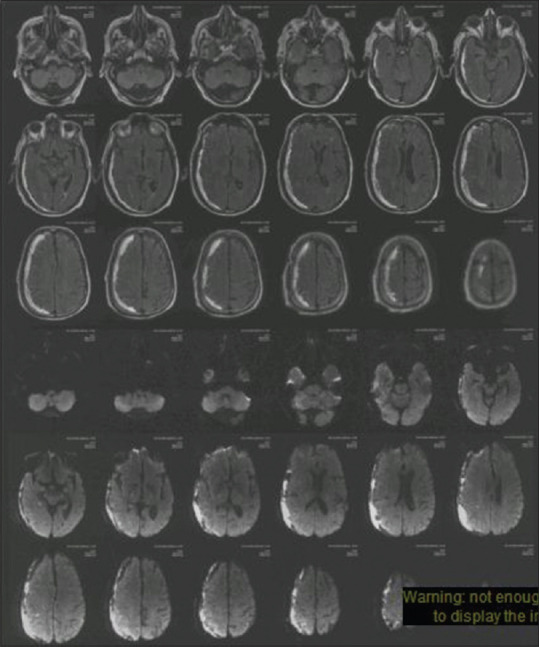
MRI of the brain axial FLAIR and DWI sequences showing a right frontoparietal subdural hematoma. No acute infarct is seen
He developed increasing drowsiness 1-week post-discharge along with occasional 'no-no' head nodding and had to be readmitted to the intensive care unit on day 10 of discharge due to unresponsiveness to stimuli. The Glasgow Coma Scale (GCS) score was E3V3M5 upon admission along with Cheyne-Stokes breathing pattern without paucity of the limb movements. Computed tomography (CT) of the head revealed no significant changes [Figure 2] and the EEG revealed diffuse theta slowing (not responsive to midazolam). A metabolic cause was considered and investigated. Routine investigations (including liver function tests, Human Immunodeficiency Virus (HIV), Hepatitis B and C), thyroid function, arterial blood gas analysis, and cultures were within normal limits. The ultrasound of the abdomen was normal (no evidence of fatty liver/hepatitis or cirrhosis). The drug levels could not be done due to the absence of their assays and serum ammonia was tested as a last resort. Remarkably, it was found to be elevated (197 μmol/L; normal <60 μmol/L). An autoimmune hepatitis workup was ordered subsequently and was also found to be negative.
Figure 2.
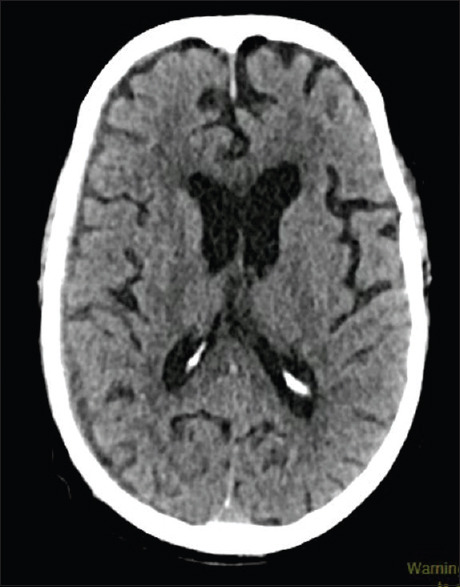
NCCT of the head showing a nearly resolved right subdural hematoma without any parenchymal insult
An iatrogenic cause was suspected and since levetiracetam is known to rarely cause hyperammonemia, brivaracetam was considered the culprit. Lamotrigine was added and brivaracetam was gradually tapered and stopped. Levetiracetam, although tolerated well previously, was not restarted for fear of a similar side effect. Simultaneously, the measures for decreasing hyperammonemia were instituted (rifaximin and lactulose). His consciousness started improving from day 3 (coinciding with a fall in blood ammonia levels) and he regained full sensorium on day 14 (blood ammonia: 21 μmol/L). He continues to be independent without any seizures or deficits 3 months post-discharge.
An extensive literature review did not reveal any case of brivaracetam-induced encephalopathy and we report the first such occurrence. Our case was challenging to manage as he suffered from rarities abound. Subdural hematoma is a known risk factor for status epilepticus with a prevalence of 0.5%.[1] Valproate and phenytoin were not used in this patient due to their Cytochrome P450 (CYP) inhibiting and inducing properties, respectively,[2] because of his multiple comorbidities and treatments and the eventual need for anticoagulation due to non-valvular atrial fibrillation (CHADS2VaSc score-4).[3] Phenytoin was not given its adverse effect on cardiac conduction.[4] Although lacosamide can have similar conduction defects, it seemed like the safer alternative between the two and was well-tolerated.[5] Levetiracetam is a relatively well-tolerated anti-seizure medication (ASM). Hyperammonemia has been reported extremely rarely[6] and is relatively commoner in individuals with renal failure. Our patient tolerated levetiracetam well but developed hyperammonemic encephalopathy with brivaracetam, and had a complete recovery with its cessation.
The pathophysiology of hyperammonemia in this setting is poorly understood and yet to be elucidated clearly. A meta-analysis of pivotal brivaracetam trials showed that although brivaracetam has some inhibitory effect on the CYP systems, no clinically relevant changes in the plasma drug concentrations occur.[7] It is unlikely to cause clinically meaningful drug-drug interactions, and therefore, no dosage adjustments are required when co-administered with common ASMs (carbamazepine, lacosamide, levetiracetam, lamotrigine, oxcarbazepine, phenytoin, phenobarbitone, valproate, topiramate, pregabalin, and zonisamide).[7] Although the interaction of brivaracetam 200 mg/day with clobazam could not be commented upon in this meta-analysis (due to the patient number being less than 10 in this group), the forest plot showed an absence of significant alteration of plasma concentrations of clobazam upon co-administration.[7] However, this CYP2C19 inhibition of brivaracetam leading to increased levels of clobazam and subsequent encephalopathy might be a plausible mechanism in our patient which needs further studying in a larger patient population.
Chronic brivaracetam has not been associated with clinically apparent liver injury (with significant elevations in the serum aminotransferase values) to date.[8] However, its older congener levetiracetam, with a similar mechanism of action, has been rarely associated with hepatocellular injury due to a hypersensitivity reaction manifesting between 1 and 20 weeks after treatment initiation.[8] Similar hepatocellular injury-causing hypersensitivity reactions can theoretically occur with brivaracetam and its limited use might have precluded their clinical occurrence or missed due to a lack of suspicion or attribution to other potential causes. Our patient developed an adverse effect 1 week into brivaracetam treatment and early diagnosis and the treatment may have prevented significant hepatocellular injury and led to complete recovery.
Restarting anticoagulation was also a complicated situation with the absence of clear guidelines. Apixaban was restarted after 4 weeks of surgery without any bleeding complications.
Newer ASMs, although promising, lack long-term data on their adverse effect profiles. Their use might be counter-productive in challenging situations like ours and should be a last resort rather than the first choice. Older ASMs are time-tested and the therapeutic monitoring available for them also helps. Relegating them to the bench because of newer ASMs is akin to mending something which is not broken!
Learning points
1. Brivaracetam can cause encephalopathy.
2. Switching from older to newer anti-seizure medications is unwarranted unless it is done for a specific reason.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
All faculty members, residents and nursing staff who were involved in managing this case.
REFERENCES
- 1.Seifi A, Asadi-Pooya AA, Carr K, Maltenfort M, Emami M, Bell R, et al. The epidemiology, risk factors, and impact on hospital mortality of status epilepticus after subdural hematoma in the United States. Springerplus. 2014;3:332. doi: 10.1186/2193-1801-3-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246–55. doi: 10.1111/j.1365-2125.2005.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 4.Brown G, Jang W, Peets A, Ramanathan K. Phenytoin-induced cardiac conduction abnormalities. J Card Crit Care TSS. 2020;4:140–3. [Google Scholar]
- 5.Chinnasami S, Rathore C, Duncan JS. Sinus node dysfunction: An adverse effect of lacosamide. Epilepsia. 2013;54:e90–3. doi: 10.1111/epi.12108. [DOI] [PubMed] [Google Scholar]
- 6.Verma R, Lalla R, Patil TB. Levetiracetam induced encephalopathy in a patient with normal renal function: An unusual clinical encounter. Ann Indian Acad Neurol. 2013;16:727–9. doi: 10.4103/0972-2327.120430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley BD, Chanteux H, Nicolas J-Marie, Laloyaux C, Gidal B, Stockis A. A review of the drug-drug interactions of the antiepileptic drug Brivaracetam. Epilepsy Res. 2020;163:106327. doi: 10.1016/j.eplepsyres.2020.106327. [DOI] [PubMed] [Google Scholar]
- 8.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Brivaracetam. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548532/ Updated 2017 Oct 02. [PubMed] [Google Scholar]


