Abstract
Delayed language development may be an early indicator of autism spectrum disorder (ASD). Early intervention is critical for children with ASD, and the present study presents pilot data on a clinical trial of omega-3 and -6 fatty acid supplementation and language development, a secondary trial outcome, in children at risk for ASD. We randomized 31 children to receive an omega-3 and -6 supplement or a placebo for 3 months, and measured their language abilities at baseline and after supplementation. Gesture use, but not word production, increased for children in the treatment group more than children in the placebo group. These results suggest possible effectiveness of omega-3 and -6 supplementation for language development in children at risk for ASD.
Keywords: Children born preterm, Autism spectrum disorder, Language development, MacArthur Bates communicative development inventory, Omega-3 fatty acids, Omega-6 fatty acids
Introduction
Delays in language development are a hallmark of autism spectrum disorder (ASD, for review: Groen et al. 2008), and understanding the language development of children at risk for ASD is crucial to early interventions. Gesture use is an early marker of language development (Cattani et al. 2010; Gordon and Watson 2015; LeBarton et al. 2015; LeBarton and Iverson 2016; Parlade and Iverson 2015; Sansavini et al. 2011), and studying gesture use can provide an earlier window into language difficulties than word comprehension or use. Children with ASD or those who are considered high risk for ASD use fewer gestures than typically developing children (Gordon and Watson 2015; LeBarton and Iverson 2016), and gesture use in children with ASD has been linked to later language abilities (Gordon and Watson 2015; Mundy et al. 1987). Children who were later diagnosed with ASD also displayed less coordinated communication from 12 to 18 months, defined as using gestures with vocalizations, than peers who were later diagnosed with a language delay (Parlade and Iverson 2015). Gesture use, therefore, represents an important early indicator of ASD risk that may also help distinguish among related diagnoses.
Infants born preterm represent a population at risk for both ASD (Johnson et al. 2010; Kuzniewicz et al. 2014; Pritchard et al. 2016; Verhaeghe et al. 2016) and early childhood language delays (Cattani et al. 2010; Foster-Cohen et al. 2007; Sansavini et al. 2011; Spittle et al. 2017). In both children with ASD and children born preterm, later language difficulties have been linked to worse scores on neonatal assessments such as the Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS, Spittle et al. 2017), altered brain activity such as delayed N400 when processing spoken language (Coderre et al. 2017), and early gross and fine motor abilities (Mody et al. 2017), indicating an early neurological basis for later language delays. Investigating gesture use in children born preterm may help parents and clinicians identify those at risk for ASD or language delay diagnoses and intervene earlier.
Interventions designed to prevent language deficits should address both altered neurological development (such as that set in motion by premature birth) and provide behavioral adaptations that will help children develop similarly to their typically developing peers. With a focus on prevention, nutritional support of the brain has been shown to be key to preventing later deficits in children born preterm, and a Cochrane review concluded that supplementation during infancy with omega-3 fatty acids was beneficial to children born preterm (Simmer et al. 2008). Omega-3 fatty acids are one family of polyunsaturated fatty acids (PUFA) that accrete rapidly in the brain during gestation, particularly in the third trimester (Martinez 1992; Martinez and Mougan 1998). Infants born preterm possibly benefit from supplementation with omega-3 fatty acids because they miss this period of considerable omega-3 fatty acid accretion in utero.
Deficiencies in omega-3 fatty acids have been shown to affect neuronal structure and function by reducing neurite elongation (Ikemoto et al. 1997), reducing neuron size in the hippocampus (Ahmad et al. 2002a, b), and reducing basal acetylcholine levels and muscarinic receptor binding in the hippocampus (Aid et al. 2003), and all three changes are associated with worse working memory performance in animals (Catalan et al. 2002; Moriguchi et al. 2000). Additionally, deficiencies in omega-3 fatty acids have been linked to increased serotonin receptor density (Delion et al. 1994) and increased serotonin receptor binding (du Bois et al. 2006) along with decreased dopaminergic receptor density, decreased dopamine levels, and decreased dopamine presynaptic terminals (Delion et al. 1994; Zimmer et al. 2000), and both omega-3 fatty acid deficiency and changes in dopamine and serotonin have been linked to depression (McNamara 2010; McNamara et al. 2007, 2010; McNamara and Liu 2011; Weiser et al. 2015), attention-deficit/hyperactivity disorder (Jucaite et al. 2005; Spencer et al. 2007), and bipolar disorder (Patrick and Ames 2015; Schneider et al. 2012).
There is some limited evidence that supplementation with omega-3 fatty acids reduces language deficits (Johnson et al. 2016; Meldrum et al. 2012; Richardson and Montgomery 2005) and ASD symptoms (Amminger et al. 2007; Parletta et al. 2016; Keim et al. under review). Specifically, supplementation with omega-3 and -6 fatty acids was related to increased gesture use at 12 and 18 months (Meldrum et al. 2012), phonological decoding and visual analysis, two measures considered indicative of neural processing of written material (Johnson et al. 2016), and children who received omega-3 fatty acid supplementation were no longer behind their peers in reading level (Richardson and Montgomery 2005). However, other studies have found no effect on any included measures of language ability in typically-developing children (Auestad et al. 2001; Lauritzen et al. 2005; Scott et al. 1998). For ASD symptoms, studies have shown reductions in hyperactivity (Amminger et al. 2007) and stereotypy (Amminger et al. 2007; Bent et al. 2014), and there has been early evidence of reductions in total ASD symptoms from one cross-sectional study (Parletta et al. 2016) and the report of primary outcomes of the supplementation trial being reported here (Keim et al. under review). However, other studies have reported no effects on hyperactivity or stereotypy (Bent et al. 2014; Mankad et al. 2015) or total ASD symptoms (Voigt et al. 2014), leading to a lack of definitive recommendations as to whether and how to supplement children at risk for poor outcomes.
Omega-3 and -6 fatty acids have complementary roles in neuronal structure and function, with omega-6 fatty acids linked to protein kinase activity (Schaechter and Benowitz 1993), ion currents (Fang et al. 2011), and the induction of long-term potentiation (LTP), a process associated with consolidation of memory and learning (Bliss and Collingridge 1993; Kato et al. 1991; Lynch and Voss 1994). Omega-3 and -6 fatty acids are metabolized using the same metabolic resources (Lee et al. 2016; Nakamura and Nara 2004), and there is evidence of competition for metabolic resources (Bourre et al. 1993, 1989; Gibson et al. 2013; Harnack et al. 2009). Omega-3 and -6 fatty acids at the top of the metabolic chain (Supplementary Fig. 1) are processed through several desaturase and elongase steps to produce long chain fatty acids further down the pathway, such as eicosapentaenoic acid (EPA, omega-3), docosahexaenoic acid (DHA, omega-3), and arachidonic acid (ARA, omega-6), which are fatty acids that have been strongly linked to human health (for review: Bowen et al. 2016; Dyall and Michael-Titus 2008; Harris 2006; Innis 2014; Kris-Etherton et al. 2010; Lapillonne and Moltu 2016). There is some evidence that supplementation with a combination of omega-3 and omega-6 fatty acids is more beneficial to cognitive function than supplementation with omega-3 fatty acids alone (Koletzko et al. 2008; Willatts and Forsyth 2000).
The present study was a pilot randomized clinical trial (RCT) that aimed to determine the feasibility of a full-scale trial of the effect of omega-3 and -6 fatty acid supplementation on ASD symptoms in children born preterm who were at increased risk for a diagnosis of ASD. A secondary goal of the trial, and the focus of the analyses reported here, was to explore treatment effects on language abilities in these children at higher risk for ASD.
Methods
Setting and Participants
The Preemie Tots trial was a pilot, single-site, double-blinded, RCT (NCT01683565) examining the effect of an omega-3 and -6 supplement on development in preterm infants considered at risk for ASD based on pre-specified screening criteria. The study, conducted from November 2012 to March 2015, was reviewed and approved by the Institutional Review Board at Nationwide Children’s Hospital (NCH), Columbus, OH, and each child’s primary caregiver provided written informed consent.
Children 18–38 months old who (1) were born at fewer than 30 completed gestational weeks, and either (2) admitted to the NCH neonatal intensive care unit (NICU) after birth or (3) had a Neonatology Clinic follow-up visit at NCH were recruited for the study. Primary caregivers of children who were potentially eligible completed a questionnaire that included the Pervasive Developmental Disorders Screening Test – II, Stage 2 (PDDST-II), the Brief Infant Toddler Social and Emotional Assessment (BITSEA), and one joint attention item from the Ages and Stages Questionnaire, “Looks in the direction you are pointing, when you are pointing at something”. Children who scored >5 on the PDDST-II, below the 15th percentile on the BITSEA competence scale, did not respond to their name, or did not respond to joint attention bids were further assessed for trial eligibility (n = 201).
Toddlers who met the above criteria had to weigh between the 5th and 95th percentiles of the World Health Organization (WHO) growth standards for age and sex, and the primary caregiver had to be proficient in English. Children were excluded if they consumed high-DHA foods, such as omega-3 fatty acid supplements, fatty fish, or beverages with added DHA, more than twice per week, were known to be unable to tolerate venipuncture, or had been diagnosed with any of the following conditions: fragile X, Rett syndrome, Angelman syndrome, tuberous sclerosis complex, deafness, blindness, quadriplegic cerebral palsy, fish or canola oil allergy, type 1 diabetes, or a bleeding disorder. Children with a score of <70 on the Bayley Scales of Infant and Toddler Development (Bayley) clinical assessment (within the past year) or who had had a recent non-febrile seizure without a clear and resolved etiology were also excluded. In all, 106 children met the inclusion criteria. The enrollment goal was 40 children, and due to funding limitations, 31 children were ultimately enrolled in the trial.
Procedures
Children were randomized to one of four color-coded groups (two colors for treatment, two colors for placebo) using a varying block size of multiples of 4 with 1:1 allocation to treatment and placebo. All investigators and staff remained blinded to participant allocation throughout the trial, and a statistician at a separate institution prepared the randomization scheme. Twins were allocated to the same group to avoid accidental treatment crossover. The treatment group received 3 months of an oral omega-3, -6, and -9 fatty acid supplement that included, as a single liquid daily dose of 2.5 ml, 338 mg EPA, 225 mg DHA, 280 mg total omega-6 fatty acids (including 83 mg GLA), and 306 mg total omega-9 fatty acids (Omega-3-6-9 Junior™, Nordic Naturals, Inc., Watsonville, CA). The placebo group received 3 months of oral canola oil, also as a single liquid daily dose of 2.5 ml, which contained 129 mg palmitic acid, 39 mg stearic acid, 513 mg linoleic acid (LA, omega-6), 225 mg alpha-linolenic acid (ALA, omega-3), and 1346 mg oleic acid. Both oils were lemon-flavored (lemon oil flavor added to the placebo by NCH Investigational Drug Services (IDS)), and both products were packaged identically and distributed to the families through the hospital pharmacy.
Baseline Study Visit (Day 0)
The primary caregiver completed questionnaires on the following: demographics, developmental delays, diagnoses, and prescriptions and supplements (if any), the child’s diet focused on number of servings of fatty fish, moderately fatty fish, and white fish/shellfish each month, and child behavior which included the Toddler Behavior Assessment Questionnaire – Short Form (TBAQ-SF), the Child Behavior Checklist (CBCL), the MacArthur-Bates Communicative Development Inventory (CDI; to assess language), the Vineland Adaptive Behavior Scales (VABS; to assess socialization), and the Infant/Toddler Sensory Profile (ITSP; to assess sensory processing). Children’s weight, height, and head circumference were measured, and a blood sample for fatty acid quantification was obtained by a trained phlebotomist. Primary caregivers were given an adherence diary to record whether the child received the provided oil each day over a 2-week period. The families received all adherence diaries at the beginning of the study, and they returned the diaries at the second and final study visits.
Second Study Visit (45 Days Post-Randomization)
Children randomized into the RCT returned for a second study visit that occurred 45 +/− 9 days post-randomization. Data collection at the second study visit provided a time to review the adherence diary and answer any questions. The primary caregiver completed the Willett Food Frequency Questionnaire (FFQ, Willett et al. 1985) about the child’s typical diet, and the child’s weight, height, and head circumference were measured. The child also provided another blood sample for fatty acid quantification.
Final Study Visit (3 Months Post-Randomization)
Children randomized into the RCT returned for a final study visit that took place 90 +/− 28 days post-randomization. The ASD screening questionnaires (e.g., BITSEA and PDDST) and baseline study visit questionnaires were repeated. The child’s weight, height, and head circumference were again measured, and the child provided a final blood sample for fatty acid quantification.
Data Reduction and Statistical Analyses
The data used for the current analyses were the CDI language assessments at the baseline study visit and final study visit and treatment allocation. The CDI is a widely used measure of language development (Alexander Pan et al. 2004; Arriaga et al. 2008; Bruckner et al. 2007; Dale 1991; Fenson et al. 2000; Rescorla 1991; Ring and Fenson 2000) that includes a Words and Gestures form for ages 8–18 months, a Words and Sentences form for ages 16–30 months, and a CDI-III for use with children 30–37 months old. Given the delays in language development commonly seen in children born preterm, a combination of the short form of the CDI Words and Gestures and Words and Sentences forms was created for use in the present study. By collecting data on both gesture and word use, we ensured that we collected as comprehensive a view of each child’s language development as possible. Due to the use of a modified set of questions from both the Words and Gestures and Words and Sentences forms, the data were scored following the standard CDI scoring system with slight modifications. A gesture score was calculated by summing the number of Yes responses to the gesture use questions (Supplementary Table 1, 12 gestures total), a word score was calculated by summing the number of words the primary caregiver indicated the child used (Supplementary Table 1, 100 words total), and a words and gestures score was calculated by summing both gestures and words used.
An important element of gesture use is the functional utility of the gesture. Deictic gestures are considered those that are bound to context and usually include a specific referent in the environment (e.g., pointing to obtain a specific object), whereas representational gestures are not bound to context and usually represent a referent that may or may not be present (e.g., shaking head for ‘no’, Garton 1985). Representational gestures, therefore, tend to be used for more general social communication. To explore potential differences in deictic and representational gesture use in the present sample, the 12 gestures from the CDI forms were divided into deictic gestures (5) and representational gestures (7) as shown in Supplementary Table 1, and the sums of each type of gesture were included in analyses. Deictic gestures were very commonly endorsed, especially at the final visit with only two children in the placebo group having only four deictic gestures and the rest of the children having all five deictic gestures. Therefore, a logistic regression was run predicting deictic gestures with completed weeks’ gestation, BITSEA ASD scale scores, and PDDST scores to obtain the probability of having all five deictic gestures. These probabilities were also included as outcome variables in the model. Additionally, the children’s scores were compared to normative data available through Wordbank (Frank et al. 2016).
All analyses were conducted with SAS software (v9.4, SAS Institute, Cary, NC). All analyses were conducted according to intent-to-treat (all randomized children were included), and no interim analyses were conducted. Due to our small sample size and the inclusion of twins, each set of twins was analyzed as a single observation by calculating the mean of the pair for all variables. We used mixed effects regression according to the method described by Winkens et al. (2007) to compare changes in language abilities (continuous variables for total gestures, total words, total words and gestures, deictic gestures, representational gestures, and the probability of having all deictic gestures) in the treatment and placebo groups controlling for baseline scores. Treatment-by-time interaction terms were included as fixed effects to serve as estimates of the treatment effect. No covariates were included in the models. Data were first assessed for violations of the general linear model, and only the distributional issues with deictic gestures mentioned above were found. Continuous variables are reported as mean ± standard deviation (SD), and categorical variables are reported as percentages.
Results
Participant Characteristics
The treatment and placebo groups did not differ on any demographic or anthropometric measures at the baseline study visit, including gestational weeks at birth, height, weight, sex, maternal education, or monthly family income (Table 1). The toddlers in both groups grew across the 3-month intervention, and both height and weight increased similarly in the treatment and placebo groups. As reported in the primary outcomes paper (Keim et al. under review), the toddlers in the treatment and placebo groups had similar risk for ASD at baseline, and supplementation with omega-3 and -6 fatty acids significantly improved BITSEA ASD scale scores across the intervention. Toddlers in the treatment group also had increased DHA, EPA, and total omega-3 blood levels and decreased ARA levels and omega-6 to omega-3 fatty acid ratios at the final study visit compared to the toddlers in the placebo group whose fatty acid levels did not change significantly. Overall, the randomization worked to create two similar samples at baseline, and the supplementation with omega-3 and -6 fatty acids predictably altered fatty acid blood levels.
Table 1.
Descriptive statistics for the Preemie Tots sample
| Baseline |
Final visit |
|||
|---|---|---|---|---|
| Treatment (n = 14) | Placebo (n = 13) | Treatment (n = 12) | Placebo (n = 12) | |
| Gestational weeks | 26.5 (1.7) | 26.9 (1.7) | ||
| Corrected age (months) | 30.8 (5.9) | 29.6 (6.2) | 33.9 (6.5) | 32.4 (6.1) |
| Weight (lbs) | 12.9 (2.5)a | 12.0 (2.3)a | 13.4 (2.8)b | 12.4 (2.2)b |
| Height (cm) | 88.3 (7.0)a | 85.2 (7.0)a | 91.1 (7.8)b | 88.9 (7.5)b |
| Family monthly income | ||||
| <$1667 | 38.5% (5) | 10.0% (1) | ||
| $1667–$2917 | 23.1% (3) | 20.0% (2) | ||
| $2917–$3378 | 7.7% (1) | 20.0% (2) | ||
| >$3378 | 30.8% (4) | 50.0% (5) | ||
| Maternal education | ||||
| <A.S. degree | 57.1% (8) | 69.2% (9) | ||
| A.S. degree or beyond | 42.9% (6) | 30.8% (4) | ||
| Female | 38.5% (5) | 10.0% (1) | ||
| Ever breastfed | 92.3% (12) | 90.0% (9) | ||
| Participant diagnosed with ASD | 7.7% (1) | 30.0% (3) | ||
| Sibling diagnosed with ASD | 15.4% (2) | 0 | 23.1% (3)^ | |
| BITSEA ASD score | 7.1 (3.7)c | 6.5 (4.1)c | 5.3 (2.6)d | 7.5 (3.3)c |
| PDDST score | 6.79 (2.2) | 5.2 (2.5) | 6.0 (2.6) | 5.4 (2.7) |
| VABS play | 17.7 (8.8) | 19.8 (11.9) | 22.8 (8.3) | 25.0 (6.7) |
| VABS relate | 28.7 (10.7)a | 28.9 (10.0)a | 40.1 (9.4)b | 23.8 (11.7)a |
| DHA (% mmol) | 4.9 (1.1)c | 5.1 (1.2)c | 6.6 (1.1)d | 5.6 (1.0)c |
| EPA (% mmol) | 0.7 (0.4)c | 0.9 (0.5)c | 1.6 (0.4)d | 0.9 (0.5)c |
| ARA (% mmol) | 16.8 (1.4)c | 16.1 (1.6)c | 15.7 (1.7)d | 16.4 (1.0)c |
| Omega-3 (% mmol) | 7.3 (1.0)c | 8.0 (1.3)c | 9.6 (1.1)c | 8.2 (0.8)c |
| Omega-6 (% mmol) | 42.8 (5.9) | 51.5 (22.7) | 42.7 (5.8) | 42.4 (4.8) |
| Omega-6/Omega-3 Ratio^* | 6.1 (1.4)a | 6.6 (3.0)a | 4.5 (0.9)b | 5.1 (0.8)b |
Different letters in a row signify significant differences (p < 0.05)
ASD autism spectrum disorder, BITSEA brief infant toddler social emotional assessment, PDDST pervasive developmental disorders screening test-II, VABS Vineland Adaptive Behavior Scales, DHA docosahexaenoic acid, EPA eicosapentaenoic acid, ARA arachidonic acid
Significant difference from the baseline to final study visits
Significant difference between treatment groups
One child had a sibling diagnosed with ASD between the baseline and final study visits
The present sample was quite different from typically developing children in their gesture and word use. Based on normative data from WordBank, the 50th percentile of 30-month-olds who completed the English CDI Words and Sentences form produced 541 words (79.6% of the 680 words queried). In the present sample, the toddlers produced a mean of 36 words (SD = 33.2, range 2.5–100, 36% of words queried) at the baseline visit and a mean of 42.9 words (SD = 31.4, range 9.5–100, 42.9% of the words queried) at the final study visit. Based on the percentages of words endorsed, on extrapolation, the current sample would have endorsed around 244 words on the full Words and Sentences form at baseline and around 291 words at the final visit. These production scores place them between the 10th and 20th percentile for 28-month-olds at baseline and the final visit. The children in the present sample were performing closer to typically-developing 18-month-olds, yet their average age was 30 months.
As has been reported by others, the emergence of word use in the Preemie Tots sample followed a similar pattern to word use among typically developing children, simply at a delay (Cattani et al. 2010; Sansavini et al. 2011). The top ten most-endorsed words in the Preemie Tots sample were very similar to highly endorsed words in typically-developing 28-month-olds, such as ‘bye’, ‘hi’, ‘no’, and ‘ball’. However, the caregivers still endorsed these words at lower rates. The caregivers of 97–99% of typically developing 28-month-olds endorsed all of the top ten words, whereas only 79% of caregivers endorsed ‘ball’ and only 78% endorsed ‘dog’ in our sample.
Effect of Omega-3 and -6 Supplementation on Language in Preterm Infants
Children in the treatment and placebo groups did not differ on any language measures at baseline (Table 2). Children who received an omega-3 and -6 supplement for 3 months increased their combined gesture and word use from baseline significantly more than children who received the placebo oil (F(1,26) = 5.37, p < 0.05, Table 2). The difference between the treatment and placebo groups in combined gesture and word use was driven by significant differences in gesture use as the treatment group produced significantly more gestures than the placebo group after supplementation (F(1, 26) = 5.47, p < 0.05, Fig. 1a and Table 2), but there were no significant differences between the treatment and placebo group in word use alone over the course of the intervention (F(1,26) = 0.01, p > 0.05, Fig. 1b and Table 2). Instead, all children increased somewhat in their word use over the 3 months (F(1,26) = 7.05, p < 0.05, Fig. 1b and Table 2). For specific types of gestures, children in the treatment group increased their representational gesture use compared to children who received the placebo (F(1,22) = 4.72, p < 0.05, Fig. 1d and Table 2). There were no differences in deictic gesture use between the two groups neither when comparing the sums of deictic gestures (F(1,22) = 2.08, p > 0.05, Fig. 1c and Table 2) nor when comparing the probabilities of having all five deictic gestures (F(1,22) = 0.5, p > 0.05, Table 2). These results indicate that supplementation with omega-3 and -6 fatty acids increased broader social communicative gesture use among children born preterm.
Table 2.
Comparison of Preemie Tots CDI scores in treatment and placebo groups across the study
| Baseline |
Final visit |
Change from baseline to final visit |
||||
|---|---|---|---|---|---|---|
| Treatment (n = 14) | Placebo (n = 13) | Treatment (n = 12) | Placebo (n = 12) | Treatment | Placebo | |
| CDI words and gestures | 12.5 (2.4) | 12.1 (1.4) | 12.9 (1.5)*^ | 11.0 (2.3)^ | 0.4τ | −1.1τ |
| CDI gestures | 9.6 (2.3) | 9.1 (1.4) | 10.9 (1.4)* | 9.0 (2.3) | 1.3 | 0.1 |
| CDI words | 40.7 (37.7) | 31.0 (28.2) | 45.8 (33.4)^ | 39.7 (30.4)^ | 5.1τ | 8.7τ |
| Deictic gestures | 4.8 (0.4) | 4.5 (0.7) | 5.0 (0) | 4.8 (0.5) | 0.2 | 0.3 |
| Representational gestures | 5.3 (1.4) | 4.7 (1.8) | 6.2 (1.1)* | 4.3 (2.3) | 0.9 | −0.4 |
| Probability of having all deictic gesturesZ | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0 | 0 |
All values represent the count of words or gestures or both except the probability of having all deictic gestures
CDI MacArthur-Bates communicative development inventory
Significantly different from the placebo group at the same time point (p < 0.05).
Significantly different from baseline in the same group (p < 0.05)
Significantly different change from baseline to final visit
At baseline, one toddler in the placebo group had only three deictic gestures, and three toddlers in the placebo group and two toddlers in the treatment group had only four deictic gestures. The rest had all five. At the final visit, all children in the treatment group had all five deictic gestures, and two children in the placebo group only had four deictic gestures. Therefore, the model was run after first creating a binary variable where 0 = did not have all deictic gestures and 1 = had all deictic gestures. The likelihood of having all deictic gestures was then predicted by weeks of completed gestation, BITSEA ASD scores, and PDDST scores. The likelihoods were output as a separate variable and predicted by time and treatment group with the original mixed model. The values in the table represent the likelihood that a child in each group at each data collection time had all deictic gestures
Fig. 1.
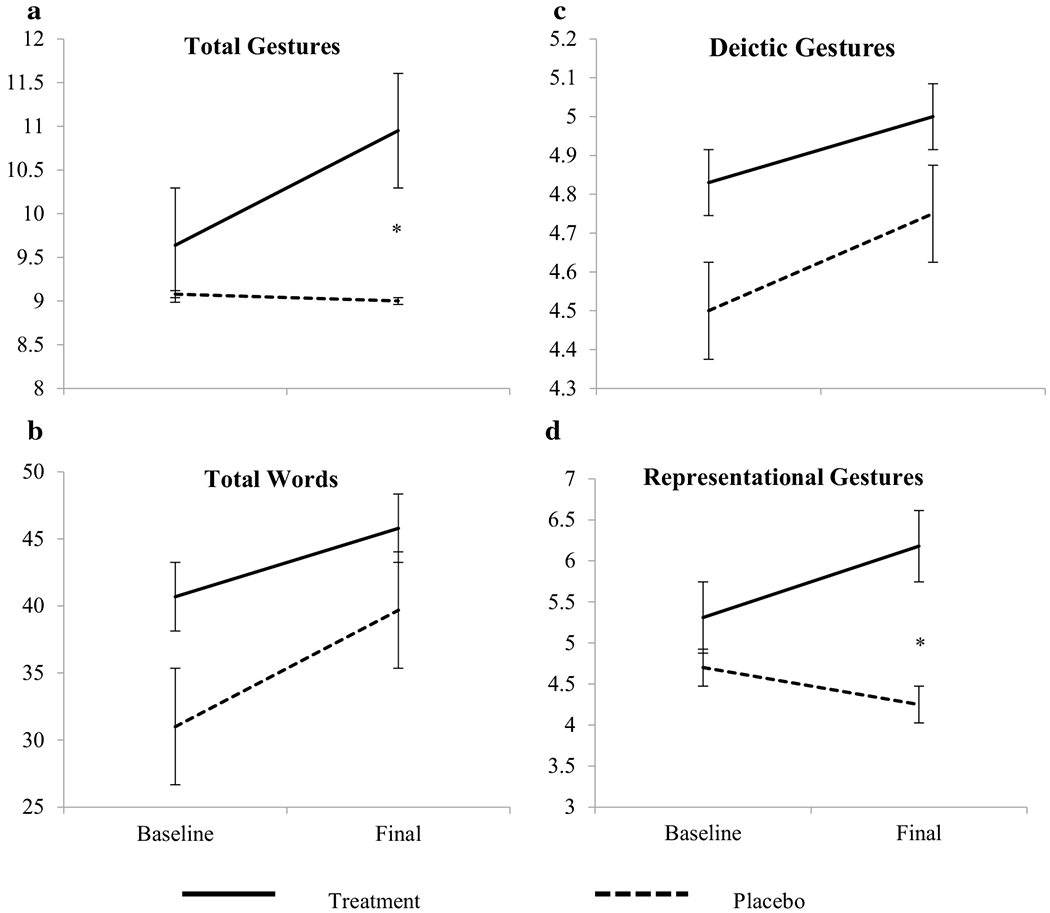
CDI gesture and word scores across time in the treatment and placebo groups. a Total gesutres, b total words, c deictic gestures, and d representational gestures. Dotted lines indicate the placebo group, and solid lines indicate the treatment group. Asterisk denotes statistically a significant difference (p < 0.05). Bars indicate standard error
Discussion
The present study demonstrated that supplementation with omega-3 and -6 fatty acids increased representational gesture use in children 18–36 months old who were born preterm and had a high risk for ASD. Word use was not altered by supplementation. These results indicate that very early language development might, on confirmation, improve in children born preterm at high risk for ASD using omega-3 and -6 fatty acids. This finding is especially important because gesture use is considered an early indicator of language development that is linked to later language abilities (Cattani et al. 2010; Gordon and Watson 2015; LeBarton et al. 2015; LeBarton and Iverson 2016; Parlade and Iverson 2015; Sansavini et al. 2011) and can serve as an early indicator of possible language delays (Gordon and Watson 2015; LeBarton and Iverson 2016; Parlade and Iverson 2015). This study provides support for a larger trial to confirm the usefulness of omega-3 and -6 fatty acid supplementation to promote language development in children born preterm.
The present study adds to the body of literature indicating that omega-3 and -6 fatty acid supplementation is effective for improving cognitive functions in children born preterm (Clandinin et al. 2005; Fewtrell et al. 2004; Henriksen et al. 2008; Simmer et al. 2008; Willatts and Forsyth 2000). There is evidence that omega-6 and omega-3 fatty acids are maximally processed when administered in combination, and such combinations may be important in improving developmental outcomes (Gibson et al. 2013; Harnack et al. 2009). Over-supplementation with omega-3 fatty acids leads to detrimental consequences (Wainwright et al. 1999, 1997), and the addition of omega-6 fatty acids provided in the supplement used in this study may afford a more optimal metabolic environment. Supplementation with omega-6 fatty acids has been controversial. Previous work predominantly used linoleic acid (LA, omega-6) in supplements, and demonstrated that adding omega-6 fatty acids reduced the amount of longer chain omega-3 PUFAs in the blood and may have contributed to worse cardiovascular outcomes in several adult samples (Barden et al. 2016; Farvid et al. 2014; Serhan and Savill 2005). However, the treatment group in the present study received omega-6 fatty acids in the form of gamma-linoleic acid (GLA, omega-6), and actually had significantly reduced ARA blood levels in addition to the increased DHA levels. The use of GLA in the supplement may have reduced the need to use delta-6 desaturase to process LA to GLA. In doing so, the delta-6 desaturase would be available to process omega-3 fatty acids instead, further enhancing omega-3 availability.
The present study adds important information about how omega-3 and -6 fatty acid supplementation may affect gesture use in children born preterm. As gesture use is an important early indicator of language development, the use of omega-3 and -6 supplementation to help children at risk for language delays is promising. Omega-3 and -6 fatty acid supplements are readily available and easy for families to administer. In this study, specifically representational gesture use was improved with omega-3 and -6 fatty acid supplementation. The use of gestures requires coordination of communication goals and the motor system to produce the appropriate gesture. Omega-3 and -6 fatty acids have been shown to affect executive functions in older, typically developing children (Sheppard and Cheatham 2017), which are higher order cognitive functions that similarly require coordination among brain areas. Both deictic and representational gesture use require the former type of coordination (motor and communication goals), but representational gesture use may require more complex cognitive process coordination to represent the most abstract goal. Due to their complementary roles in neuronal function, it may be that we found effects on representational gesture use in this smaller sample because of the greater coordination of brain activity required. This greater coordination was in turn more highly affected by supplementation with omega-3 and -6 fatty acids. Supplementation provided to children born preterm may be particularly effective at aiding the development of skills that require coordination among brain areas.
The effect of the omega-3 and -6 fatty acid supplementation also may not directly target language abilities but social communication more broadly. The fact that representational gestures were more affected by supplementation than deictic gestures may reflect the fact that supplementation with omega-3 and -6 fatty acids affects overall social communication. This interpretation is supported by the correlations between representational gesture use and VABS Relate scale scores (rs = 0.7 and 0.8, ps < 0.05 for baseline and final visit, respectively) and the lack of correlations between VABS Relate scale scores and deictic gesture use (rs = 0.1 and 0.4, ps > 0.05 for baseline and final visit, respectively). VABS Relate scale scores also increased significantly in the treatment group across time (Table 1), supporting a potential effect of omega-3 and -6 supplementation on overall social communication. However, deictic gesture use, and not representational gesture use, has been previously linked to later language abilities in children with ASD (Ozcaliskan et al. 2016). The present study did not employ a measure designed to provide a comprehensive look at specific types of gestures, and therefore effects on deictic gesture use could simply be masked by the use of a less sensitive tool. Another possible explanation of the results could be that omega-3 and -6 fatty acid supplementation aided brain development more generally, and helped children born preterm progress faster in social communication more broadly. Omega-3 and -6 fatty acids are incorporated into different phospholipid pools in cell membranes (Chalon et al. 1998; Clandinin et al. 1991; Jumpsen et al. 1997a, b), and those phospholipid pools are not distributed uniformly in the brain (Breckenridge et al. 1973). By supplementing with omega-3 and -6 fatty acids, more diffuse effects may have occurred than would have been seen with solely omega-3 fatty acid supplementation.
The participants in this study were also at risk for an ASD diagnosis by virtue of their preterm birth. The improvement seen in more social communicative gestures may have occurred because omega-3 and -6 fatty acids have been linked to ASD symptoms (Amminger et al. 2007; Bent et al. 2014; Parletta et al. 2016; Keim et al. under review). The improvement seen in this study may have been related to the participants demonstrating improvements in ASD symptoms and not specific to language. However, the correlations between BITSEA ASD scale scores and all CDI scores were generally weak (ranging from −0.02 to −0.22 at baseline and 0.01 to −0.33 at the final visit), indicating that the changes reported in the BITSEA ASD scale scores and the changes in CDI scores reported here could represent two different effects. An interesting question for future prospective research is how to select children at risk for ASD for intervention. The children in the present study were screened using two standardized screening tools (BITSEA and PDDST) in addition to two key components of ASD (response to joint attention and response to name). Other studies have recruited children at risk due to having a sibling with ASD (e.g., Parlade and Iverson 2015). Both methods of identifying children at risk have their advantages, but they may also represent two different ASD etiologies that may or may not benefit from different treatment. Only three participants in the present study had a sibling diagnosed with ASD, and this sample may therefore be different from other samples commonly studied as at-risk for ASD. Future studies should examine the relevance of these differences on potential outcomes as efforts continue to be made to identify indicators of developmental challenges and intervene earlier.
The present study has limitations to address in future work. The sample size for this pilot was quite small, and the results need to be replicated in a larger sample. The children in this study were not screened specifically for meeting criteria for ASD but to capture children at risk through preterm birth and broader symptom scores on commonly used ASD screeners. The modification of the CDI is a weakness in that the data are not as directly comparable to studies that employed the standard instruments. However, the modifications proved to be a strength in that a large portion of the sample would have received scores in the bottom 5th percentile on the age-appropriate Words & Sentences measure, and it allowed for study of language development across developmental periods that bridged two versions of the measure. Merging the two questionnaires and including all gestures allowed us to analyze the different types of gestures and allowed for variability in scores for this sample of children known to have delayed language development.
In summary, this pilot study offers initial, suggestive evidence of the efficacy of omega-3 and -6 fatty acid supplementation in improving aspects of early language development in children at risk for ASD, specifically gesture use. The use of a combined omega-3 and -6 fatty acid supplement may be important for providing preterm children with an appropriate balance of omega-3 and -6 fatty acids that allows for the most efficient use of metabolic resources and the greatest production of fatty acids known to be important for brain development, especially DHA and EPA. The effects were seen in the use of representational gestures, which may also indicate general improvements in social communication rather than language, per se. Future research with larger samples over a longer period of time will help clarify the findings reported here. Studies that stratify children by other important factors, such as baseline ASD symptomatology and reason for at-risk status (e.g., sibling with a diagnosis, genetic predisposition, premature birth) will help clarify the role of omega-3 and -6 fatty acids in language abilities in children at risk for ASD.
Supplementary Material
Acknowledgments
We would like to thank the families who participated in the study and Yvette Bean, Kendra Heck, Chenali Jayadeva, Julia Less, and Kamma Smith of Nationwide Children’s Hospital for data collection and administrative support.
Funding
This study was funded by The Marci and Bill Ingram Fund for Autism Spectrum Disorders Research (no grant number), Cures Within Reach (no grant number), the National Center for Advancing Translational Sciences/NIH (UL1TR001070), and internal support from the Research Institute at Nationwide Children’s Hospital.
Footnotes
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. Nordic Naturals provided the investigational product, and Welsh, Holme, & Clark Co., Inc. provided canola oil at no cost. Neither the study sponsors nor product providers had a role in the study design.
Conflict of interest All authors declare that they have no conflicts of interest.
Ethical Approval All procedures performed in this study were in accordance with institutional ethical standards and with the 1964 Helsinki declaration and its later amendments.
Informed Consent Written informed consent (parental permission for the children) was obtained from all individual participants included in this study.
Electronic supplementary material The online version of this article (doi:10.1007/s10803-017-3249-3) contains supplementary material, which is available to authorized users.
References
- Ahmad A, Moriguchi T, & Salem N (2002a). Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatric Neurology, 26, 210–218. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Murthy M, Moriguchi T, Salem N, & Greiner RS (2002b). A decrease in cell size accompanies a loss of docosahexaenoate in the rat hippocampus. Nutritional Neuroscience, 5, 103–113. doi: 10.1080/10284150290018973. [DOI] [PubMed] [Google Scholar]
- Aid S, Vancassel S, Poumes-Ballihaut C, Chalon S, Guesnet P, & Lavialle M (2003). Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. Journal of Lipid Research, 44, 1545–1551. doi: 10.1194/jlr.M300079-JLR200. [DOI] [PubMed] [Google Scholar]
- Pan BA, Rowe ML, Spier E, & Tamis-Lemonda C (2004). Measuring productive vocabulary of toddlers in low-income families: Concurrent and predictive validity of three sources of data. Journal of Child Language, 31, 587–608. doi: 10.1017/s0305000904006270. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Berger GE, Schafer MR, Klier C, Friedrich MH, & Feucht M (2007). Omega-3 fatty acids supplementation in children with autism: A double-blind randomized, placebo-controlled pilot study. Biological Psychiatry, 61, 551–553. doi: 10.1016/j.biopsych.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Arriaga RI, Fenson L, Cronan T, Pethick SJ (2008). Scores on the MacArthur Communicative Development Inventory of children from lowand middle-income families. Applied Psycholinguistics, 19, 209 doi: 10.1017/s0142716400010043. [DOI] [Google Scholar]
- Auestad N, et al. (2001). Growth and development in term infants fed long-chain polyunsaturated fatty acids: A double-masked, randomized, parallel, prospective, multivariate study. Pediatrics, 108, 372–381. doi: 10.1542/peds.108.2.372. [DOI] [PubMed] [Google Scholar]
- Barden AE, Mas E, & Mori TA (2016). n-3 Fatty acid supplementation and proresolving mediators of inflammation. Current Opinion in Lipidology, 27, 26–32. doi: 10.1097/MOL.0000000000000262. [DOI] [PubMed] [Google Scholar]
- Bent S, et al. (2014). Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. Journal of the American Academy of Child and Adolescent Psychiatry, 53, 658–666. doi: 10.1016/j.jaac.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, & Collingridge GL (1993). A synaptic model of memory: Long-term potentiation in the hippocampus. Nature, 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Bourre JM, et al. (1989). The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. Journal of Nutrition, 119, 1880–1892. [DOI] [PubMed] [Google Scholar]
- Bourre JM, et al. (1993). Function of dietary polyunsaturated fatty acids in nervous system. Prostaglandins, Leukotrienes and Essential Fatty Acids, 48, 5–15. [DOI] [PubMed] [Google Scholar]
- Bowen KJ, Harris WS, & Kris-Etherton PM (2016). Omega-3 fatty acids and cardiovascular disease: Are there benefits? Current Treatment Options in Cardiovascular Medicine, 18, 69. doi: 10.1007/s11936-016-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge WC, Morgan IG, Zanetta JP, & Vincendon G (1973). Adult rat brain synaptic vesicles II. Lipid composition. Biochimica et Biophysica Acta, 320, 681–686. [DOI] [PubMed] [Google Scholar]
- Bruckner C, Yoder P, Stone W, & Saylor M (2007). Construct validity of the MCDI-I receptive vocabulary scale can be improved: Differential item functioning between toddlers with autism spectrum disorders and typically-developing infants. Journal of Speech Language and Hearing Research, 50, 1631–1638. [DOI] [PubMed] [Google Scholar]
- Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, & Salem N (2002). Cognitive deficits in docosahexaenoic acid-deficient rats. Behavioral Neuroscience, 116, 1022–1031. doi: 10.1037/0735-7044.116.6.1022. [DOI] [PubMed] [Google Scholar]
- Cattani A, Bonifacio S, Fertz M, Iverson JM, Zocconi E, & Caselli MC (2010). Communicative and linguistic development in preterm children: a longitudinal study from 12 to 24 months. International Journal of Language & Communication Disorders, 45, 162–173. doi: 10.3109/13682820902818870. [DOI] [PubMed] [Google Scholar]
- Chalon S, et al. (1998). Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. Journal of Nutrition, 128, 2512–2519. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, et al. (2005). Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. The Journal of pediatrics, 146, 461–468. doi: 10.1016/j.jpeds.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Cheema S, Field J, Garg ML, Venkatraman J, & Clandinin TR (1991). Dietary fat: Exogenous determination of membrane structure and cell function. FASEB Journal, 5, 2761–2769. [DOI] [PubMed] [Google Scholar]
- Coderre EL, Chernenok M, Gordon B, & Ledoux K (2017). Linguistic and non-linguistic semantic processing in individuals with autism spectrum disorders: An ERP study. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-016-2985-0. [DOI] [PubMed] [Google Scholar]
- Dale PS (1991). The validity of a parent report measure of vocabulary and syntax at 24 months. Journal of Speech and Hearing Research, 34, 565–571. [DOI] [PubMed] [Google Scholar]
- Delion S, Chalon S, Herault J, Guilloteau D, Besnard J-C, & Durand G (1994). Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. Journal of Nutrition, 124, 2466–2476. [DOI] [PubMed] [Google Scholar]
- du Bois TM, Deng C, Bell W, & Huang XF (2006). Fatty acids differentially affect serotonin receptor and transporter binding in the rat brain. Neuroscience, 139, 1397–1403. doi: 10.1016/j.neuroscience.2006.02.068. [DOI] [PubMed] [Google Scholar]
- Dyall SC, & Michael-Titus AT (2008). Neurological benefits of omega-3 fatty acids. Neuromolecular Medicine, 10, 219–235. doi: 10.1007/s12017-008-8036-z. [DOI] [PubMed] [Google Scholar]
- Fang YJ, Zhou MH, Gao XF, Gu H, & Mei YA (2011). Arachidonic acid modulates Na+ currents by non-metabolic and metabolic pathways in rat cerebellar granule cells. The Biochemical Journal, 438, 203–215. doi: 10.1042/BJ20110569. [DOI] [PubMed] [Google Scholar]
- Farvid MS, et al. (2014). Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation, 130, 1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Pethick SJ, Renda C, Cox JL (2000). Short-form versions of the MacArthur communicative development inventories. Applied Psycholinguistics, 21, 95–116. [Google Scholar]
- Fewtrell MS, et al. (2004). Randomized, double-blind trial of long-chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. The Journal of Pediatrics, 144, 471–479. doi: 10.1016/j.jpeds.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Foster-Cohen S, Edgin JO, Champion PR, & Woodward LJ (2007). Early delayed language development in very preterm infants: Evidence from the MacArthur-Bates CDI. Journal of Child Language, 34, 655. doi: 10.1017/s0305000907008070. [DOI] [PubMed] [Google Scholar]
- Frank MC, Braginsky M, Yurovsky D, Marchman VA (2016). Wordbank: An open repository for developmental vocabulary data. Journal of Child Language, 1–18. doi: 10.1017/S0305000916000209. [DOI] [PubMed] [Google Scholar]
- Garton AF (1985). The production of this and that by young children. First Language, 6, 29–39. [Google Scholar]
- Gibson RA, Neumann MA, Lien EL, Boyd KA, & Tu WC (2013). Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. Prostaglandins Leukotrienes and Essential Fatty Acids, 88, 139–146. doi: 10.1016/j.plefa.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Gordon RG, & Watson LR (2015). Brief report: Gestures in children at risk for autism spectrum disorders. Journal of Autism and Developmental Disorders, 45, 2267–2273. doi: 10.1007/s10803-015-2390-0. [DOI] [PubMed] [Google Scholar]
- Groen WB, Zwiers MP, van der Gaag RJ, & Buitelaar JK (2008). The phenotype and neural correlates of language in autism: An integrative review. Neuroscience and Biobehavioral Reviews, 32, 1416–1425. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Harnack K, Andersen G, & Somoza V (2009). Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and docosahexaenoic acid as affected by the ratio of n6/n3 fatty acids. Nutrition Metabolism, 6, 8. doi: 10.1186/1743-7075-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS (2006). The omega-6/omega-3 ratio and cardiovascular disease risk: Uses and abuses. Current Atherosclerosis Reports, 8, 453–459. [DOI] [PubMed] [Google Scholar]
- Henriksen C, et al. (2008). Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics, 121, 1137–1145. doi: 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- Ikemoto A, Kobayashi T, Watanabe S, & Okuyama H (1997). Membrane fatty acid modifications of PC12 cells by arachidonate or docosahexaenoate affect neurite outgrowth but not norepinephrine release. Neurochemical Research, 22, 671–678. [DOI] [PubMed] [Google Scholar]
- Innis SM (2014). Omega-3 fatty acid biochemistry: Perspectives from human nutrition. Military Medicine, 179, 82–87. doi: 10.7205/MILMED-D-14-00147. [DOI] [PubMed] [Google Scholar]
- Johnson M, Fransson G, Ostlund S, Areskoug B, & Gillberg C (2016). Omega 3/6 fatty acids for reading in children: A randomized, double-blind, placebo-controlled trial in 9-year-old mainstream schoolchildren in Sweden. Journal of Child Psychology and Psychiatry and Allied Disciplines. doi: 10.1111/jcpp.12614. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessey E, Wolke D, & Marlow N (2010). Autism spectrum disorders in extremely preterm children. Journal of Pediatrics, 156, 525–531. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Fernell E, Halldin C, Forssberg H, & Farde L (2005). Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: Association between striatal dopamine markers and motor hyperactivity. Biological Psychiatry, 57, 229–238. doi: 10.1016/j.biopsych.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Jumpsen J, Lien EL, Goh YK, & Clandinin MT (1997a). During neuronal and glial cell development diet n-6 to n-3 fatty acid ratio alters the fatty acid composition of phosphatidylinositol and phosphatidylserine. Biochimica et Biophysica Acta, 1347, 40–50. [DOI] [PubMed] [Google Scholar]
- Jumpsen J, Lien EL, Goh YK, & Clandinin MT (1997b). Small changes of dietary (n-6) and (n-3)/fatty acid content ratio alter phosphatidylethanolamine and phosphatidylcholine fatty acid composition during development of neuronal and glial cells in rats. Journal of Nutrition, 127, 724–731. [DOI] [PubMed] [Google Scholar]
- Kato K, Uruno K, Saito K, & Kato H (1991). Both arachidonic acid and 1-oleoyl-2-acetyl glycerol in low magnesium solution induce long-term potentiation in hippocampal CA1 neurons in vitro. Brain Research, 563, 94–100. [DOI] [PubMed] [Google Scholar]
- Keim SA, et al. (under review). Omega-3 and -6 fatty acid supplementation may benefit autism symptoms based on parent report in preterm toddlers. The Journal of Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletzko B, et al. (2008). The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. Journal of Perinatal Medicine, 36, 5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton P, Fleming J, & Harris WS (2010). The debate about n-6 polyunsaturated fatty acid recommendations for cardiovascular health. Journal of the American Dietetic Association, 110, 201–204. doi: 10.1016/j.jada.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, & Croen LA (2014). Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. The Journal of Pediatrics, 164, 20–25. doi: 10.1016/j.jpeds.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Lapillonne A, & Moltu SJ (2016). Long-chain polyunsaturated fatty acids and clinical outcomes of preterm infants. Annals of Nutrition and Metabolism, 69(Suppl 1), 35–44. doi: 10.1159/000448265. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Jorgensen MH, Olsen SF, Straarup EM, & Michaelsen KF (2005). Maternal fish oil supplementation in lactation: Effect on developmental outcome in breast-fed infants. Reproduction Nutrition Development, 45, 535–547. doi: 10.1051/rnd:2005044. [DOI] [PubMed] [Google Scholar]
- LeBarton ES, Goldin-Meadow S, & Raudenbush S (2015). Experimentally-induced Increases in Early gesture lead to increases in spoken vocabulary. Journal of Cognition and Development, 16, 199–220. doi: 10.1080/15248372.2013.858041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBarton ES, & Iverson JM (2016). Gesture development in toddlers with an older sibling with autism. International Journal of Language & Communication Disorders, 51, 18–30. doi: 10.1111/1460-6984.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Lee H, Kang S, Park WJ (2016). Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients, 8, 23. doi: 10.3390/nu8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA, & Voss KL (1994). Membrane arachidonic acid concentration correlates with age and induction of long-term potentiation in the dentate gyrus in the rat. European Journal of Neuroscience, 6, 1008–1014. [DOI] [PubMed] [Google Scholar]
- Mankad D, et al. (2015). A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Molecular Autism, 6, 18. doi: 10.1186/s13229-015-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M (1992). Tissue levels of polyunsaturated fatty acids during early human development. Journal of Pediatrics, 120, S129–S138. [DOI] [PubMed] [Google Scholar]
- Martinez M, & Mougan I (1998). Fatty acid composition of human brain phospholipids during normal development. Journal of Neurochemistry, 71, 2528–2533. [DOI] [PubMed] [Google Scholar]
- McNamara RK, et al. (2007). Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biological Psychiatry, 62, 17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McNamara RK (2010). DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. The Journal of Nutrition, 140, 864–868. doi: 10.3945/jn.109.113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, & Pandey GN (2010). Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. Journal of Affective Disorders, 126, 303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, & Liu Y (2011). Reduced expression of fatty acid biosynthesis genes in the prefrontal cortex of patients with major depressive disorder. Journal of Affective Disorders, 129, 359–363. doi: 10.1016/j.jad.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum SJ, D’Vaz N, Simmer K, Dunstan JA, Hird K, & Prescott SL (2012). Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: A randomised controlled trial. The British Journal of Nutrition, 108, 1443–1454. doi: 10.1017/S0007114511006878. [DOI] [PubMed] [Google Scholar]
- Mody M, et al. (2017). Communication deficits and the motor system: exploring patterns of associations in autism spectrum disorder (ASD). Journal of Autism and Developmental Disorders, 47, 155–162. doi: 10.1007/s10803-016-2934-y. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Greiner RS, & Salem N (2000). Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. Journal of Neurochemistry, 75, 2563–2573. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, & Sherman T (1987). Nonverbal communnication and play correlates of language development in autistic children. Journal of Autism and Developmental Disorders, 17, 349–364. [DOI] [PubMed] [Google Scholar]
- Nakamura MT, & Nara TY (2004). Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annual Review of Nutrition, 24, 345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- Ozcaliskan S, Adamson LB, & Dimitrova N (2016). Early deictic but not other gestures predict later vocabulary in both typical development and autism. Autism, 20, 754–763. doi: 10.1177/1362361315605921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlade MV, & Iverson JM (2015). The development of coordinated communication in infants at heightened risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 2218–2234. doi: 10.1007/s10803-015-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parletta N, Niyonsenga T, Duff J (2016). Omega-3 and omega-6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS ONE. doi: 10.4226/78/572fdf0edfb74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick RP, & Ames BN (2015). Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. The FASEB Journal, 29, 2207–2222. doi: 10.1096/fj.14-268342. [DOI] [PubMed] [Google Scholar]
- Pritchard MA, et al. (2016). Autism in toddlers born very preterm. Pediatrics, 137, e20151949. doi: 10.1542/peds.2015-1949. [DOI] [PubMed] [Google Scholar]
- Rescorla L (1991). Identifying expressive language delay at two. Topics in Language Disorders, 11, 14–20. [Google Scholar]
- Richardson AJ, & Montgomery P (2005). The Oxford-Durham study: A randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics, 115, 1360–1366. doi: 10.1542/peds.2004-2164. [DOI] [PubMed] [Google Scholar]
- Ring ED, Fenson L (2000). The correspondence between parent report and child performance for receptive and expressive vocabulary beyond infancy. First Language, 20, 141–159. [Google Scholar]
- Sansavini A, et al. (2011). Longitudinal trajectories of gestural and linguistic abilities in very preterm infants in the second year of life. Neuropsychologia, 49, 3677–3688. doi: 10.1016/j.neuropsychologia.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, & Benowitz LI (1993). Activation of protein kinase C by arachidonic acid selectively enhances the phosphorylation of GAP-43 in nerve terminal membranes. The Journal of Neuroscience, 13, 4361–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, DelBello MP, McNamara RK, Strakowski SM, & Adler CM (2012). Neuroprogression in bipolar disorder. Bipolar Disorders, 14, 356–374. doi: 10.1111/j.1399-5618.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- Scott DT, Janowsky JS, Carroll RE, Taylor JA, Auestad N, & Montalto MB (1998). Formula supplementation with long-chain polyunsaturated fatty acids: Are there developmental benefits? Pediatrics, 102, e59–e59. doi: 10.1542/peds.102.5.e59. [DOI] [PubMed] [Google Scholar]
- Serhan CN, & Savill J (2005). Resolution of inflammation: The beginning programs the end. Nature Immunology, 6, 1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Sheppard KW, & Cheatham CL (2017). Executive functions and the omega-6-to-omega-3 fatty acid ratio: A cross-sectional study. The American Journal of Clinical Nutrition, 105, 32–41. doi: 10.3945/ajcn.116.141390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer K, Schulzke S, Patole SK (2008). Longchain polyunsaturated fatty acid supplementation in preterm infants (Review). The Cochrane Library, 1, 1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, et al. (2007). Further evidence of dopamine transporter dysregulation in ADHD: A controlled PET imaging study using altropane. Biological Psychiatry, 62, 1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittle AJ, et al. (2017). Neurobehaviour at term-equivalent age and neurodevelopmental outcomes at 2 years in infants born moderate-to-late preterm. Developmental Medicine and Child Neurology, 59, 207–215. doi: 10.1111/dmcn.13297. [DOI] [PubMed] [Google Scholar]
- Verhaeghe L, Dereu M, Warreyn P, De Groote I, Vanhaesebrouck P, & Roeyers H (2016). Extremely preterm born children at very high risk for developing autism spectrum disorder. Child Psychiatry and Human Development, 47, 729–739. doi: 10.1007/s10578-015-0606-3. [DOI] [PubMed] [Google Scholar]
- Voigt RG, et al. (2014). Dietary docosahexaenoic acid supplementation in children with autism. Journal of Pediatric Gastroenterology and Nutrition, 58, 715–722. doi: 10.1097/MPG.0000000000000260. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Jalali E, Mutsaers M, Bell R, & Cvitkovic S (1999). An imbalance of dietary essential fatty acids retards behavioral development in mice. Physiology and Behavior, 66, 833–839. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Xing H-C, Mutsaers L, McCutcheon D, & Kyle D (1997). Arachidonic acid offsests the effects of mouse brain and behavior of a diet with low (n–6):(n–3) ratio and very high levels of docosahexaenoic acid. Journal of Nutrition, 127, 184–193. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Wynalda K, Salem N Jr., Butt CM (2015). Dietary DHA during development affects depression-like behaviors and biomarkers that emerge after puberty in adolescent rats. Journal of Lipid Research, 56, 151–166. doi: 10.1194/jlr.M055558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willatts P, Forsyth JS (2000). The role of long-chain polyunsaturated fatty acids in infant cognitive development. Prostaglandins Leukotrienes and Essential Fatty Acids, 63, 95–100. [DOI] [PubMed] [Google Scholar]
- Willett W, et al. (1985). Reproducibility and validity of a semiquan-titative food frequency questionnaire. American Journal of Epidemiology, 122, 51–65. [DOI] [PubMed] [Google Scholar]
- Winkens B, van Breukelen GJ, Schouten HJ, & Berger MP (2007). Randomized clinical trials with a pre- and a post-treatment measurement: Repeated measures versus ANCOVA models. Contemporary Clinical Trials, 28, 713–719. doi: 10.1016/j.cct.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, & Chalon S (2000). Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neuroscience Letters, 284, 25–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


