Abstract
Objectives
To compare radiographic bone changes, following alveolar ridge preservation (ARP) using Guided Bone Regeneration (GBR), a Socket Seal (SS) technique or unassisted socket healing (Control).
Material and methods
Patients requiring a single rooted tooth extraction in the anterior maxilla, were randomly allocated into: GBR, SS and Control groups (n= 14/). Cone Beam Computed Tomography (CBCT) images were recorded post‐extraction and at 4 months, the mid‐buccal and mid‐palatal alveolar ridge heights (BARH/PARH) were measured. The alveolar ridge width, cross‐sectional socket and alveolar‐process area changes, implant placement feasibility, requirement for bone augmentation and post‐surgical complications were also recorded.
Results
BARH and PARH was found to increase with the SS (0.65 mm ± 1.1/0.65 mm ± 1.42) techniques, stabilise with GBR (0.07 mm ± 0.83/0.86 mm ±1.37) and decrease in the Control (−0.52 mm ± 0.8/−0.43 mm ± 0.83). Statistically significance was found when comparing the GBR and SS BARH (p = .04/.005) and GBR PARH (p = .02) against the Control. GBR recorded the smallest reduction in alveolar ridge width (−2.17 mm ± 0.84), when compared to the Control (−2.3 mm ± 1.11) (p = .89). A mid‐socket cross‐sectional area reduction of 4% (−2.27 mm2 ± 11.89), 1% (−0.88 mm2 ± 15.48) and 13% (−6.93 mm2 ± 8.22) was found with GBR, SS and Control groups (GBR vs. Control p = .01). The equivalent alveolar process area reduction was 8% (−7.36 mm2 ± 10.45), 6% (−7 mm2 ± 18.97) and 11% (−11.32 mm2 ± 10.92). All groups supported implant placement, with bone dehiscence noted in 57% (n = 4), 64%(n = 7) and 85%(n = 12) of GBR, SS and Control cases (GBR vs. Control p = .03). GBR had a higher risk of swelling and mucosal colour change, with SS associated with graft sequestration and matrix breakdown.
Conclusion
GBR ARP was found to be more effective at reducing radiographic bone dimensional changes following tooth extraction.
Keywords: alveolar bone dimensions, alveolar ridge preservation, bone healing complications and visual analogue pain scores, cone beam computerised tomography, guided bone regeneration, optical scanning, randomised controlled trial, socket seal
1. INTRODUCTION
The clinical need for a prosthetically driven implant restorations, as well as the need for adequate bone dimensions at the implant site, has led to an increased interest in the physiological changes that occur during healing and remodelling of the extraction socket.
Healing of the extraction socket has been demonstrated to lead to extensive vertical and horizontal tissue resorption (Demircan & Demircan, 2015), with the risks associated with dimensional change particularly evident in the anterior maxilla (Araujo et al., 2015). Alveolar Ridge Preservation (ARP) techniques have evolved as a clinically relevant protocol, under the assumption that their adoption promotes favourable tissue healing, whilst limiting bone and soft tissue dimensional change (Avila‐Ortiz et al., 2019; Iocca et al., 2017; MacBeth et al., 2016; Mardas et al., 2015; Retzepi & Donos, 2010). This reduced tissue loss influences the requirement for future alveolar ridge augmentation at implant placement and simplifies the consequential implant surgical procedures (De Ris et al., 2013; Horváth et al., 2013; Vignoletti et al., 2014; Wang & Lang, 2012).
Although there was recognition that Guided Bone Regeneration (GBR) and Socket Seal (SS) ARP techniques (Avila‐Ortiz et al., 2019; Darby et al., 2008; Horváth et al., 2013; Jonker et al., 2021; Sapata et al., 2020) can be used to better preserve ridge dimensions in comparison to unassisted socket healing, the recent Systematic Reviews by Atieh et al. (2021) and Couso‐Queiruga et al. (2021) concluded that a lack of consensus was still present, when considering the impact of ARP on tissue dimensional change. The clinical differences attributed to the use of different barrier materials and ARP techniques remained unclear, particularly when considering the differences in the contour of the healed alveolar ridge (Jonker et al., 2021), the requirement for additional bone augmentation at implant placement and the patient or surgical complications attributed to specific ARP procedures.
The requirement for additional longitudinal studies was also highlighted in the systematic review undertaken by Avila‐Ortiz et al. (2020), indicated with a need for researchers to investigate the influence of the local gingival phenotype, volumetric bone dimensional changes and patient‐reported outcome measures following ARP treatment.
The ability of various ARP techniques to preserve the dimensions of the alveolar ridge has been examined, using different intra‐oral measurement techniques. These procedures have included direct examination of study casts (Pietrokovski & Massler, 1967), comparison of two dimensional (2‐D) radiographs, linear examination of the osseous ridge following surgical exposure (Huynh‐Ba et al., 2010), ultrasound assessment (Chan et al., 2017), direct intra‐surgical measurement using a rulers or probe (Schropp et al., 2003; Spray et al., 2000) and cast measurement using digital callipers (Katranji et al., 2007). Cone Beam Computerised Tomography (CBCT) techniques (Cavalcanti et al., 1999) have now been proposed as a superior method to detect dimensional and area (mm2) changes at the healed extraction socket, as they are accurate and easy to use, have high resolution and follow non‐invasive procedures. They are also able to produce multi‐planar images for dimensional analysis (Fickl et al., 2009; Jemt & Lekholm, 2003; Schneider et al., 2014; Thoma et al., 2010; Wälivaara et al., 2007).
The aim of this Randomised Controlled Trial (RCT) was to compare the radiographic linear and cross‐sectional bone area dimensional changes, at a tooth extraction site, following ARP using SS or GBR ARP technique, when compared to unassisted healing.
1.1. Null hypothesis
There is no difference in the alveolar bone dimensions and healing characteristics, when unassisted healing is compared with ARP at a tooth extraction site.
2. MATERIALS AND METHODOLOGY
2.1. Study population
This was a single‐centre, prospective, randomised, controlled, single blind clinical trial, that compared CBCT radiographic dimensional changes, socket healing characteristics and pain experience, following SS or GBR ARP, with unassisted healing acting as the control. The study was conducted in full accordance with the ethical principles of the Declaration of Helsinki (version, 2008) and ISO 14,155, and was independently reviewed and approved by the Ministry of Defence Research Ethics Committee (MODREC). All procedures were performed between 2015 and 2019. CONSORT guidelines for reporting clinical trials were followed (http//:www.consort‐statement.org/).
2.2. Inclusion criteria
Military patients attending a specialist secondary care referral practice, presenting with a terminal prognosis maxillary single rooted incisor, canine or premolar tooth, requiring extraction and prosthetic replacement using an implant supported restoration. Extraction could be precipitated due to trauma, periodontitis, endodontic complication or unrestorable caries. Fifty‐two patients were originally screened for eligibility, with 43 patients enrolled in the study.
The eligibility criteria included male or female military patients, aged 18 years to 55 years of age (mean 32 years, ± 9.6) who were systemically fit and well. Patients with a previous diagnosis of periodontitis were required to have successfully completed a course of periodontal treatment before enrolment, with disease stability demonstrated over a 6‐month period. A moderate to thick gingival phenotype and a FMPS of below 15% and a FMBS below 10% was also required at study baseline. The gingival phenotype was assessed using a probe as described by Jepsen et al. (2018). It was assumed that the periodontal probe (Hu‐Frieddy, Chicago, IL, USA) would be visible when the phenotype was thin (gingival tissue ≤1 mm) and not visible when thick (gingival tissue >1 mm). The accepted characteristics for the extraction socket included, a buccal socket wall, with less than 3 mm or 25% of the coronal mid‐buccal vertical bone wall lost. The integrity of the buccal socket wall was assessed clinically and using the CBCT radiograph following tooth extraction. Adequate mesio‐distal space was required for implant placement.
2.3. Exclusion criteria
The exclusion criteria for the study included smokers, pregnant or lactating females and patients with uncontrolled diabetes, active systemic illness, infection or patient who had undergone recent periodontal regenerative, access or gingival surgical treatment. Patients prescribed phenytoin, dihydropyridine, calcium antagonists, cyclosporine and anticoagulant therapy, or with a history of a severe bruxing/clenching habit, alcoholism, chronic drug abuse and psychological disorders were also excluded.
Local exclusion factors included the presence of a clinically symptomatic periapical radiolucency, acute abscesses, chronic sinus tracts and a residual periodontal pocket depth of >5 mm, at the completion of the pre‐treatment periodontal therapy.
2.4. Patient enrolment
On the patient's first visit, the medical history, dental status, full mouth PPD, FMBS, REC and MFPS scores were recorded. All clinical measurements were documented by a single, previously calibrated examiner, using a manual UNC‐15 periodontal probe with light probing force (20 gr/N). Periodontal indices were recorded at six sites; mesio‐buccal (MB), mid‐buccal (MidB), disto‐buccal (DB), mesio‐ palatal (MP), mid‐palatal (Midp) and disto‐ palatal (DP) around the dentition.
An upper and lower alginate impression (Imprint., 3 M, UK) was taken to record the baseline morphology of the extraction site. The working cast was fabricated using Type IV stone (GC Corp., Tokyo, Japan).
2.5. Study outcomes
The primary Outcome evaluated was the change in the radiographic vertical dimension of the buccal alveolar ridge height (BARH), following ARP using a SS or GBR technique when compared with unassisted healing. Dimensional changes were recorded at the baseline of tooth extraction and following 4 months healing.
The Secondary Outcomes measured included: (i) The vertical change in the mesial and distal BARH, (ii) The vertical change in the mesial, mid and distal palatal alveolar ridge height (PARH), (iii) The change in horizontal radiographic socket dimensions, (iv) The radiographic thickness of the buccal bone plate at a position 5 mm and 10 mm below the reference stent, (v) Radiographic socket and alveolus process cross‐sectional area changes, (vi) The requirement for additional bone augmentation at implant placement (4 month), (vii) Healing complications at the extraction socket, (viii) Pain scores during initial healing and (viii) Intra‐oral clinical parameters including full‐mouth bleeding (FMBS) and plaque scores (FMPS), probing pocket depths (PPD) and mucosal recession (REC).
2.6. Surgical and radiographic protocol
2.6.1. Minimally invasive tooth extraction
One hour prior to tooth extraction, patients were prescribed a course of 500 mg of Amoxicillin, which was continued three times daily for the following 5‐day post‐operative period. In the case of a reported allergy to penicillin, 500 mg of erythromycin was prescribed one hour before treatment and a dose of 250 mg prescribed four times daily for 5 days as an alternative. A 0.2% chlorhexidine rinse was administered before treatment, with Paracetamol 500 mg (2 tablets) prescribed for post‐operative pain control.
A circumferentially surgical incision was undertaken within the confines of the gingival sulcus, separating the periodontal attachment apparatus from the root of the tooth. Extraction of the tooth was facilitated using a luxator periotome and extraction forceps (Mardas et al., 2010), with care taken to preserve the integrity of the socket bone and gingival tissue boundary. A piezosurgery bone cutting insert was used, if ankylosis of the root was observed. Curettage of the socket was then performed to remove residual granulation tissue. If the integrity of the socket wall was noted to be fractured or displaced following visual or tactile assessment of the socket, or only a two walled socket configuration remained, predisposing to socket regeneration rather that ridge augmentation, then the patient was excluded.
The patient was then enrolled sequentially into the study, with the operative clinician provided with an envelope from an independent administrator, detailing the treatment allocation of either SS, GBR or Control. The envelope sequence was created from a master randomisation list that was held at the Defence Centre for Rehabilitative Dentistry, with the operator blinded to the allocation prior to treatment. As premolar and canine teeth are known covariates, which may influence bone dimensional changes, a separate stratified block randomisation method (groups of two) was used to address this risk of bias. This methodology resulted in the SS and Control groups including two premolar teeth, with the GBR group containing one canine and one premolar. This equal distribution of non‐incisor dentition reduced the risk of bias from an imbalanced distribution of dissimilar teeth. All subsequent CBCT and clinical assessments were undertaken with the assessor blinded to the ARP treatment allocation.
2.6.2. ARP technique
In the GBR group, the extraction socket was filled with a xenograft bone substitute (DBBM) (Bio‐Oss®; Geistlich Biomaterials, Wollhusen, Switzerland) up to the pre‐extraction level of the buccal and lingual/palatal alveolus bone plate. A localised tissue flap was then raised circumpherentially around the socket rim, to allow placement of a collagen barrier membrane (Bio‐Gide®, Geistlich Biomaterials, Wollhusen, Switzerland) 2–3 mm onto the adjacent alveolar bone surface. The extension of the flap in the mesial and distal interproximal areas was designed to avoid complete detachment of the adjacent gingival papilla. The localised mucosal flap was then replaced without major coronal advancement and secured in place with Ethylon® 6(0) (Johnson & Johnson Medical N.V., Belgium) cross‐mattress sutures. The sutures were placed in both the mesio‐distal and bucco‐palatal direction, to allow maximum stabilisation of the exposed membrane (Figure 1).
FIGURE 1.
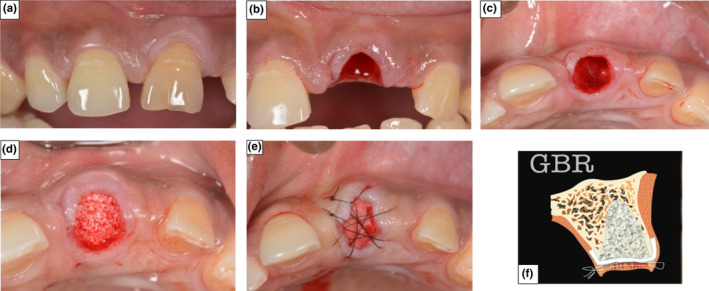
Photographs demonstrating Surgical Protocol for ARP using GBR technique (a) The incisor in position 21 prior to extraction. (b) Atraumatic tooth extraction following incision of the gingival tissue. (c) De‐epithelialization of the gingival tissue collar and localised flap raised. (d) Socket filled with a xenograft bone substitute. (e) The collagen membrane was sutured in place to seal the socket aperture. (f) Graphical representation of ARP using GBR
In the SS group, de‐epithelialization of the gingival tissue collar was undertaken using a high‐speed, round, coarse diamond bur, with the extraction socket filled with the same xenograft bone substitute (Bio‐Oss®) and the coronal aspect of the grafted socket covered with a cut to shape collagen matrix (Mucograft® Seal; Geistlich Biomaterials, Wollhusen, Switzerland) according to the manufacturer guidelines. The Mucograft® matrix was held in place, by suturing the top layer to the gingival tissue using single interrupted Ethylon® 6–0 sutures (Figure 2).
FIGURE 2.
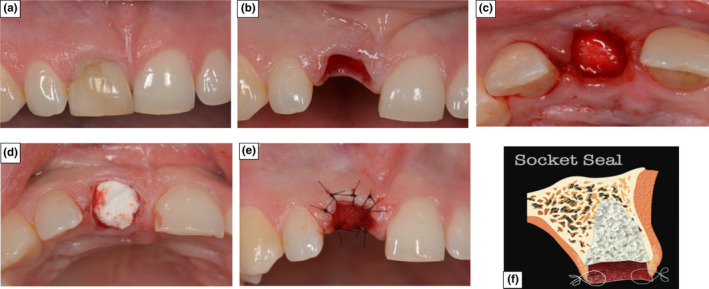
Photographs demonstrating Surgical Protocol for ARP using SS technique (a) The incisor in position 11 prior to extraction. (b) Atraumatic tooth extraction with de‐epithelialization of the gingival tissue collar. (c) Socket filled with a xenograft bone substitute. (d) A collagen matrix placed over the xenograft bone substitute (e) The collagen matrix was sutured in place to seal the socket aperture. (f) Graphical representation of ARP using SS
In the control group, haemostasis and clot stabilisation was achieved by the direct application of pressure to the extraction site for 5 min using a rolled sterile gauze pack, soaked in saline (Figure 3).
FIGURE 3.
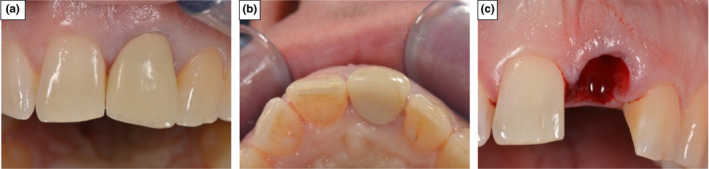
Control patient demonstrating unassisted socket healing protocol. (a) and (b) Pictures of the incisor in position 21 prior to extraction. (c) Socket left to form primary clot, prior to application of sterile pack
2.6.3. Post‐Operative instructions
The patients were instructed not to wear the immediate tooth replacement for 24 h and to avoid strenuous physical activities for 72 h, to prevent disruption or displacement of the primary clot. After 24 h, a 0.2% chlorhexidine‐di‐gluconate mouthwash was initiated, three time per day, with a modified brushing and oral care programme resumed in the upper maxilla at 72 h and respected for a further 11 days. Suture removal was scheduled for 14 days, with a dental hygienist visit providing tooth debridement and oral hygiene reinforcement at 2 weeks and again at 8 weeks.
2.6.4. Manufacture of the radiographic reference stent
On the pre‐surgical models, a thermoplastic matrix was manufactured extending three teeth either side of the extraction site. The tooth planned for extraction was sectioned from the cast, with the model trimmed to the buccal and palatal gingival contour. The thermoplastic material was then adjusted to the outlined gingival contour and filled internally with a barium sulphate radiopaque filler. This stent was designed to be used as a stable radiographic reference, to enable the measurement and comparison of dimensional changes immediately after extraction and following 4 months of healing. Reference points were marked with a depressed vertical grove in the mesial (M), mid (MID) and distal (D) areas of the buccal and palatal aspects of the stent, to ensure consistence in the vertical orientation and measurement position (Figure 4).
FIGURE 4.
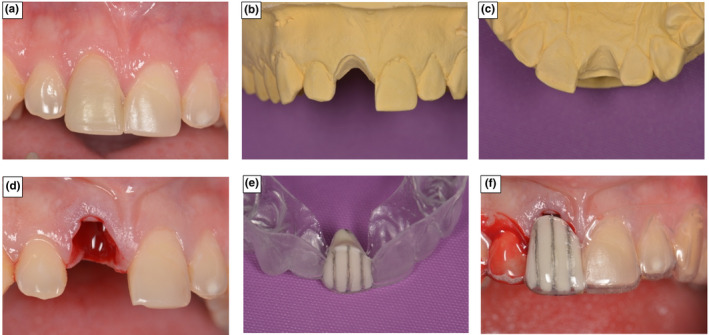
Manufacture of radiographic Measurement stent at extraction site. (a) The incisor in position 11 prior to extraction. (b) 11 sectioned from the cast and buccal aspect trimmed to gingival margin position (c) Palatal aspect trimmed to gingival margin contour. (d) Extraction socket immediately following tooth removal. (e) Radiographic reference stent constructed to marked gingival contour. (f) Gingival margin positional change, immediately following tooth removal
The patients were asked to wear the stent during CBCT imaging, with radiographic images taken immediately after tooth extraction (primary) and at 4‐months healing (secondary), prior to implant placement. The CBCT images were captured using a Carestream 9300 x‐ray unit, with the patient's inter‐arch position stabilised with a local customised index. An image field size of 5 cm by 5 cm, at a voxel size of 200 μm, scanning time 12 s, tube voltage of 60–90 KV and a frequency 140 KHz was selected (Figure 5).
FIGURE 5.
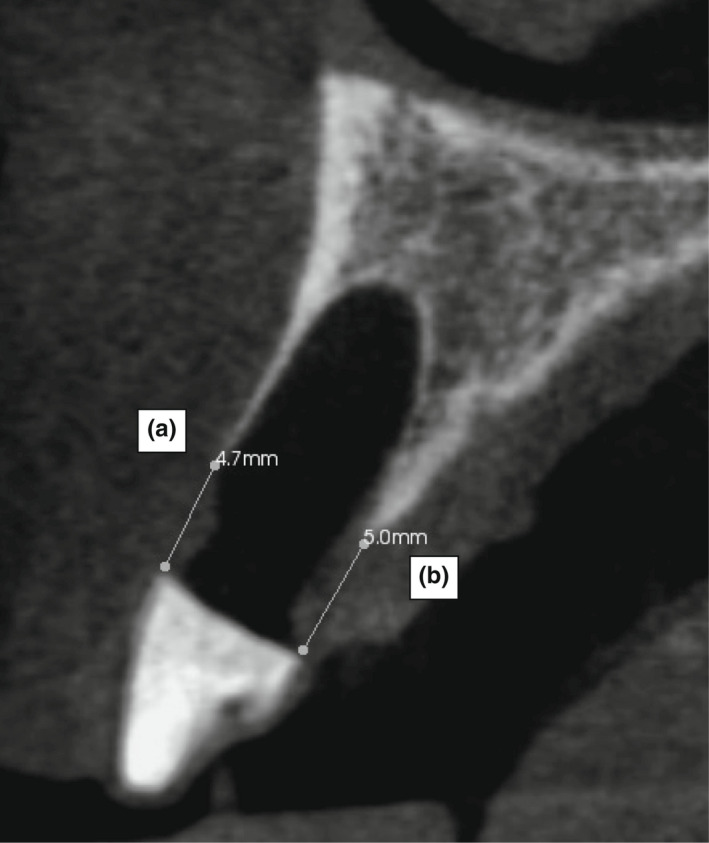
CBCT radiographic measurement of the Mid BARH and PARH. (a) Buccal alveolar ridge height (BARH): The distance from the buccal alveolar bone crest to the base of the reference measurement stent. (b) Palatal alveolar crest ridge height (PARH): The distance from the palatal alveolar bone crest to the base of the reference measurement stent
2.7. Primary outcome measurement
2.7.1. Buccal alveolar ridge height
Post extraction and 4‐month CBCT DIACOM image files were imported into the OnDemand3D software suite (Version 1.0.10.5385‐ Cybermed, USA). The Profile Measurement Tool was selected to measure the vertical distance between the crest of the alveolar ridge and the base of the radiographic stent. The Profile Measurement Tool assessed the variation in bone/tissue grey‐scale pixel density along a demarcated line, to aid in the detection of the edge of an anatomical surface, when partly mineralised bone was under investigation.
The BARH was determined by measuring the distance from the base of the radiographic stent to the uppermost point of the alveolar bone crest in the Mid‐buccal aspect of the extraction socket. The primary outcome measure was assessed as the change in the radiographic vertical BARH measurement in the ARP test groups and control group, following 4‐months healing.
2.8. Secondary outcome measures
2.8.1. Mesial and distal buccal alveolar ridge height dimensions (BARH)
The change in the radiographic alveolar bone height was assessed at the M, Mid and D positions of the radiographic stent at 4‐months healing.
2.8.2. Palatal alveolar ridge height
The radiographic palatal alveolar ridge height (PARH) was again measured after 4‐months socket healing. The PARH recorded the distance from the base of the radiographic stent to the uppermost point of the alveolar bone crest in the M, Mid and D positions, on the palatal aspect of the extraction socket.
2.8.3. Horizontal alveolar ridge width
The horizontal Alveolar Ridge Width (ARW) was measured using the Profile Measurement Tool at the M, Mid and D positions, at a distance of 5 mm (the Cervical ARW ‐ CARW) and 10 mm (the apical ARW‐ AARW), from the radiographic stent (Figure 6). The dimensional change in the CARW and AARW measurements were assessed following 4‐months healing.
FIGURE 6.
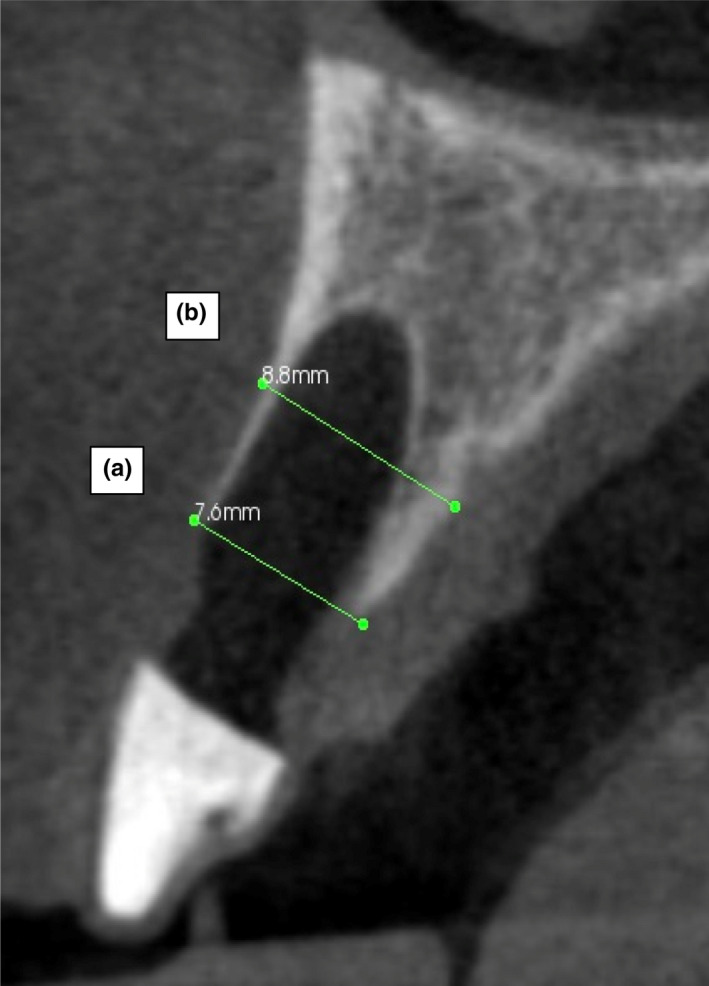
CBCT radiographic measurement of the alveolar ridge width. (a) Coronal alveolar ridge width (CARW): The external width of the alveolar ridge at a distance 5mm from the radiographic stent. (b) Apical alveolar ridge width (AARW): The external width of the alveolar ridge at a distance of 10 mm from the index
2.8.4. Buccal socket wall thickness
The thickness of buccal socket wall was recorded at 5 mm and 10 mm below the radiographic stent using the profile measurement tool (Figure 7).
FIGURE 7.
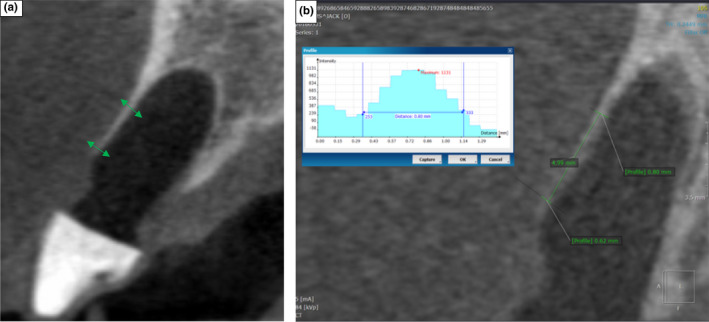
CBCT Images demonstrating the 5 and 10 mm buccal socket measurement positions (a) and the grey scale histogram (b) produced by the Profile Measurement Tool, which was used to assist in the measurement of the buccal socket wall thickness
2.8.5. Cross‐sectional socket and alveolus process area measurements
The OnDemand3D software allows for the superimposition of two CBCT images, imported as separate DICOM files, through a registration and merging function. Alignment of the CBCT images was achieved through a three‐staged process. Initially, the primary and secondary images were fused using an auto registration tool, in an attempt to align the axial, sagittal and coronal planes. The fused images were then checked for accuracy of registration on a monitor. Errors in alignment were corrected using a mutual information algorithm, with the procedure repeated to obtain the best fit.
Once the registration alignment was established, the secondary DICOM data set was reconfigured using the resliced tool, to conform to the axial, sagittal and coronal configuration of the primary image. Merger of both the primary and secondary images was undertaken using two different colour masks, to allow for differentiation and accurate visual assessment of the alveolar bone changes over the 4‐month healing period (Figure 8).
FIGURE 8.
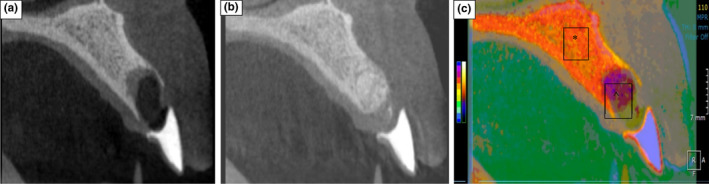
Superimposed CBCT Images demonstrating Alveolar bone change following 4‐months healing. (a) primary CBCT image taken after tooth extraction. (b) Secondary CBCT image taken at 4‐month healing. (c) Merged primary and secondary CBCT images, visualised using different colour masks. (*) orange colour represents original bone profile. (^) purple overlay outlines the residual morphology of the alveolar ridge when the secondary CBCT image was taken (4‐months)
The initial CBCT image was used to outline the internal surface of the extraction socket and the extent of the original alveolar process supporting the root of the tooth. The apical aspect of the socket was used as the base of the alveolar process, with the base determined as the bisecting plane, drawn parallel to the bucco‐palatal coronal socket orientation. The socket (SA) and alveolar process (APA) cross‐sectional area (mm2) was then calculated. The merged image was then examined, using the primary image socket and alveolar process outline as a reference. The level of bone infill (mm2) in the extraction socket and the change in the cross‐sectional area of the outlined alveolar process were then calculated (Figure 9).
FIGURE 9.
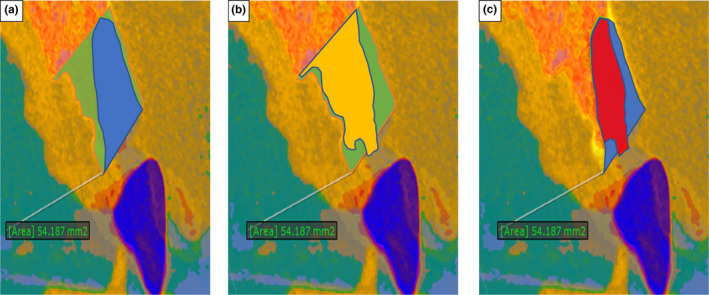
Measurement of socket and alveolar process cross‐sectional area (mm2.). (a) Primary socket (blue) and alveolar process area (green). (b) Primary alveolar process outline (green) and secondary healed outline (yellow). (c) Primary socket outline (blue), secondary healed outline (red)
2.8.6. Implant placement feasibility and requirement for additional bone grafting at implant placement
Implant placement feasibility was determined according to the ability of the operator to adopt a prosthetically driven, implant placement protocol. A surgical stent was used to determine the desired three‐dimensional spatial relationship between the prosthetic restoration and the implant fixture. The outcome was expressed as the percentage of cases, where the idealised surgical protocol could be achieved, whilst safeguarding adequate implant primary stability and bone coverage. The primary stability of the dental implants was assessed through the ability to obtain an insertion torque of more than 30N and the surgeons’ perception of implant stability at placement (O'Sullivan et al., 2004).
The number of cases with evidence of dehiscence or fenestration defects, which required GBR to cover the exposed implant threads, was recorded (Figure 10). Cases that required GBR for contour augmentation to facilitate optimal aesthetics and prosthetic reconstruction, in the absence of a fenestration or dehiscence defect were also measured. Aesthetic GBR contour augmentation was undertaken, when less that 2 mm of buccal bone remained, or when the residual buccal bone profile had a horizontal discrepancy or marked asymmetry when compared to the contralateral tooth. The results were presented as a frequency distribution percentage for each augmentation outcome.
FIGURE 10.
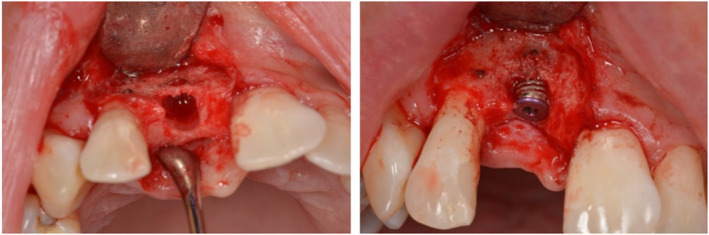
Pictures of surgical osteotomy site, where additional GBR was required at implant placement to cover exposed implant threads (dehiscence)
2.8.7. Post‐operative surgical complications
An evaluation of the extraction site was undertaken at the 2‐week control visit. The presence of side effects or patient complaints was recorded as being dichotomously present or absent in a case report form. The complications logged included suppuration at graft site, the presence of swelling, persistent pain in the grafted area swelling, expulsion/sequestration of grafted material, tissue reaction to graft material, resorption and remodelling of the graft, colour and tissue morphological changes and clefting of the gingival tissue. Recession of the gingival tissue, sensitivity from the adjacent dentition, chronic pain, local infection, loss of and dehiscence of the membrane was also recorded.
2.8.8. Pain intensity scores (Visual Analogue Scale)
The patient's pain intensity score was recorded using a visual analogue scale (VAS) at 2 weeks and at 8 weeks healing (Figure 11).
FIGURE 11.
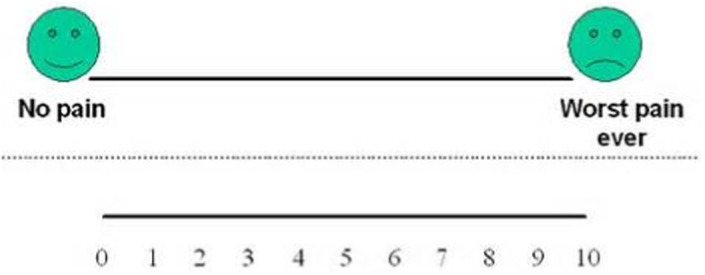
VAS used for pain assessment with recording scale detailed below
Patients were asked to mark on the analogue scale, the point that they felt represented their perceived perception of their current pain state. The VAS score was measured in millimetres from the left‐hand end of the line to the point that the patient marked.
The patient's pain experience was classified according to the following threshold values: no pain (0–4 mm), mild pain (5–44 mm), moderate pain (45–74 mm) and severe pain (75–100 mm) (Jensen et al., 2003).
2.9. Power calculation
The systematic review by MacBeth et al. (2016) indicated a standardised mean difference of 0.74–0.796 mm in the alveolar ridge height, when comparing ARP and unassisted healing studies. The value (0.8 mm) was therefore used as the minimal difference or effect size that wished to be identified in the power calculation.
Assuming a CBCT radiographic vertical change of −0.5 mm in the unassisted control group, with a standard deviation of 0.9 mm (Jung et al., 2013) and an effect size of 0.8 mm, then a three‐group study would require a sample size of 13 per group to produce an 80% power at an alpha level of 5% (Georgiev, 2016). In this study, a sample size of 14 patients per group was used to allow for patient dropouts.
2.10. Statistical analysis and randomisation
All data were entered in a computer database, proofed for entry error and loaded in the SPSS statistical software package (v.22). Tissue dimensions at tooth extraction and at 4 months healing were recorded as a mean ± standard deviation for all three groups.
Data normality was assessed by the Shapiro–Wilk test and Levene's test of variance. If the data was normally distributed, the differences between the groups will be assessed using parametric methods. If the assumptions were not fulfilled, non‐parametric tests were used instead. Significance was set at p < .05. All statistical results were limited to three decimal places.
2.10.1. Parametric tests
Independent sample t‐tests, or a one‐way fixed effect ANOVA assessment were used to examine the differences in means between the three test groups. If a significant difference was observed between the groups from the one‐way ANOVA, a Tukey's honestly significant difference (HSD) post hoc analysis was performed to check which specific groups differed.
2.10.2. Non‐parametric tests
An independent Wilcoxon rank sum test and a Kruskal–Wallis one‐way analysis of variance, was used to assess the differences between test groups. If a significant difference was observed between the groups, a post hoc analysis was performed using a Bonferroni correction for multiple comparisons and adjusted p‐values were presented.
An intra‐class correlation coefficient was used to measure the level of reliability between CBCT radiographic measurements (10 sets), repeated over a 10‐day interval.
2.11. Study registration
The registration of the RCT was undertaken with the Royal Air Force Military Centre for Aviation Medicine (643/MODREC/15). As the study ethical approval was initiated in 2015, there was no requirement at that time to register the study with https://www.clinicaltrials.gov.
3. RESULTS
3.1. Study population
The study population consisted of 43 individuals, 42 male and one female. One male patient was lost from the study, due to military deployment. The average age of enrolled patients was 32 years, with an age range of 27–53 years. Fourteen patients were allocated in each of the SS, GBR and Control groups. FMPS and FMBS scores were recorded at less than 10%, for all patients during treatment.
3.2. Tooth extraction position
All patients underwent extraction of a single rooted tooth in the upper anterior maxilla (15–25 position), with 36 central incisor teeth, one canine and five premolar teeth removed. The five premolars, were observed to have an oval root morphology and fused roots at the apex, conforming to the inclusion criteria.
3.3. Primary outcome measures
3.3.1. Buccal alveolar ridge height dimension
After 4 months of healing, analysis of the CBCT images revealed that the Mid‐BARH increased in the SS group (0.65 mm ± 1.1), stabilised with GBR (0.07 mm ± 0.83), with a reduction recorded in the Control group (−0.52 mm ± 0.8) (Table 1).
TABLE 1.
Alveolar ridge dimensions at tooth extraction and dimensional changes at 4‐month healing (mm)
| Test protocol | M‐BARH | Mid‐BARH | D‐BARH | M‐PARH | Mid‐PARH | D‐PARH | M‐CARW | Mid‐CARW | D‐CARW | M‐AARW | Mid‐AARW | D‐AARW | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GBR | Mean | 3.49 | 3.31 | 3.42 | 3.95 | 3.90 | 3.62 | 7.74 | 8.23 | 8.01 | 8.16 | 8.47 | 8.16 |
| SD | 0.68 | 0.50 | 0.84 | 0.93 | 0.83 | 1.16 | 1.11 | 1.09 | 1.08 | 1.00 | 1.10 | 1.13 | |
| SS | Mean | 3.55 | 3.64 | 3.51 | 3.76 | 4.04 | 3.79 | 8.35 | 8.83 | 8.66 | 8.53 | 8.41 | 8.92 |
| SD | 0.88 | 0.86 | 0.75 | 1.00 | 1.11 | 0.90 | 1.10 | 1.15 | 1.12 | 1.28 | 2.43 | 1.10 | |
| Control | Mean | 3.90 | 3.94 | 3.74 | 3.87 | 4.05 | 4.04 | 8.37 | 9.00 | 8.47 | 8.15 | 9.10 | 8.45 |
| SD | 0.78 | 0.66 | 0.74 | 0.87 | 0.68 | 0.79 | 1.41 | 1.19 | 1.05 | 2.01 | 1.22 | 2.22 | |
| Dimension Change GBR 4‐month | Mean | −0.24 | −0.07 | −0.27 | −0.50 | −0.86 | −0.33 | −1.73 | −2.17 | −1.89 | −0.71 | −0.96 | −0.77 |
| SD | 0.90 | 0.83 | 0.87 | 1.13 | 1.37 | 1.52 | 0.88 | 0.84 | 0.64 | 1.08 | 0.34 | 0.61 | |
| Dimension Change – SS 4‐months | Mean | −0.27 | −0.65 | −0.11 | −0.02 | −0.65 | −0.08 | −3.03 | −2.36 | −2.91 | −0.80 | −0.86 | −0.76 |
| SD | 1.35 | 1.10 | 0.99 | 1.61 | 1.42 | 1.21 | 2.25 | 2.76 | 1.99 | 0.72 | 1.48 | 0.64 | |
| Dimension Change Control 4‐months | Mean | 0.59 | 0.52 | 0.52 | 0.39 | 0.43 | 0.33 | −1.85 | −2.30 | −1.57 | −1.22 | −0.82 | −0.71 |
| SD | 1.03 | 0.80 | 0.84 | 1.26 | 0.83 | 0.75 | 2.48 | 1.11 | 2.43 | 1.42 | 0.76 | 0.47 | |
|
One way ANOVA p‐Value |
0.09 | 0.007 | 0.07 | 0.263 | 0.02 | 0.362 | 0.2 | 0.96 | 0.165 | 0.46 | 0.938 | 0.955 | |
| F Test | 2.574 | 5.6 | 2.49 | 1.384 | 4.23 | 1.045 | 1.679 | 0.038 | 1.894 | 0.791 | 0.06 | 0.045 | |
| Tukey HSD |
GBR Vs. Control p = .04 SS Vs. Control p = .005 |
GBR Vs. Control p = .03 | |||||||||||
A negative result in BARH and PARH indicates alveolar ridge augmentation. (p<three decimal places).
Abbreviations: AARW, apical alveolar ridge width; BARH, buccal alveolar ridge height; CARW, cervical alveolar ridge width; D, distal; PARH, palatal alveolar ridge height; M, mesial; Mid, middle.
When examining BARH measurements using a one‐way ANOVA calculation, a statistically significant difference in Mid‐BARH was found when comparing GBR vs. Control (p = .04) and SS vs. Control (p = .005). No statistical difference was present when examining the outcome of GBR and SS. The intra‐class correlation co‐efficient demonstrated a measurement reliability of 0.91 with a CI (0.86–0.95).
3.4. Secondary outcome measures
3.4.1. Mesial and Distal Buccal alveolar ridge height dimensions (BARH)
The GBR group reported more height gain in the Mesial and Distal BARH measurement sites (6%), when compared with the SS and Control groups.
3.4.2. Palatal alveolar ridge height dimension
Comparison of CBCT images at 4‐month healing, revealed an increase in the Mid‐PAH of 0.65mm (±1.1) with SS and an increase of 0.86 mm (±1.37) when undertaking GBR. A statistically significant difference was observed between GBR and the Control in the Mid‐PARH dimension (p = .03).
In the Control group, resorption of the palatal alveolar bone crest occurred, resulting in a Mid‐BARH reduction of −0.43 mm (±0.83) (Table 1). Palatally, all three groups demonstrated the greatest dimensional change in the Mid‐PARH position, when compared to the Mesial and Distal proximal sites.
3.4.3. Horizontal coronal and apical alveolar ridge width dimensions (CARW and AARW)
At 4‐months healing, the GBR group recorded a Mid‐CARW reduction of −2.17 mm (±0.84), with the SS group demonstrated the greatest Mid‐CARW change of −2.36 mm (±2.76). The Control was found to have a dimensional change of −2.30 mm (±1.11).
These figures represent an individual Mid‐socket CARW mean change of 26.4% for GBR, 26.7% when using the SS technique and 27.5% with unassisted healing. The SS technique reported a greater CARW reduction in the M and D positions, when compared to the Mid‐socket region, with this observation reversed in the GBR and Control groups.
The Mid‐socket AARW reduction was similar for the GBR, SS and Control groups (−0.96 mm (±0.34) for GBR, −0.86 mm (±1.48) for SS and −0.82 mm (±0.76) for Control). These measurements equated to a mean AARW reduction of 10%, with only negligible differences in the ARRW found, when the Mid and M and D socket dimensions were compared.
The ANOVA analysis revealed no statistical difference in the CARW and AARW dimensional changes at 4‐months healing when comparing groups.
3.4.4. Buccal alveolar plate (socket) thickness
A mean mid‐buccal alveolar socket thickness of 1.02 mm (±0.32) was recorded at the coronal aspect of the extraction socket for the enrolled patients, with a thickness of 1.04 mm (±0.29) measured at 5 mm and 1.02 mm (±0.27) at 10 mm. No evidence of buccal socket dehiscence or fenestration was noted in the CBCT images, with no statistic differences found, when comparing the buccal socket wall thickness in the GBR, SS and Control groups.
At 4‐months healing, only two patients in all three test groups, demonstrated evidence of retention of an aspect of the original buccal socket contour in the coronal 4 mm. These patients had a socket wall thickness of 1.9 mm and 2.4 mm. All other patients demonstrated loss of the buccal alveolar bone plate.
3.4.5. Socket area (SA) and alveolar process cross‐sectional area (APA)
The mid SA and APA was 51.34 mm2 (±13.09) and 94.45 mm2 (±26.6) in the GBR group, 58.86mm2 (±12.32) and 110.50 mm2 (±33.61) with SS, and 54.28 mm2 (±14.8) and 102.37 mm2 (±30.75) in the Control.
At 4‐months healing, the GBR, SS and Control groups all demonstrated a reduction in the Mid‐SA measurement. The Mid‐SA was reduced by 4% (−2.27 mm2 ± 11.89) in the GBR group, 1% (−0.88 mm2 ± 15.48) when using SS and 13% (−6.93MM2 ± 8.22) in the Control. The GBR group demonstrated an increase in the SA in the M‐SA (0.22 mm2 ± 7.88) and D‐SA (0.02 mm2 ± 8.29) reference positions.
A Kruskal–Wallis One‐way ANOVA analysis, with post hoc Bonferroni calculation revealed a statistical difference in the SA when comparing the GBR and Control groups (p = .01) at 4‐months.
The Control group demonstrated the greatest SA reduction at 4‐months healing in the M, Mid and D positions. ANOVA analysis did not reveal a statistical difference in the SA changes at 4 months, when comparing the SS, and Control groups.
A reduction in the APA was again found in GBR, SS and Control groups at 4‐months healing. A 8% (−7.36mm2 ± 10.45) reduction was observed in the Mid‐APA when using GBR, with a 6% (−7mm2 ± 18.97) reduction for SS group and a 11% (−11.32mm2 ± 10.92) reduction in the Control. The Control group demonstrated greater APA loss in the M (16%) and D (12%) positions, when compared to the Mid‐socket area (Table 2).
TABLE 2.
SA and APA at tooth extraction, and area changes at 4‐month healing (mm2)
| ARP procedure | M‐SA | Mid‐SA | D‐SA | M‐APA | Mid‐APA | D‐APA | |
|---|---|---|---|---|---|---|---|
| GBR | Mean | 44.62 | 51.34 | 45.23 | 86.26 | 94.45 | 85.28 |
| SD | 9.95 | 13.09 | 12.75 | 26.77 | 26.60 | 28.28 | |
| SS | Mean | 53.31 | 58.86 | 57.35 | 101.43 | 110.50 | 108.72 |
| SD | 12.15 | 12.32 | 13.14 | 28.86 | 33.61 | 30.56 | |
| Control | Mean | 48.49 | 54.28 | 48.24 | 91.01 | 102.37 | 100.58 |
| SD | 15.99 | 14.80 | 13.51 | 29.87 | 30.75 | 26.45 | |
| Area change GBR | Mean | 0.22 | −2.27 | 0.02 | −6.50 | −7.36 | −8.72 |
| SD | 7.88 | 11.89 | 8.29 | 12.50 | 10.45 | 11.82 | |
| Area change SS | Mean | 2.45 | −0.88 | −3.36 | −1.61 | −7.00 | −10.24 |
| SD | 17.91 | 15.48 | 14.47 | 22.87 | 18.97 | 15.51 | |
| Area change ‐ Control | Mean | −7.80 | −6.93 | −4.00 | −14.76 | −11.32 | −12.14 |
| SD | 5.71 | 8.22 | 7.60 | 6.39 | 10.92 | 6.85 | |
| Kruskal–Wallis | 0.32 | 0.05 | 0.19 | 0.067 | 0.83 | 0.786 | |
| H Test | 2.26 | 5.4 | 3.31 | 6.28 | 0.389 | 0.482 | |
| Bonferroni Correction |
GBR Vs. Control p = .01 |
||||||
Abbreviations: APA, alveolar process area; D, distal; M, mesial; Mid, middle; SA, socket area.
3.5. Implant placement feasibility and requirement for additional bone grafting at implant placement
All patients in the GBR, SS and Control groups (100%), were able to realise implant placement according to a prosthetically driven protocol, whilst still achieving adequate primary stability. In the GBR group, 57% (eight patients) required bone augmentation at implant placement, with 28.5% (4‐patients) undergoing augmentation due to bone dehiscence or for aesthetic contour augmentation. In the SS group, 64% (nine patients) required augmentation at implant placement, with 50% (seven patients) due to implant dehiscence and 14% (two patients) to facilitate contour augmentation. No cases of implant fenestration were documented in the GBR or SS groups. The Chi‐square statistic was used to examine the difference in effect size when bone augmentation was required due to bone dehiscence or fenestration. A significant difference was found when comparing the GBR and Control group (p = .03).
The Control group required bone augmentation at the time of implant placement in 85% (12 patients), with 71% (10 patients) due to the presence of bone dehiscence and 14% (two patients) due to a fenestration defect. No bone augmentation was undertaken in the Control group for contour augmentation alone.
3.6. Post‐operative surgical complications
Post‐operative complications were regularly reported when using the GBR and SS ARP techniques (Table 3). Sloughing and localised breakdown of the collagen membrane was observed in 28% (4) of GBR patients, with loss of the membrane integrity predominantly recorded in the proximal areas. Graft sequestration was noted in 21% (3) of these cases. Inflammation was reported in the 49% (7) of the GBR group, resulting in localised mucosal colour change in 42% (6) of patients during the initial stages of healing. These colour changes were resolved at the 8‐week review.
TABLE 3.
Complications associated with SS and GBR ARP and tooth extraction at 2‐weeks
| ARP procedure | Suppuration | Inflammation / swelling | Persistent pain | Expulsion or sequestration of graft/ bone | Tissue reaction | Resorption of the graft | Colour changes | Clefting of the gingival tissue | Recession | Sensitivity | Chronic pain | Local or systemic infection |
Loss of the membrane |
Dehiscence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GBR | 7 | 3 | 6 | 3 | 1 | 4 | ||||||||
| SS | 3 | 1 | 7 | 4 | 4 | 2 | 1 | 1 | 6 | |||||
| Control | 2 | 1 | 4 | 1 | 8 | 3 |
Partial breakdown of the collagen matrix occurred prior to suture removal in 43% (6) of SS cases, with complete loss of the seal observed in 7% (1) patients. Loss of graft particles was reported in all of these cases. Inflammation 21% (3) and colour change 28% (4) was less frequently observed when using SS ARP and was again resolved at 8 weeks.
One patient experienced a dry socket in the Control group, with delayed healing, pain and localised infection recorded in this case. Recession of the gingival margin was noted in 56% (8) of patients and was the most common outcome. The recession was found to be associated with a higher level of tooth sensitivity, with 21% (3) of patient recording this complication. Initial colour changes were also seen in 28% (4) patients (Figure 12).
FIGURE 12.
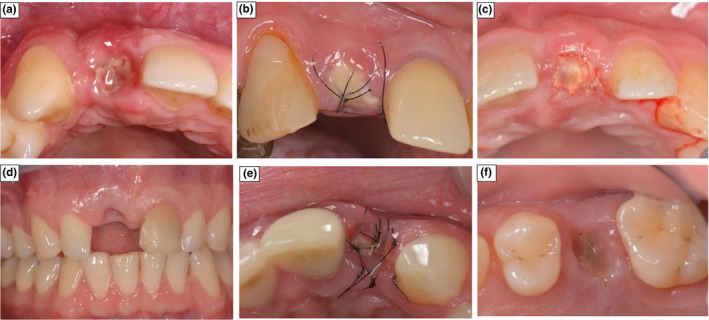
Pictures of socket healing at 2‐weeks demonstrating local Complications. (a) Colour change with GBR group. (b) Dehiscence of the membrane with SS. (c) Dehiscence of the membrane with GBR. (d) Tissue recession with Control. (e) Sequestration of graft with SS. (f) Loss of the membrane with SS
3.7. Visual analogue pain scores
At two weeks healing, four patients recorded a moderate level of residual pain (45–74 mm), with two of these cases associated with more complex surgical tooth removal and one associated with localised infection. No singular ARP technique or Control was found to be associated with increased pain scores for the patient. The SS technique was associated with a slightly higher level of residual discomfort at suture removal (2 weeks). GBR patient's experienced no pain at 8 weeks, with the SS and Control recording mild pain, but at the lower limit of this grading (Table 4). No statistical difference was recorded between the VAS pain scores for the SS, GBR and control groups at suture removal (p = .62/F = 1.58) and 8‐week review (p = .71/F = 1.47).
TABLE 4.
VAS recorded following tooth extraction and ARP
| ARP procedure | VAS score at Suture Removal | Patient Observations | VAS score at 8 weeks review |
|---|---|---|---|
| GBR | 2.3 SD 1.37 | One patient above 6 | 0.4 SD 0.3 |
| SS | 2.6 SD 1.67 | Two patients above 6 | 0.5 SD 0.4 |
| Control | 2.21 SD 1.47 | One patient above 6 | 0.56 SD 0.3 |
|
One way ANOVA p‐Value |
p = .62 | p = .71 | |
| F Test | 1.58 | 1.47 | |
| Tukey HSD |
Abbreviation: VAS, visual analogue score (pain).
4. DISCUSSION
Healing at an extraction site is characterised by re‐organisation, proliferation and maturation of the oral tissues, resulting in dimensional changes to the alveolar bone and gingival tissues Avila Ortiz et al. (2019, 2020). The amount of horizontal and vertical alveolar dimensional change is directly interlinked, as vertical crestal resorption can occur as a direct result of damage to the extraction socket, or due to a complex pattern of osteoclastic remodelling activity on either the inner and outer socket wall (Araujo & Lindhe, 2005), leading to both vertical and horizontal dimensional changes. This RCT established that GBR and SS ARP techniques, were effective in maintaining the vertical ARH, creating a more clinically favourable condition prior to implant placement.
4.1. Vertical dimension
Both GBR and SS ARP techniques, resulted in either stability or a vertical gain in the Mid BARH and Mid PARH dimensions when compared with the height loss experience in the Control group. Whilst the overall increase in the BARH at the GBR group was small, the dimensional preservation in the vertical alveolar ridge height was at a level that could be considered both clinically and statistically relevant (p = .04). The SS technique recorded a greater mean BARH gain than GBR, but there was a higher level of variance in the measurements, alluding to the potential for more complications with this technique. This variation may be due to the SS ARP technique allowing for a degree of over‐extension of the bone graft above the coronal boundaries of the extraction socket, but this over extension being affected by a greater level of graft dehiscence during healing. The dehiscence risk affecting the predictability of the healed outcome. The GBR technique was restricted in its ability to enhance the vertical alveolar crestal contour, as it required the barrier membrane to be extended onto the peripheral bone surface. The superior occlusive properties of the barrier during early healing, may allow for more predictable bone formation (Calciolari et al., 2018; Donos et al., 2015; Retzepi & Donos, 2010).
Whilst the buccal bone contour has a direct impact on future implant treatment, the need for bone augmentation is also influenced by the three‐dimensional ridge topography. As the external ridge contour is influenced by the PARH, the ridge augmentation experience with GBR may provide a beneficial effect on both surgical implant therapy and future aesthetic outcomes for the patient.
Recent systematic reviews by Avila Ortiz et al. (2019) and Troiano et al. (2018) have supported the outcomes of this RCT’s, indicating ARP procedures effectively reduced the level of vertical alveolar ridge high loss by between 1.65 mm to 1.72 mm, when compared to an unassisted healing. Similar levels of vertical alveolar ridge height conservancy were also reported when using GBR (Barone et al., 2008; Cardaropoli & Cardaropoli, 2008; Crespi et al., 2009; Jung et al., 2013) and SS (Jung et al., 2013) ARP techniques. When using a SS procedure, Coomes et al. (2014) and Neiva et al. (2008) described a smaller level of alveolar ridge height preservation, with an overall reduction in vertical dimension. The reason that this RCT may have reported vertical height stability with GBR and a gain, in the SS group, may be attributed to the described advantages of the grafting protocol, the atraumatic extraction technique utilised (Thoma et al., 2010) and the flat or scalloped thick phenotype (Avila Ortiz et al., 2019, Thoma et al., 2020) required for patient inclusion.
Vertical BARH and PARH dimensional changes were found to be more extensive in the buccal and palatal mid‐socket region, when compared to the proximal areas of both the ARP test and the Control groups. Excluding the grafting limitations already discussed, the difference in the morphometric dimensions of these results, correspond with the outcome of the systematic review undertaken by Tan et al. (2012) where a weighted mean, Mid BARH reduction of 1.24 mm, at 3 to 7‐months, with only a 0.8 mm to 0.84 mm loss proximally, in patients with unassisted healing was reported. It was suggested that the different rates of bone remodelling, could be attributed to the proximal bone dimension being partially determined by the blood supply from the interdental and periodontal ligament space of neighbouring teeth (Al‐Hezaimi et al., 2011) with the additional vasculature contributing to the stabilisation of the proximal bone and a reduce risk of bone resorption.
4.2. Horizontal dimensions
This study reported a horizontal Mid‐socket width reduction of −2.3 mm (±1.11) or 25.5% in the Control group, which is slightly less that the outcomes (2.6 mm to 4.6 mm, and 3.87 mm ± 0.82) reported in the systematic reviews undertaken by Ten Heggeler et al. (2011) and Van der Weijden et al. (2009). The difference was attributed to the inclusion of multiple rooted teeth, with the 2.3mm (± 1.1) CARW reduction found in this study, considered representative of the dimensional change for single rooted teeth in the maxillary dentition.
Comparison of the SS, GBR and Control Mid‐CARW and AARW measurements, indicated a similar level of horizontal socket width reduction in all groups, at 4 months healing. Whilst ARP may offer a clinically relevant reduction in alveolar width changes during socket healing, reducing the need for subsequent bone augmentation at implant placement, the magnitude of the reduction in horizontal alveolar dimensional change was variable (Avila‐Ortiz et al., 2019). Whilst other studies have reported great conservancy of width dimensions, following ARP with a GBR (Aimetti et al., 2009; Barone et al., 2013b; Barone et al., 2008; Jung et al., 2013) or SS (Jung et al., 2013; Meloni et al., 2015) techniques, the high level of heterogeneity in the published data may account for the observed statistical differences.
Another difference may be attributed to the reference position used to measure the horizontal socket width. The socket width measurements are often taken at a more coronal positions (3 mm), as this region has been found to suffer more extensive dimensional change (Araujo et al., 2015). This study recorded the horizontal socket width at 5 and 10 mm below the radiographic reference stent, as a more superficial position was not found to be associated with repeated retention of the buccal and palatal alveolar bone socket walls.
4.3. Area measurements
The SA and ARA cross‐sectional changes are representative of the extent of socket alveolus bone resorption, bone regeneration and the amount of residual graft matrix visible on the radiograph. The measurements described the healing pattern at the extraction socket and provide insight into whether additional bone grafting would be needed at implant placement.
Both the GBR and SS techniques reported a small loss of Mid‐SA (1% and 4%) and Mid ‐APA (6% and 8%) area, when compared to the area changes of 13% and 11% observed in the Control. GBR demonstrating a statistical difference to the Control in the Mid‐SA area. A similar level of SA reduction (3%) was reported by Araujo et al. (2015), but this same study also indicated a higher ARA reduction of 25%. The observed difference may be because of variations in the selected outline of the alveolar ridge, differences in extraction techniques and the patient phenotype characteristics outlined previously. The importance and effect of using different alveolar ARA boundaries can be appreciated by comparing the mean 102.49 mm2 (±13.48) ARA reduction observed in this study, with the 99.1 ± 30.1 mm2. ARP reduction found by Misawa (2016), who used a similar outline for analysis of the anterior maxillary alveolar ridge.
Whilst the SS group appeared to record a lower SA reduction, a higher level of horizontal SA dimensional change occurred with this technique, requiring greater vertical SA augmentation, to offset the buccal tissue resorption. Although GBR was observed to suffer a slightly higher level of SA change, when compared to SS, it was observed to have suffered a lower level of buccal tissue resorption and only vertical bone augmentation on the palatal aspect.
The ARA changes reflected the characteristics of the SA bone augmentation and healing pattern, recording only a small area of coronal palatal bone resorption, with the majority of the alveolar bone loss recorded in the crestal 4mm of the buccal socket wall for the SS, GBR and Control groups. This localised area of bone morphological change confirms the findings by Araujo and Lindhe (2005), Araujo et al. (2015) and Tomasi et al. (2010). Whilst it was anticipated that the need to raise a small flap to facilitate membrane placement for GBR might be associated with a higher level of horizontal bone resorption, it was observed that that the SS procedure suffered a greater level of alveolar ridge width dimensional change This difference in resorption rates may be attributed to the GBR membrane offering greater protection to the grafted matrix in the bundle bone area, with improved bone healing characteristics (Retzepi & Donos, 2010).
4.4. Implant bone augmentation
Whilst all GBR, SS and Control groups facilitated implant placement according to a prosthetically driven protocol, the requirement for additional bone augmentation was reduced in the GBR (3) and SS groups (4). The lower number of GBR patients, who required bone augmentation due to a dehiscence or fenestration defect at implant placement was statistically different to the control (p = .03), supporting the assumption that GBR was more effective at protecting the augmented buccal socket wall dimension (Mardas et al., 2015).
4.5. Pain and complications
This study indicated that patient's experienced only mild pain following tooth extraction, with no difference noted in the patient's perceived pain experience during the initial 2 weeks of healing following ARP with either a GBR or SS technique. This low level of pain experience has been reported in several RCT’s (Barone et al., 2013a; Camargo et al., 2000; Festa et al., 2013; Jung et al., 2013) and documented in the systematic review undertaken by Atieh et al. (2021).
Pain, oedema and erythema were the most common surgical complications reported in both GBR and SS ARP test groups and the Control, with the frequency of these complications slightly higher when using GBR (Cook & Mealey, 2013; Mardas et al., 2010; Pinho 2006) and SS (Fiorellini et al., 2005; Karaca et al., 2015) procedures. Temporary colour change and membrane/collagen matrix exposure with sequestration of the graft matrix was observed in both SS and GBR procedures and was attributed to the additional surgical trauma and the loss of a suture. When dehiscence of the collagen membrane and collagen matrix was reported, the loss of the biomaterial integrity was observed to be very localised and only caused limited graft sequestration. At 8 weeks healing, no observed differences were seen in the SS, GBR and Control groups.
4.6. New developments in study methodology
4.6.1. Alveolar bone measurements
This study used an innovative combination of optical scans, superimposed CBCT radiographs and overlaid mesh images to undertake comparative analysis of dimensional changes following two different ARP techniques and unassisted healing. Whilst the use of superimposed or fused images (Fickl et al., 2008a) has been documented, the accuracy of recorded measurements is influenced by the quality of the CBCT scans and their ability to display anatomical features. The image display is affected by the field of view, tube voltage and amperage, partial volume averaging, the presence of noise or artefacts on the image (Molen, 2010), soft tissue factors, voxel size and spatial resolution (Molen, 2010; Patcas et al., 2012).
The accuracy of measurement recorded by CT and CBCT machines has been reported on by several authors (Loubele et al., 2008). Although it can be concluded that CBCT systems render anatomical measurements reliably and are an appropriate tool for linear measurements (Patcas et al., 2012), the level of accuracy and inter‐operator error may be affected by visual limitations, or when measuring small cross‐sectional bone dimensions (Cao et al., 2017; Leung et al., 2010; Patcas et al., 2012; Wood et al., 2013). This is particularly important when immature or newly forming bone tissue may have a reduced bone density (Januario et al., 2011; Marmulla et al., 2005). To minimise this risk of CBCT interpretation errors, this study utilised a grey scale pixel density to delineate the bone margin. This was particularly useful when determining the position and thickness of the buccal wall, as this anatomical surface was often visually indistinct.
4.6.2. Study limitations
The restricting of enrolled patients, to those with a moderate to thick gingival phenotype (Cook et al., 2011; Chappuis et al., 2017; Thoma et al.,2020), may have influenced the level of vertical and horizontal socket remodelling experienced in this study. An additional factor is the bias associated with the predominately male population group. Gender differences in alveolar bone dimensions have been chronicled by Lee et al. (2010) and El Nahass et al. (2015), with the CBCT buccal socket thickness measurements of 1.04 mm at 5 mm and 1.02 mm at 10 mm recorded in this study, higher than the population average reported by Tsigarida et al. (2020). As baseline buccal bone thickness has been demonstrated as being a predictor for buccal bone resorption (Araujo & Lindhe, 2005; Araujo et al., 2015; Tomasi et al., 2010), the above average buccal socket thickness may have resulted in reduced horizontal socket dimensional change.
A post hoc power calculation indicated that this RCT population had a low power (p = .255) to detect a 1 mm horizontal size reduction of the CARW and AARW measurements for the SS and GBR test groups. These results suggest that a larger population size is required to demonstrate a statistical difference between ARP and Control groups when examining the width changes in the alveolar ridge. if one truly exists.
Whilst a stratified randomisation model reduced the risk of variation in buccal socket thickness risk, the inclusion of both central incisor, canine and premolars in this study, may have introduced an element of bias, as different resorption patterns could have been experienced by these teeth. If a larger sample population was investigated, a liner statistical analysis approach would have been preferred, as it would allow variation in the bone dimensions at different tooth sites.
5. CONCLUSION
Referenced and aligned CBCT images offered an advantage when reviewing bone dimensional changes. Comparison of ARP techniques indicated that GBR and SS were effective in limiting vertical alveolar (buccal and palatal) bone loss, when compared with an unassisted healing Control Group. Furthermore, ARP procedures may decrease the need for further ridge augmentation during implant placement.
AUTHOR CONTRIBUTION
Neil Mac Beth: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (lead); Project administration (lead); Resources (lead); Writing – original draft (lead). Nikolaos Donos: Conceptualization and data analysis; Supervision (lead); Validation (lead); Writing – review & editing (equal). Nikos Mardas: Contributions and Conceptualization; Formal analysis (equal); Validation (equal); Writing – review & editing (equal).
CONFLICT OF INTEREST
The authors declare that there is nothing to disclose.
HUMAN RIGHTS STATEMENTS AND INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Ethical approval for the study was provided by the UK MOD Research Ethics Committee (643/MODREC/15). Informed consent was obtained from all patients for being included in the study.
Supporting information
Supplementary Material
MacBeth, N. D. , Donos, N. , & Mardas, N. (2022). Alveolar ridge preservation with guided bone regeneration or socket seal technique. A randomised, single‐blind controlled clinical trial. Clinical Oral Implants Research, 33, 681–699. 10.1111/clr.13933
Clinical Trial Registration: This RCT was not registered with the public trials registry as it was initiated prior to 2017. A full copy of the trial protocol can be obtained from the Ministry of Defence Research Ethics Committee (643MODREC/15).
Funding information
NM received support from the Military of Defence Surgeon Generals Research Strategy Group for the costs of the PhD programme and the histological analysis of the alveolar bone samples. The funders had no role in the study, data collection and analysis, decision to publish or preparation of the manuscript
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the finding of this paper are available within the article and its supplementary material. Raw data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aimetti, M. , Romano, F. , Griga, F. B. , & Godio, L. (2009). Clinical and histologic healing of human extraction sockets filled with calcium sulfate. International Journal of Oral and Maxillofacial Implants, 24, 902–909. [PubMed] [Google Scholar]
- Al‐Hezaimi, K. , Levi, P. , Rudy, R. , Al‐Jandan, B. , & Al‐Rasheed, A. (2011). An extraction socket classification developed using analysis of bone type and blood supply to the buccal bone in monkeys. International Journal of Periodontics and Restorative Dentistry, 31, 421. [PubMed] [Google Scholar]
- Araujo, M. G. , & Lindhe, J. (2005). Dimensional ridge alterations following tooth extraction. An experimental study in the dog. Journal of Clinical Periodontology, 32, 212–218. [DOI] [PubMed] [Google Scholar]
- Araujo, M. G. , Silva, C. O. , Misawa, M. , & Sukekava, F. (2015). Alveolar socket healing: What can we learn? Periodontology 2000, 68, 122–134. [DOI] [PubMed] [Google Scholar]
- Atieh, M. A. , Alsabeeha, N. H. , Payne, A. G. , Ali, S. , Faggion, C. M. J. , & Esposito, M. (2021). Interventions for replacing missing teeth: Alveolar ridge preservation techniques for dental implant site development. Cochrane Database Systematic Review, 4, CD010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila‐Ortiz, G. , Chambrone, L. , & Vignoletti, F. (2019). Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 46(Suppl 21), 195–223. [DOI] [PubMed] [Google Scholar]
- Avila‐Ortiz, G. , Gonzalez‐Martin, O. , Couso‐Queiruga, E. , & Wang, H. L. (2020). The peri‐implant phenotype. Journal of Periodontology, 91, 283–288. [DOI] [PubMed] [Google Scholar]
- Barone, A. , Aldini, N. N. , Fini, M. , Giardino, R. , Calvo Guirado, J. L. , & Covani, U. (2008). Xenograft versus extraction alone for ridge preservation after tooth removal: A clinical and histomorphometric study. Journal of Periodontology, 79, 1370–1377. [DOI] [PubMed] [Google Scholar]
- Barone, A. , Ricci, M. , Tonelli, P. , Santini, S. , & Covani, U. (2013a). Tissue changes of extraction sockets in humans: a comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clinical Oral Implants Research, 24, 1231–1237. [DOI] [PubMed] [Google Scholar]
- Barone, A. , Todisco, M. , Ludovichetti, M. , Gualini, F. , Aggstaller, H. , Torres‐Lagares, D. , Rohrer, M. D. , Prasad, H. S. , & Kenealy, J. N. (2013b). A prospective, randomized, controlled, multicenter evaluation of extraction socket preservation comparing two bovine xenografts: clinical and histologic outcomes. The International Journal of Periodontics & Restorative Dentistry, 33, 795–802. [DOI] [PubMed] [Google Scholar]
- Calciolari, E. , Ravanetti, F. , Strange, A. , Mardas, N. , Bozec, L. , Cacchioli, A. , Kostomitsopoulos, N. , & Donos, N. (2018). Degradation pattern of a porcine collagen membrane in an in vivo model of guided bone regeneration. Journal of Periodontal Research, 53, 430–439. [DOI] [PubMed] [Google Scholar]
- Camargo, P. M. , Lekovic, V. , Weinlaender, M. , Klokkevold, P. R. , Kenney, E. B. , Dimitrijevic, B. , Nedic, M. , Jancovic, S. , & Orsini, M. (2000). Influence of bioactive glass on changes in alveolar process dimensions after exodontia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 90, 581–586. [DOI] [PubMed] [Google Scholar]
- Cao, D. , Zhu, L. , Chen, Y. , Xie, L. , Yan, B. , & Sun, Z. (2017). Buccally impacted maxillary canines increase the likelihood of root separation in adjacent first premolars. Oral Diseases, 23, 36–41. [DOI] [PubMed] [Google Scholar]
- Cardaropoli, D. , & Cardaropoli, G. (2008). Preservation of the postextraction alveolar ridge: A clinical and histologic study. International Journal of Periodontics & Restorative Dentistry, 28. [PubMed] [Google Scholar]
- Cavalcanti, M. G. P. , Haller, J. W. , & Vannier, M. W. (1999). Three‐dimensional computed tomography landmark measurement in craniofacial surgical planning: Experimental validation in vitro. Journal of Oral and Maxillofacial Surgery, 57, 690–694. [DOI] [PubMed] [Google Scholar]
- Chan, H. L. , Wang, H. L. , Fowlkes, J. B. , Giannobile, W. V. , & Kripfgans, O. D. (2017). Non‐ionizing real‐time ultrasonography in implant and oral surgery: A feasibility study. Clinical Oral Implants Research, 28, 341–347. [DOI] [PubMed] [Google Scholar]
- Chappuis, V. , Araujo, M. G. , & Buser, D. (2017). Clinical relevance of dimensional bone and soft tissue alterations post‐extraction in esthetic sites. Periodontology 2000, 73, 73–83. [DOI] [PubMed] [Google Scholar]
- Cook, D. C. , & Mealey, B. L. (2013). Histologic comparison of healing following tooth extraction with ridge preservation using two different xenograft protocols. Journal of Periodontology, 84, 585–594. [DOI] [PubMed] [Google Scholar]
- Cook, D. R. , Mealey, B. L. , Verrett, R. G. , Mills, M. P. , Noujeim, M. E. , Lasho, D. J. , Cronin, R. J. Jr. (2011). Relationship between clinical periodontal biotype and labial plate thickness: an in vivo study. International Journal of Periodontics & Restorative Dentistry, 31, 345–354. [PubMed] [Google Scholar]
- Coomes, A. M. , Mealey, B. L. , Huynh‐Ba, G. , Barboza‐Arguello, C. , Moore, W. S. , & Cochran, D. L. (2014). Buccal bone formation after flapless extraction: a randomized, controlled clinical trial comparing recombinant human bone morphogenetic protein 2/absorbable collagen carrier and collagen sponge alone. Journal of Periodontology, 85, 525–535. [DOI] [PubMed] [Google Scholar]
- Couso‐Queiruga, E. , Stuhr, S. , Tattan, M. , Chambrone, L. , & Avila‐Ortiz, G. (2021). Post‐extraction dimensional changes: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 48, 127–145. [DOI] [PubMed] [Google Scholar]
- Crespi, R. , Cappare, P. , & Gherlone, E. (2009). Dental implants placed in extraction sites grafted with different bone substitutes: Radiographic evaluation at 24 months. Journal of Periodontology, 80, 1616–1621. 10.1902/jop.2009.090156 [DOI] [PubMed] [Google Scholar]
- Darby, I. , Chen, S. , & de Poi, R. (2008). Ridge preservation: What is it and when should it be considered. Australian Dental Journal, 53, 11–21. [DOI] [PubMed] [Google Scholar]
- De Ris, V. , Clementini, M. , Vittorini, G. , Mannocci, A. , & De Sanctis, M. (2013). Alveolar ridge preservation techniques. A systematic review and meta‐analysis of histological and histomorphometric data. Clinical Oral Implants Research, 26, 50–68. [DOI] [PubMed] [Google Scholar]
- Demircan, S. , & Demircan, E. (2015). Dental cone beam computed tomography analyses of the anterior maxillary bone thickness for immediate implant placement. Implant Dentistry, 24, 664–668. [DOI] [PubMed] [Google Scholar]
- Donos, N. , Dereka, X. , & Mardas, N. (2015). Experimental models for guided bone regeneration in healthy and medically compromised conditions. Periodontology 2000, 68, 99–121. [DOI] [PubMed] [Google Scholar]
- El Nahass, H. , & Naiem, S. N. (2015). Analysis of the dimensions of the labial bone wall in the anterior maxilla: a cone‐beam computed tomography study. Clinical Oral Implants Research, 26, e57–61. [DOI] [PubMed] [Google Scholar]
- Festa, V. M. , Addabbo, F. , Laino, L. , Femiano, F. , & Rullo, R. (2013). Porcine‐derived xenograft combined with a soft cortical membrane versus extraction alone for implant site development: a clinical study in humans. Clinical Implant Dentistry and Related Research, 15, 707–713. [DOI] [PubMed] [Google Scholar]
- Fickl, S. , Schneider, D. , Zuhr, O. , Hinze, M. , Ender, A. , Jung, R. E. , & Hürzeler, M. B. (2009). Dimensional changes of the ridge contour after socket preservation and buccal overbuilding: An animal study. Journal of Clinical Periodontology, 36, 442–448. [DOI] [PubMed] [Google Scholar]
- Fickl, S. , Zuhr, O. , Wachtel, H. , Bolz, W. , & Huerzeler, M. (2008a). Tissue alterations after tooth extraction with and without surgical trauma: a volumetric study in the beagle dog. Journal of Clinical Periodontology, 35, 356–363. [DOI] [PubMed] [Google Scholar]
- Fiorellini, J. P. , Howell, T. H. , Cochran, D. , Malmquist, J. , Lilly, L. C. , Spagnoli, D. , Toljanic, J. , Jones, A. , & Nevins, M. (2005). Randomized study evaluating recombinant human bone morphogenetic protein‐2 for extraction socket augmentation. Journal of Periodontology, 76, 605–613. [DOI] [PubMed] [Google Scholar]
- Georgiev, G. Z. (2016) "Sample Size Calculator", [online] Available at: https://www.gigacalculator.com/calculators/power‐sample‐size‐calculator.php URL [Accessed Date: 05 Jan, 2022]. [Google Scholar]
- Horváth, A. , Mardas, N. , Mezzomo, L. , Needleman, I. , & Donos, N. (2013). Alveolar ridge preservation. A systematic review. Clinical Oral Investigations, 17, 341–363. [DOI] [PubMed] [Google Scholar]
- Huynh‐Ba, G. , Pjetursson, B. E. , Sanz, M. , Cecchinato, D. , Ferrus, J. , Lindhe, J. , & Lang, N. P. (2010). Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clinical Oral Implants Research, 21, 37–42. [DOI] [PubMed] [Google Scholar]
- Iocca, O. , Farcomeni, A. , Pardiñas Lopez, S. , & Talib, H. S. (2017). Alveolar ridge preservation after tooth extraction: A Bayesian Network meta‐analysis of grafting materials efficacy on prevention of bone height and width reduction. Journal of Clinical Periodontology, 44, 104–114. 10.1111/jcpe.12633 [DOI] [PubMed] [Google Scholar]
- Januario, A. L. , Duarte, W. R. , Barriviera, M. , Mesti, J. C. , Araujo, M. G. , & Lindhe, J. (2011). Dimension of the facial bone wall in the anterior maxilla: a cone‐beam computed tomography study. Clinical Oral Implants Research, 22, 1168–1171. [DOI] [PubMed] [Google Scholar]
- Jemt, T. , & Lekholm, U. (2003). Measurements of buccal tissue volumes at single‐implant restorations after local bone grafting in maxillas: A 3‐year clinical prospective study case series. Clinical Implant Dentistry and Related Research, 5, 63–70. [DOI] [PubMed] [Google Scholar]
- Jensen, M. P. , Chen, C. , & Brugger, A. M. (2003). Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. Journal of Pain, 4, 407–414. 10.1016/S1526-5900(03)00716-8 [DOI] [PubMed] [Google Scholar]
- Jepsen, S. , Caton, J. G. , Albandar, J. M. , Bissada, N. F. , Bouchard, P. , Cortellini, P. , Demirel, K. , de Sanctis, M. , Ercoli, C. , Fan, J. , Geurs, N. C. , Hughes, F. J. , Jin, L. , Kantarci, A. , Lalla, E. , Madianos, P. N. , Matthews, D. , McGuire, M. K. , Mills, M. P. , … Yamazaki, K. (2018). Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology, 45, S219–S229. [DOI] [PubMed] [Google Scholar]
- Jonker, B. P. , Gil, A. , Naenni, N. , Jung, R. E. , Wolvius, E. B. , & Pijpe, J. (2021). Soft tissue contour and radiographic evaluation of ridge preservation in early implant placement: A randomized controlled clinical trial. Clinical Oral Implants Research, 32, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, R. E. , Zaugg, B. , Philipp, A. O. H. , Truninger, T. C. , Siegenthaler, D. W. , & Hammerle, C. H. F. (2013). A prospective, controlled clinical trial evaluating the clinical radiological and aesthetic outcome after 5 years of immediately placed implants in sockets exhibiting periapical pathology. Clinical Oral Implants Research, 24, 839–846. [DOI] [PubMed] [Google Scholar]
- Karaca, C. , Er, N. , Gulsahi, A. , & Koseoglu, O. T. (2015). Alveolar ridge preservation with a free gingival graft in the anterior maxilla: volumetric evaluation in a randomized clinical trial. International Journal of Oral and Maxillofacial Surgery, 44, 774–780. [DOI] [PubMed] [Google Scholar]
- Katranji, A. , Misch, K. , & Wang, H.‐L. (2007). Cortical bone thickness in dentate and edentulous human cadavers. Journal of Periodontology, 78, 874–878. [DOI] [PubMed] [Google Scholar]
- Lee, S. L. , Kim, H. J. , Son, M. K. , & Chung, C. H. (2010). Anthropometric analysis of maxillary anterior buccal bone of Korean adults using cone‐beam CT. The Journal of Advanced Prosthodontics, 2, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, C. C. , Palomo, L. , Griffith, R. , & Hans, M. G. (2010). Accuracy and reliability of cone‐beam computed tomography for measuring alveolar bone height and detecting bony dehiscences and fenestrations. American Journal of Orthodontics and Dentofacial Orthopedics, 137, S109–S119. [DOI] [PubMed] [Google Scholar]
- Loubele, M. , van Assche, N. , Carpentier, K. , Maes, F. , Jacobs, R. , van Steenberghe, D. , & Suetens, P. (2008). Comparative localized linear accuracy of small‐field cone‐beam CT and multislice CT for alveolar bone measurements. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics, 105, 512–518. [DOI] [PubMed] [Google Scholar]
- Macbeth, N. , Trullenque‐Eriksson, A. , Donos, N. , & Mardas, N. (2016). Hard and soft tissue changes following alveolar ridge preservation: A systematic review. Clinical Oral Implants Research, 28(8), 982–1004. 10.1111/clr.12911 [DOI] [PubMed] [Google Scholar]
- Mardas, N. , Chadha, V. , & Donos, N. (2010). Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine‐derived xenograft: A randomized, controlled clinical trial. Clinical Oral Implants Research, 21, 688–698. [DOI] [PubMed] [Google Scholar]
- Mardas, N. , Trullenque‐Eriksson, A. , Macbeth, N. , Petrie, A. , & Donos, N. (2015). Does ridge preservation following tooth extraction improve implant treatment outcomes: A systematic review: Group 4: Therapeutic concepts & methods. Clin Oral Implants Res, 26(Suppl 11), 180–201. [DOI] [PubMed] [Google Scholar]
- Marmulla, R. , Wortche, R. , Muhling, J. , & Hassfeld, S. (2005). Geometric accuracy of the NewTom 9000 cone beam CT. Dentomaxillofacial Radiology, 34, 28–31. [DOI] [PubMed] [Google Scholar]
- Meloni, S. M. , Tallarico, M. , Lolli, F. M. , Deledda, A. , Pisano, M. , & Jovanovic, S. A. (2015). Postextraction socket preservation using epithelial connective tissue graft vs porcine collagen matrix. 1‐year results of a randomised controlled trial. European Journal of Oral Implantology, 8, 39–48. [PubMed] [Google Scholar]
- Molen, A. D. (2010). Considerations in the use of cone‐beam computed tomography for buccal bone measurements. American Journal of Orthodontics and Dentofacial Orthopedics, 137, S130–S135. [DOI] [PubMed] [Google Scholar]
- Neiva, R. F. , Tsao, Y.‐P. , Eber, R. , Shotwell, J. , Billy, E. , & Wang, H.‐L. (2008). Effects of a putty‐form hydroxyapatite matrix combined with the synthetic cell‐binding peptide P‐15 on alveolar ridge preservation. Journal of Periodontology, 79, 291–299. [DOI] [PubMed] [Google Scholar]
- O'Sullivan, D. , Sennerby, L. , Jagger, D. , & Meredith, N. (2004). A comparison of two methods of enhancing implant primary stability. Clinical Implant Dentistry and Related Research, 6, 48–57. [DOI] [PubMed] [Google Scholar]
- Patcas, R. , Muller, L. , Ullrich, O. , & Peltomaki, T. (2012). Accuracy of cone‐beam computed tomography at different resolutions assessed on the bony covering of the mandibular anterior teeth. American Journal of Orthodontics and Dentofacial Orthopedics, 141, 41–50. [DOI] [PubMed] [Google Scholar]
- Pietrokovski, J. , & Massler, M. (1967). Ridge remodeling after tooth extraction in rats. Journal of Dental Research, 46, 222–231. [DOI] [PubMed] [Google Scholar]
- Retzepi, M. , & Donos, N. (2010). Guided Bone Regeneration: Biological principle and therapeutic applications. Clinical Oral Implants Research, 21, 567–576. [DOI] [PubMed] [Google Scholar]
- Sapata, V. M. , Llanos, A. H. , Cesar Neto, J. B. , Jung, R. E. , Thoma, D. S. , Hämmerle, C. H. F. , Pannuti, C. M. , & Romito, G. A. (2020). Deproteinized bovine bone mineral is non‐inferior to deproteinized bovine bone mineral with 10% collagen in maintaining the soft tissue contour post‐extraction: A randomized trial. Clinical Oral Implants Research, 31(3), 294–301. 10.1111/clr.13570 [DOI] [PubMed] [Google Scholar]
- Schneider, D. , Schmidlin, P. R. , Philipp, A. , Annen, B. M. , Ronay, V. , Hämmerle, C. H. , Attin, T. , & Jung, R. E. (2014). Labial soft tissue volume evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. Journal of Clinical Periodontology, 41, 612–617. [DOI] [PubMed] [Google Scholar]
- Schropp, L. , Kostopoulos, L. , & Wenzel, A. (2003). Bone healing following immediate versus delayed placement of titanium implants into extraction sockets: A prospective clinical study. International Journal of Oral and Maxillofacial Implants, 18, 189–199. [PubMed] [Google Scholar]
- Spray, J. R. , Black, C. G. , Morris, H. F. , & Ochi, S. (2000). The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Annals of Periodontology, 5, 119–128. 10.1902/annals.2000.5.1.119 [DOI] [PubMed] [Google Scholar]
- Tan, W. L. , Wong, T. L. , Wong, M. C. , & Lang, N. P. (2012). A systematic review of post‐extractional alveolar hard and soft tissue dimensional changes in humans. Clinical Oral Implants Research, 23(Suppl 5), 1–21. [DOI] [PubMed] [Google Scholar]
- Ten Heggeler, J. M. , Slot, D. E. , & van der Weijden, G. A. (2011). Effect of socket preservation therapies following tooth extraction in non‐molar regions in humans: a systematic review. Clin Oral Implants Res, 22, 779–788. [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Bienz, S. P. , Lim, H.‐C. , Lee, W. Z. , Hämmerle, C. H. F. , & Jung, R. E. (2020). Explorative randomized controlled study comparing soft tissue thickness, contour changes, and soft tissue handling of two ridge preservation techniques and spontaneous healing two months after tooth extraction. Clinical Oral Implants Research, 31, 565–574. [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Jung, R. E. , Schneider, D. , Cochran, D. L. , Ender, A. , Jones, A. A. , Görlach, C. , Uebersax, L. , Graf‐Hausner, U. , & Hämmerle, C. H. (2010). Soft tissue volume augmentation by the use of collagen‐based matrices: A volumetric analysis. Journal of Clinical Periodontology, 37, 659–666. [DOI] [PubMed] [Google Scholar]
- Tomasi, C. , Sanz, M. , Cecchinato, D. , Pjetursson, B. , Ferrus, J. , Lang, N. P. , & Lindhe, J. (2010). Bone dimensional variations at implants placed in fresh extraction sockets: a multilevel multivariate analysis. Clinical Oral Implants Research, 21, 30–36. [DOI] [PubMed] [Google Scholar]
- Troiano, G. , Zhurakivska, K. , & Lo Muzio, L. , Laino, L. , Cicciù, M. , & Lo Russo, L. (2018). Combination of bone graft and resorbable membrane for alveolar ridge preservation: A systematic review, meta‐analysis, and trial sequential analysis. Journal of Periodontology, 89, 46–57. [DOI] [PubMed] [Google Scholar]
- Tsigarida, A. , Toscano, J. , De Brito Bezerra, B. , Geminiani, A. , Barmak, A. B. , Caton, J. , Papaspyridakos, P. , & Chochlidakis, K. (2020). Buccal bone thickness of maxillary anterior teeth. A systematic review and meta‐ analysis. Journal of Clinical Periodontology, 47. [DOI] [PubMed] [Google Scholar]
- van der Weijden, F. , Dell'Acqua, F. , & Slot, D. E. (2009). Alveolar bone dimensional changes of post‐extraction sockets in humans: a systematic review. Journal of Clinical Periodontology, 36, 1048–1058. [DOI] [PubMed] [Google Scholar]
- Vignoletti, F. , Nunez, J. , & Sanz, M. (2014). Soft tissue woud healing at teeth, dental implants and the edentulous ridge whne using barrier membranes, growth and differentiation factors and soft tissue substitutes. Journal of Clinical Periodontology, 41, S23–S35. [DOI] [PubMed] [Google Scholar]
- Wälivaara, D.‐Å. , Isaksson, S. , & Rosén, S. (2007). Description of a technique for evaluation of three‐dimensional shape alterations in soft tissue after intra oral bone reconstruction. British Journal of Oral and Maxillofacial Surgery, 45, 382–386. [DOI] [PubMed] [Google Scholar]
- Wang, R. E. , & Lang, N. (2012). Ridge preservation after tooth extraction. Clinical Oral Implants Research, 23, 147–156. [DOI] [PubMed] [Google Scholar]
- Wood, R. , Sun, Z. , Chaudhry, J. , Tee, B. C. , Kim, D. G. , Leblebicioglu, B. , & England, G. (2013). Factors affecting the accuracy of buccal alveolar bone height measurements from cone‐beam computed tomography images. American Journal of Orthodontics and Dentofacial Orthopedics, 143, 353–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The authors confirm that the data supporting the finding of this paper are available within the article and its supplementary material. Raw data that supports the findings of this study are available from the corresponding author upon reasonable request.


