Abstract
Objective
This study tested the hypothesis, in a prospective cohort study design, that maternal saturated free fatty acid (sFFA) concentration during pregnancy is prospectively associated with offspring (newborn) hypothalamic (HTH) microstructure and to explore the functional relevance of this association with respect to early‐childhood body fat percentage (BF%).
Methods
In N = 94 healthy newborns (born mean 39.3 [SD 1.5] weeks gestation), diffusion‐weighted magnetic resonance imaging was performed shortly after birth (25.3 [12.5] postnatal days), and a subgroup (n = 37) underwent a dual‐energy x‐ray absorptiometry scan in early childhood (4.7 [SD 0.7] years). Maternal sFFA concentration during pregnancy was quantified in fasting blood samples via liquid chromatography‐mass spectrometry. Infant HTH microstructural integrity was characterized using mean diffusivity (MD). Multiple linear regression was used to test the association between maternal sFFA and HTH MD, accounting for newborn sex, age at scan, mean white matter MD, and image quality. Multiple linear regression models also tested the association between HTH MD and early‐childhood BF%, accounting for breastfeeding status.
Results
Maternal sFFA during pregnancy accounted for 8.3% of the variation in newborn HTH MD (β‐std = 0.25; p = 0.006). Furthermore, newborn HTH MD prospectively accounted for 15% of the variation in early‐childhood BF% (β‐std = 0.32; p = 0.019).
Conclusions
These findings suggest that maternal overnutrition during pregnancy may influence the development of the fetal hypothalamus, which, in turn, may have clinical relevance for childhood obesity risk.
Study Importance.
1. What is already known?
A convergent body of preclinical experimental evidence suggests that maternal overnutrition during pregnancy can alter offspring hypothalamic microstructural development, with adverse consequences for the regulation of body weight and metabolism.
What does this study add?
In humans, using a prospective cohort study design, we report an association between maternal saturated free fatty acid concentration during pregnancy and newborn hypothalamic microstructure.
In a small subgroup of children followed from birth through early childhood, we observed a prospective association between newborn hypothalamic microstructure and body fat percentage, supporting the functional importance of this newborn phenotype in humans.
How might these results change the direction of research or the focus of clinical practice?
These observations provide evidence for the influence of maternal overnutrition during pregnancy on the developing human fetal hypothalamus.
This work provides further mechanistic evidence that the management of maternal overnutrition, before and/or during pregnancy, is important for the primary prevention of childhood obesity.
INTRODUCTION
Childhood obesity represents a major public health challenge. Children with obesity are substantially more likely to have obesity as adults and to develop obesity‐related disorders at earlier ages (1) and of greater severity (2). Moreover, once established, obesity is difficult to reverse, underscoring the importance of primary prevention (3, 4).
Obesity is a complex, multifactorial phenotype (5). The importance of the structural and functional integrity of brain regions, particularly hypothalamic (HTH) nuclei, in the regulation of energy balance and its influence on obesity risk are well established. Preclinical and translational imaging findings support the premise that the typical structure (e.g., astrogliosis, axonal density, median eminence permeability) and function (e.g., leptin, insulin, and glucose sensing) of the human HTH is altered in obesity (6, 7, 8, 9, 10, 11). However, it remains unclear as to what degree the observed differences in the HTH of those with obesity relative to individuals with normal weight are a cause, consequence, or both of obesity. A rapidly growing and convergent body of preclinical evidence suggests that the HTH exhibits developmental plasticity, particularly during the fetal period, as it adapts to variation in maternal nutritional state (e.g., under/overnutrition) (12, 13, 14, 15, 16, 17, 18). This process may then influence postnatal metabolism and growth. However, specific effectors of this process in humans are, so far, largely unknown.
Maternal saturated free fatty acid (sFFA) concentrations during pregnancy represent a plausible biological candidate linking maternal overnutrition to variation in fetal HTH development, in that they may function as both a sensor of the maternal state and as an effector of HTH development (after crossing the placenta and blood‐brain barrier via passive diffusion and/or active transport) (19, 20). Circulating FFAs (both saturated and unsaturated) in the fasting state arise almost entirely from adipose tissue stores (21), thereby representing an indicator, or sensor, of maternal obesity and of previous dietary fat quality (22, 23). Experimental evidence in rodents suggests that sFFA‐induced inflammation in the HTH can alter anorexigenic signaling (24) and that maternal obesity‐induced increases in circulating sFFAs blunt offspring HTH axonal outgrowth, with downstream implications for fat deposition (25). This concept is also indirectly supported in humans by two observations: (1) maternal FFA concentrations during pregnancy are associated with offspring body fat percentage (BF%) in early childhood (26); and (2) maternal prepregnancy obesity is associated with alterations in child HTH function (cerebral blood flow in response to glucose challenge), that, in turn, are predictive of subsequent weight gain (27).
Based on these considerations, the goal of this study was to examine the prospective association of maternal sFFA concentrations during pregnancy with infant human HTH microstructure, as indexed by mean diffusivity (MD). HTH MD represents the degree to which water can freely diffuse within the HTH and constitutes a replicable neuroimaging phenotype of obesity in human adults (28, 29). MD is reflective of, but not specific to, microstructural states at the cellular level (e.g., gliosis, axonal density, cytosis, angiogenesis) (30). Based on findings in rodents that sFFAs reduce HTH axonal outgrowth, and because any decrease in cell bodies and cell walls would also represent an associated decrease in barriers to diffusion (thus allowing for an increased capacity for diffusion processes), we hypothesized that maternal sFFA concentrations during pregnancy would be positively associated with infant HTH MD (i.e., fewer barriers to diffusion).
To address this hypothesis, we conducted a prospective, longitudinal study in a cohort of mother‐infant dyads from early pregnancy through birth until approximately 5 years of age. We quantified maternal circulating concentrations of sFFAs in early (13.1 [1.8], 20.5 [1.4], and 30.5 [1.4] weeks gestation) pregnancy. We also acquired infant brain magnetic resonance imaging (MRI) scans shortly after birth to quantify HTH MD. The importance of imaging the newborn brain derives from the logic that brain circuitry at this time is not yet influenced by postnatal obesogenic factors (31). In order to explore the functional consequences of interindividual variation in newborn HTH MD, we quantified early‐childhood BF% via dual‐energy x‐ray absorptiometry (DXA). Notably, early childhood represents the age/developmental stage at which body composition, particularly BF%, is predictive of subsequent child, adolescent, and adult obesity risk (32). Associations between maternal sFFA concentrations and infant HTH MD, as well as between infant HTH MD and early‐childhood BF%, were tested using multiple linear regression models that accounted for the effects of key covariates and potentially confounding factors.
METHODS
Study population
Mother‐child dyads were part of a prospective cohort study at the University of California, Irvine's Development, Health and Disease Research Program, designed to investigate the effects of maternal conditions among healthy pregnant women on offspring development. The study enrolled pregnant women attending antenatal care at clinics affiliated with the University of California, Irvine Medical Center in Orange County, California, between 2011 and 2015. The present analysis included 94 women enrolled between March 2011 and December 2013 whose children underwent a brain MRI scan shortly after birth. Exclusion criteria were as follows: mother less than 18 years of age; nonsingleton/intrauterine pregnancy; type 1 diabetes; maternal use of psychotropic medications or systemic corticosteroids during pregnancy; infant birth before 34 weeks gestation (n = 7 healthy late preterm born children, i.e., between 34 and 37 weeks gestational age); and infant congenital, genetic, or neurologicical disorder. Race/ethnicity, education, and household income of the maternal participants were assessed by self‐report at the first study visit. Demographic characteristics are presented in Table 1. MRI was successfully performed in the offspring shortly after birth (N = 96 total; n = 2 rejected based on visual inspection) and DXA imaging (n = 37) in early childhood. Measures of maternal sFFA concentration were available for all women whose children participated in the postnatal imaging assessments (infant MRI and early‐childhood DXA). The Institutional Review Board of the University of California, Irvine approved all study procedures, and all parents provided written informed consent.
TABLE 1.
Demographic information
| Maternal age (y), mean (SD) | 27.8 (5.5) |
| Maternal prepregnancy BMI, continuous | 27.4 (6.6) |
| Maternal prepregnancy BMI category, % | |
| Underweight | 3.2 |
| Normal weight | 44.7 |
| Overweight | 25.5 |
| Obesity | 25.5 |
| Maternal race/ethnicity, % | |
| White non‐Hispanic | 39.4 |
| White Hispanic | 34.0 |
| Asian | 7.5 |
| Other | 19.1 |
| Household highest level of maternal education, % | |
| High school or test equivalent | 22.2 |
| Vocational school or some college | 41.2 |
| Associate's degree | 5.8 |
| Bachelors or graduate level degree | 30.7 |
| Gross annual household income, % | |
| <$15,000 | 10.0 |
| $15,000–$29,999 | 20.0 |
| $30,000–$49,999 | 22.2 |
| $50,000–$100,000 | 40.0 |
| >$100,000 | 7.8 |
| Birth characteristics | |
| Gestational age at birth (N = 94) (wk), mean (SD) | 39.3 (1.5) |
| Weight at birth (g), mean (SD) | 3,347 (485) |
| Length at birth, (in), mean (SD) | 50.1 (2.7) |
| Infant MRI | |
| Postnatal age at scan (d), mean (SD) | 25.3 (12.5) |
| Weight at scan (kg), mean (SD) | 3.99 (0.65) |
| Childhood DXA | |
| Age at scan (n = 37) (y), mean (SD) | 4.7 (0.7) |
Note: Demographic information for the full sample (N = 94 mothers or the subset of n = 37 children with follow‐up DXA imaging) with available maternal sFFA and MRI measurements. There were no significant differences in key demographics between the full sample and the DXA subset.
Abbreviations: DXA, dual‐energy x‐ray absorptiometry; MRI, magnetic resonance imaging; sFFA, saturated free fatty acid.
sFFA collection
Following an overnight fast, maternal morning blood samples were collected at each of three study visits in early, mid‐, and late pregnancy (13.1 [1.8]; 20.5 [1.4]; and 30.5 [1.4] weeks gestation, respectively). Maternal sFFA quantification was performed at Ludwig‐Maximilian University Munich in Germany, as reported elsewhere (33). Briefly, blood (10 mL) was drawn by standard venipuncture, centrifuged for plasma aliquots (0.5 mL), and stored at −80 °C prior to analysis. Samples were then assayed using liquid chromatography‐tandem mass spectrometry analysis performed for identification of nonesterified fatty acid concentration (34), and the 14 quantified saturated fatty acids were selected for aggregation across pregnancy (10:0, 11:0, 12:0, 13:0, 14:0, 15:0, 16:0, 17:0, 18:0, 19:0, 20:0, 22:0, 24:0, 26:0). All 42 measures (14 constituents × 3 visits) were first z scored (measure dimension), followed by median replacement (across individual, 14.8% missingness) to avoid missingness bias from abundant/sparse constituents (e.g., palmitic acid). Average maternal sFFA concentration across pregnancy was used as the primary exposure to improve signal‐to‐noise through signal averaging (35) and, more closely, represent trait, as opposed to state, sFFA concentration.
MRI assessments
Newborn MRI scans were acquired during natural sleep using a 12‐channel head‐receive coil (Siemens 3‐T Tim Trio, Siemens AG, Munich, Germany). After feeding and soothing, infants were placed in a CIVCO beaded pillow (Orange City, Iowa). This pillow covers the infant's body and head, becomes rigid under vacuum, and provides a comforting swaddle, motion prevention, and hearing protection when used in conjunction with standard foam earplugs. Participants were monitored for heart rate and oxygen saturation via a pulse oximeter attached to the foot. The single‐shell 42‐direction diffusion‐weighted protocol (echo planar imaging [EPI], repetition time [TR]/echo time [TE] = 8,900/83 milliseconds, field of view [FoV] = 256 × 224 × 150 mm, resolution = 2 × 2 × 2 mm, partial Fourier = 6/8, GRAPPA (GeneRalized Autocalibrating Partial Parallel Acquisition) Phase Encoding Acceleration Factor = 2, 42 unique directions at b = 1,000 s/mm2, 7 at b = 0) was 7 minutes and 43 seconds in duration.
MRI data processing
Diffusion‐weighted data were preprocessed (Figure 1) in accordance with the developing Human Connectome Project (dHCP) pipeline (36). Data were corrected for motion, signal dropout, and eddy current, including outlier slice detection and replacement using a Gaussian process prediction via a nonparametric approach, and slice to volume correction. Data quality measures were extracted using FMRIB (Functional MRI of the Brain) Software Library (FSL) QUAD (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/eddyqc/UsersGuide), screened for extreme outliers, and retained for use as a potentially confounding factor (mean framewise displacement). Scalar maps were derived using the fully preprocessed diffusion‐weighted data (DTIFIT), including b‐vector rotation to reflect coregistration transformation matrices. Specifically, whole‐brain MD and fractional anisotropy (FA) were fit for hypothesis testing and registration to a common template, respectively. An age‐specific sample‐based template in common Montreal Neurological Institute (MNI) space was derived using a three‐stage process as per the standardized FSL TBSS pipeline (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS). First, a representative participant was nonlinearly registered (FSL FNIRT, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FNIRT/UserGuide) to the FMRIB FA template. Second, a sample‐based template was derived via nonlinear registration to the representative participant in MNI/FMRIB FA space (all participants) and averaged for a final sample‐based mean FA image template. Third, all participants were registered to this sample average. Of note, qualitative assessment of registration consistency in the vicinity of the HTH verified proper alignment, presumably aided by the high signal contrast along the HTH‐third ventricle interface. HTH was defined using a high resolution atlas published in 2018 (37). The high resolution atlas was first transformed into MNI space (a common space aligned with the FA template) and the HTH region of interest (ROI) defined via a threshold of 0.6 based on visual inspection (Figure 1 shows HTH ROI alignment with MD atlas). Finally, this bilateral ROI was used to extract the primary outcome for each infant (median HTH MD).
FIGURE 1.
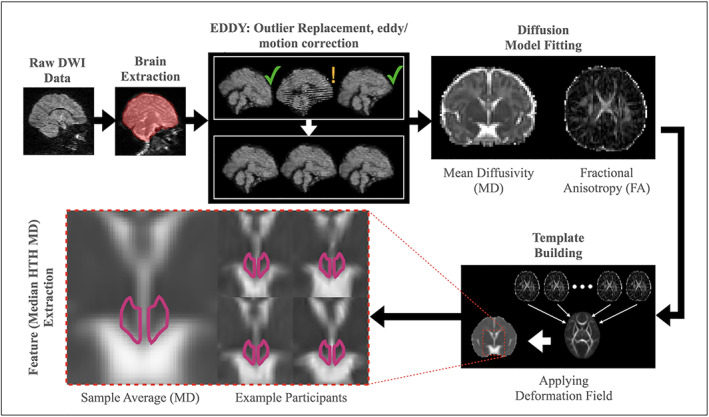
Image processing overview. Image processing included a community standard preprocessing pipeline (e.g., FSL's eddy) followed by a three‐dimensional atlas‐based region of interest (ROI) HTH definition (magenta outline, bottom left). HTH MD was summarized by the median value. Inset images are composed of relative high (top) and low (bottom) sample median HTH MD images. DWI, diffusion‐weighted imaging; FSL, FMRIB Software Library; HTH, hypothalamic; MD, mean diffusivity; ROI, region of interest [Color figure can be viewed at wileyonlinelibrary.com]
DXA assessments
A whole‐body DXA scan was obtained using a Hologic Discovery Scanner (A, QDR 4500 series, Hologic Inc., Bedford, Massachusetts) in pediatric scan mode. Calibration using Hologic's anthropomorphic Spine QC Phantom was performed before each scan. Children were scanned awake in a supine position, wearing a standardized light cotton short and shirt set provided by the study team. If the child moved during the scan, a single repeat was performed. Global BF% was defined as 100 times the ratio of global fat mass to the sum of global fat mass and global fat‐free mass. BF% was residualized for age at scan and offspring sex.
Covariates
Our a priori selection of the covariates was based on theoretical considerations and findings from the available literature on possible associations with either only the outcome of interest (to improve model precision) or with both the outcome and primary predictor of interest (to address potential confounding). Gestational age at birth was determined by best obstetric estimate with a combination of last menstrual period and early uterine size and was confirmed by obstetric ultrasonographic biometry before 15 weeks using standard clinical criteria. Infant sex was abstracted from the medical record. Socioeconomic status (SES) was summarized using a two‐component score composed of household income and highest level of maternal education. Each component was categorized on a five‐level scale and averaged across components (mean = 3.2 [1.0], range = 1–5). Obstetric risk (OB risk) was a binary value indicating the presence of one or more of the following risk factors extracted from the medical record: preeclampsia, hypertension, diabetes, severe anemia, severe infection, vaginal bleeding. Infant feeding practices were assessed via monthly maternal interviews. A composite measure of feeding practice categorized offspring with greater than 75% of the first 6 months of life spent feeding as breastfed, less than 25% of the first 6 months of life spent feeding as formula‐fed, and intermediate values as mixed feeding practice.
Statistical analysis
Multiple linear regression models were used to examine the relationship between maternal sFFA concentration during pregnancy and offspring infant HTH MD by first using a parsimonious regression analysis based on known or likely associations with infant HTH MD (Equation 1), followed by a post hoc “full” model accounting for additional confounding factors (SES, ethnicity, OB risk). The rationale for the a priori inclusion of whole‐brain white matter MD (binary mask, FA > 0.2) and framewise displacement is based on the premise that associations should be present above and beyond global MD associations and limit noise‐induced variation, respectively. Acronyms for Equations 1 and 2 are as follows: HTHMD = hypothalamus mean diffusivity; sFFA = saturated free fatty acid concentration; GA = gestational age at birth; SA = postnatal age at scan, WMMD = global white matter mean diffusivity; FD = framewise displacement.
| (1) |
A bivariate linear regression model was used to examine the relationship between covariate‐adjusted offspring infant HTH MD (Equation 2a is the equivalent to Equation 1 without the sFFA term, rearranged) and early‐childhood age and sex‐adjusted BF% (Equation 2b) beginning with a parsimonious analysis (Equation 2c), followed by a post hoc full model accounting for breastfeeding status in addition to SES, ethnicity, and OB risk.
| (2a) |
| (2b) |
| (2c) |
Finally, because OB risk represents a clinically and biologically heterogenous set of risk factors that may not be accurately represented by a single binary risk variable, we repeated each full model excluding those mothers identified as belonging to the OB risk group (N sFFA‐HTH = 32 [34% of analytic sample] and N HTH‐BF% = 14 [38% of analytic sample] excluded). The relative frequency of OB risk factors (presence of any risk factor at any study visit) within this (N = 94) sample are as follows: preeclampsia = 3.2%; hypertension = 5.3%; diabetes = 6.4%; severe anemia = 7.5%; severe infection = 6.4%; and vaginal bleeding = 17.0%. There were no cases of fetal growth restriction or chronic hypertension included in the study (e.g., all participants identified as hypertensive for analytical purposes exhibited hypertension at only a single visit). Furthermore, a sensitivity analysis repeating the models with OB risk conditions as separate predictors in the model was performed (Supporting Information Table S2). In addition, a model covarying for maternal prepregnancy BMI was tested in order to provide additional information on the role of maternal sFFA concentration during pregnancy in fetal HTH microstructural development, when accounting for maternal prepregnancy BMI (Supporting Information Table S3).
Data sharing plans
STROBE guidelines (STrengthening the Reporting of OBservational studies in Epidemiology; https://www.strobe-statement.org/index.php?id=available-checklists) were followed and the checklist provided as part of the review process. Furthermore, in accordance with the Material Design Analysis Reporting (MDAR) (38) guidelines promoting access to underlying data and code, deidentified and unprocessed MRI data used in the context of this study are publicly available through the National Institute of Mental Health Data Archive Collection #1890 (https://nda.nih.gov/edit_collection.html?id=1890). Deidentified and unprocessed metabolomic and associated (e.g., covariates) measures can be made available upon request and through the completion of the necessary Data Use Agreement materials. Derived measures and statistical models used here are made publicly available via a Github repository (https://github.com/jerodras/sffa-hypothalamus.git).
RESULTS
Study population in brief
A total of 94 mother‐child dyads were included for study. MRI was successfully performed in the infants shortly after birth (N = 96 total; n = 2 rejected based on visual inspection) and DXA imaging (n = 37) in early childhood. Measures of maternal sFFA concentration were available for all women whose children participated in the postnatal imaging assessments (infant MRI and early‐childhood DXA).
Descriptive findings
Maternal FFA concentrations during pregnancy in the present cohort have been described in detail elsewhere (23). In brief, raw sFFA measures across the three pregnancy visits were 7.14 (2.51), 6.82 (2.32), and 7.56 (2.40) mmol/L, respectively, whereas mean z‐transformed sFFA across pregnancy was −0.09 (0.34), normally distributed (Lilliefors nonuniform null‐hypothesis test; p = 0.13), and visit‐specific sFFA concentrations were correlated across pregnancy visits (r 1st,2nd = 0.48; r 2nd,3rd = 0.39; r 1st,3rd = 0.38; intraclass correlation coefficient = 0.40), consistent with existing literature (35). In addition, average maternal sFFA concentrations across pregnancy were associated with maternal prepregnancy BMI (p < 10−5, see Supporting Information Table S1 for a categorical breakdown), but not gestational weight gain (p > 0.1), in a manner consistent with previously published findings from this cohort (23).
Mean infant HTH MD was 0.0017 (0.0001) mm2/s, normally distributed (Lilliefors nonuniform null‐hypothesis test; p = 0.11) and negatively associated with postmenstrual age at scan (partial R 2 = 6.5%; p = 0.013), but not sex, in a manner consistent with the literature on early white matter developmental trajectories (39).
Mean BF% in early childhood was 27.6% (5.6%), normally distributed (Lilliefors nonuniform null‐hypothesis test; p = 0.39), and negatively associated with age at scan (partial R 2 = 10.9%; p = 0.014), but not sex, in a manner consistent with the adiposity rebound period.
Maternal sFFA concentration during pregnancy is associated with offspring infant HTH MD
Maternal sFFA concentration during pregnancy was positively associated with infant HTH MD (Table 2; Figure 2; parsimonious regression model: F 7,94 = 6.46, p < 0.001, model‐adjusted R 2 = 26.1%) above and beyond factors accounting for age‐related growth (age and global variation in MD) and sex. Maternal sFFA remained significant when considering the potentially confounding factors of SES, ethnicity, and OB risk (Table 2; full regression model: F 10,94 = 6.55, p < 0.001, model‐adjusted R 2 = 34.9%), including those analyses conducted while excluding mothers in the OB risk group. Furthermore, maternal sFFA remained significant when adjusting for individual OB risk conditions (Supporting Information Table S2) and, separately, prepregnancy BMI (Supporting Information Table S3). Maternal sFFA concentration during pregnancy explained ~8% of the interindividual variance in infant HTH MD.
TABLE 2.
Parsimonious and full model comparison: maternal sFFA concentration during pregnancy and infant HTH MD
| Parsimonious model | Full model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Independent variable |
|
|
p |
|
|
p | ||||
| sFFA concentration | 0.25 | 8.3% | 0.006 | 0.23 | 8.1% | 0.008 | ||||
| Gestational age at birth | 0.13 | 1.0% | 0.361 | 0.00 | 0.0% | 0.943 | ||||
| Postnatal age at scan | 0.17 | 1.7% | 0.226 | 0.06 | 0.2% | 0.665 | ||||
| Infant sex (male) | 0.11 | 0.4% | 0.543 | 0.09 | 0.3% | 0.612 | ||||
| White matter MD | 0.57 | 14.4% | <0.001 | 0.47 | 11.1% | 0.002 | ||||
| QC (framewise displacement) | 0.22 | 6.3% | 0.017 | 0.15 | 3.2% | 0.097 | ||||
| Socioeconomic status | na | na | na | 0.21 | 5.3% | 0.033 | ||||
| Hispanic (yes) | na | na | na | 0.56 | 10.9% | 0.002 | ||||
| OB risk (yes) | na | na | na | −0.34 | 4.3% | 0.056 | ||||
Note: Maternal sFFA concentration during pregnancy was associated with infant HTH MD.
Abbreviations: HTH, hypothalamic; MD, mean diffusivity; na, not applicable; OB, obesity; QC, quality control; sFFA, saturated free fatty acid.
FIGURE 2.
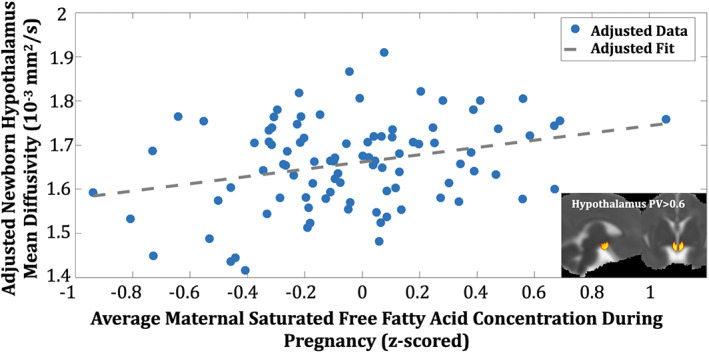
Maternal saturated free fatty acid (sFFA) concentration during pregnancy and infant hypothalamic MD (HTH MD). Maternal sFFA was linearly associated with newborn HTH MD (HTH region of interest inset) [Color figure can be viewed at wileyonlinelibrary.com]
Offspring infant HTH MD is associated with early‐childhood BF%
Higher adjusted (Equation 2a) infant HTH MD was associated with higher adjusted (Equation 2b) early‐childhood BF% (Table 3; Figure 3; parsimonious regression model: F 2,37 = 6.06, p = 0.019, model‐adjusted R 2 = 12.3%). Post hoc testing suggested that the association between newborn HTH MD and early‐childhood BF% remained significant when considering potential pre‐ and postnatal confounding factors (Table 3; full regression model: F 7,37 = 2.47, p = 0.054, model‐adjusted R 2 = 17.0%), including those analyses conducted while excluding mothers in the OB risk group. Newborn HTH MD independently explained ~14% to 17% of variance in early‐childhood BF%.
TABLE 3.
Parsimonious and full model comparison: offspring HTH MD and early‐childhood BF%
| Parsimonious model | Full model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Independent variable |
|
|
p |
|
|
p | ||||
| HTH MD | 0.32 | 14.8% | 0.019 | 0.41 | 17.2% | 0.037 | ||||
| Breastfeeding status | na | na | na | 0.22 | 5.0% | 0.210 | ||||
| SES | na | na | na | 0.26 | 7.4% | 0.125 | ||||
| Hispanic (yes) | na | na | na | 0.63 | 9.0% | 0.090 | ||||
| Obstetric risk (yes) | na | na | na | −0.23 | 1.5% | 0.493 | ||||
Note: Infant HTH MD was associated with early‐childhood BF%.
Abbreviations: BF%, body fat percentage; HTH, hypothalamic; MD, mean diffusivity; na, not applicable; SES, socioeconomic status.
FIGURE 3.
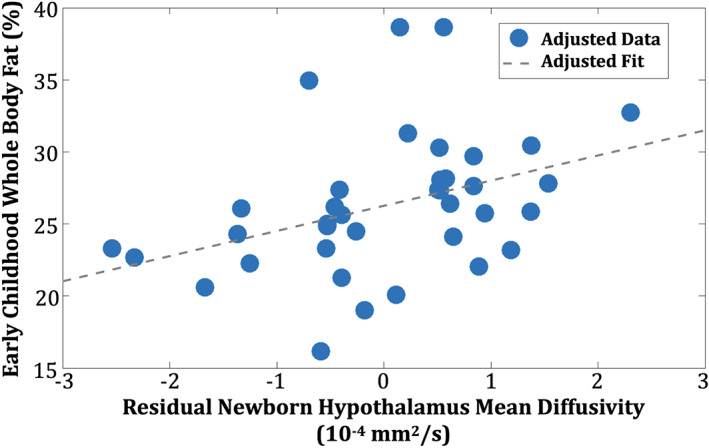
Infant hypothalamic mean diffusivity (HTH MD) is associated with early‐childhood body fat percentage (BF%). Residualized newborn HTH MD (Equation 2a) was linearly associated with earlychildhood BF% [Color figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
This work represents, to the best of our knowledge, the first report in humans of a prospective association between maternal sFFA concentrations during pregnancy and newborn HTH MD, a clinically relevant (28) indicator of HTH microstructural integrity. Furthermore, we provide proof‐of‐concept evidence for the functional relevance of this infant neurological phenotype by demonstrating an association between infant HTH MD and early‐childhood BF%. The direction of the observed associations is consistent with previous findings relating higher maternal sFFA concentrations during pregnancy with reduced offspring axonal outgrowth in rodents (25) and human literature linking maternal energy substrate availability with offspring HTH functioning (27) and adiposity in early childhood (26). Furthermore, the observed associations remained significant after controlling for key prenatal and postnatal covariates.
In terms of the magnitude of effects, a 1‐SD difference in maternal sFFA concentrations during pregnancy corresponded with a +0.25‐SD (95% CI: 0.17‐0.33; MD = 2.9 × 10−5 mm2/s) difference in infant HTH MD. Given the paucity of human infant HTH neuroimaging data, it is difficult to put the magnitude of this effect into any direct context. However, the direction of this effect is consistent with recent observations in rodents (25). Specifically, dams fed a diet rich in sFFAs demonstrated increased circulating sFFA concentrations and produced offspring with a reduced density of leptin responsive cells in the HTH. Furthermore, explants from the offspring HTH demonstrated marked decreases in axonal projections when directly exposed to sFFAs ex vivo. Because any decrease in cell bodies represents a decrease in barriers to diffusion, it follows that diffusion (i.e., MD) at the microstructural level would increase in the presence of elevated sFFA concentrations, as observed here.
In terms of the potential clinical significance of the observed effects, a 1‐SD difference in HTH MD corresponded to a +0.32‐SD (95% CI: 0.19‐0.45; BF% = 1.8%) difference in BF% in early childhood. The lower bound of this association is consistent with (overlapping 95% CI) reported estimates in adults, measured using similar techniques and validated in two large independent samples (testing and validation samples both +0.14‐SD change per SD unit of BMI) (28). Potential explanations for the larger effect size observed in our study are several‐fold, including a more proximal measure of adiposity (DXA BF% vs. BMI) (40), decreased exposure to obesogenic factors commonly seen in adulthood (41) (thus a more direct reflection of HTH‐regulated homeostatic processes), and/or, more simply, an uncertain estimate due to the limited longitudinal sample size in early childhood.
The direction of the current finding is conceptually consistent with two key pieces of human empirical evidence. First, in a large sample of mother‐child dyads, a positive association was reported between maternal FFA concentrations and early‐childhood adiposity (26). Second, maternal prepregnancy BMI has been associated with early‐childhood HTH response to oral glucose administration (cerebral blood flow measures using MRI), and the magnitude of this response was predictive of subsequent weight gain (27). Importantly, our study extends these human findings with our observation that the effects of excess maternal energy substrate on the newborn HTH are antecedent to those of the postnatal obesogenic environment and the emergence of early‐childhood fat gain.
Obesity has been labeled a “heritable neurobehavioral disorder that is highly sensitive to environmental conditions.” (42). Because of the rapid developmental changes the brain undergoes during embryonic and fetal life, prenatal environmental exposures are especially salient in altering the developing brain with downstream implications on feeding behavior and energy homeostasis. Fetal brain development culminates in the formation, segregation (differentiation/specialization), and integration (forming a network of networks) of key energy homeostasis networks (e.g., satiety) by the time of birth, at which time the foundational setting of these brain networks likely plays an especially meaningful role. Therefore, despite the small (in absolute terms) longitudinal sample size available for understanding the functional relevance of the observed newborn neurological phenotype (HTH MD), we assert that the observed association between newborn HTH MD and BF% in early childhood is of significant clinical relevance and that it warrants targeted external replication efforts, particularly given that this finding reaffirms a large body of preclinical evidence in support of the HTH being critical for energy homeostasis and showing a high degree in developmental plasticity and, consequently, phenotypic variation resulting from in utero exposures (12, 13, 14, 15, 16, 17, 18). Although it is unlikely that detailed measures of maternal sFFA concentrations across pregnancy such as those used here will be available for replication using large‐scale, publicly available, longitudinal neuroimaging data, partial replication using more readily available but distal biomarkers such as maternal prepregnancy BMI could help further characterize the role of the HTH in the intergenerational transmission of obesity risk.
Limitations of this study include the lack of cellular specificity provided by standard diffusion tensor imaging (DTI) measures, peripheral measures of circulating maternal sFFAs, partial characterization of the gestational milieu, and the specific focus on the HTH. Because DTI models reflect diffusion processes that are nonspecific to the cellular processes that underlie signal variation (43), there are plausible alternative explanations of variation in HTH MD. For example, increased gliosis, as previously reported in MRI‐based studies of HTH inflammation (29) and/or greater permeability of the median eminence (7), would naturally tend toward increased HTH MD because of associated increases in tissue fluid. A second limitation of this study is the lack of more proximal measures of fetal sFFA exposure. Although the serial measurement approach used here provides a comprehensive and stable characterization of maternal sFFA concentrations across pregnancy, the collection and quantification of sFFA concentrations in cord blood and/or amniotic fluid could provide additional insight into the degree of association between maternal circulation and fetal exposure around the time of birth. The current study did not collect or consider several other potentially important ligands known to play a role in the ontogeny of the fetal HTH, including leptin and adiponectin. Future efforts should be aimed at characterizing the gestational milieu in a more comprehensive fashion in order to gain a more complete understanding of relative sFFA importance. Finally, the HTH is well established to be the primary central regulator of energy homeostasis. However, there are several extrahypothalamic brain systems (e.g., interoception, salience, reward) with demonstrated relevance in the context of both maternal exposures during pregnancy and offspring obesity risk (44). Although the current study was focused on testing a hypothesis‐based translation of experimental data from the animal literature, large‐scale longitudinal developmental neuroimaging studies such as the upcoming HEALthy Brain and Child Development (45) study represent the ideal platform for conducting a more discovery‐based approach to identifying key extrahypothalamic brain systems in the context of the fetal programming of obesity.
To conclude, the current study provides evidence linking maternal sFFA concentrations during pregnancy with infant HTH MD and supports, in a preliminary fashion, the clinical relevance of this finding with respect to childhood adiposity/obesity risk. Importantly, circulating sFFA concentrations are modifiable through diet, exercise (46), tauroursodeoxycholic (a naturally occurring bile acid) supplementation (25), and/or a glucagon‐like peptide‐1 receptor agonist (e.g., semaglutide) (47). Therefore, this work holds promise in contributing to an improved understanding of plausible intervention targets for the primary prevention of childhood obesity.
CONFLICT OF INTEREST
PMT receives partial grant support from Biogen, Inc., for research unrelated to this manuscript. The other authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
JMR, PMT, LEG, KLL, SE, PDW, and CB contributed to the conceptualization of this project; JMR, BK, and KLL contributed to the methodology used; JMR, LEG, SE, PDW, and CB contributed to the analysis; JMR, LEG, KLL, TGO, SE, PDW, BK, and CB provided valuable resources; and all coauthors contributed to the writing process.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank the study participants for their enthusiastic participation and the research staff at the Development, Health and Disease Research Program for their dedicated efforts on this project.
Rasmussen JM, Thompson PM, Gyllenhammer LE, et al. Maternal free fatty acid concentration during pregnancy is associated with newborn hypothalamic microstructure in humans. Obesity (Silver Spring). 2022;30(7):1462‐1471. doi: 10.1002/oby.23452
Funding informationSupport for this work was provided by National Institute of Child Health and Human Development (grant numbers R01 HD060628, K99 HD100593); National Institute of Mental Health (grant number R01 MH091351); National Institute for Diabetes and Digestive and Kidney Diseases (grant number R21 DK118578); and National Institutes of Health (UG3OD023349). BK is the Else Kröner Senior professor of Pediatrics at Ludwig‐Maximillian University, financially supported by the Else Kröner Fresenius Foundation and the Ludwig‐Maximillian University Medical Faculty and Hospitals.
Contributor Information
Jerod M. Rasmussen, Email: rasmussj@uci.edu.
Claudia Buss, Email: claudia.buss@charite.de.
REFERENCES
- 1. Dabelea D, Harrod CS. Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr Rev. 2013;71(suppl 1):S62‐S67. doi: 10.1111/nure.12061 [DOI] [PubMed] [Google Scholar]
- 2. Forgat‐Campagna A, Narayan KV. Type‐2 diabetes in children: exemplifies the growing problem of chronic diseases. BMJ. 2001;322:377‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Speakman JR. The evolution of body fatness: trading off disease and predation risk. J Exp Biol. 2018;221(Pt suppl 1):jeb167254. doi: 10.1242/jeb.167254 [DOI] [PubMed] [Google Scholar]
- 4. Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38:267‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finegood DT, Merth TDN, Rutter H. Implications of the foresight obesity system map for solutions to childhood obesity. Obesity (Silver Spring). 2010;18(suppl 1):S13‐S16. [DOI] [PubMed] [Google Scholar]
- 6. Kreutzer C, Peters S, Schulte DM, et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes. 2017;66:2407‐2415. [DOI] [PubMed] [Google Scholar]
- 7. Razolli DS, Moura‐Assis A, Bombassaro B, Velloso LA. Hypothalamic neuronal cellular and subcellular abnormalities in experimental obesity. Int J Obes (Lond). 2019;43:2361‐2369. [DOI] [PubMed] [Google Scholar]
- 8. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF‐kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thaler JP, Yi C‐X, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schneeberger M, Dietrich MO, Sebastián D, et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valdearcos M, Douglass JD, Robblee MM, et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2018;26:185‐197.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim DW, Glendining KA, Grattan DR, Jasoni CL. Maternal obesity leads to increased proliferation and numbers of astrocytes in the developing fetal and neonatal mouse hypothalamus. Int J Dev Neurosci. 2016;53:18‐25. [DOI] [PubMed] [Google Scholar]
- 13. Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo‐pituitary‐adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gali Ramamoorthy T, Begum G, Harno E, White A. Developmental programming of hypothalamic neuronal circuits: impact on energy balance control. Front Neurosci. 2015;9:126. doi:10.3389/fnins.2015.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang G‐Q, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high‐fat diet and fetal programming: increased proliferation of hypothalamic peptide‐producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107‐12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dearden L, Ozanne SE. Early life origins of metabolic disease: developmental programming of hypothalamic pathways controlling energy homeostasis. Front Neuroendocrinol. 2015;39:3‐16. [DOI] [PubMed] [Google Scholar]
- 17. Dearden L, Buller S, Furigo IC, Fernandez‐Twinn DS, Ozanne SE. Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways. Mol Metab. 2020;42:101079. doi:10.1016/j.molmet.2020.101079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lippert RN, Brüning JC. Maternal metabolic programming of the developing central nervous system: unified pathways to metabolic and psychiatric disorders. Biol Psychiatry. 2022;91:898‐906. [DOI] [PubMed] [Google Scholar]
- 19. Larqué E, Demmelmair H, Gil‐Sánchez A, et al. Placental transfer of fatty acids and fetal implications. Am J Clin Nutr. 2011;94(6 suppl):1908S‐1913S. [DOI] [PubMed] [Google Scholar]
- 20. Le Foll C. Hypothalamic fatty acids and ketone bodies sensing and role of FAT/CD36 in the regulation of food intake. Front Physiol. 2019;10:1036. doi:10.3389/fphys.2019.01036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441‐2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hellmuth C, Demmelmair H, Schmitt I, Peissner W, Bluher M, Koletzko B. Association between plasma nonesterified fatty acids species and adipose tissue fatty acid composition. PLoS ONE. 2013;8:e74927. doi: 10.1371/journal.pone.0074927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hellmuth C, Lindsay KL, Uhl O, et al. Association of maternal prepregnancy BMI with metabolomic profile across gestation. Int J Obes (Lond). 2017;41:159‐169. [DOI] [PubMed] [Google Scholar]
- 24. Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park S, Jang A, Bouret SG. Maternal obesity‐induced endoplasmic reticulum stress causes metabolic alterations and abnormal hypothalamic development in the offspring. PLoS Biol 2020;18:e3000296. 10.1371/journal.pbio.3000296 [DOI] [PMC free article] [PubMed]
- 26. Gademan MGJ, Vermeulen M, Oostvogels AJJM, et al. Maternal prepregancy BMI and lipid profile during early pregnancy are independently associated with Offspring's body composition at age 5‐6 years: the ABCD study. PLoS ONE. 2014;9:e94594. doi: 10.1371/journal.pone.0094594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Page KA, Luo S, Wang X, et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care. 2019;42:1473‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas K, Beyer F, Lewe G, et al. Higher body mass index is linked to altered hypothalamic microstructure. Sci Rep. 2019;9:17373. doi:10.1038/s41598‐019‐53578‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spindler M, Özyurt J, Thiel CM. Automated diffusion‐based parcellation of the hypothalamus reveals subunit‐specific associations with obesity. Sci Rep. 2020;10:22238. doi:10.1038/s41598‐020‐79289‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beaulieu C. The basis of anisotropic water diffusion in the nervous system ‐ a technical review. NMR Biomed. 2002;15:435‐455. [DOI] [PubMed] [Google Scholar]
- 31. Rasmussen JM, Graham AM, Entringer S, et al. Maternal interleukin‐6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage. 2019;185:825‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cunningham SA, Kramer MR, Narayan KMV. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:403‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindsay KL, Hellmuth C, Uhl O, et al. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS ONE. 2015;10:e0145794. doi: 10.1371/journal.pone.0145794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hellmuth C, Weber M, Koletzko B, Peissner W. Nonesterified fatty acid determination for functional lipidomics: comprehensive ultrahigh performance liquid chromatography–tandem mass spectrometry quantitation, qualification, and parameter prediction. Anal Chem. 2012;84:1483‐1490. [DOI] [PubMed] [Google Scholar]
- 35. Zeleniuch‐Jacquotte A, Chajès V, Van Kappel AL, Riboli E, Toniolo P. Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr. 2000;54:367‐372. [DOI] [PubMed] [Google Scholar]
- 36. Bastiani M, Andersson JLR, Cordero‐Grande L, et al. Automated processing pipeline for neonatal diffusion MRI in the developing human connectome project. Neuroimage. 2018;185:750‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pauli WM, Nili AN, Tyszka JM. A high‐resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data. 2018;5:180063. doi: 10.1038/sdata.2018.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macleod M, Collings AM, Graf C, et al. The MDAR (Materials Design Analysis Reporting) framework for transparent reporting in the life sciences. Proc Natl Acad Sci USA. 2021;118:e2103238118. doi: 10.1073/pnas.2103238118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rasmussen JM, Kruggel F, Gilmore JH, et al. A novel maturation index based on neonatal diffusion tensor imaging reflects typical perinatal white matter development in humans. Int J Dev Neurosci. 2017;56:42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kennedy AP, Shea JL, Sun G. Comparison of the classification of obesity by BMI vs. dual‐energy X‐ray absorptiometry in the Newfoundland population. Obesity (Silver Spring). 2009;17:2094‐2099. [DOI] [PubMed] [Google Scholar]
- 41. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905‐2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239‐254. [DOI] [PubMed] [Google Scholar]
- 44. Rasmussen JM, Thompson PM, Entringer S, Buss C, Wadhwa PD. Fetal programming of human energy homeostasis brain networks: issues and considerations. Obes Rev. 2022;23:e13392. doi: 10.1111/obr.13392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Volkow ND, Gordon JA, Freund MP. The healthy brain and child development study‐shedding light on opioid exposure, COVID‐19, and health disparities. JAMA Psychiatry. 2021;78:471‐472. [DOI] [PubMed] [Google Scholar]
- 46. Gemmink A, Schrauwen P, Hesselink MKC. Exercising your fat (metabolism) into shape: a muscle‐centred view. Diabetologia. 2020;63:1453‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sorli C, Harashima S‐I, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251‐260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


