Abstract
Transcutaneous spinal stimulation (TSS) is a non-invasive neuromodulation technique that has been used to facilitate the performance of voluntary motor functions such as trunk control and self-assisted standing in individuals with spinal cord injury. Although it is hypothesized that TSS amplifies signals from supraspinal motor control networks, the effect of TSS on supraspinal activation patterns is presently unknown. The purpose of this study was to investigate TSS-induced activity in supraspinal sensorimotor regions during a lower-limb motor task. Functional magnetic resonance imaging (fMRI) was used to assess changes in neural activation patterns as eleven participants performed mimicked-standing movements in the scanner. Movements were performed without stimulation, as well as in the presence of (1) TSS, (2) stimulation applied to the back muscle, (3) paresthesia stimulation, and (4) neuromuscular electrical stimulation. TSS was associated with greater activation in subcortical and cortical sensorimotor regions involved in relay and processing of movement-related somatosensory information (e.g., thalamus, caudate, pallidum, putamen), as compared to the other stimulation paradigms. TSS also resulted in deactivation in both nucleus accumbens and posterior parietal cortex, suggesting a shift toward somatosensory feedback-based mechanisms and more reflexive motor control. Together, these findings demonstrate that spinal stimulation can alter the activity within supraspinal sensorimotor networks and promote the use of somatosensory feedback, thus providing a plausible neural mechanism for the stimulation-induced improvements of sensorimotor function observed in participants with neurological injuries and disorders.
Keywords: Neuromodulation, Neuroimaging, Spinal Cord, Standing, Sensorimotor network, Brain-Spinal Connectome
Introduction
In combination with physical rehabilitation, spinal stimulation can promote the recovery of voluntary motor functions such as trunk control, self-assisted standing, and stepping in individuals with spinal cord injury (SCI) (Angeli et al., 2018; Gill et al., 2018; Wagner et al., 2018). Both invasive epidural spinal stimulation (ESS) and non-invasive transcutaneous spinal stimulation (TSS) are effective neuromodulation techniques for enabling motor functions in SCI (Gerasimenko et al., 2015; Minassian et al., 2016; Taylor et al., 2021). Importantly, even a single session of ESS or TSS improves voluntary motor function in individuals with neurological injuries and disorders, such as SCI and multiple sclerosis (MS) (Grahn et al., 2017; Rath et al., 2018; Roberts et al., 2021; Sayenko et al., 2019).
Although the neural mechanisms underlying improvements in voluntary function in the presence of spinal stimulation are not well understood, activation of sensorimotor networks in the spinal cord is hypothesized to play a central role (Minassian and Hofstoetter, 2016; Taccola et al., 2018). Previous studies have suggested various underlying mechanisms of the interaction between supraspinal and spinal networks. Some have proposed that spinal stimulation may alter spinal network excitability in a way that facilitates the integration of descending commands from voluntary movement centers in the brain (Gill et al., 2018; Harkema et al., 2011). Others propose that spinal stimulation may facilitate the use of a movement control strategy wherein feedback-based corrections are implemented using primarily subcortical and spinal sensorimotor circuits (Edgerton et al., 2018; Gerasimenko et al., 2018; Gerasimenko et al., 2017). Finally, our recent findings suggest that the descending drive (residual in case of SCI) elevates spinal interneuronal network excitability, while ESS or TSS targets specific populations of inter- and/or motoneurons and serve to regain given functions (Calvert et al., 2021; Steele et al., 2021). However, the effect of TSS on the activity of supraspinal sensorimotor networks during voluntary movement remains unknown.
Previous neuroimaging and electrophysiological studies in humans and animals have revealed that many supraspinal regions are involved in the initiation and control of lower-limb movements (Christensen and Grey, 2013; Christensen et al., 2007; Ciccarelli et al., 2005; Drew and Marigold, 2015; Francis et al., 2009; Jahn et al., 2004; la Fougere et al., 2010; Takakusaki, 2017). Specifically, neuroimaging studies in humans have demonstrated that the performance of lower-limb motor tasks is associated with changes in activation of various regions in the brainstem, cerebellum, basal ganglia and sensorimotor cortices (Jahn et al., 2004; la Fougere et al., 2010; Sahyoun et al., 2004; Zwergal et al., 2012). These regional activities are hypothesized to represent a supraspinal motor control network that plays a critical role in balance, postural control, and locomotion (Jahn et al., 2008; Jahn et al., 2009). Although many of these regions have been repeatedly shown to be involved in lower-limb motor control across multiple studies, the patterns of activation can vary depending on the task requirements. For example, lower-limb motor tasks involving multisensory integration and movement corrections, such as voluntary plantarflexion or dorsiflexion with a visual feedback, have been associated with greater activation of cortical regions, such as the premotor, motor and posterior parietal cortex (PPC) (Christensen et al., 2007; Culham et al., 2006; Francis et al., 2009; Saradjian et al., 2019). In contrast, greater activation of subcortical sensorimotor regions, such as the basal ganglia and cerebellum, has been associated with feedback-based adjustments to limb perturbations, and the processing of somatosensory information for feedback-based motor control (Bonzano et al., 2013; Christensen and Grey, 2013; Ciccarelli et al., 2005; Francis et al., 2009; MacIntosh et al., 2004).
The purpose of the present study was to use functional magnetic resonance imaging (fMRI) to test whether TSS is associated with altered neural activation patterns in the supraspinal sensorimotor network during lower-limb motor tasks. To date, no other studies have examined the effect of spinal stimulation on supraspinal sensorimotor network activity during a voluntary movement task in humans. We compared changes in activation in supraspinal regions of interest (ROIs) during exposure to TSS and four other stimulation paradigms: No-stimulation, sham, paresthesia, and neuromuscular electrical stimulation, applied during bilateral isometric plantarflexion representing mimicked-standing. We hypothesized that TSS will be associated with altered activation in both cortical and subcortical regions involved in the relay and processing of movement-related sensory information.
Methods
Participants:
Thirteen neurologically-healthy participants were initially enrolled in the study. Two participants were excluded due to interference in the fMRI data caused by dental implants. Thus, data from eleven participants (four women; age: range= 28–40 years, mean (SD) = 28 (6.1) years; height mean (SD) = 171 (12) cm; weight mean (SD) = 73 (14) kg) were analyzed. Participants provided written informed consent before the experiment, and the Institutional Review Board at Houston Methodist Research Institute approved all procedures. The experiment took place over two sessions (see below for details) and lasted approximately five hours in total.
Stimulation paradigms:
In order to assess the effects of TSS-induced spinal interneuronal network activity on the supraspinal sensorimotor network, the experimental paradigms also included No-Stimulation (NO-STIM), sham (SHAM), paresthesia (PARA), and neuromuscular electrical (NMES) stimulation (Figure 1). The SHAM, PARA, and NMES paradigms were incorporated to control for more localized sensorimotor activity which may also occur during TSS, including somatosensory perception of the stimulation on the skin (SHAM), lower-limb paresthesia produced by TSS (PARA), and lower-limb muscle contraction (NMES) (Hofstoetter et al., 2020; Manson et al., 2020).
Figure 1.
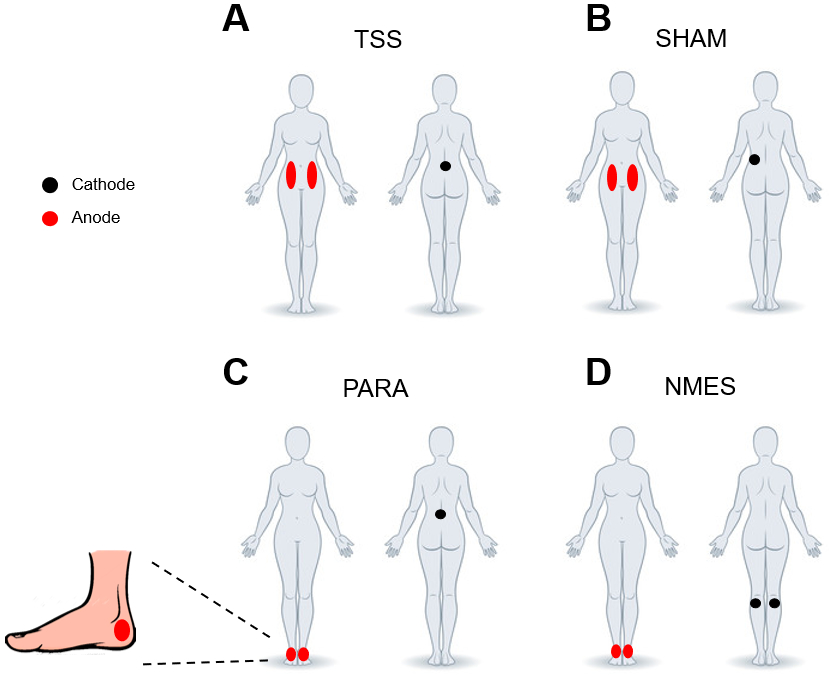
Electrode configurations for each stimulation paradigm: (A) transcutaneous spinal stimulation (TSS), (B) sham stimulation (SHAM), (C) paresthesia stimulation (PARA), (D) neuromuscular electrical stimulation (NMES). Black circles indicate the position of the cathode and red circles indicate the position of the anode electrodes.
TSS was administered using a round (5 cm diameter) conductive self-adhesive electrode (PALS; Abelard Manufacturing Co Ltd., USA) placed between the spinous processes of the L1 and L2 vertebrae as the cathode. Two oval (5×10 cm) self-adhesive electrodes (PALS; Axelgaard Manufacturing Co Ltd., USA) were placed symmetrically over the abdomen as the anodes. For the SHAM paradigm, stimulation was delivered through a cathode placed ~4–6 cm to the left of the TSS electrode. The placement of the anodes for the SHAM paradigm was the same as during TSS. The characteristics of the PARA and NMES paradigms, including the location, frequency, and pulse width, were empirically selected in pilot tests to match the participants’ sensation of tingling sensation and increased muscle tone in the lower limbs, occurring during TSS. For the PARA paradigm, the cathode position was the same as during TSS, while the anodes electrodes (5 cm diameter) were placed posterior to the medial malleolus on the right and left foot. For the NMES paradigm, the cathodes (5 cm in diameter) were placed between the lateral and medial heads of the gastrocnemius muscle just below the popliteal fossa. The anode electrodes were placed the same as in the PARA paradigm. For all stimulation paradigms, electrical stimulation was delivered using the DS8R biphasic constant current stimulator (Digitimer Ltd., UK). The duration of asymmetric (80%) biphasic square-wave pulses used for the TSS and SHAM paradigms was 500 μs, whereas the pulse duration used for the PARA and NMES stimulation paradigms was 200 μs.
MRI:
Images were acquired on a Siemens MAGNETOM Vida 3T scanner (Siemens Healthineers, Germany) with a 64-channel head coil (Nova Medical Inc., USA). Each run included a high-resolution structural T1-weighted scan (sagittal direction, 0.7 mm3 isotropic resolution) and five T2*-weighted functional scans (one scan for each stimulation paradigm) in an axial orientation (repetition time [TR] = 3000 ms, echo time [TE] = 30 ms, spatial resolution = 2.8 mm3 isotropic, flip angle [FA] = 90°).
Procedure:
Participants attended two experimental sessions (mean (SD) time between the two sessions = 7.2 (4.9) days):
1). Neurophysiological Assessment Session:
The goals were to: 1) Characterize participants’ muscle responses to TSS; 2) Determine the stimulation intensity for the TSS, PARA, and NMES paradigms; and 3) Practice the mimicked-standing task with and without stimulation.
Surface electromyograms (EMG) were recorded bilaterally from the following lower-limb muscles: Vastus lateralis (VL), medial hamstrings (MH), medial gastrocnemius (MG), tibialis anterior (TA) and soleus (SOL). Trigno Avanti wireless surface EMG electrodes (Delsys Inc., USA) were placed longitudinally over the muscle bellies. EMG data were amplified using a Trigno Avanti amplifier (gain: 909; bandwidth: 20–450 Hz) and recorded at a sampling frequency of 2,000 Hz using a PowerLab data acquisition system (ADInstruments, New Zealand). An MR-compatible apparatus Exolab (Antex Lab LLC, Russia) was used to support the hip, knee, and ankle joints at 155, 90, and 90 degrees, respectively, and to measure force output during isometric plantarflexion (Figure 2).
Figure 2.
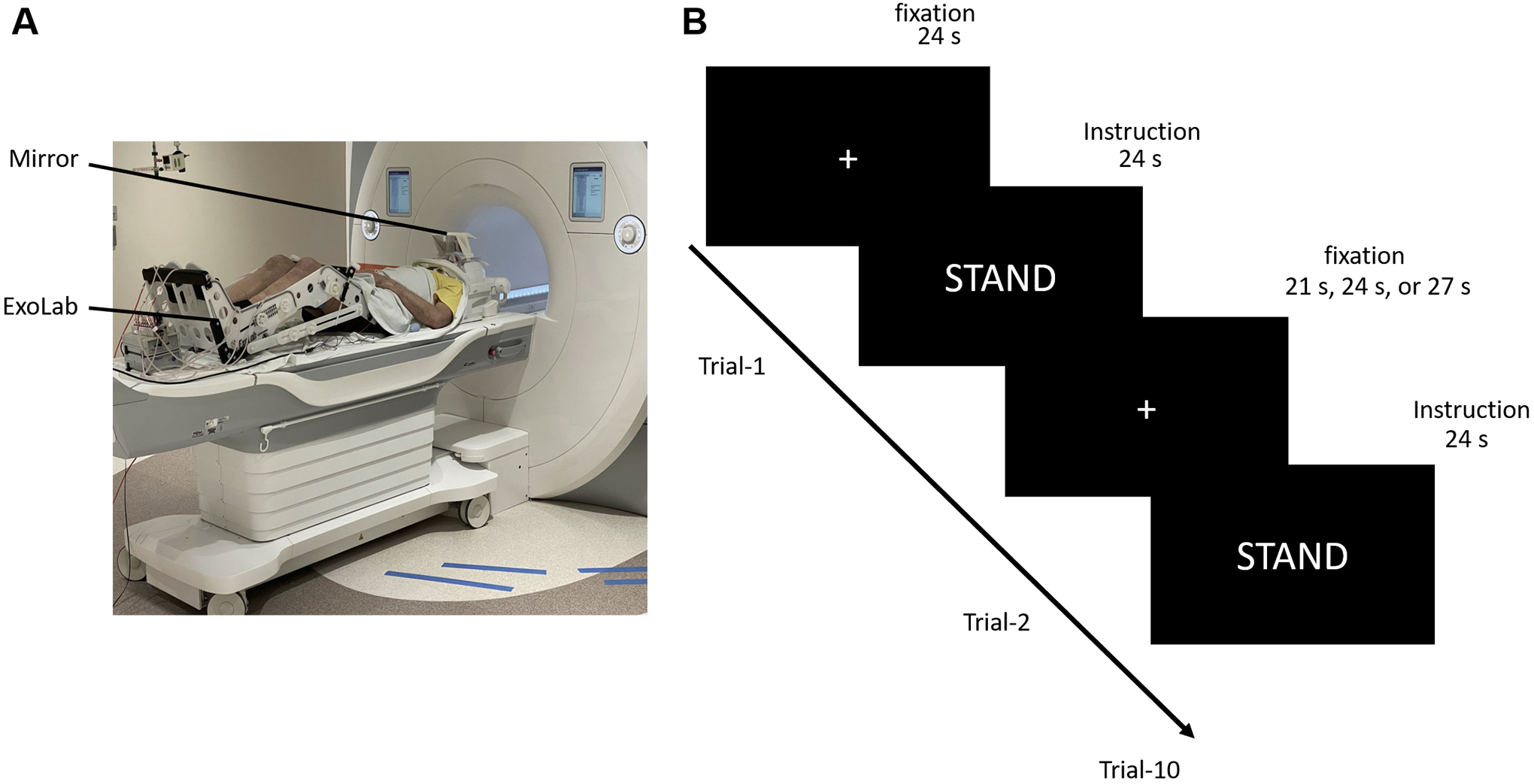
View of (A) experimental apparatus and (B) the paradigm used for the fMRI protocol. Participants were positioned on the scanner bed with both legs placed in the Exolab apparatus, and viewed an LCD monitor through a mirror attached to the head coil. Each fMRI scan was implemented in a block design. Specifically, participants started each scanning run by viewing a fixation cross for 24 s (“fixation, no-movement period”). After this, an on-screen instruction to “stand” was presented to the participant for 24 s (“movement block”). A fixation period then followed and was displayed for either 21, 24 or 27 s. There were 10 stimulation blocks in total.
The TSS cathode placement over the caudal portion of the lumbosacral enlargement was verified by inducing motor responses emerging first in the distal muscles (i.e., SOL) (Atkinson et al., 2020; Sayenko et al., 2015). Targeting specifically this region of the spinal cord was critical in our experiments as it is encompasses the interneuronal network and motor pools projecting to the lower limb muscles (Grahn et al., 2017; Sayenko et al., 2019). During this test, double-pulse TSS consisting of two 500 μs biphasic pulses with an inter-stimulus interval of 50 ms, was delivered every ~5 s. The attenuation of the amplitude of the second response in the pair due to post-activation depression confirmed that our responses were evoked by stimulation of afferent fibers within the dorsal roots entering the lumbar spinal cord (Andrews et al., 2015; Hofstoetter et al., 2019; Steele et al., 2021), and transsynaptic transmission of the stimuli onto the motor pools, as opposed to direct excitation of efferents. Stimulation intensity was incrementally increased from the SOL motor threshold (mean (SD) = 37 (15) mA) to the maximum tolerated intensity (mean (SD) = 135 (34.8) mA). Testing within the range of intensities was critical to gauge the relationship between the motor threshold and maximum responses induced by TSS and stimulation intensity used during fMRI.
After spinally evoked motor potentials were recorded, participants were instructed to contract their knee extensor and plantarflexor muscles to push against the foot plate of the Exolab, and maintain the force level as if they were standing in one position (i.e., mimicked-standing). Participants were instructed to produce the same level of force each time, which was approximately 10 to 15% of the maximum force (Masani et al., 2013; Vette et al., 2010; Winter et al., 1998), and monitored in real-time by the experimenter using Exolab apparatus. Importantly, participants were also instructed to keep their upper body (i.e., neck and torso) as still as possible when performing these movements, and their head movements were monitored by the experimenter. Participants practiced this task until they were able to achieve the desired contractions with no head motion.
The participants were then exposed to continuous TSS with the frequency 30 Hz, which is most commonly utilized during therapeutic interventions (Taylor et al., 2021). Stimulation intensity was gradually increased (~ 5 mA every 5–10 s) to the level which participants could tolerate without severe discomfort. Then, participants were exposed to the SHAM stimulation, the intensity of which was adjusted so that it matched the participant’s perception of TSS. After that, 60 Hz PARA or NMES were delivered at the intensity to match somatosensory sensation during TSS, i.e., increased muscle tone or a tingling sensation in the lower limbs, occurring during TSS (Hofstoetter et al., 2020; Manson et al., 2020). Participants were asked to verbally report when they felt: 1) the onset of stimulation; 2) tingling in their lower-limbs (for TSS and PARA); 3) contractions in their core or lower-limbs (for TSS and NMES), and 4) the stimulation became uncomfortable (i.e., they could not tolerate the stimulation for more than 30 s). After the stimulation intensity was determined for each paradigm, participants practiced the movement at least eight times in the presence of each stimulation paradigm.
2). Scanning Session:
The participant’s head was placed in the head coil and the lower-limbs were positioned in the Exolab apparatus (Figure 2A). Following a structural scan, participants completed five functional scans. Each functional scan was implemented in a block design and included 10 movement blocks with an on-screen “STAND” instruction for 24 s, as well as fixation (no-movement) periods of 21, 24 or 27 s between the blocks (Figure 2B). The first scan was always during the NO-STIM paradigm. The order of subsequent functional scans during TSS, SHAM, PARA, and NMES was randomized between participants. Stimulation was delivered to participants at the same time as the “STAND” instruction. Each scan consisted of 167 volumes and was approximately 9 min in length. After each functional scan, participants’ perceived comfort was assessed by a numerical rating scale (NRS), wherein a rating of 1 signified that they felt no discomfort during the scan, and a rating of 10 meant that they could not perform another scan in those same conditions (no participant reported a score above 8).
Data Analysis
Neurophysiological Assessment:
During double-pulse TSS, motor threshold for each participant was determined based on the first stimulation amplitude that caused motor activation which resulted in EMG amplitudes exceeding 20 μV in both left and right SOL muscles (Calvert et al., 2019). The motor threshold was used to normalize the stimulation intensity during 30 Hz TSS for comparison across participants.
Stimulation
Stimulation intensities used during functional scans for each paradigm were compared using a repeated-measures ANOVA. Post-hoc tests were performed using Bonferroni-corrected paired t-tests.
NRS scores
NRS scores for perceived comfort were analyzed using the four-stimulation paradigms (i.e., TSS, SHAM, PARA, NMES) repeated-measures Friedman’s ANOVA. Post-hoc tests were performed using paired Wilcoxon-signed rank tests with a Hochberg correction.
Force Output
Force Output during mimicked-standing was quantified using the left foot sensor on the Exolab apparatus, and analyzed using a five paradigms (i.e., NO-STIM, TSS, SHAM, PARA, NMES) repeated-measures ANOVA. Post-hoc tests were performed using Bonferroni-corrected paired t-tests.
FMRI Data
FMRI Data were processed using the Analysis of Functional Neuroimaging (AFNI) software package (http://afni.nimh.nih.gov/afni/; version AFNI_19.1.00 ‘Caligula’). A standard preprocessing pipeline was performed, including de-spiking, slice timing correction, motion correction (motion was censored at 0.3 mm), volume alignment (functional images were aligned to the minimum outlier), warping to standard space (the MNI152 template was used), co-registration and spatial smoothing using an 8mm full-width-at-half-maximum (FWHM) Gaussian kernel. Very little data were censored due to motion (0.004 % or Forty-three volumes across all participants were excluded due to motion artifacts). No participant had more than 1% of volumes excluded per run (mean number of TRs excluded = 0.78, range = 0–7) and there were no differences in motion between the utilized paradigms (i.e., NO-STIM, TSS, SHAM, PARA, NMES) as determined by the repeated-measures ANOVA, (F(4,12) = 1.59, p = .24).
After preprocessing, a generalized linear model was used to analyze the hemodynamic response during the mimicked-standing task in each stimulation paradigm. Contrasts between the NO-STIM paradigm and the four stimulation paradigms, as well as contrasts between TSS and the other three stimulation paradigms, were also modelled at the individual level. For group-level comparisons, ROI-analyses were performed to examine stimulation-induced altered activation in task-related regions during mimicked-standing. Several ROIs were identified based on previous studies examining lower-limb motor control in humans (see Supplementary Tables S1–S3 for citations) and indicated in Supplementary Figure S1. These studies also included work on lower-limb motor tasks, real and imagined postural control tasks, epidural spinal stimulation, and peripheral electrical stimulation that is similar to the NMES paradigm employed in the present study. In the brainstem and cerebellum, the task-related ROIs included the midbrain, as well as the cerebellar vermis (lobes 8, 9, 10), and the cerebellar hemispheres (4, 6, and 8). Subcortical sensorimotor ROIs included areas of the basal ganglia (e.g., caudate, nucleus accumbens, pallidum, putamen) and sensory processing and relay areas (e.g., insula, thalamus, amygdala). Cortical ROIs included sensorimotor processing regions such as the: Primary motor cortex (M1), PPC, premotor cortex, the supplementary motor area (SMA), dorsolateral prefrontal cortex, the inferior frontal gyrus (IFG), anterior cingulate cortex, primary somatosensory cortex (SI) and secondary somatosensory cortex (SII). The ROI coordinates obtained from previous studies were verified using the MNI152 atlas and functional neuroimaging meta-analyses generated by http://www.neurosynth.org.
Because of the large number of ROIs used in lower-limb motor control, a Bayesian multilevel modelling (BML) was used to assess changes in task-related brain activation patterns during mimicked-standing. Chen et al. (2019) demonstrated that ROI analyses using BML had significantly better detection sensitivity as compared to the conventional null-hypothesis significance testing. To summarize, traditional approaches to group-level ROI analyses use independent general linear models (GLM) to examine changes in levels of activation in each ROI. The GLM approach may provide an accurate fit and estimate of activity in each region, but the GLM is based on the assumption of independence, and thus corrections for multiple comparisons must be applied. Other research teams (Cox et al., 2017; Noble et al., 2020) have also noted that corrections applied to GLM derived activations unfairly penalize ROIs of smaller sizes, and thus do not provide the best estimate measure of group-level activations. In contrast, BML models regional activations using partial pooling (no assumption of complete independence) and adaptively regularizes activations using a Gaussian prior distribution. The activation within each ROI is pulled toward the center of the distribution resulting in better overall model fit (Chen et al., 2019). Estimates of activity using BML, therefore, have more predictive validity than the traditional GLM. Furthermore, because all regions are included in one model, BML controls for multiplicity more systematically than corrections used for GLM estimates. The use of an informative prior also allows for conservative estimates for confidence when performing multiple comparisons (Gelman et al., 2012; Han, 2020). In addition, the BML provides an easily interpretable measure of effect size for levels of activation in an ROI. Given the advantages of BML, and the biases found in conventional parametric approaches (Eklund et al., 2016), we employed BML as our main analysis to detect differences in task-related activations between stimulation paradigms. Changes in the percentage of blood oxygenation level-dependent (BOLD) signals that were positive and negative were interpreted as either activation or deactivation, respectively.
A sphere with a radius of 6 mm or 12 mm, depending on the region, was created for each ROI. Mean percent signal change from each ROI was obtained from individual-level contrast maps for comparisons of interest (i.e., NO-STIM vs. each stimulation paradigm, and TSS vs. each other paradigm). BML was used to identify regions showing evidence of differences in activation between stimulation paradigms. “Very strong evidence” was defined as contrast differences with the 97.5% confidence interval (greater than 97.5% for activations and less than 2.5% for deactivations); “Strong evidence” was defined as contrast differences within the 95% quantile range (greater than 95% and less than 5%); and “Moderate evidence” was defined as contrast differences in the 90% quantile range (greater than 90% and less than 10%). It should be noted that the results of the BML represent an update to the prior distribution based on the collected data. Thus, all results could be considered meaningful, and as each posterior distribution represents an update of the prior probability distribution, the thresholds defined above to delineate evidence levels are in some ways arbitrary.
Results
Stimulation Intensity:
There was a main effect of stimulation paradigm (F(3,30) = 25.4, p < 0.001). Post-hoc tests revealed that the stimulation intensity used during TSS (mean (SD) = 27.7 (6.8) mA) was higher than the intensities used during SHAM (mean (SD) = 23.2 (4.6) mA, t(10) = −3.19, p = .010), PARA (mean (SD) = 18.4 (3.6) mA, t(10) = −4.39, p = .001) and NMES (mean (SD) = 13.7 (4.2) mA, t(10) = −7.18, p < .0001). The intensity used during SHAM was also higher than the intensity used during both NMES (t(10) = −5.54, p =.0002), and PARA (t(10) = −2.66, p = .024). Finally, the intensity used during PARA was significantly higher than the intensity used during NMES (t(10) = −5.71, p =.0002).
TSS stimulation intensity during fMRI scans:
For TSS stimulation (30 Hz), participants tolerated a mean stimulation intensity of 53% of their motor threshold (range (SD) = 27–85 (16) %) detected using double-pulse TSS.
NRS Score:
The Friedman’s ANOVA revealed a significant main effect of stimulation paradigm (X2(3) = 29.4, p < .001). Participants felt significantly more discomfort when performing the mimicked-standing task with SHAM stimulation (mean (SD) = 4.1 (2.0)) as compared to TSS (mean (SD) = 2.7 (1.2), Z = 36, p = .024); PARA (mean (SD) = 1.2 (1.1); Z = 0, p = .018), or NMES (mean (SD) = 1.2 (0.8), Z = 0, p = .018). Also, participants perceived TSS as more uncomfortable than both PARA (Z = 0, p = .021) and NMES (Z = 0, p = 0.020).
Force Output:
There was no significant effect of stimulation paradigm on force output during mimicked-standing (F(4,40) = 2.234, p = 0.08), suggesting that participants did not modify their mimicked-standing force throughout the entire experimental protocol (Supplementary Table S4).
FMRI Data:
Below, we summarize activation results in the brainstem, cerebellar, subcortical, and cortical ROIs, and report two types of comparisons within each section: 1) Change in activation during the NO-STIM paradigm (relative to the no-movement period). This analysis was performed to relate the change in activation in our mimicked-standing paradigm to those of previous fMRI studies that employed lower-limb movements; 2) Change in activation during TSS and other (NO-STIM, SHAM, PARA, NMES) paradigms, to address the main hypothesis. Complete statistical outputs for the BML are outlined in Supplementary Tables S5–S9.
Brainstem and Cerebellum
Mimicked-Standing with NO-STIM:
Our analyses yielded strong evidence for deactivation in the midbrain ROI and moderate evidence for increased activation in the cerebellar vermis (8) (Supplementary Table S5).
Mimicked-Standing with TSS
Mimicked-Standing with TSS was associated with relatively higher levels of activation in the midbrain as compared to the other paradigms (Figure 3, Supplementary Table S6): There was strong evidence for greater activation during TSS as compared to the SHAM paradigm, and moderate evidence for greater activation as compared to the NO-STIM paradigm. In the cerebellar vermis (9), moderate evidence for increased activation was found in response to TSS as compared to the SHAM paradigm. In the left cerebellar hemisphere 8, we found moderate evidence for increased activation in response to TSS as compared to the NMES and PARA paradigms. In the right hemisphere 8, the analysis revealed moderate evidence for increased activation during TSS as compared to the NMES paradigm.
Figure 3.
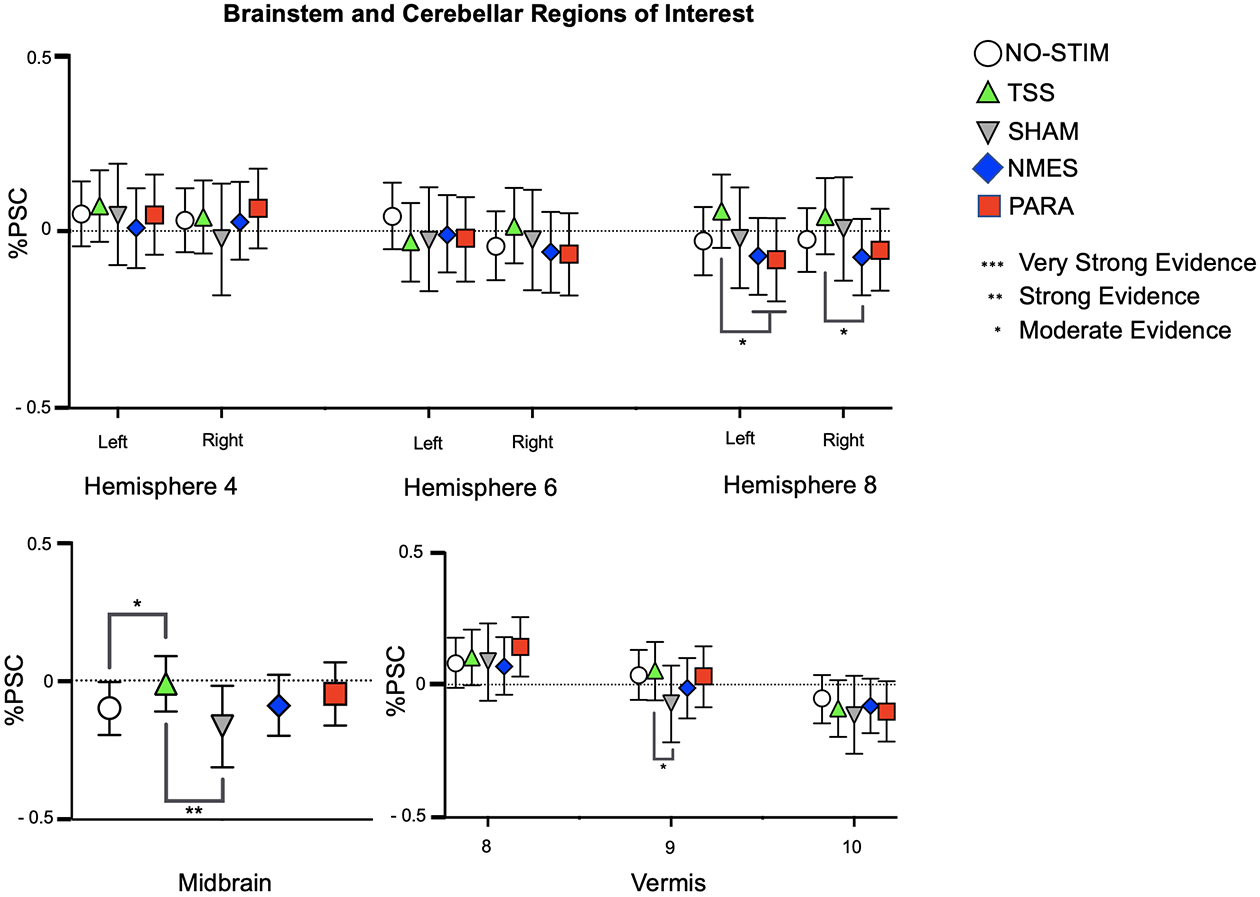
Patterns of activation and deactivation in ROIs located in the cerebellum and brainstem. BOLD signal changes are plotted as mean percent signal change (%PSC) from baseline for each condition. The strength of differences outputted by the BML for the comparisons between NO-STIM and TSS are indicated by the asterisks above the lines, whereas the asterisks below the lines indicate the strength of the differences outputted for comparisons between TSS and the NMES, PARA, and SHAM. Error bars indicate 95% confidence interval.
Subcortical Regions
Mimicked-Standing with NO-STIM:
Our analyses of the NO-STIM paradigm yielded very strong evidence for deactivation in the amygdala ROI (Supplementary Table S7).
Mimicked-Standing with TSS:
Overall, our analyses revealed that exposure to TSS during mimicked-standing was associated with increased activation in subcortical sensorimotor ROIs in the basal ganglia (e.g., pallidum and putamen) and the thalamus (Figure 4, Supplementary Table S8). In the left caudate nucleus, there was very strong evidence for increased activation during TSS as compared to the NMES, PARA, and SHAM paradigms. In the right caudate, there was strong evidence for increased activation during TSS as compared to NMES. There was also very strong evidence in both the right and left pallidum for increased activation when participants were exposed to TSS versus SHAM, NMES and PARA paradigms. Furthermore, the analyses revealed strong evidence for increased activation during TSS in comparison to the NO-STIM paradigm. This result is similar to what was found in the left and right putamen, as there was very strong evidence for increased activation during TSS as compared to all other paradigms.
Figure 4:
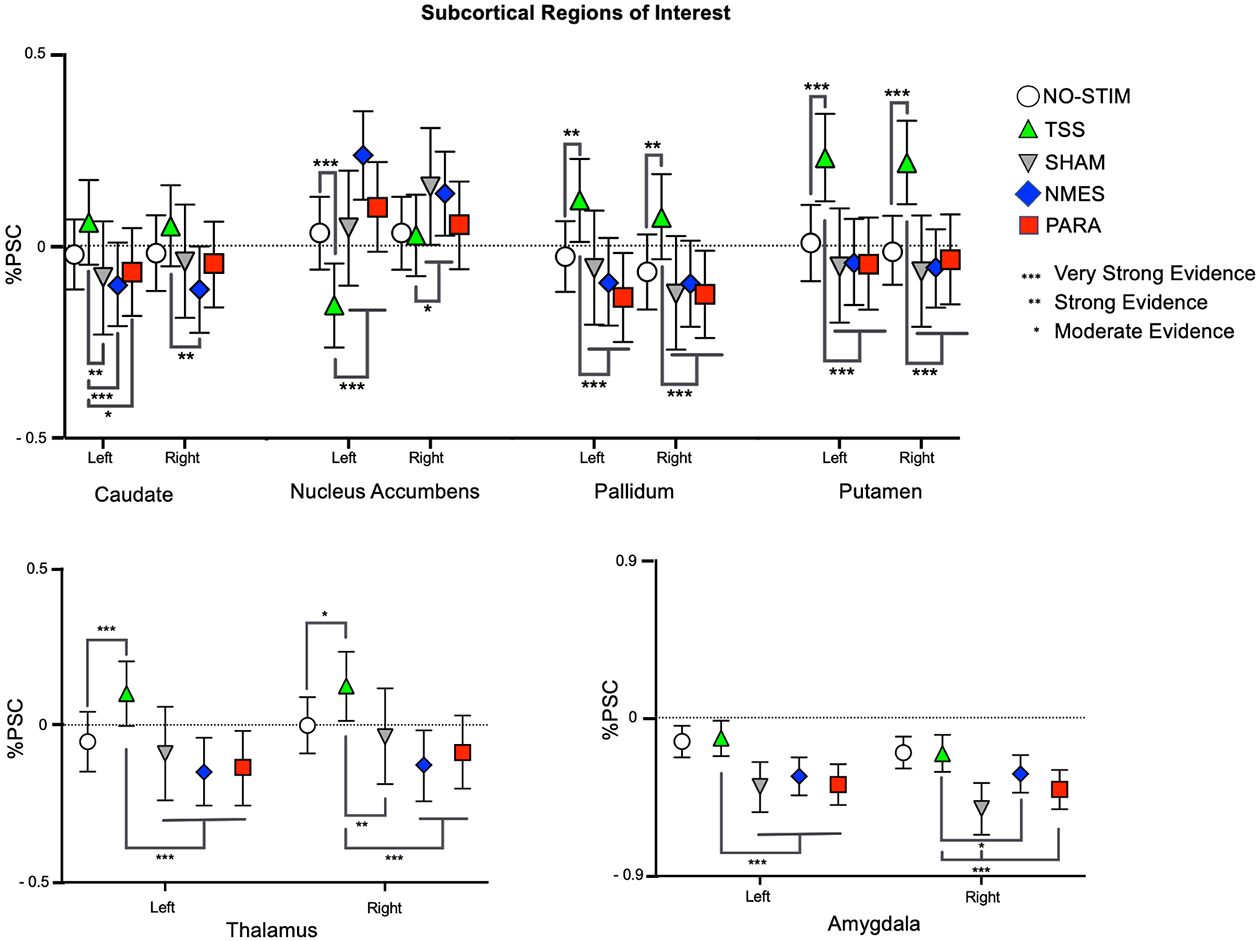
Patterns of activation and deactivation in subcortical ROIs. BOLD signal changes are plotted as mean percent signal change (%PSC) from baseline for each condition. The strength of differences outputted by the BML for the comparisons between NO-STIM and TSS are indicated by the asterixis above the lines, whereas the asterixis below the lines indicate the strength of the differences between TSS and the NMES, PARA, and SHAM. Error bars indicate 95% confidence interval.
In contrast to the aforesaid regions, TSS during mimicked-standing was associated with a greater deactivation in the nucleus accumbens ROI (another basal ganglia region). Overall, there was moderate to very strong evidence for deactivation when participants were exposed to TSS versus all other paradigms. In the left nucleus accumbens, there was very strong evidence for deactivation in response to TSS compared to all other paradigms. In the right nucleus accumbens, there was moderate evidence for deactivation in response to TSS compared to SHAM and NMES.
In the thalamus ROI, analyses yielded moderate to very strong evidence for increased activation in response to TSS, compared to all other paradigms. In the left thalamus, there was very strong evidence for increased activation in response to TSS compared to all other paradigms. In the right thalamus ROI, analyses revealed very strong evidence for increased activation in response to TSS as compared to the NMES and PARA, strong evidence for an increase compared to the SHAM, and moderate evidence for increased activation in comparison to the NO-STIM paradigm.
Lastly, analyses yielded moderate to very strong evidence for increased activation in the amygdala in response to TSS compared to the other stimulation paradigms, but comparable levels of activation to the NO-STIM paradigm. In the left amygdala, there was very strong evidence for increased activation in response to TSS as compared to the SHAM, NMES, and PARA. In the right amygdala, there was very strong evidence for more activation in response to TSS as compared to both the SHAM and PARA and moderate evidence for increased activation in comparison to the NMES. In both the right and left amygdala, there were comparable levels of activation between TSS and the NO-STIM paradigms.
Cortical Regions
Mimicked-Standing with NO-STIM:
As expected, mimicked-standing with NO-STIM activated numerous cortical regions associated with voluntary motor control. There was very strong evidence for increased bilateral activation in both the primary motor cortex, supplementary motor area, and the PPC. There was also very strong evidence for increased activation in the left SI, and moderate evidence for increased activation in both the right SI and the left SII. In contrast to the aforesaid regions, the analyses also yielded moderate evidence that mimicked-standing was associated with deactivation of the right parahippocampal ROI (Supplementary Table S9).
Mimicked-Standing with TSS:
The analyses of the cortical ROIs yielded evidence for increased activation in sensorimotor cortical regions and deactivation in the PPC during TSS, whereas all other stimulation paradigms and the NO-STIM condition resulted in increased activation in the PPC. In both the primary motor and premotor cortices there were comparable levels of activation between TSS and all other paradigms (Figure 5, Supplementary Table S10).
Figure 5:
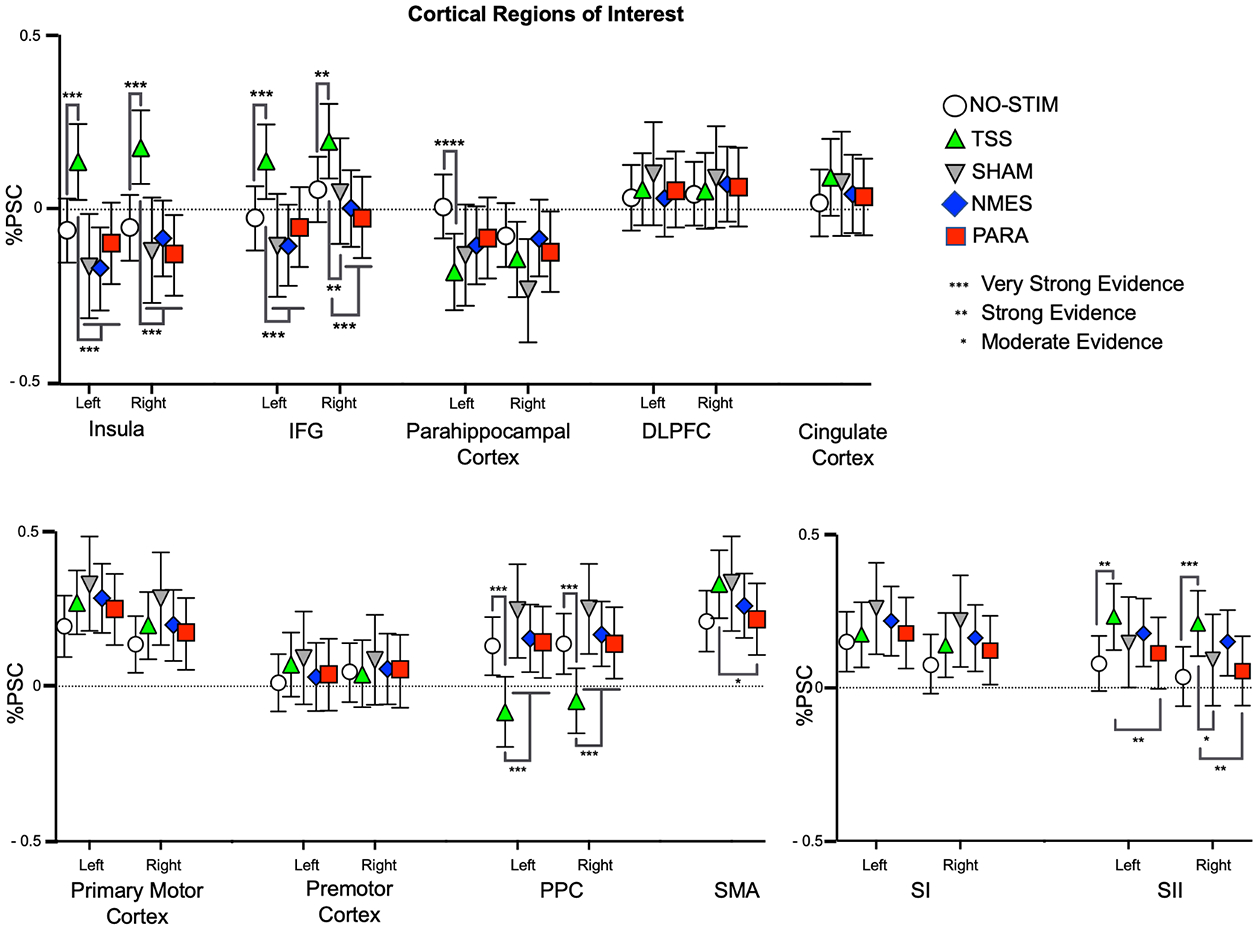
Patterns of activation and deactivation in cortical ROIs. BOLD signal changes are plotted as mean percent signal change (%PSC) from baseline for each condition. The strength of differences outputted by the BML for the comparisons between NO-STIM and TSS are indicated by the asterixis above the lines, whereas the asterixis below the lines indicate the strength of the differences between TSS and the NMES, PARA, and SHAM. Error bars indicate 95% confidence interval.
In the left SII ROI, the analyses revealed strong evidence for increased activation during TSS as compared to the NO-STIM and PARA. For the right SII, the analyses revealed very strong evidence for increased activation in response to TSS as compared to the NO-STIM paradigm, as well as moderate and strong evidence as compared to the SHAM and PARA, respectively. In the insula ROI, there was very strong evidence for increased activation in both the left and right insula during TSS as compared to all other stimulation paradigms. In contrast, in the left parahippocampal gyrus ROI, there was also very strong evidence for a deactivation during TSS as compared to NO-STIM.
Finally, the analyses revealed increased activation in the IFG during mimicked-standing in the presence of TSS compared to the other paradigms. For the left IFG ROI, there was very strong evidence of increased activation in response to TSS relative to the NO-STIM, SHAM, PARA, and NMES. For the right IFG ROI, there was very strong evidence for increased activation during TSS as compared to the NMES and PARA, and strong evidence for increased activation during TSS as compared to both the NO-STIM and SHAM paradigms.
Discussion
The purpose of this study was to explore whether and how TSS alters activity in supraspinal sensorimotor networks during mimicked-standing. In agreement with our hypotheses, we found that TSS increased activation in both subcortical sensorimotor ROIs (e.g., the pallidum, putamen, and thalamus) and cortical sensory processing ROIs (e.g., insula, SI, and SII) when compared to the other stimulation paradigms. We have also demonstrated that mimicked-standing in the presence of TSS resulted in a deactivation in the nucleus accumbens and the PPC. Overall, our results provide evidence that TSS facilitates the use of a somatosensory feedback-based movement control network during lower-limb motor tasks. Here, we discuss how the task-related activation patterns seen in the NO-STIM condition correspond to findings in the literature, and then contextualize how the changes in activation during TSS indicate a shift toward somatosensory feedback-based sensorimotor control processes.
Mimicked-standing results in activation in brainstem, cerebellar, and cortical sensorimotor ROIs
The results of the present study suggest that mimicked-standing engaged cerebellar and cortical regions associated with the performance of lower-limb motor tasks. Specifically, we found that mimicked-standing was associated with increased activation in cerebellar vermis. It is consistent with the previous findings that the cerebellum relays joint position information during lower-limb movement control (de Almeida et al., 2015; Jahn et al., 2004; Nakata et al., 2019; Trinastic et al., 2010; Wei et al., 2020). Importantly, similar to previous fMRI studies that employed lower-limb movements (Ciccarelli et al., 2005; Drew and Marigold, 2015; la Fougere et al., 2010; Nakata et al., 2019; Volz et al., 2015), we found that mimicked-standing was associated with increased activation in several cortical and subcortical regions associated with voluntary motor control (e.g., primary motor cortex, PPC, SI, and SII). Also, similar to previous studies examining both real and imagined postural tasks (Jahn et al., 2004; la Fougere et al., 2010; Zwergal et al., 2012), we observed increased activation in the SMA.
In contrast to other studies with real and imagined posture- and locomotor-like tasks (Jahn et al., 2008; Jahn et al., 2004; Trinastic et al., 2010), we found deactivation in the amygdala, midbrain, and parahippocampal cortex. In addition, we did not find evidence for increased activation in subcortical sensorimotor regions (Ciccarelli et al., 2005; Goble et al., 2011; Goble et al., 2012). These inconsistences between our and previous experiments are most likely attributable to differences in the motor task requirements. Specifically, the supine position of the participants during our experiments eliminated the need for active maintenance of posture and balance, and thus the deactivation in midbrain and the absence of activation of subcortical sensorimotor regions may reflect a diversion of resources based on the task at hand. Similarly, Jahn et al. (2009) suggested that the deactivation of the parahippocampal regions could be indicative of a shift in resources from visuomotor networks during postural control to networks that use primarily non-visual sources. Taken together, the activation patterns in supraspinal sensorimotor control networks during our mimicked-standing task closely resemble those from previous experiments using voluntary lower limb movements.
Exposure to TSS during mimicked-standing can facilitate feedback-based movement control
In the present study, exposure to TSS during mimicked-standing altered activation in several subcortical and cortical ROIs (see Figure 6 for a visualization and Table 1 for a summary of changes). Importantly, when compared to the other stimulation paradigms, it appears that TSS facilitates activation in ROIs associated with the relay of movement-related sensory information (e.g., midbrain, thalamus, pallidum, putamen, and SII). These findings are in agreement with previous fMRI experiments that have found that activation in subcortical regions (e.g., thalamus and pallidum) was associated with the transmission and utilization of movement-related somatosensory information from the lower-limbs (Ciccarelli et al., 2005; Goble et al., 2011; Goble et al., 2012). Specifically, Goble et al. (2011) found that increased activation of the thalamus, pallidum and putamen was positively correlated with improvements in postural control during standing with eyes closed in older adults. The authors concluded that increases in activation in subcortical regions was linked to the effective transmission of somatosensory information for movement control.
Figure 6:
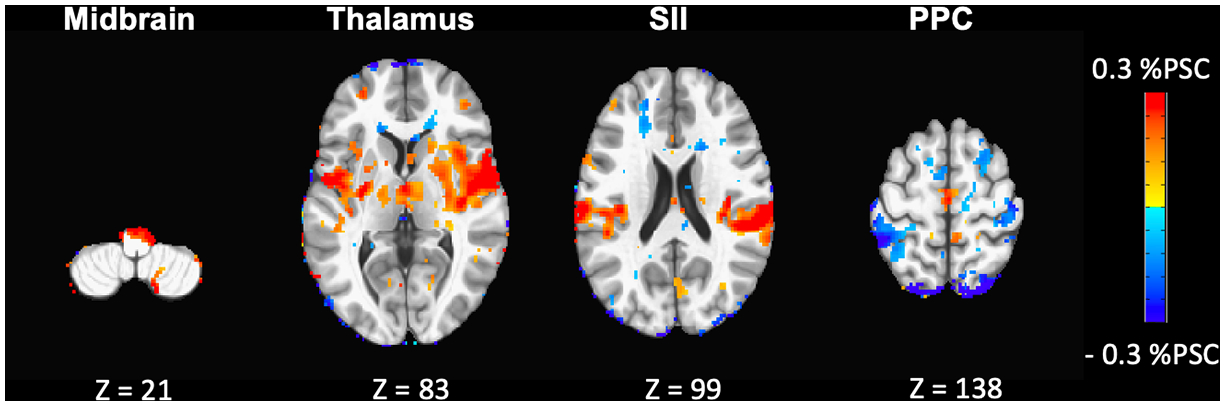
Visual summary of changes in supraspinal activation during exposure to TSS. Axial contrast maps outputted from a mixed-effect model analysis (using 3dMEMA AFNI program; p<0.05, uncorrected) depict the differences in supraspinal activation between the TSS and NO-STIM paradigms (slice numbers are displayed below each image).
Table 1:
Summary of alterations in cortical and subcortical activation patterns during exposure to TSS. TSS was associated with greater activation in subcortical and cortical sensorimotor regions involved in relay and processing of movement-related somatosensory information. TSS also resulted in deactivation in both nucleus accumbens and posterior parietal cortex (PPC), suggesting a shift toward somatosensory feedback-based mechanisms and more reflexive motor control.
| ROI Activations | ROI Deactivations |
|---|---|
| Midbrain | Nucleus Accumbens |
| Pallidum | PPC |
| Putamen | |
| Thalamus | |
| SII | |
| Insula | |
| IFG |
The bilateral increase in activation in IFG and SII ROIs further supports the idea that TSS may facilitate cortical somatosensory information processing during lower-limb motor tasks. Previous studies have associated activation in the IFG and SII with better performance on tactile detection tasks (Hagen et al., 2002; Spitzer et al., 2014), perception of body position (Ticini et al., 2009), and lower-limb motor control (Christensen and Grey, 2013; Joa et al., 2012). Importantly, other studies on NMES have associated increased activation of SII with movement-related proprioceptive processing (Qiu et al., 2015; Wegrzyk et al., 2017). In the present study, there were similar increases in activation of SII during the movement in both the TSS and NMES paradigms. In addition, activation of the insula has been consistently associated with the processing of sensory information related to body position during motor tasks (Christensen et al., 2007; Gentile et al., 2011; Karnath and Baier, 2010).Thus, it is possible that TSS during motor performance facilitates the processing of ascending somatosensory information from the lower-limbs.
The idea that TSS-induced spinal network activation may facilitate sensory information processing has received both experimental and theoretical support (Gerasimenko et al., 2018; Gerasimenko et al., 2017; Illis et al., 1980; Moreno-López and Hollis, 2021; Roberts et al., 2021; Taccola et al., 2018). In our recent study, participants with multiple sclerosis (MS) had significant improvements in their balance during standing with eyes closed (thus relying on primarily proprioception) in the presence of sub-threshold TSS as compared to the no-stimulation condition (Roberts et al., 2021). These observations and the findings from the current study provide support that exposure to TSS during voluntary action may shift movement control toward somatosensory feedback-based processes.
On the other hand, these findings seem to contrast with a recent computational and clinical study by Formento et al. (2018) that proposed that ESS in humans with SCI may result in occurrence of “antidromic collisions” due to bi-directional depolarization of proprioceptive axons, thus hindering functional signal integration for neuroaugmentation. Specifically, the authors reported that cancellation of proprioceptive information during ESS applied at 1.5× motor threshold, alters the conscious perception of joint position and movement velocity during passive knee motions (Formento et al., 2018). The discrepancy between these and our data can be explained by several differences in their study and our own. First, it is possible that submotor threshold intensity in our experiments did not interfere with the ascending proprioceptive input. This possibility is also supported by the above observations on the effects of submotor threshold TSS in individuals with MS (Roberts et al., 2021). Second, there was also a significant difference in the motor tasks employed in the experiments of Formento et al. (2018), with the requirement of conscious perception of the passive movement of the knee joint, vs. the trained and stereotyped isometric motions in our experiments. In addition, ascending transmission of proprioceptive inputs can differ between individuals with SCI and neurologically intact participants. Finally, it is entirely possible that the synergistic integration between spinal and supraspinal somatosensory network depends on more complex interactions than proprioceptive ascending pathways alone.
Exposure to TSS during mimicked-standing can facilitate shift toward more reflexive control networks
Our results demonstrated that TSS caused deactivation in areas associated with visuomotor control (e.g., nucleus accumbens and PPC), suggesting that exposure to TSS can promote feedback-based mechanisms to engage in more reflexive motor control. The strongest evidence for a shift toward more reflexive control networks is the deactivation in the PPC when participants performed mimicked-standing in the presence of TSS. This deactivation was specific only to the PPC, whereas in other regions associated with the initiation and relay of motor commands (e.g., primary motor cortex), activation occurred. Previous studies on human sensorimotor control have highlighted the role of the PPC in multisensory integration (e.g., visual, auditory, vestibular) during movement planning and control (Blangero et al., 2005; Buneo and Andersen, 2006; Creem-Regehr, 2009; Culham et al., 2006; Hinton et al., 2019). In contrast, deactivation/disruptions of the PPC is suggested to be indicative of a shift toward sensorimotor control mechanisms that rely primarily on somatosensory (proprioceptive) feedback (Christensen et al., 2007; Cooke et al., 2014; Sahyoun et al., 2004).
Thus, the deactivation of PPC in combination with increased activation in the thalamus and SII further indicates that TSS may promote the use of somatosensory feedback for lower-limb motor control. Taken together with previous works (Gerasimenko et al., 2016; Gerasimenko et al., 2018; Gerasimenko et al., 2017), we conclude that TSS-induced activation of spinal sensorimotor networks decreased the demand of resources attributed to anticipatory conscious movement planning processes, and increased contribution of reflexive, automatic control networks.
No change in amygdala activation during TSS indicates differences in perception between TSS and alternative stimulation paradigms
In both the right and left amygdala, we found moderate to very strong evidence for greater deactivation during SHAM, NMES and PARA, as compared to TSS. In fact, there were comparable levels of activation between TSS and NO-STIM paradigms. Previous fMRI studies have found that amygdala deactivation usually occurs after repeated exposure to noxious, surprising, or emotionally disturbing stimuli (Deogaonkar et al., 2016; Simons et al., 2014; Yin et al., 2018). Although the perception of stimulation intensity evoked by TSS and the other stimulation paradigms was similar, TSS resulted in less discomfort. This is not surprising, given that unpleasant sensations associated with electrical stimulation can be due to other factors besides the activation of skin nociceptors, and include muscle contractions (Manson et al., 2020). Given that the stimulating TSS electrode was located on the midline of the spine, less intense muscle contractions were involved than, for instance, during SHAM (located above the paraspinal muscles), and thus the perception of TSS was less uncomfortable. In addition, it is plausible that TSS uniquely alters sensory processing relative to alternative stimulation paradigms, resulting in differences in sensory perception.
Limitations
Although the results of the present study provide initial evidence that exposure to TSS can engage supraspinal sensorimotor networks, further work using larger (as well as clinical) populations is needed to extend our results and conclusions about the mechanisms underlying functional improvements during exposure to TSS to clinical populations. The stimulation intensity used during 30 Hz TSS was below the motor threshold intensity due to discomfort experienced by neurologically intact participants. Consequently, stimulation intensities during the alternative paradigms were optimized for participant sensation rather than to elicit motor activation. The effects of stimulation intensities closer to or above motor threshold (i.e., those used during therapeutic interventions) can have different effects on supraspinal sensorimotor network.
Conclusion
In the present study, we found that exposure to TSS during a lower-limb motor task increased activation of subcortical sensorimotor processing regions in the basal ganglia, and of cortical regions associated with movement-related sensory information processing. Furthermore, TSS led to deactivation in subcortical and cortical regions associated with voluntary action planning and control. These novel findings demonstrate that during voluntary movement attempts, spinal stimulation can facilitate the engagement of cortical and subcortical sensorimotor networks associated with the use of somatosensory feedback, thus providing a plausible neural mechanism for the stimulation-induced improvements of sensorimotor function observed in participants with neurological injuries and disorders.
Supplementary Material
Supplementary Figure S1: Spherical ROIs mapped onto an MNI52 template.
Acknowledgements
Sources of funding for the work reported here include Mission Connect Grant #019-102 - Jerry Johnston Andrew Award, the National Institutes of Health grant 1 R01 NS119587-01A1, Craig H. Neilsen Research Grant (733278), and Wings for Life Foundation (227). In addition, this work was in part supported by philanthropic funding from Paula and Rusty Walter and the Walter Oil & Gas Corporation. The funders were not involved in the design of the study, the collection, analysis, and interpretation of the experimental data, the writing of this article, or the decision to submit this article for publication. The authors gratefully acknowledge Vi Phan and Lien Phan for their assistance with the neuroimaging data collection. The authors would also like to acknowledge the reviewers of this paper for their thoughtful suggestions that significantly improved this report.
References
- Andrews JC, Stein RB, Roy FD, 2015. Post-activation depression in the human soleus muscle using peripheral nerve and transcutaneous spinal stimulation. Neurosci Lett 589, 144–149. [DOI] [PubMed] [Google Scholar]
- Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, Ferreira CK, Harkema SJ, 2018. Recovery of over-ground walking after chronic motor complete spinal cord injury. New England Journal of Medicine 379, 1244–1250. [DOI] [PubMed] [Google Scholar]
- Atkinson DA, Sayenko DG, D’Amico JM, Mink A, Lorenz DJ, Gerasimenko YP, Harkema S, 2020. Interlimb conditioning of lumbosacral spinally evoked motor responses after spinal cord injury. Clin Neurophysiol 131, 1519–1532. [DOI] [PubMed] [Google Scholar]
- Blangero A, Rossetti Y, Honoré J, Pisella L, 2005. Influence of gaze direction on pointing to unseen proprioceptive targets. Advances in Cognitive Psychology 1, 9. [Google Scholar]
- Bonzano L, Tacchino A, Saitta L, Roccatagliata L, Avanzino L, Mancardi GL, Bove M, 2013. Basal ganglia are active during motor performance recovery after a demanding motor task. Neuroimage 65, 257–266. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA, 2006. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44, 2594–2606. [DOI] [PubMed] [Google Scholar]
- Calvert JS, Gill ML, Linde MB, Veith DD, Thoreson AR, Lopez C, Lee KH, Gerasimenko YP, Edgerton VR, Lavrov IA, Zhao KD, Grahn PJ, Sayenko DG, 2021. Voluntary Modulation of Evoked Responses Generated by Epidural and Transcutaneous Spinal Stimulation in Humans with Spinal Cord Injury. Journal of Clinical Medicine 10, 4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JS, Manson GA, Grahn PJ, Sayenko DG, 2019. Preferential activation of spinal sensorimotor networks via lateralized transcutaneous spinal stimulation in neurologically intact humans. J Neurophysiol 122, 2111–2118. [DOI] [PubMed] [Google Scholar]
- Chen G, Xiao Y, Taylor P, Rajendra J, Riggins T, Geng F, Cox R, 2019. Handling Multiplicity in Neuroimaging Through Bayesian Lenses with Multilevel Modeling. Neuroinformatics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MS, Grey MJ, 2013. Modulation of proprioceptive feedback during functional electrical stimulation: an fMRI study. European Journal of Neuroscience 37, 1766–1778. [DOI] [PubMed] [Google Scholar]
- Christensen MS, Lundbye-Jensen J, Petersen N, Geertsen SS, Paulson OB, Nielsen JB, 2007. Watching your foot move—An fMRI study of visuomotor interactions during foot movement. Cerebral Cortex 17, 1906–1917. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Toosy A, Marsden J, Wheeler-Kingshott C, Sahyoun C, Matthews P, Miller D, Thompson A, 2005. Identifying brain regions for integrative sensorimotor processing with ankle movements. Experimental brain research 166, 31–42. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Goldring AB, Baldwin MK, Recanzone GH, Chen A, Pan T, Simon SI, Krubitzer L, 2014. Reversible deactivation of higher-order posterior parietal areas. I. Alterations of receptive field characteristics in early stages of neocortical processing. J Neurophysiol 112, 2529–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI clustering in AFNI: false-positive rates redux. Brain connectivity 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creem-Regehr SH, 2009. Sensory-motor and cognitive functions of the human posterior parietal cortex involved in manual actions. Neurobiology of learning and memory 91, 166–171. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavina-Pratesi C, Singhal A, 2006. The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia 44, 2668–2684. [DOI] [PubMed] [Google Scholar]
- de Almeida PMD, Vieira A.I.C.M.d.F., Canário NIS, Castelo-Branco M, de Castro Caldas AL, 2015. Brain activity during lower-limb movement with manual facilitation: an fMRI study. Neurology research international 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deogaonkar M, Sharma M, Oluigbo C, Nielson DM, Yang X, Vera-Portocarrero L, Molnar GF, Abduljalil A, Sederberg PB, Knopp M, Rezai AR, 2016. Spinal Cord Stimulation (SCS) and Functional Magnetic Resonance Imaging (fMRI): Modulation of Cortical Connectivity With Therapeutic SCS. Neuromodulation 19, 142–153. [DOI] [PubMed] [Google Scholar]
- Drew T, Marigold DS, 2015. Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol 33, 25–33. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Gerasimenko Y, Gad P, Sayenko D, 2018. Basic Concepts Underlying Activity-Dependent Mechanisms in the Rehabilitation of Sensory-Motor Function After Spinal Cord Injury. In: Kirshblum S, Lin VW (Eds.), Spinal Cord Medicine. Springer Publishing Company, New York, pp. 890–896. [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formento E, Minassian K, Wagner F, Mignardot JB, Le Goff-Mignardot CG, Rowald A, Bloch J, Micera S, Capogrosso M, Courtine G, 2018. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci 21, 1728–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S, Lin X, Aboushoushah S, White TP, Phillips M, Bowtell R, Constantinescu CS, 2009. fMRI analysis of active, passive and electrically stimulated ankle dorsiflexion. Neuroimage 44, 469–479. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J, Yajima M, 2012. Why we (usually) don’t have to worry about multiple comparisons. Journal of research on educational effectiveness 5, 189–211. [Google Scholar]
- Gentile G, Petkova VI, Ehrsson HH, 2011. Integration of visual and tactile signals from the hand in the human brain: an FMRI study. Journal of neurophysiology 105, 910–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Gad P, Sayenko D, McKinney Z, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Shigueva T, Tomilovskaya E, Kozlovskaya I, Edgerton VR, 2016. Integration of sensory, spinal, and volitional descending inputs in regulation of human locomotion. J Neurophysiol 116, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Reggie Edgerton V, 2015. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med 58, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Sayenko D, Gad P, Kozesnik J, Moshonkina T, Grishin A, Pukhov A, Moiseev S, Gorodnichev R, Selionov V, Kozlovskaya I, Edgerton VR, 2018. Electrical Spinal Stimulation, and Imagining of Lower Limb Movements to Modulate Brain-Spinal Connectomes That Control Locomotor-Like Behavior. Front Physiol 9, 1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Sayenko D, Gad P, Liu CT, Tillakaratne NJK, Roy RR, Kozlovskaya I, Edgerton VR, 2017. Feed-Forwardness of Spinal Networks in Posture and Locomotion. Neuroscientist 23, 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, Beck LA, Sayenko DG, Van Straaten MG, Drubach DI, Veith DD, Thoreson AR, Lopez C, Gerasimenko YP, Edgerton VR, Lee KH, Zhao KD, 2018. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 24, 1677–1682. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Doumas M, Wenderoth N, Swinnen SP, 2011. Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. Journal of Neuroscience 31, 16344–16352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Van Hecke W, Sunaert S, Wenderoth N, Swinnen SP, 2012. The neural basis of central proprioceptive processing in older versus younger adults: an important sensory role for right putamen. Human Brain Mapping 33, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn PJ, Lavrov IA, Sayenko DG, Van Straaten MG, Gill ML, Strommen JA, Calvert JS, Drubach DI, Beck LA, Linde MB, Thoreson AR, Lopez C, Mendez AA, Gad PN, Gerasimenko YP, Edgerton VR, Zhao KD, Lee KH, 2017. Enabling Task-Specific Volitional Motor Functions via Spinal Cord Neuromodulation in a Human With Paraplegia. Mayo Clin Proc 92, 544–554. [DOI] [PubMed] [Google Scholar]
- Hagen MC, Zald DH, Thornton TA, Pardo JV, 2002. Somatosensory processing in the human inferior prefrontal cortex. Journal of neurophysiology 88, 1400–1406. [DOI] [PubMed] [Google Scholar]
- Han H, 2020. Implementation of Bayesian multiple comparison correction in the second-level analysis of fMRI data: with pilot analyses of simulation and real fMRI datasets based on voxelwise inference. Cognitive neuroscience 11, 157–169. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR, 2011. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DC, Thiel A, Soucy JP, Bouyer L, Paquette C, 2019. Adjusting gait step-by-step: Brain activation during split-belt treadmill walking. Neuroimage 202, 116095. [DOI] [PubMed] [Google Scholar]
- Hofstoetter US, Freundl B, Binder H, Minassian K, 2019. Recovery cycles of posterior root-muscle reflexes evoked by transcutaneous spinal cord stimulation and of the H reflex in individuals with intact and injured spinal cord. PLoS One 14, e0227057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstoetter US, Freundl B, Danner SM, Krenn MJ, Mayr W, Binder H, Minassian K, 2020. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J Neurotrauma 37, 481–493. [DOI] [PubMed] [Google Scholar]
- Illis LS, Sedgwick EM, Tallis RC, 1980. Spinal cord stimulation in multiple sclerosis: clinical results. J Neurol Neurosurg Psychiatry 43, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, Brandt T, 2008. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage 39, 786–792. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T, 2004. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage 22, 1722–1731. [DOI] [PubMed] [Google Scholar]
- Jahn K, Wagner J, Deutschlander A, Kalla R, Hufner K, Stephan T, Strupp M, Brandt T, 2009. Human hippocampal activation during stance and locomotion. Annals of the New York Academy of Sciences 1164, 229–235. [DOI] [PubMed] [Google Scholar]
- Joa K-L, Han Y-H, Mun C-W, Son B-K, Lee C-H, Shin Y-B, Ko H-Y, Shin Y-I, 2012. Evaluation of the brain activation induced by functional electrical stimulation and voluntary contraction using functional magnetic resonance imaging. Journal of NeuroEngineering and Rehabilitation 9, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H-O, Baier B, 2010. Right insula for our sense of limb ownership and self-awareness of actions. Brain Structure and Function 214, 411–417. [DOI] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K, 2010. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage 50, 1589–1598. [DOI] [PubMed] [Google Scholar]
- MacIntosh BJ, Mraz R, Baker N, Tam F, Staines WR, Graham SJ, 2004. Optimizing the experimental design for ankle dorsiflexion fMRI. Neuroimage 22, 1619–1627. [DOI] [PubMed] [Google Scholar]
- Manson GA, Calvert JS, Ling J, Tychhon B, Ali A, Sayenko DG, 2020. The relationship between maximum tolerance and motor activation during transcutaneous spinal stimulation is unaffected by the carrier frequency or vibration. Physiological Reports 8, e14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masani K, Sayenko DG, Vette AH, 2013. What triggers the continuous muscle activity during upright standing? Gait Posture 37, 72–77. [DOI] [PubMed] [Google Scholar]
- Minassian K, Hofstoetter US, 2016. Spinal Cord Stimulation and Augmentative Control Strategies for Leg Movement after Spinal Paralysis in Humans. CNS Neurosci Ther 22, 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian K, McKay WB, Binder H, Hofstoetter US, 2016. Targeting Lumbar Spinal Neural Circuitry by Epidural Stimulation to Restore Motor Function After Spinal Cord Injury. Neurotherapeutics 13, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-López Y, Hollis ER, 2021. Sensory Circuit Remodeling and Movement Recovery After Spinal Cord Injury. Frontiers in Neuroscience 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H, Domoto R, Mizuguchi N, Sakamoto K, Kanosue K, 2019. Negative BOLD responses during hand and foot movements: An fMRI study. PLoS One 14, e0215736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S, Scheinost D, Constable RT, 2020. Cluster failure or power failure? Evaluating sensitivity in cluster-level inference. Neuroimage 209, 116468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Yi W, Xu J, Qi H, Du J, Wang C, He F, Ming D, 2015. Event-related beta EEG changes during active, passive movement and functional electrical stimulation of the lower limb. Ieee Transactions on Neural Systems and Rehabilitation Engineering 24, 283–290. [DOI] [PubMed] [Google Scholar]
- Rath M, Vette AH, Ramasubramaniam S, Li K, Burdick J, Edgerton VR, Gerasimenko YP, Sayenko DG, 2018. Trunk Stability Enabled by Noninvasive Spinal Electrical Stimulation after Spinal Cord Injury. J Neurotrauma 35, 2540–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Atkinson DA, Manson GA, Markley R, Kaldis T, Britz GW, Horner PJ, Vette AH, Sayenko DG, 2021. Transcutaneous spinal cord stimulation improves postural stability in individuals with multiple sclerosis. Multiple sclerosis and related disorders 52, 103009. [DOI] [PubMed] [Google Scholar]
- Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM, 2004. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. Neuroimage 21, 568–575. [DOI] [PubMed] [Google Scholar]
- Saradjian AH, Teasdale N, Blouin J, Mouchnino L, 2019. Independent early and late sensory processes for proprioceptive integration when planning a step. Cerebral Cortex 29, 2353–2365. [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Atkinson DA, Dy CJ, Gurley KM, Smith VL, Angeli C, Harkema SJ, Edgerton VR, Gerasimenko YP, 2015. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J Appl Physiol 118, 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayenko DG, Rath M, Ferguson AR, Burdick JW, Havton LA, Edgerton VR, Gerasimenko YP, 2019. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma 36, 1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D, 2014. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 35, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer B, Goltz D, Wacker E, Auksztulewicz R, Blankenburg F, 2014. Maintenance and manipulation of somatosensory information in ventrolateral prefrontal cortex. Human Brain Mapping 35, 2412–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AG, Atkinson DA, Varghese B, Oh J, Markley RL, Sayenko DG, 2021. Characterization of Spinal Sensorimotor Network Using Transcutaneous Spinal Stimulation during Voluntary Movement Preparation and Performance. Journal of Clinical Medicine 10, 5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccola G, Sayenko D, Gad P, Gerasimenko Y, Edgerton VR, 2018. And yet it moves: Recovery of volitional control after spinal cord injury. Prog Neurobiol 160, 64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, 2017. Functional Neuroanatomy for Posture and Gait Control. J Mov Disord 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C, McHugh C, Mockler D, Minogue C, Reilly RB, Fleming N, 2021. Transcutaneous spinal cord stimulation and motor responses in individuals with spinal cord injury: A methodological review. PLoS One 16, e0260166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticini LF, Klose U, Nägele T, Karnath H-O, 2009. Perfusion imaging in Pusher syndrome to investigate the neural substrates involved in controlling upright body position. PLoS One 4, e5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinastic JP, Kautz SA, McGregor K, Gregory C, Bowden M, Benjamin MB, Kurtzman M, Chang YL, Conway T, Crosson B, 2010. An fMRI study of the differences in brain activity during active ankle dorsiflexion and plantarflexion. Brain Imaging and Behavior 4, 121–131. [DOI] [PubMed] [Google Scholar]
- Vette AH, Masani K, Nakazawa K, Popovic MR, 2010. Neural-mechanical feedback control scheme generates physiological ankle torque fluctuation during quiet stance. IEEE Trans Neural Syst Rehabil Eng 18, 86–95. [DOI] [PubMed] [Google Scholar]
- Volz LJ, Eickhoff SB, Pool E-M, Fink GR, Grefkes C, 2015. Differential modulation of motor network connectivity during movements of the upper and lower limbs. Neuroimage 119, 44–53. [DOI] [PubMed] [Google Scholar]
- Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seanez I, Caban M, Pirondini E, Vat M, McCracken LA, Heimgartner R, Fodor I, Watrin A, Seguin P, Paoles E, Van Den Keybus K, Eberle G, Schurch B, Pralong E, Becce F, Prior J, Buse N, Buschman R, Neufeld E, Kuster N, Carda S, von Zitzewitz J, Delattre V, Denison T, Lambert H, Minassian K, Bloch J, Courtine G, 2018. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71. [DOI] [PubMed] [Google Scholar]
- Wegrzyk J, Ranjeva J-P, Fouré A, Kavounoudias A, Vilmen C, Mattei J-P, Guye M, Maffiuletti NA, Place N, Bendahan D, 2017. Specific brain activation patterns associated with two neuromuscular electrical stimulation protocols. Scientific reports 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zou T, Lv Z, Fan Y, 2020. Functional MRI reveals locomotion-control neural circuits in human brainstem. Brain sciences 10, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K, 1998. Stiffness control of balance in quiet standing. J Neurophysiol 80, 1211–1221. [DOI] [PubMed] [Google Scholar]
- Yin S, Liu Y, Petro NM, Keil A, Ding M, 2018. Amygdala Adaptation and Temporal Dynamics of the Salience Network in Conditioned Fear: A Single-Trial fMRI Study. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K, 2012. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiology of aging 33, 1073–1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Spherical ROIs mapped onto an MNI52 template.


