Abstract
During infection, Yersinia enterocolitica exports Yop proteins via a type III secretion pathway. Secretion is activated when the environmental concentration of calcium ions is below 100 μM (low-calcium response). Yersiniae lacking yopN (lcrE), yscB, sycN, or tyeA do not inactivate the type III pathway even when the concentration of calcium is above 100 μM (calcium-blind phenotype). Purified YscB and SycN proteins form cytoplasmic complexes that bind a region including amino acids 16 to 100 of YopN, whereas TyeA binds YopN residues 101 to 294. Translational fusion of yopN gene sequences to the 5′ end of the npt reporter generates hybrid proteins that are transported by the type III pathway. The signal necessary and sufficient for the type III secretion of hybrid proteins is located within the first 15 codons of yopN. Expression of plasmid-borne yopN, but not of yopN1–294-npt, complements the calcium-blind phenotype of yopN mutants. Surprisingly, yopN mutants respond to environmental changes in calcium concentration and secrete YopN1–294-Npt in the absence but not in the presence of calcium. tyeA is required for the low-calcium regulation of YopN1–294-Npt secretion, whereas sycN and yscB mutants fail to secrete YopN1–294-Npt in the presence of calcium. Experiments with yopN-npt fusions identified two other signals that regulate the secretion of YopN. yopN codons 16 to 100 prevent the entry of YopN into the type III pathway, a negative regulatory effect that is overcome by expression of yscB and sycN. The portion of YopN encoded by codons 101 to 294 prevents transport of the polypeptide across the bacterial double membrane envelope in the presence of functional tyeA. These data support a model whereby YopN transport may serve as a regulatory mechanism for the activity of the type III pathway. YscB/SycN binding facilitates the initiation of YopN into the type III pathway, whereas TyeA binding prevents transport of the polypeptide across the bacterial envelope. Changes in the environmental calcium concentration relieve the TyeA-mediated regulation, triggering YopN transport and activating the type III pathway.
Pathogenic Yersinia species (Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica) possess a virulence plasmid-encoded type III secretion mechanism that allows bacterial escape from phagocytic killing (8). Twenty-one Y. enterocolitica ysc genes (Yop secretion; yscCDEFGIJKLNOPQRSTUVWXY) encode components of a secretion machinery that transports 14 proteins across the bacterial double membrane envelope (Yersinia outer proteins; YopBDEHMNOPQRT, YscM1, YscM2, and LcrV) (8). During infection of tissue culture cells, Y. enterocolitica transports LcrV and YopBDR into the extracellular medium (type III secretion) (20, 23) and injects YopEHMNOPT and YscM1 into the cytoplasm of eukaryotic cells (type III targeting) (5, 6, 12, 16, 20, 26–28). The locations of YopQ and YscM2 during infection are unknown. Type III transport of Yop proteins is regulated in response to environmental signals (21). Serum factors trigger type III secretion of LcrV and YopBDR, while a low-calcium signal induces the type III targeting of YopEHMNOPT and YscM1 (21). During infection of animal or cultured host cells, the extracellular calcium concentration is high (1.2 mM). Under these conditions, the low-calcium signal is presumably generated by the cytoplasm of eukaryotic cells (100 nM) (4, 21). Mutations in several genes affect the type III program and either abolish Yop targeting (Not phenotype [no type III targeting]), prevent the specific injection of Yops into eukaryotic cells (Los phenotype [loss of type III targeting specificity]), or alter the rate of Yop targeting (UP or DOWN phenotype). For example, knockout mutations in yopD and lcrV cause a Not phenotype (22, 23), whereas knockout mutations in yopN, tyeA, and lcrG result in a Los phenotype (7, 10, 20). Knockout mutations in yopQ or yscM1 and yscM2 cause an UP phenotype, and knockout mutations in sycH cause a DOWN phenotype (6, 14).
When multiplying in laboratory media, wild-type Yersinia secretes YopEHMNOPTQ via the type III pathway in the absence but not in the presence (≥100 μM) of calcium ions (21, 25). Secretion of YopBDR and LcrV is also activated by low calcium; however, small amounts of these polypeptides are secreted even in the presence of environmental levels of calcium (21, 23). The molecular mechanisms that allow Yersinia to sense calcium ions and to regulate the type III machinery are not known. YopN (LcrE) appears to be involved in regulating the low-calcium response (Lcr), as yopN (lcrE) mutants secrete Yop proteins in both the presence and absence of calcium (11, 32). yopN is the first gene in the yopN-tyeA-sycN operon (15, 17). tyeA (translocation of YopE A) and sycN (secretion of YopN chaperone) encode small cytoplasmic polypeptides that each bind to YopN (9, 17). yscB (Yop secretion), the second gene in the virC operon (1), encodes the third YopN binding protein (18). SycN and YscB have been proposed to act as secretion chaperones that initiate YopN into the type III pathway (9). The role of TyeA in the presumed YopN-mediated repression of the type III pathway has not yet been described.
YopN is a substrate for type III targeting. It is proposed here that YopN transport may act as a mechanism for the regulation of the type III pathway. However, regulation may occur by another mechanism, and YopN transport could be the consequence, but not the cause, of activating the type III pathway. This report sought to distinguish between these possibilities by exploring YopN transport under various conditions. It is shown here that the signal necessary and sufficient for the type III secretion of hybrid proteins resides in the first 15 codons of yopN. The yopN secretion signal is functional in the absence but not in the presence of environmental calcium ions. The presence of codons 16 to 100 of yopN acts as a second signal and confers a property to YopN that prevents entry of the polypeptide into the type III pathway in the presence of environmental calcium ions. Expression of sycN and yscB, which specify two factors that bind YopN residues 16 to 100, allows the initiation of YopN into the type III pathway even in the presence of calcium ions. A third functional signal, located within yopN codons 101 to 294, prevents transport of YopN across the bacterial double membrane envelope. Proper function of this inhibitory signal requires expression of tyeA, encoding a cytoplasmic protein that binds this portion of YopN between residues 101 and 294. The data support a model whereby the SycN/YscB-mediated initiation and the TyeA-mediated inhibition of transport allow yersiniae to shut down the type III secretion pathway. Reduced amounts of the environmental calcium ions, presumably encountered during insertion of the YscF needle complex into host cells (13, 21), may relieve the TyeA-mediated inhibition. The activated type III pathway may subsequently transport YopN as well as YopEHMOPT into the cytosol of eukaryotic cells.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Y. enterocolitica strains W22703 (wild type), VTL1 (yopN1), and LC7 (tyeA2) have been described elsewhere (7, 20). Y. enterocolitica strains OK5 (yscB1), OK2 (sycN1), and LC8 (tyeA yscB1) were generated by allelic exchange. The yscB1 allele is a replacement of codons 18 to 79 of yscB with an HpaI site (GTTAAC) followed by a two-nucleotide (GC) insertion. yscB1 was constructed from PCR products and amplified with the primers YscB-XbaI (5′-AATCTAGAAGGTCAGATTTCATGGCAGAA-3′), YscB-HpaI-R (5′-AACGTTAACGTTATTTCCTTCACGT-3′), YscB-HpaI-F (5′-AAGTTAACGCGCCATTTTCTGTACCGT-3′), and YscB-XhoI (5′-AACTCGAGCAAACCAAATTGTAAAGAGAGG-3′). The PCR fragments were cut with XbaI-BamHI and BamHI-XhoI and cloned into plasmid pLC28 cut with XbaI-XhoI, thereby generating plasmid pOK43. sycN1 is a replacement of codons 43 to 75 with a BamHI site (GGATCC). sycN1 was constructed from PCR products and amplified with the primers Orf2-XbaI (5′-AATCTAGAACTCTGCAGTCTATAGCTGAT-3′), Orf2-BamHI-R (5′-AAGGATCCTTGAGCCATCTCTAATTGAA-3′), Orf2-BamHI-F (5′-AAGGATCCGCGCTAACGCTCACGGCGGC-3′), and Orf2-KpnI (5′-AAGGTACCAAGATCGCCGTTCAGTTGTTG-3′). The PCR fragments were cut with XbaI-BamHI and BamHI-KpnI and cloned into plasmid pLC28 cut with XbaI-KpnI, thereby generating plasmid pOK44. Plasmids pOK43 and pOK44 were mated into Y. enterocolitica W22703, and mutants with double-crossover mutations were isolated. Y. enterocolitica LC8 (tyeA 2yscB1) was generated by mating pOK43 into Y. enterocolitica LC7 (tyeA2). For expression of yscBHis6, tyeAHis6 and sycNHis6 (encoding proteins with a six-histidyl tag at the C terminus), PCR-amplified fragments were cut with NdeI-BamHI and cloned into pET33b, generating pLC250 (sycNHis6), pLC251 (yscBHis6), and pLC252 (tyeAHis6), respectively. These three plasmids were transformed into Escherichia coli BL21 (DE3).
To express wild-type yscB (pLC205), sycN (pOK17), tyeA (pLC186), yopN (pSM19), and yopNHis6 (pSM20), coding sequences were PCR amplified with specific primer pairs, cut with NdeI and BamHI, and cloned into the low-copy-number vector pDA234 (pSC101 derivative) cut with NdeI-BamHI. Ligated DNAs were electroporated into yersiniae, and transformants were selected on chloramphenicol plates. Insertions of DNA between the NdeI and BamHI sites of pDA234 places the gene of interest under the control of the tac promoter. Yersiniae carrying specific plasmids were grown in tryptic soy broth (TSB) supplemented with 20 μg of chloramphenicol per ml, and gene expression from the tac promoter was induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to the culture medium. Primers employed for the cloning reactions were YscB-NdeI (5′-AACATATGCAAAATTTACTAAAAAACTTGGC-3′) YscB-BamHI (5′-AAGGATCCTTACTTAATTCCACCCCACGCGAG-3′), SycN-NdeI (5′-AACATATGAGTTGGATTGAACCCATCATT-3′), SycN-BamHI (5′-AAGGATCCTCACGGCGCAAGCACCTC-3′), TyeA-NdeI (5′-AACATATGTCCCCTATACTAGGTTATTGGA-3′), TyeA-BamHI (5′-AAGGTACCAACAGATGCACGACGAGATC-3′), YopN-NdeI (5′-AACATATGACGACGCTTCATAACCTATCT-3′), and YopN-BamHI (5′-AAAGGATCCTCAGAAAGGTCGTAAGCC-3′) or YopNHis6-BamHI (5′-AAAGGATCCTCAGTGATGGTGATGGTGATGGAAAGGTCGTAAGCC-3′).
Yersinia secretion of Yop proteins.
Yersiniae were grown in TSB supplemented either with or without 5 mM calcium chloride. Overnight cultures of Yersinia were diluted 1:50 into 30 ml of fresh TSB medium, grown for 2 h at 26°C, and induced at 37°C for 3 h. Cultures were centrifuged at 10,500 × g for 15 min, and the supernatant was separated from the cell pellet. Proteins in both fractions were precipitated with trichloroacetic acid (TCA), washed in acetone, and suspended in sample buffer. Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. Immunoreactive species were quantified as chemiluminescent signals on X-ray film using laser densitometry scanning. The fusion proteins YopN1–294-Npt (pDA82), YopN1–100-Npt (pDA83), YopN1–15-Npt (pDA85), and YopNΔ•2–15-Npt (pDA111) have been described elsewhere (2). Plasmids pLC248 (YopE1–15-YopN16–294-Npt) and pLC249 (YopQ1–15-YopN16–294-Npt) were generated by the insertion of coding sequence for yopN16–294 flanked by KpnI sites into pDA46(YopE1–15-Npt) (2) or pDA184 (YopQ1–15-Npt) (3) cut with KpnI.
Yersinia infection of HeLa tissue cultures.
Overnight cultures of Yersinia were diluted 1:20 into 20 ml of fresh TSB and grown for 2 h at 26°C with shaking. Bacteria were sedimented at 8,000 × g for 10 min and suspended in phosphate-buffered saline (PBS). HeLa cells were grown to 80% confluence in 75-cm2 tissue culture flasks with Dulbecco's modified Eagle medium and 10% fetal bovine serum. Prior to infection, cells were washed twice with PBS, covered with 10 ml of Dulbecco's modified Eagle medium, and warmed to 37°C for 30 min. Aliquots of HeLa cells were counted, and each flask was infected with yersiniae at a multiplicity of infection of 10 and incubated for 3 h at 37°C with 5% CO2. Culture medium was decanted and centrifuged at 32,000 × g for 15 min to separate soluble proteins from nonadherent bacteria in the sediment. HeLa cells as well as adherent bacteria were suspended into 10 ml of 1% digitonin in PBS and shaken at 26°C for 20 min. HeLa cells were scraped off tissue culture flasks and vortexed, and samples were centrifuged at 32,500 × g for 15 min. A 7-ml aliquot was withdrawn and precipitated with methanol-chloroform while the remaining supernatant was discarded. The sediment was suspended in 10 ml of 1% SDS in PBS, and a 7-ml aliquot was precipitated with methanol-chloroform. Protein precipitates were solubilized in sample buffer, separated by SDS-PAGE, and analyzed by immunoblotting with specific antiserum. Immunoreactive species were quantified as chemiluminescent signals on X-ray film using laser densitometry scanning. Linearity of chemiluminescent signals is limited to a narrow concentration range and was calibrated by determining the signal strength of a dilution series of purified protein with known concentration.
Purification of TyeA, SycN, and YscB.
yscB and sycN were cloned into pQE30 (Qiagen) to yield plasmids pCT37 and pCT220, respectively. Primers carrying abutted BamHI sites were used for PCR amplification: YscB01 (5′-AAGGATCCCAAAATTTACTAAAAAACTTGGCT-3′), YscB02 (5′-AAGGATCCATTCCACCCCACGCGAGAC-3′), Orf2B6H5′ (5′-AAGGATCCATGAGTTGGATTGAACCCATC-3′), and Orf2B6H3′ (5′-AAGGATCCCGGCGCAAGCACCTCTTG-3′). After digestion with BamHI, the DNA fragments were cloned into pQE30 cut with BamHI and transformed into E. coli XL1-Blue. Recombinants were verified by restriction digestion and DNA sequencing. pLC182, encoding His-tagged TyeA, and the purification procedure for purification of TyeA have been described (7). One liter of E. coli XL1-Blue carrying pCT37 or pCT220 was grown to mid-log phase at 37°C and induced with 1 mM IPTG for 3 h, and the cells were harvested by centrifugation at 6,000 × g for 15 min. Samples were suspended in 20 ml of buffer A (6 M guanidinium hydrochloride, 0.1 M NaH2PO4, 0.01 M Tris-HCl4 [pH 8.0]) and incubated with vortexing for 1 h on ice. Insoluble material was removed with two centrifugation steps, each at 33,000 × g for 15 min. A 1-ml column of nickel-nitriloacetic acid (Ni-NTA) Sepharose was preequilibrated with 10 ml of buffer A and loaded with the supernatant of crude E. coli extracts. The column was washed with 10 ml of buffer A, 10 ml of buffer B (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris-HCl [pH 8.0]), and 20 ml of buffer C (same as buffer B, but pH 6.3). SycN and YscB were eluted with 4 ml of buffer E (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris-HCl [pH 4.5]). Purified proteins were used to immunize rabbits to obtain polyclonal antibodies.
Binding of YscB, SycN, and TyeA to YopN6His.
Overnight cultures of Y. enterocolitica W22703 (wild-type yopN) or VTL1(pSM20) (yopN6His), grown in TSB supplemented with 20 μg of chloramphenicol per ml, were diluted into fresh medium (10 ml of culture into 500 ml of TSB). Bacteria were grown for 2 h at 26°C and induced for 3 h at 37°C. Cells were harvested and suspended in 10 ml of TN buffer (50 mM Tris-HCl, 150 mM NaCl [pH 7.5]) and broken by a single passage through a French pressure cell at 14,000 lb/in2. Unbroken cells were removed by centrifugation at 6,000 × g for 15 min, and the supernatant was subjected to ultracentrifugation at 100,000 × g for 45 min. Supernatants were subjected to affinity chromatography on Ni-NTA Sepharose preequilibrated with TN buffer. The column was washed with one column volume of wash buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM imidazole [pH 7.5]) followed by two column volumes of wash buffer with 15% glycerol. Proteins were eluted with 4 ml of 50 mM Tris-HCl–250 mM imidazole (pH 7.5). Eluted proteins were concentrated by methanol-chloroform precipitation and suspended in 0.1 volume of sample buffer. Proteins were separated by SDS-PAGE and analyzed by immunoblotting.
Copurification assay.
E. coli XL1-Blue strains carrying pLC250, pLC251, pLC252, and pHTT24 were induced for the production of SycN6His, YscB6His, TyeA6His, and SrtA6His, respectively (31). Proteins were purified under denaturing conditions (see above) and retained on Ni-NTA Sepharose. The resin was washed with buffer and by stepwise lowering of the concentration of urea (7, 6, 5, and 4 M urea in 0.1 M NaH2PO4–0.01 M Tris-HCl, pH 8.0). Aliquots (40 μl each) of Ni-NTA Sepharose beads were employed in cosedimentation experiments with Yersinia extracts. Yersinia cells (1012 CFU from 500 ml of culture) carrying plasmids that contain yopN-npt fusions were grown for 2 h at 26°C and induced for type III secretion by a temperature shift to 37°C in TSB lacking calcium. Cells were harvested by centrifugation, suspended in 10 ml of TNGT buffer (50 mM Tris-HCl, 150 mM NaCl, 15% glycerol, 0.5% Triton X-100, 10 mM imidazole [pH 7.5]), and broken by a single passage through a French pressure cell at 14,000 lb/in2. Unbroken cells were removed by centrifugation at 6,000 × g for 15 min, and the supernatant was subjected to ultracentrifugation at 100,000 × g for 45 min. Aliquots (500 μl) of the supernatant as well as a buffer control were added to the Ni-NTA Sepharose containing immobilized SycN6His, YscB6His, SycN6His/YscB6His, TyeA6His, or SrtA6His. Samples were incubated for 30 min at room temperature with shaking and washed three times with TNGT buffer. Proteins retained on the beads were solubilized in hot sample buffer and separated by SDS-PAGE. Proteins were either visualized by Coomassie staining or analyzed by immunoblotting.
RESULTS
Role of tyeA, sycN, and yscB in YopN secretion.
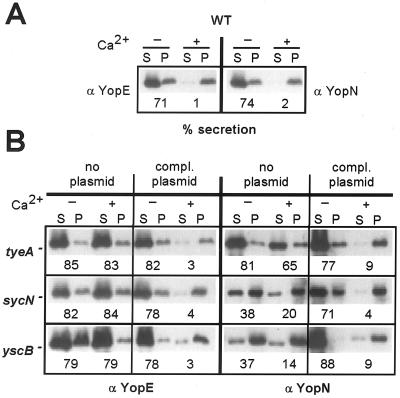
Deletion mutations in tyeA, sycN, and yscB were introduced by allelic exchange into the virulence plasmid of Y. enterocolitica W22703. The wild-type strain W22703 and its isogenic tyeA, sycN, and yscB variants were grown in TSB for 3 h at 37°C in the presence or absence of 5 mM calcium chloride. Cultures were centrifuged at 10,000 × g, and the extracellular medium was separated from the bacterial sediment. Proteins in both fractions were precipitated with TCA and solubilized in SDS sample buffer. Proteins were separated on SDS-PAGE and analyzed by immunoblotting with specific antibodies (antiYopE or antiYopN). As expected, Y. enterocolitica W22703 secreted 71% of the total amount of YopE and 74% of YopN into the extracellular medium when grown in the absence of calcium (Fig. 1A). In the presence of calcium, Y. enterocolitica W22703 transported very little YopE (1%) or YopN (2%). Growth of the tyeA, sycN, and yscB mutant strains in the absence or presence of calcium resulted in about 80% YopE secretion (Fig. 1B). The tyeA mutant strain secreted 81 and 65% of YopN in the absence and presence of calcium, respectively. When the sycN and yscB mutant strains were grown in the presence or absence of calcium, 38 and 20% (sycN) and 37 and 14% (yscB) of YopN were found in the extracellular medium. Thus, compared to results with the tyeA strain or other calcium-blind mutant strains (10), YopN secretion by the sycN and yscB mutants is somewhat reduced. Defects in calcium regulation of type III secretion of tyeA, sycN, and yscB mutants as well as the reduced secretion of YopN in sycN and yscB strains were complemented when variants were transformed with plasmids that restored the expression of tyeA, sycN, and yscB wild-type alleles (Fig. 1B). Together these results confirm earlier observations that nonpolar mutations in tyeA, sycN, and yscB cause a calcium-blind phenotype for type III secretion (9, 17, 18). Furthermore, nonpolar mutations in sycN and yscB result in a partial defect in YopN secretion and a calcium-blind phenotype for the secretion of YopE (9).
FIG. 1.
tyeA, sycN, and yscB mutant Y. enterocolitica strains display a calcium-blind phenotype as well as defects in the regulation of YopN secretion. (A) Y. enterocolitica strain W22703 (wild type [WT]) was grown in TSB in the presence or absence of 5 mM calcium chloride and induced for type III secretion by a temperature shift to 37°C. Cultures were centrifuged, and the extracellular medium was separated with the supernatant (S) from the bacterial pellet (P). Proteins were precipitated with TCA, suspended in sample buffer, separated on SDS-PAGE, and analyzed by immunoblotting with specific antibody (anti-YopE and anti-YopN). Immunoreactive signals were quantified by densitometry scanning, and the amount of secreted polypeptide was calculated. (B) Y. enterocolitica LC4 (tyeA), OK2 (sycN), and OK5 (yscB) carried either no plasmid or plasmids expressing wild-type tyeA, sycN, or yscB under the control of the IPTG-inducible tac promoter.
Fate of YopN during HeLa cell infection with tyeA, sycN, and yscB mutants.
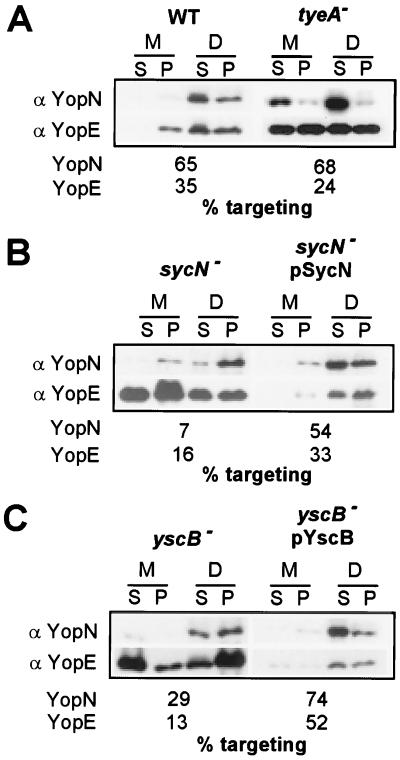
Yersinia type III targeting of YopE and YopN into the cytosol of eukaryotic cells was measured by infecting HeLa cells. Briefly, tissue cultures in medium containing 1.8 mM calcium were infected with Y. enterocolitica W22703 (wild type) and tyeA, sycN, and yscB mutant strains for 3 h. The growth medium was decanted and centrifuged, separating nonadherent bacteria (pellet) from the extracellular medium (supernatant). Yersiniae and HeLa cells adherent to the culture flasks were extracted with digitonin, a detergent that binds to cholesterol and disrupts the eukaryotic plasma membrane (20). Samples were centrifuged to separate the soluble components of HeLa cell extracts (digitonin supernatant) from the bacterial pellet (digitonin pellet). Proteins in all fractions were precipitated with chloroform-methanol, solubilized in sample buffer, separated on SDS-PAGE, and analyzed by immunoblotting. As a control for correct fractionation, samples were analyzed with anti-p130cas and anti-SycE. p130cas resides in the eukaryotic cytosol and is solubilized by digitonin extraction of tissue culture cells (data not shown). Yersiniae resist solubilization with digitonin, causing SycE to sediment with the bacteria after centrifugation of tissue culture extract (data not shown). After HeLa cell infection with Y. enterocolitica W22703, 65% of YopN and 35% of YopE were solubilized by extraction with digitonin (Fig. 2A). Although the tyeA mutant strain retained the ability to target 68% of YopN and 24% of YopE into the cytosol of HeLa cells, large amounts of YopN and YopE were observed in the extracellular medium (Fig. 2A). These results confirm an earlier observation that tyeA mutants display a Los (loss of type III targeting specificity) phenotype for the type III targeting of YopN and YopE and secrete larger amounts of these polypeptides than wild-type yersiniae (7).
FIG. 2.
tyeA, sycN, and yscB mutant Y. enterocolitica strains display a Los phenotype. HeLa cells were infected with Y. enterocolitica strains W22703 (wild type [WT]), LC4 (tyeA), OK2 (sycN), and OK5 (yscB). After incubation for 3 h at 37°C, the tissue culture medium (M) was decanted and centrifuged to separate secreted proteins from those present within nonadherent bacteria. HeLa cells as well as adherent yersiniae were extracted with digitonin (D), a detergent that solubilizes the eukaryotic plasma membrane but not the bacterial envelope. Extracts were centrifuged to separate proteins solubilized from the HeLa cytoplasm from those that sediment with the bacteria. Proteins were precipitated with chloroform-methanol and analyzed by immunoblotting with anti-YopE and anti-YopN. Immunoreactive signals were quantified by densitometry scanning, and the amount of targeted polypeptide (protein present in the digitonin supernatant) was calculated.
The sycN mutant also displayed the Los phenotype for YopE targeting, as this polypeptide was found in all fractions of infected tissue culture cells (Fig. 2B). However, during sycN mutant infection of HeLa cells, only 7% of YopN was found in the supernatant of digitonin extracts and no YopN was observed in the extracellular medium. This result suggests that sycN mutant yersiniae target only small amounts of YopN into HeLa cells and do not secrete YopN into the extracellular medium. Similar results were observed with the yscB mutant strain: 29% of YopN was targeted and no YopN secretion was observed (Fig. 2C). Both the reduced targeting of YopN and the Los phenotype of YopE were complemented when sycN and yscB mutants were transformed with plasmids that restored the expression of sycN and yscB wild-type alleles.
The yscB mutant phenotype is epistatic over that of tyeA.
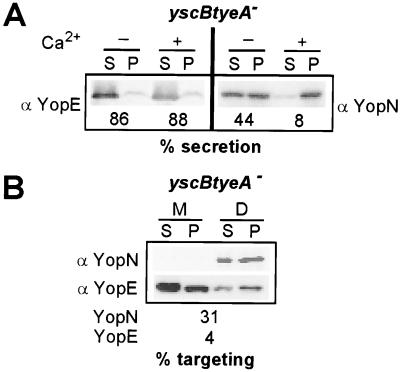
tyeA and yscB/sycN seem to play opposing roles during the type III transport of YopN, as knockout mutations in these genes either increase or decrease YopN secretion. We wondered what the YopN secretion phenotype of a yscB tyeA double-mutant strain might be. Y. enterocolitica LC8, a yscB tyeA double-mutant strain, was generated by allelic exchange and grown in TSB at 37°C. Strain LC8 secreted 86 and 88% of YopE in the absence and presence of calcium, respectively (Fig. 3A). Moreover, 44 and 8% of YopN were secreted in the absence and presence of calcium (Fig. 3A). During infection of tissue culture cells with the yscB tyeA mutant strain, YopE was secreted into the extracellular medium as well as injected into the cytosol of HeLa cells (Fig. 3B). In contrast, 31% of YopN was found targeted into the cytosol of HeLa cells, and YopN was not secreted into the extracellular medium (Fig. 3B). These results suggest that the yscB tyeA double-mutant strain cannot regulate the type III secretion of YopE in response to environmental calcium changes (calcium-blind phenotype). In contrast, type III secretion of YopN by the double-mutant strain is regulated in response to calcium. We conclude that the role of yscB in YopN secretion and targeting is epistatic over that of tyeA. Further, yscB must act before tyeA in regulating the secretion of YopN. tyeA and yscB tyeA mutants seem capable of sensing calcium, as the type III secretion of YopN in these strains is regulated in response to changing environmental concentrations of this ion.
FIG. 3.
The phenotype of yscB knockout mutations on the regulation of YopN secretion is epistatic over that of tyeA knockout mutations. (A) Y. enterocolitica strain LC8 (yscB tyeA) was grown in TSB in the presence or absence of 5 mM calcium chloride and induced for type III secretion by a temperature shift to 37°C. Cultures were centrifuged, and the extracellular medium was separated with the supernatant (S) from the bacterial pellet (P). Proteins were precipitated with TCA, suspended in sample buffer, separated on SDS-PAGE, and analyzed by immunoblotting with specific antibody (anti-YopE and anti-YopN). (B) HeLa cells were infected with Y. enterocolitica strain LC8, and samples were subjected to digitonin fractionation. Proteins were precipitated with chloroform-methanol and analyzed by immunoblotting with anti-YopE and anti-YopN. Abbreviations are as in Fig. 2.
Secretion signal of yopN.
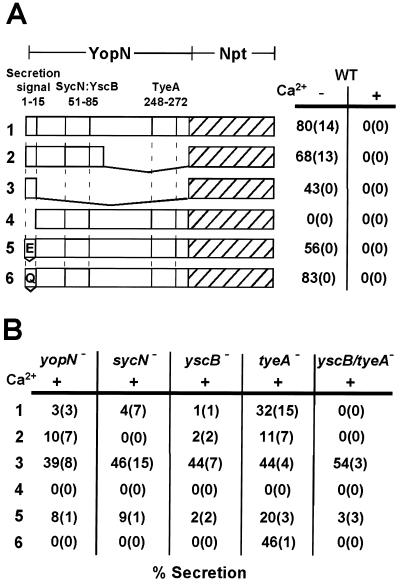
The secretion signal of YopN has been mapped by fusing the open reading frame of the npt (neomycin phosphotransferase) reporter gene to the 3′ end of yopN coding sequences (2). Fusion to full-length yopN resulted in expression of YopN1–294-Npt, a hybrid protein that is secreted by the type III pathway. Figure 4A shows that 80% of YopN1–294-Npt was secreted when Y. enterocolitica W22703(pDA82) was grown in the absence of calcium. No secretion of YopN1–294-Npt occurred when bacteria were grown in the presence of calcium. Thus, the secretion of YopN1–294-Npt resembles that of wild-type YopN (74% secretion without Ca2+ and 2% secretion with Ca2+). As reported previously, fusion of 3′ truncations of yopN to npt resulted in secretion, because 68% of YopN1–100-Npt and 43% of YopN1–15-Npt were found in the supernatant of low-calcium-induced Y. enterocolitica W22703 carrying various plasmids (Fig. 4A) (2). Fusion of mutant yopN lacking codons 2 to 15 to npt resulted in a hybrid protein, YopNΔ2–15-Npt, that was not secreted by the type III pathway in either the presence or absence of calcium ions (Fig. 4A). These results, taken together with previously published data (2), suggest that codons 1 to 15 of yopN encode a signal that is necessary and sufficient for the type III secretion of fused npt reporter sequences.
FIG. 4.
The role of SycN, YscB, and TyeA in the regulated secretion of YopN. (A) Secretion of YopN-Npt hybrids was measured by expressing plasmid-borne yopN-npt translational fusions in Y. enterocolitica W22703 (wild type [WT]) and growing bacteria in TSB with (+) or without (−) 5 mM calcium chloride (Ca2+). Bacterial cultures were centrifuged, culture medium and bacterial sediment were separated, and protein secretion was measured by immunoblotting with anti-Npt. Numbers indicate the average amount of secreted polypeptide (standard deviations in parentheses) obtained from three independent experiments. The drawing depicts the primary structure of hybrid polypeptides: (1) YopN-Npt, full-length yopN; (2) YopN1–100-Npt, codons 1 to 100 of yopN; (3) YopN1–15-Npt, codons 1 to 15 of yopN; (4) YopN15–294-Npt; yopN codons 2 to 15 deleted; (5) YopE1–15-YopN-Npt, replacement of yopN codons 1 to 15 with yopE codons 1 to 15; (6) YopQ1–15-YopN-Npt, replacement of yopN codons 1 to 15 with yopQ codons 1 to 15. (B) Secretion of YopN-Npt hybrids was measured by expressing plasmid-borne yopN-npt translational fusions in Y. enterocolitica VTL1 (yopN), OK2 (sycN), OK5 (yscB), LC7 (tyeA), or LC8 (tyeA yscB) and growing bacteria in TSB with 5 mM calcium chloride (Ca2+).
Previous work described the effect on secretion following mutation of the yopN secretion signal by insertion or deletion of nucleotides immediately following the AUG start codon (2). The frameshifted yopN sequences were fused to npt with reciprocal nucleotide insertions or deletions at the fusion site, thereby restoring translation of npt in the correct open reading frame. yopN secretion signals carrying −1, +1, −2, or +2 frameshift mutations were functional and allowed secretion of the mutant polypeptides (2). These results suggest that mRNA sequences encoding YopN amino acids 1 to 15 may provide the signal that leads to the type III secretion of YopN or YopN-Npt. Similar secretion signals have been identified within yopE and yopQ (2, 3). Here we asked whether the first 15 codons of yopE and yopQ are functional when substituting for the yopN secretion signal. Plasmids pLC248 and pLC249 encode the YopE1–15-YopN16–294-Npt and YopQ1–15-YopN16–294-Npt hybrids, respectively. Both hybrids were exported under low-calcium conditions in a manner resembling that of YopN1–294-Npt, suggesting that the first 15 codons of yopE and yopQ can substitute for the secretion signal of yopN (Fig. 4A).
Role of yopN, tyeA, sycN, and yscB during the secretion of YopN-Npt fusions.
yopN-npt-bearing plasmids were transformed into several mutant strains. Strain VTL1(pDA82) (yopN) secreted YopN1–294-Npt in the absence of calcium but not in the presence of calcium (Fig. 4B). This was a surprising result, as strains VTL1 and VTL1(pDA82) both display a calcium-blind phenotype for the secretion of YopE and other Yops (data not shown). Expression of wild-type yopN, but not of yopN1–294-npt, reversed the calcium-blind phenotype of strain VTL1 (data not shown). Further, the expression of yopN1–294-npt in wild-type strain W22703 did not affect the calcium regulation of Yop secretion (data not shown). These results suggest that yopN1–294-npt cannot fulfill the regulatory function of wild-type yopN. As YopN1–294-Npt is secreted in the absence but not in the presence of 5 mM calcium chloride, it appears that the yopN mutant strain is capable of sensing and responding to environmental calcium changes.
Expression of yopN1–294-npt in sycN and yscB mutant strains resulted in calcium-regulated secretion of YopN1–294-Npt, as observed for the yopN mutant strain (Fig. 4B). In contrast, the tyeA mutant strain secreted YopN1–294-Npt when growing both in the presence and in the absence of calcium ions (Fig. 4B). These data are consistent with a repressor function of TyeA for YopN secretion when yersiniae are exposed to an environment containing 5 mM calcium ions. Expression of yopN1–15-npt in yopN, tyeA, sycN, yscB, and yscB tyeA mutants resulted in type III secretion of YopN1–15-Npt in the presence of calcium ions (Fig. 4B). Thus, recognition of the yopN secretion signal occurs when yersiniae are grown in the presence and absence of calcium ions. Further, the combined function of yopN, tyeA, sycN, and yscB seems necessary to prevent the secretion of YopN and other Yops in the presence of calcium.
When expressed together with Gst-TyeA in E. coli, YopN amino acids 248 to 272 are required for copurification of YopN with Gst-TyeA (17). If one assumes that the TyeA binding site is located within the C-terminal half of YopN, truncation of 3′ yopN coding sequences should result in shortened polypeptides that are not subject to TyeA-mediated repression of secretion. This was tested, and 10% (11%) of YopN1–100-Npt and 39% (44%) of YopN1–15-Npt were found in the supernatants of yopN and tyeA (data in parentheses) mutant yersiniae grown in the presence of calcium (Fig. 4B). These data reveal no significant difference for the secretion of truncated YopN fusions in yopN and tyeA mutant strains, consistent with the binding of TyeA repressor to C-terminal YopN residues (101 to 294). The yopN mutant strain secreted YopN1–100-Npt at a lower level than YopN1–15-Npt. Presumably, yopN codons 16 to 100 reduce YopN initiation into the secretion pathway when bacteria reside in an environment with calcium ions (Fig. 4B). yopE codons 16 to 100, encoding the binding site for the SycE chaperone, are alone sufficient to initiate YopE into the secretory pathway when yersiniae are induced by low calcium. The analogous region of yopN, i.e., codons 16 to 100, does not function as an independent secretion signal, as YopNΔ2–15-Npt was not secreted in the presence or absence of calcium (Fig. 4).
In contrast to yopN mutants, sycN and yscB knockout strains did not secrete YopN1–100-Npt when growing in the presence of calcium (Fig. 4B). These results are consistent with the hypothesis that SycN and YscB binding to the polypeptide are required for initiation of YopN into the secretion pathway when bacteria are grown in the presence of calcium. Further, within a region of yopN codons 16 to 100 there appears to be a signal that is inhibitory to the secretion of YopN and that can be overcome by the binding of YscB and SycN to a polypeptide region within amino acids 16 to 100. One simple explanation for the data is a model whereby YopN residues 16 to 100 assume a conformation that is incompatible with type III secretion. Binding of YscB/SycN to this region may reverse the presumed hindrance of type III secretion. Jackson and colleagues showed that cross-linked YopN and YscB can be coimmunoprecipitated with antisera raised against either polypeptide (18). YopN residues 50 to 85 are required for the interaction between YopN and YscB. Further, SycN and YscB associate in a manner that allows cross-linking of the two polypeptides even in the absence of YopN (9). This association seems a prerequisite for YopN binding, as YscB and SycN alone cannot be cross-linked to YopN when the other chaperone is absent (9). Thus, previous data support the view that YscB and SycN form a heterodimeric complex that binds amino acid residues 50 to 85 of YopN (9). If so, YopN1–100-Npt, lacking the TyeA binding site of YopN, should be transported in a YscB/SycN-dependent fashion. This was indeed observed. The data therefore suggest that binding of YscB/SycN to YopN residues 1 to 100 promotes initiation of the polypeptide into the type III secretion pathway in presence of calcium.
In vivo binding of YopN to SycN/YscB and TyeA.
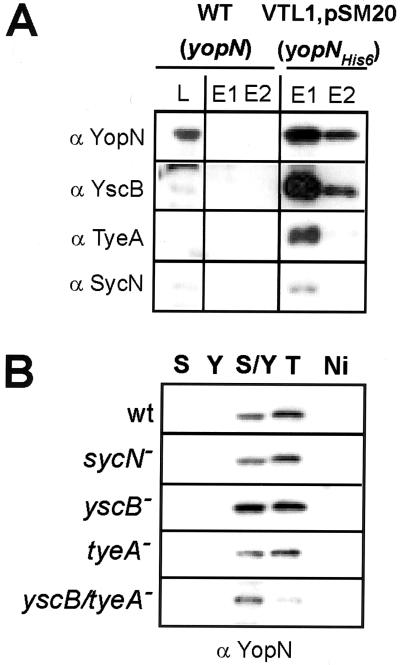
Previous attempts to demonstrate direct binding between YopN, SycN, YscB, and TyeA have failed (9, 15). We sought to establish an assay system that permits measurements of YopN binding to YscB/SycN and TyeA. YopNHis6 bears a six-histidyl tag appended to the C terminus of YopN. Expression of plasmid-borne yopNHis6 in the yopN mutant strain VTL1 restored calcium regulation of type III secretion to wild-type levels (data not shown). This result suggested that yopNHis6 functions in a manner similar to that of wild-type yopN. Crude cell extracts of Y. enterocolitica W22703 (wild-type yopN) and VTL1(pSM20) (yopNHis6) were subjected to affinity chromatography on Ni-NTA Sepharose and eluted with 0.25 M imidazole. YopNHis6, but not YopN, was eluted by the addition of imidazole, suggesting that six-histidyl-tagged YopN was retained on Ni-NTA Sepharose (Fig. 5). Immunoblotting of eluted extracts revealed coelution of SycN, YscB, and TyeA with YopNHis6 but not with wild-type YopN (Fig. 5A). These experiments suggest an association between SycN, YscB, TyeA, and YopN in crude Yersinia extracts that can be preserved during affinity chromatography.
FIG. 5.
SycN/YscB and TyeA copurify with YopNHis6. (A) Y. enterocolitica wild-type (WT) strain W22703 and Y. enterocolitica VTL1(pSM20) (yopN), expressing yopNHis6, were grown in TSB without calcium chloride. Bacteria were harvested and broken in a French press, and extracts were subjected to ultracentrifugation at 100,000 × g. The supernatant (lysate [L]) was applied to chromatography on Ni-NTA Sepharose. Bound proteins were eluted with buffer containing 250 mM imidazole (E1 and E2) and, together with a lysate sample, analyzed by immunoblotting with specific antibody. (B) Cleared lysates obtained from Y. enterocolitica strains W22703, OK2 (sycN), OK5 (yscB), LC4 (tyeA), or LC8 (tyeA yscB) were subjected to chromatography on Ni-NTA Sepharose (Ni) or Ni-NTA Sepharose precharged with purified SycNHis6 (S), YscBHis6 (Y), SycNHis6/YscBHis6 (S/Y), or TyeAHis6 (T). Proteins were eluted and analyzed by immunoblotting with anti-YopN.
We wondered whether SycNHis6, YscBHis6, or TyeAHis6 purified from E. coli can bind YopN molecules in crude Yersinia extracts. Purified SycNHis6, YscBHis6, TyeAHis6, or an equimolar mixture of SycNHis6 and YscBHis6 was immobilized on Ni-NTA Sepharose (Fig. 5B). The resins were then incubated with crude Yersinia extracts and washed. Bound protein was eluted with SDS sample buffer and analyzed by immunoblotting. YopN bound to purified YscBHis6/SycNHis6 complexes as well as to purified TyeAHis6. YopN binding to purified SycNHis6, YscBHis6, or Ni-NTA Sepharose alone was not observed. Binding of YscBHis6/SycNHis6 and TyeAHis6 to YopN molecules within various mutant strains was measured (Fig. 5B). The presence or absence of endogenous YscB, SycN, or TyeA in crude Yersinia extracts did not interfere with the binding of YopN to purified YscBHis6/SycNHis6 or TyeAHis6. We do not know whether purified YscBHis6/SycNHis6 and TyeAHis6 are capable of displacing prebound YscB/SycN and TyeA from YopN polypeptides. Nevertheless, the data reveal that the mutant Yersinia extracts contain YopN molecules that can be captured by affinity chromatography using immobilized YscBHis6/SycNHis6 or TyeAHis6.
YopN binding sites for SycN/YscB and TyeA.
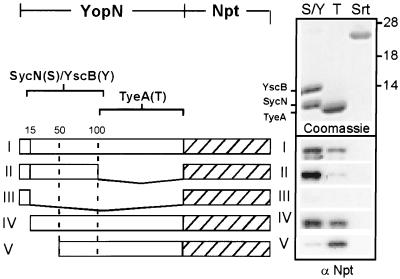
YopN-Npt fusion proteins were analyzed for their ability to be retained by affinity chromatography on immobilized SycNHis6/YscBHis6, TyeAHis6 or staphylococcal sortase (SrtAHis6) (31), a control for binding to an irrelevant protein (Fig. 6). YopN1–294-Npt (full-length YopN) bound YscBHis6/SycNHis6 and TyeAHis6 but not sortase. However, YopN1–100-Npt was retained on YscBHis6/SycNHis6 resin but not on TyeAHis6 resin. Further truncation of YopN sequences abolished binding of YscBHis6/SycNHis6, as YopN1–15-Npt was not retained by any of the three resins examined. YopN16–294-Npt bound to both YscBHis6/ SycNHis6 and TyeAHis6, suggesting that the first 15 residues of YopN are dispensable for its interaction with the regulatory factors. Finally, deletion of the first 50 residues in YopN51–294-Npt reduced binding to YscBHis6/SycNHis6 but not binding to TyeAHis6. Together these data suggest that the binding site for YscBHis6/SycNHis6 is located at residues 16 to 100, whereas the binding site for TyeAHis6 is located at residues 101 to 294 of YopN.
FIG. 6.
Binding sites of SycN/YscB and TyeA on YopN polypeptide. Cleared lysates were obtained by recovery of the supernatant of ultracentrifuged French press extracts from Y. enterocolitica W22703 harboring pDA82 (YopN-Npt) (I); pDA83 (YopN1–100-Npt) (II); pDA85 (YopN1–15-Npt) (III); pDA111 (YopN15–290-Npt) (IV); and pDA159 (YopN50–290-Npt) (V). Samples were applied to affinity chromatography on Ni-NTA Sepharose containing immobilized SycNHis6/YscBHis6 (S), TyeAHis6 (T), or SrtAHis6 (Srt [staphylococcal sortase as a control]). Proteins were eluted and analyzed by immunoblotting with anti-Npt. The top panel shows a Coomassie-stained SDS-PAGE gel of eluted SycNHis6/YscBHis6, TyeAHis6, and SrtAHis6. Numbers on the right correspond to molecular weight markers (in thousands).
DISCUSSION
Previous work identified lcrE (yopN) as a gene that is required for the low-calcium regulation of the Yersinia type III pathway (32). Others have proposed that YopN may act as a sensor for calcium ions on the bacterial surface (11). This study shows the following. (i) yopN mutant yersiniae are capable of regulating the type III secretion of YopN1–294-Npt in response to a low-calcium signal. (ii) The signal necessary and sufficient for the type III secretion of fused npt reporter proteins is located within codons 1 to 15 of yopN. (iii) In wild-type yersiniae, the secretion signal of yopN is functional in the absence but not in the presence of calcium. (iv) In yopN, yscB, sycN, and tyeA mutants, the yopN secretion signal is operational even in the presence of calcium. (v) Calcium regulation of YopN secretion requires fully functional yopN, sycN, tyeA, and yscB genes. (vi) yopN codons 16 to 100 contain a negative regulatory signal for the initiation of YopN into the secretion pathway, which can be modulated by the binding of YscB/SycN to amino acid residues 16 to 100. (vii) yopN codons 101 to 294 include a signal that prevents YopN secretion in the presence of calcium; proper function of this signal requires tyeA, encoding a cytoplasmic protein that binds YopN residues 101 to 290. (viii) The role of yscB in YopN secretion is epistatic over that of tyeA. (ix) During the infection of tissue cells, sycN and yscB mutants display a Los phenotype for the targeting of YopE. (x) tyeA mutant strains, but not yscB, sycN, or yscB tyeA variants, display a Los phenotype for the type III targeting of YopN. (xi) Purified SycN and YscB bind to a region within YopN residues 16 to 100, and purified TyeA binds to a region within residues 101 to 294.
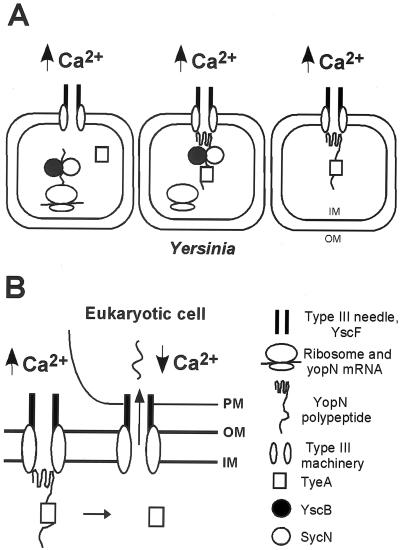
Figure 7A shows a hypothetical model for the regulated secretion of YopN. When environmental calcium concentrations are high (>80 μM), the secretion signal of yopN initiates YopN into the type III pathway. yopN codons 16 to 100 exert a negative regulatory effect on YopN transport, which is relieved by the binding of YscB/SycN to its site within YopN amino acid residues 16 to 100. yopN codons 101 to 294 prevent the secretion of YopN across the bacterial envelope in a manner that requires binding of TyeA to YopN residues 101 to 294. One explanation for the negative regulatory effect of codons 16 to 100 on initiation of YopN secretion is that the encoded polypeptide has evolved to resist transport by the type III pathway. Such property could be overcome by the binding of YscB/SycN to amino acid residues 16 to 100. It has not yet been established whether under noninducing conditions YscB/SycN and TyeA bind YopN for a prolonged period. Nevertheless, entry of YopN into the secretion pathway via YscB/SycN binding seems to be a prerequisite for the regulatory function of yopN, sycN, tyeA, and yscB on the type III machinery. A likely explanation for the negative regulatory effect of yopN codons 101 to 294 on YopN secretion is that this signal encodes the binding site for TyeA. TyeA binding presumably hinders further progression of YopN along the type III pathway and prevents secretion across the bacterial envelope. Although YscB, SycN, and TyeA appear to reside in the cytoplasm (7), a precise subcellular location of YopN/YscB/SycN or YopN/TyeA and a precise stage(s) at which the type III pathway may be arrested cannot yet be discerned. It is assumed here that YscB/SycN-mediated initiation and TyeA-mediated arrest of YopN transport represent physiological stages of the type III pathway. Further, it is assumed here that the YopN-mediated regulation of the type III pathway is limited to the recognition and targeting of YopEHMNOPT, as small amounts of YopBDR and LcrV are known to be secreted even under high-calcium conditions (21, 23).
FIG. 7.
Hypothesis for the regulated transport of YopN by the type III machinery of Y. enterocolitica. (A) In the presence of extracellular calcium ions (≥100 μM, ↑Ca2+), the secretion signal (yopN codons 1 to 15) and the binding of YscB/SycN to YopN amino acid residues 15 to 100 initiate YopN into the type III pathway. Further progression of YopN along the secretion pathway is stalled by the binding of TyeA to YopN amino acid residues 101 to 294. In the arrested state, the YopN-TyeA complex may act as a repressor for the type III targeting of YopEHMOPT by the Yersinia type III machinery. (B) Insertion of the YscF type III needle into the cytosol of eukaryotic cells may transmit a low-calcium signal (<100 μM calcium, ↓Ca2+), resulting in the dissociation of TyeA from YopN polypeptide and in the type III targeting of YopN across bacterial inner membranes (IM) and outer membranes (OM) as well as the plasma membrane (PM) of eukaryotic cells.
Anderson and Schneewind suggested that codons 1 to 15 of yop mRNA function as a signal for the type III secretion of Yop proteins (2). Karlinsey et al. hypothesized that secretion chaperones or pilots such as SycN and YscB act as regulators for ribosomal translation of their cognate secretion substrate in a type III pathway that also recognizes mRNA signals (19). Sory et al. and Lloyd et al. proposed that the type III machinery recognizes Yop peptide sequences as a signal for substrate recognition and secretion (24, 29, 30). These models vary considerably in predicting the mode of substrate recognition. It should be noted that the experiments conducted thus far have measured exclusively the type III secretion of mutationally altered gene products or fused reporter proteins. The generated data do permit some conclusions about secretion signals and substrate recognition. However, direct interactions between Yop proteins and the type III machinery as well as the in vitro transport of Yop proteins by the type III machinery have not yet been studied. Furthermore, previous work has not yet revealed a precise molecular mechanism for substrate recognition (e.g., binding of specific Yop residues to specific type III machinery components).
This study employed molecular genetic techniques to describe three regions of yopN that play separate roles in substrate recognition and transport: codons 1 to 15, 16 to 100, and 101 to 294. The observation that codons 1 to 15 of yopN can be frameshifted without loss of function suggests that yopN mRNA may be involved in signaling secretion of the encoded polypeptide. yopN codons 16 to 100 inhibit entry of YopN into the type III pathway. The function of this signal is overcome by expression of sycN and yscB, encoding two factors that bind to YopN residues 16 to 100. Finally, yopN codons 101 to 294 signal the inhibition of YopN secretion by the type III pathway. Expression of tyeA, specifying a regulatory factor that binds YopN within residues 101 to 294, is a prerequisite for proper function of the signal encoded by yopN codons 101 to 294. Although it seems likely that the signal for the inhibition of initiation of YopN is recognized as amino acid residues 16 to 100 and the signal for the inhibition of secretion is recognized as amino acid residues 101 to 294, the experiments do not provide definitive proof for the presumed mode of signal recognition.
The hypothesis depicted in Fig. 7 implies that the TyeA-mediated arrest of YopN transport is reversed once bacteria encounter a low-calcium environment. Two models can be entertained. (i) Once the extracellular calcium concentration drops below a threshold level, all newly synthesized YopN molecules may be refractory to tyeA-mediated repression. This regulation could be achieved by the posttranslational control of gene function (e.g., chemical decoration of TyeA or YopN). One important prediction of this model is that the continuous expression of yopN is required to provide for the repression of the type III pathway. Such a pathway would require additional mechanisms of gene regulation that either modify yopN expression or alter the function of yopN and thereby regulate the type III machinery. (ii) YopN (SycN/YscB and TyeA)-mediated repression may be static, and once an inhibitory complex has been assembled it needs removal by a mechanism that is coupled to sensing low extracellular calcium concentrations. This model resembles somewhat the previously proposed “bathtub-plug model” for YopN function (11). To garner supporting evidence for this model, one would need to reveal the physical identity of the “plug”, or inhibitory complex. Previous work suggests that YopN and YopN-TyeA do not associate with the membrane envelope of Y. enterocolitica (7). This observation does not exclude the existence of an inhibitory complex. However, together with the notion that type III machines are capable of transporting small amounts of YopBDR and LcrV in the presence of calcium, it seems unlikely that YopN, together with SycN/YscB and TyeA, can physically “plug up” the entire type III pathway (21, 23).
It is reported here and elsewhere that yopN mutant yersiniae are capable of sensing and responding to extracellular calcium ions (21). What is the mechanism whereby yersiniae sense extracellular calcium? Hoiczyk and Blobel described the YscF needle structure of the Y. enterocolitica type III machinery (13). The hollow YscF needle is assembled even when bacteria are grown in the presence of calcium and is capable of puncturing the plasma membrane of mammalian cells (13). It is speculated here that the YscF needle not only is involved in transporting Yop proteins but may also permit measurement of extracellular calcium ions. During the infection of tissue culture cells, the low-calcium signal for the transport of YopE (or other effector Yops) appears to be generated by the eukaryotic cytoplasm (21). It is conceivable that after penetration of the plasma membrane, the needle structure may transmit the low-calcium signal to type III machines that respond by transporting YopN as well as YopEHMOPT into the eukaryotic cytosol (Fig. 7B).
ACKNOWLEDGMENTS
We thank Deborah Anderson, Sarkis Mazmanian, Christina Tam, and Hung Ton-That for reagents and members of our laboratory for critical reading of the manuscript.
This work was supported by U.S. Public Health Service grant AI42797 from the NIH-NIAID, Infectious Diseases Branch. L.W.C. was supported by graduate student fellowships from the Microbial Pathogenesis Training Grant at UCLA (US PHS AI07323) and from the Warsaw Family Fellowship Fund.
REFERENCES
- 1.Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. doi: 10.1111/j.1365-2958.1995.mmi_18020343.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D M, Schneewind O. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol Microbiol. 1999;31:1139–1148. doi: 10.1046/j.1365-2958.1999.01254.x. [DOI] [PubMed] [Google Scholar]
- 4.Barrero M J, Montero M, Alvarez J. Dynamics of [Ca2+] in the endoplasmic reticulum and cytoplasm of intact HeLa cells. J Biol Chem. 1997;272:27694–27699. doi: 10.1074/jbc.272.44.27694. [DOI] [PubMed] [Google Scholar]
- 5.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5–1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 6.Cambronne E D, Cheng L W, Schneewind O. LcrQ/YscM1, regulators of the Yersinia yop virulon, are injected into host cells by a chaperone dependent mechanism. Mol Microbiol. 2000;37:263–273. doi: 10.1046/j.1365-2958.2000.01974.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L W, Schneewind O. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for the specific targeting of YopT, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J Bacteriol. 2000;182:3183–3190. doi: 10.1128/jb.182.11.3183-3190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M-P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day J B, Plano G V. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol Microbiol. 1998;30:777–789. doi: 10.1046/j.1365-2958.1998.01110.x. [DOI] [PubMed] [Google Scholar]
- 10.DeBord K, Lee V T, Schneewind O. Roles of LcrG and LcrV during the type III targeting of effector Yops by Yersinia enterocolitica. J Bacteriol. 2001;183:4588–4598. doi: 10.1128/JB.183.15.4588-4598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsberg A, Viitanen A-M, Skunik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 12.Hakansson S, Gaylov E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoiczyk E, Blobel G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Natl Acad Sci USA. 2001;98:4669–4674. doi: 10.1073/pnas.071065798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmstrom A, Petterson J, Rosqvist R, Hakansson S, Tafazoli F, Fallman M, Magnusson K-E, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 15.Iriarte M, Cornelis G R. Identification of SycN, YscX, and YscY, three new elements of the Yersinia yop virulon. J Bacteriol. 1999;181:675–680. doi: 10.1128/jb.181.2.675-680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iriarte M, Cornelis G R. YopT, a new Yersinia effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 17.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson M W, Day J B, Plano G V. YscB of Yersinia pestis functions as a specific chaperone for YopN. J Bacteriol. 1998;180:4912–4921. doi: 10.1128/jb.180.18.4912-4921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlinsey J E, Lonner J, Brown K L, Hughes K T. Translation/secretion coupling by type III secretion systems. Cell. 2000;102:487–497. doi: 10.1016/s0092-8674(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 20.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee V T, Mazmanian S K, Schneewind O. A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. J Bacteriol. 2001;183:4970–4978. doi: 10.1128/JB.183.17.4970-4978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee V T, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee V T, Tam C, Schneewind O. Yersinia enterocolitica type III secretion. LcrV, a substrate for type III secretion, is required for toxin-targeting into the cytosol of HeLa cells. J Biol Chem. 2000;275:36869–36875. doi: 10.1074/jbc.M002467200. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd S A, Norman M, Rosqvist R, Wolf-Watz H. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol Microbiol. 2001;39:520–531. doi: 10.1046/j.1365-2958.2001.02271.x. [DOI] [PubMed] [Google Scholar]
- 25.Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills S D, Boland A, Sory M-P, van der Smissen P, Kerbouch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persson C, Nordfelth R, Holmstrom A, Hakansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 28.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schesser K, Fritzh-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sory M-P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ton-That H, Liu G, Mazmanian S K, Faull K F, Schneewind O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci USA. 1999;96:12424–12429. doi: 10.1073/pnas.96.22.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]