Abstract
Objectives
The aim of this study was to describe the prevalence of coronary artery ectasia (CAE) in patients with ST‐elevation myocardial infarction (STEMI) and to compare the long‐term outcome of subjects with and without CAE undergoing emergent coronary angiography.
Background
The prognostic impact of CAE in STEMI patients has been poorly investigated.
Methods
This retrospective, single‐center, study included consecutive patients with STEMI undergoing emergent coronary angiography from January 2012 to December 2017. The primary endpoint was the assessment of recurrent myocardial infarction (MI) in patients with versus those without CAE at the longest available follow‐up. The propensity score weighting technique was employed to account for potential selection bias between groups.
Results
From 1,674 patients with STEMI, 154 (9.2%) had an angiographic evidence of CAE; 380 patients were included in the no CAE group. CAE patients were more often males and smokers, and showed a lower prevalence of diabetes than no CAE patients. After percutaneous coronary intervention, the corrected thrombolysis in MI frame count (p < .001) and the myocardial blush grade (p < .001) were significantly lower in CAE than in no CAE patients.
The mean follow‐up was 1,218.3 ± 574.8 days. The adjusted risk for the primary outcome resulted significantly higher in patients with CAE compared to those without (adjusted HR: 1.84; p = .017). No differences in terms of all‐cause and cardiac death were found between groups.
Conclusions
In this study, STEMI patients with CAE had a distinct clinical and angiographic profile, and showed a significantly higher risk of recurrent MI than those without CAE.
Keywords: coronary artery ectasia, outcome, percutaneous coronary intervention, ST‐elevation myocardial infarction
1. INTRODUCTION
Coronary artery ectasia (CAE) is defined as a diffuse or focal dilation of an epicardial coronary artery, which diameter exceeds at least 1.5 times the normal adjacent segment. 1 This is a relatively uncommon angiographic finding and its prevalence ranges widely across studies (from 1.2 to 4.9%), depending on the patients' clinical presentation and the definition of CAE. 1 , 2 Although often used interchangeably, the terms ectasia and aneurysm identify two different anatomical phenotypes: The first one refers to a diffuse dilation, which involves more than 50% of the length of the vessel; the second one defines a focal vessel dilation. 3 The most frequently reported classification of CAE, based on the extension in the coronary tree, was proposed by Markis et al. 4
Although the etiopathogenesis of CAE is still unclear, atherosclerosis seems to be the underpinning mechanism of coronary dilation in the majority of cases. 4 , 5 Beyond the morphological findings of coronary tree, coronary angiography is able to detect blood flow turbulences in the dilated vessels, which have been hypothesized to increase the risk of thrombotic and embolic events, independently from coexisting stenoses. 6
The abnormal coronary dilation, often associated with high thrombus burden, may be a challenge for percutaneous coronary intervention (PCI), particularly in patients presenting with ST‐elevation myocardial infarction (STEMI). Although primary PCI is the standard of care to restore coronary flow in these patients, 7 periprocedural drawbacks, such as distal thrombus embolization and stent malapposition, may affect device and procedural success. 8 These issues are mostly relevant in STEMI patients with CAE, which remain a demanding challenge for the interventional cardiologist and a concern for the clinician during follow‐up.
To date, few studies have investigated the prevalence and prognostic impact of CAE in patients with STEMI who undergo emergent coronary angiography. Whether CAE may influence the clinical course and prognosis in this high‐risk clinical setting is still debated.
The aim of the present study was to describe the prevalence of CAE in patients with STEMI and to compare the long‐term outcome of subjects with and without CAE who underwent emergent coronary angiography.
2. MATERIALS AND METHODS
2.1. Study population
From January 2012 to December 2017, all consecutive patients with STEMI undergoing emergent coronary angiography at our hospital were retrospectively collected by recruiting cases from our Institutional acute coronary syndrome (ACS) registry. All the angiographic images were examined by three experienced operators (C. B., L. E., and G. F.) in order to identify patients with CAE.
CAE was defined as a diffuse or focal dilation of an epicardial coronary artery exceeding by 1.5 times the normal adjacent segment diameter. Patients with history of Kawasaki disease, systemic vasculitis or connective tissue disorder were excluded.
In the present analysis, the study population was categorized into two groups according to the presence or not of CAE. Due to the need for retrospective re‐evaluation of coronary angiographies to assess measures potentially related to the exposure, we restricted the comparator group to an adequate cohort for the analysis (CAE‐no CAE ratio of 1:2). 9 , 10 From our institutional dataset, we randomly selected a control group 2.5 times the CAE group to account for the potential patient loss in the comparator.
In all patients, baseline demographic, clinical, echocardiographic, laboratory, angiographic, and PCI procedural findings were collected. The study was approved by our local Ethical Committee. The investigation conforms to the principles outlined in the Declaration of Helsinki. All patients were informed of the nature and aims of the study and asked to sign an informed consent for the anonymous management of their individual data.
2.2. Angiographic and periprocedural evaluation
Markis classification was adopted to define CAE extension as follows: Diffuse ectasia of two or three vessels was classified as type I, diffuse ectasia in one vessel and focal dilation in another vessel as type II, diffuse ectasia of one vessel only and focal aneurysm as type III and IV, respectively. 4 Whether the culprit vessel or segment (if identifiable) were ectatic was reported for each patient. Multivessel disease was defined as the presence of a stenosis ≥70% in two or more coronary arteries. 11 Primary PCI (pPCI) was performed according to the standard technique. Data on maximum diameter and total length of stents implanted in the infarct‐related artery (IRA) were collected. The use of thrombus aspiration and/or glycoprotein IIb/IIIa inhibitors during the procedure was also reported.
The postprocedural coronary flow grade was systematically assessed by two independent operators (C. B. and L. E.) and reported according to the thrombolysis in myocardial infarction (TIMI) classification. 12 The TIMI frame count was assessed in each patient who underwent pPCI, as the number of cine frames required for contrast medium to reach the respective distal landmarks of the epicardial coronary arteries. To take into account for the longer length of left anterior descending (LAD) compared with the left circumflex (LCx) and right coronary artery (RCA), this value was divided by 1.7 in the case of LAD, and reported as corrected TIMI frame count. 13 Myocardial blush grade (MBG) in the treated vessel was assessed at the end of the procedure using the semiquantitative densitometric method: MBG 0 denoted no contrast density or persistent blush or staining in the territory supplied by the IRA; MBG 1 denoted minimal contrast density; MBG 2 denoted moderate contrast density but less than contralateral or ipsilateral non‐IRA, and MBG 3 denoted normal contrast density, comparable with that obtained during angiography of a contralateral or ipsilateral non‐IRA. 14
2.3. Follow up and study outcome measures
Follow‐up data were obtained through routine visit in the dedicated outpatient clinic of our Institution.
In some cases, information was obtained by telephone interview of the patient, of the treating physicians or the next of kin.
Clinical outcome was assessed at the longest available follow‐up. The primary study outcome was the recurrence of myocardial infarction (MI) in CAE versus no CAE groups. Recurrent MI was defined by the presence of angina symptoms with typical ECG changes and elevated cardiac troponin levels with at least one value above the 99th percentile upper reference limit. 15
The secondary study outcomes were the occurrence of cardiac and all‐cause death in CAE versus no CAE groups. Other study outcomes included the rate of revascularization, stent thrombosis, in‐stent restenosis.
2.4. Statistical analysis
All consecutive patients with CAE enrolled during the study period were included in the analysis. After excluding patients with angiographic evidence of CAE, we randomly selected 25% of no CAE cases as controls to achieve approximately a no CAE/CAE ratio of 2.5.
Distribution of continuous data was tested with the Kolmogorov–Smirnov and the Shapiro–Wilk test. Normally distributed variables were expressed as mean ± SD, whereas non‐normal distributed ones as median and interquartile range (IQR). Categorical variables were reported as numbers and percentages. Continuous normally‐distributed variables were compared by using the Student t‐test; differences between non‐normally distributed variables were tested with the Mann–Whitney U test. Categorical variables were compared with chi‐squared test, or Fisher exact test, when appropriate.
Cumulative event rates were estimated by the Kaplan–Meier method and the Log‐Rank test was performed for comparisons between CAE and no CAE patients. The unadjusted and adjusted hazard ratios (HR) for the outcomes of interest were calculated using the Cox proportional hazard regression model and presented as HR with their 95% confidence intervals (CI). We used the propensity score weighting technique to account for potential selection bias between the two study groups (average effect weights). The propensity score model was developed using a non‐parsimonious approach and by incorporating all the pre‐procedural covariates potentially related to the outcome or the angiographic evidence of CAE regardless of their statistical significance or collinearity with other variables included in the model. The following baseline covariates were included in the propensity score model: Sex, age, hypertension, obesity, hyperlipidemia, diabetes, family history of coronary artery disease, smoking status, prior MI, prior PCI, prior coronary artery bypass graft, treated vessel. After weighting, standardized mean differences (SMD) were calculated to assess the balance for all covariates included in the model and SMD >0.1 was considered significant.
For all test, a p value <.05 was considered statistically significant. Analyses were performed by using the R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). The Sample function was used for random selection and the WeightIt package for propensity score weighting analysis.
3. RESULTS
3.1. Baseline demographic and clinical characteristics
From 1,674 patients with STEMI retrospectively identified, 154 (9.2%) had an angiographic evidence of CAE (CAE group); 380 patients were included in the no CAE group. The baseline demographic and clinical characteristics of the study population are reported in Table 1. There was no difference in terms of age between groups (64.6 ± 12.0 vs. 62.3 ± 13.7 years, p = .069). CAE patients were more often males (90.9 vs. 72.6%, p < .001) and smokers (72.1 vs. 62.4%, p = .042), and showed a lower prevalence of diabetes (11.7 vs. 25.8%, p = .001) compared to no CAE patients. The other risk factors were comparable between groups. A history of prior MI was reported in 15.6 and 13.3% of CAE and no CAE patients (p = .589), respectively. There was no difference in terms of Killip class, left ventricular ejection fraction at admission as well as of the laboratory parameters collected between groups.
Table 1.
Baseline demographic and clinical characteristics of CAE and no CAE groups
| CAE (N = 154) | No CAE (N = 380) | p | SMD | |
|---|---|---|---|---|
| Age, years | 64.6 ± 12.0 | 62.3 ± 13.7 | .069 | 0.179 |
| Male sex, N (%) | 140 (90.9) | 276 (72.6) | <.001 | 0.487 |
| BMI, kg/m 2 | 28.66 (4.27) | 28.36 (8.98) | .690 | 0.043 |
| Systolic AP, mmHg | 120.7 ± 22.7 | 120.4 ± 23.2 | .909 | 0.013 |
| Diastolic AP, mmHg | 77.6 ± 14.8 | 76.8 ± 14.6 | .594 | 0.058 |
| Hypertension, N (%) | 98 (63.6) | 231 (60.8) | .607 | 0.059 |
| Obesity, N (%) | 59 (38.3) | 115 (30.3) | .090 | 0.170 |
| Hyperlipidemia, N (%) | 64 (41.6) | 160 (42.1) | .985 | 0.011 |
| Diabetes, N (%) | 18 (11.7) | 98 (25.8) | .001 | 0.367 |
| History of CAD, N (%) | 62 (40.3) | 170 (44.7) | .396 | 0.091 |
| Smoking status, N (%) | 111 (72.1) | 237 (62.4) | .042 | 0.208 |
| Prior MI, N (%) | 24 (15.6) | 50 (13.3) | .589 | 0.064 |
| Prior PCI, N (%) | 20 (13.0) | 47 (12.5) | .993 | 0.015 |
| Prior CABG, N (%) | 2 (1.3) | 4 (1.1) | 1.000 | 0.023 |
| Killip class, N (%) | .059 | 0.277 | ||
| 1 | 132 (85.7) | 293 (77.1) | ||
| 2 | 6 (3.9) | 34 (8.9) | ||
| 3 | 6 (3.9) | 11 (2.9) | ||
| 4 | 10 (6.5) | 42 (11.1) | ||
| LVEF, N (%) | ||||
| > 45% | 104 (67.5) | 229 (60.3) | .141 | 0.152 |
| 45–35% | 41 (26.6) | 114 (30.0) | .501 | 0.075 |
| < 35% | 9 (5.8) | 36 (9.5) | .232 | 0.137 |
| Peak troponin, pg/ml | 39.9 (8.1, 87.9) | 35.7 (10.1, 92.8) | .726 | 0.110 |
| Creatinine, mg/dl | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.2) | .154 | 0.022 |
| eGFR, ml/min | 79.5 ± 26.8 | 76.9 ± 24.6 | .299 | 0.101 |
| Hb, g/dl | 14.3 ± 1,7 | 14.0 ± 2.0 | .240 | 0.133 |
| Total cholesterol, mg/dl | 176.4 ± 52.7 | 182.1 ± 47.3 | .280 | 0.115 |
| HDL‐C, mg/dl | 43.2 ± 12.5 | 45.5 ± 14.1 | .119 | 0.177 |
| LDL‐C, mg/dl | 107.8 ± 44.1 | 110.2 ± 40.5 | .606 | 0.055 |
| Triglicerides, mg/dl | 131.2 ± 72.3 | 130.4 ± 71.2 | .917 | 0.011 |
Note: Continuous normally‐distributed variables are expressed as mean ± SD; continuous asymmetrically‐distributed variables are reported as median (IQR); categorical variables are expressed as number and percentage.
Abbreviations: AP, arterial pressure; CABG, coronary artery by‐pass graft; CAD, coronary artery disease; CAE, coronary artery ectasia; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HDL‐c, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary artery intervention; SD, standard deviation; SMD, standardized mean difference.
3.2. Angiographic and periprocedural characteristics
The baseline angiographic and periprocedural characteristics of the study population are reported in Table 2. CAE involved the RCA in 79.2% of cases, LAD in 40.3%, and LCx in 35.1%. The left main was ectatic in only 2.6% of patients. Markis 3 (75, 48.7%) and 1 (44, 28.6%) were the most frequent phenotypes. The IRA was ectatic in 89 (57.8%) patients, and the infarct‐related segment was ectatic in 55 (35.7%). Most of patients underwent PCI (96.1 vs. 97.6%, p = .497); at least one stent was implanted in 87.0 and 88.7% in CAE and no CAE group, respectively, (p = .693). PCI in the RCA was more frequently performed in CAE than in no CAE patients (30.8 vs. 41.6%, p = .023); LAD was more commonly treated in no CAE group (45.5 vs. 62.1%, p < .001). As expected, stent diameter was significantly higher in CAE patients (p < .001), but no difference in total stent length was observed between groups (p = .292). The glycoprotein IIb/IIIa inhibitors were more frequently administered in patients with angiographic evidence of CAE (27.3 vs. 18.2%, p = .025) than in no CAE group. Although TIMI flow grade was not statistically different between groups, the corrected TIMI frame count resulted higher in CAE than in no CAE patients (15.5 ± 8.9 vs. 12.5 ± 7.8, p < .001). After PCI, MBG was significantly lower in CAE as compared to no CAE group (p < .001).
Table 2.
Angiographic characteristics of the study population (N = 534)
| CAE (N = 154) | No CAE (N = 380) | p | SMD | |
|---|---|---|---|---|
| Ectatic vessel, N (%) | ||||
| Left main | 4 (2.6) | — | — | — |
| Left anterior descending | 62 (40.3) | — | — | — |
| Left circumflex | 54 (35.1) | — | — | — |
| Right coronary artery | 122 (79.2) | — | — | — |
| Markis classification, N (%) | ||||
| 1 | 44 (28.6) | — | — | — |
| 2 | 16 (10.4) | — | — | — |
| 3 | 75 (48.7) | — | — | — |
| 4 | 19 (12.3) | — | — | — |
| Ectatic infarct‐related artery, N (%) | 89 (57.8) | — | — | — |
| Ectatic infarct‐related segment, N (%) | 55 (35.7) | — | — | — |
| PCI, N (%) | 148 (96.1) | 371 (97.6) | .497 | 0.088 |
| Treated vessel, N (%) | ||||
| Left main | 2 (1.3) | 8 (2.1) | .787 | 0.062 |
| Left anterior descending | 70 (45.5) | 236 (62.1) | .001 | 0.339 |
| Left circumflex | 26 (16.9) | 45 (11.8) | .157 | 0.144 |
| Right coronary artery | 64 (41.6) | 117 (30.8) | .023 | 0.226 |
| Stent implantation, N (%) | 134 (87.0) | 337 (88.7) | .693 | 0.051 |
| Stent diameter, N (%) | <.001 | 0.766 | ||
| 2 mm | 1 (0.7) | 2 (0.6) | ||
| 2,25 mm | 6 (4.5) | 10 (3.0) | ||
| 2,5 mm | 11 (8.2) | 38 (11.3) | ||
| 2,75 mm | 14 (10.4) | 75 (22.3) | ||
| 3 mm | 38 (28.4) | 117 (34.8) | ||
| 3,25 mm | 0 | 1 (0.3) | ||
| 3,5 mm | 25 (18.7) | 79 (23.5) | ||
| 4 mm | 30 (22.4) | 13 (3.9) | ||
| 4,5 mm | 8 (6.0) | 1 (0.3) | ||
| 5 mm | 1 (0.7) | 0 | ||
| Total stent length, mm | 25.0 ± 11.0 | 26.3 ± 11.7) | .292 | 0.109 |
| MVD, N (%) | 71 (46.1) | 175 (46.1) | 1.000 | 0.001 |
| Glycoprotein IIb/IIIa inhibitors, N (%) | 42 (27.3) | 69 (18.2) | .025 | 0.219 |
| Thrombus aspiration, N (%) | 51 (33.1) | 118 (31.1) | .717 | 0.044 |
| Angiographic assessment after PCI | ||||
| TIMI frame count | 25.7 ± 14.8 | 20.49 ± 12.7 | <.001 | 0.378 |
| Corrected TIMI frame count | 15.5 ± 8.9 | 12.5 ± 7.8 | <.001 | 0.357 |
| TIMI flow, N (%) a | .283 | 0.186 | ||
| 0 | 5 (3.4) | 8 (2.2) | ||
| 1 | 4 (2.8) | 5 (1.4) | ||
| 2 | 34 (23.4) | 66 (18.4) | ||
| 3 | 102 (70.3) | 280 (78.0) | ||
| MBG, N (%) b | <.001 | 0.576 | ||
| 0 | 21 (14.6) | 17 (4.8) | ||
| 1 | 26 (18.1) | 43 (12.1) | ||
| 2 | 51 (35.4) | 89 (25.1) | ||
| 3 | 46 (31.9) | 205 (57.9) | ||
Abbreviations: CAE, coronary artery ectasia; MBG, myocardial blush grade; MVD, multivessel disease; PCI, percutaneous coronary artery intervention; SMD, standardized mean difference; TIMI, thrombolysis in myocardial infarction.
Data available in 504 of 534 patients.
Data available in 498 of 534 patients.
3.3. Long‐term clinical outcome
The mean follow‐up time was 1,218.3 ± 574.8 days. The recurrence of MI was reported in 80 patients: 30 in CAE (19.5%) and 50 in no CAE (13.2%) group. Stent thrombosis occurred in six patients, with no statistical difference in CAE vs. no CAE groups (2 vs. 4, p = 1.000; Supplemental Table 1). Both stent thromboses in patients with CAE occurred after 1 year from the index PCI.
The unadjusted risk for MI recurrence was not statistically different between groups (HR: 1.49; 95% CI 0.94–1.35; p = .088; Figure 1). After propensity score weighting, the risk for MI recurrence resulted significantly higher in patients with CAE as compared to those without (adjusted HR: 1.84; 95% CI 1.11–3.05; p = .017).
Figure 1.
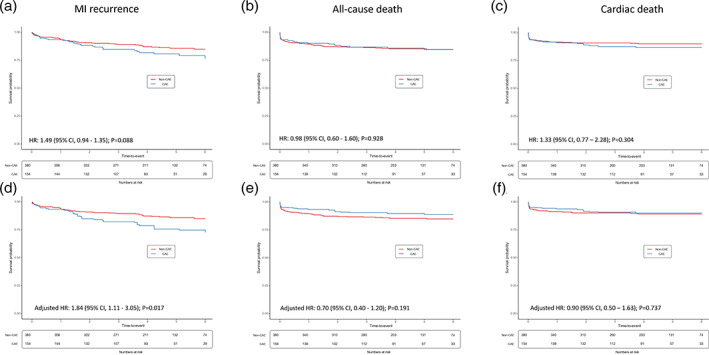
Kaplan–Meier survival free from the study outcomes at unadjusted (panels a–c) and adjusted analyses (panels d–f). CAE, coronary artery ectasia; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction
All‐cause death occurred in 79 cases (22 in CAE and 57 in no CAE); 34 patients died during the index hospitalization. Cardiac death was reported in 58 cases. There was no difference in the risk for all‐cause death (adjusted HR: 0.70; 95% CI 0.40–1.20; p = .191) as well as for cardiac death (adjusted HR: 0.90; 95% CI 0.50–1.63; p = .737) between groups.
Supplemental Figures 1 and 2 depict the survival free from MI recurrence and all‐cause death stratified for the Markis phenotype. Of note, no statistical difference was observed between groups for both the study outcomes.
Also, no statistical difference was observed between CAE patients with versus without ectatic IRA (EIRA) in terms of survival free from MI recurrence and all‐cause death (Supplemental Figures 3 and 4 groups).
Comparisons for the other outcome measures of interest between CAE versus no CAE group, and EIRA versus non‐EIRA are reported in Supplemental Tables 1 and 2. No statistical differences were found between groups.
4. DISCUSSION
The main findings of the present study can be summarized as follows: (a) in this STEMI population, CAE was detected by coronary angiography in 9.2% of cases; (b) CAE patients, compared to no CAE, were more often males and smokers, but showed a lower prevalence of diabetes; (c) in CAE population, RCA was the most common IRA and post‐procedural TIMI frame count and MBG were significantly lower than in no CAE group; (d) after balancing for potential confounders, Cox regression analysis revealed a significantly higher risk of recurrent MI in CAE patients compared to no CAE at long term.
The prevalence data reported in CAE are still sparse, ranging from 1.2 to 4.9% in all‐comers patients' cohorts. The higher proportion of CAE observed in our population may be related to the selective recruitment of STEMI patients and to the inclusion of all patients with angiographic evidence of CAE both in the culprit or non‐culprit vessel. Indeed, we found a prevalence of EIRA of 5.7%, which was consistent with previous observational findings in STEMI cohorts. 16 , 17
Consistently with previous studies, a significantly higher percentage of diabetes among patients without CAE was observed, whereas male sex and smoking were prevalent in CAE group. 18 , 19 Since impaired glycemic control induce negative arterial vessel remodeling, some authors hypothesized that the lower prevalence of diabetic patients in CAE population may be associated with the compensatory vessel enlargement observed in CAE. 19 , 20
In this study, RCA was involved in CAE in the majority of cases, followed by LAD and LCx; although the reason remains unknown, this topographic distribution has been already described in previous studies. 21 , 22 We also found a significantly higher adoption of glycoprotein IIb/IIIa inhibitors during primary PCI in CAE as compared to no CAE group. This wider use of intravenous antiplatelet agents has been already reported in an Italian cohort of patients with STEMI and angiographic evidence of EIRA, 23 and may be explained by the high thrombus burden in CAE patients which prompted the use of glycoprotein IIb/IIIa inhibitors as a bailout strategy. 24 Of interest, our study showed a lower procedural success in terms of post‐PCI MBG in the CAE group, despite the absence of any significant difference in TIMI flow grade. Similar angiographic findings have been reported by Erden et al, 17 who described a lower MBG in patients with EIRA undergoing primary PCI compared to non‐EIRA patients. A MBG <3 reflects an impaired myocardial perfusion, often related to distal embolization of thrombotic material, even if the epicardial coronary flow is adequately restored. Indeed, Henriques et al demonstrated that MBG is a strong predictor of mortality in patients with TIMI 3 flow after primary PCI. 25
Although abnormal coronary dilation is associated with flow disturbances, enhanced thrombogenicity and therapeutic challenges for both clinicians and interventionalists, the clinical impact of CAE in patients admitted for STEMI has been poorly investigated. To the best of our knowledge, the literature provides neither definite proofs of prognostic significance nor recommendations on the best treatment of CAE in STEMI patients.
In our study the adjusted risk of recurrent MI at long‐term follow‐up was significantly higher in CAE compared to no CAE group. Ipek et al, in a retrospective study on 1,655 STEMI patients, showed no difference in terms of in‐hospital and 1 year mortality as well as in terms of revascularization between patients with and without EIRA; of note, they found a higher rate of no reflow after PCI in patients with EIRA. 16 However, these findings were limited by the unadjusted statistical analysis, the relatively small sample size, the paucity of adverse events, and the short‐term follow‐up time. Fujii et al 26 documented a crude value of lower mortality in CAE patients compared to no CAE at 1 year after STEMI. However, the propensity‐matched analysis between groups did not detect any statistically significant difference, confirming the hypothesis that these findings could be the result of different baseline characteristics between the two populations. On the other hand, a recent propensity score matching‐based study reported a significantly higher risk of cardiac death and non‐fatal MI in 51 patients with CAE compared to no CAE group, supporting the role of CAE as a strong predictor of adverse cardiac events in this clinical setting. 27
In this heterogeneous conceptual framework of conflicting evidence, our study provides further information on the prognostic impact of CAE: The evidence of a higher risk of recurrent MI than in no CAE group, emphasizes the importance of this finding, captured by angiography in the emergency setting, during long‐term management of these patients.
Nevertheless, the higher recurrence of MI did not result in increased all‐cause and cardiac death. We may hypothesize that the relatively short follow‐up time along with the limited number of patients included in this analysis may have affected the capability to detect differences for these hard adverse events.
This study seems to support the hypothesis of a prognostic significance of CAE and, consequently, encourages any clinical and interventional efforts for the optimal management of this particular population of STEMI patients.
4.1. Study limitations
The present study has several limitations. Beyond the observational retrospective study design, the small sample size and the single‐center recruitment of patients might have affected the generalizability of our findings. However, to the best of our knowledge, this is the largest propensity score weighting study evaluating the prognostic role of CAE in patients with STEMI. Moreover, we deliberately decided to not further extend the enrollment back in the years and to circumscribe the evaluation to patients who underwent contemporary treatment of STEMI, as recommended by the current guidelines (i.e., use of potent antiplatelet agents and newer generation drug‐eluting stents). Another limitation concerns the lack of data on antithrombotic treatment after discharge. Given the absence of specific recommendation by current guidelines, we did not consider a confounding role of antithrombotic therapy between CAE and no CAE patients very likely, and we hypothesize that most of the patients were treated with dual antiplatelet therapy as per the standard of care. 28 Eventually, a systematic use of intracoronary imaging techniques might have provided interesting data for the assessment of mechanism of recurrent MI. However, this was a real‐world study which reflects contemporary clinical practice; although helpful in many cases, current guidelines do not recommend the systematic use of intracoronary imaging for PCI guidance in patients with recurrent MI.
5. CONCLUSIONS
In conclusions, despite comparable rates of all‐cause and cardiac death, STEMI patients with angiographic evidence of CAE showed a higher risk of recurrent MI at long‐term follow up compared to a propensity‐weighted group of no CAE STEMI. Additionally, in CAE patients undergoing primary PCI was reported a higher rate of reperfusion failure assessed by a poorer postprocedural myocardial blush grade. Thus, CAE represents an additional risk in the clinical setting of STEMI and its therapeutic management remains challenging. Further studies are required to get the correct answer to this unmet need.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Supplemental Figure 1 Kaplan–Meier survival free from recurrent MI in patients with CAE according to the Markis Class.
Supplemental Figure 2 Kaplan–Meier survival free from all‐cause death in patients with CAE according to the Markis Class.
Supplemental Figure 3 Kaplan–Meier survival free from recurrent MI in CAE patients with versus those without EIRA.
EIRA, ectatic infarct‐related artery.
Supplemental Figure 4 Kaplan–Meier survival free from recurrent MI in CAE patients with versus those without EIRA.
EIRA, ectatic infarct‐related artery.
Supplemental Table 1 Explorative outcomes between no CAE versus CAE patients.
Supplemental Table 2. Explorative outcomes between patient with non‐ectatic vs. ectatic culprit vessel.
Baldi C, Silverio A, Esposito L, et al. Clinical outcome of patients with ST‐elevation myocardial infarction and angiographic evidence of coronary artery ectasia. Catheter Cardiovasc Interv. 2022;340–347. 10.1002/ccd.29738
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Swaye PS, Fisher LD, Litwin P, et al. Aneurysmal coronary artery disease. Circulation. 1983;67(1):134‐138. [DOI] [PubMed] [Google Scholar]
- 2. Hartnell GG, Parnell BM, Pridie RB. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br Heart J. 1985;54(4):392‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Syed M, Lesch M. Coronary artery aneurysm: a review. Prog Cardiovasc Dis. 1997;40:77‐84. [DOI] [PubMed] [Google Scholar]
- 4. Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976;37(2):217‐222. [DOI] [PubMed] [Google Scholar]
- 5. Antoniadis AP, Chatzizisis YS, Giannoglou GD. Pathogenetic mechanisms of coronary ectasia. Int J Cardiol. 2008;130(3):335‐343. [DOI] [PubMed] [Google Scholar]
- 6. Kruger D, Stierle U, Herrmann G, Simon R, Sheikhzadeh A. Exercise‐induced myocardial ischemia in isolated coronary artery ectasias and aneurysms ("dilated coronopathy"). J Am Coll Cardiol. 1999;34(5):1461‐1470. [DOI] [PubMed] [Google Scholar]
- 7. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13‐20. [DOI] [PubMed] [Google Scholar]
- 8. Stone GW, Peterson MA, Lansky AJ, Dangas G, Mehran R, Leon MB. Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J Am Coll Cardiol. 2002;39(4):591‐597. [DOI] [PubMed] [Google Scholar]
- 9. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. da Costa BR, Gahl B, Jüni P. Tools & techniques‐statistics: propensity score techniques. EuroIntervention. 2014;10(6):761‐767. [DOI] [PubMed] [Google Scholar]
- 11. Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381(15):1411‐1421. [DOI] [PubMed] [Google Scholar]
- 12. TIMI Study Group . The thrombolysis in myocardial infarction (TIMI) trial: phase I findings. N Engl J Med. 1985;312:932‐936. [DOI] [PubMed] [Google Scholar]
- 13. Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879‐888. [DOI] [PubMed] [Google Scholar]
- 14. vant Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle myocardial infarction study group. Circulation. 1998;97(23):2302‐2306. [DOI] [PubMed] [Google Scholar]
- 15. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA. White HD; executive group on behalf of the joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/world heart federation (WHF) task force for the universal definition of myocardial infarction. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231‐2264. [DOI] [PubMed] [Google Scholar]
- 16. Ipek G, Gungor B, Karatas MB, et al. Risk factors and outcomes in patients with ectatic infarct‐related artery who underwent primary percutaneous coronary intervention after ST elevated myocardial infarction. Catheter Cardiovasc Interv. 2016;88(5):748‐753. [DOI] [PubMed] [Google Scholar]
- 17. Erden I, Erden EC, Ozhan H, Karabulut A, Ordu S, Yazici M. Outcome of primary percutaneous intervention in patients with infarct‐related coronary artery ectasia. Angiology. 2010;61(6):574‐579. [DOI] [PubMed] [Google Scholar]
- 18. Demopoulos VP, Olympios CD, Fakiolas CN, et al. The natural history of aneurysmal coronary artery disease. Heart. 1997;78(2):136‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinar Bermúdez E, López Palop R, Lozano Martínez‐Luengas I, et al. Ectasia coronaria: prevalencia, características clínicas y angiográficas [Coronary ectasia: prevalence, and clinical and angiographic characteristics]. Rev Esp Cardiol. 2003;56(5):473‐479. [DOI] [PubMed] [Google Scholar]
- 20. Androulakis AE, Andrikopoulos GK, Kartalis AN, et al. Relation of coronary artery ectasia to diabetes mellitus. Am J Cardiol. 2004;93(9):1165‐1167. [DOI] [PubMed] [Google Scholar]
- 21. Zografos TA, Korovesis S, Giazitzoglou E, et al. Clinical and angiographic characteristics of patients with coronary artery ectasia. Int J Cardiol. 2013;167(4):1536‐1541. [DOI] [PubMed] [Google Scholar]
- 22. Gunasekaran P, Stanojevic D, Drees T, et al. Prognostic significance, angiographic characteristics and impact of antithrombotic and anticoagulant therapy on outcomes in high versus low grade coronary artery ectasia: a long‐term follow‐up study. Catheter Cardiovasc Interv. 2019;93(7):1219‐1227. [DOI] [PubMed] [Google Scholar]
- 23. Campanile A, Sozzi FB, Consonni D, et al. Primary PCI for the treatment of ectatic infarct‐related coronary artery. Minerva Cardioangiol. 2014;62(4):327‐333. [PubMed] [Google Scholar]
- 24. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119‐177. [DOI] [PubMed] [Google Scholar]
- 25. Henriques JP, Zijlstra F, vant Hof AW, et al. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation. 2003;107(16):2115‐2119. [DOI] [PubMed] [Google Scholar]
- 26. Fujii T, Sakai K, Kimura M, et al. Coronary flow improvement following unsuccessful primary percutaneous coronary intervention in ST‐elevation myocardial infarction with diffuse ectatic coronary artery. Eur Heart J Acute Cardiovasc Care. 2017;6(7):623‐631. [DOI] [PubMed] [Google Scholar]
- 27. Doi T, Kataoka Y, Noguchi T, et al. Coronary artery ectasia predicts future cardiac events in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2017;37(12):2350‐2355. [DOI] [PubMed] [Google Scholar]
- 28. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213‐260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Kaplan–Meier survival free from recurrent MI in patients with CAE according to the Markis Class.
Supplemental Figure 2 Kaplan–Meier survival free from all‐cause death in patients with CAE according to the Markis Class.
Supplemental Figure 3 Kaplan–Meier survival free from recurrent MI in CAE patients with versus those without EIRA.
EIRA, ectatic infarct‐related artery.
Supplemental Figure 4 Kaplan–Meier survival free from recurrent MI in CAE patients with versus those without EIRA.
EIRA, ectatic infarct‐related artery.
Supplemental Table 1 Explorative outcomes between no CAE versus CAE patients.
Supplemental Table 2. Explorative outcomes between patient with non‐ectatic vs. ectatic culprit vessel.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


