Abstract
Background
The natural history of peridevice leak (PDL) following left atrial appendage occlusion (LAAO) is unknown. This study sought to investigate changes of PDL from 2 until 12 months after LAAO, using cardiac computed tomography (CT), and to assess the potential association between persistent PDL and clinical outcomes
Methods
Single‐center observational study of Amplatzer LAAO implants between 2010 and 2017 (n = 206). Patients with 2 and 12 months cardiac CT were included in the study (n = 153). Images were blindly analyzed. PDL was characterized by frequency and size at the device disc, lobe, and left atrial appendage contrast patency. Patients were followed for the composite outcome of ischemic stroke, transient ischemic attack, systemic embolism, or all‐cause death. Median follow up from LAAO was 3.1 (2.3–4.3) years.
Results
Contrast patency was present in 101 (66%) and 72 (47%) (p < 0.001) at 2 and 12 months, respectively. PDL was identified at the disc in 103 (67%) patients at 2 months versus 93 (61%) at 12 months (p = 0.08), and at the lobe in 29 (19%) at both time points. PDL area at the disc did not change significantly over time, area: −8.95 mm (95% confidence interval [CI]: −18.9; 1.01) p = 0.08. Permanent atrial fibrillation was independently associated with persistent PDL. Persistent versus no PDL was associated with a 62% worse clinical outcome, however not statistically significant, hazard ratio (HR): 1.62 (95% CI: 0.9–2.93), p = 0.11.
Conclusion
Persistent PDL was frequently observed following LAAO with Amplatzer devices. The PDL frequency and size appeared unchanged between 2 and 12 months. Persistent PDL was not significantly associated with worse clinical outcomes, yet this needs further delineation in future studies.
Keywords: CLLA–closure, left atrial appendage, SHDI–structural heart disease intervention, STR–stroke, TTE/TEE
Abbreviations
- AF
atrial fibrillation
- CT
computed tomography
- DRT
device‐related thrombosis
- LAA
left atrial appendage
- LAAO
left atrial appendage occlusion
- PDL
peridevice leak
- TEE
transesophageal echocardiography
- TIA
transient ischemic attack
1. INTRODUCTION
An increasing number of transcatheter left atrial appendage occlusion (LAAO) procedures are performed to mitigate the stroke and bleeding risk in selected high‐risk patients with atrial fibrillation (AF). 1 The efficacy and safety have been demonstrated in both randomized trials 2 , 3 and real‐world registries. 4 , 5 Currently, various clinical trials are ongoing to investigate efficacy and safety as a direct alternative to oral anticoagulation in broader populations of AF patients. However, many unresolved issues remain. For instance, the temporal change in frequency and clinical implications of residual peridevice leak (PDL) are uncertain.
The natural history of PDL is still debated. Clinical studies indicate both a decrease or increase in the frequency of PDL over time. 6 TEE has been used as the primary imaging modality in prior studies assessing PDL. However, cardiac computed tomography (CT) is increasingly used for follow‐up after LAAO and data suggest that CT is more sensitive and accurate than TEE in diagnosing and categorizing PDL. 7 , 8 Limited data on cardiac CT delineation of PDL overtime is available. Adequately powered outcome studies are currently missing, yet, smaller studies have not reported a significant association between PDL and the risk of thromboembolic adverse events. 7 , 9 , 10 Therefore, the long‐term progression and clinical management of PDL is inadequately defined. The purpose of this study was threefold: (1) To investigate the temporal changes of PDL determined by cardiac CT at 2 and 12 months after LAAO with the Amplatzer devices. (2) To identify predictors of persistent PDL, and (3) To assess the potential clinical implications of persistent PDL.
2. METHODS
2.1. Study design and population
This was a single‐center observational cohort study of consecutive patients undergoing LAAO with the Amplatzer Cardiac Plug or Amulet (Abbott) at Aarhus University Hospital, Denmark. The clinical setup has been described in detail. 7 , 11 , 12 , 13 Briefly, by institutional practice all patients are scheduled for 2 months post‐LAAO follow‐up including both transesophageal echocardiography (TEE) and cardiac CT. Patients undergoing LAAO between 2010 and January 2017 (n = 206) were also scheduled to undergo repeated TEE and cardiac CT at 12‐months follow‐up. Cardiac CT was not performed in patients with a glomerular filtration rate <30 ml/min. Patients undergoing successful LAAO with both 2 and 12 months cardiac CT during this period formed the basis of this study (n = 153).
2.2. LAAO procedure
All patients underwent preprocedural cardiac CT for exclusion of LAA thrombus, and for device sizing and procedural planning. 14 , 15 Procedures were performed either in general anesthesia with TEE guidance or local anesthesia with intracardiac echocardiography (ICE) from the left atrium. 12 Ultrasound‐guided double femoral vein access was obtained with a medial 9F Terumo sheath for the ICE catheter, and a lateral 6F (and later 12/14F) sheath for the delivery system. Transseptal puncture was performed inferoposteriorly, with a single‐transseptal hole for both ICE catheter and delivery sheath. Once the delivery sheath was positioned in the LAA, a selective contrast angiogram was obtained to confirm anatomy and sizing. The device was deployed under echocardiographic and fluoroscopic guidance, and device position and sealing were confirmed by color Doppler and contrast angiogram. The femoral access was closed using a figure‐8‐suture. Patients were discharged on low‐dose aspirin monotherapy after a transthoracic echocardiogram confirming the stability of the device and absent pericardial effusion.
2.3. Imaging protocol
Follow‐up cardiac CT images were acquired using a Siemens Somatom Definition Flash or Force scanner (Siemens Healthcare). The CT acquisition protocol has previously been described. 7 , 14 , 15 Briefly, a prospective electrocardiogram‐gated high‐pitch single‐heart beat spiral acquisition (Flash) was used with automated tube current modulation (CareDose 4D) with tube voltage set between 70 and 140 kV. The scans aimed for a diastolic phase in heart rates below 70 beats/min, and a systolic phase in heart rates above 70 beats/min. A single‐contrast injection (350 mg I/ml iodine concentration) of 40–60 ml was administered through an antecubital vein. Images were reconstructed using a 0.75 mm slice thickness and a medium soft Bv40 kernel. All images were analyzed using the syngo.via platform (Siemens Healthcare). The algorithm to detect and characterize PDL has been described 7 and is illustrated in Figure 1. In brief, the analysis strategy is based on contrast gaps adjacent to the device at the level of the disc, and the proximal, mid, and distal cross‐sectional views of the device lobe. The absence of contrast patency was determined based on Hounsfield attenuation <100 in the distal LAA, or LAA/left atrium Hounsfield ratio <0.25. 16 PDL severity was categorized into three grades 7 (Figure 1). All images were analyzed in a blinded fashion.
Figure 1.
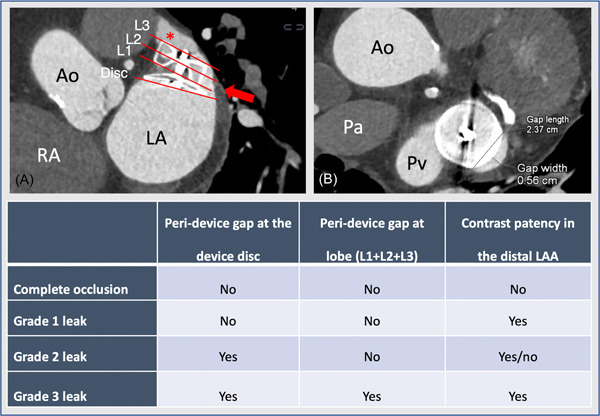
Peridevice leak sizing and categorization by cardiac computed tomography. Illustration of the assessment of PDL by cardiac CT. (A) Illustrates the assessment of peridevice leak at the disc (Disc), proximal (L1), mid (L2), and distal (L3) cross‐sectional view of the device. (B) A cross‐sectional view of the device, with measurement of peridevice leak width and length. The table illustrates the categorization of PDL severity. Ao, ascending aorta; CT, computed tomography; LA, left atrium; Pa, pulmonary artery; PDL, peridevice leak; Pv, left upper pulmonary vein; RA, right atrium [Color figure can be viewed at wileyonlinelibrary.com]
2.4. Clinical outcomes and follow‐up
Outcomes were assessed by follow‐up through the Danish National Patient Registry and the Civil Registration System. The Danish administrative and health registries contain patient‐level data on all hospital admissions and outpatient visits, and vital status linked to each citizen through a unique social security number assigned at birth.
The clinical outcome was a composite of ischemic stroke, systemic embolism, transient ischemic attack (TIA), or all‐cause mortality. Clinical outcomes were defined according to discharge diagnoses in the Danish National Health Registry using the International Classification of Diseases, 10th edition (ICD‐10) for ischemic stroke (I63, I64), TIA (G458, G459), or systemic embolism (I74). Mortality was captured from the Danish Civil Registration System.
Patients were considered at risk from the time of 12‐month cardiac CT with censoring at time of the first event or at end of follow‐up 4‐years after 12‐months cardiac CT. Median (interquartile range [IQR]) follow‐up was 2.1 (1.3–3.3) years after the second cardiac CT.
2.5. Statistical analyses
The distribution of data was assessed by QQ‐plot and histograms. Continuous variables were expressed as mean with standard deviation (SD), or median with IQR and were compared using the Student t test or Mann–Whitney U test for unpaired data, and paired t test or Wilcoxon signed‐rank test for paired data. Categorical variables were expressed as counts and percentages, and groups were compared using the Fisher's exact test or McNemar test as appropriate.
A multivariable logistic model was used to identify predictors of persistent PDL. Variables identified following univariable logistic regression of p < 0.15 was included in the final multivariable logistic regression model. Clinical outcome analyses were based on time‐to‐event analysis using the Kaplan–Meier estimator with a Cox proportional regression model for hazard ratios (HRs). Follow‐up started at the time of 12‐month cardiac CT with administrative censoring 4 years after the cardiac CT. A multivariable Cox regression model included adjustment for age, permanent AF, chronic kidney disease, CHA2DS2‐VASc score, and device size. A two‐tailed p < 0.05 was considered statistically significant. Statistical analyses were performed using STATA (STATA IC, version 17, StataCorp).
3. RESULTS
A total of 153 patients had both 2 and 12 months cardiac CT available for analysis and thus were included in this study (Figure 2). Baseline characteristics are summarized in Table 1. LAAO was technically successful in all patients, with an Amplatzer Amulet device implanted in 110 (72%) patients and Amplatzer Cardiac Plug in 43 (28%). Procedures were performed with local anesthesia and ICE in 93 (61%) cases (Table 2). Baseline characteristics and outcomes for excluded patients are summarized in Supporting Information: Tables S1 and S2.
Figure 2.
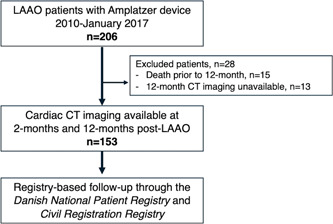
Flow chart of patients
Table 1.
Baseline characteristics
| Total cohort, n = 153 | ||
|---|---|---|
| Age (years) |
|
|
| Female gender | 50 (33) | |
| Body mass index (kg/m2) | 26.9 4.2 | |
| Permanent atrial fibrillation | 69 (45) | |
| Congestive heart failure | 27 (18) | |
| LVEF (%) | 60 (50–60) | |
| Hypertension | 125 (82) | |
| Diabetes mellitus | 26 (17) | |
| Previous stroke or TIA | 69 (45) | |
| Peripheral vascular disease | 65 (42) | |
| History of bleeding | 127 (83) | |
| Chronic kidney disease, Stage 3–5 | 11 (7) | |
| Creatinine (µmol/L) | 85 (72–103) | |
| CHA2DS2‐VASc score | 4.1 1.6 | |
| HAS‐BLED score | 3.8 1.0 | |
| Primary indication for LAAO | ||
| ICH | 63 (41) | |
| GI bleeding | 31 (20) | |
| Urogenital bleeding | 12 (7) | |
| Other spontaneous bleeding | 15 (10) | |
| Cerebral amyloid angiopathy | 10 (6) | |
| Stroke despite OAC | 9 (8) | |
| High bleeding risk | 13 (8) |
Note: Data are presented as mean ± SD, median (IQR), or frequency (%).
Abbreviations: GI, gastrointestinal; HAS‐BLEDICH, intracranial hemorrhage; LAAO, left atrial appendage occlusion; LVEF, left ventricular ejection fraction; OAC, oral anticoagulation; TIA, transient ischemic attack.
Table 2.
Procedural data
| Total cohort, n = 153 | |
|---|---|
| LAA morphology | |
| Chicken wing | 66 (43) |
| Cactus | 45 (30) |
| Windsock | 38 (24) |
| Cauliflower | 4 (3) |
| LAA orifice diameter (mm) | 30.6 5.7 |
| LAA landing zone diameter (mm) | 22.3 4.2 |
| LAA depth (mm) | 19.5 |
| Technical success | 153 (100) |
| Implanted device | |
| Amplatzer cardiac plug | 43 (28) |
| Amplatzer amulet | 110 (72) |
| Mean device size (mm) | 24.3 4.2 |
| Procedural guidance | |
| Transesophageal echo | 60 (39) |
| Intracardiac echo | 93 (61) |
| Procedure time (min) | 47 (36–62) |
| Device repositioning required | 21 (12) |
Data are presented as mean ± SD, median (IQR), or frequency (%).
Abbreviations: IQR, interquartile range; LAA, left atrial appendage.
3.1. Cardiac CT acquisition
At the time of CT acquisition, 96 (63%) and 98 (64%) patients were in AF at 2 and 12 months, respectively, p = 0.61. The median (IQR) heart rate was 71 (64–83) and 71 (61–85) beats per minutes, p = 0.40. Mean (SD) contrast amount used was 65 (9.6) and 62 (7.3) ml at 2 and 12 months, p = 0.002. A left ventricular diastolic phase was acquired in 111 (72%) at 2 months, and 99 (65%) at 12 months, p = 0.18. The scans were acquired using a mean (SD) voltage of 107 (15.4) and 103 (16.2) kV at 2 and 2 months, p < 0.001. Mean (SD) milliampere‐seconds were 374 (116) and 418 (122) mAs at 2 and 12 months scan acquisition, p < 0.001. The median (IQR, range) radiation exposure associated with the 2 and 12 months acquisition was 1.7 (1.1–2.1, 0.5–11.6) and 1.5 (1.1–2.0, 0.5–13.1) mSv, respectively, p = 0.24.
3.2. PDL change over time
The first and second clinical follow‐up cardiac CT scans were performed at a median (IQR) of 55 (48–63) and 365 (359–380) days after LAAO. Contrast patency was more frequent at the 2 versus 12 months follow‐up, 101 (66%) versus 72 (47%) (p < 0.001). PDL at the disc was detected in 103 (67%) patients at 2 months and 93 (61%) patients at 12 months (p = 0.08), while PDL at the device lobe was present in 29 (19%) at both follow‐up times The PDL grading at 2 and 12 months is summarized in Table 3. The PDL grade was downgraded to a less severe grade in 32 (21%) patients, unchanged in 108 (70%), and progressed to a more severe PDL grade in 13 (8%) (Figure 3). Late signs of PDL developed in 3 (2%) patients having complete occlusion at 2 months, while 6 (4%) patients with only contrast patency (Grade 1 leak) at 2 months demonstrated signs of PDL at the disc and/or lobe at 12 months follow‐up (Table 3). Mean (SD, 95% CI) change in PDL grade over time was −0.25 (SD 0.9, 95% CI: −0.40; −0.11), p < 0.001.
Table 3.
Peridevice leak grading at 2 and 12 months
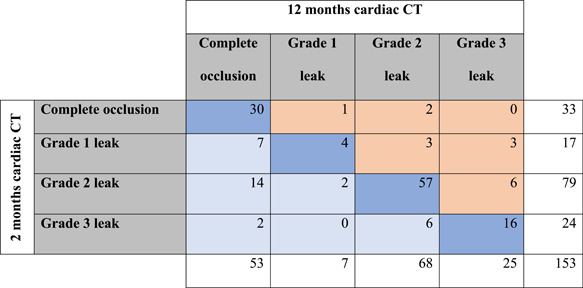
|
Note: Peridevice leak severity classification at 2 and 12 months follow‐up. Light blue indicates down‐graded leak severity at 12 months compared to 2 months, dark blue indicates the severity is unchanged and light orange indicates upgrading of peridevice leak severity at 12 months.
Figure 3.
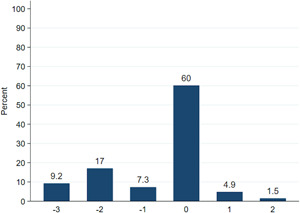
Change in peridevice leak grade from 2 to 12 months cardiac computed tomography (CT). The x axis denotes the absolute change in peridevice leak grade from 2 to 12 months CT scan. A negative number indicates a less severe grading at 12 months, while a positive number indicates a more severe grading at 12 months scan [Color figure can be viewed at wileyonlinelibrary.com]
The median PDL area at the disc was 58mm2 (29–104) at 2 months and 60 mm2 (36–111) at 12 months ( area: −8.95 mm [95% CI: −18.9; 1.01] p = 0.08). The mean (SD) PDL width at the disc was 3.2 mm at 2 months, and 3.2 .3 mm at 12 months ( width: −0.03 mm [95% CI: −0.35; 0.28] p = 0.84). The PDL length was 12.2 ± 10.5 mm at 2 months and 11.5 10.8 mm at 12 months ( length: −0.75 mm [95% CI: −1.82; 0.32], p = 0.17).
Hounsfield unit LAA/Left atrium ratio was higher at 2 versus 12 months, median (IQR) 0.56 (0.19–0.83) versus 0.28 (0.14–0.71) at 12 months, p < 0.001.
All 153 patients had 2 months TEE performed, with 139 (91%) patients undergoing repeated TEE at 12 months follow‐up. By TEE, PDL was present in 58 (38%) at 2 months and in 43 (38%) at 12 months (p = 0.37). Mean (SD) PDL size (Color Doppler width) was 2.9 ± 1.7 versus 2.4 ± 1.4 mm at 2 and 12 months, respectively (p = 0.07).
3.3. Predictors of persistent PDL
Patients with persistent PDL (CT Grade 2–3) at 12 months follow‐up were more likely to have permanent AF, higher CHA2DS2‐VASc score, larger LAA diameters, and received a larger device implant (Table 4). In a multivariable analysis, only permanent AF was significantly associated with persistent PDL at 12 months of cardiac CT (Table 5).
Table 4.
Predictors of persistent peridevice leak at 12 months
| No PDL (N = 60) | New or persistent | p value | |||
|---|---|---|---|---|---|
| Grade 2–3 PDL (N = 93) | |||||
| Age, years |
|
|
0.11 | ||
| Female gender | 13 (25) | 37 (37) | 0.15 | ||
| Body mass index, kg/m2 | 26.9 | 26.9 4.5 | 0.91 | ||
| Permanent atrial fibrillation | 14 (26) | 55 (55) | <0.01 | ||
| Congestive heart failure | 8 (15) | 19 (19) | 0.66 | ||
| LVEF, % | 60 (55–60) | 60 (50–60) | 0.35 | ||
| Hypertension | 41 (77) | 84 (84) | 0.38 | ||
| Diabetes mellitus | 9 (17) | 17 (17) | 0.99 | ||
| Previous stroke or TIA | 23 (43) | 46 (46) | 0.87 | ||
| History of bleeding | 41 (77) | 86 (86) | 0.18 | ||
| Chronic kidney disease, Stage 3–5 | 8 (15) | 3 (3) | 0.02 | ||
| CHA2DS2‐VASc score | 3.8 1.4 | 4.3 1.6 | 0.07 | ||
| HAS‐BLED score | 3.8 1.1 | 3.9 0.8 | 0.48 | ||
| Amplatzer Amulet implant | 39 (74) | 71 (71) | 0.85 | ||
| Implanted device size, mm | 23.1 ± 3.9 | 25 ± 4.2 | 0.01 | ||
| LAA orifice diameter, mm | 29.1 ± 6.2 | 31.5 ± 5.3 | 0.01 | ||
| LAA landing zone diameter, mm | 20.9 ± 3.8 | 23.1 ± 4.2 | <0.01 | ||
| Time from LAAO to 12‐month CT, days | 366 (360–385) | 364 (358–379) | 0.22 |
Note: Data are presented as mean ± SD, median (IQR), or frequency (%).
Abbreviations: CT, computed tomography; LAA, left atrial appendage; LAAO, left atrial appendage occlusion; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack.
Table 5.
Multivariable analysis for predictors of persistent peridevice leak at 12 months
| Risk ratio | p value | |
|---|---|---|
| Age 75 years | 1.23 (1.00–1.50) | 0.05 |
| Permanent atrial fibrillation | 1.26 (1.01–1.60) | 0.04 |
| Device size > 25 mm | 1.16 (0.97–1.40) | 0.11 |
| Orifice diameter (per 1‐mm increase) | 1.02 (0.99–1.03) | 0.06 |
| Chronic kidney disease | 0.45 (0.17–1.18) | 0.10 |
Note: Multivariable logistic regression model illustrating predictors of persistent peridevice leak at 12 months follow‐up CT.
3.4. Clinical outcomes
Median follow‐up after the 12 months cardiac CT was 2.1 years (IQR: 1.3–3.3). The composite outcome occurred in 52 (34%) patients between 12 months visit and the last‐known follow up. A 63% higher risk of the combined outcome in the PDL versus no PDL group was observed but did not reach statistical significance, HR 1.63 (95% CI: 0.90–2.93), p = 0.11 (Figure 4). The adjusted HR for the occurrence of the clinical composite outcome was 1.65 (95% CI: 0.86–3.17), p = 0.13. Individual outcomes are listed in Table 6. Persistent PDL as classified by TEE imaging did not show significant association to the composite outcome, HR 1.11 (95% CI: 0.61–2.00) (Supporting Information: Figure S1).
Figure 4.
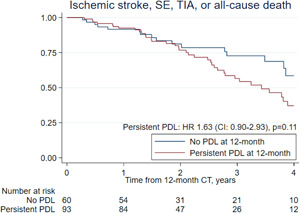
Kaplan–Meier analysis for clinical outcome of persistent peridevice leak. CI, 95% confidence interval; HR, hazard ratio; PDL, peridevice leak; SE, systemic embolism; TIA, transient ischemic attack [Color figure can be viewed at wileyonlinelibrary.com]
Table 6.
Clinical outcomes stratified by presence of persistent peridevice leak at 12 months
| No PDL or Grade 1 PDL | New or persistent Grade 2–3 PDL | |||
|---|---|---|---|---|
| n = 60 | n = 93 | |||
| Events, n (%) | Events per 100 patient‐years | Events, n (%) | Events per 100 patient‐ years | |
| Composite outcome | 16 (27) | 11.2 (6.9–18.4) | 36 (39) | 17.3 (12.4–23.9) |
| Ischemic stroke | 4 (6.6) | 2.9 (1.1–7.9) | 11 (12) | 5.3 (2.9–9.5) |
| Transient ischemic attack | 3 (5) | 2.1 (0.6–6.5) | 0 | 0 |
| All‐cause death | 9 (15) | 7.1 (3.7–13.7) | 25 (27) | 13.3 (8.9–19.6) |
Note: Event rates are presented at events per 100 patients‐years (95% confidence interval).
Abbreviations: CI, 95% confidence interval; HR, hazard ratio; PDL, peridevice leak; SE, systemic embolism; TIA, transient ischemic attack.
4. DISCUSSION
This study demonstrates that PDLs are persistent at 12 months in most patients already demonstrating signs of PDL early after LAAO. The PDL size did not significantly decrease over time, while progression or newly detected PDL was a rare event. Patients with persistent PDL were more likely to have permanent AF. Finally, we observed a 63% increased risk of the composite endpoint at 4 years follow‐up in patients with persistent PDL at 12 month imaging follow‐up, although this difference did not reach statistical significance.
Reports from the PROTECT‐AF trial, utilizing the Watchman device, indicated a reduction in PDL frequency from 41% to 32% between the post‐LAAO 45 days and 12 months TEE. The size of the PDL appeared unchanged over time. 9 A small study reporting data from 30 patients undergoing repeated 3 and 12 months cardiac CT after Amplatzer or Watchman implantations, documented a significant reduction in contrast patency. 17 However, the study did not report the size or presence of PDL at the disc or lobe. Consistent with these data, we found a significant reduction in the frequency of contrast patency over time. On the other hand, Cochet et al. reported that contrast patency varies according to which phase of the cardiac cycle the CT scan is acquired. 18 In our study, we did not see a reduction in the frequency of PDL at the disc, nor a reduction in the PDL size from 2 till 12 months. These findings were consistent with both cardiac CT and TEE imaging. A study performing TEE at a median (range) of 3.1 (1–7.5) years after LAAO reported a decrease in PDL frequency from 63% at 6 weeks to 46% at the end of follow‐up. 19 Although some data indicate a small reduction in PDL over time, studies consistently report that nearly half the patients have persistent PDL years after LAAO.
A few patients in our cohort had documented progression or new PDL at 12 months. Similar findings were observed in a study utilizing TEE follow‐up. 19 It should be acknowledged that this finding, in our cohort and prior cohorts, 19 represents very few patients, and it cannot be excluded that such findings may represent limitations or measurement uncertainty of the applied imaging modalities rather than atrial remodeling. For instance, the applied imaging CT acquisition protocol utilizing a high‐pitch single‐heart beat acquisition could affect the presence of contrast patency, 18 or capture a cardiac phase where a PDL is not fully demasked as a consequence of the atrial contractility. 20 However, in the present data set we could not document any obvious association between these findings and CT acquisition variances. Although a small, nonsignificant difference was observed in the cardiac phase obtained at 2 and 12 months. Factors that could affect PDL detection by TEE are likely operator dependent, for instance, Nyquist limit settings or the use of two‐dimensional or three‐dimensional imaging. 20 Whether atrial remodeling causes progression or new PDL requires further investigations.
Studies have interpreted contrast patency without signs of PDL as indicative of device permeability due to incomplete endothelialization. 8 , 21 , 22 Similarly, complete occlusion on cardiac CT might be indicative of neo‐endothelialization of the atrial device surface. 8 , 21 , 22 Based on these assumptions, a significant proportion of patients could still have incomplete device endothelialization 12 months post‐LAAO. It should be acknowledged that the Hounsfield unit thresholds for contrast patency and the association with neo‐endothelialization have not been confirmed by autopsy or surgical studies. However, case reports with confirmation of incomplete device‐endothelialization during cardiac surgery one to two years after LAAO seems to support the assumptions from CT studies. 23 Currently, most knowledge of device endothelialization stems from animal studies indicating that complete endothelialization is achieved during the initial 3 months from implant. 24 , 25 These findings have been extrapolated into clinical practice, but the current data seems to challenge our understanding of the device endothelialization process.
Larger LAA dimensions, larger implanted LAAO devices, chronic kidney disease, and permanent AF were more common among patients with persistent PDL at 12 months. Only permanent AF remained significantly associated to persistent PDL in the multivariable model. The causal mechanisms behind this association remain unknown, nevertheless, similar associations have been reported by a recently presented study. 26 Additionally, nonparoxysmal AF have been associated with device‐related thrombosis (DRT). 27
Implications of delayed endothelialization and PDL remain uncertain. 7 , 9 , 10 A recent multicenter study reported a significantly higher risk of stroke or TIA in patients with PDL compared to those without on 45 days post‐LAAO TEE. Our present analysis indicates that patients with persistent PDL 12 months after LAAO have an increased risk of thromboembolism or death, although not reaching statistical significance. This interpretation is supported by a recently presented study reporting a significant association between persistent PDL and higher risk of thromboembolism in the PROTECT‐AF and PREVAIL cohorts. 26 Adding to the potential adverse effects of PDL, up to 30% of DRT are diagnosed later than 6 months from LAAO implant 27 , 28 and PDL appears to be more common among patients with DRT. 27 Whether post‐LAAO signs of contrast patency and PDL should warrant prolonged antiplatelet/anticoagulation therapy or attempts of percutaneous closure is unclear, but the present study clearly indicates they do not spontaneously resolve. Future studies aiming to further delineate the association between PDL delayed device endothelialization, and the risk of DRT and thromboembolic events are warranted to guide the management of PDL.
4.1. Limitations
This was a single‐center observational cohort study with inherent limitations such as potential selection bias and lack of generalizability to other practice settings using other LAAO platforms. The study is further limited by restricted sample size. The clinical outcomes were evaluated based on registry follow up without adjudication of events, however, the Danish registries contain highly validated and reliable outcome data. 29 , 30 , 31
5. CONCLUSION
The present study shows that most patients having PDL at early imaging follow‐up after LAAO with the Amplatzer devices, demonstrate persistent PDL at 12 months follow‐up. The PDL size was unchanged from 2 till 12 months. Persistent PDL was associated with worse clinical outcomes, however, not statistically significant. The study data questions the need for repeated imaging after LAAO, however, the clinical consequence of PDL requires confirmation in larger studies.
CONFLICTS OF INTEREST
Dr. Korsholm has received speaker's honorarium from Abbott and Boston Scientific, and a travel grant from Boston Scientific. Dr. Nielsen‐Kudsk is a consultant/proctor for Abbott and Boston Scientific. The remaining authors have nothing to declare.
Supporting information
Supplementary information.
Supplementary information.
Supplementary information.
Korsholm K, Jensen JM, Nørgaard BL, Nielsen‐Kudsk JE. Temporal changes and clinical significance of peridevice leak following left atrial appendage occlusion with Amplatzer devices. Catheter Cardiovasc Interv. 2022;99:2071‐2079. 10.1002/ccd.30178
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study will be available from the corresponding author upon reasonable request.
REFERENCES
- 1. Freeman JV, Varosy P, Price MJ, et al. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol. 2020;75(13):1503‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osmancik P, Herman D, Neuzil P, et al. Left atrial appendage closure versus direct oral anticoagulants in high‐risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75(25):3122‐3135. [DOI] [PubMed] [Google Scholar]
- 3. Reddy VY, Doshi SK, Kar S, et al. 5‐year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70(24):735‐1097. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen‐Kudsk JE, Korsholm K, Damgaard D, et al. Clinical outcomes associated with left atrial appendage occlusion versus direct oral anticoagulation in atrial fibrillation. JACC Cardiovasc Interv. 2021;14(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 5. Boersma LV, Ince H, Kische S, et al. Evaluating real‐world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology: final 2‐year outcome data of the EWOLUTION Trial focusing on history of stroke and hemorrhage. Circ Arrhythm Electrophysiol. 2019;12(4):e006841. [DOI] [PubMed] [Google Scholar]
- 6. Raphael CE, Friedman PA, Saw J, Pislaru SV, Munger TM, Holmes DR. Residual leaks following percutaneous left atrial appendage occlusion: assessment and management implications. EuroIntervention. 2017;13(10):1218‐1225. [DOI] [PubMed] [Google Scholar]
- 7. Korsholm K, Jensen JM, Nørgaard BL, et al. Peridevice leak following Amplatzer left atrial appendage occlusion: cardiac computed tomography classification and clinical outcomes. JACC Cardiovasc Interv. 2021;14(1):83‐93. [DOI] [PubMed] [Google Scholar]
- 8. Qamar SR, Jalal S, Nicolaou S, Tsang M, Gilhofer T, Saw J. Comparison of cardiac computerized tomography angiography and trans‐esophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention. 2019;15:663‐670. [DOI] [PubMed] [Google Scholar]
- 9. Viles‐Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;Mar 6 59(10):923‐929. [DOI] [PubMed] [Google Scholar]
- 10. Saw J, Tzikas A, Shakir S, et al. Incidence and clinical impact of device‐associated thrombus and peri‐device leak following left atrial appendage closure with the Amplatzer cardiac plug. JACC Cardiovasc Interv. 2017;10(4):391‐399. [DOI] [PubMed] [Google Scholar]
- 11. Korsholm K, Jensen JM, Nørgaard BL, Nielsen‐Kudsk JE. Detection of device‐related thrombosis following left atrial appendage occlusion: a comparison between cardiac computed tomography and transesophageal echocardiography. Circ Cardiovasc Interv. 2019;12(9):e008112. [DOI] [PubMed] [Google Scholar]
- 12. Korsholm K, Jensen JM, Nielsen‐Kudsk JE. Intracardiac echocardiography from the left atrium for procedural guidance of transcatheter left atrial appendage occlusion. JACC Cardiovasc Interv. 2017;10(21):2198‐2206. [DOI] [PubMed] [Google Scholar]
- 13. Korsholm K, Nielsen KM, Jensen JM, Jensen HK, Andersen G, Nielsen‐Kudsk JE. Transcatheter left atrial appendage occlusion in patients with atrial fibrillation and a high bleeding risk using aspirin alone for post‐implant antithrombotic therapy. EuroIntervention. 2016;12(17):2075‐2082. [DOI] [PubMed] [Google Scholar]
- 14. Korsholm K, Jensen JM, Nielsen‐Kudsk JE. Cardiac computed tomography for left atrial appendage occlusion: acquisition, analysis, advantages, and limitations. Interv Cardiol Clin. 2018;7(2):229‐242. [DOI] [PubMed] [Google Scholar]
- 15. Korsholm K, Berti S, Iriart X, et al. Expert recommendations on cardiac computed tomography for planning transcatheter left atrial appendage occlusion. JACC Cardiovasc Interv. 2019;13(3):277‐292.31678086 [Google Scholar]
- 16. Saw J, Lopes JP, Reisman M, McLaughlin P, Nicolau S, Bezerra HG. Cardiac computed tomography angiography for left atrial appendage closure. Can J Cardiol. 2016;32(8):1033.e1‐1033.e9. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen A, Gallet R, Riant E, et al. Peridevice leak after left atrial appendage closure: incidence, risk factors, and clinical impact. Can J Cardiol. 2019;35(4):405‐412. [DOI] [PubMed] [Google Scholar]
- 18. Cochet H, Iriart X, Sridi S, et al. Left atrial appendage patency and device‐related thrombus after percutaneous left atrial appendage occlusion: a computed tomography study. Eur Heart J Cardiovasc Imaging. 2018;19(12):1351‐1361. [DOI] [PubMed] [Google Scholar]
- 19. Staubach S, Schlatterbeck L, Mörtl M, et al. Long‐term transesophageal echocardiography follow‐up after percutaneous left atrial appendage closure. Heart Rhythm. 2020;17(5 Pt A):728‐733. [DOI] [PubMed] [Google Scholar]
- 20. Korsholm K, Jensen JM, Nørgaard BL, Saw J, Nielsen‐Kudsk JE. Reply: alternative hypothesis for the discrepancy in peridevice leak detection. JACC Cardiovasc Interv. 2021;14(8):925‐926. [DOI] [PubMed] [Google Scholar]
- 21. Behnes M, Akin I, Sartorius B, et al. ‐LAA Occluder View for post‐implantation evaluation (LOVE)‐standardized imaging proposal evaluating implanted left atrial appendage occlusion devices by cardiac computed tomography. BMC Med Imaging. 2016;16(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saw J, Fahmy P, DeJong P, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging. 2015;16:1198‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma SP, Singh D, Nakamura D, Gopinathannair R, Lakkireddy D. Incomplete endothelialization of WatchmanTM Device: predictors and implications from two cases. J Atr Fibrillation. 2019;11(5):2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwartz RS, Holmes DR, Van Tassel RA, et al. Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv. 2010;3(8):870‐877. [DOI] [PubMed] [Google Scholar]
- 25. Kar S, Hou D, Jones R, et al. Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc Interv. 2014;7(7):801‐809. [DOI] [PubMed] [Google Scholar]
- 26. Reddy V, Holmes DR, Doshi SK, et al. ABSTRACT 16212: peri‐device leak after left atrial appendage closure: impact on long‐term clinical outcomes. Circulation. 2021;144(25):e564‐e593.34928705 [Google Scholar]
- 27. Simard T, Jung RG, Lehenbauer K, et al. Predictors of device‐related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol. 2021;78(4):297‐313. [DOI] [PubMed] [Google Scholar]
- 28. Sedaghat A, Vij V, Al‐Kassou B, et al. Device‐related thrombus after left atrial appendage closure: data on thrombus characteristics, treatment strategies, and clinical outcomes from the EUROC‐DRT‐registry. Circ Cardiovasc Interv. 2021;14(5):e010195. [DOI] [PubMed] [Google Scholar]
- 29. Wildenschild C, Mehnert F, Thomsen RW, et al. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2014;6:27‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vest‐Hansen B, Riis AH, Christiansen CF. Registration of acute medical hospital admissions in the Danish National Patient Registry: a validation study. Clin Epidemiol. 2013;5(1):129‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary information.
Supplementary information.
Data Availability Statement
The data that supports the findings of this study will be available from the corresponding author upon reasonable request.


