Abstract
Androgen excess in women typically presents clinically with hirsutism, acne or androgenic alopecia. In the vast majority of cases, the underlying aetiology is polycystic ovary syndrome (PCOS), a common chronic condition that affects up to 10% of all women. Identification of women with non‐PCOS pathology within large cohorts of patients presenting with androgen excess represents a diagnostic challenge for the endocrinologist, and rare pathology including nonclassic congenital adrenal hyperplasia, severe insulin resistance syndromes, Cushing's disease or androgen‐secreting tumours of the ovary or adrenal gland may be missed in the absence of a pragmatic screening approach. Detailed clinical history, physical examination and biochemical phenotyping are critical in risk‐stratifying women who are at the highest risk of non‐PCOS disorders. Red flag features such as rapid onset symptoms, overt virilization, postmenopausal onset or severe biochemical disturbances should prompt investigations for underlying neoplastic pathology, including dynamic testing and imaging where appropriate. This review will outline a proposed diagnostic approach to androgen excess in women, including an introduction to androgen metabolism and provision of a suggested algorithmic strategy to identify non‐PCOS pathology according to clinical and biochemical phenotype.
Keywords: adrenocortical carcinoma, androgen excess, androstenedione, ovarian hyperthecosis, polycystic ovary syndrome, testosterone
1. INTRODUCTION
Androgen excess is defined as clinical or biochemical evidence of elevated androgenic steroids in women, 1 typically presenting with clinical features such as hirsutism, alopecia or acne. 2 Polycystic ovary syndrome (PCOS) represents the underlying diagnosis in the vast majority of adolescent girls and reproductive‐aged women presenting with androgen excess, and has a background population prevalence of at least 10%. 3 More severe biochemical disturbance may result in overt virilization; however, this phenomenon is typically due to pathology other than PCOS, and more likely to represent monogenic or neoplastic adrenal, ovarian and pituitary disease. Identifying patients at risk of harbouring non‐PCOS pathology represents a diagnostic challenge for the endocrinologist, with careful clinical and biochemical phenotyping critical to guide clinical judgement on the requirement for further investigations such as imaging, or definitive interventions such as surgery.
Androgen excess is a cardinal clinical and biochemical characteristic of PCOS and is a central component in both the androgen excess and PCOS (AE‐PCOS) Society and earlier National Institute of Health (NIH) diagnostic criteria. 4 , 5 , 6 Although application of the Rotterdam consensus criteria leads to a phenotypic subgroup with ovulatory dysfunction and PCO morphology without androgen excess, the existence of a non‐hyperandrogenic PCOS subgroup is not universally accepted by all clinicians . 7 Androgen excess has been increasingly associated with adverse chronic health consequences in women, with increased rates of disorders of glucose regulation, nonalcoholic fatty liver disease and cardiovascular disease, which closely correlate with circulating androgen burden, observed in women with PCOS. 8 , 9 , 10
This review aims to provide objective guidance for clinicians in their approach to the patient presenting with clinical or biochemical evidence of androgen excess. Particular emphasis will be placed on delineating clinical and biochemical risk factors for non‐PCOS pathology, with a proposed algorithmic approach to determine the requirement for dynamic testing and imaging strategies where appropriate (Table 1).
Table 1.
Summary of previously published studies that aimed to define the frequency of disorders observed in women that had serum androgens measured for clinically suspected androgen excess
| First author | Year of publication | n | Menopausal status | Setting | Presentation | Androgens measured (method) | PCOS n (%) | Idiopathic hirsutism n (%) | CAH n (%) | Androgen secreting tumour n (%) | Other n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Elhassan 11 | 2018 | 1205 | Pre‐ and postmenopausal (881 and 324 women, respectively | Endocrinology department | Clinical hyperandrogenism, oligomenorrhoea, subfertility | DHEAS, T and A4 (LC‐MS/MS) | Premenopausal 270 (89%) | ‐ | Premenopausal 18 (6%) | Premenopausal ACC 4 (1.3%) | Premenopausal 6 (2%) |
| Postmenopausal 7 (9.3%) | |||||||||||
| Postmenopausal 22 (29.3%) | |||||||||||
| Postmenopausal ACC 11 (14.7%); VOT 2 (2.7%) | |||||||||||
| Postmenopausal 0 | |||||||||||
| Di Fede 12 | 2010 | 152 | Premenopausal | Endocrinology department | Mild hirsutism (Ferriman–Gallwey score 8–15) | DHEAS, T and A4 (immunoassay) | 72 (47.4%) | 77 (50.6%) | 3 (2.0%) | ‐ | ‐ |
| Karrer‐Voegeli 13 | 2009 | 318 | Premenopausal | Endocrinology department | Skin manifestations of androgen excess | DHEAS, T and A4 (immunoassay) | 62 (27.6%) | ‐ | 4 (1.8%) | VOT 2 (0.9%) | Idiopathic hyper‐androgenism (hirsutism or elevated androgens) |
| 101 (44.3%) | |||||||||||
| Escobar‐Morreale 14 | 2008 | 270 | Premenopausal | Endocrinology department | Oligo‐/amenorrhoea and/or skin manifestations of androgen excess | DHEAS, T and A4 (immunoassay) | 171 (63.3%) | 24 (8.9%) | 6 (2.2%) | ‐ | Idiopathic hyper‐androgenism |
| 61 (22.6%) | |||||||||||
| Hyper‐ prolactinaemia | |||||||||||
| 2 (0.7%) | |||||||||||
| Fanta 15 | 2008 | 298 | Premenopausal | Obstetrics and gynaecology department | Oligo‐/amenorrhoea and/or skin manifestations of androgen excess and biochemical androgen excess | DHEAS, T and A4 (immunoassay) | 290 (97.3%) | ‐ | 8 (2.7%) | ‐ | ‐ |
| Carmina 16 | 2006 | 950 | Premenopausal | Two endocrinology departments | Skin manifestations of androgen excess | DHEAS and T (immunoassay) | 685 (72.1%) | 72 (7.6%) | 41 (4.3%) | VOT 2 (0.2%) | Idiopathic hyper‐androgenism |
| 150 (15.8%) | |||||||||||
| Azziz 17 | 2004 | 873 | Premenopausal | Obstetrics and gynaecology reproductive endocrinology department | Oligo‐/amenorrhoea and/or skin manifestations of androgen excess | DHEAS and T (immunoassay) | 716 (82.0%) | 39 (4.7%) | 24 (2.2%) | VOT 2 (0.2%) | Hyper‐ androgenic insulin‐resistant acanthosis nigricans |
| 33 (3.1%) | |||||||||||
| Glintborg 18 | 2004 | 340 | Premenopausal | Endocrinology department | Hirsutism | DHEAS and T (immunoassay) | 134 (39.4%) | 201 (59.1%) | 2 (0.6%) | VOT 1 (0.3%) | Prolactinoma |
| 1 (0.3%) | |||||||||||
| CD 1 (0.3%) | |||||||||||
| Unluhizarci 19 | 2004 | 168 | Premenopausal | Endocrinology department | Hirsutism | DHEAS, T and A4 (immunoassay) | 96 (57.1%) | 27 (16.0%) | 12 (7.1%) | ACC 3 (1.8%) | CD 1 (0.6%) |
| O'Driscoll 20 | 1994 | 350 | Pre‐ and postmenopausal (322 and 28 women respectively) | Endocrinology department | Skin manifestations of androgen excess | DHEAS, T and A4 (immunoassay) | 170 (60.0%) of the 282 women who had an ultrasound scan | ‐ | 3 (0.8%) | ACC 1 (0.2%) | Cortisone reductase deficiency 1 (0.2%) |
| VOT 1 (0.2%) | |||||||||||
| Two (12.0%) of the 17 postmenopausal women who were scanned had PCO** |
Note: Only studies with >100 patients were included.
Abbreviations: ACC, adrenocortical carcinoma; CAH, congenital adrenal hyperplasia; CD, Cushing's disease; DHEAS, dehydroepiandrosterone sulphate; PCO, polycystic ovary; PCOS, polycystic ovary syndrome; VOT, virilizing ovarian tumour.
Adapted from J Clin Endocrinol Metab 2018;103(3):1214–1223. doi:10.1210/jc.2017-0242611-20
2. ANDROGEN METABOLISM IN WOMEN
The ovaries, adrenal glands and peripheral tissues all play key roles in androgen metabolism in women (Figure 1). Androgenic precursors such as dehydroepiandrosterone (DHEA), its sulphated ester DHEAS, and androstenedione (A4) are secreted by the zona reticularis of the adrenal cortex, and activated to more potent androgens such as testosterone (T) and 5α‐dihydrotestosterone (DHT) in the ovaries and peripheral tissues. Although T may be produced and secreted by the adrenal gland in low concentrations, serum T levels are generally considered representative of ovarian androgen generation, while DHEA and DHEAS production is primarily reflective of adrenal androgen production. 21 Debate persists over the predominant source of A4 production, with likely relatively equivalent contributions from the adrenals and ovaries. 22
Figure 1.
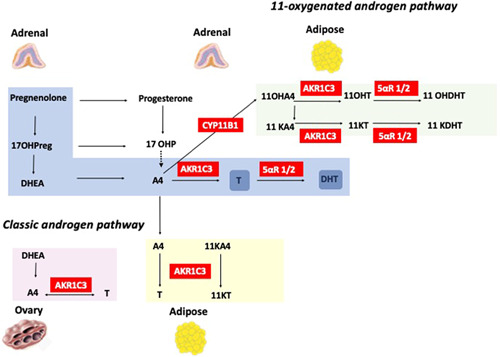
Adrenal, ovarian and peripheral androgen metabolism. Both classic and 11‐oxygenated androgen pathways are demonstrated. Androgenic precursors are secreted predominantly by the adrenal glands and activated to potent androgens in the ovaries and peripheral tissues. 5αR 1/2, 5α‐reductase type 1 and 2; 11OHA4, 11β‐hydroxyandrostenedione; 11OHT, 11β‐hydroxytestosterone; 11OHDHT, 11‐hydroxydihydrotestosterone; 11KA4, 11‐ketoandrostenedione; 11KT, 11‐ketotestosterone; 11KDHT, 11‐ketodihydrotestosterone; 17OHP, 17‐hydroxyprogesterone; A4, androstenedione; AKR1C3, aldoketoreductase type 1C3; CYP11B1, 11β‐hydroxylase type 1; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; T, testosterone [Color figure can be viewed at wileyonlinelibrary.com]
The zona reticularis of the adrenal cortex is responsible for adrenal androgen precursor synthesis, 23 which is under the control of pituitary corticotropin (ACTH). 24 The adrenal gland produces several 19 carbon (C19) steroids, with DHEA and DHEAS produced in the greatest quantity. 25 The majority of DHEA is converted to A4 via oxidation of the 3‐beta‐hydroxyl group through the action of 3β‐hydroxysteroid dehydrogenase type 2.
Androgen synthesis in the ovarian follicular unit occurs primarily in the thecal cells and is regulated by pituitary luteinizing hormone (LH) secretion. Ovarian thecal cells can generate DHEA from cholesterol and pregnenolone through the actions of cholesterol side‐chain cleavage enzyme and 17α‐hydroxylase, with subsequent conversion to A4 and T. 26 Venous sampling studies have confirmed significant gradients between ovarian vein and peripheral samples for T and A4, which persist even after menopause, with significantly greater relative production of T. 27 Insulin is a potent regulator of ovarian androgen generation, acting directly on thecal cell steroidogenesis; it also potentiates the ovarian steroidogenic response to LH. 28 Insulin plays a further role in peripheral androgen metabolism, suppressing sex hormone‐binding globulin (SHBG) production at the level of the liver, thereby increasing circulating free testosterone concentrations. Previous work from our group has also highlighted the role of insulin in adipose androgen generation in women with PCOS through upregulation of AKR1C3 expression and activity 29 ; indeed, adipose tissue itself is a source of androgen production in simple obesity. 30 Hyperinsulinaemia in PCOS and simple obesity is therefore likely to be a key driver of the androgen excess phenotype in these conditions.
Androgenic precursors such as DHEA and A4 may undergo activation to more potent active androgens such as T and DHT in the periphery, depending on the expression of androgen activating enzymes in local tissues. 3βHSD1 and 2 are expressed in skin, adipose, liver, brain and breast and may convert DHEA to A4. 31 A4 may then undergo downstream conversion to T by the action of AKR1C3 in peripheral tissues; this enzyme is highly expressed in adipose tissue and a key contributor to androgen excess in the context of both simple obesity and PCOS. 26 , 32 , 33 T may bind directly to the androgen receptor, but its effect can be amplified by enzymatic reduction to DHT, the most potent androgen, by isoforms of the enzyme 5α‐reductase (5αR). Systemic 5αR activity is upregulated in women with PCOS. 34 , 35
In the adrenal gland, cytochrome P450 11β‐hydroxylase type 1 (CYP11B1) has the ability to convert A4 to the 11‐oxygenated androgen precursor 11β‐hydroxyandrostenedione (11OHA4). 36 This is converted to 11‐ketoandrostenedione (11KA4) by 11β‐hydroxysteroid dehydrogenase type 2 (11βHSD2) at the level of the kidney. 11KA4 is activated to 11‐ketotestosterone (11KT) in peripheral tissues by AKR1C3; indeed, 11KA4 is a preferred substrate for AKR1C3 compared to A4. 37 , 38 11‐Oxygenated androgens constitute the majority of circulating androgens in PCOS, premature adrenarche and CAH. 39 , 40 , 41 11KT and 11‐ketodihydrotestosterone (11KDHT) are potent androgens that bind and activate the androgen receptor with the same affinity as counterparts T and DHT in the classic pathway. 42 Circulating 11KT levels do not decline with age in women, a phenomenon that may have implications for metabolic health in the postmenopausal phase of life. 43
3. CLINICAL EVALUATION AND DIFFERENTIAL DIAGNOSIS
3.1. History
Clinical features of androgen excess in women include hirsutism, acne and alopecia. 44 A focused history will include assessment of the severity and duration of symptoms as well as rapidity of onset. Establishing how much time is spent removing hair from face, trunk and limbs may also give an indication of the severity of hirsutism, as the modified Ferriman–Gallwey index (mFG) may be confounded by cosmetic measures taken preconsultation. It should be emphasized that acne is highly prevalent in non‐hyperandrogenic women, particularly in adolescence, with up to 20% of individuals in their midteens and 15% of those in their early 20 s reporting the presence of acne. 45
Long‐standing mild to moderate symptoms of androgen excess are usually due to PCOS, whereas rapid virilization suggests non‐PCOS pathology (Figure 2). Androgen excess may be linked to menstrual disturbances such as oligomenorrhoea (defined as menses occurring at intervals longer than 35 days apart or less than 8 periods per year) either causally or in the context of underlying PCOS, and so a full menstrual history should be obtained. Cardiovascular risk factors including obesity, cigarette smoking, dyslipidaemia, hypertension and impaired glucose tolerance should be considered. 10 Patients should be asked about a family history of congenital adrenal hyperplasia (CAH), severe insulin resistance (SIR) syndromes, impaired glucose tolerance or PCOS. It is important to determine fertility plans as the majority of systemic pharmacological treatments for androgen excess are not compatible with trying to conceive due to teratogenic risk. Lastly the patient should be asked about signs and symptoms consistent with overt virilization such as clitoral enlargement or voice deepening.
Figure 2.
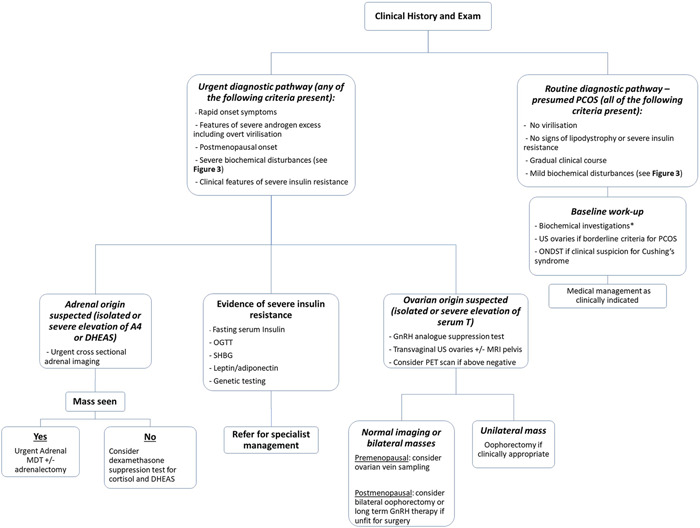
Clinical approach to the patient with androgen excess. A typical peripubertal and indolent presentation is in keeping with PCOS. Severe biochemical disturbances, rapid onset symptoms and other red flag clinical features such as virilization should prompt investigation for non‐PCOS adrenal or ovarian pathology. *Baseline biochemical workup: FSH, LH, estradiol, 17‐hydroxyprogesterone (17OHP), testosterone (T), dehydroepiandrosterone sulphate (DHEAS), androstenedione (A4), sex hormone‐binding globulin (SHBG) and prolactin. FSH, follicle‐stimulating hormone; LH, luteinizing hormone; OGTT, oral glucose tolerance test; ONDST, overnight dexamethasone suppression test; PCOS, polycystic ovary syndrome; PET, positron emission tomography
3.2. Physical exam
Anthropometric characteristics including BMI, waist circumference and blood pressure should be documented. An mFG 46 is used to grade hirsutism. A score of 0–4 is assigned to nine areas of the body depending on the density of terminal‐end hairs, giving a final score out of 36. There is wide inter‐racial variability and cut‐off values are somewhat arbitrary, but a value ≥8 is generally considered at the threshold for hirsutism. The mFG may not always be accurate as many women will undertake cosmetic measures to remove excess hair growth in advance of clinic consultation. The presence of other features such as acne and/or androgenic alopecia should be documented. Clinical examination should also assess for acanthosis nigricans and skin tags, which are clinical features of SIR, as well as cushingoid features such as striae, bruising and proximal muscle weakness. Adipose tissue distribution should be examined to look for signs of lipodystrophy. Examination of external genitalia may not be appropriate unless the patient reports specific clinical signs such as clitoromegaly or other features of overt virilization.
3.3. Differential diagnosis
3.3.1. PCOS
The vast majority of women of reproductive age presenting with clinical signs of androgen excess will have a diagnosis of PCOS, which affects up to 10% of all women. 3 The typical age of onset of PCOS is between ages of 15 and 25 years, with slowly progressive signs and symptoms. However, caution should be exercised in making a diagnosis in the adolescent phase, as many girls will experience irregular menstrual cycles that will subsequently normalize. 46 PCOS is now increasingly considered a chronic metabolic disorder, and many of the long‐term health consequences are closely correlated with the severity of androgen excess. 46 Typical clinical presentations include hirsutism, acne, alopecia, weight gain, subfertility or menstrual disturbance. A transvaginal ovarian ultrasound is indicated in women without oligomenorrhoea to ensure that Rotterdam criteria are met for diagnostic purposes. However, this is usually not appropriate in younger girls with hymen intacta. The typical biochemical picture observed is mild‐to‐moderate elevations in serum T, A4 and/or DHEAS. 11 , 47 Overweight or obesity is observed in over 70% of women with PCOS. In women with regular periods and without polycystic ovarian morphology on ultrasound, a diagnosis of idiopathic hirsutism/androgen excess should be considered.
3.3.2. Congenital adrenal hyperplasia
CAH is a rare monogenic form of androgen excess with a prevalence rate of ~1:14,000 to 1:18,000 births, depending on the population studied. 48 21‐Hydroxylase deficiency due to a homozygous or compound heterozygous mutation in the CYP21A2 gene on chromosome 6 accounts for 95% of cases and results in defective conversion of 17‐hydroxyprogesterone (17OHP) to 11‐deoxycortisol 49 ; this leads to glucocorticoid and mineralocorticoid deficiency in most cases, alongside adrenal androgen excess due to a compensatory rise in ACTH. Affected female infants are typically diagnosed at birth due to ambiguous genitalia, while male infants usually present after 2–4 weeks with a salt‐wasting adrenal crisis. In so‐called nonclassic CAH (NCCAH), there is typically 20%–70% residual activity of the 21‐hydroxylase enzyme, resulting in a less severe phenotype, with adrenal androgen excess but preserved glucocorticoid and mineralocorticoid production. 50 NCCAH typically presents in adolescence or even adulthood, with hirsutism, acne or ovulatory dysfunction in women often clinically indistinguishable from PCOS. 51 Clinical history should focus on family history or any history of familial consanguinity as a risk factor for inherited metabolic disease. Confirmatory biochemical testing for NCCAH is outlined in the section on diagnostics. In certain ethnic groups, the incidence of consanguinity in parents of patients with CAH is relatively high. One study looking at consanguinity rates in Saudi Arabia showed positive consanguinity between parents in 22.2% of cases. Of the cases with a history of consanguinity, 38% were first degree, and a family history of CAH was present in 25.6%. 52
3.3.3. Adrenocortical carcinoma
Adrenocortical carcinoma (ACC) is a rare malignant tumour of the adrenal cortex with a clinical incidence between 0.7 and 2.0 per million per year. 53 Presentation of ACC varies from incidental detection on imaging to clinically overt syndromes of hormone excess due to malignant secretion of steroids from malignant tissue. 54 Most ACCs are biochemically active, secreting both precursor and active adrenal corticosteroids from at least one histological zone of the adrenal cortex. 55 Affected women may present with clinical signs of severe androgen excess due to adrenal androgen hypersecretion. ACC should be considered in all women presenting with severe biochemical disturbances as highlighted in Figures 3 and 4, 11 in particular preferential elevation of adrenal androgen precursors, or with rapid onset androgen excess, including virilization. Our 2018 paper on biochemical phenotype as a predictor of non‐PCOS pathology highlighted that 9/9 postmenopausal women with an elevation of DHEAS levels above 20 μmol/L on radioimmunoassay (RIA) had an underlying diagnosis of ACC. 43 Patients with malignant cosecretion of cortisol and other glucocorticoids may also demonstrate features of Cushing's syndrome clinically. Patients presenting with the above clinical and biochemical features must undergo urgent cross‐sectional imaging of the abdomen to screen for an adrenal mass.
Figure 3.
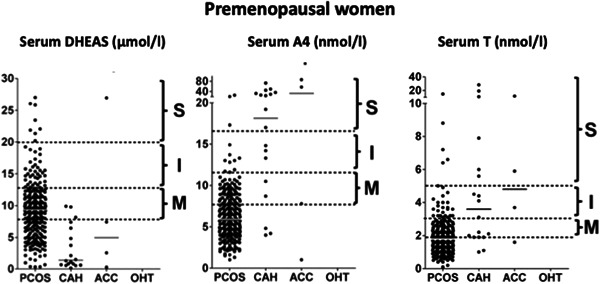
Severity of androgen excess according to diagnosis and androgen measured in premenopausal women on a single assay. 11 Arbitrary stratification of elevated serum androgen levels into mild (M), intermediate (I), and severe (S) was applied, with grouping of values according to underlying diagnosis. Median values for each diagnosis are denoted by a solid black line. ACC, adrenocortical carcinoma; CAH, congenital adrenal hyperplasia; OHT, ovarian hyperthecosis; PCOS, polycystic ovary syndrome (reference J Clin Endocrinol Metab 2018;103(3):1214–1223. doi:10.1210/jc.2017-02426)
Figure 4.
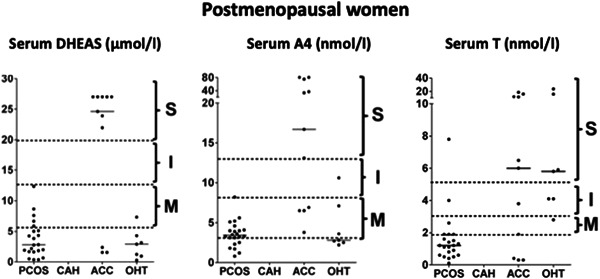
Severity of androgen excess according to diagnosis and androgen measured in postmenopausal women on a single assay. 11 Arbitrary stratification of elevated serum androgen levels into mild (M), intermediate (I) and severe (S) was applied, with grouping of values according to the underlying diagnosis. Median values for each diagnosis are denoted by a solid black line. PCOS, polycystic ovary syndrome; CAH, congenital adrenal hyperplasia; ACC, adrenocortical carcinoma; OHT, ovarian hyperthecosis (reference J Clin Endocrinol Metab 2018;103(3):1214–1223. doi:10.1210/jc.2017-02426)
3.3.4. Syndromes of SIR
Monogenic disorders of insulin signalling or adipose tissue function resulting in SIR syndromes may also present with androgen excess in women of reproductive age. 28 Severe hyperinsulinaemia can drive ovarian androgen generation by acting synergistically with pituitary LH secretion; high insulin levels may also stimulate ovarian cystic enlargement and rupture. An incorrect diagnosis of PCOS is often made in such patients due to a combination of ovulatory dysfunction, androgen excess and severe PCO on ultrasound. Clinical indicators of monogenic SIR include the presence of acanthosis nigricans, a strong family history of type 2 diabetes and SIR in the setting of a relatively normal BMI. A fasting serum insulin >150 pmol/L and/or a peak insulin >1500 pmol/L during oral glucose tolerance test are highly suspicious for SIR. Serum testosterone levels are often significantly elevated (>10 nmol/L), sometimes with overt virilization.
SIR is categorized as due to either primary defects in insulin signalling, such as insulin receptor (INSR) mutations, or due to primary adipose tissue dysfunction, such as lipodystrophy. Women with INSR mutations typically present with severe androgen excess in the context of a family history of diabetes; they are protected against dyslipidaemia and hepatic steatosis due to a postreceptor phenomenon. 56 Unlike lipodystrophy and acquired insulin resistance SHBG, adiponectin and leptin levels may be normal or even elevated. Patients with lipodystrophy present with regional abnormalities of fat distribution or absence of adipose tissue. In contrast to primary SIR, there is significant dyslipidaemia and hepatic steatosis, with low SHBG, leptin and adiponectin levels. Lipodystrophy may be partial or complete, and congenital and acquired variants exist. The androgen excess phenotype is biochemically indistinguishable from that observed in INSR mutations, with predominant ovarian hyperandrogenism and severe elevation of serum testosterone.
3.3.5. Cushing's disease
Cushing's disease (CD) is a rare cause of androgen excess in women caused by a corticotroph pituitary adenoma, and accounts for 1% and 4% of pre‐ and postmenopausal women, respectively, presenting with androgen excess in our recent series. 11 Discriminant clinical features of hypercortisolism include violaceous abdominal striae and proximal muscle weakness; the presence of suppressed gonadotropins and oestradiol, bruising, osteoporosis and hypertension should also raise the index of suspicion. 57 Serum levels of DHEAS and A4 may be elevated due to autonomous ACTH secretion. With Cushing's disease, the clinical course is typically much more indolent than that observed with hypercortisolism due to an ACC, and may be misdiagnosed as PCOS in many cases.
3.3.6. Ovarian hyperthecosis
Ovarian hyperthecosis (OHT) results from overproduction of androgens in the ovarian stromal cells and can result in severe androgen excess. 44 The pathophysiology has not been fully elucidated, but a typical histological finding is ovarian stromal hyperplasia with cellular luteinization. It is due to a complex interplay between pituitary LH secretion, ovarian stroma proliferation and possibly increased serum insulin levels in the context of insulin resistance. The majority of women are postmenopausal and complain of gradual but severe features of androgen excess, with features of overt virilization such as voice deepening and clitoromegaly sometimes observed. In general, the onset is slow and progressive, which can help to distinguish it from androgen‐secreting tumours that also present in postmenopausal women. 58 Biochemically these women will have significantly elevated testosterone levels and relatively normal adrenal androgens. 59 It is frequently associated with insulin resistance or T2DM in postmenopausal women, and postmenopausal gonadotropin levels are usually preserved.
3.3.7. Virilizing ovarian tumours
Virilizing ovarian tumours (VOTs) are a rare but important cause of androgen excess, as early diagnosis of these potentially malignant tumours may improve outcomes. Similarly to OHT, VOTs present with severe androgen excess and virilizing signs are seen in up to 50% of women, with a rapid clinical course. 60 VOTs are rare accounting for 5% of all ovarian tumours. Common androgen‐secreting ovarian tumours include Leydig cell neoplasms, thecomas, stromal luteoma and Sertoli‐Leydig cell tumours. 61 It can be challenging to discriminate between OHT and VOT on biochemical grounds alone. In one study evaluating the biochemical profile in the differential diagnosis of VOT versus OHT, the VOT group showed higher T and E2 levels and lower gonadotropin levels compared to the OHT group. In the same study, mean serum T levels were 20 versus 6.2 nmol/L in the VOT and OHT groups, respectively. The authors have suggested a T > 10 nmol/to be more specific for VOT; in both VOT and OHT there was >50% suppression following gonadotropin‐releasing hormone (GnRH). However, there was a significant overlap in the hormonal levels noted. 62
4. DIAGNOSTIC WORK‐UP
Following a targeted history and exam, appropriate biochemical and radiological investigations should be instituted. A recommended diagnostic approach and decision tool on the requirement for dynamic testing and imaging is summarized in Figure 2.
4.1. Biochemistry—baseline and dynamic testing
Initial biochemical screening of a patient with androgen excess should include a baseline reproductive profile taken in the early follicular phase between Days 2 and 5 of the menstrual cycle. However, this may not be possible in patients with oligo‐ or amenorrhoea. Baseline testing should include LH, follicle‐stimulating hormone (FSH), oestradiol, T, DHEAS, A4, SHBG, 17‐OHP, prolactin and thyroid function tests.
4.1.1. Testosterone
Serum total T remains the most commonly measured androgen in clinical practice in the evaluation of suspected androgen excess, 63 and is recommended by most consensus statements as the first‐line biochemical investigation. 64 However, the diagnostic utility of serum T measurement is limited by a number of key factors. Serum T circulates bound to SHBG and other proteins. 65 Therefore measured total serum T is affected by fluctuating SHBG levels, and suppression of hepatic SHBG output due to hyperinsulinaemia and obesity in PCOS may significantly interfere with the interpretability of serum total T levels. T circulates in the low nanomolar range in females and may be below the limit of quantitation in many assays. Liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) is a highly sensitive and specific tool for the quantification of very low abundance steroids. However, LC‐MS/MS is limited by cost and operator skill and is therefore not universally available in all centres. The severity of androgen excess is often poorly correlated with circulating total serum T levels. 66 Measurement of total T with direct RIA can be inaccurate and unreliable due to substantial cross‐reactivity, 27 , 28 while estimation of free T using equilibrium dialysis is analytically challenging, not universally available and produces highly variable results.
Calculation of free T, bioavailable T and the free androgen index (FAI) is recommended in the 2018 PCOS consensus guidelines for the biochemical work‐up of patients with PCOS. 67 FAI is calculated using the formula Tx100/SHBG. This helps to partially mitigate the biochemical impact of low SHBG levels due to hyperinsulinaemia observed in women with PCOS, who may manifest a normal total serum T level on direct measurement. However caution must be exercised in applying this to all patient cohorts, and FAI becomes increasingly inaccurate when SHBG levels drop below 30 nmol/L as is frequently observed in PCOS. 60
Serum T is generally reflective of ovarian androgen generation. Although it may also be elevated in ACC, this rarely occurs in isolation in this context, with co‐elevation of adrenal androgen precursors almost invariably observed 11 Isolated severe T elevations in postmenopausal women are typically seen in OHT, although rare androgen‐producing tumours such as Leydig cell tumours or ovarian thecomas should also be considered. Based on our published data, serum T levels below 5 nmol/L measured using LC‐MS/MS in premenopausal women do not warrant adnexal or adrenal imaging, as these are almost invariably due to PCOS (Figure 3). Conversely, we recommend ovarian imaging in all women with serum T > 5nmol/l, regardless of menopausal status.
4.1.2. Androstenedione (A4)
A4 is a weak androgenic precursor that circulates in higher nanomolar concentrations than serum T and may therefore have enhanced diagnostic utility for the work up of women with androgen excess. Unlike T, A4 is not influenced by variables such as SHBG; therefore, it may be a more reliable marker of PCOS‐related androgen excess. 47 Current consensus guidelines for the investigation of women with suspected PCOS do not routinely recommend measurement of A4, but evidence of its utility continues to grow. Our published data show that up to 25% of women with a normal serum T who had clinical features of androgen excess had an elevated serum A4. 47 Both the ovary and adrenal produce A4, but the relative contribution of each to the circulating A4 pool remains somewhat contentious. Severe A4 elevations in premenopausal women (>15 nmol/L on LC‐MS/MS assays) are typically seen in CAH or ACC while the latter is much more likely in postmenopausal women with levels above 10 nmol/L on LC‐MS/MS (Figures 3 and 4).
4.1.3. DHEAS
DHEAS is water soluble and is present in large quantities circulating in the micromolar range in serum. It may be measured with either RIA or LC‐MS/MS; however, use of the latter is not critical due to its significantly higher circulating concentration than serum T in women. Severe DHEAS elevation in premenopausal women is still most likely to be attributable to PCOS, but in the postmenopausal phase of life the likelihood of ACC is extremely high (Figures 3 and 4). Nonetheless, we recommend imaging all premenopausal women with DHEAS elevations >20 μmol/L, and in all postmenopausal women with levels in excess of 5 μmol/L presenting with androgen excess.
4.1.4. 11‐Oxygenated androgens
11‐Oxygenated androgens are currently not routinely available in most clinical biochemistry laboratories. Their wider clinical availability in the future may represent an additional diagnostic tool for the clinician in the evaluation of androgen excess. Areas for future research include the utility of 11‐oxygenated androgens to differentiate between adrenal and ovarian hyperandrogenism, as biomarkers of disease control in CAH, and in discriminating between nonclassic CAH and more severe forms of PCOS. It also remains to be elucidated whether 11‐oxygenated androgens may play a role as surrogate markers of metabolic risk in women with PCOS. 11OHA4 can only be generated by adrenal CYP11B1 activity, and therefore in theory should be helpful in discriminating between underlying adrenal and ovarian pathology in the work up of androgen excess.
4.1.5. Dexamethasone suppression testing
Dexamethasone suppression testing with measurement of adrenal androgens may be clinically indicated in patients with significant adrenal androgen excess atypical for PCOS and in whom cross‐sectional adrenal imaging has not revealed an abnormality. Suppression of DHEAS by dexamethasone confirms adrenal androgen hypersecretion remains under the control of ACTH as might be observed in PCOS or idiopathic adrenal hyperandrogenism, rather than due to an autonomous process such as occult tumour hypersecretion. The half‐life of DHEAS is such that a 96‐h dexamethasone suppression test may be necessary (0.5 mg every 6 h), with 96 h of glucocorticoid exposure deemed optimal in comparison to 24, 48 or 72 h in some studies. 35 , 68
4.1.6. GnRH analogue test
GnRH analogue testing is a valuable diagnostic tool in the work‐up of suspected ovarian hyperandrogenism, particularly in women with a preferential elevation of serum testosterone as observed in postmenopausal women with OHT. 69 GnRH analogue testing involves administering a single dose of a GnRH analogue such as leuprorelin (3.75 mg) or triptorelin (3 mg) intramuscularly or subcutaneously. Ovarian secretion of androgens is gonadotropin‐dependent even in the context of OHT, and suppression of gonadotropins and consequently T secretion after GnRH analogue therapy strongly supports a suspected diagnosis of benign ovarian hyperandrogenism. GnRH analogues induce prolonged activation of GnRH receptors in the pituitary gonadotrophs, leading to desensitization and resultant downregulation of these receptors. The net effect after 21–28 days is suppression of LH and FSH secretion and inhibition of oestrogen and androgen secretion. Suppression of T by more than 50% at 28 days after administration of a GnRH analogue suggests a benign ovarian source of hyperandrogenism. Postmenopausal women who have suppressed T after GnRH analogue therapy should undergo bilateral oophorectomy unless there is a clear unilateral lesion; in premenopausal women who do not have a radiologically defined unilateral lesion, joint ovarian and adrenal vein sampling should be considered.
There is limited data on whether GnRH analogue testing can differentiate between OHT and VOTs in the literature. Theoretically, the severity of androgen excess with VOTs may be more severe and less likely to be under gonadotropin regulation; therefore, this diagnosis should be considered in patients with a strong suspicion of ovarian hyperandrogenism who fail to suppress their testosterone after GnRH agonist. In very rare cases of adrenal hyperandrogenism, androgen secretion may fall under the control of LH or even GnRH. If the GnRH analogue adequately suppresses testosterone, long‐term GnRH agonist treatment can be considered for use in patients with an increased risk for surgery due to comorbidities or who are unwilling to undergo bilateral oophorectomy.
4.1.7. Short synacthen test
Routine measurement of serum 17OHP in the early follicular phase is a sensitive but poorly specific screening strategy for NCCAH. 50 Initial screening tests should include early morning serum 17OHP, T and A4, which may be markedly elevated. DHEAS levels tend to be relatively normal. Depending on the assay, the proposed cut‐off value for basal 17OHP levels as a prompt for confirmatory testing varies between 5 and 12 nmol/L. 70 Measurement of 17OHP 30 min after ACTH stimulation (with synthetic ACTH 250 μg i.v. or i.m) is the gold standard for confirmatory diagnosis. 71 The Endocrine Society Guidelines recommend a stimulated 17OHP cut‐off value of 30 nmol/L for NCCAH diagnosis. 72
4.1.8. Ovarian and adrenal venous sampling
In postmenopausal women with unequivocal evidence of ovarian hyperandrogenism on GnRH analogue testing, bilateral oophorectomy is generally appropriate, as fertility preservation and maintenance of oestrogen levels is usually not a key consideration. Ovarian hyperthecosis is usually bilateral, and many VOTs are radiologically occult. However, ovarian venous sampling may be considered in premenopausal women with severe ovarian hyperandrogenism beyond that deemed typical for PCOS, especially if imaging is not helpful at locating a discrete culprit unilateral ovarian lesion such as thecoma or Leydig cell tumour. Ovarian vein sampling can lateralize and therefore direct targeted unilateral surgical oophorectomy while preserving fertility. 73 This may be conducted in tandem with adrenal vein sampling in situations where there is equivocal evidence of ovarian versus adrenal hyperandrogenism. Drawbacks include invasiveness, requirement for specialist experienced operator input and lack of availability outside tertiary centres.
4.2. Imaging
Adrenal imaging for androgen excess should be considered in premenopausal women with severe biochemical disturbances, especially preferential elevation of adrenal androgens DHEAS and A4, and any postmenopausal woman with new‐onset androgen excess. 74 The initial investigation of choice for those with a suspected adrenal mass is an unenhanced computed tomography (CT) scan of the adrenal glands. An unenhanced CT adrenal with a cut‐off of over 20 HU has a 99% sensitivity for ACC, but specificity is poor. 75 In older patients, the likelihood of clinically insignificant incidental adrenal lesions is much higher and careful multidisciplinary team involvement should guide decision making in these equivocal cases to avoid unwarranted surgical intervention.
In premenopausal women with mild clinical and biochemical androgen excess with oligomenorrhoea, ovarian imaging is typically not required as the Rotterdam criteria threshold for a diagnosis of PCOS will already be met; in cases without menstrual dysfunction, imaging to assess ovarian morphology is clinically indicated to help establish a diagnosis of PCOS. 46 The preferred imaging modality is a transvaginal ultrasound, but this is usually not appropriate in younger girls or adolescents.
A transvaginal ultrasound should be the first‐line radiological investigation for pre‐ and postmenopausal women with suspected ovarian hyperandrogenism and severely elevated serum T. Bulky ovaries bilaterally may be observed in women with OHT, but the finding is relatively subjective and has a sensitivity as low as 33%. VOTs may be radiologically occult on ultrasound in up to 45% of cases in one series. 62 Pelvic magnetic resonance imaging has been reported as the best radiological discriminator between VOT and OHT by identifying a unilateral ovarian nodule, although positron emission tomography scan may also have some further diagnostic utility. However, imaging is often nondiagnostic as many VOTs can be less than 1 cm in size. 76
5. SUMMARY
PCOS is the underlying cause of androgen excess in the vast majority of women presenting to primary and secondary care, and is associated with a significant metabolic risk burden. A targeted history and exam are required to identify those patients who harbour non‐PCOS pathology, in particular amongst those presenting with atypical or red flag features such as rapid onset symptoms or overt virilization. Systematic interrogation of circulating serum T and adrenal androgen precursors, in tandem with other markers such as 17OHP and gonadotropins, can identify signature patterns suspicious for underlying adrenal, pituitary and ovarian disease, and guide imaging strategies accordingly. Serum T levels below 5 nmol/L measured using LC‐MS/MS in premenopausal women typically do not warrant further investigation in women with clinical features otherwise consistent with polycystic ovary syndrome. Early multidisciplinary input from colleagues in radiology and clinical biochemistry is key to achieve an early diagnosis and understand the limitations of imaging and biochemical testing, respectively.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
This study is supported by a Wellcome‐HRB Irish Clinical Academic Training (ICAT) Fellowship to LC and an HRB Emerging Clinician Scientist Award to MOR (ECSA‐2020‐001). Open access funding provided by IReL.
Cussen L, McDonnell T, Bennett G, Thompson CJ, Sherlock M, O'Reilly MW. Approach to androgen excess in women: clinical and biochemical insights. Clin Endocrinol (Oxf). 2022;97:174‐186. 10.1111/cen.14710
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this paper.
REFERENCES
- 1. Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—Part 2. Endocr Pract. 2015;21(12):1415‐1426. [DOI] [PubMed] [Google Scholar]
- 2. Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71(5):847.e1‐e10. quiz 857‐8. [DOI] [PubMed] [Google Scholar]
- 3. Knochenhauer ES, Key TJ, Kahsar‐Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078‐3082. [DOI] [PubMed] [Google Scholar]
- 4. Rosenfield RL. Puberty and its disorders in girls. Endocrinol Metab Clin North Am. 1991;20(1):15‐42. [PubMed] [Google Scholar]
- 5. Rotterdam EA‐SPCWG . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 6. Azziz R, Carmina E, Dewailly D, et al. The androgen excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456‐488. [DOI] [PubMed] [Google Scholar]
- 7. Carmina E. Diagnosis of polycystic ovary syndrome: from NIH criteria to ESHRE‐ASRM guidelines. Minerva Ginecol. 2004;56(1):1‐6. [PubMed] [Google Scholar]
- 8. Kumarendran B, O'Reilly MW, Manolopoulos KN, et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018;15(3):e1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumarendran B, Sumilo D, O'Reilly MW, et al. Increased risk of obstructive sleep apnoea in women with polycystic ovary syndrome: a population‐based cohort study. Eur J Endocrinol. 2019;180(4):265‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod Update. 2010;16(4):347‐363. [DOI] [PubMed] [Google Scholar]
- 11. Elhassan YS, Idkowiak J, Smith K, et al. Causes, patterns, and severity of androgen excess in 1205 consecutively recruited women. J Clin Endocrinol Metab. 2018;103(3):1214‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Fede G, Mansueto P, Pepe I, Rini GB, Carmina E. High prevalence of polycystic ovary syndrome in women with mild hirsutism and no other significant clinical symptoms. Fertil Steril. 2010;94(1):194‐197. [DOI] [PubMed] [Google Scholar]
- 13. Karrer‐Voegeli S, Rey F, Reymond MJ, Meuwly JY, Gaillard RC, Gomez F. Androgen dependence of hirsutism, acne, and alopecia in women: retrospective analysis of 228 patients investigated for hyperandrogenism. Medicine. 2009;88(1):32‐45. [DOI] [PubMed] [Google Scholar]
- 14. Escobar‐Morreale HF, Sanchon R, San Millan JL. A prospective study of the prevalence of nonclassical congenital adrenal hyperplasia among women presenting with hyperandrogenic symptoms and signs. J Clin Endocrinol Metab. 2008;93(2):527‐533. [DOI] [PubMed] [Google Scholar]
- 15. Fanta M, Cibula D, Vrbikova J. Prevalence of nonclassic adrenal hyperplasia (NCAH) in hyperandrogenic women. Gynecol Endocrinol. 2008;24(3):154‐157. [DOI] [PubMed] [Google Scholar]
- 16. Carmina E, Rosato F, Janni A, Rizzo M, Longo RA. Extensive clinical experience: relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab. 2006;91(1):2‐6. [DOI] [PubMed] [Google Scholar]
- 17. Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89(2):453‐462. [DOI] [PubMed] [Google Scholar]
- 18. Glintborg D, Henriksen JE, Andersen M, et al. Prevalence of endocrine diseases and abnormal glucose tolerance tests in 340 Caucasian premenopausal women with hirsutism as the referral diagnosis. Fertil Steril. 2004;82(6):1570‐1579. [DOI] [PubMed] [Google Scholar]
- 19. Unluhizarci K, Gokce C, Atmaca H, Bayram F, Kelestimur F. A detailed investigation of hirsutism in a Turkish population: idiopathic hyperandrogenemia as a perplexing issue. Exp Clin Endocrinol Diabetes. 2004;112(9):504‐509. [DOI] [PubMed] [Google Scholar]
- 20. O'Driscoll JB, Mamtora H, Higginson J, Pollock A, Kane J, Anderson DC. A prospective study of the prevalence of clear‐cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol. 1994;41(2):231‐236. [DOI] [PubMed] [Google Scholar]
- 21. Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3‐S5. [DOI] [PubMed] [Google Scholar]
- 22. Miller KK, Sesmilo G, Schiller A, Schoenfeld D, Burton S, Klibanski A. Androgen deficiency in women with hypopituitarism. J Clin Endocrinol Metab. 2001;86(2):561‐567. [DOI] [PubMed] [Google Scholar]
- 23. Idkowiak J, Lavery GG, Dhir V, et al. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165(2):189‐207. [DOI] [PubMed] [Google Scholar]
- 24. Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr. 1977;90(5):766‐770. [DOI] [PubMed] [Google Scholar]
- 25. Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography‐tandem mass spectrometry analysis of human adrenal vein 19‐carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92(8):3040‐3043. [DOI] [PubMed] [Google Scholar]
- 28. Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin‐related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535‐582. [DOI] [PubMed] [Google Scholar]
- 29. O'Reilly MW, Kempegowda P, Walsh M, et al. AKR1C3‐mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327‐3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity—a site‐specific role for 17beta‐hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183(2):331‐342. [DOI] [PubMed] [Google Scholar]
- 31. Konings G, Brentjens L, Delvoux B, et al. Intracrine regulation of estrogen and other sex steroid levels in endometrium and non‐gynecological tissues; pathology, physiology, and drug discovery. Front Pharmacol. 2018;9:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277‐284. [DOI] [PubMed] [Google Scholar]
- 33. O'Reilly M, Gathercole L, Capper F, Arlt W, Tomlinson J. Effect of insulin on AKR1C3 expression in female adipose tissue: in‐vivo and in‐vitro study of adipose androgen generation in polycystic ovary syndrome. Lancet. 2015;385(Suppl 1):S16. [DOI] [PubMed] [Google Scholar]
- 34. Stewart PM, Shackleton CH, Beastall GH, Edwards CR. 5 alpha‐reductase activity in polycystic ovary syndrome. Lancet. 1990;335(8687):431‐433. [DOI] [PubMed] [Google Scholar]
- 35. Fassnacht M, Schlenz N, Schneider SB, Wudy SA, Allolio B, Arlt W. Beyond adrenal and ovarian androgen generation: Increased peripheral 5 alpha‐reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(6):2760‐2766. [DOI] [PubMed] [Google Scholar]
- 36. Strushkevich N, Gilep AA, Shen L, et al. Structural insights into aldosterone synthase substrate specificity and targeted inhibition. Mol Endocrinol. 2013;27(2):315‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barnard M, Quanson JL, Mostaghel E, Pretorius E, Snoep JL, Storbeck KH. 11‐Oxygenated androgen precursors are the preferred substrates for aldo‐keto reductase 1C3 (AKR1C3): implications for castration resistant prostate cancer. J Steroid Biochem Mol Biol. 2018;183:192‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schiffer L, Bossey A, Kempegowda P, et al. Peripheral blood mononuclear cells preferentially activate 11‐oxygenated androgens. Eur J Endocrinol. 2021;184(3):353‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Reilly MW, Kempegowda P, Jenkinson C, et al. 11‐Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(3):840‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turcu AF, Nanba AT, Chomic R, et al. Adrenal‐derived 11‐oxygenated 19‐carbon steroids are the dominant androgens in classic 21‐hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rege J, Turcu AF, Kasa‐Vubu JZ, et al. 11‐ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589‐4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11beta‐Hydroxydihydrotestosterone and 11‐ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013;377(1‐2):135‐146. [DOI] [PubMed] [Google Scholar]
- 43. Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE, Turcu AF. 11‐Oxygenated C19 steroids do not decline with age in women. J Clin Endocrinol Metab. 2019;104(7):2615‐2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Braithwaite SS, Erkman‐Balis B, Avila TD. Postmenopausal virilization due to ovarian stromal hyperthecosis. J Clin Endocrinol Metab. 1978;46(2):295‐300. [DOI] [PubMed] [Google Scholar]
- 45. Rea JN, Newhouse ML, Halil T. Skin disease in Lambeth. A community study of prevalence and use of medical care. Br J Prev Soc Med. 1976;30(2):107‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Reilly MW, Taylor AE, Crabtree NJ, et al. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. J Clin Endocrinol Metab. 2014;99(3):1027‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21‐hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043‐4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pang SY, Wallace MA, Hofman L, et al. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21‐hydroxylase deficiency. Pediatrics. 1988;81(6):866‐874. [PubMed] [Google Scholar]
- 50. Tusie‐Luna MT, Traktman P, White PC. Determination of functional effects of mutations in the steroid 21‐hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem. 1990;265(34):20916‐20922. [PubMed] [Google Scholar]
- 51. Chrousos GP, Loriaux DL, Mann DL, Cutler GB, Jr. Late‐onset 21‐hydroxylase deficiency mimicking idiopathic hirsutism or polycystic ovarian disease. Ann Intern Med. 1982;96(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 52. Milyani AA, Al‐Agha AE, Al‐Zanbagi M. Initial presentations and associated clinical findings in patients with classical congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2018;31(6):671‐673. [DOI] [PubMed] [Google Scholar]
- 53. Kerkhofs TM, Verhoeven RH, Van der Zwan JM, et al. Adrenocortical carcinoma: a population‐based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49(11):2579‐2586. [DOI] [PubMed] [Google Scholar]
- 54. Terzolo M, Ali A, Osella G, Mazza E. Prevalence of adrenal carcinoma among incidentally discovered adrenal masses. A retrospective study from 1989 to 1994. Gruppo Piemontese Incidentalomi Surrenalici. Arch Surg. 1997;132(8):914‐919. [DOI] [PubMed] [Google Scholar]
- 55. Arlt W, Biehl M, Taylor AE, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96(12):3775‐3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parker VE, Semple RK. Genetics in endocrinology: genetic forms of severe insulin resistance: what endocrinologists should know. Eur J Endocrinol. 2013;169(4):R71‐R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meczekalski B, Szeliga A, Maciejewska‐Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37(8):677‐682. [DOI] [PubMed] [Google Scholar]
- 59. Friedman CI, Schmidt GE, Kim MH, Powell J. Serum testosterone concentrations in the evaluation of androgen‐producing tumors. Am J Obstet Gynecol. 1985;153(1):44‐49. [DOI] [PubMed] [Google Scholar]
- 60. Keevil BG, Adaway J, Fiers T, Moghetti P, Kaufman JM. The free androgen index is inaccurate in women when the SHBG concentration is low. Clin Endocrinol. 2018;88(5):706‐710. [DOI] [PubMed] [Google Scholar]
- 61. Outwater EK, Wagner BJ, Mannion C, McLarney JK, Kim B. Sex cord‐stromal and steroid cell tumors of the ovary. Radiographics. 1998;18(6):1523‐1546. [DOI] [PubMed] [Google Scholar]
- 62. Yance VRV, Marcondes JAM, Rocha MP, et al. Discriminating between virilizing ovary tumors and ovary hyperthecosis in postmenopausal women: clinical data, hormonal profiles and image studies. Eur J Endocrinol. 2017;177(1):93‐102. [DOI] [PubMed] [Google Scholar]
- 63. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745‐2749. [DOI] [PubMed] [Google Scholar]
- 64. Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91(11):4237‐4245. [DOI] [PubMed] [Google Scholar]
- 65. Pardridge WM. Serum bioavailability of sex steroid hormones. Clin Endocrinol Metab. 1986;15(2):259‐278. [DOI] [PubMed] [Google Scholar]
- 66. Legro RS, Schlaff WD, Diamond MP, et al. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95(12):5305‐5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Arlt W, Justl HG, Callies F, et al. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83(6):1928‐1934. [DOI] [PubMed] [Google Scholar]
- 69. Pascale MM, Pugeat M, Roberts M, et al. Androgen suppressive effect of GnRH agonist in ovarian hyperthecosis and virilizing tumours. Clin Endocrinol (Oxf). 1994;41(5):571‐576. [DOI] [PubMed] [Google Scholar]
- 70. Azziz R, Hincapie LA, Knochenhauer ES, Dewailly D, Fox L, Boots LR. Screening for 21‐hydroxylase‐deficient nonclassic adrenal hyperplasia among hyperandrogenic women: a prospective study. Fertil Steril. 1999;72(5):915‐925. [DOI] [PubMed] [Google Scholar]
- 71. Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non‐classic CAH due to 21‐hydroxylase deficiency. Eur J Endocrinol. 2019;180(3):R127‐R145. [DOI] [PubMed] [Google Scholar]
- 72. Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21‐hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(9):4133‐4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dennedy MC, Smith D, O'Shea D, McKenna TJ. Investigation of patients with atypical or severe hyperandrogenaemia including androgen‐secreting ovarian teratoma. Eur J Endocrinol. 2010;162(2):213‐220. [DOI] [PubMed] [Google Scholar]
- 74. Idkowiak J, Elhassan YS, Mannion P, et al. Causes, patterns and severity of androgen excess in 487 consecutively recruited pre‐ and post‐pubertal children. Eur J Endocrinol. 2019;180(3):213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bancos I, Taylor AE, Chortis V, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE‐ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tng EL, Tan JMM. Gonadotropin‐releasing hormone analogue stimulation test versus venous sampling in postmenopausal hyperandrogenism. J Endocr Soc. 2021;5(1):bvaa172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this paper.


