Abstract
Scope
This study aims to assess in rats whether normalizing maternal diet during lactation prevents the harmful effects of western diet (WD) consumption during the whole perinatal period on the lipidomic profile in maternal milk and offspring plasma.
Methods and Results
Control dams (CON‐dams), fed with standard diet (SD); WD‐dams, fed with WD prior and during gestation and lactation; and reversion dams (REV‐dams), fed as WD‐dams but moved to SD during lactation are followed. Lipidomic analysis is performed in milk and plasma samples from pups. Milk of WD‐dams presents a different triacylglycerol composition and free fatty acid (FA) profile compared to CON‐dams, including an increased ratio of pro‐inflammatory to anti‐inflammatory long‐chain polyunsaturated FA. Such alterations, which are also present in the plasma of their offspring, are widely reversed in the milk of REV‐dams and the plasma of their pups. This is related with the recovery of control adiponectin expression levels in the mammary gland, and the presence of decreased expression of pro‐inflammatory factors.
Conclusion
Implementing a healthy diet during lactation prevents early alterations in the plasma lipidome of pups associated to the maternal intake of an obesogenic diet, which may be related to the normalization of milk lipid content and the inflammatory state in the mammary gland.
Keywords: inflammation, lipidomics, milk composition, suckling, western diet
Implementing a healthy diet during lactation prevents harmful effects of the maternal intake of an obesogenic diet during the whole perinatal period on the lipidomic profile in maternal milk and offspring plasma. The reversion of such alterations, which include different triacylglycerol and free fatty acid (FA) profiles, and an increased ratio of pro‐inflammatory to anti‐inflammatory long‐chain polyunsaturated FA, may be related to the normalization of the inflammatory state in the mammary gland.
1. Introduction
Nutritional environment during fetal and early postnatal development is crucial in the programming of later metabolic health.[ 1 , 2 ] It is widely known that suboptimal nutrition during gestation is an important risk factor for the development of obesity in adulthood,[ 3 , 4 ] but lactation is showing to be a critical window of development with remarkable relevance in the health programming of the offspring.[ 5 ] Noteworthy, both maternal obesity and the intake of an obesogenic diet during lactation have been related to a detrimental and persistent impact on the development of the neonates.[ 6 , 7 , 8 ] Although the specific mechanisms involved are only partially understood,[ 9 ] milk quality may represent one of the key factors.[ 10 ] Thus, intervention studies in rodents have shown that the intake of an obesogenic diet during the perinatal period negatively affects the structure and function of the mammary gland and therefore the composition of milk,[ 11 ] including altered metabolism‐regulating hormones and fatty acids (FA) profiles.[ 12 , 13 ]
Milk lipids, represented by triacylglycerols (TG) in approximately 98%, provide most of the energy needed for offspring during early postnatal life[ 14 ] and play essential roles in the structural and functional programming of developing tissues that are key for later energy homeostasis.[ 15 ] Milk TG are composed by FA that derive from different sources. They can be endogenously synthesized in the mammary gland but also come from maternal diet or the mobilization of fat reserves.[ 16 , 17 , 18 ] Thus, besides the indirect effect of the intake of a high‐fat diet on de novo FA synthesis by the mammary gland, the type and/or quantity of fat that mothers consume during lactation directly influence the FA composition of breast milk.[ 19 , 20 ]
In this line, it is plausible to consider that the consumption of an unbalanced diet during the perinatal period could program a higher susceptibility of the offspring to suffer from obesity in adulthood by affecting the lipid content of breast milk.[ 10 ] For example, previous studies in Yucatan pigs have shown that changes in the lipid profile due to a western diet (WD) consumption could lead to disturbances in the neuronal connections of the offspring early in life, triggering negative and permanent effects in different metabolic processes.[ 21 ] Moreover, the consumption of a cafeteria diet during lactation in rats has been shown to affect the composition of maternal milk, including alterations in the TG profile,[ 8 ] and this has been related with later metabolic disruptions in their pups, characterized by increased adiposity without effect on body weight,[ 8 ] and an impaired response to fasting conditions in different tissues involved in energy homeostasis.[ 22 ]
Considering the impact of maternal overnutrition and obesity during the perinatal period on milk composition, and its negative outcomes on the offspring development, it is of interest to study possible strategies to prevent such adverse effects. In this regard, we have recently demonstrated that some alterations in milk composition that are associated with the maternal intake of WD during gestation, specifically on total protein content and levels of metabolic hormones, could be reversed by the implementation of a healthy diet during the lactation period.[ 13 ] However, the effects of such dietary intervention on the lipidomic profile of maternal milk and its potential impact on the offspring are not known. Thus, here we aimed to assess, in rats, whether the implementation of a standard diet during lactation, after the exposure to a WD prior to and during gestation, is able to normalize alterations in the milk lipid profile that could be potentially involved in the offspring's risk of suffering from chronic diseases later in life.
2. Results
2.1. Phenotypic Traits of Dams and Pups during Lactation
Maternal phenotypic traits during pre‐gestation, gestation, and lactation and of their offspring during the suckling period have been previously described.[ 13 ] A summary of most relevant traits of dams during the lactation period and of male and female pups during the suckling period are shown in Table 1 . WD exposure for 1 month prior gestation resulted in higher body weight and fat mass (8% and 66%, respectively) compared to controls.[ 13 ] However, at LD5, WD‐ and reversion (REV)‐dams displayed greater fat mass and fat percentage than control (CON)‐dams (one‐way analysis of variance [ANOVA]), with no differences in body weight. At the end of lactation (LD21), WD‐dams showed lower body weight than CON‐ and REV‐dams, suggesting that the negative energy balance that characterizes the lactation period due to milk production was apparently more marked in WD fed dams.[ 13 ] Only REV‐dams displayed significantly higher fat mass and fat percentage than CON‐dams (one‐way ANOVA). Body weight of WD‐dams decreased from LD5 to LD21, but not in CON‐ and REV‐dams (one‐way ANOVA), and the decrease in fat mass and fat percentage was higher in WD‐ and REV‐dams compared to CON‐dams (one‐way ANOVA). WD‐dams displayed higher circulating insulin and free FA (NEFA) levels than CON‐ and REV‐dams, whereas REV‐dams showed higher leptin levels and higher rWAT weight than CON‐dams (one‐way ANOVA). No differences were found regarding adiponectin levels.
Table 1.
Phenotypic traits of dams and pups during lactation
| A) Dams | CON | WD | REV |
|---|---|---|---|
| LD5 | |||
| Body weight [g] | 285 ± 9 | 284 ± 8 | 291 ± 10 |
| Fat mass [g] | 31.6 ± 2.8 a | 48.6 ± 4.7 b | 52.1 ± 6.3 b |
| [%] | 11.1 ± 0.9 a | 16.9 ± 1.2 b | 17.6 ± 1.6 b |
| LD21 | |||
| Body weight [g] | 304 ± 9 a | 283 ± 5 b | 311 ± 7 a |
| Fat mass [g] | 30 ± 2 a | 32 ± 2 a | 41 ± 4 b |
| [%] | 9.7 ± 0.8 a | 11.3 ± 0.7 ab | 13.2 ± 1.0 b |
| Δ Body weight | |||
| (from LD5 to LD21 [g]) | 36.0 ± 4.4 a | −3.4 ± 5.2 b | 26.1 ± 6.6 a |
| Δ Fat mass | |||
| (from LD5 to LD21 [g]) | −2.1 ± 1.4 a | −16.6 ± 3.2 b | −10.8 ± 3.0 b |
| (From LD5 to LD21 [%]) | −1.3 ± 0.4 a | −5.6 ± 0.8 b | −4.4 ± 0.8 b |
| Mammary gland [g] | 6.51 ± 0.53 | 7.82 ± 0.49 | 7.58 ± 0.35 |
| Retroperitoneal WAT [g] | 2.35 ± 0.26 a | 2.98 ± 0.24 ab | 3.73 ± 0.49 b |
| Insulin [µg L–1] | 1.39 ± 0.25 a | 2.20 ± 0.30 b | 1.38 ± 0.21 a |
| Leptin [pg mL–1] | 1130 ± 215 a | 1645 ± 200 ab | 1956 ± 219 b |
| Adiponectin [ng mL–1] | 5440 ± 1656 | 6091 ± 1310 | 7571 ± 1254 |
| NEFA (nmol µL–1] | 0.25 ± 0.05 a | 0.40 ± 0.05 b | 0.27 ± 0.03 a |
| B) Pups | CON | WD | REV | Two‐way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | ||
| PND1 | |||||||
| Body weight [g] | 6.9 ± 0.1 a | 6.5 ± 0.1 | 6.9 ± 0.1 a | 6.5 ± 0.2 | 6.5 ± 0.1 b | 6.3 ± 0.1 | – |
| PND15 | |||||||
| A | B | C | S, MD | ||||
| Body weight [g] | 28.0 ± 0.4 | 26.9 ± 0.4 | 31.7 ± 0.5 | 30.5 ± 0.4 | 26.4 ± 0.6 | 26.0 ± 0.4 | |
| Glucose [mg dL–1] | 115 ± 3 | 117 ± 5 | 123 ± 4 | 127 ± 4 | 119 ± 2 | 121 ± 3 | – |
| A | B | A | MD | ||||
| Leptin [pg mL–1] | 437 ± 69 | 396 ± 63 | 1373 ± 239 | 1537 ± 279 | 543 ± 63 | 460 ± 84 | |
| PND21 | |||||||
| A | B | C | S, MD | ||||
| Body weight [g] | 40.7 ± 0.7 | 39.1 ± 0.6 | 49.0 ± 0.6 | 46.4 ± 0.7 | 37.3 ± 0.8 | 36.2 ± 0.6 | |
| A | B | C | MD | ||||
| Fat mass [g] | 3.0 ± 0.1 | 2.9 ± 0.1 | 6.4 ± 0.2 | 6.2 ± 0.2 | 2.5 ± 0.1 | 2.5 ± 0.1 | |
| A | B | C | MD | ||||
| [%] | 7.2 ± 0.2 | 7.4 ± 0.2 | 13.2 ± 0.3 | 13.4 ± 0.3 | 6.8 ± 0.3 | 6.7 ± 0.3 | |
| A | B | C | S, MD | ||||
| Δ Body weight (from PND1 to PND21 [g]) | 33.8 ± 0.7 | 32.3 ± 0.6 | 41.9 ± 0.7 | 40.2 ± 0.6 | 31.1 ± 0.7 | 30.3 ± 0.6 | |
| A | B | A | MD | ||||
| Glucose [mg dL–1] | 139 ± 3 | 137 ± 4 | 151 ± 3 | 147 ± 5 | 135 ± 4 | 137 ± 4 | |
| A | B | A | S, MD | ||||
| Leptin [pg mL–1] | 1374 ± 146 | 1364 ± 125 | 4476 ± 454 | 3234 ± 344 | 1597 ± 220 | 1094 ± 129 | |
Phenotypic traits of CON‐, WD‐, and REV‐dams during lactation (n = 8–10) (A), and of their male and female offspring. During the suckling period (n = 18–26 for body weight‐related parameters, and n = 8–10 for glucose and leptin levels) (B). Data are mean ± SEM. Statistics: two‐way ANOVA was performed to analyze the effects of sex and/or maternal diet, and one‐way ANOVA was carried out to determine differences between groups. ANOVA was followed by a LSD post hoc test. Symbols: S, effect of sex; MD, effect of maternal diet; A ≠ B ≠ C (p < 0.05, two‐way ANOVA); a ≠ b (p < 0.05, one‐way ANOVA). ANOVA, analysis of variance; CON, control; LD, lactation day; LSD, least significant difference; NEFA, non‐esterified fatty acids; PND, postnatal day; REV, reversion; SEM, standard error of the mean; WAT, white adipose tissue; WD, western diet.
Regarding pups, male REV‐pups, but not females, showed lower body weight than CON‐ and WD‐pups at PND1 (one‐way ANOVA). At PND15 and PND21, both male and female WD‐pups displayed higher body weight than CON‐ and REV‐pups, and REV‐pups also displayed lower body weight than CON‐pups (two‐way ANOVA). The same trend was found when considering fat mass and fat percentage (at PND21), and body weight increase during the suckling period (from PND1 to PND21). Since no differences have been described in the energy content or composition of the milk of REV‐dams in relation to the controls,[ 13 ] the lower body weight gain in REV‐pups could be tentatively attributed to a lower milk intake. However, this has not been specifically measured. WD‐pups showed higher circulating leptin (at PND15 and PND21) and glucose (at PND21) levels than CON‐ and REV‐pups (two‐way ANOVA). In all the groups, leptin levels increased about three‐fold between PND15 and PND21.
2.2. Triacylglycerol Composition of Maternal Milk and the Plasma of Their Offspring
Principal component analysis (PCA) was used to compare the TG profile in milk samples of CON‐, WD‐, and REV‐dams at LD5, LD10, and LD15, including all the TG intensities obtained in the lipidomic analysis (Figure 1 , and Supplementary Figure 1A for details). At the three time points, milk of WD‐dams was clearly separated from that of CON‐ and REV‐dams, but no separation between CON‐ and REV‐dams was found. Of note, the first and second components explained the 85.6%, 82.6%, and 78.6% of variance between experimental groups on LD5, LD10, and LD15, respectively. Figure 2 shows milk partial TG composition based on the total number of carbon atoms and double bonds in the three acyl chains. In both cases, WD‐dams generally displayed a different profile than CON‐dams, which was largely normalized in REV‐dams. Regarding the total number of carbon atoms (Figure 2A), WD‐dams showed a reduced signal for TG with lower number of carbon atoms (32–40) and an increased signal for those with higher number (48–54) compared to CON‐dams throughout the whole lactation period (one‐way ANOVA). Interestingly, this profile was normalized to control values in REV‐dams, with few exceptions on LD5. In addition, WD‐dams also showed a different profile in TG composition according to the total number of double bonds in comparison with CON‐dams (Figure 2B). Specifically, WD‐dams presented lower proportion of saturated TG species and higher of TG with one to three double bonds than CON‐dams (one‐way ANOVA). REV‐dams, at LD5, also presented lower proportion of saturated TG species and higher of TG with one double bond than CON‐dams, but these values were normalized at LD10 and LD15 (one‐way ANOVA). During the whole lactation, the proportion of TG with two and three double bonds in REV‐dams was not significantly different from that of CON‐dams (one‐way ANOVA). Notably, WD‐dams also showed lower signal of TG with six double bonds than CON‐dams at the three time points, which was totally normalized in REV‐dams at LD10, and partially at LD15 (one‐way ANOVA).
Figure 1.
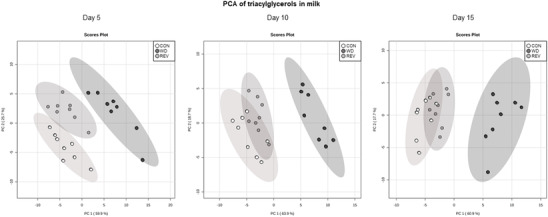
PCA including signal of triacylglycerol species detected by electrospray ionization in milk samples of CON‐, WD‐, and REV‐dams at lactation day 5, 10, and 15 (n = 8). CON, control; PCA, principal component analysis; REV, reversion; WD, western diet.
Figure 2.
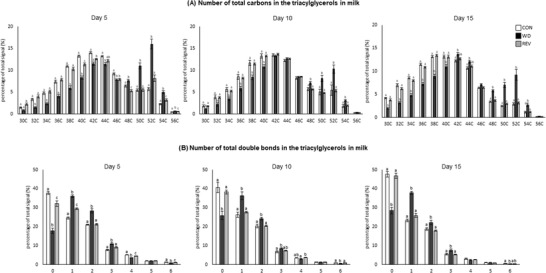
Total number of carbon atoms a) and double bonds b) in the three acyl chains of the most abundant TG in the milk of CON‐, WD‐, and REV‐dams at lactation days 5, 10, and 15. TG species with 58 and 60 carbon atoms are not represented as they are not representative and no differences between groups have been found. Data are mean ± SEM (n = 8). Statistics: one‐way ANOVA followed by Bonferroni post hoc test was performed to determine differences between groups. Symbols: a ≠ b ≠ c (p < 0.05). ANOVA, analysis of variance; CON, control; PCA, principal component analysis; REV, reversion; SEM, standard error of the mean; TG, triacylglycerols; WD, western diet.
Figure 3 shows the PCA of plasma TG profile in male and female pups of the three experimental groups at PND15 (see Supplementary Figure S1B for details). Samples from WD‐pups were clearly separated from those of CON‐ and REV‐pups, but no separation between samples of CON‐ and REV‐pups was observed. In this case, the first two components explained 79.3% of the variance between experimental groups, when considering both sexes together, and 82.8% and 77.3% in male and female pups, respectively, when separated. TG composition in plasma of pups at PND15 is shown in Figure 4 . Both male and female WD‐pups displayed a percentage of TG with low (34–44) and high (56–62) carbon atoms significantly lower than that of CON‐pups, but an increase in the proportion of those with a carbon number between 48 and 52 (one‐way ANOVA) (Figure 4A). Regarding the number of double bonds (Figure 4B), WD‐pups showed a lower signal for TG‐containing saturated FA and of TG with a higher number of double bonds (4 and between 6 and 12), but a higher proportion of TG with one to three double bonds, compared to CON‐pups (one‐way ANOVA). Interestingly, all these significant differences were normalized to control values in both male and female REV‐pups (one‐way ANOVA).
Figure 3.
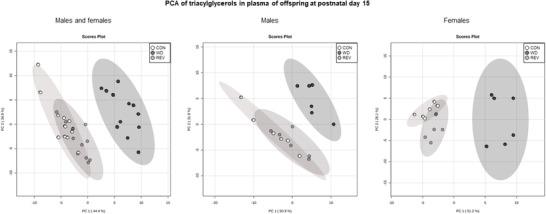
PCA including signal of triacylglycerol species detected by electrospray ionization in plasma samples of CON‐, WD‐, and REV‐pups at postnatal day 15 (n = 6). CON, control; PCA, principal component analysis; REV, reversion; WD, western diet.
Figure 4.
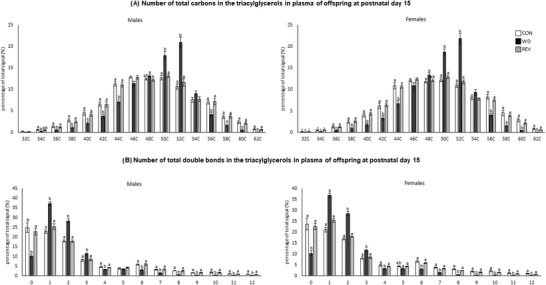
Total number of carbon atoms a) and double bonds b) in the three acyl chains of the most abundant TG in the plasma of CON‐, WD‐, and REV‐pups at postnatal day 15. TG species with 30 carbon atoms are not represented as they are not representative and no differences between groups have been found. Data are mean ± SEM (n = 6). Statistics: one‐way ANOVA followed by Bonferroni post hoc test was performed to determine differences between groups. Symbols: a ≠ b (p < 0.05). ANOVA, analysis of variance; CON, control; REV, reversion; SEM, standard error of the mean; TG, triacylglycerols; WD, western diet.
Partial TG composition in the plasma of the offspring regarding the number of carbon atoms and of double bonds was positively correlated with that of maternal milk, with the exceptions of TG with 42, 44, 46, 56, or 58 carbon atoms, and those with five double bounds (see Supplementary Table S3). In the case of TG with 44 carbon atoms, a negative correlation was found between the percentage of this species in both maternal milk and plasma.
2.3. Fatty Acid Composition of Maternal Milk and the Plasma of Their Offspring
Figure 5A shows milk free FA profile from CON‐, WD‐, and REV‐dams. In comparison with CON‐dams, milk of WD‐dams presented a higher percentage of palmitoleic (C16:1) and oleic acid (C18:1) and a lower percentage of linoleic (C18:2), α‐linolenic (C18:3), eicosadienoic (C20:2), eicosapentaenoic acid (commonly known as EPA, C20:5), docosatetraenoic (C22:4), docosapentaenoic (C22:5), and docosahexaenoic acid (known as DHA, C22:6) (one‐way ANOVA). These differences were already observed at LD5 and were generally maintained throughout the lactation period. In milk of REV‐dams at LD5, the percentage of C18:1 was also higher than in CON‐dams, but lower than in WD‐dams, and those of C18:2, C20:5, C22:5, and C22:6 were lower than in CON‐dams but not as much as in WD‐dams (one‐way ANOVA). At LD10 and LD15, the differences between REV‐ and CON‐dams were no longer observed and the free FA profile in milk from both groups of dams was practically the same, maintaining the aforementioned differences with WD‐dams (one‐way ANOVA).
Figure 5.
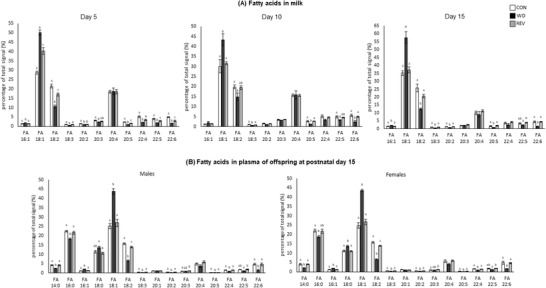
Percentage of total signal of free fatty acids in the milk of CON‐, WD‐, and REV‐dams at lactation days 5, 10, and 15 a), and in the plasma of their male and female offspring at postnatal day 15 b). Data are mean ± SEM (n = 6–8). Statistics: one‐way ANOVA followed by Bonferroni post‐hoc test was performed to determine differences between groups. Symbols: a ≠ b ≠ c (p < 0.05). ANOVA, analysis of variance; CON, control; REV, reversion; SEM, standard error of the mean; WD, western diet.
Free FA profile in the plasma of male and female pups at PND15 is presented in Figure 5B. In general terms, male and female WD‐pups also showed a different profile from those of CON‐ and REV‐dams. Specifically, WD‐pups showed a lower percentage of myristic acid (C14:0), palmitic acid (C16:0), C18:2, C18:3, C20:2, C20:5, C22:4, and C22:6, and a higher proportion of C16:1, stearic acid (C18:0) (only females), and C18:1 compared to CON‐ and REV‐pups (one‐way ANOVA). CON‐ and REV‐pups generally showed a similar profile, but REV‐pups showed a lower percentage of C18:2 and a higher percentage of C20:3 compared to their controls (one‐way ANOVA).
Free FA profile in the plasma of the offspring, considering both males and females, was positively correlated with that of maternal milk for most of the species, with the exception of docosatetraenoic acid (see Supplementary Table S3).
Figure 6 shows the ratio of pro‐inflammatory arachidonic acid (AA, C20:4) to anti‐inflammatory EPA and DHA (AA/(EPA + DHA) ratio) in both maternal milk (Figure 6A) and plasma of the offspring (Figure 6B). The AA/(EPA + DHA) ratio was increased in the milk of WD‐dams compared to that of CON‐dams during the whole lactation period (one‐way ANOVA). In the case of REV‐dams, at LD5, the ratio was lower than in WD‐dams, but still higher than in CON‐dams (one‐way ANOVA). However, it was completely normalized to the values of CON‐dams at LD10 and LD15 (one‐way ANOVA). In both male and female offspring, the AA/(EPA + DHA) ratio in the plasma of WD‐pups at PND15 was also higher than in CON‐ and REV‐pups (one‐way ANOVA).
Figure 6.
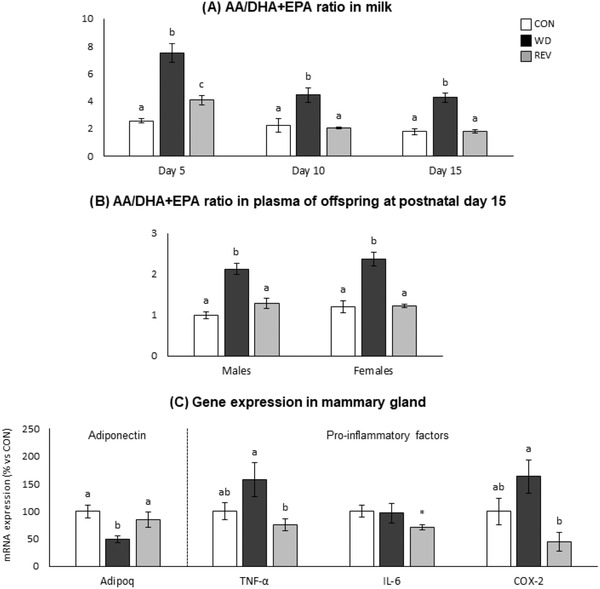
AA/(EPA + DHA) ratio in the milk of CON‐, WD‐, and REV‐dams at lactation days 5, 10, and 15 a), and in the plasma of their male and female offspring at postnatal day 15 b), and mRNA expression of anti‐inflammatory (adiponectin) and pro‐inflammatory (TNF‐α, IL‐6, and COX‐2) factors in the mammary gland of dams at the end of lactation (day 21) (C). Data are mean ± SEM (n = 6–10). Statistics: one‐way ANOVA followed by a LSD post hoc test was performed to determine differences between groups, and Mann–Whitney U test was used for single comparisons. Symbols: a ≠ b ≠ c (p < 0.05, one‐way ANOVA); *different from CON‐dams (p < 0.05, Mann–Whitney U test). AA, arachidonic acid; ANOVA, analysis of variance; CON, control; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LSD, least significant difference; REV, reversion; SEM, standard error of the mean; WD, western diet.
2.4. Gene Expression in Mammary Gland and rWAT of Dams
Figure 6C shows expression levels of anti‐inflammatory (adiponectin, Adipoq) and pro‐inflammatory (TNF‐α, IL‐6, and COX‐2) factors in the mammary gland of dams at LD21. WD‐dams displayed significantly lower Adipoq expression levels than CON‐ and REV‐dams (one‐way ANOVA), whereas REV‐dams presented lower mRNA levels of TNF‐α and COX‐2 than WD‐dams (one‐way ANOVA), and of IL‐6 compared to CON‐dams (Mann–Whitney U test).
Expression levels of genes related to lipogenesis, lipolysis, FA uptake and oxidation, and insulin and leptin signaling were also studied in dams at LD21, both in the mammary gland and in the rWAT, to ascertain potential differences in their regulation between both tissues (Figure 7 ). In mammary gland, REV‐dams presented higher Fasn mRNA levels than CON‐dams, and lower Lipe mRNA levels than CON‐ and WD‐dams (one‐way ANOVA). WD‐dams showed higher Lpl expression levels than CON‐dams (Mann–Whitney U test). Regarding rWAT, and compared to CON‐dams, WD‐dams displayed higher expression levels of Lipe and Cd36, and REV‐dams higher expression levels of Lpl and Insr (one‐way ANOVA). Both WD‐ and REV‐dams showed increased Lepr mRNA levels than CON‐dams (one‐way ANOVA).
Figure 7.
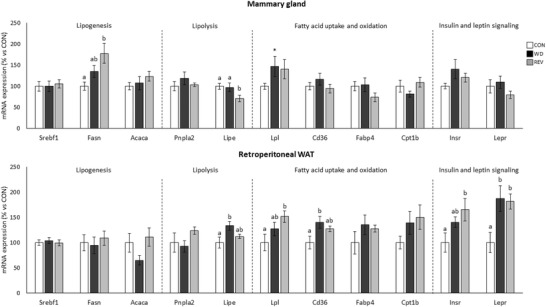
Gene expression of selected genes related to lipid metabolism in mammary gland and retroperitoneal white adipose tissue of CON‐, WD‐, and REV‐dams at the end of lactation (day 21). Data are mean ± SEM (n = 8–10). Statistics: one‐way ANOVA followed by an LSD post hoc test was performed to determine differences between groups, and Mann–Whitney U test was used for single comparisons. Symbols: a ≠ b (p < 0.05, one‐way ANOVA); *different from CON‐dams (p < 0.05, Mann–Whitney U test). ANOVA, analysis of variance; CON, control; LSD, least significant difference; REV, reversion; SEM, standard error of the mean; WD, western diet.
3. Discussion
Understanding how maternal nutritional environment during the perinatal period can affect the composition of breast milk, and whether this may have an impact on the offspring phenotype, is an interesting approach to make dietary recommendations that favor an optimal growth and development from early stages of life. In this study, we have focused on the effects of the diet normalization during lactation on the lipidomic profile of maternal milk in diet‐induced obese dams, as well as in the plasma of their pups at late lactation. Partial TG composition profile, regarding the number of carbon atoms and of double bounds, as well as free FA profile, was different in milk of WD‐dams compared to that of CON‐ and REV‐dams, while practically no differences were found between milk of CON‐ and REV‐dams, particularly from LD10. This highlights the relevance of the implementation of a healthy diet during lactation on the prevention of the alterations in the milk TG and free FA profile in diet‐induced obese dams.
Differences in TG composition between milk from CON‐ and WD‐dams, but not between CON‐ and REV‐dams in terms of both number of carbon atoms and double bonds, were already observed at LD5 and maintained throughout the whole lactation period, indicating that the standard diet feeding in REV‐dams for only 5 days following delivery was sufficient to reverse the effects of the WD feeding before delivery on the TG composition. Such differences are clearly illustrated in the PCA carried out with normalized TG peak intensities of maternal milk at the three time points during lactation. Regarding the number of carbon atoms, the reduced proportion of TG with lower number (less than 40 carbon atoms) and the increased proportion of TG with higher number (more than 48C) observed in the milk of WD‐dams in comparison to CON‐dams is in accordance with previous studies showing that maternal consumption of a high‐fat diet during the perinatal period was associated with changes in the FA proportion in milk, with lower levels of short‐ and medium‐chain FA and higher levels of long‐chain ones.[ 12 , 23 ] FA distribution according to the length of their chains may give information on the origin of TG that are present in breast milk.[ 17 , 24 ] It is known that de novo synthesis in the mammary gland produces FA up to 14 carbon atoms,[ 25 ] so the reduced proportion of TG with lower number of carbon atoms in the milk of WD‐dams could be reflecting a lower FA synthesis in the mammary gland of these rats. Thus, as previously described in dams fed a high‐fat[ 26 ] or a cafeteria diet[ 24 ] during lactation, WD consumption during this period may be associated with a disrupted de novo lipogenesis in the mammary gland. In this line, it is plausible to consider that the FA that make up the TG present in the milk of WD‐dams may come mainly from the diet or the mobilization of their own fat reserves. In fact, WD‐dams displayed, compared to CON‐dams, significantly higher Lpl mRNA levels in the mammary gland, which may be related with the upregulating effects of insulin on the expression and activity of LPL,[ 27 ] although no differences were found regarding the expression levels of this gene in the rWAT. On the other hand, the presence of increased expression levels of the Lipe gene in the rWAT of WD‐dams would suggest increased TG mobilization of their fat stores, probably related with the lipolysis‐stimulating effects of leptin, and hence increased FA supply for milk production. This is in accordance with the presence of higher circulating levels of free FA in WD‐dams at the end of lactation. Moreover, the greater reduction in their fat mass content and fat percentage during the lactation period in WD‐dams in comparison with their controls also suggest an increased mobilization of their fat stores.
The changes in the milk TG composition caused by the consumption of WD in the pre‐gestation period and during gestation and lactation were not observed in rats that were moved to a standard diet during the lactation period. The partial TG profile in REV‐dams, in terms of number of carbon atoms, was fully normalized to the values observed in CON‐dams. Gene expression profile in the mammary gland of these animals, particularly the higher expression levels of Fasn and lower expression levels of Lipe in comparison to their controls, could be reflecting an increased lipogenic and a reduced lipolytic activity, respectively, which could contribute, at least partially, to the normalization of the milk TG profile in REV‐dams. Notably, expression levels of lipid metabolism related genes in the rWAT of REV‐dams were normalized to control levels, although they displayed increased Lpl mRNA levels compared with controls, in agreement with the presence of increased Insr mRNA levels. In these animals, the presence of higher Insr mRNA levels in comparison with CON‐dams, in absence of increased circulating insulin levels, could be contributing to the preservation of insulin sensitivity, considering that they were exposed to WD prior to and during the gestational period. The effects of implementing a standard diet were also evident when looking at the TG composition in terms of number of double bonds. From LD5, and maintained throughout the whole lactation period, the profile in WD‐dams was different to that of CON‐dams, displaying a lower proportion of saturated TG species, as well as an increased percentage of TG with one to three double bonds. Of note, this altered profile was generally normalized in REV‐dams, highlighting that milk TG composition was directly influenced by the diet consumed during lactation, rather than the obesity status.
Lipidomic analysis was also performed on plasma samples from male and female pups at PND15. As seen regarding maternal milk, PCA performed with normalized TG peak intensities in plasma of the offspring at PND15 showed a clear separation between CON‐ and WD‐pups, with no differences between CON‐ and REV‐pups, both in males and females. Moreover, partial TG composition in the plasma offspring regarding the number of carbon atoms and of double bonds was positively correlated with the above‐described composition in maternal milk, with few exceptions. Most of the changes observed in milk during the whole lactation period, particularly the presence of decreased levels of TG with a lower number of carbon atoms (between 34 and 40), and increased levels of TG with a higher number of carbon atoms (between 48 and 52) in WD‐dams compared to their controls, but not in REV‐dams, were also observed in the plasma of the offspring, although a causal relationship cannot be established. Different studies have shown that long‐chain FA, but not medium‐ or short‐chain ones, plays an important role in the activation of pro‐inflammatory pathways. For example, the incubation of myotubes with palmitic and stearic acids has been related to a higher gene and protein expression of cytokines such as TNF‐α and IL‐6, stimulating inflammatory processes that are ultimately related to an insulin‐resistant state.[ 28 , 29 ] Instead, medium‐chain FA has been shown to be the optimal energy source for the proper development in neonates,[ 26 ] so their reduced levels in WD‐pups may contribute, at least in part, to an impaired growth. In fact, as previously described,[ 13 ] and confirmed here, WD‐pups presented an altered phenotype, already evident at mid lactation, as they displayed a rapid post‐natal growth‐up and higher adiposity, along with hyperleptinemia and higher circulating glucose levels, compared to CON‐pups. In this sense, it should be noted that the above‐mentioned metabolic alterations were not observed in REV‐pups, which displayed a plasma TG profile similar to that of CON‐pups. Moreover, it is noteworthy that the plasma TG profile in REV‐pups, also in terms of double bounds, was fully normalized to that of CON‐pups, in both males and females. This is in accordance with the results obtained by Alexandre‐Gouabau et al.,[ 12 ] in which the lower content of TG with saturated medium‐chain FA, together with the higher content of TG with unsaturated long‐chain FA, in the milk of dams fed with WD during gestation and lactation, were associated to a blood lipidome of their offspring with reduced levels of TG containing saturated FA. Thus, our results suggest that the plasma TG composition in pups during lactation is reflecting both the maternal diet during this period and the metabolic status of the animals already at this early age.
Besides differences in milk TG composition, here we show changes in the proportions of free FA in the milk of dams exposed to a WD before and during the gestation and lactation period, which are practically normalized when a standard diet is implemented during lactation. Moreover, such changes in maternal milk composition are generally observed in the plasma of the offspring at LD15. Some differences in maternal milk composition regarding free FA could be related, at least in part, to differences in the FA composition of the diets consumed during lactation. This is particularly evident in the case of oleic acid (C18:1), which amount in the WD is just over ten times higher than in standard diet, and this may account for the increased proportion of this FA in the milk of WD‐dams during the whole lactation period, compared to CON‐dams. Notably, the consumption of an excess of this FA in adult rats has been linked to a greater amount in adipose tissue,[ 30 ] which suggests that the higher proportion of oleic acid observed in the milk of WD‐dams may be attributable not only to the diet during lactation but also to the FA composition of their fat reserves, since these animals also consumed WD one month prior and during gestation. In fact, at LD5, but not later on, the milk of REV‐dams also had a higher proportion of oleic acid than that of controls (although not as high as that of WD‐dams), despite consuming the same standard diet as controls, supporting that this FA in milk would also derive from maternal fat reserves accumulated during the period of exposure to WD. Noteworthy, both male and female WD‐pups also displayed a marked increase in the proportion of oleic acid in plasma at LD15, compared to CON‐pups, which was totally reversed in REV‐pups.
Dietary essential polyunsaturated FA (PUFA), which include linoleic acid (LA, C18:2), α‐linolenic acid (ALA, C18:3), and their long‐chain derivatives (LC‐PUFA), are of special interest for adequate development during fetal and early postnatal life.[ 31 ] In this sense, it is remarkable that WD‐dams displayed significantly lower proportion of free LA and ALA than CON‐dams throughout the whole lactation period, and this can explain the presence of lower proportions of these FA in the plasma of both male and female WD‐pups at PND15. Notably, the proportions of these PUFA were normalized, at least partially, in the milk of REV‐dams. It could be speculated that the dietary deficiency of these essential FA early in life in WD‐pups may contribute to the inappropriate programming and development of these animals, which is in accordance with the metabolic alterations observed in WD‐pups at mid lactation. Moreover, ALA is precursor of the long‐chain n‐3 PUFA EPA (C20:5) and DHA (C22:6),[ 32 ] whose proportions were also decreased in the milk of WD‐dams. It has been previously described that maternal obesity induced by a high‐fat diet also causes, among other changes, a reduction in the levels of EPA and DHA in milk.[ 11 ] Notably, EPA and DHA levels were also lower in the plasma of WD‐pups, compared to CON‐pups, at PN15, and a positive correlation was also found between their proportions in maternal milk and in the plasma of the offspring. During this period, EPA and DHA are crucial for the proper development and function of the immune system and cognitive function, respectively.[ 33 , 34 , 35 , 36 ] Low levels of DHA in milk have been shown to negatively affect neural cell membranes and impair the cognitive function of pups,[ 33 , 37 ] and such early alterations may also increase the predisposition of animals to different metabolic disorders in adulthood, including increased fat accumulation.[ 38 ] In this sense, it could be considered that the lower levels of EPA and DHA in the milk of WD‐dams may partially contribute to the metabolic malprogramming occurring in these pups. Moreover, in absence of differences in the proportion of AA (C20:4), the presence in an increased ratio of pro‐inflammatory AA to anti‐inflammatory DHA and EPA (AA/(EPA + DHA) ratio) in milk of WD‐dams, as well as in the plasma of their offspring, in comparison with controls, could be associated with the increased adiposity of pups at the end of the suckling period, as previously described in other animal models.[ 39 ] In humans, an increased AA/(EPA + DHA) ratio in breastmilk has also been associated with increased fat accumulation in their infants.[ 40 ] Interestingly, it is remarkable that the normalization of the diet during lactation prevents the changes in the proportion of milk EPA and DHA, since their relative levels were fully normalized to the values of CON‐dams from LD10, as well as the AA/(EPA + DHA) ratio. The proportions of EPA and DHA and the AA/(EPA + DHA) ratio were also normalized to control levels in plasma of REV‐pups, both males and females, at PND15, which may contribute, at least partially, to counteract the detrimental effects caused by prenatal exposure to WD. Also in relation to the inflammatory state, maternal adiponectin deficiency in lactating animals has been related to increased production of inflammatory cytokines in the mammary gland and, to some extent, to systemic inflammation in the offspring.[ 41 ] Despite no differences in circulating adiponectin were found at LD21, the significantly lower Adipoq expression levels in the mammary gland of WD‐dams would be reflecting specific detrimental effects of the WD consumption in this tissue. It is noteworthy that expression levels of this gene were fully normalized in REV‐dams, which could be associated, at least in part, with the amelioration of metabolic disorders shown in REV‐pups compared to WD‐pups. In fact, the lower expression of the pro‐inflammatory cytokines IL‐6 and TNF‐α in the mammary gland of REV‐dams compared to CON‐ and WD‐dams, respectively, supports the improvement of the inflammatory state of the mammary gland of these animals despite being exposed to WD during gestation. These changes may also account for changes in the expression of COX‐2, an isoform of COX, which is the rate‐limiting enzyme in the conversion of AA to prostaglandins.[ 42 ] Unlike COX‐1, which is constitutively active and mediates normal physiological function, COX‐2 is responsible for producing inflammatory prostaglandins such as prostaglandin E2, and its expression has been shown to be induced by a variety of stimuli, including TNF‐α and IL‐6.[ 42 , 43 ] Interestingly, the expression pattern of COX‐2 shown in the mammary gland, with elevated expression levels in WD‐dams and lower expression in REV‐dams, mimics that found for the TNF‐α, and, partially, for IL‐6.
To sum up, we show here an altered lipidomic profile, including changes in TG composition and free FA proportions, in the milk of dams exposed to a WD before and during gestation and lactation, which seems to be mainly related to the obesogenic diet during lactation, and to a lesser extent to maternal fat stores, rather than the obese status per se. Notably, most of the changes in the milk lipid content were also observed in the plasma of their offspring, and such alterations, including the presence of an increased AA/(EPA + DHA) ratio, may favor a pro‐inflammatory state, and an increased fat accretion, among other adverse outcomes. Interestingly, the improvement of maternal diet during lactation, despite the previous exposure to WD, prevents most of the aforementioned alterations in the lipid profile, as well as normalizes mammary gland expression of the anti‐inflammatory cytokine adiponectin and the pro‐inflammatory factors TNF‐α and COX‐2, and may be associated, to some extent, with an improved metabolic programming in the offspring. These findings may have some relevant implications, since both epidemiological and experimental evidence show that nutritional environment during fetal life is critical for proper growth and development later in life, but our results also suggest that dietary improvement during lactation could be an interesting strategy for the prevention of metabolic disorders in the offspring caused by maternal obesity and/or exposure to unbalanced diet during prenatal stages. However, we must be cautious in extrapolating these findings to humans, due to interspecies differences, especially regarding the greater complexity and different composition of the human diet in comparison with commercial diets for rodents. For example, it must be considered the low lipid content of the standard diet for rodents compared to the human diet. It also remains to be determined whether a longer period of exposure to WD, probably more similar to the human situation, could affect the effectiveness of implementing a healthier diet during lactation observed in the present study.
4. Experimental Section
Animals and Experimental Design
The study was based on samples previously obtained.[ 13 ] The animal protocol was reviewed and approved by the Bioethical Committee of the University of the Balearic Islands (Exp. 2018/13/AEXP, January 23, 2019), and measures for the use and care of laboratory animals of the University were followed.
Virgin female Wistar rats were housed under controlled conditions, which were 22°C and a 12 h light‐dark period, and with free access to food and water. Rats were divided into two groups depending on the diet they were provided 1 month before being mated with male rats: dams fed with a standard chow diet (SD; 3.3 kcal g–1, with 8.4% calories from fat, 72.4% from carbohydrates, and 19.3% from protein; Safe, Augy, France) or with a high‐fat and high‐sucrose diet (WD; 4.7 kcal g–1, with 40.0% calories from fat, 43.0% from carbohydrates, and 17.0% from proteins; Research Diets, New Brunswick, USA). FA composition of both diets was detailed in Supplementary Table S1. After mating, pregnant dams were housed individually and fed with their previously assigned diets during gestation. After delivery, litters were adjusted to 10 pups per dam at postnatal day (PND) 1, trying to keep five males and five females whenever possible. Throughout lactation, dams fed with SD continued with this diet (CON‐dams, n = 8), and of those fed WD, half continued to be fed WD (WD‐dams, n = 9) and the other half were moved to SD (REV‐dams, n = 10). Milk samples from dams were collected at three time points of lactation (lactation day [LD] 5, LD10, and LD15) as previously described.[ 13 ] At PND15 and PND21, blood from the offspring of CON‐, WD‐, and REV‐dams (CON‐pups, WD‐pups, and REV‐pups, respectively) was collected in a capillary from the end of the tail, and plasma was obtained by centrifugation (1000 g). Plasma samples from the same litter and sex obtained at PND15 were pooled. Dams were sacrificed by decapitation at LD21 during the first 2 h of the beginning of the light cycle. Mammary gland and retroperitoneal white adipose tissue (rWAT) were removed and frozen in liquid nitrogen. Collected milk, plasma, and tissue samples were stored at −80°C until further analysis.
Lipidomics
Milk samples (at LD5, LD10, and LD15) and plasma samples from offspring (at PND15) were extracted using a biphasic solvent system of cold methanol (Fisher Scientific, Waltham, MA, USA), methyl tert‐butyl ether (MTBE) (Sigma–Aldrich, St. Louis, MO, USA), and 10% methanol.[ 44 ] In more detail, 165 and 600 µL of methanol and MTBE (respectively) containing a mixture of odd chain and deuterated lipid internal standards were added to 25 µL of milk or plasma sample. After shaking for 30 s, 165 µL of 10% methanol with deuterated polar metabolite internal standards were added, shaken again for 30 s, and centrifuged at 16 000 g for 10 min at 4°C. For the analysis of lipid species in plasma samples, an aliquot of 100 µL of the upper organic phase was collected, evaporated using a SpeedVac (Savant SPD121P, Thermo Scientific, USA) and resuspended in 100 µL of methanol with [12‐[(cyclohexylamino) carbonyl]amino]‐dodecanoic acid (CUDA) internal standard (Cayman Chemical, Ann Arbor, MI, USA). In the case of milk samples, one aliquot of 10 µL of the upper organic phase was collected, evaporated, and resuspended in 300 µL of methanol with CUDA for the analysis of abundant TG, and another aliquot of 100 µL of the upper phase was also collected, evaporated, and resuspended in 100 µL of 80% methanol with CUDA for the analysis of minor polar lipid species.[ 44 ] The resuspended aliquots were shaken for 30 s, centrifuged at 16 000 g for 5 min at 4°C, and analyzed using lipidomics platform.
Experimental samples, together with quality control (constituted by a set of diluted pools of all experimental samples) and blank samples, were randomized and measured. The LC‐MS system consisted of a Vanquish UHPLC System (Thermo Fisher Scientific, Bremen, Germany) coupled to a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). An Acquity UPLC BEH C18 column (50 × 2.1 mm; 1.7 µm) coupled to an Acquity UPLC BEH C18 VanGuard pre‐column (5 × 2.1 mm; 1.7 µm) (Waters, Milford, MA, USA) was used for lipids separation, and later detection was performed in positive and negative electrospray ionization (ESI) mode.
RNA Extraction
Total RNA was extracted from mammary gland and rWAT of dams at LD21 using TriPure Reagent (Roche Diagnostic Gmbh, Mannheim, Germany) according to the instructions of the manufacturer. Isolated RNA was quantified using the NanoDrop ND‐1000 spectrophotometer (NanoDrop Technologies Llc, Wilmington, DE, USA) and its integrity confirmed using 1% agarose gel electrophoresis.
Real‐Time Quantitative Polymerase Chain Reaction (RT‐qPCR) Analysis
RT‐qPCR was used to measure mRNA expression levels of sterol regulatory element binding transcription factor 1 (Srebf1), FA synthase (Fasn), acetyl‐CoA carboxylase alpha (Acaca), patatin‐like phospholipase domain containing 2 (Pnpla2), lipase E hormone sensitive type (Lipe), lipoprotein lipase (Lpl), Cd36 molecule (Cd36), FA binding protein 4 (Fabp4), carnitine palmitoyltransferase 1b (Cpt1b), insulin receptor (Insr) and leptin receptor (Lepr) in mammary gland and rWAT, and adiponectin (Adipoq), tumor necrosis factor alpha (TNF‐α), interleukin 6 (IL‐6), and cyclooxygenase 2 (COX‐2, also known as Prostaglandin‐endoperoxide synthase 2 [PTGS2]) in mammary gland. Total RNA (0.25 µg in a final volume of 5 µL) was denatured at 65°C for 10 min and then reverse transcribed to cDNA using MuLV reverse transcriptase (Applied Biosystem, Madrid, Spain) at 25°C for 10 min, 37°C for 50 min, and 70°C for 15 min. RT‐qPCR reactions were performed from diluted cDNA template (1/5 for mammary gland and 1/10 for rWAT), forward and reverse primers (10 µM) and Power SYBER Green PCR Master Mix (Applied Biosystems, CA, USA), using the Applied Biosystems StepOne Plus Real‐time PCR Systems (Applied Biosystems) with the following temperature profile: 95°C for 10 min, and 40 two‐temperature cycles at 95°C for 15 s, and 60°C for 1 min. A melting curve was produced after each run according to the instructions of the manufacturer to confirm the purity of the products. The threshold cycle was determined by the StepOne software v2.2.2 and the relative expression of each gene was expressed as a percentage of CON dams, using the 2−ΔΔCt method. Guanosine diphosphate dissociation inhibitor (Gdi) was used as reference gene. Primers were obtained from Sigma (Madrid, Spain) and its sequences and amplicon size are detailed in Supplementary Table S2.
Statistical Analysis
Data were represented as the mean ± SEM. Differences between experimental groups were analyzed by one‐way ANOVA, followed by Bonferroni or a least significant difference (LSD) post hoc test, and single comparisons between groups were carried out by Mann–Whitney U test. The relation between two variables was assessed using the Pearson's correlation coefficient. The specific tests used for each comparison are indicated in the figure legends. Normality and homogeneity of variances of the data were evaluated by Shapiro–Wilk and Bartlett test, respectively. Analyses were carried out with SPSS for Windows (SPSS, Chicago, IL, USA), with the threshold of significance at p < 0.05. PCAs were performed after data normalization and scaling using MetaboAnalyst 5.0 software.[ 45 ]
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
C.P., M.P., A.P., J.K., and O.K. designed research. P.C., C.A.P., and M.P. conducted research. P.C., O.K., C.A.P., M.P., and C.P. analyzed data. P.C., O.K., J.K., C.A.P., A.P., M.P., and C.P. participated in the discussion of the results. P.C. and C.P. wrote the first version of the paper. All authors revised the paper, and approved the final manuscript.
Supporting information
Supporting Information
Acknowledgements
This research was supported by Proyecto PGC2018‐097436‐B‐I00 financed by MCIN/AEI/10.13039/501100011033/and by “FEDER Una manera de hacer Europa”; and by grant from the Czech Academy of Sciences (Lumina quaeruntur LQ200111901). The Research Group “Nutrigenomics, Biomarkers and Risk Evaluation” (NuBE) received financial support from Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y Nutrición, CIBERobn, and is a member of the European Research Network of Excellence NuGO (The European Nutrigenomics Organization, EU Contract: no. FP6‐506360). The authors would like to acknowledge the Metabolomics Core Facility at the Institute of Physiology of the Czech Academy of Sciences for lipidomics profiling.
Castillo P., Kuda O., Kopecky J., Pomar C. A., Palou A., Palou M., Picó C., Reverting to a Healthy Diet during Lactation Normalizes Maternal Milk Lipid Content of Diet‐Induced Obese Rats and Prevents Early Alterations in the Plasma Lipidome of the Offspring. Mol. Nutr. Food Res. 2022, 66, 2200204. 10.1002/mnfr.202200204
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Langley‐Evans S. C., Proc. Nutr. Soc. 2006, 65, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suzuki K., J. Dev. Orig. Health Dis. 2018, 9, 266. [DOI] [PubMed] [Google Scholar]
- 3. Picó C., Palou M., Priego T., Sánchez J., Palou A., Front. Physiol. 2012, 3, 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marciniak A., Patro‐Małysza J., Kimber‐Trojnar Ż., Marciniak B., Oleszczuk J., Leszczyńska‐Gorzelak B., Taiwan. J. Obstet. Gynecol. 2017, 56, 133. [DOI] [PubMed] [Google Scholar]
- 5. Plagemann A., Harder T., Schellong K., Schulz S., Stupin J. H., Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 641. [DOI] [PubMed] [Google Scholar]
- 6. Zambrano E., Ibáñez C., Martínez‐Samayoa P. M., Lomas‐Soria C., Durand‐Carbajal M., Rodríguez‐González G. L., Arch. Med. Res. 2016, 47, 1. [DOI] [PubMed] [Google Scholar]
- 7. Wahlig J. L., Bales E. S., Jackman M. R., Johnson G. C., McManaman J. L., MacLean P. S., Obesity (Silver Spring) 2012, 20, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pomar C. A., Van Nes R., Sánchez J., Picó C., Keijer J., Palou A., Int. J. Obes. (Lond). 2017, 41, 1279. [DOI] [PubMed] [Google Scholar]
- 9. Skowronski A. A., Shaulson E. D., Leibel R. L., LeDuc C. A., Int. J. Obes. (Lond). 2022, 46, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Picó C., Reis F., Egas C., Mathias P., Matafome P., Eur. J. Clin. Invest. 2021, 51, e13482. [DOI] [PubMed] [Google Scholar]
- 11. Bautista C. J., Montanõ S., Ramirez V., Morales A., Nathanielsz P. W., Bobadilla N. A., Zambrano E., Br. J. Nutr. 2016, 115, 538. [DOI] [PubMed] [Google Scholar]
- 12. Alexandre‐Gouabau M. C., David‐Sochard A., Royer A. L., Parnet P., Paillé V., Int. J. Mol. Sci. 2020, 21, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pomar C. A., Castillo P., Palou M., Palou A., Picó C., J. Nutr. Biochem. 2022, 109043. [DOI] [PubMed] [Google Scholar]
- 14. Grote V., Verduci E., Scaglioni S., Vecchi F., Contarini G., Giovannini M., Koletzko B., Agostoni C., Eur. J. Clin. Nutr. 2016, 70, 250. [DOI] [PubMed] [Google Scholar]
- 15. Innis S. M., Early Hum. Dev. 2007, 83, 761. [DOI] [PubMed] [Google Scholar]
- 16. Innis S. M., Am. J. Clin. Nutr. 2014, 99, 734S. [DOI] [PubMed] [Google Scholar]
- 17. Neville M. C., Picciano M. F., Annu. Rev. Nutr. 1997, 17, 159. [DOI] [PubMed] [Google Scholar]
- 18. Demmelmair H., Baumheuer M., Koletzko B., Dokoupil K., Kratl G., Adv. Exp. Med. Biol. 2001, 501, 169. [DOI] [PubMed] [Google Scholar]
- 19. Keikha M., Bahreynian M., Saleki M., Kelishadi R., Breastfeed. Med. 2017, 12, 517. [DOI] [PubMed] [Google Scholar]
- 20. Bravi F., Wiens F., Decarli A., Dal Pont A., Agostoni C., Ferraroni M., Am. J. Clin. Nutr. 2016, 104, 646. [DOI] [PubMed] [Google Scholar]
- 21. Val‐Laillet D., Besson M., Guérin S., Coquery N., Randuineau G., Kanzari A., Quesnel H., Bonhomme N., Bolhuis J. E., Kemp B., Blat S., Le Hüerou‐Luron I., Clouard C., FASEB J. 2017, 31, 2037. [DOI] [PubMed] [Google Scholar]
- 22. Pomar C. A., Castro H., Picó C., Palou A., Sánchez J., Mol. Nutr. Food Res. 2019, 63, 1900504. [DOI] [PubMed] [Google Scholar]
- 23. Del Prado M., Villalpando S., Gordillo J., Hernández‐Montes H., J. Nutr. 1999, 129, 1574. [DOI] [PubMed] [Google Scholar]
- 24. Pomar C. A., Kuda O., Kopecky J., Rombaldova M., Castro H., Picó C., Sánchez J., Palou A., Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158556. [DOI] [PubMed] [Google Scholar]
- 25. Smith S., J. Dairy Sci. 1980, 63, 337. [DOI] [PubMed] [Google Scholar]
- 26. Saben J. L., Bales E. S., Jackman M. R., Orlicky D., MacLean P. S., McManaman J. L., PLoS ONE 2014, 9, e98066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramos P., Martín‐Hidalgo A., Herrera E., Endocrinology 1999, 140, 1089. [DOI] [PubMed] [Google Scholar]
- 28. Hommelberg P. P. H., Plat J., Langen R. C. J., Schols A. M. W. J., Mensink R. P., Am. J. Physiol. Endocrinol. Metab. 2009, 296, E114. [DOI] [PubMed] [Google Scholar]
- 29. Weigert C., Brodbeck K., Staiger H., Kausch C., Machicao F., Häring H. U., Schleicher E. D., J. Biol. Chem. 2004, 279, 23942. [DOI] [PubMed] [Google Scholar]
- 30. Lladó I., Pons A., Palou A., Biochem. Mol. Biol. Int. 1996, 40, 295. [DOI] [PubMed] [Google Scholar]
- 31. Schuchardt J. P., Huss M., Stauss‐Grabo M., Hahn A., Eur. J. Pediatr. 2010, 169, 149. [DOI] [PubMed] [Google Scholar]
- 32. Khor G. L., Tan S. S., Stoutjesdijk E., Ng K. W. T., Khouw I., Bragt M., Schaafsma A., Dijck‐Brouwer D. A. J., Muskiet F. A. J., Nutrients 2020, 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heird W. C., Lapillonne A., Annu. Rev. Nutr. 2005, 25, 549. [DOI] [PubMed] [Google Scholar]
- 34. Vernon R. G., J. Dairy Res. 2005, 72, 460. [DOI] [PubMed] [Google Scholar]
- 35. Ganapathy S., Indian Pediatr. 2009, 46, 785. [PubMed] [Google Scholar]
- 36. Lauritzen L., Hansen H. S., Jorgensen M. H., Michaelsen K. F., Prog. Lipid Res. 2001, 40, 1. [DOI] [PubMed] [Google Scholar]
- 37. Diau G. Y., Hsieh A. T., Sarkadi‐Nagy E. A., Wijendran V., Nathanielsz P. W., Brenna J. T., BMC Med. 2005, 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zambrano E., Martínez‐Samayoa P. M., Rodríguez‐González G. L., Nathanielsz P. W., J. Physiol. 2010, 588, 1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monks J., Orlicky D. J., Stefanski A. L., Libby A. E., Bales E. S., Rudolph M. C., Johnson G. C., Sherk V. D., Jackman M. R., Williamson K., Carlson N. E., MacLean P. S., McManaman J. L., Nutr. Diabetes 2018, 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rudolph M. C., Young B. E., Lemas D. J., Palmer C. E., Hernandez T. L., Barbour L. A., Friedman J. E., Krebs N. F., Maclean P. S., Int. J. Obes. (Lond). 2017, 41, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jin Z., Du Y., Schwaid A. G., Asterholm I. W., Scherer P. E., Saghatelian A., Wan Y., Endocrinology 2015, 156, 1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nørregaard R., Kwon T. H., Frøkiær J., Kidney Res. Clin. Pract. 2015, 34, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kam P. C. A., See A. U. L., Anaesthesia 2000, 55, 442. [DOI] [PubMed] [Google Scholar]
- 44. Tsugawa H., Ikeda K., Takahashi M., Satoh A., Mori Y., Uchino H., Okahashi N., Yamada Y., Tada I., Bonini P., Higashi Y., Okazaki Y., Zhou Z., Zhu Z. J., Koelmel J., Cajka T., Fiehn O., Saito K., Arita M., Arita M., Nat. Biotechnol. 2020, 38, 1159. [DOI] [PubMed] [Google Scholar]
- 45. Pang Z., Chong J., Zhou G., De Lima Morais D. A., Chang L., Barrette M., Gauthier C., Jacques P. É., Li S., Xia J., Nucleic Acids Res. 2021, 49, W388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


