Abstract
Myristica fragrans (Houtt.) is an evergreen tree native to the Maluku Islands, Indonesia. M. fragrans kernel is extensively used in Indian traditional medicines to treat various diseases. Several studies attempt to compile and interpret the pharmacological potential of Myristica fragrans (Houtt.) aqueous and various chemical extracts. Thus, the pharmacological potential of nutmeg essential oil has not been reviewed phytochemically and pharmacologically. Therefore, the present study aimed to share appropriate literature evidence regarding the plant essential oil chemical composition and therapeutic potential of Myristica fragrans essential oil (MFEO). MFEO of leaf, mace, kernel, and seed were used worldwide as potential Ayurvedic medicine and fragrance. MFEO extracted by various methods and oil yield was 0.7–3.2, 8.1–10.3, 0.3–12.5, and 6.2–7.6% in leaf, mace, seed, and kernel. The primary chemical constituents of MFEO were sabinene, eugenol, myristicin, caryophyllene, β‐myrcene, and α‐pinene. Clinical and experimental investigations have confirmed the antioxidant, antimicrobial, antiinflammatory, anticancer, antimalarial, anticonvulsant, hepatoprotective, antiparasitic, insecticidal, and nematocidal activities of MFEO. It is the first attempt to compile oil yield, composition, and the biological activities of MFEO. In future, several scientific investigations are required to understand the mechanism of action of MFEO and their bioactive constituents.
Keywords: biological activities, chemical composition, essential oil, Myristica fragrans , myristicin, nutmeg, sabinene
1. INTRODUCTION
Nutmeg (Myristica fragrans Houtt.) belongs to the Myristicaceae family. The plant is native to the Maluku Islands of Indonesia; however, it is extensively distributed to Grenada, India, Sri Lanka, Mauritius, South Africa, and the USA (Francis, James, Varughese, & Nair, 2019). The nutmeg seed has outer, red arils called mace and an inner, brown kernel called nutmeg, both of which are used as a spice (Abourashed & El‐Alfy, 2016; Periasamy, Karim, Gibrelibanos, Gebremedhin, & Gilani, 2016). In traditional medicine, different parts of the plant mentioned traditionally are used to cure various diseases. However, in Indian Ayurvedic medicine, nutmeg has been used to treat anxiety, nausea, diarrhea, cholera, stomach cramps, parasites, paralysis, and rheumatism and is also used as an aphrodisiac (Gils & Cox, 1994; Ziyatdinova, Ziganshina, Cong, & Budnikov, 2016). Furthermore, in Pakistan traditional medicine, the nutmeg plant has been used to treat hypertension (Malik et al., 2018).
The essential oil (EO) of M. fragrans is a colorless‐to‐light yellow liquid with a distinct spicy odor (Nikolic et al., 2021). Several types of research on the plant's EO have been conducted in various parts of the world (Ashokkumar, Vellaikumar, Murugan, Dhanya, & Aiswarya, 2022; Atta‐ur‐Rahman et al., 2000; Dupuy et al., 2013; Ogunwande, Olawore, Adeleke, & Ekundayo, 2003). Hydrodistillation, steam distillation, supercritical fluid extraction, microwave, and ultrasound‐assisted techniques were used to extract the MFEO (Ashokkumar et al., 2022; Azwanida, 2015). The oil yield of nutmeg kernels ranged from 5 to 15% (Barceloux, 2009). MFEO has chiefly monoterpenes (sabinene, β‐pinene, β‐terpineol, p‐menth‐8‐en‐1‐ol, and terpinen‐4‐ol), phenylpropene (eugenol, methyl eugenol, and myristicin), sesquiterpenes (germacrene D and β‐bergamotene), and other constituents (Atta‐ur‐Rahman et al., 2000; Dupuy et al., 2013; Francis et al., 2019). The major constituents of leaf were sabinene, eugenol, myristicin, caryophyllene, and β‐myrcene. Sabinene, α‐pinene, β‐pinene, d‐limonene, and 3‐carene were predominant constituents of mace. The major constituents of the kernel and seed were sabinene, α‐pinene, β‐pinene, d‐limonene, and β‐myrcene (Ashokkumar et al., 2022).
Several scientific reports say that MFEO has potential antioxidant, antimicrobial, antiinflammatory, antiulcer, anticancer, aphrodisiac, and various other activities (Das et al., 2020; Hiranrat & Hiranrat, 2019; Kholibrina & Aswandi, 2021; Nikolic et al., 2021; Özkan et al., 2018; Purkait, Bhattacharya, Bag, & Chattopadhyay, 2018; Thileepan, Thevanesam, & Kathirgamanathar, 2018; Valente, Jham, Jardim, Dhingra, & Ghiviriga, 2015). The pharmacological potential of M. fragrans crude extracts/various organic chemical extracts was reviewed and published by several researchers, but the pharmacological activities of MFEO and their active constituents were not yet compiled. Therefore, the present study aimed to provide an overview and critically analyze the reported botanical description, essential oil yield, chemical composition of MFEO, and its biological and pharmacological potentials conferring to published literature up to March 2022 and identify the remaining gaps for further investigations. Furthermore, the review seeks to take peoples and researchers attention to the wide‐ranging biological features of MFEO and its active ingredients to improve their use in the future.
2. MATERIALS AND METHODS
A comprehensive review of the literature on M. fragrans EO yield, chemical composition, pharmacology, and biological studies was conducted, with data compiled using a variety of search engines and publishing houses, including Science Direct, Springer, PubMed, Google Scholar, Taylor and Francis, Frontiers, and NCBI. Other literature sources, such as Wikipedia, ethnobotanical publications, and various online domains, were also examined to obtain as much information about M. fragrans as possible. Myristica fragrans, nutmeg, mace, therapeutic uses of Myristica fragrans essential oil, phytochemicals of Myristica fragrans essential oil, pharmacological activities of Myristica fragrans essential oil, botany of Myristica fragrans, and numerous synonyms were also utilized in the literature search.
3. BOTANICAL DESCRIPTION
Myristica fragrans is an evergreen tree, grown up to 20–25 ft high with greyish brown soft bark and spreading branches (Periasamy et al., 2016). The plant is grown well in warm, humid climate, with an elevation of 1,000 m above sea level with 150–250 cm rainfall. The plant has been reported to have four chromosome numbers such as 2n = 38 (Dhamayanthi & Krishnamoorthy, 1999), 2n = 41 (Nair, 2019), 2n = 42 (Purseglove et al., 1981), and 2n = 44 (Nair, 2019). However, chromosome number 2n = 44 is predominant at the nutmeg seedling stage (Nair, 2019). The leaves are aromatic, dark green, glossy above, alternate, oblong, glabrous, and acuminate (Periasamy et al., 2016). Flowers are usually dioecious, occasionally monoecious with variable sex expression, small axillary, sub‐umbellate racemes, compound, or sometimes forked (Haldankar et al., 2007). The pedicels and peduncles have a glabrous appearance. The fruit has a fleshy pericarp and is spherical; the fruit skin is yellowish and splits into two longitudinal valves. The mace (aril) is a fleshy scarlet aril that is laciniate, folded, and envelops the nut when wet; when dry, it is considerably hornier, yellowish‐brown in color, and highly brittle (Wallis, 1985). The nut is oval or broadly ovate, with a hard, rough, dark‐brown, glossy shell that is pale and smooth on the inside and is approximately half a line thick. When young, the seed/kernel is oval, pale brown, and soft, but it quickly shrivels and has irregular, vertical lines or furrows on its surface and rich in oil (Naeem, Rehman, Mushtaq, & Ghania, 2016; Periasamy et al., 2016). The tree bears fruit all year, but the best time to harvest is between April and November. The morphological identification of whole plant, leaves, fruit, seed, rind, kernel, and mace (aril) of M. fragrans is shown in Figure 1.
FIGURE 1.

Morphological identification of Myristica fragrans Houtt. (a) The young tree; (b) leaves of the plant; (c) fruit; (d) seed; (e) rind; (f) kernel (nutmeg); (g) mace (aril); (h) ground powder of mace; and (i) mace essential oil
3.1. MFEO yield
The essential oil yield of M. fragrans leaves, mace, seed, and kernel were 0.7–3.2, 8.1–10.3, 0.3–12.5, and 6.2–7.6%, respectively. The variation in EO depends upon the source and extraction method (Table 1). MFEO was extracted by various methods including, hydrodistillation (Ashokkumar et al., 2022; Carolina & Maman, 2016; Ibrahim et al., 2020; Mickus et al., 2021; Muchtaridi et al., 2010; Nikolic et al., 2021; Waman, 2020), steam distillation (Al‐Jumaily & Al‐Amiry, 2012; Carolina & Maman, 2016; Purseglove et al., 1981), microwave‐assisted hydrodistillation (Sagarika et al., 2018), and CO2 supercritical fluid extraction (Hanif et al., 2010). Ashokkumar et al. (2022) found that the Western Ghats, southern India, grown nutmeg leaf EO yield (3.2%) was greater than that found in Bogor, West Java, Indonesia, grown nutmeg leaf (0.7%; Carolina & Maman, 2016). Furthermore, the yields of nutmeg seed oil grown in South India (5.2%) were lower than those grown in Brazil (7.1%; Valente et al., 2015). The differences in EO yield of nutmeg might be due to changes in soil type, location, origin, extraction methods, and environmental conditions (Ashokkumar et al., 2022).
TABLE 1.
Yield of essential oil from various parts of Myristica fragrans
| Parts | Extraction method | Volatile oil (%) | References |
|---|---|---|---|
| Leaf | Hydrodistillation | 3.2 | Ashokkumar et al. (2022) |
| Leaf | Steam distillation | 0.7 | Carolina and Maman (2016) |
| Mace | Hydrodistillation | 8.1 | Ashokkumar et al. (2022) |
| Mace | Hydrodistillation | 10.3 | Muhammad et al. (2016) |
| Kernel | Hydrodistillation | 6.2 | Ashokkumar et al. (2022) |
| Kernel | Hydrodistillation | 7.6 | Muhammad et al. (2016) |
| Seed | Hydrodistillation | 5.2 | Ashokkumar et al. (2022) |
| Seed | Hydrodistillation | 5.1–7.2 | Waman (2020) |
| Seed | Hydrodistillation | 6.9 | Muchtaridi, Subarnas, Apriyantono, and Mustarichie (2010) |
| Seed | Hydrodistillation | 5.8 | Ibrahim, Cantrell, Jeliazkova, Astatkie, and Zheljazkov (2020) |
| Seed | Hydrodistillation | 7.1 | Nikolic et al. (2021) |
| Seed | Hydrodistillation | 8.4 | Mickus et al. (2021) |
| Seed | Steam distillation | 4.5–7.5 | Al‐Jumaily and Al‐Amiry (2012) |
| Seed | Steam distillation | 0.3 | Carolina and Maman (2016) |
| Seed | Steam distillation | 12.5 | Purseglove, Brown, Green, and Robbins (1981) |
| Seed | Microwave‐assisted hydrodistillation | 3.8–5.8 | Sagarika et al. (2018) |
| Kernel | CO2 supercritical fluid extraction | 5.9 | Hanif et al. (2010) |
Among the extraction methods, classical hydrodistillation by Clevenger apparatus is a widely used method of volatile oil estimation than other techniques. Owing to the prominence of nutmeg essential oil yield have been reviewed and summarized in the Table 1.
3.2. MFEO chemical composition
Studies in recent research report showed that Western Ghats of South India grown nutmeg leaf oil (3.2% v/w) was found to have sabinene (17.2%), eugenol (16.6%), myristicin (9.1%), caryophyllene (8.8%), α‐pinene (5.4%), β‐pinene (6.4%), limonene (5.0%), β‐myrcene (4.7%), copaene (3.2%), germacrene D (3.0%), and 3‐Carene (2.7%), while mace oil (8.1% v/w) had sabinene (38.4%), α‐pinene (8.2%), β‐pinene (7.6%), limonene (7.1%), myristicin (5.9%), 3‐carene (5.1%), 4‐carene (4.2%), safrole (3.9%), β‐phellandrene (3.6%), and terpinen‐4‐ol (3.0%) as the major constituents (Ashokkumar et al., 2022). Furthermore, the Western Ghats (South India) grown nutmeg kernel (without rind/shell) are predominantly comprised of sabinene, α‐pinene, β‐pinene, limonene, and β‐myrcene (Table 2). However, these predominant chemical components were greater than those found in Pakistan grown nutmeg kernel oil (Atta‐ur‐Rahman et al., 2000). The higher concentration of oil components could be due to changes in soil type, location, season, and cultivars (Ashokkumar et al., 2021a; Ashokkumar et al., 2022). However, mace oil was rich in γ‐terpinene, safrole, terpinen‐4‐ol, α‐pinene, sabinene, and myristicin (Muhammad et al., 2016). Likewise, seeds from Pakistan contained EO consisting of sabinene, followed by α‐pinene, cymene, terpinen‐4‐ol, elemicin, and safrole (Table 2). Interestingly, the other nutmeg seed essential oil from Andaman & Nicobar Islands, India, extracted by hydrodistillation method, contained higher myristicin, sabinene, α‐thujene, α‐pinene, 4‐terpineol, limonene, γ‐terpinene, and elemicin (Waman, 2020). In a recent study, the Western Ghats (South India) grown nutmeg seed (with rind/shell) oil was predominantly composed of sabinene (27.7%) (Ashokkumar et al., 2022) and it was found to have a two‐fold lower concentration of sabinene (52.8%) in Grenada grown nutmeg seed EO (Mickus et al., 2021). The molecular structures of major EO constituents isolated from various parts of M. fragrans were drawn by ChemDraw software as shown in Figure 2.
TABLE 2.
Major constituents of Myristica fragrans essential oils from various geographical origins
| Geographical origins | Parts | Constituents | Reference |
|---|---|---|---|
| India (Western Ghats) | Leaf | Sabinene (17.2%), eugenol (16.6%), myristicin (9.1%), caryophyllene (8.8%), α‐pinene (5.4%), β‐pinene (6.4%), limonene (5.0%), β‐Myrcene (4.7%), copaene (3.2%), germacrene D (3.0%) | Ashokkumar et al. (2022) |
| India (Western Ghats) | Mace | Sabinene (38.4%), α‐pinene (8.2%), β‐pinene (7.6%), limonene (7.1%), myristicin (5.9%), 3‐carene (5.1%), 4‐carene (4.2%), safrole (3.9%), β‐phellandrene (3.6%), terpinen‐4‐ol (3.0%) | Ashokkumar et al. (2022) |
| Pakistan | Mace | γ‐Terpinene (19.1%), safrole (18.2%), terpinen‐4‐ol (12.7%), α‐pinene (11.6%), sabinene (11.2%), myristicin (7.5%) | Muhammad et al. (2016) |
| Sri Lanka | Kernel | Sabinene (43.4%), α‐pinene (17.5%), β‐pinene (12.1%), α‐phellandrene (4.3%), limonene (3.2%), terpinen‐4‐ol (3.5%), myristicin (3.0%) | Sarath‐Kumara, Jans, and Dharmadasa (1985) |
| India (Western Ghats) | Kernel | Sabinene (38.0%), α‐pinene (19.2%), β‐pinene (14.9%), limonene (7.2%), β‐myrcene (3.4%) | Ashokkumar et al. (2022) |
| India (Western Ghats) | Seed | Sabinene (27.7%), α‐pinene (21.8%), β‐pinene (18.2%), limonene (6.4%), β‐myrcene (2.9%) | Ashokkumar et al. (2022) |
| Indonesia (West Java) | Seed | Sabinene (21.4%), α‐pinene (10.2%), myristicin (10.6%), 4‐terpineol (13.9%), safrole (4.3%), γ‐terpinene (4.0%) | Muchtaridi et al. (2010) |
| India (Andaman & Nicobar Islands) | Seed | Myristicin (20.3%), sabinene (19.3%), α‐thujene (12.1%), α‐pinene (9.5%), 4‐terpineol (7.1%), limonene (5.9%), γ‐terpinene (4.1%), elemicin (4.0%) | Waman (2020) |
| Pakistan | Seed | Sabinene (18.9%), α‐pinene (15.8%), cymene (15.2%), terpinen‐4‐ol (11.7%), elemicin (11.5%), safrole (6.2%) | Muhammad et al. (2016) |
| Brazil | Seed | β‐Pinene (12.4–26.0%), sabinene (9.1–25.0%), α‐pinene (10.5–14.1%), myristicin (10.9%), γ‐terpinene (8.5%), limonene (6.3%), terpinen‐4‐ol (3.5%) | Valente, Jham, Dhingra, and Ghiviriga (2011); Cossetin et al. (2021) |
| Grenada | Seed | Sabinene (52.8%), α‐pinene (13.5%), α‐terpinyl acetate (6.0%), limonene (7.0%), γ‐terpinene (4.1%), β‐pinene (3.6%), | Mickus et al. (2021) |
FIGURE 2.
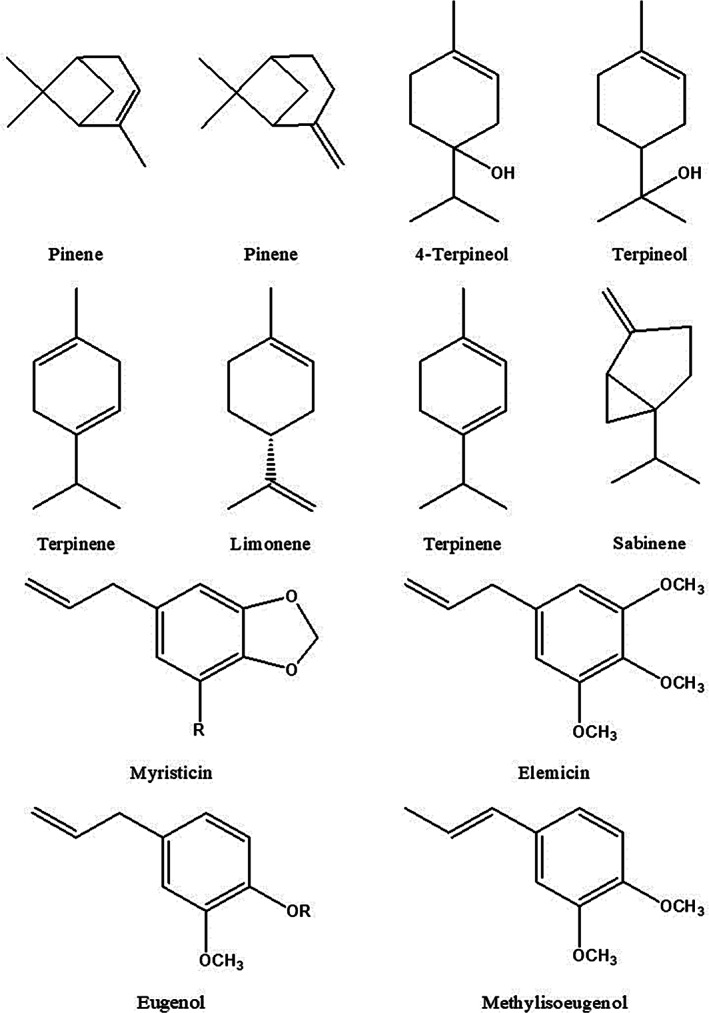
Some major compounds of Myristica fragrans Houtt. essential oils
The yield of minor EO constituents of South India grown nutmeg mace are α‐phellandrene (1.9%), γ‐asarone (1.4%), p‐cymene (1.1%), α‐thujene (1.0%), methyleugenol (0.6%), caryophyllene (0.5%), α‐terpineol (0.4%), eugenol (0.4%), copaene (0.4%), β‐linalool (0.3%), germacrene‐D (0.3%), β‐terpineol (0.2%), and γ‐elemene (0.2%), while kernel oil had 3‐carene (1.8%), terpinen‐4‐ol (1.7%), copaene (1.7%), α‐thujene (1.5%), isoterpinolene (1.3%), γ–terpinene (1.2%), β‐terpineol (0.8%), γ‐asarone (0.8%), α‐phellandrene (0.8%), γ‐elemene (0.7%), myristicin (0.7%), p‐cymene (0.7%), 4‐carene (0.7%), caryophyllene (0.5%), p‐menth‐8‐en‐1‐ol (0.5%), safrole (0.5%), α‐terpinyl acetate (0.4%), β‐phellandrene (0.3%), camphene (0.3%), and α‐cubebene (0.3%) (Ashokkumar et al., 2022). Furthermore, the minor constituents of nutmeg seed essential oil are terpinen‐4‐ol (2.4%), copaene (2.4%), γ‐asarone (2.3%), β‐phellandrene (2.2%), myristicin (1.9%), α‐thujene (1.3%), 3‐carene (1.2%), β‐terpineol (1.1%), γ‐terpinene (1.0%), isoterpinolene (0.9%), p‐menth‐8‐en‐1‐ol (0.9%), γ‐elemene (0.8%), safrole (0.7%), methyleugenol (0.7%), 4‐carene (0.7%), p‐cymene (0.7%), α‐bergamotene (0.6%), α‐phellandrene (0.5%), camphene (0.4%), and α‐terpinyl acetate (0.4%) (Ashokkumar et al., 2022; Mickus et al., 2021; Waman, 2020). The variation in the minor components of MFEO might be due to the EO of four plant parts of nutmeg used.
4. BIOLOGICAL AND PHARMACOLOGICAL APPLICATIONS OF MFEO
The pharmacological activities of M. fragrans extracts have been extensively reviewed (Asgarpanah & Kazemivash, 2012; Barceloux, 2009; Gupta, 2020; Jaiswal, Kumar, Singh, & Singh, 2009), which was not covered by pharmacological activities of MFEO. Therefore, we decided to review the pharmacological studies of MFEO and its active constituents up to 2022 that were not covered by previous reviews. The chemical constituents of various parts of MFEO are affected by several factors. However, different therapeutic applications such as antioxidant, antimicrobial, anticancer, and other miscellaneous activities are reported for MFEO and its active constituents in various literature. Furthermore, biological and pharmacological potential oil MFEO and its bioactive constituents were summarized in Tables 3 and 4, respectively. The potential biological and pharmacological activities of MFEO were diagrammatically presented in Figure 3.
TABLE 3.
Biological activities of Myristica fragrans essential oil and its bioactive components
| Biological activities | MFEO/active constituent | In vitro/in vivo | Target/ model | Control(s) | IC50/dosage | Results/remarks | Reference |
|---|---|---|---|---|---|---|---|
| Antioxidant activity | MFEO | In vitro | DPPH assay | Negative: Ethanol | EC50: 1.35 mg/ml | Good antioxidant activity observed after 20 min incubation | Nikolic et al. (2021) |
| Antioxidant activity |
Elemicin 4‐terpineol Myristicin |
In vitro | DPPH (Trolox equivalents) assay | Negative: Methanol |
IC50:11.78 μM/g IC50:1.48 μM/g IC50:3.24 μM/g |
The most potential antioxidant compound in the DPPH test was elemicin | Adiani, Gupta, Chatterjee, Variyar, and Sharma (2015) |
| Antioxidant activity | MFEO | In vitro | DPPH assay | Negative: Ethanol | 0.2–20% concentration | Antioxidant activity was increased in a dose‐dependent manner. | Matulyte et al. (2020) |
| Antibacterial activity | MFEO | In vitro | Pasteurella multocida, E. faecalis, and S. mutans | Negative: DMSO | MIC: 0.2–1.0% | The concentration 0.2%, 0.5%, and 1% effectively suppress the growth of P. multocida, E. faecalis, and S. mutans, respectively. | Matulyte et al. (2020) |
| Antibacterial activity | MFEO | In vitro | Escherichia coli ATCC 25922 | Positive: Gentamicin | MIC: 2.5% | MFEO have 10.89 mm inhibitory effects at 2.5% | Ansory, Fitriani, and Nilawati. (2020) |
| Antibacterial activity | MFEO | In vitro | Bacillus cereus, L. Monocytogenes, and Micrococcus luteus | Negative: DMSO |
MIC: 83.3 μg/ml MIC: 79.2 μg/ml MIC: 88.1 μg/ml |
The inhibition zone was 8.1, 9.0, and 8.2 mm, with the best inhibitory effect against B. cereus, L. monocytogenes, and M. luteus. | Purkait et al. (2018) |
| Antimicrobial activity | MFEO | In vitro | B. subtilis, C. albicans, E. aerogenes, E. faecalis, E. durans, E. faecium, E. coli, K. pneumoniae, and L. innocua, | – | MIC: 3.2 μg/ml −12.5 μg/ml | MFEO had displayed significant antimicrobial activity against all the microorganism. | Özkan et al. (2018) |
| Antibacterial activity | MFEO | In vitro | S. aureus, B. subtilis, E. coli, S. typhi, K. pneumonia, P. aeruginosa, and B. pumilus | Negative: Sterile water |
MIC: 0.05% MBC: 0.1% |
MFEO inhibited both Gram‐positive and Gram‐negative bacteria equally well | Cui et al. (2015) |
| Antifungal activity | MFEO | In vitro | Aspergillus flavus and A. ochraceus | Negative: Ethanol |
MIC: 0.1% MIC: 0.3% |
A. flavus and A. ochraceus growth were suppressed by 43 and 65%, respectively, at a concentration of 0.1% of MFEO. 0.3% inhibited A. flavus and A. ochraceus growth by 84 and 79%, correspondingly |
Valente et al. (2015) |
| Antifungal activity | MFEO | In vitro | Candida tropicalis ATCC 13803, C. krusei ATCC 6258, C. albicans ATCC 90028, C. glabrata ATCC 90030, C. parapsilosis ATCC 22019, C. albicans | Negative: Sterile water |
MIC: 0.31–2.5 μg/ml MBC: 0.31–2.5 μg/ml |
Inhibition zone ranged between 8.3 and 30 mm with anticandidal activity against all tested Candida sp. | Thileepan et al. (2018) |
| Antimicrobial activity | MFEO | In vitro | C. albicans ATCC2091, S. aureus ATCC 25923, B. cereus ATCC 11778, B. luteus, E. coli ATCC 25922, K. pneumoniae ATCC 700603 | Positive: Nystatin | MIC: 0.1% | The inhibition zone was 28, 14, 14, 23, 14, and 15 mm with the best inhibitory effect against C. albicans, S. aureus, B. cereus, B. luteus, E. coli, and K. pneumoniae, respectively | Nikolic et al. (2021) |
| Antimalarial activity | MFEO | In vitro | Plasmodium falciparum D6 | – | MIC: 16 μg/ml | MFEO showed some antimicrobial activities | Ibrahim et al. (2020) |
| Fumigant activity | MFEO | In vivo | Callosobruchus maculatus | Negative: Untreated beans | LC50: 30 μl/L for 24 hr | 100% mortality of adults C. maculatus were observed | Alibabaie and Safaralizadeh (2015) |
|
Larvicidal activity |
MFEO | In vivo | Aedes aegypti | Negative: Distilled water |
LC50: 110.1 μg/ml for 24 hr |
Significant larvicidal activity was noticed | Carolina and Maman (2016) |
| Insecticidal activity | MFEO | In vivo | Lasioderma serricorne | Negative: Hexane | LD50: 19.3 mg/adult for 24 hr | MFEO exhibited strong contact toxicity against L. serricorne | Shu‐Shan et al. (2014) |
| Insecticidal activity | Elemicin | In vivo | Lasioderma serricorne | Negative: Hexane | LD50: 9.8 mg/adult for 24 hr | Elemicin showed strong contact toxicity against L. serricorne | Shu‐Shan et al. (2014) |
| Insecticidal activity | MFEO | In vivo | Chrysomya albiceps and Musca domestica | – | LC50: 2.2 μg/ml LC50: 8.6 μg/ml | Topical application of MFEO was toxic to C. albiceps and M. domestica | Cossetin et al. (2021) |
Note: Not reported; EC₅₀, Half maximal effective concentration; IC₅₀, Half maximal inhibitory concentration; MFEO, Myristica fragrans essential oil; LC50, Lethal concentration 50%; LD50, Lethal dose 50%; MIC, Minimum inhibition concentration; MBC, Minimum bactericide concentration.
TABLE 4.
Pharmacological activities of Myristica fragrans essential oil and its active components
| Biological activities | MFEO/active constituent | In vitro/in vivo | Target/model | Control(s) | IC, LD50/dosage | Results/remarks | Reference |
|---|---|---|---|---|---|---|---|
| Cytotoxic activity | MFEO | In vitro | Vero cell line | – | IC50: 24.8 μg/ml | Low cytotoxicity observed against Vero cell line | Piaru et al. (2012b) |
| Anticancer activity | MFEO | In vitro | Human colon adenocarcinoma cell line (undifferentiated Caco‐2 cells) | Positive: Myristicin | IC50: 250 μg/ml | MFEO were found to have a considerable inhibitory effect on the growth of a colon cancer cell line | Piras et al. (2012) |
| Antiinflammatory activity | MFEO | In vitro | hTERT‐immortalized foreskin fibroblast cell line, BJ‐5ta treated with viral dsRNR mimetic poly I:C |
Negative: 96% ethanol |
LD50: 1 mg/ml | Nutmeg essential oils and hydrolats have shown significant antiinflammatory effect protecting cell viability | Matulyte et al. (2020) |
| Hepatoprotective activity | MFEO | In vivo | Male albino mice | – | 500, 1,000, mg/kg for 24 hr | MFEO‐treated mice showed substantial alteration in the biochemical indicators of liver function in a dose‐dependent manner | Al‐Jumaily & Al‐Amiry, 2012 |
| Antiinflammatory activity | MFEO | In vivo | Complete Freund's adjuvant (CFA)‐injected rats (30 mg/kg/day) | Positive: Diclofenac | 20 mg/kg/day | MFEO potentially alleviated the CFA injection‐induced joint swelling, mechanical allodynia of rats through inhibition of COX‐2 expression, and blood substance P level | Zhang et al. (2016) |
| Anticonvulsant activity | MFEO | In vivo | Male mice | Positive: Valproic acid | LD50:2150 μL/kg for 24 hr | Substantial anticonvulsant activity was observed | Wahab, Ul Haq, Ahmed, Khan, and Raza (2009) |
| Anticonvulsant activity | α‐Terpineol | In vivo | Genetic absence epilepsy rats from Strasbourg (GAERS) rats | Positive: Diazepam | 10, 20, 50 mg/kg, i.p. | Intraperitoneal dose 10 mg/kg was less effective to control seizures and spike and wave discharges (SWDs) in GAERS rats, but 20 mg/ kg and 50 mg/kg (i.p.) decreased the number of seizure episodes and number of SWDs | Islam et al. (2012) |
Note: Not reported; IC50, inhibitory concentration, LD50, lethal concentration; MFEO, Myristica fragrans essential oil.
FIGURE 3.
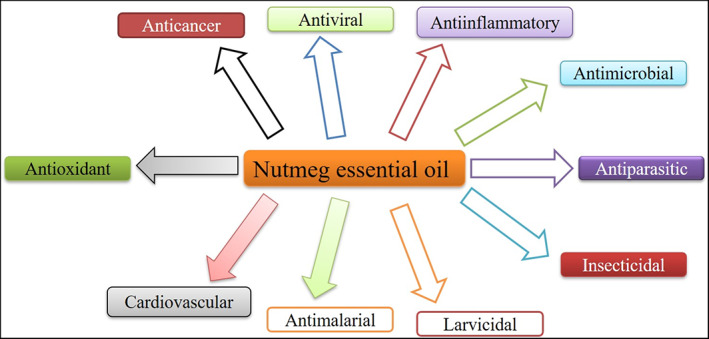
Diagrammatic representation of potential biological and pharmacological activities of M. fragrans essential oil
4.1. Antioxidant activities
The antioxidant activity of MFEO can be dignified through testing chemical assays like DPPH (2,2‐diphenyl‐1‐picrylhydrazyl), ferric reducing/antioxidant power assay (FRAP), inhibition of lipid peroxidation, and bleaching of β‐carotene (Gupta, Bansal, Babu, & Maithil, 2013). The nutmeg essential oil showed 88.7% inhibition in linoleic acid oxidation with an EC50 dosage of 181.4 μg/ml (Piaru et al., 2012a). Matulyte et al. (2020) noticed that pure MFEO and MFEO with 1% of magnesium aluminometasilicate had similar antioxidant activity for EO concentrations of 0.2–20%. Also, this study noted that antioxidant activity was increased in a dose‐dependent manner.
In another study, Adiani et al. (2015) assessed the antioxidant potential of MFEO bioactive constituents like elemicin, myristicin, 4‐terpineol, and sabinene, and among them, elemicin was an effective antioxidative agent confirmed by DPPH assay. In a recent study, MFEO with an EC50 dosage of 1.35 mg/ml showed significant antioxidant activity after 20 min of incubation (Nikolic et al., 2021). However, most studies of antioxidant activity based on chemical testing, such as DPHH assays, are no longer relevant to pharmacology. For these effects to be regarded as clinically relevant, their potential under in vitro conditions must be investigated (Harnly, 2017).
4.2. Antimicrobial activity
Nowadays, the utilization of EO and its natural compounds for controlling food‐borne bacteria and fungi microorganisms in food and by‐products have increased the demand for food industries due to its green policies. The essential oils of spices have been reported to inhibit microorganism growth (Ashokkumar et al., 2020a, 2020b, 2021b) and are insoluble in water. To date, only a few studies were conducted to investigate the antimicrobial activity of MFEO. The MFEO with 0.5% concentration was sufficient to complete suppression in the growth of E. faecalis, and S. mutans even 0.2% concentration effective to suppression of Pasteurella multocida growth. Thus, this study results also suggested that the combination of MFEO and magnesium aluminometasilicate (1%) widens the spectrum of antimicrobial activity (Matulyte et al., 2020). According to Soni, Sharma, and Jasuja (2016), dilution of MFEO from 8 to 14 μl (25% v, v) had significant antibacterial activity against Gram‐negative bacterial strains E. coli, P. vulgaris, and K. pneumoniae and Gram‐positive bacterial strains like S. aureus, and B. subtilis using agar well‐diffusion method. Özkan et al. (2018) also found that the EO of nutmeg has potent antibacterial activity against S. typhimurium with MIC value of 1.6 μg/ml.
Nurjanah, Putri, and Sugiarti (2017) examined Indonesia grown nutmeg seed oil for antibacterial activity by disc diffusion method using different concentrations of MFEO, 20, 40, 60, 80, and 100%, and highest inhibition zone was observed on 60% concentration for S. aureus, S. epidermis, S. dysenteriae, and S. typhi. The Brazilian nutmeg EO (MIC: 0.1%) had inhibited the growth of Colletotrichum gloeosporioides (98%), C. musa (97%), Fusarium oxysporum (75%), F. semitectum (78%), Aspergillus niger (71%), and A. glaucus (60%). Also, 0.3% concentration of MFEO increased the inhibition growth from 85% to 100% (Valente et al., 2011). In a recent study, Ansory et al. (2020) observed that Indonesian nutmeg EO, MIC: 1.2% and MBC: 2.5%, substantially inhibited Shigella sp. growth. The majority of studies focusing on the antimicrobial activity of MFEO have been conducted using the disc diffusion method. However, because of its flaws, the method needs to be paired with the more relevant MIC assay (Van Vuuren, 2008).
4.3. Antiinflammatory and analgesic activity
Inflammation plays an imperative role in the human body. The antiinflammatory and analgesic effects of MFEO were investigated using rats injected with Complete Freund's adjuvant (30 mg/kg/day). Daily intraperitoneal injections of nutmeg oil [CFA + Nitric oxide (NO) high, 20 mg/kg/day and CFA + NO low, 10 mg/kg/day] may reduce CFA injection caused by joint swelling, mechanical allodynia, and heat hyperanalgesia in rats by inhibiting COX‐2 expression and blood substance P levels (Zhang et al., 2016). In a recent study, Matulyte et al. (2020) investigated the antiinflammatory activity of MFEO and hydrolats in the hTERT‐immortalized foreskin fibroblast cell line, BJ‐5ta, treated with viral dsRNR mimetic Poly I: C. The study results reported that both the MFEO and hydrolats had shown significant antiinflammatory effects. Only a few studies have looked into MFEO's antiinflammatory and analgesic properties, and they were all done in animal models rather than humans. Future research should look into the bioactivity of MFEO in different clinical studies with people.
4.4. Insecticidal and nematicide activities
Gotke, Maheshwari, and Mathur (1990) noticed that the mace oil effectively controlled root‐knot nematode (Meloidogyne incognita) than nutmeg kernel oil. MFEO substantially inhibits T. castaneum eggs hatching and following survival of larvae in the concentration range 1.4–3.2 mg/cm2. The production of F1 progeny was completely suppressed at MFEO concentration of 0.35 g/100 g wheat for Sitophilus zeamais, and 1.05 g/100 g rice for Tribolium castaneum. The feeding deterrence index 7% and 33% was observed for Tribolium castaneum and Sitophilus zeamais, respectively, at a concentration of 20 g nutmeg oil/100 ml (Huang, Tan, Kini, & Ho, 1997). The concentration of 10 mg/ml of nutmeg EO revealed highest fumigant action and contact toxicity against whitefly adults and whitefly nymphs, respectively (Wagan, Wang, Hua, & Cai, 2017). MFEO also had significant antifeedant activity against larvae of Lymantria dispar (Kostic et al., 2013). The oviposition of cowpea storage bruchid (Callosobruchus maculates) was effectively inhibited by nutmeg oil, and 60% mortality and 85% mortality were observed at 2% dosage on the 3rd day after application and 7‐day post‐treatment, respectively. Another study also reported that MFEO inhibited adult emergence of C. maculates (Adedire, 2002). In a recent study, adult house fly (Musca domestica) and Chrysomya albiceps were subjected to MFEO impregnated paper with LC50 values of 2.74 g/ml and 3.65 g/ml, respectively. As a result, the findings demonstrated that MFEO has insecticidal activity and can be utilized to control M. domestica and C. albiceps (Cossetin et al., 2021). So far, only a few studies have investigated the insecticidal properties of MFEO, so more research is needed in this promising biological field.
4.5. Miscellaneous activities
Myristicin is one of the vital chemical compounds of MFEO. Ingestion of myristicin attributed various antagonistic effects, including vomiting, gastrointestinal, tiredness, cardiovascular, and hypotension (Grover, Khandkar, Vats, Dhunnoo, & Das, 2002). In mice, myristicin‐suppressed lipopolysaccharide/d‐galactosamine (LPS/D‐GalN) induced increases in serum TNF and hepatic DNA fragmentation, indicating that it possesses significant hepatoprotective effect (Morita et al., 2003). Besides, myristicin had potential apoptotic, anthelmintic, and insecticidal activities (Lopez et al., 2015; Martins et al., 2014). Furthermore, most of these effects are limited in the literature, with no logical follow‐up investigations that further validate outcomes. MFEO showed antiamoebic activity against Entamoeba hystolytica (De Blasi, Debrot, Menoud, Gendre, & Schowing, 1990). In another study, MFEO showed significant antiangiogenic activity with IC50 of 77.6 μg/ml compared with Morida citrifolia oil which exhibits IC50 of 109.3 μg/ml (Piaru et al., 2012a). At a concentration of 200 g/ml, MFEO has significant antiangiogenic properties. Antiangiogenesis is a process that inhibits the creation of new blood vessels in tumors in order to slow their growth (Al‐Rawi et al., 2011). Kholibrina and Aswandi (2021) reported that the combined EOs of nutmeg, citronella, and benzoin significantly reduced hypertension. However, this study lacks systematic experiment protocols like positive and negative control and dosages. Therefore, future studies need to conduct the experiment as systematically as possible to confirm the hypertensive activity of the MFEO. Furthermore, some additional pharmacological potential of MFEO, and their active constituents was also summarized in Table 4.
5. CONCLUSION AND FUTURE OUTLOOKS
In this review, we summarize the knowledge of botanical description, oil yield, chemical composition and biological activities of M. fragrans EO. The plant essential oil yield and chemical compositions focused on mace, leaves, and kernels, but barks and roots have been neglected or have received less attention. Among the various essential oil extraction methods, hydrodistillation is predominantly used for essential oil extraction. Chromatographic techniques are used for the identification of chemical constituents from essential oil. The primary chemical constituents of MFEO were sabinene, eugenol, myristicin, caryophyllene, β‐myrcene, α‐pinene, β‐pinene, D‐limonene, and 3‐carene. These predominant chemical constituents of nutmeg can serve as a novel potential natural source, which can be used for food, perfumery, and pharmaceutical industries. Our literature survey found that MFEO can protect people from several diseases due to their potent biological activities. Among the previous biological and pharmacological studies, most of them did not report positive and negative controls details (Tables 3 and 4). Hence, future studies need to conduct systematic studies using cell and animal models. Clinical and experimental investigations have confirmed the antioxidant, antibacterial, antimalarial, antifungal, anticonvulsant, antiinflammatory, analgesic, anticancer, apoptotic, anthelmintic, antiangiogenic, antiamoebic, and insecticidal activities of MFEO. However, these clinical studies were conducted only in animals and cell lines, and no human clinical trials have been conducted. As a result, future research should concentrate on MFEO's and their active constituents' pharmacological activities in humans. Future research should look at the toxicity, bioavailability, and pharmacokinetics of MFEO to find the chemical components responsible for its activities and expand the existing medical application of M. fragrans. We believe that the information provided or discussed here will increase public awareness of MFEO and be valuable for future research.
AUTHOR CONTRIBUTIONS
Kaliyaperumal Ashokkumar, Jesus Simal‐Gandara, and Muthusamy Murugan conceived and designed the review. Kaliyaperumal Ashokkumar and Jesus Simal‐Gandara wrote the manuscript. Mannananil Krishnankutty Dhanya and Arjun Pandian collected literature and drew molecular structures of major nutmeg essential oil compounds by ChemDraw software. Jesus Simal‐Gandara and Muthusamy Murugan edited the manuscript. All the authors reviewed and approved this version of the manuscript.
FUNDING INFORMATION
Funding for open access charge: Universidade de Vigo/CISUG.
CONFLICT OF INTEREST
The authors declare no conflict of interest in this article.
Ashokkumar, K. , Simal‐Gandara, J. , Murugan, M. , Dhanya, M. K. , & Pandian, A. (2022). Nutmeg ( Myristica fragrans Houtt.) essential oil: A review on its composition, biological, and pharmacological activities. Phytotherapy Research, 36(7), 2839–2851. 10.1002/ptr.7491
Funding information Universidade de Vigo
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abourashed, E. A. , & El‐Alfy, A. T. (2016). Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt.). Phytochemistry Reviews, 15(6), 1035–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adedire, C. (2002). Use of nutmeg Myristica fragrane (Houtt.) powder and oil for the control of cowpea storage bruchid, Callosobruchus maculatus Fabricius. Journal of Plant Diseases and Protection, 109(2), 193–199. [Google Scholar]
- Adiani, V. , Gupta, S. , Chatterjee, S. , Variyar, P. S. , & Sharma, A. (2015). Activity guided characterization of antioxidant components from essential oil of nutmeg (Myristica fragrans). Journal of Food Science and Technology, 52, 221–230. 10.1007/s13197-013-1034-7 [DOI] [Google Scholar]
- Alibabaie, M. , & Safaralizadeh, M. (2015). Fumigant toxicity of nutmeg seed essential oil (Myristica fragrans Houtt.) (MF, Myristicaceae) on cowpea weevil, Callosobruchus maculatus F. (Coleoptera: Bruchidae). In Chakravarthy A. (Ed.), New horizons in insect science: Towards sustainable pest management (pp. 127–133). New Delhi: Springer. [Google Scholar]
- Al‐Jumaily, E. F. , & Al‐Amiry, M. H. A. (2012). Extraction and purification of terpenes from nutmeg (Myristica fragrans). Journal of Al‐Nahrain University, 15(3), 151–160. [Google Scholar]
- Al‐Rawi, S. S. , Ibrahim, A. H. , Abdur Rahman, N. N. N. , Nama, M. M. B. , Majid, A. M. S. A. , & Kadir, M. O. (2011). The effect of supercritical fluid extraction parameters on the nutmeg oil extraction and its cytotoxic and antiangiogenic properties. Procedia Food Science, 1, 1946–1952. [Google Scholar]
- Ansory, H. M. , Fitriani, I. N. , & Nilawati, A. (2020). Chemical separation and antibacterial activity of nutmeg seed essential oil against Shigella sp. and Escherichia coli ATCC 25922. Materials Science and Engineering, 846, 012005. 10.1088/1757-899X/846/1/012005 [DOI] [Google Scholar]
- Asgarpanah, J. , & Kazemivash, N. (2012). Phytochemistry and pharmacologic properties of Myristica fragrans Hoyutt.: A review. African Journal of Biotechnology, 11(65), 12787–12793. [Google Scholar]
- Ashokkumar, K. , Murugan, M. , Dhanya, M. K. , Sathyan, T. , Raj, S. , & Nimisha, M. (2020). Traditional uses, phytochemistry and pharmacological properties of Zingiber officinale essential oil and extracts. In Mishra N. (Ed.), Ethnopharmacological investigation of Indian spices (pp. 62–84). Hershey, PA: IGI Global Publisher. 10.4018/978-1-7998-2524-1.ch005 [DOI] [Google Scholar]
- Ashokkumar, K. , Murugan, M. , Dhanya, M. K. , & Warkentin, T. D. (2020). Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. Journal of Ethnopharmacology, 246, 112244. 10.1016/j.jep.2019.112244 [DOI] [PubMed] [Google Scholar]
- Ashokkumar, K. , Murugan, M. , Dhanya, M. K. , & Warkentin, T. D. (2021). Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin Phytoscience, 7(52). 10.1186/s40816-021-00292-2 [DOI] [Google Scholar]
- Ashokkumar, K. , Vellaikumar, S. , Murugan, M. , Dhanya, M. K. , & Aiswarya, S. (2022). Compositional variation in the leaf, mace, kernel, and seed essential oil of nutmeg (Myristica fragrans Houtt.) from the Western Ghats, India. Natural Product Research, 36(1), 432–435. 10.1080/14786419.2020.1771713 [DOI] [PubMed] [Google Scholar]
- Ashokkumar, K. , Vellaikumar, S. , Murugan, M. , Dhanya, M. K. , Karthikeyan, A. , Ariharasutharsan, G. , … Aiswarya, S. (2021). GC/MS analysis of essential oil composition from selected seed spices. National Academy Science Letters, 44, 503–506. 10.1007/s40009-021-01066-7 [DOI] [Google Scholar]
- Atta‐ur‐Rahman , Choudhary, M. L. , Farooq, A. , Ahmed, A. , Zafar, M. I. , Demirci, B. , … Baser, K. H. C. (2000). Antifungal activities and essential oil constituents of some spices from Pakistan. Journal of the Chemical Society of Pakistan, 22, 60–65. [Google Scholar]
- Azwanida, N. N. (2015). A review on the extraction methods use in medicinal plants, principle strength and limitation. Medicinal & Aromatic Plants, 4, 3–8. [Google Scholar]
- Barceloux, D. G. (2009). Nutmeg (Myristica fragrans Houtt.). Disease‐a‐Month, 55, 373–379. [DOI] [PubMed] [Google Scholar]
- Carolina, A. , & Maman, M. (2016). Larvicidal activity of essential oils from the leaves and fruits of nutmeg (Myristica fragrans Houtt) against Aedes aegypti (Diptera: Culicidae) . Turkish Journal of Agriculture‐Food Science and Technology, 4(7), 552–556. [Google Scholar]
- Cossetin, L. F. , Santi, E. M. T. , Garlet, Q. I. , Matos, A. F. I. M. , De Souza, T. P. , Loebens, L. , … Monteiro, S. G. (2021). Comparing the efficacy of nutmeg essential oil and a chemical pesticide against Musca domestica and Chrysomya albiceps for selecting a new insecticide agent against synantropic vectors. Experimental Parasitology, 225, 108104. 10.1016/j.exppara.2021.108104 [DOI] [PubMed] [Google Scholar]
- Cui, H. , Zhang, X. , Zhou, H. , Zhao, C. , Xiao, Z. , Lin, L. , & Li, C. (2015). Antibacterial properties of nutmeg oil in pork and its possible mechanism. Journal of Food Safety, 35(3), 370–377. [Google Scholar]
- Das, S. , Kumar Singh, V. , Kumar Dwivedy, A. , Kumar Chaudhari, A. , Upadhyay, N. , Singh, A. , … Dubey, N. K. (2020). Assessment of chemically characterised Myristica fragrans essential oil against fungi contaminating stored scented rice and its mode of action as novel aflatoxin inhibitor. Natural Product Research, 34(11), 1611–1615. 10.1080/14786419.2018.1519826 [DOI] [PubMed] [Google Scholar]
- De Blasi, V. , Debrot, S. , Menoud, P. A. , Gendre, L. , & Schowing, J. (1990). Amoebicidal effect of essential oils in vitro. Journal of Toxicology and Clinical Experimental, 10(6), 361–373. [PubMed] [Google Scholar]
- Dhamayanthi, K. P. M. , & Krishnamoorthy, B. (1999). Somatic chromosome number in nutmeg (Myristica fragrans Houtt.). Journal of Spices and Aromatic Crops, 8(2), 205–2206. [Google Scholar]
- Dupuy, N. , Molinet, J. , Mehl, F. , Nanlohy, F. , Le Dréau, Y. , & Kister, J. (2013). Chemometric analysis of mid infrared and gas chromatography data of Indonesian nutmeg essential oils. Industrial Crops and Products, 43, 596–601. [Google Scholar]
- Francis, S. K. , James, B. , Varughese, S. , & Nair, M. S. (2019). Phytochemical investigation on Myristica fragrans stems, bark. Natural Product Research, 33, 1204–1208. [DOI] [PubMed] [Google Scholar]
- Gils, C. V. , & Cox, P. A. (1994). Ethnobotany of nutmeg in the Spice Islands. Journal of Ethnopharmacology, 42, 117–124. [DOI] [PubMed] [Google Scholar]
- Gotke, N. , Maheshwari, M. L. , & Mathur, V. K. (1990). Nematicidal activity of Myristica fragrans against Meloidogyne incognita . Indian Perfumer, 34(2), 105–107. [Google Scholar]
- Grover, J. K. , Khandkar, S. , Vats, V. , Dhunnoo, Y. , & Das, D. (2002). Pharmacological studies on Myristica fragrans antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods and Findings in Experimental and Clinical Pharmacology, 24, 675–680. [DOI] [PubMed] [Google Scholar]
- Gupta, A. D. , Bansal, V. K. , Babu, V. , & Maithil, N. (2013). Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). Journal of Genetic Engineering & Biotechnology, 11, 25–31. [Google Scholar]
- Gupta, E. (2020). Elucidating the phytochemical and pharmacological potential of Myristica fragrans (nutmeg). In Mishra N. (Ed.), Ethnopharmacological investigation of Indian spices (pp. 52–61). Hershey, PA: IGI Global Publisher. [Google Scholar]
- Hanif, M. A. , Bhatti, H. N. , Jamil, M. S. , Anjum, R. M. , Jamil, A. , & Khan, M. M. (2010). Antibacterial and antifungal activities of essential oils extracted from medicinal plants using CO2 supercritical fluid extraction technology. Asian Journal of Chemistry, 22(10), 7787–7798. [Google Scholar]
- Haldankar, P.M. , Khandekar, R.G. , & Joshi, G.D. (2007). Effect of growth regulators on germination and seedling growth in nutmeg. South Indian Horticulture, 55, 315‐319. [Google Scholar]
- Harnly, J. (2017). Antioxidant methods. Journal of Food Composition and Analysis, 64, 145–146. [Google Scholar]
- Hiranrat, A. , & Hiranrat, W. (2019). Myristigranol, a new diarylpropane derivative from the wood of Myristica fragrans . Natural Product Research, 33, 2958–2963. [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Tan, J. M. W. L. , Kini, R. M. , & Ho, S. H. (1997). Toxic and antifeedant action of nutmeg oil against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Journal of Stored Products Research, 33(4), 289–298. [Google Scholar]
- Ibrahim, M. A. , Cantrell, C. L. , Jeliazkova, E. A. , Astatkie, T. , & Zheljazkov, V. D. (2020). Utilization of nutmeg (Myristica fragrans Houtt.) seed hydrodistillation time to produce essential oil fractions with varied compositions and pharmacological effects. Molecules, 25(3), 565. 10.3390/molecules25030565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M. R. , Muthuraju, S. , Tarmizi, C. H. , Zulkifli, M. M. , Osman, H. , Mohamad, H. , & Abdullah, J. M. (2012). Anticonvulsant activity of α‐terpineol isolated from Myristica fragrans in GAERS model of absence epilepsy. ASM Science Journal, 6, 95–102. [Google Scholar]
- Jaiswal, P. , Kumar, P. , Singh, V. K. , & Singh, D. K. (2009). Biological effects of Myristica fragrans . RBS Annual Review of Biomedical Sciences, 11, 21–29. [Google Scholar]
- Kholibrina, C. R. , & Aswandi, A. (2021). The aromatherapy formulation of essential oils in reducing stress and blood pressure on human. IOP Conference Series: Earth and Environmental Science, 914, 012072. 10.1088/1755-1315/914/1/012072 [DOI] [Google Scholar]
- Kostic, I. , Petrovic, O. , Milanovic, S. , Popovic, Z. , Stankovic, S. , Todorovic, G. , & Kostic, M. (2013). Biological activity of essential oils of Athamanta haynaldii and Myristica fragrans to gypsy moth larvae. Industrial Crops and Products, 41, 17–20. [Google Scholar]
- Lopez, V. , Gerique, J. , Langa, E. , Berzosa, C. , Valero, M. S. , & Gomez‐Rincon, C. (2015). Antihelmintic effects of nutmeg (Myristica fragrans) on Anisakis simplex L3 larvae obtained from Micromesistius potassou . Research in Veterinary Science, 100, 148–152. [DOI] [PubMed] [Google Scholar]
- Malik, K. , Ahmad, M. , Bussmann, R. W. , Tariq, A. , Ullah, R. , Alqahtani, A. S. , … Shah, S. N. (2018). Ethnobotany of anti‐hypertensive plants used in northern Pakistan. Frontiers in Pharmacology, 9, 789. 10.3389/fphar.2018.00789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, C. , Doran, C. , Silva, I. C. , Miranda, C. , Rueff, J. , & Rodrigues, A. S. (2014). Myristicin from nutmeg induces apoptosis via the mitochondrial pathway and down regulates genes of the DNA damage response pathways in human leukaemia K562 cells. Chemico‐Biological Interactions, 218, 1–9. [DOI] [PubMed] [Google Scholar]
- Matulyte, I. , Jekabsone, A. , Jankauskaite, L. , Zavistanaviciute, P. , Sakiene, V. , Bartkiene, E. , … Bernatoniene, J. (2020). The essential oil and hydrolats from Myristica fragrans seeds with magnesium aluminometasilicate as excipient: Antioxidant, antibacterial, and anti‐inflammatory activity. Food, 9(1), 37. 10.3390/foods9010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickus, R. , Jančiukė, G. , Raškevičius, V. , Mikalayeva, V. , Matulytė, I. , Marksa, M. , … Skeberdis, V. A. (2021). The effect of nutmeg essential oil constituents on Novikoff hepatoma cell viability and communication through Cx43 gap junctions. Biomedicine & Pharmacotherapy, 135, 111229. 10.1016/j.biopha.2021.111229 [DOI] [PubMed] [Google Scholar]
- Morita, T. , Jinno, K. , Kawagishi, H. , Arimoto, Y. , Suganuma, H. , Inakuma, T. , & Sugiyama, K. (2003). Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d‐galactosamine‐induced liver injury. Journal of Agricultural and Food Chemistry, 51, 1560–1565. [DOI] [PubMed] [Google Scholar]
- Muchtaridi , Subarnas, A. , Apriyantono, A. , & Mustarichie, R. (2010). Identification of compounds in the essential oil of nutmeg seeds (Myristica fragrans Houtt.) that inhibit locomotor activity in mice. International Journal of Molecular Sciences, 11, 4771–4781. 10.3390/ijms11114771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad, I. S. , Mahmood, A. , Ayesha, R. , Zahoor, Q. S. , Muhammad, A. Q. , Sania, M. , & Amir, A. (2016). Chemical composition of the essential oils of nutmeg and mace by GC‐FID/MS indigenous to Pakistan and evaluation of their biological activities. Latin American Journal of Pharmacy, 35(10), 2176–2184. [Google Scholar]
- Naeem, N. , Rehman, R. , Mushtaq, A. , & Ghania, J. B. (2016). Nutmeg: A review on uses and biological properties. International Journal of Chemical and Biochemical Sciences, 9, 107–110. [Google Scholar]
- Nair, R. R. (2019). Chromosome number is different sex types and open pollinated seedlings of nutmeg (Myristica fragrans Houtt.). Journal of Plantation Crops, 47(3), 197–201. [Google Scholar]
- Nikolic, V. , Nikolic, L. , Dinic, A. , Gajic, I. , Urosevic, M. , Stanojevic, L. , … Danilovic, B. (2021). Chemical composition, antioxidant and antimicrobial activity of nutmeg (Myristica fragrans Houtt.) seed essential oil. Journal of Essential Oil‐Bearing Plants, 24(2), 218–227. 10.1080/0972060X.2021.1907230 [DOI] [Google Scholar]
- Nurjanah, S. , Putri, I. L. , & Sugiarti, D. P. (2017). “Antibacterial activity of nutmeg oil” in 2nd international conference on sustainable agriculture and food security: A comprehensive approach. KnE Life Sciences, 2(3), 563–569. doi: 10.18502/kls.v2i6.1076 [DOI] [Google Scholar]
- Ogunwande, I. A. , Olawore, N. O. , Adeleke, K. A. , & Ekundayo, O. (2003). Chemical composition of essential oil of Myristica fragrans Houtt (nutmeg) from Nigeria. Journal of Essential Oil‐Bearing Plants, 6, 21–26. [Google Scholar]
- Özkan, O. E. , Olgun, Ç. , Güney, B. , Gür, M. , Güney, K. , & Ateş, S. (2018). Chemical composition and antimicrobial activity of Myristica fragrans & Elettaria cardamomum essential oil. Kastamonu Uni., Orman Fakültesi Dergisi, 18(2), 225–229. [Google Scholar]
- Periasamy, G. , Karim, A. , Gibrelibanos, M. , Gebremedhin, G. , & Gilani, A. H. (2016). Nutmeg (Myristica fragrans Houtt.) oils. In Preedy V. R. (Ed.), Essential oils in food preservation, flavor and safety (pp. 607–616). New York, NY: Academic Press. [Google Scholar]
- Piaru, S. P. , Mahmud, R. , Abdul Majid, A. M. , & Mahmoud Nassar, Z. D. (2012). Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia . Asian Pacific Journal of Tropical Medicine, 5(4), 294–298. 10.1016/S1995-7645(12)60042-X [DOI] [PubMed] [Google Scholar]
- Piaru, S. P. , Mahmud, R. , & Perumal, S. (2012). Determination of antibacterial activity of Myristica fragrans Houtt. Using tetrazolium microplate assay and its cytotoxic activity against Vero cell line. International Journal of Pharmacology, 8(6), 572–576. 10.3923/ijip.2012.572.576 [DOI] [Google Scholar]
- Piras, A. , Rosa, A. , Marongiu, B. , Atzeri, A. , Dess'i, M. A. , Falconieri, D. , & Porcedda, S. (2012). Extraction and separation of volatile and fixed oils from seeds of Myristica fragrans by supercritical CO2: Chemical composition and cytotoxic activity on Caco‐2 cancer cells. Journal of Food Science, 77(4), C448–C453. 10.1111/j.1750-3841.2012.02618.x [DOI] [PubMed] [Google Scholar]
- Purkait, S. , Bhattacharya, A. , Bag, A. , & Chattopadhyay, R. R. (2018). Antibacterial and antioxidant potential of essential oils of five spices. Journal of Food Quality and Hazards Control, 5, 61–71. [Google Scholar]
- Purseglove, J. W. , Brown, E. G. , Green, C. L. , & Robbins, S. R. J. (1981). Nutmeg and Mace (pp. 174–228). Longman, London: Spices. [Google Scholar]
- Sagarika, N. , Prince, M. V. , Kothakota, A. , Pandiselvam, R. , Sreeja, R. , & Mathew, S. M. (2018). Characterization and optimization of microwave assisted process for extraction of nutmeg (Myristica fragrans Houtt.) mace essential oil. Journal of Essential Oil‐Bearing Plants, 21(4), 895–904. 10.1080/0972060X.2018.1517613 [DOI] [Google Scholar]
- Sarath‐Kumara, S. J. , Jans, E. R. , & Dharmadasa, H. M. (1985). Some physical and chemical characteristics of Sri Lankan nutmeg oil. Journal of the Science of Food and Agriculture, 36, 93–100. [Google Scholar]
- Shu‐Shan, D. , Kai, Y. , Cheng‐Fang, W. , Chun‐Xue, Y. , Zhu‐Feng, G. , Shan‐Shan, G. , … Zhi‐Long, L. (2014). Chemical constituents and activities of the essential oil from Myristica fragrans against cigarette beetle Lasioderma serricorne . Chemistry and Biodiversity, 11, 1449–1456. [DOI] [PubMed] [Google Scholar]
- Soni, R. , Sharma, G. , & Jasuja, N. D. (2016). Essential oil yield pattern and antibacterial and insecticidal activities of Trachyspermum ammi and Myristica fragrans . Scientifica, 2016, 1428194. 10.1155/2016/1428194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thileepan, T. , Thevanesam, V. , & Kathirgamanathar, S. S. (2018). Anticandidal activity of essential oils of Myristica fragrans and Syzygium aromaticum . Journal of Innovations in Pharmaceutical and Biological Sciences, 5(3), 35–38. [Google Scholar]
- Valente, V. M. M. , Jham, G. N. , Dhingra, O. D. , & Ghiviriga, I. (2011). Composition and antifungal activity of the Brazilian Myristica fragrans Houtt essential oil. Journal of Food Safety, 31, 197–202. [Google Scholar]
- Valente, V. M. M. , Jham, G. N. , Jardim, C. M. , Dhingra, O. D. , & Ghiviriga, I. (2015). Major antifungals in nutmeg essential oil against aspergillus flavus, and A. ochraceus . Journal of Food Research, 4(1), 51–57. [Google Scholar]
- Van Vuuren, S. F. (2008). Antimicrobial activity of south African medicinal plants. Journal of Ethnopharmacology, 119, 462–472. [DOI] [PubMed] [Google Scholar]
- Wagan, T. A. , Wang, W. , Hua, H. , & Cai, W. (2017). Chemical constituents and toxic, repellent, and oviposition‐deterrent effects of ethanol‐extracted Myristica fragrans (myristicaceae) oil on Bemisia tabaci (Hemiptera: Aleyrodidae). Florida Entomologist, 100(3), 594–601. [Google Scholar]
- Wahab, A. , Ul Haq, R. , Ahmed, A. , Khan, R. A. , & Raza, M. (2009). Anticonvulsant activities of nutmeg oil of Myristica fragrans . Phytotherapy Research, 23(2), 153–158. [DOI] [PubMed] [Google Scholar]
- Wallis, T. E. (1985). Text book of pharmacognosy (5th ed., p. 652). New Delhi, India: CBS Publishers and Distributors. [Google Scholar]
- Waman, A. A. (2020). Essential oil composition of clove and nutmeg from Andaman and Nicobar Islands, India. Indian Journal of Biochemistry & Biophysics, 57, 95–100. [Google Scholar]
- Zhang, W. K. , Tao, S. S. , Li, T. T. , Li, Y. S. , Li, X. J. , Tang, H. B. , … Wan, C. J. (2016). Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX‐2 expression and substance P release in vivo. Food & Nutrition Research, 60(1), 30849. 10.3402/fnrv60.30849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyatdinova, G. , Ziganshina, E. , Cong, P. N. , & Budnikov, H. (2016). Ultrasound‐assisted micellar extraction of phenolic antioxidants from spices and antioxidant properties of the extracts based on coulometric titration data. Analytical Methods, 8, 7150–7157. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


